Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a highly debilitating disease with heterogeneous constitutional and neurological complaints. Infection-like symptoms often herald disease onset, but no pathogen or immune defect has been conclusively linked. In this issue of the JCI, Mandarano et al. illuminate bioenergetic derangements of ME/CFS T cell subsets. CD4+ and CD8+ T cells had impaired resting glycolysis. CD8+ cells additionally showed activation-related metabolic remodeling deficits and decreased mitochondrial membrane potential; a subset had increased resting mitochondrial mass. Immune senescence and exhaustion paradigms offer only partial explanations. Hence, unique mechanisms of disrupted immunometabolism may underlie the complex neuroimmune dysfunction of ME/CFS.
The case for neuroimmune dysregulation in disease
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a common but underdiagnosed multisystem and disabling disease. Diagnoses are established clinically by the presence of unexplained severe fatigue, postexertional malaise, and unrefreshing sleep with orthostatic intolerance or cognitive impairment. At illness onset, infection-like signs and symptoms (fever, lymphadenopathy, sore throat, myalgia, general malaise) often make an acute and occasionally dramatic entrance and sometimes persist or recur, supporting the concept that some portion of disease risk represents an infectious process or host immune vulnerability. Immune, inflammatory, infectious, and allergic processes are frequent themes connecting the diverse clinical features additionally present in ME/CFS (gastrointestinal, pain, skin/mast cell abnormalities) (1).
An immune or infectious diathesis is also supported by spatiotemporal clustering of outbreaks as well as alteration of immune cell numbers and function (e.g., NK cells), immune profiles, and autoimmune parameters (2, 3). Serologic and molecular studies have as yet failed to confirm a specific infectious trigger. Certain infectious agents (e.g., Epstein-Barr virus) are nonetheless commonly reported as disease precursors (3, 4). The presence of infection-like features in some patients with ME/CFS remains suggestive of enhanced vulnerability for dysregulated immune cell responses to infectious exposures. Alternatively, immune molecules secreted by infection-exposed immune cells may accentuate or prolong host CNS responses. The latter model of neuroimmune disruption as a potential contributor to ME/CFS pathogenesis resonates with animal model and human investigations seeking to understand “acute sickness responses” — a coordinated set of autonomic, physiologic, metabolic, inflammatory, neurocognitive, and neurovegetative responses to immune and infectious stimuli — and their role in disease and resiliency (5). Consistent with this model, “brain fog,” a component of neurocognitive impairment commonly occurring with infection that clouds mentation and limits attentional capacity, is one of the most frequently reported symptoms (up to 85% of subjects) and a major driver of ME/CFS disability (6). The exertion-sensitive neurocognitive impairment of ME/CFS may relate to deficits in cellular energy metabolism, as increased cerebrospinal fluid (CSF) lactate is reported (4). Additional metabolic defects potentially related to ME/CFS clinical features (postexertional malaise, irritable bowel syndrome) have also been identified through plasma metabolomics (4, 7–10).
T cell dysfunction: through the immunometabolism looking glass
The relatively recent lens of immunometabolism is rapidly illuminating molecular drivers that govern the metabolic reprogramming capacity of immune cells as they adapt to environmental challenges (microbial, traumatic, bioenergetic) (11). CD4+ and CD8+ T cells reprogram metabolism upon activation to increase glycolysis, forgoing the efficiency of oxidative phosphorylation to prioritize short-term biosynthetic needs to fuel rapid proliferation and effector function development. CD8+ cells typically undergo greater metabolic remodeling than do CD4+ cells (11–13).
Mandarano et al., in this issue of the JCI, use immunometabolism as a lens to focus on metabolic remodeling capacity in CD4+ and CD8+ T cell subsets in ME/CFS (14). Their findings indicate differential involvement of metabolic defects in CD4+ and CD8+ T cell subsets in ME/CFS. Mandarano and colleagues identify reduced mitochondrial membrane potential in ME/CFS CD8+ T cells both at rest and upon activation. CD8+ T cell subsets from patients with ME/CFS are more strikingly impaired than CD4+ subsets in their capacity to meet energy needs either at baseline (resting state) or upon activation with anti-CD3/anti-CD28 antibodies and IL-2. Metabolic deficiencies of CD4+ T cells in ME/CFS were instead largely restricted to glycolysis of resting CD4+ T cells. Prior work showed hypometabolism in different immune cell subsets (3, 4, 15); however, another study that failed to find changes in glycolysis used PBMCs (16). Although no differences in mitochondrial mass were found in either CD4+ or CD8+ cell subsets as a whole, a subset of resting CD8+ cells with increased mitochondrial mass was identified (14). The current study also reports differences in correlations of plasma cytokines with the assessed T cell metabolic parameters, often showing unanticipated inverse associations of proinflammatory cytokines with CD8+ glycolytic metabolism in patients with ME/CFS but not in controls (14). This evidence suggests unique patterns of impairment in mitochondrial and glycolytic metabolism in T cell subsets in ME/CFS.
Accumulating evidence illustrates the insights that immune cell metabolism can provide with respect to immune-mediated diseases (infection, autoimmunity, diabetes, obesity). Recent studies on immunometabolism of brain-resident immune cells (microglia) also support a possible role in neurodegenerative disorder pathogenesis (17). Although not specifically focused on immunometabolic control mechanisms, a new study of Alzheimer’s disease suggests that CD8+ effector memory CD45RA-reexpressing (EMRA) T cells are present in increased numbers in peripheral blood and CSF, associate inversely with impaired cognition, and have enhanced T cell receptor signaling (18). This highly differentiated T cell subset typically displays several senescence characteristics, including decreased proliferation and telomerase activity and increased DNA damage (13). Far less is known about the role of immune cell metabolism in complex disorders affecting both peripheral systems and the brain. The current work from the Hanson laboratory defines previously unexplored patterns in peripheral CD4+ and CD8+ T cell metabolism, while raising fundamental questions about underlying mechanisms. Notably, these observed differences were not explained by changes in activation-induced expression of the early activation marker CD69 or GLUT1, the surface glucose transporter.
CD8+ T cell metabolic impediments: immune senescence, exhaustion, or aging?
ME/CFS shares features with disorders that are accompanied by accelerated aging. One feature of senescence, premature telomere attrition, was found in ME/CFS, particularly among women younger than 45 years (19). Although different T cell subsets (e.g., exhaustion-prone CD8+ T cell subsets, as in chronic viral infection) might have driven this finding, the studies used DNA derived from whole blood, not isolated immune cells. It is also possible that illness duration was greater in this younger subset of women with ME/CFS despite their younger chronological age. Factors associated with long-term illness might promote more prominent reductions in telomere length. In a study of plasma immune profiles, controlling for age, patients with ME/CFS with a longer duration of illness (>3 years) had lower levels of immune molecules relative to those of patients with short-duration disease (≤3 years) and healthy controls (20). Reductions in circulating immune molecules among patients with long-duration ME/CFS suggested the possibility of immune exhaustion, potentially reflecting recurrent episodes of viral reactivation or other prolonged antigenic challenge, but contributions from immunosenescence are undetermined. It is unclear whether the CD8+ T cell metabolic defects observed here relate to immunosenescence. Vulnerability to immunosenescence varies for CD4+ and CD8+ T cells, with CD8+ T cells acquiring an immunosenescent phenotype more rapidly than their CD4+ T cell counterparts. Greater mitochondrial mass of CD4+ T cells is implicated, but senescent CD4+ EMRA T cells also take up more lipid and glucose than do CD8+ EMRA T cells, promoting metabolic flexibility in fueling cellular processes in CD4+ T cells through either oxidative or glycolytic metabolism and facilitating proliferation (13). Age effects may also occur independently of disease duration or processes that accelerate the acquisition of senescence characteristics. A study of naive CD8+ T cells from older individuals found reduced mitochondrial respiration despite greater mitochondrial mass in older individuals’ cells (12). Age, independent of factors regulating immune senescence or exhaustion, may compromise the metabolic state of old naive CD8+ T cells and diminish the capacity for clonal expansion in the course of immune responses.
The significance of the reduced mitochondrial membrane potential in CD8+ T cells that Mandarano and colleagues observed is unclear (14). Such reductions are characteristic of T cell exhaustion and can be observed with chronic viral infection (13). A proposed role for chronic antigenic stimulation in ME/CFS (via viral reactivation, intracellular bacteria, or pathogen- or danger-associated molecular patterns) raises the alternate possibility that ME/CFS immune cells may be more likely to become exhausted. Hyperpolarization of mitochondrial membranes is maintained through increased local production of reactive oxygen species (ROS). ROS have adverse effects as well as essential anti-infective roles. Reduced mitochondrial membrane potential in CD8+ T cells might also reflect “reductive stress,” impairing CD8+ T cell control over intracellular pathogens or repair mechanisms. Differences in redox regulation are also possible.
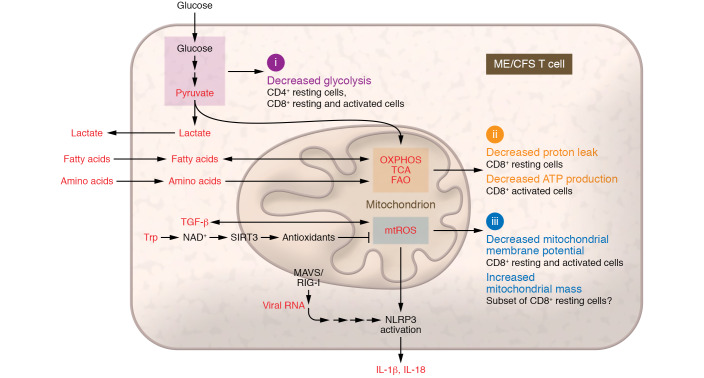
The interplay of metabolic regulators and mediators of the innate immune system in fine-tuning energy substrate consumption and conservation pathways may help illuminate how these processes become impaired in chronic viral infections, cancer, and autoimmune diseases. Energy-intensive dynamic adaptations to fluctuating energy supply and demand must strike a balance between anabolic and catabolic processes; mitochondrial processes require tightly controlled structural remodeling (fission/fusion) to maintain supplies of fuel sources in a metabolically efficient manner that is responsive to the micro- and macroenvironments (Figure 1).
Figure 1. A model of potential metabolic and mitochondrial derangements in CD4+ and/or CD8+ T cell subsets from patients with ME/CFS.
Affected T cells show (i) deficits in basal glycolysis that may interplay with (ii) abnormalities in mitochondrial metabolism, with decreased proton leak at rest and reduced ATP production in the CD8+ subset upon activation. (iii) Resting and activated CD8+ cells also show decreased mitochondrial membrane potential, and a resting CD8+ subset may display increased mitochondrial mass. Alterations in factors that have been previously shown (red) suggest that deficits in bioenergetics, metabolic reprogramming, and mitochondrial dynamics may vary across more specific CD4+ and CD8+ T cell subsets, including subsets with features of immune aging, senescence, or exhaustion (3, 4, 7–10, 15, 16). FAO, fatty acid oxidation; MAVS, mitochondrial antiviral signaling protein; mtROS, mitochondrial reactive oxygen species; NAD+, nicotinamide adenine dinucleotide; NLRP3, NOD-, LRR- and pyrin domain–containing protein 3 (inflammasome); OXPHOS, oxidative phosphorylation; RIG-I, retinoic acid–inducible gene I; SIRT3, NAD-dependent deacetylase sirtuin-3; TCA, tricarboxylic acid cycle; TGF-β, transforming growth factor β; Trp, tryptophan.
Conclusions
Researchers from the Hanson laboratory extend immunometabolism approaches to CD4+ and CD8+ T cell subsets in ME/CFS, providing important insights into potential cell subset–specific defects in bioenergetics and mitochondrial function. T cell subset–specific assays showed both CD4+ and CD8+ T cells to be hypometabolic at rest with reduced glycolysis; CD8+ T cells were additionally impaired in activated mitochondrial respiratory and glycolytic responses. CD8+ cells as a whole also had reduced mitochondrial membrane potential (14). Patterns of metabolic dysfunction in CD4+ and CD8+ T cells overlapped only partially with features of immunosenescence and immune exhaustion and could reflect the interaction of age with either of these processes after an inciting event. The contributions of intracellular signaling pathways and alternatively fueled pathways of energy metabolism merit further examination in conjunction with studies of additional immune cell subsets and a broader continuum of disease. Immunometabolism studies appear to hold promise for elucidating mechanisms that contribute to neuroimmune dysfunction in ME/CFS.
Acknowledgments
Support for related work from NIH grants U54 AI138370 and R56 AI120724, the Chronic Fatigue Initiative of the Hutchins Family Foundation, the Microbe Discovery Project, and The Korein Foundation is gratefully acknowledged.
Version 1. 02/10/2020
Electronic publication
Version 2. 03/02/2020
Print issue publication
Footnotes
Conflict of interest: The author is coinventor on a patent assigned to Columbia University titled “Autism-associated biomarkers and uses thereof” (US patent number 9050276) and on US patent applications (US patent application numbers 20170328917, 20150329909, 20150219674, and 20120207726).
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(3):1102–1105. https://doi.org/10.1172/JCI134985.
References
- 1.Haney E, et al. Diagnostic methods for myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med. 2015;162(12):834–840. doi: 10.7326/M15-0443. [DOI] [PubMed] [Google Scholar]
- 2.Curriu M, et al. Screening NK-, B- and T-cell phenotype and function in patients suffering from chronic fatigue syndrome. J Transl Med. 2013;11:68. doi: 10.1186/1479-5876-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweetman E, et al. Current research provides insight into the biological basis and diagnostic potential for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Diagnostics (Basel) 2019;9(3):E73. doi: 10.3390/diagnostics9030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomberg J, Gottfries CG, Elfaitouri A, Rizwan M, Rosén A. Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol. 2018;9:229. doi: 10.3389/fimmu.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piraino B, Vollmer-Conna U, Lloyd AR. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun. 2012;26(4):552–558. doi: 10.1016/j.bbi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komaroff AL, Buchwald D. Symptoms and signs of chronic fatigue syndrome. Rev Infect Dis. 1991;13(Suppl 1):S8–S11. doi: 10.1093/clinids/13.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 7.Fluge Ø, et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight. 2016;1(21):e89376. doi: 10.1172/jci.insight.89376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagy-Szakal D, et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci Rep. 2018;8(1):10056. doi: 10.1038/s41598-018-28477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain A, Ruppert D, Levine SM, Hanson MR. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst. 2017;13(2):371–379. doi: 10.1039/C6MB00600K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naviaux RK, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. 2016;113(37):E5472–E5480. doi: 10.1073/pnas.1607571113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moskowitz DM, et al. Epigenomics of human CD8 T cell differentiation and aging. Sci Immunol. 2017;2(8):eaag0192. doi: 10.1126/sciimmunol.aag0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callender LA, Carroll EC, Bober EA, Akbar AN, Solito E, Henson SM. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell. 2020;19(2):e13067. doi: 10.1111/acel.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandarano, et al. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J Clin Invest. 2020;130(3):1491–1505. doi: 10.1172/JCI132185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mensah FFK, et al. CD24 expression and B cell maturation shows a novel link with energy metabolism: potential implications for patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Immunol. 2018;9:2421. doi: 10.3389/fimmu.2018.02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomas C, Brown A, Strassheim V, Elson JL, Newton J, Manning P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS One. 2017;12(10):e0186802. doi: 10.1371/journal.pone.0186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch MA. Can the emerging field of immunometabolism provide insights into neuroinflammation? Prog Neurobiol. 2020;184:101719. doi: 10.1016/j.pneurobio.2019.101719. [DOI] [PubMed] [Google Scholar]
- 18.Gate D, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577(7790):399–404. doi: 10.1038/s41586-019-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajeevan MS, Murray J, Oakley L, Lin JS, Unger ER. Association of chronic fatigue syndrome with premature telomere attrition. J Transl Med. 2018;16(1):44. doi: 10.1186/s12967-018-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornig M, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1(1):e1400121. doi: 10.1126/sciadv.1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]