Abstract
BACKGROUND
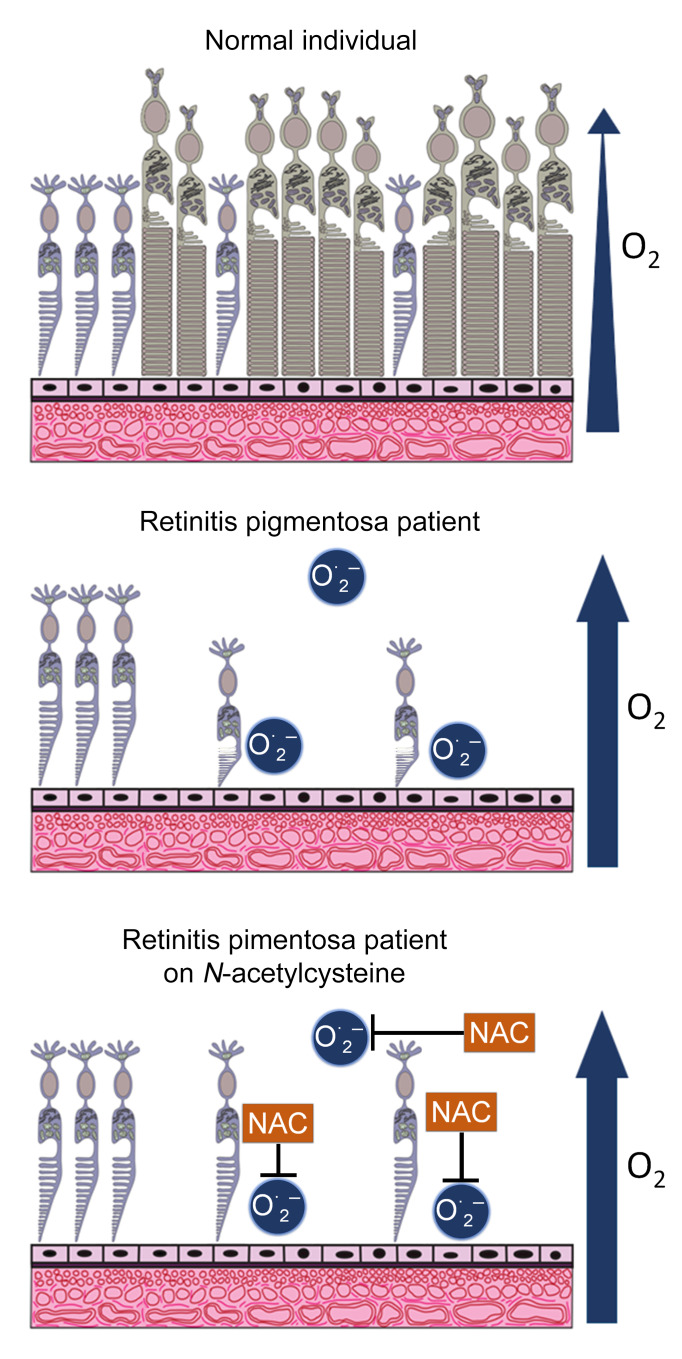
In retinitis pigmentosa (RP), rod photoreceptors degenerate from 1 of many mutations, after which cones are compromised by oxidative stress. N-acetylcysteine (NAC) reduces oxidative damage and increases cone function/survival in RP models. We tested the safety, tolerability, and visual function effects of oral NAC in RP patients.
METHODS
Subjects (n = 10 per cohort) received 600 mg (cohort 1), 1200 mg (cohort 2), or 1800 mg (cohort 3) NAC bid for 12 weeks and then tid for 12 weeks. Best-corrected visual acuity (BCVA), macular sensitivity, ellipsoid zone (EZ) width, and aqueous NAC were measured. Linear mixed-effects models were used to estimate the rates of changes during the treatment period.
RESULTS
There were 9 drug-related gastrointestinal adverse events that resolved spontaneously or with dose reduction (maximum tolerated dose 1800 mg bid). During the 24-week treatment period, mean BCVA significantly improved at 0.4 (95% CI: 0.2–0.6, P < 0.001), 0.5 (95% CI: 0.3–0.7, P < 0.001), and 0.2 (95% CI: 0.02–0.4, P = 0.03) letters/month in cohorts 1, 2, and 3, respectively. There was no significant improvement in mean sensitivity over time in cohorts 1 and 2, but there was in cohort 3 (0.15 dB/month, 95% CI: 0.04–0.26). There was no significant change in mean EZ width in any cohort.
CONCLUSION
Oral NAC is safe and well tolerated in patients with moderately advanced RP and may improve suboptimally functioning macular cones. A randomized, placebo-controlled trial is needed to determine if oral NAC can provide long-term stabilization and/or improvement in visual function in patients with RP.
TRIAL REGISTRATION
FUNDING
Mr. and Mrs. Robert Wallace, Mr. and Mrs. Jonathan Wallace, Rami and Eitan Armon, Marc Sumerlin, Cassandra Hanley, and Nacuity Pharmaceuticals, Inc.
Keywords: Neuroscience, Ophthalmology
Keywords: Drug therapy
Introduction
Retinitis pigmentosa (RP) is a group of diseases in which one of many different mutations causes degeneration of rod photoreceptors. The mutated gene may be differentially expressed in rods versus other cells, which is the case for RHO (rhodopsin), or it may be widely expressed, but plays a more critical role in rod function and survival than in other cells. There is extraordinary genetic heterogeneity within the group of diseases that cause the RP phenotype, because of the large number RP disease genes in which there may be several different pathogenic mutations. Thus, while the RP phenotype is quite common, occurring with a prevalence of approximately 1:4000 (1), each of the disease-causing mutations is quite rare (2).
Night blindness is a prominent feature of the RP phenotype, because rod photoreceptors provide vision when there is little illumination. In animal models of RP, cone cell death does not begin until the vast majority of rods have degenerated. This appears to be the case in the human disease as well, and as the gradual degeneration of cones occurs, there is constriction of the visual fields usually occurring over decades. Loss of peripheral vision is debilitating because it makes it difficult to orient in space, which is necessary for driving and important for general mobility. The macula, which has the highest cone density, is maintained late into the disease so that visual acuity loss is a relatively late feature in most cases. The central island of functional cones gets progressively smaller, resulting in tunnel vision and severe disability. Finally, compromise of the central island results in blindness.
There are several hypotheses as to why mutation-induced rod photoreceptor cell death invariably leads to the gradual dysfunction and death of cone photoreceptors. While there are likely to be several contributing factors, there is compelling evidence suggesting that oxidative stress is an important initiating event (3). Rods constitute 95% of the cells in the outer retina and as they degenerate, oxygen consumption is reduced, and the level of tissue oxygen is markedly increased (4). In a pig model of RP, after rods die, several markers of oxidative damage occur in cones and increase over time as cone cell death occurs (5). In rodent models of RP, antioxidants reduce markers of oxidative damage and promote cone function and survival (6, 7). There is also evidence of excess oxidative stress in the eyes of patients with RP (8, 9).
N-acetylcysteine (NAC) is a derivative of L-cysteine that neutralizes reactive oxygen species itself, but is also converted to cysteine that is used to biosynthesize glutathione, a major component of the endogenous antioxidant defense system (10). When given in a timely manner, NAC is a life-saving treatment for acetaminophen overdose because it prevents severe oxidative damage from aldehydes formed in the liver during metabolic breakdown of acetaminophen (11, 12). In the rd10 mouse model of RP, oral administration of NAC promoted prolonged cone survival and maintenance of cone electroretinographic function (13). Herein, we report a dose-ranging study to assess the safety, tolerability, and effects of NAC treatment on multiple parameters of visual function in patients with RP.
Results
Patient screening and enrollment.
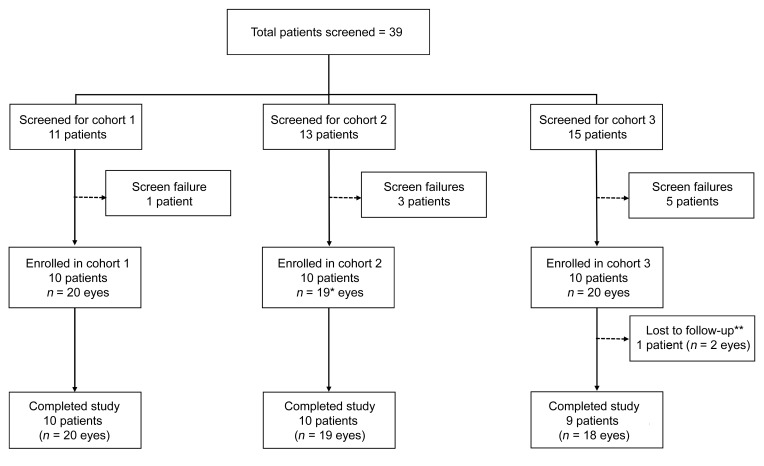
Thirty subjects with a clinical diagnosis of RP with symptoms of night blindness and peripheral visual field constriction were enrolled in the study. Patients were ineligible if they had cone-rod dystrophy or any inherited retinal degeneration other than RP or their retinal degeneration was so advanced that they were unable to perform study tests. A total of 39 patients were screened for the trial and 9 were determined to be ineligible (Figure 1). Both eyes of each eligible subject were evaluated for all outcomes unless 1 eye had a concurrent disease that could contribute to loss of visual function; this was true for 1 subject in cohort 2 who had previously had a central retinal vein occlusion in 1 eye.
Figure 1. Patient enrollment and disposition.
Patients who had previously been diagnosed to have retinitis pigmentosa (RP) at the Wilmer Eye Institute or were referred or self-referred with that diagnosis were screened. There were 9 screen failures defined as a patient who signed the consent form, completed at least 1 study assessment or procedure, and was found ineligible or chose not to participate. Five patients were ineligible because their fixation was compromised to the point that they could not perform macular sensitivity testing, 1 patient had poorly controlled blood pressure, and 3 patients decided not to participate after the screening visit. *One patient in cohort 2 had only one eligible eye because he had previously had a central retinal vein occlusion in his other eye. **One patient in cohort 3 who lived a long distance away decided to exit the study after the week 12 visit because a family member was diagnosed with a terminal illness that required constant attention and precluded the patient making monthly trips for study visits.
Baseline demographics and ocular characteristics.
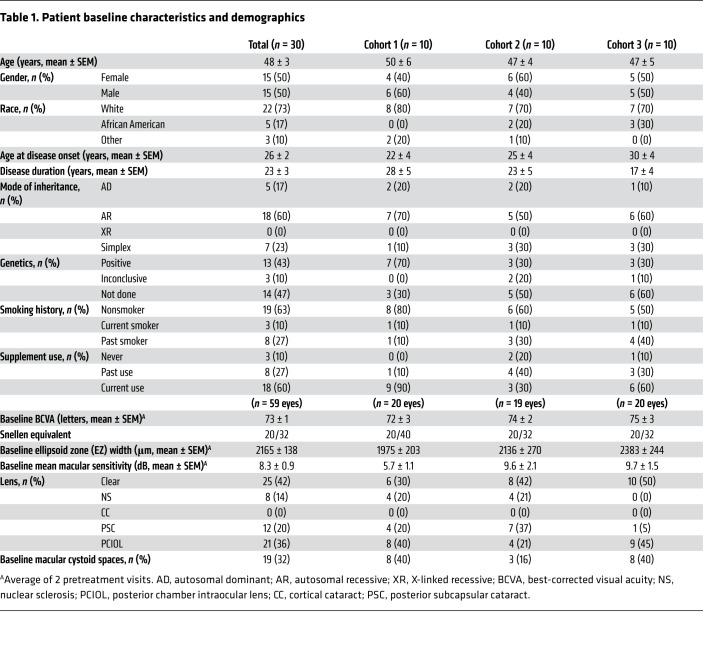
Fifty percent of the 30 subjects were female, 73% were white, the mean age at baseline was 48 ± 3 years, and the 3 cohorts were similar with regard to these demographics (Table 1). The mean age at disease onset judged by patient’s recollection of the first onset of symptoms was 22, 25, and 30 years, and duration of disease was 28, 23, and 17 years in cohorts 1, 2, and 3, respectively. The mode of inheritance was autosomal dominant in 17%, autosomal recessive in 60%, and simplex in 23%. Genetic testing was not required for study eligibility, but results of genetic testing were available for 53% of patients and a causative mutation was identified in 43% (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI132990DS1).
Table 1. Patient baseline characteristics and demographics.
The mean baseline best-corrected visual acuity (BCVA) was 72 (Snellen equivalent 20/40), 74 (20/32), and 75 (20/32) in Early Treatment Diabetic Retinopathy Study (ETDRS) letter score (14) in cohorts 1, 2, and 3, respectively (Table 1). Ellipsoid zone (EZ) width is a measurement obtained from a high-resolution spectral domain–optical coherence tomography (SD-OCT) scan (Spectralis, Heidelberg Engineering) through the fovea and corresponds to remaining photoreceptors with intact inner and outer segments (15–17). The mean baseline EZ width was 1975, 2136, and 2383 μm in cohorts 1, 2, 3, respectively. The baseline mean macular sensitivity (MMS) determined using the Macular Integrity Assessment Instrument (MAIA, CenterVue) was 5.7, 9.6, and 9.7 decibels (dB), respectively. Posterior subcapsular cataracts are common in patients with RP; 8 (40%), 4 (21%), and 9 (45%) eyes of subjects in cohorts 1, 2, and 3 had had cataract surgery with posterior chamber intraocular lens (PCIOL), and 4 (20%), 7 (37%), and 1 (5%) eyes had mild posterior subcapsular cataract that were not visually significant. Cystoid spaces in the macula, often referred to as cystoid macular edema, are also common in RP and were present in 8 (40%), 3 (16%), and 8 (40%) of eyes in cohorts 1, 2, and 3.
Patient disposition.
Subjects were examined every 4 weeks for a total of 36 weeks of follow-up including 24 weeks of treatment with NAC and 12 weeks of posttreatment observation. One subject in cohort 3 had a family medical emergency that necessitated exiting the trial at week 12. As noted above, 1 subject in cohort 2 had only 1 eligible eye, because he had had a central retinal vein occlusion in the other eye; therefore, data are available for 19 eyes in cohort 2. The MAIA microperimetry protocol includes randomly timed presentation of stimuli over the optic nerve, a physiological blind spot, which should have a sensitivity of 0. The manufacturer indicates that subjects who respond to 30% or more of stimuli presented within the blind spot are unreliable and unable to perform the test. Three subjects met this criterion because they responded to 83% or more of all stimuli presented within the blind spot during all MAIA tests they received. There are no macular sensitivity data available for these subjects (1 in cohort 1 and 2 in cohort 2), but all other outcome data are available. Thus, microperimetry data are available for 18 eyes in cohort 1, 15 eyes in cohort 2, and 20 eyes through week 12 in cohort 3 and 18 eyes thereafter.
Safety and tolerability of oral NAC.
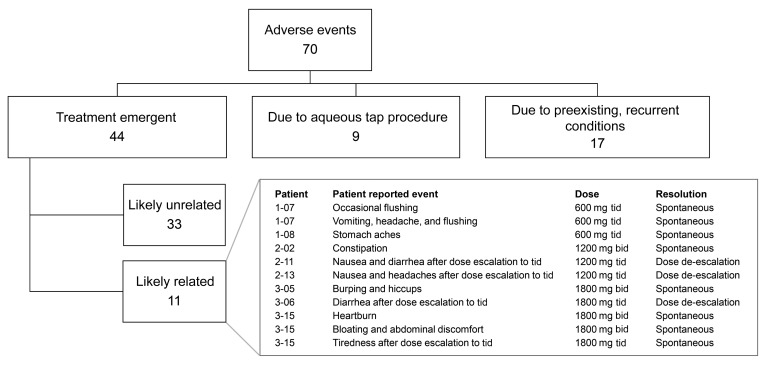
A total of 70 adverse events (AEs) occurred during the 36-week duration of the trial (Figure 2). Nine AEs were related to the aqueous tap procedure and included conjunctival injection, tearing, and/or ocular irritation that were mild and resolved within a day. Seventeen AEs were related to preexisting, recurrent conditions such as seasonal allergies, arthritis, or migraine headaches. There were 44 treatment-emergent AEs, of which 11 were judged to be drug-related, and 9 of these involved gastrointestinal symptoms. Nausea or stomach upset occurred in 8 subjects, diarrhea in 6, heartburn in 1, constipation in 1, and an episode of vomiting in 1. Symptoms occurred during tid dosing in 7 subjects and during bid dosing in 4 patients. Symptom severity was moderate in 2 subjects in cohort 2 and 1 subject in cohort 3 soon after dose escalation to tid dosing; dosing was subsequently reduced to bid and the symptoms resolved. The remainder of the events were mild, brief, and resolved spontaneously without any change in dosing.
Figure 2. Adverse events.
There were 11 adverse events that were judged likely related to study medication and 9 were related to the gastrointestinal tract. Eight of the adverse events were transient and resolved spontaneously without change in study medication dosage and 3 resolved after dosing was reduced from 3 times a day (tid) to twice a day (bid).
Aqueous and plasma NAC levels.
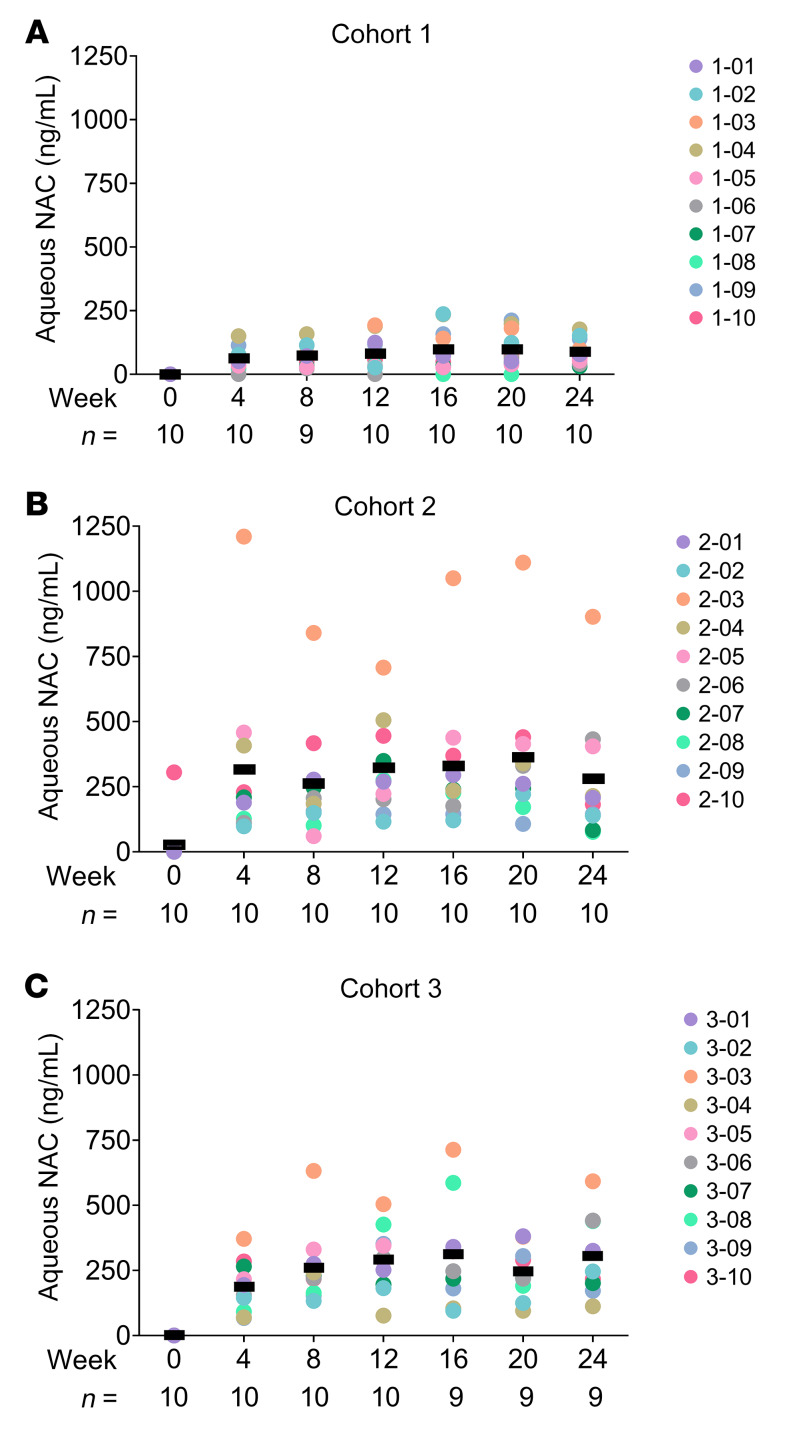
In cohort 1, the mean aqueous NAC level during the 600-mg-bid dosing period was 63, 75, and 80 ng/mL at weeks 4, 8, and 12, respectively, and during the 600-mg-tid dosing period was 98, 98, and 87 ng/mL at weeks 16, 20, and 24 (Figure 3A). One cohort 2 subject had an aqueous NAC level of 305 ng/mL at screening but when this was repeated at baseline the level was below the lower limit of detection (20 ng/mL). This patient denied taking NAC prior to enrollment. The mean aqueous NAC levels in cohort 2 during the 1200-mg-bid dosing period were 315, 264, and 323 ng/mL at weeks 4, 8, and 12, respectively, and were 329, 364, and 278 ng/mL during the 1200-mg-tid dosing at weeks 16, 20, and 24 (Figure 3B). These values were skewed by particularly high aqueous NAC levels in patient 2-03. In cohort 3, mean aqueous NAC levels were 186, 259, and 292 ng/mL during 1800-mg-bid dosing at weeks 4, 8, and 12, and were 312, 245, and 305 ng/mL at weeks 16, 20, and 24 during 1800-mg-tid dosing (Figure 3C). At all posttreatment visits (week 28, 32, and 36 visits), aqueous NAC was below the lower limit of detection (20 ng/mL) in all subjects except the 1 early-termination subject for whom it was not tested.
Figure 3. N-acetylcysteine (NAC) levels in aqueous humor.
The dose of NAC was 600 mg twice a day for 12 weeks and then 600 mg 3 times a day for the subsequent 12 weeks in cohort 1 (A), 1200 mg twice a day for 12 weeks and then 1200 mg 3 times a day for the subsequent 12 weeks in cohort 2 (B), and 1800 mg twice a day for 12 weeks and then 1800 mg 3 times a day for the subsequent 12 weeks in cohort 3 (C). At each visit during the treatment period, an aqueous sample was obtained and NAC concentration was measured by LC-MS. The colored circles corresponding to patient identifiers show aqueous NAC levels in study eyes at each visit. Bars show the mean for each time point. The number of eyes (n) for each time point is shown along the x axis. Aqueous NAC was below the limit of quantification (20 ng/mL) for all subjects (except for the cohort 3 subject who exited at week 12 for whom no samples were available) at weeks 28, 32, and 36.
There was no statistically significant difference in the mean aqueous NAC level during the bid dosing period (week 4, 8, and 12 visits) compared with during the tid dosing period (week 16, 20, and 24 visits). The difference was 18.35 (95% CI: –22.0 to 58.69), 3.38 (95% CI: –81.56 to 88.31), and 41.56 (95% CI: –15.07 to 98.19) ng/mL in cohorts 1, 2, and 3, respectively. During the bid dosing period, there was a statistically significant difference in the mean aqueous NAC level comparing cohort 2 with cohort 1 (difference = 311.91, 95% CI: 169.34–454.49) ng/mL and comparing cohort 3 with cohort 1 (difference = 241.60, 95% CI: 185.27–297.93) ng/mL, but there was no statistically significant difference in the mean aqueous NAC level between cohort 2 and cohort 3 (difference = 70.31, 95% CI: –82.99 to 223.62) ng/mL. Findings were similar when comparing the mean aqueous NAC level between the 3 cohorts during the tid dosing period.
In each cohort, plasma NAC levels were measured 1 hour after dosing at 3 visits: baseline, week 12, and week 24. There was considerable variability in plasma NAC levels among subjects 1 hour after receiving the same oral dose of NAC, but the 3 measurements in each subject were similar and the mean plasma NAC level 1 hour after a particular NAC dose was similar each time it was measured. One hour after 600 mg of NAC, the mean plasma NAC level was 1705.40, 1705.63, and 1799.20 ng/mL at baseline, week 12, and week 24 in cohort 1 (Supplemental Figure 1A). One hour after 1200 mg of NAC, the mean plasma NAC level was 7887.14, 7782.86, and 7388.75 ng/mL in cohort 2 (Supplemental Figure 1B). One hour after 1800 mg of NAC, the mean plasma NAC level was 6081.43, 8831.11, and 9216.25 in cohort 3 (Supplemental Figure 1C). A predose measurement was obtained at the week 12 and 24 visits, providing a measurement in the midst of treatment and approximately 12 hours after the most recent dose. For cohort 1, the mean predose measurement was 302.70 ng/mL at week 12 and 392.75 ng/mL at week 24; in cohort 2, the mean predose levels at weeks 12 and 24 were 1424.88 and 991.88 ng/mL; and in cohort 3, they were 1025.33 and 1077.78 ng/mL at weeks 12 and 24, respectively.
Longitudinal analysis of change in BCVA during the treatment period.
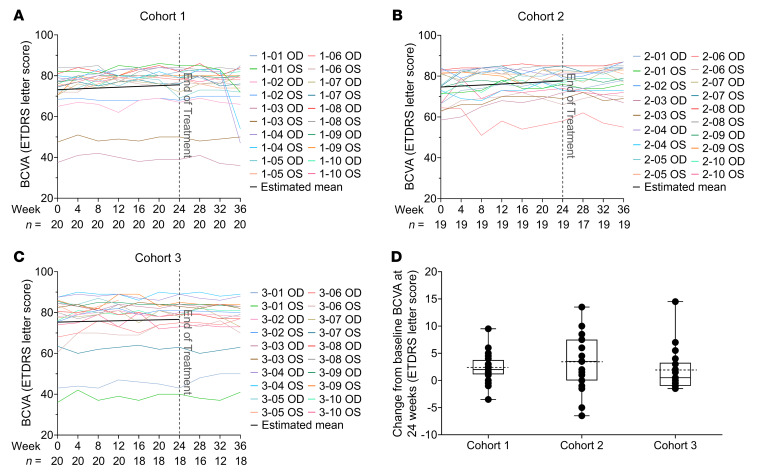
All cohorts had statistically significant improvements in mean BCVA by linear mixed-effects models (Figure 4 and Table 2). The rate of improvement was 0.40 letters/month (95% CI: 0.20–0.59, P < 0.001) in cohort 1 (Figure 4A), 0.49 letters/month (95% CI: 0.29–0.69, P < 0.001) in cohort 2 (Figure 4B), and 0.22 letters/month (95% CI: 0.02–0.43, P = 0.03) in cohort 3 (Figure 4C). The rates of change were not significantly different between the 3 cohorts (P > 0.05). Comparing the baseline and week 24 visit, in cohort 1, the mean BCVA improved 2.33 letters (95% CI: 1.17–3.48; P < 0.001), and 3 eyes (15%) improved 5 or more letters (Figure 4D). In cohort 2, the mean BCVA improved 3.28 letters (95% CI: 0.93–5.64; P = 0.006), and 7 eyes (37%) improved 5 or more letters. In cohort 3, the mean BCVA improved 1.83 letters (95% CI: –0.45 to 4.11; P = 0.12), and 3 eyes (17%) improved 5 or more letters. The mean rate of BCVA change per month during the week 24 to week 36 posttreatment observation period is shown in Supplemental Table 2. There was a statistically significant decline in cohort 1, which had experienced the greatest improvement during treatment, but this was due to a large decline in 1 subject and small declines in other subjects (Supplemental Table 2). The BCVA at baseline, week 12, and week 24 for each eye of all subjects is shown in Supplemental Figure 2A.
Figure 4. Change in best-corrected visual acuity (BCVA) during NAC treatment.
A trained visual acuity examiner measured BCVA on a standardized chart at 4 meters in letter score using a validated protocol established in the Early Treatment Diabetic Retinopathy Study (ETDRS). The BCVA at each study visit is shown for each eligible right (OD) and left (OS) eye in cohorts 1 (A), 2 (B), and 3 (C). In each plot, each colored line reflects the BCVA values of 1 eye over time. At each visit, the number of eyes with BCVA values (n) is shown along the x axis. The black line is the estimated trend of change over the 24-week treatment period in that cohort as estimated by linear mixed-effects model. There was a statistically significant improvement in mean BCVA over time during the 24-week treatment period in each cohort: 0.40 letters/month (95% CI: 0.20–0.59, P < 0.001) in cohort 1, 0.49 letters/month (95% CI: 0.29–0.69, P < 0.001) in cohort 2, and 0.22 letters/month (95% CI: 0.02–0.43, P = 0.03) in cohort 3. Box plots of the mean change in BCVA between baseline and week 24 showed a significant improvement in mean BCVA of 2.33 (95% CI: 1.17–3.48) letters (P < 0.001) in cohort 1, and 3.28 (95% CI: 0.93–5.64) letters (P = 0.006) in cohort 2 by linear mixed-effects model (D). In cohort 3, the improvement of 1.83 letters (95% CI: –0.45 to 4.11, P = 0.12) was not statistically significant. In each box plot, the box upper and lower borders correspond to the 25th to 75th percentile, the solid line in the middle of the box is the median, and the dotted line is the mean of the data.
Table 2. Rates of change in BCVA, mean sensitivity, and EZ width during NAC treatment.
Longitudinal analysis of change in mean macular sensitivity during the treatment period.
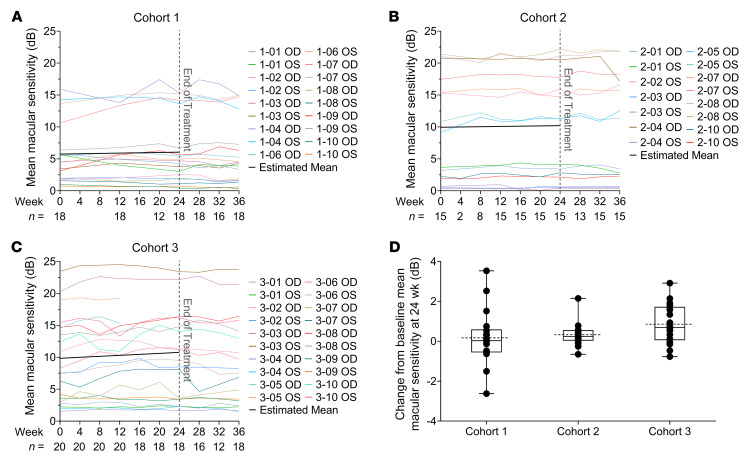
Microperimetry or fundus-driven perimetry uses eye-tracking technology to measure retinal sensitivity in a Cartesian grid of equidistant points covering the central 20 degrees of the visual field centered on the fovea. In this study, MAIA was used. Patients with RP typically have good fixation until late in the disease, allowing repeated measurements at the exact same location over time. Mean macular sensitivity (MMS) of the values obtained at each of the 68 loci tested is a reflection of sensitivity throughout the central 20 degrees. There was no statistically significant improvement in MMS during the 24-week treatment period in cohort 1 (rate of change = 0.05 dB/month, 95% CI: –0.07 to 0.17, P = 0.38) or cohort 2 (rate of change = 0.04 dB/month, 95% CI: –0.08 to 0.16 dB/month, P = 0.48; Figure 5 and Table 2). In cohort 3, there was a statistically significant improvement in MMS of 0.15 dB/month (95% CI: 0.04–0.26; P = 0.008). In cohort 1, there was no significant difference between MMS at baseline and week 24 (change = 0.17 dB, 95% CI: –0.62 to 0.96, P = 0.67); however, there were statistically significant improvements in MMS between baseline and week 24 in cohort 2 (change = 0.33 dB, 95% CI: 0.02–0.64, P = 0.04) and cohort 3 (change = 0.85 dB, 95% CI: 0.29–1.42, P = 0.003; Figure 5D and Table 2). The mean rate of change per month in MMS during the week 24 to week 36 posttreatment observation period is shown in Supplemental Table 2. There was a statistically significant decline in cohort 3, which had shown the greatest improvement during treatment, with no significant change for cohorts 1 and 2 (Supplemental Table 2). The MMS at baseline, week 12, and week 24 for each eye of each subject able to perform microperimetry is shown in Supplemental Figure 2B.
Figure 5. Change in mean macular sensitivity during NAC treatment.
Macular sensitivity was measured at 68 loci in the central 20° using the Macular Integrity Assessment Instrument (MAIA) at each study visit, and the mean sensitivity (MS) for the 68 loci is shown. The average of the 2 pretreatment tests was used as the baseline MS value and along with subsequent values are plotted for each eligible eye in cohort 1 (A), cohort 2 (B), and cohort 3 (C) (each colored line is for 1 eye). The black line shows the estimated trend of MS change during the treatment period using the linear mixed-effects model. There was no statistically significant improvement in MS during the 24-week treatment period in cohort 1 (rate of change = 0.05 dB/month, 95% CI: –0.07 to 0.17, P = 0.38) or cohort 2 (rate of change = 0.04 dB/month, 95% CI: –0.08 to 0.16 dB/month, P = 0.48), but in cohort 3, there was a statistically significant improvement in MS of 0.15 dB/month (95% CI: 0.04–0.26; P = 0.008). (D) Box plots of the mean change in MS between baseline and week 24 showed no significant improvement in cohort 1 (change = 0.17 dB, 95% CI: –0.62 to 0.96, P = 0.67) by linear mixed-effects model, but there were statistically significant improvements in mean MS between baseline and week 24 in cohort 2 (change = 0.33 dB, 95% CI: 0.02–0.64, P = 0.04) and cohort 3 (change = 0.85 dB, 95% CI: 0.29–1.42, P = 0.003). In each box plot, the box upper and lower borders correspond to the 25th to 75th percentile, the solid line in the middle of the box is the median, and the dotted line is the mean of the data.
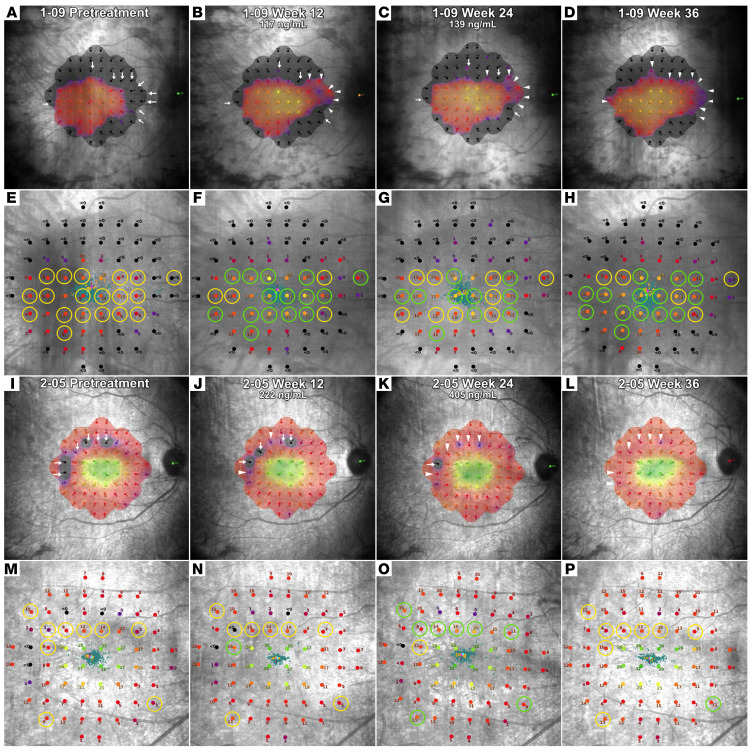
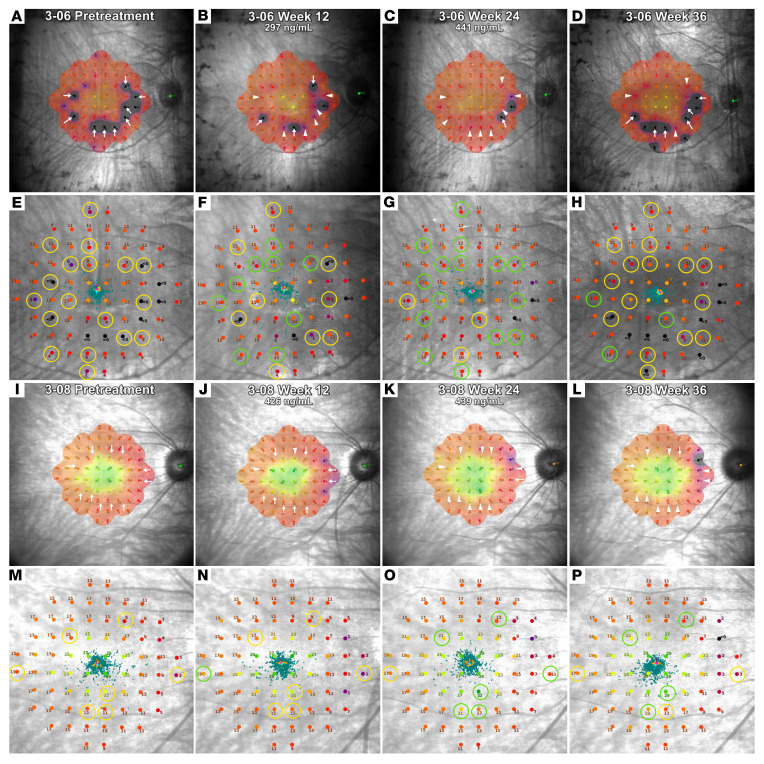
Change in macular sensitivity is also qualitatively assessed by visual comparison of pretreatment and posttreatment sensitivity heatmaps generated by the MAIA software. This was done for the 51 eyes that had valid MAIA test results available at both the baseline and the 24-week visits. The heatmaps and fundus images with sensitivity readings at each locus obtained at the baseline and week 12 and week 24 visits are shown in Figures 6 and 7 and Supplemental Figures 3–49. Additionally, the week 36 heatmaps and images are also shown to demonstrate macular sensitivity observed 12 weeks after stopping NAC. Figure 6, A–D and I–L, and Figure 7, A–D and I–L show regions of improved macular sensitivity during treatment (arrowheads). Figure 6, E–H and M–P, and Figure 7, E–H and M–P show fundus images with sensitivity at each measurement locus. Circled loci improved 6 dB or greater during treatment, which based upon test-retest variability of 3 to 4 dB, is unlikely due to chance (18–20). The 4 subjects shown in Figures 6 and 7 had large improvements in macular sensitivity during treatment with NAC that partially regressed after treatment cessation. A detailed explanation of changes seen in Figures 6 and 7 is provided in Supplemental Data.
Figure 6. Heatmaps and fundus images from microperimetry testing during treatment and posttreatment observation in a patient from cohort 1 and a patient from cohort 2.
The heatmaps generated by the Macular Integrity Assessment Instrument software (A–D and I–L) and fundus images showing the sensitivity value at each retinal location (E–H and M–P) obtained pretreatment, during treatment at weeks 12 and 24, and after treatment at week 36 are shown for 1 eye for a cohort 1 patient (1-09) and 1 eye for a cohort 2 patient (2-05). Two pretreatment tests were obtained and the one with the highest mean sensitivity is shown. In heatmaps, the arrows point to regions where the retinal sensitivity improved after the onset of treatment. A change from an arrow to an arrowhead indicates improvement had occurred, and any subsequent decline is noted by a change from arrowhead to arrow. The loci circled in yellow on the pretreatment fundus images (E and M) show loci at which sensitivity increased equal to or more than 6 dB after the onset of treatment. On subsequent fundus images, green circles indicate that sensitivity at that location was increased equal to or more than 6 dB from pretreatment and a change back to yellow indicates a decline below the 6 dB threshold. In both of these eyes, there was a large improvement in sensitivity during treatment that was mostly sustained in A–H and partially sustained in I–P 3 months after treatment ended.
Figure 7. Heatmaps and fundus images from microperimetry testing during treatment and posttreatment observation in 2 patients from cohort 3.
The heatmaps generated by the Macular Integrity Assessment Instrument software (A–D and I–L) and fundus images showing the sensitivity value at each retinal location (E–H and M–P) obtained pretreatment, during treatment at weeks 12 and 24, and after treatment at week 36 are shown for 1 eye of patients 3-06 and 3-08 in cohort 3. Two pretreatment tests were obtained and the one with the highest mean sensitivity is shown. In heatmaps, the arrows point to regions where the retinal sensitivity improved after the onset of treatment. A change from an arrow to an arrowhead indicates improvement had occurred, and any subsequent decline is noted by a change from arrowhead to arrow. The loci circled in yellow on the pretreatment fundus images (E and M) show loci at which sensitivity increased equal to or more than 6 dB after the onset of treatment. On subsequent fundus images, green circles indicate that sensitivity at that location was increased equal to or more than 6 dB from pretreatment and a change back to yellow indicates a decline below the 6 dB threshold. In the right eye of patient 3-06, there was a large improvement in macular sensitivity during treatment that regressed after treatment. In the right eye of patient 3-08, there was a large improvement in macular sensitivity that was partially sustained 3 months after stopping treatment.
There were 3 other eyes, all from patients in cohort 3, that showed substantial improvements in sensitivity similar in magnitude to that of the eyes illustrated in Figures 6 and 7 (Supplemental Figures 42, 43, and 45). In cohort 1, 9 eyes showed substantial improvement in MMS and heatmaps (Figure 6, A–H, and Supplemental Figures 5, 7, 8, 9, 11, 12, 13, and 14) and 9 eyes showed little change or worsening (Supplemental Figures 3, 4, 6, 10, 15, 16, 17, 18, and 19). In cohort 2, 8 eyes showed substantial improvement in MMS and heatmaps (Figure 6, I–P, and Supplemental Figures 20, 21, 22, 25, 29, and 32) and 7 eyes showed little change or worsening (Supplemental Figures 23, 24, 26–28, 31, and 33). In cohort 3, 16 eyes showed improvement in MMS and heatmaps (Figure 7, A–D, and Supplemental Figures 34–38, 40–46, and 48–49) and 2 eyes showed little change or worsening (Supplemental Figures 39 and 47). Maintenance of sensitivity improvement during the 3-month posttreatment observation period was variable, with substantial maintenance of benefit in 4 eyes (Figure 6, A–H and I–P, Figure 7, I–P, and Supplemental Figure 14), and the remainder showing regression ranging from little to nearly complete. In general, patients with advanced disease and small central islands of remaining visual field at baseline tended to show little or no change in macular sensitivity during NAC treatment (Supplemental Figures 15, 16, 18, 19, 23, 24, 26, 33, 40, and 41). Eyes with excellent macular sensitivity at baseline also had limited improvement, possibly due to a ceiling effect (Supplemental Figures 28–31, 38, and 39).
Change in macular cystoid spaces during the treatment period.
Eight eyes in cohort 1, 3 in cohort 2, and 8 in cohort 3 had cystoid spaces in the macula at baseline. Extrafoveal cystoid spaces do not affect visual acuity; 12 eyes had 1 or more small extrafoveal cystoid space(s) (Supplemental Figure 50; 1-03OD, 1-04OD, 1-06OS, 1-09OD, 2-01OD, 2-05OD, 2-05OS, 3-02OD, 3-03OD, 3-06OD, 3-06OS, 3-07OD, and 3-10OD) and 2 eyes had a group of moderate-sized extrafoveal cystoid spaces (1-04OS and 1-09OS). Four eyes had cystoid spaces involving the fovea that likely had some impact on visual acuity (Supplemental Figure 50; 1-07OD, 1-07OS, 3-07OS, and 3-10OS). During oral NAC treatment, there was reduction in size and number of cystoid spaces that may have contributed to the 1.5-letter improvement in visual acuity in 1-07OD and 1-07OS. There was an increase in size and number of cystoid spaces that may have contributed to the loss of 1.0 letter in BCVA in 3-10OS, and despite some improvement in size and number of cystoid spaces, there was loss of 0.5 letters in 3-07OS. During the 24-week treatment period there was discernable reduction in size and/or number of cystoid spaces in 10 eyes, increase in size or number of cystoid spaces in 6 eyes, and no discernable change in 3 eyes. A sensitivity analysis estimating the rates of BCVA and MMS change among eyes that did not have cystoid spaces at baseline (n = 40) showed statistically significant improvements in BCVA during the treatment period in cohorts 1 and 2, and statistically significant improvement in macular sensitivity for cohort 3 (Supplemental Table 3), similar to what was seen for the entire population.
Longitudinal analysis of change in EZ width during the treatment period.
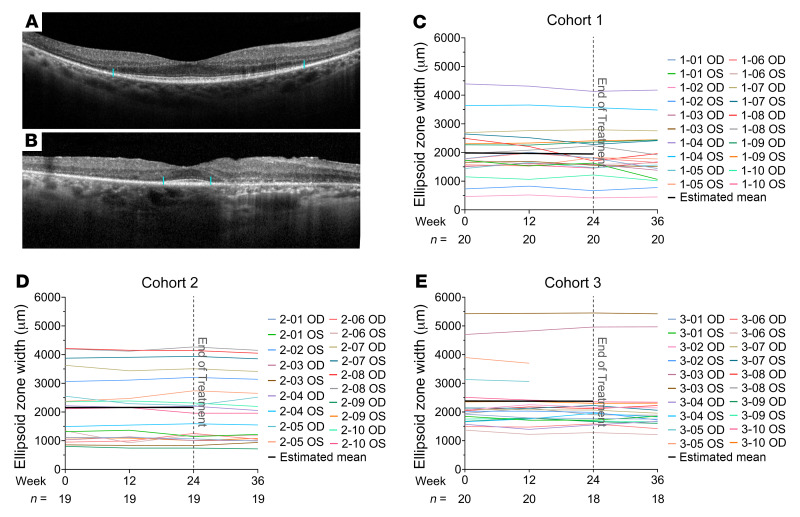
The EZ line is a distinct line visualized in SD-OCT scans above the line generated by the retinal pigmented epithelium, and width of the EZ is a measure of the integrity of photoreceptors. In the region around the fovea it is generated by intact cone inner and outer segments and its absence indicates that cones in that area lack outer segments or have degenerated. As cone cell death proceeds in patients with RP, there is gradual reduction in the width of the EZ through the fovea, and hence EZ width is a marker of cone survival (17, 21). Figure 8A shows a baseline SD-OCT scan of a patient with moderately advanced loss of EZ width and Figure 8B shows a baseline SD-OCT of a patient with advanced loss of EZ width. During the 24 weeks of treatment, there was no significant change in mean EZ width in cohort 1, 2, or 3 (Figures 8, C–E, and Table 2). The mean rate of change per month of EZ width during the week 24 to week 36 posttreatment observation period is shown in Supplemental Table 2. There was no statistically significant decline in the rate of change of EZ width in any of the 3 cohorts. The EZ width at baseline, week 12, and week 24 for each eye of all subjects is shown in Supplemental Figure 2C.
Figure 8. Change from baseline in ellipsoid zone (EZ) width.
Spectral domain–optical coherence tomography (SD-OCT) scans through the fovea are shown for a patient with moderately severe loss of EZ width (A, margins marked by blue lines) and severe loss of EZ width (B). The longitudinal EZ width measurements at baseline and weeks 12, 24, and 36 are shown as a colored line for each eye for cohorts 1 (C), 2 (D), or 3 (E). The black line is the average trend of EZ width over the treatment period as estimated by a linear mixed-effects model in each cohort. The number (n) of eyes with EZ measurements for each time point is shown along the x axis.
Fundus autofluorescence.
Fundus autofluorescence was obtained at baseline and week 24, and there no identifiable changes in any of the subjects.
Reduced glutathione and total glutathione in aqueous humor in cohort 3 subjects.
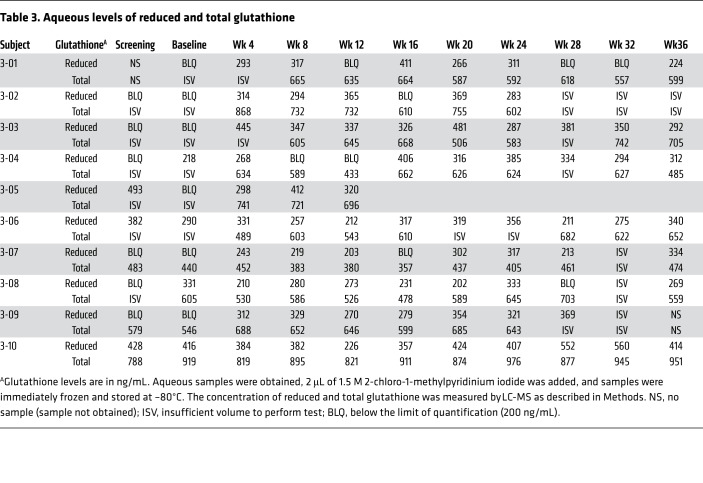
A previous study showed patients with RP have a decrease in reduced glutathione in aqueous humor compared with healthy controls (9). In the present study, it was planned to determine whether administration of NAC would increase reduced glutathione in the aqueous of patients with RP and samples were collected and stored for that purpose; however, it was learned during the trial that even in frozen samples reduced glutathione is oxidized over time unless 1.5 M 2-chloro-1-methylpyridinium iodide is added. This was not done for aqueous samples obtained for cohorts 1 and 2, but was done for cohort 3. Table 3 shows aqueous levels of reduced and total glutathione throughout the trial. In 6 of the 10 eyes of cohort 3 subjects, reduced glutathione in aqueous was undetectable or low prior to treatment and increased after oral NAC was begun. Interestingly, posttreatment samples were available for 4 of the eyes and showed that reduced glutathione persisted in aqueous for at least 3 months after stopping NAC. Two eyes had discordant pretreatment measurements, 1 below the limit of quantification (BLQ) and the other within the detectable range and not substantially lower than posttreatment measurements. The other 2 cohort 3 subjects had fairly high measurements of reduced glutathione in both pretreatment aqueous samples, with little change after the institution of oral NAC. These 4 subjects had relatively preserved central fields at baseline.
Table 3. Aqueous levels of reduced and total glutathione.
Discussion
In RP, rod photoreceptors degenerate from 1 of many different mutations. After rods degenerate, cone photoreceptors degenerate over a span of many years, resulting in gradual constriction of visual fields and eventual blindness. There is considerable evidence suggesting that oxidative damage contributes to cone dysfunction and death and that treatments, including NAC, that reduce oxidative damage promote cone function and survival (5–9, 13, 22–24). In this study we tested several oral doses of NAC for safety and tolerability, pharmacokinetics, and effects on parameters of photoreceptor integrity and visual function in patients with RP.
There were 11 AEs that were felt to probably be related to NAC and 9 of them involved the gastrointestinal system. Most were mild to moderate in severity and resolved spontaneously without interruption in dosing; however, in 3 subjects the onset of symptoms coincided with an increase in dosing frequency to tid and resolved when dosing was decreased to bid. For this reason, 1800 mg bid was determined to be the maximum tolerated dose. The doses used in this study, to our knowledge, are the highest doses of oral NAC that have been tested over an extended period and therefore these data will be useful to help guide future studies investigating NAC for other diseases throughout the body in which oxidative stress may play a pathogenic role.
Pharmacokinetic data obtained by measuring NAC levels in aqueous humor throughout the treatment period are informative because they demonstrate that good intraocular levels of NAC are obtained after oral dosing. However, there is considerable variability in aqueous and plasma NAC levels among patients, suggesting that there are substantial differences in the absorption and/or metabolism of NAC from patient to patient. This variability and the relatively small number of patients per cohort make it difficult to make firm conclusions regarding dose selection for future trials based on mean aqueous NAC levels. However, both mean levels and individual patient data suggest that tid administration of 1200 or 1800 mg NAC does not result in a substantial increase in aqueous levels compared with bid administration of 1200 or 1800 mg NAC. This observation, combined with better tolerability and convenience, favor bid dosing for future studies. Comparing between the 1200-mg-bid and 1800-mg-bid dosing, the mean aqueous NAC levels were not significantly different; since 1800 mg bid was well tolerated and improvements in macular sensitivity were greatest in cohort 3, it seems prudent not to eliminate this regimen from further testing.
Typically, BCVA is preserved until very late in the natural history of RP. As expected, the mean baseline BCVA was excellent in each of the 3 cohorts. No change in BCVA was expected, and thus it was surprising to observe a small but steady increase in BCVA during the 24-week dosing period in each cohort. The small number of patients in each cohort made it difficult to determine if this was a clinically meaningful improvement, but statistical analyses estimating the rates of BCVA change using all visits during the dosing period and the analyses estimating the change from baseline to the 24-week visit found statistically significant improvements in all cohorts, suggesting that the steady increase during the treatment period in each cohort may not be due to chance. The later onset of symptoms, shorter duration of disease, better mean BCVA at baseline, and higher mean EZ width suggest that cohort 3 subjects had less compromise of foveal cone structure and function than cohort 1 subjects. The better baseline mean BCVA in cohort 3 compared with the other 2 cohorts likely contributed to the lesser improvement in mean BCVA seen in cohort 3, since higher baseline mean BCVA has correlated with lesser improvement in previous interventional trials due to a ceiling effect (25, 26).
The possibility that the improvements in mean BCVA are related to NAC and not simply due to chance is further supported by improvement in macular sensitivity that occurred in many patients, which appeared likely to be due to treatment with NAC rather than chance for several reasons. First, microperimetry is commonly used to track progression in patients with RP and typically gradual decline is seen (27). However, in this study, many eyes showed substantial improvements that are not normally seen in untreated patients with RP. Second, in many eyes, macular sensitivity progressively improved during the treatment period and then decreased during the 12-week posttreatment observation period, although 4 eyes showed substantial maintenance of benefit for 3 months after stopping treatment. The pattern of improvement is unlikely to be due to chance because specific loci showed improvement after 12 weeks of treatment and then those same loci often showed further improvement after 24 weeks of treatment (Figures 6 and 7). Third, the benefits were dose related, because improvement in the mean sensitivity was the greatest in cohort 3. Fourth, many of the eyes that failed to show improvement in macular sensitivity had small central islands of remaining visual field at baseline, and thus may have been too advanced to improve. Fifth, some other eyes that showed little or no improvement had excellent macular sensitivity at baseline, raising the possibility of a ceiling effect.
Cystoid spaces commonly occur in the macula of patients with RP (28). In many patients the cystoid spaces are small and/or extrafoveal and have no impact on vision, but large spaces in the fovea can have a negative impact on vision. In this study, 19 eyes (32%) had 1 or more cystoid spaces in the macula, but for most eyes the spaces were small and did not involve the fovea. During NAC treatment, cystoid spaces improved in 10 eyes, worsened in 6, and were unchanged in 3. This is consistent with the spontaneous fluctuation that often occurs and provides no evidence suggesting that oral NAC has any effect on cystoid spaces. Because cystoid spaces were extrafoveal and mild in 15 of the 19 eyes with cystoid spaces at baseline and there was little change in visual acuity in the 4 eyes that had clinically significant cystoid space, fluctuations in cystoid spaces in these eyes should not have contributed to the observed improvement in BCVA in each cohort. This is further confirmed by a sensitivity analysis estimating the rates of BCVA and MMS change among eyes that did not have cystoid spaces at baseline (n = 40). In this subgroup of eyes, there was statistically significant improvements in BCVA during the treatment period in cohorts 1 and 2, and statistically significant improvement in macular sensitivity was seen for cohort 3 (Supplemental Table 3), similar to what was seen for the entire population. The estimated rates of change in the 3 cohorts were also comparable to the rates estimated using all eyes irrespective of the baseline status of cystoid spaces. This subgroup sensitivity analysis suggests that an effect on cystoid spaces did not contribute to the improvement in BCVA and macular sensitivity seen during NAC treatment.
This study suggests that some of the reduction in macular sensitivity seen in eyes of untreated patients with moderately advanced RP is likely to be due initially to cone dysfunction rather than cone cell death and that reduction of oxidative stress by administration of NAC may improve cone function, resulting in improved macular sensitivity and small improvements in visual acuity. However, patients with very advanced RP and a small central island of remaining cones may have few cones with potential for improved function and hence show little or no identifiable improvement in macular sensitivity during a 6-month treatment period. It is reasonable to hypothesize that improvement in cone function indicates an improvement in overall health of cones that could delay or prevent cone cell death. In order to test this hypothesis, a large, randomized, double-masked, placebo-controlled trial is needed, and the results of the current study provide critical information that will help design that study. Moreover, a longer follow-up period of 2 or 3 years is needed to learn the trajectories of NAC treatment effects on photoreceptor survival and parameters of visual function, and to learn whether the effects of NAC can be sustained beyond the initial few months of treatment. Electroretinograms (ERGs) provide another tool for assessing photoreceptor function and are helpful in the early stages of RP; however, the patients in this trial were all at an intermediate to relatively late stage of the disease when ERGs are extinguished. There was little or no change in fundus autofluorescence images between baseline and week 24.
It has long been known that aqueous humor from normal human eyes contains micromolar levels of glutathione almost exclusively in the reduced state (29). A previous study has suggested that the high level of oxidative stress in the retinas of RP patients can shift aqueous glutathione toward the oxidized state (9). Consistent with this, 6 of the 10 cohort 3 subjects had undetectable or low pretreatment levels of reduced glutathione. In those subjects, the levels of reduced glutathione in aqueous were increased during NAC treatment. We hypothesize that this shift in redox state in the aqueous during treatment reflects a similar shift in the retina due to reduced oxidative stress. Interestingly, reduced glutathione remained detectable in the aqueous of 5 these patients for whom it was measured for at least 3 months after cessation of treatment, suggesting that the redox state of aqueous may be altered for a time after treatment. Three subjects had relatively high reduced glutathione in aqueous in at least 1 pretreatment measurement. Those 3 subjects had relatively full central fields and may have had less oxidative stress in the retina at baseline. They also showed substantial improvement in retinal sensitivity during NAC treatment, suggesting that relatively high reduced glutathione in aqueous prior to treatment does not preclude improvement in macular sensitivity during treatment with oral NAC.
Compliance is critical for any drug trial and we assessed it in 3 ways. We had discussions with the subjects about the importance of not only taking the NAC, but trying to take it at a consistent time each day and recording the date and time of each dose in a diary that we collected. Secondly, we required subjects to save and return the foil from each blister pack. Finally, we measured aqueous and plasma NAC levels. All 3 of these assessments indicated that compliance was excellent, and of course the third assessment tool is definitive.
This study has a few limitations. First, for participants enrolled early during the study (mainly participants in cohorts 1 and 2), by our original study protocol, MAIA testing was conducted every 3 months rather than every 4 weeks, and thus macular sensitivity measurements were not available at week 4, 8, and 16 visits. We sought to balance the benefit of frequent testing, particularly for trend analysis (30), with the burden experienced by patients. During the initial stages of the study, we determined that the MAIA testing was tolerated well and was not as burdensome as we initially thought. We therefore amended the protocol to include MAIA testing at each visit thereafter. As a result, MMS measurements were not available for some visits for some cohort 1 subjects. However, such missing data generation was independent of patients’ status and their level of macular sensitivity, and thus the missing-at-random assumption is reasonable (31). Therefore, the linear mixed-effects model technique, which utilized all available data, could still provide unbiased estimates of the rates of MMS change. Second, we also found that 3 of 30 enrollees could not reliably perform the MAIA test, not due to poor fixation, but rather because when put in a testing situation in which they were expected to see a light, they thought they saw it even when it was not present. This inability to reliably perform MAIA testing appears to be related to difficulty with neuropsychological processing of the test procedure, rather than a reflection of visual function. In future trials, it will be worthwhile to perform several pretreatment macular sensitivity tests and exclude patients who are unable to reliably perform the test. Third, as a phase I, dose-ranging study, the number of subjects and hence the number of evaluable eyes per cohort was limited. This reduced our ability to evaluate factors such as smoking or past smoking (a known source of systemic oxidative stress) and a past history of dietary supplement intake, which may confound the effect of NAC on visual functions. Future studies will include prespecified subgroup analyses to evaluate history of smoking and dietary supplements.
Currently, there is no effective treatment for RP. Gene therapy has the potential to address pathogenic mutations, but there are hundreds of mutations so only a relatively small number of patients can benefit from a single gene therapy construct. Furthermore, the goal of gene therapy is to correct the mutation that causes rods to die so it must be administered relatively early in the disease when a large number of rods are still alive. In general, unless there is a family history of RP that prompts early genetic testing, patients tend to present due to night blindness or visual field loss when most rods have already been eliminated. Also, it is not possible to identify the pathogenic mutation in many patients (32). In RP, cone death occurs as a result of death of rods, rather than a result of the pathogenic mutations and therefore treatment with NAC has the potential to apply to all patients with RP irrespective of disease-causing mutation. Cone photoreceptors provide vision during daytime lighting and thus are critical for daily activities. A treatment for RP aimed at promoting cone function and survival and independent of the pathogenic mutations like the one described in this study is desperately needed. Coincidentally, in the current study population, 7 of 13 subjects for whom a pathogenic mutation had been identified had a mutation in Ush2A. In the subsequent large randomized trial, it will be important to enroll patients based on a clinical diagnosis of RP, and genotype information will be collected to assess whether genotype is an effect modifier for the efficacy of NAC on cone function.
In summary, this study suggests that oral NAC treatment at doses of 1200 mg or 1800 mg bid is safe and well tolerated in patients with RP, at least for 6 months, which is the duration of treatment in the study. Building on the extensive previous preclinical research, the current study provides sufficient encouraging preliminary clinical data to warrant the tremendous effort and expense required to test whether long-term orally administered NAC can safely slow the normally inexorable loss of cones and thus prevent blindness in RP.
Methods
Study design.
The FIGHT RP trial was a single-center, open-label clinical trial designed to test the safety and tolerability, pharmacokinetics, and effects on several parameters of visual function of several dosing regimens of NAC in patients with RP (ClinicalTrials.gov Identifier: NCT03063021). The first patient was enrolled in February 2017 and the last patient enrolled completed the final study visit in February 2019. A total of 30 patients were enrolled, 10 in each of 3 cohorts: (a) cohort 1, 600 mg NAC bid × 12 weeks followed by 600 mg NAC tid × 12 weeks; (b) cohort 2, 1200 mg NAC bid × 12 weeks followed by 1200 mg NAC tid × 12 weeks; and (c) cohort 3, 1800 mg NAC bid × 12 weeks followed by 1800 mg NAC tid × 12 weeks. The doses in cohort 1 were selected based on previous studies in patients with pulmonary fibrosis, which had demonstrated that doses of NAC up to 600 mg tid are well tolerated (33). Treatment with NAC was stopped at 24 weeks and subjects were followed for an additional 12 weeks. The trial adhered to the tenets of the Declaration of Helsinki and was conducted in accordance with the International Conference of Harmonisation E6 Guidelines for Good Clinical Practice.
Study population.
The study population was composed of men and women 18 years or older with a diagnosis of RP based on clinical phenotype and diagnostic testing. Patients were excluded if they had another retinal disease in addition to RP in both eyes that could contribute to visual dysfunction, or they had RP that was so advanced that fixation was compromised to the point that they could not perform 1 or more study tests. All outcomes were measured for both eyes except 1 eye that had another disease process that could affect visual function, but only 1 eye was selected for obtaining aqueous samples.
Study protocol.
All participants had a screening visit to determine eligibility and a baseline visit at which the first dose of NAC was administered. At both of these visits BCVA and retinal sensitivity were measured to provide 2 pretreatment assessments. Other tests included SD-OCT and autofluorescence using the Spectralis (version 6.8.1.0) and ultrawide-angle fundus photographs (Optos 200Tx Version 1.3.0.154PLC). For SD-OCT, pupils of both eyes were dilated and 49-section-volume (20° × 20°) high-resolution scans were obtained for each eye with automatic real-time image mean function of 9 or more images and a quality index ≥ 20 dB (range, 0–40 dB). The follow-up scans were marked as progression scans with screening visit set as reference. A plasma sample was obtained 1 and 2 hours after NAC administration; however, subjects and families found it to be a hardship to remain at the study site for the 2-hour postdose sample and often refused. Therefore, the protocol was amended so that after the first cohort, only a 1-hour postdose plasma sample was obtained.
Subjects returned every 4 weeks for follow-up study visits, during which they had assessment of AEs and change in concomitant medications, vital signs, measurement of BCVA, microperimetry, eye examination, and anterior chamber tap. Empty blister packs were collected and counted, study medication diaries were collected, and study medication was dispensed. SD-OCT was done at the week 12, 24, and 36 visits. Subjects were instructed not to take study medication the morning of their week 12 or 24 visit and instead it was administered at the study visit. A plasma sample was obtained predose and 1 hour postdose during the visit.
Study medication.
NAC was provided as 600 mg effervescent tablets (Zambon Pharmaceuticals). For each cohort, patients were shown how many tablets should be dissolved in a glass of water for each dose, and for the first dose this was done for each patient. They were then shown how to store empty blister packs for collection by the study team at the next visit and how to record the time and number of tablets taken for each dose in the study medication diary.
Outcomes.
The primary outcome was the safety and tolerability of NAC. This was assessed by detailed questioning and review of systems at every study visit to determine if any symptoms had emerged. Patients were also required to maintain a diary documenting when and how much NAC was taken and whether any symptoms occurred. Secondary outcomes include the longitudinal changes in BCVA, mean macular sensitivity, and width of the EZ during the 24 weeks of treatment. The EZ was identified as the hyperreflective band located between the hyperreflective layer of the retinal pigment epithelium and the lesser reflective band of the external limiting membrane. The temporal and nasal margins of the EZ were marked and the EZ width was measured using the measurement tool in the Spectralis software. Because all the follow-up scans were set as progression scans, the same section was marked in sequential visits, to determine the change in EZ width.
Measurement of NAC levels in aqueous and plasma, and reduced/oxidized glutathione in aqueous.
Samples were stored at –80°C until assayed. Assays were performed at AIT Biosciences. The d3-labeled isotope of NAC, used as an internal standard (IS) for NAC, was obtained from Toronto Research Chemicals and stored at 2°C to 8°C. Glutathione-13C2,15N used as an IS for glutathione was obtained from Sigma-Aldrich. 2-Chloro-1-methylpyridinium iodide (CMPI), tris(2-carboxyethyl) phosphine (TCEP), and disodium ethylenediamine tetraacetic acid (EDTA) were obtained from Sigma-Aldrich. Acetonitrile, methanol, ammonium bicarbonate, formic acid, ammonium hydroxide solution, and acetic acid were LC-MS grade (Thermo Fisher Scientific). Extractions were performed using a Tomtec Quadra 96 system, polypropylene 1.2-mL 96-well plates, and cap mats (Thermo Fisher Scientific). Samples were analyzed on a Waters Acquity liquid chromatograph (Waters Corporation) interfaced with a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific) and ionization in positive-ion mode. An ethylene-bridged hybrid stationary phase for hydrophilic-interaction chromatography (BEH HILIC, 2.1 × 100 mm, 1.7 μm; Waters Corporation) was used. Free or total NAC in plasma was quantified according to a previous method (34). For total NAC in aqueous humor, the same procedure was used, substituting aqueous humor for plasma, with the following modifications: 4.0 μL aliquot of ammonium hydroxide, 25 μL aliquot of 200 ng/mL NAC-d3 in 0.5 mg/mL disodium EDTA (pH 7.0), 10.0 μL of 100 mM ammonium carbonate, 5.0 μL of CMPI (60 mM), 5.0 μL of TCEP (60 mM), 300 μL of acetonitrile, and 150 μL of supernatant.
Statistics.
Subject demographic and clinical characteristics at the baseline visit were first summarized (for each participant, the secondary outcomes were measured at both the screening day and the baseline visit day when treatment was issued. The average values of measurements from these 2 visits were used to summarize the baseline and pretreatment status). AEs observed during the study period were summarized. Graphic methods such as spaghetti plots were used to present the longitudinal data of the secondary outcomes over the 36 weeks of observation.
The aqueous humor and plasma NAC levels at each visit were summarized for each cohort. In addition, linear models with generalized estimating equation (GEE) technique were used to estimate the difference in mean aqueous NAC levels during the bid dosing period (i.e., data from visits at weeks 4, 8, and 12) compared to the mean aqueous NAC levels during tid dosing (i.e., data from visits at weeks 16, 20, and 24) in each cohort. Moreover, for each dosing period (bid or tid), a linear model with GEE was used to estimate the difference in the mean aqueous levels between the 3 cohorts. A first-order autoregressive working correlation matrix was used in all GEE models (35).
Longitudinal data of the secondary outcomes during the 24-week treatment period were analyzed in 2 ways to assess the changes: first, linear mixed-effects models were used to estimate the rate of change of each outcome, using data from all visits during the treatment period. Specifically, for each outcome variable, the mean of the outcome was modeled as a linear function of time since baseline, and a main effect for cohort and an interaction effect between cohort and time was also included in the modeling. A random intercept and random slope model was first fit, where for each of the intercept and the slope parameters a random effect for participant and a random effect for eye (nested with participant) was included. An unstructured variance-covariance matrix of the random effects for the intercept and slope parameters was used. This model allowed that the individual eyes had different intercepts and rates of change and allowed different cross-sectional correlations between eyes and longitudinal correlations within an eye and across eyes. Additionally, for model parsimony, a random-intercept-only model was also fit, where a random effect for participant and a random effect for eye was included for the intercept term only. The model (random intercept and random slope vs. random intercept only) with smaller AIC (Akaike information criterion) was used as the final model to report the rate of change of that outcome variable.
The second way of assessing changes during the treatment period compared the measurements between the baseline and the last treatment visit (i.e., 24-week visit). The change of each outcome variable was summarized by cohort, and a linear model with GEE was used to test whether there was a statistically significant change while accounting for correlation between eyes within each cohort.
All statistical analysis was conducted using SAS 9.4. All P values reported were based on 2-sided tests without multiple testing adjustment. For the linear mixed-effects models, the P values were from tests using the updated Kenward and Roger method to calculate the denominator degrees of freedom (35).
Study approval.
The study was performed through an Investigator IND from the FDA and the protocol was approved by the Johns Hopkins Medicine Institutional Review Board. Written informed consent was obtained from all participants.
Author contributions
PAC designed the study protocol, performed study evaluations, collected data, analyzed and interpreted data, and wrote the first draft of and approved the manuscript. MI performed study evaluations, collected data, analyzed data, prepared figures, and edited and approved the manuscript. GH assisted in protocol design, performed study evaluations, collected data, and edited and approved the manuscript. AA and GT collected data, assisted in figure preparation, and edited and approved manuscript. DW performed study evaluations, collected data, and edited and approved manuscript. LL collected data and edited and approved the manuscript. GMW assisted in data analysis and edited and approved the manuscript. MSS performed study evaluations and edited and approved the manuscript. XK assisted in data analysis and interpretation, performed all statistical analysis, and edited and approved the manuscript.
Supplementary Material
Acknowledgments
Funding was provided by Mr. and Mrs. Robert Wallace, Mr. and Mrs. Jonathan Wallace, Rami and Eitan Armon, Marc Sumerlin, and Cassandra Hanley. The authors thank Cameron Brown and his Colleagues at AIT Biosciences, Indianapolis, Indiana, USA, for the analyses of NAC in plasma and aqueous humor, which were funded by Nacuity Pharmaceuticals, Inc.
Version 1. 12/05/2019
In-Press Preview
Version 2. 02/17/2020
Electronic publication
Version 3. 03/02/2020
Print issue publication
Footnotes
Conflict of interest: Johns Hopkins University has a licensing agreement concerning N-acetylcysteine amide with a company, Nacuity Pharmaceuticals, Inc. (Nacuity), under which the University is entitled to royalty distributions and has equity in Nacuity. GMW is also an employee of Nacuity.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(3):1527–1541.https://doi.org/10.1172/JCI132990.
Contributor Information
Peter A. Campochiaro, Email: pcampo@jhmi.edu.
Mustafa Iftikhar, Email: mustafaiftikhar92@gmail.com.
Gulnar Hafiz, Email: ghafiz1@jhmi.edu.
Anam Akhlaq, Email: aakhlaq1@jhmi.edu.
Grace Tsai, Email: gtsai8@jhmi.edu.
Dagmar Wehling, Email: dwehlin1@jhu.edu.
Lili Lu, Email: llu3@jhmi.edu.
G. Michael Wall, Email: michael@nacuity.com.
Mandeep S. Singh, Email: mandeep@jhmi.edu.
Xiangrong Kong, Email: xkong4@jhu.edu.
References
- 1.Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl. 2002;(233):1–34. doi: 10.1046/j.1395-3907.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 2.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125(2):151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campochiaro PA, Mir TA. The mechanism of cone cell death in retinitis pigmentosa. Prog Retin Eye Res. 2018;62:24–37. doi: 10.1016/j.preteyeres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41(12):3999–4006. [PubMed] [Google Scholar]
- 5.Shen J, et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203(3):457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 6.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213(3):809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Fernández de la Cámara C, et al. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS ONE. 2013;8(9):e74223. doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campochiaro PA, et al. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal. 2015;23(7):643–648. doi: 10.1089/ars.2015.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldini G, et al. N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52(7):751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 11.Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2(8035):432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 12.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, et al. N-Acetylcysteine promotes long-term survival of cones in a model of retinitis pigmentosa. J Cell Physiol. 2011;226(7):1843–1849. doi: 10.1002/jcp.22508. [DOI] [PubMed] [Google Scholar]
- 14.Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–1806. doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina (Philadelphia, Pa) 2011;31(8):1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;121(8):1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Birch DG, Locke KG, Wen Y, Locke KI, Hoffman DR, Hood DC. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013;131(9):1143–1150. doi: 10.1001/jamaophthalmol.2013.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen FK, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50(7):3464–3472. doi: 10.1167/iovs.08-2926. [DOI] [PubMed] [Google Scholar]
- 19.Cideciyan AV, et al. Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012;53(2):841–852. doi: 10.1167/iovs.11-8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong EN, Mackey DA, Morgan WH, Chen FK. Intersession test-retest variability of conventional and novel parameters using the MP-1 microperimeter. Clin Ophthalmol. 2016;10:29–42. doi: 10.2147/OPTH.S92018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai CX, Locke KG, Ramachandran R, Birch DG, Hood DC. A comparison of progressive loss of the ellipsoid zone (EZ) band in autosomal dominant and X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014;55(11):7417–7422. doi: 10.1167/iovs.14-15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usui S, et al. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther. 2009;17(5):778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usui S, et al. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009;110(3):1028–1037. doi: 10.1111/j.1471-4159.2009.06195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Channa R, et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond) 2014;28(3):269–278. doi: 10.1038/eye.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bressler SB, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130(9):1153–1161. doi: 10.1001/archophthalmol.2012.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iftikhar M, et al. Progression of retinitis pigmentosa as measured on microperimetry: The PREP-1 study. Ophthalmol Retina. 2018;2(5):502–507. doi: 10.1016/j.oret.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Liew G, et al. Prevalence of cystoid macular oedema, epiretinal membrane and cataract in retinitis pigmentosa. Br J Ophthalmol. 2019;103(8):1163–1166. doi: 10.1136/bjophthalmol-2018-311964. [DOI] [PubMed] [Google Scholar]
- 29.Riley MV, Meyer RF, Yates EM. Glutathione in the aqueous humor of human and other species. Invest Ophthalmol Vis Sci. 1980;19(1):94–96. [PubMed] [Google Scholar]
- 30.Aref AA, Budenz DL. Detecting visual field progression. Ophthalmology. 2017;124(12S):S51–S56. doi: 10.1016/j.ophtha.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 31. Little RJA, Rubin DB. Stastitical Analysis with Missing Data. Hoboken, New Jersey, USA: John Wiley & Sons; 2002. [Google Scholar]
- 32.Sullivan LS, et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Invest Ophthalmol Vis Sci. 2006;47(7):3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demedts M, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 34.King B, Vance J, Wall GM, Shoup R. Quantitation of free and total N-acetylcysteine amide and its metabolite N-acetylcysteine in human plasma using derivatization and electrospray LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1109:25–36. doi: 10.1016/j.jchromb.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 35. SAS Institute. SAS/STAT® 9.4 User’s Guide. Cary, North Carolina, USA: SAS Institute; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.