Abstract
Objective:
Heightened generalization of fear from an aversively reinforced conditioned stimulus (CS+, a conditioned danger cue) to resembling stimuli is widely accepted as a pathogenic marker of posttraumatic stress disorder (PTSD). Indeed, a distress response to benign stimuli that “resemble” aspects of the trauma is a central feature of the disorder. To date, the link between overgeneralization of conditioned fear and PTSD derives largely from clinical observations, with limited empirical work on the subject. This represents the first effort to examine behavioral and brain indices of generalized conditioned fear in PTSD using systematic methods developed in animals known as generalization gradients: the gradual decline in conditioned responding as the presented stimulus gradually differentiates from CS+.
Method:
Gradients of conditioned fear generalization were assessed using functional MRI and behavioral measures in U.S. combat veterans who served in Iraq or Afghanistan and had PTSD (N=26), subthreshold PTSD (N=19), or no PTSD (referred to as trauma control subjects) (N=17). Presented stimuli included rings of graded size, with extreme sizes serving as CS+ (paired with shock) and as a nonreinforced conditioned stimulus (CS−, a conditioned safety cue), and with intermediate sizes forming a continuum of similarity between CS+ and CS−. Generalization gradients were assessed as response slopes from CS+, through intermediate ring sizes, to CS−, with less steep slopes indicative of stronger generalization.
Results:
Relative to trauma control subjects, PTSD patients showed stronger conditioned generalization, as evidenced by less steep generalization gradients in both behavioral risk ratings and brain responses in the left and right anterior insula, left ventral hippocampus, dorsolateral and dorsomedial prefrontal cortex, and caudate nucleus. Severity of PTSD symptoms across the three study groups was positively correlated with levels of generalization at two such loci: the right anterior insula and left ventral hippocampus.
Conclusions:
The results point to evidence of brain-based markers of overgeneralized fear conditioning related to PTSD. These findings provide further understanding of a central yet understudied symptom of trauma-related psychopathology.
Keywords: PTSD, fear-conditioning, stimulus-generalization, fMRI
Introduction
Generalization of conditioned fear is a basic, cross-species, associative-learning process whereby fear acquired to a conditioned stimulus (CS+), paired with an aversive unconditioned stimulus, transfers to safe stimuli resembling the CS+ (1). Heightened levels of generalized conditioned fear have been adopted as a core feature of trauma-related psychopathology (2), and DSM-5 criteria for posttraumatic stress disorder (PTSD) include heightened distress to situations “resembling” aspects of the trauma. The pathogenic contribution of conditioned generalization to PTSD follows from the undue proliferation of trauma cues in an individual’s posttrauma environment that then increases and/or sustains PTSD symptoms.
Despite clinical consensus linking PTSD to generalized conditioned fear, to our knowledge no laboratory-based studies have tested this link using systematic generalization methods developed in animals. Such methods assess conditioned fear to both CS+ and generalization stimuli (GS) parametrically varying in similarity to CS+, and document generalization gradients, or slopes, with the highest levels of responding to CS+ and with gradually declining levels of fear to GS of decreasing perceptual similarity to CS+ (3). Through this method, the strength of generalization is indexed by the steepness of gradients, with less steep downward gradients indicating greater generalization.
In both intact animal and healthy human subjects, generalization gradients are characterized by steep downward slopes reflecting precipitous quadratic declines in conditioned responding from CS+ to the closest one to three approximations of the conditioned danger cue, followed by a leveling off of responses to remaining GS (3–5). One central aim of the present study was to assess the degree to which neural and behavioral generalization gradients in individuals with PTSD or subthreshold PTSD deviate from this pattern, with less quadratic and more gradual linear generalization gradients, reflective of overgeneralization, predicted in those with PTSD compared with those without PTSD. Candidate neural loci of such gradients derive from past animal and human studies of generalized conditioned fear.
In lower mammals, lesions of either the hippocampus (6, 7) or the cortical inputs to the hippocampus (the postrhinal or perirhinal cortex) (8) increase generalization of fear from CS+ to resembling conditioned safety cues (CS−). Findings from these studies suggest that the hippocampus is necessary for successful discrimination of CS+ from CS−, potentially attributable to hippocampally mediated pattern separation found in rodents (9) and humans (10) through which brain representations of resembling, yet distinct, sensory experiences are discriminated. Consistently, our previous functional MRI (fMRI) results demonstrate robust gradients of generalization in the human hippocampus. Specifically, activations in the left and right ventral hippocampus were strongest to GS most distinguishable from CS+ (stimuli for which pattern separation is most appropriate), with levels decreasing bilaterally as the presented stimulus became more similar to CS+ (11). These results are consistent with the proposed role of hippocampally mediated pattern separation in human conditioned fear generalization.
Additional candidate brain substrates of human generalization derive from the long-observed finding that GS elicit the same response evoked by CS+, with gradual declines as the GS differentiate from CS+ (12). Thus, fear-related brain activations to CS+ found repeatedly in the amygdala (13), anterior insula (13, 14), dorsomedial prefrontal cortex (13–15), dorsal anterior cingulate (14), inferior parietal lobule (14), and dorsolateral prefrontal cortex (16) are predicted to decrease as the presented GS diverge from CS+. Conversely, safety-related activations to CS− repeatedly found by human neuroimaging studies in the ventromedial prefrontal cortex (17), hippocampus (17), and precuneus (17) are predicted to gradually increase as the GS become less similar to CS+. Results from recent fMRI studies of fear generalization are largely consistent with these predictions and find gradually decreasing activations in the anterior insula (11, 16, 18–20), dorsomedial prefrontal cortex (11, 18, 19), inferior parietal lobule (11), and dorsolateral prefrontal cortex (11) (downward gradients) and gradually increasing activations in the ventromedial prefrontal cortex (11, 18–20), hippocampus (11, 20), and precuneus (11) (upward gradients) as the presented GS differentiate from CS+. Furthermore, several such activations have been linked to the hippocampus, as functional connectivity studies reveal stronger connectivity between the ventral hippocampus and brain areas associated with fear excitation (amygdala, anterior insula) to stimuli with more, as opposed to less, resemblance to CS+, as well as stronger connectivity between the ventral hippocampus and brain areas associated with fear inhibition (ventromedial prefrontal cortex, precuneus) to stimuli with less, as opposed to more, resemblance to CS+ (11). These connectivity findings are consistent with the proposal that exposure to GS elicits hippocampal activation of neural substrates of either fear excitation or fear inhibition, depending on the degree of similarity between the GS and CS+ (11).
The central goal of the present work was to elucidate PTSD-related perturbations in the aforementioned neural and behavioral substrates of generalization. To this end, a previously validated fMRI generalization paradigm (11) was applied in trauma survivors to test predictions that PTSD is associated with 1) less steep downward gradients of behavioral generalization indicative of overgeneralization; 2) less steep downward and upward neural gradients of generalization instantiated in brain areas associated with fear excitation and fear inhibition, respectively; and 3) heightened functional connectivity between the ventral hippocampus and fear excitation areas of the brain (anterior insula, dorsomedial prefrontal cortex, and amygdala) and with reduced functional connectivity between the ventral hippocampus and fear-inhibitory areas of the brain (ventromedial prefrontal cortex) while processing safe stimuli resembling CS+.
Methods
Participants were 71 male U.S. combat veterans of the wars in Iraq and Afghanistan categorized into three groups: those with PTSD (N=26), those with subthreshold PTSD (N=23), and those without PTSD, referred to as trauma control subjects (N=22). The PTSD group comprised veterans meeting criteria for PTSD as assessed by the Clinician-Administered PTSD Scale (CAPS) (21). Participants with subthreshold PTSD and trauma control subjects did not meet criteria for PTSD and were defined by CAPS scores from 20 to 39 and from 0 to 19, respectively, as recommended (22). Participants who did not meet study conditions (four with PTSD, two with subthreshold PTSD, and one trauma control subject) were excluded from the analyses because they had no fear responses available for generalization. In addition, two PTSD and one trauma control subject were removed because of excessive head motion during scanning. Final analyses were conducted on 20 PTSD, 21 subthreshold PTSD, and 20 trauma control subjects. Applied exclusion criteria are listed in the data supplement that accompanies the online edition of this article. (The data supplement also includes sample characteristics, shown in Tables S1 and S2; generalization paradigm parameters; and MRI image acquisition, methods for individual- and group-level analyses of fMRI data, and analysis of behavioral data.) All participants provided written informed consent after receiving a complete description of the study.
Results
Behavioral Findings
Figure 1 displays generalization gradients across groups. A 3×5 (group-by-stimulus type) interaction emerged (F=2.81, df=8, 112, p=0.007), indicating that patterns of generalization, as indexed by online risk ratings, differed across groups. Furthermore, group-by-stimulus type interactions, with levels of group reflecting each possible pair of groups, revealed a PTSD/trauma control subject-by-stimulus type interaction (F=4.97, df=4, 35, p=0.003) and a subthreshold PTSD/trauma control subject-by-stimulus type interaction (F=2.70, df=4, 36, p=0.05). There was no significant PTSD/subthreshold PTSD-by-stimulus type interaction. The significant group-by-stimulus type interactions, when defining group as PTSD subjects versus trauma control subjects, and as subthreshold PTSD subjects versus trauma control subjects, were attributable to more gradual linear declines in gradients in PTSD relative to trauma control subjects (F=7.42, df=1, 38, p=0.01) and to more gradual linear declines in subthreshold PTSD relative to trauma control subjects (F=4.60, df=1, 39, p=0.04), respectively.
FIGURE 1.
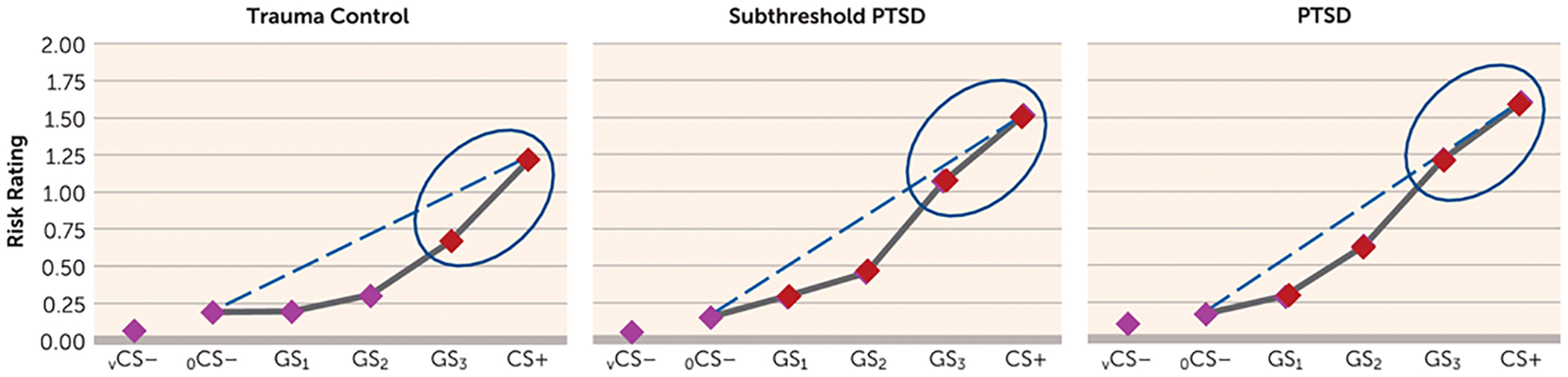
Generalization Results Across Groups for Ratings of Perceived Risk of Shock in a Study of Neural Substrates of Overgeneralized Conditioned Feara
a Perceived risk of shock (0=no risk, 1=some risk, 2=high risk) was assessed during conditioned danger cues (CS+), generalization stimuli (GS3, GS2, GS1), and conditioned safety cues (ring-shaped [oCS−] or V-shaped [vCS−]), forming a continuum of similarity between CS+ and CS−. Generalized conditioned fear was evidenced by the main effects of stimulus type in each group (all p values <0.0001), reflecting downward gradients of perceived risk as stimuli differentiated from CS+. Dotted lines indicate hypothetical linear decreases in responding from CS+ to oCS− with which to visualize the deviation of gradients from linearity in each group. Such deviations reflect a significantly stronger linear component in the generalization gradient of posttraumatic stress disorder (PTSD) subjects relative to trauma control subjects (p=0.01) and of subthreshold PTSD subjects relative to trauma control subjects (p=0.04), indicating more gradual, linear declines indicative of overgeneralization in PTSD. To identify the point on the continuum of similarity at which perceived risk ceased to generalize for each group, planned comparisons contrasting oCS− against CS+ and GS3, GS2, and GS1 were computed. Red data points signify stimulus types eliciting increased risk ratings relative to oCS− after applying Hochberg’s adjustment for multiple tests (23). In trauma control subjects, perceived risk was elevated from oCS− to CS+ (p<0.0001) and GS3 (p<0.0001), but not GS2 (p=0.06) or GS1 (p=0.91). By contrast, in PTSD and subthreshold PTSD subjects, perceived risk was elevated from oCS− to CS+ (all p values <0.0001), GS3 (all p values <0.0001), GS2 (all p values <0.0001), and GS1 (all p values ≤0.016). Thus, while trauma control subjects generalized perceived risk only to one degree of differentiation from CS+ (i.e., GS3), those with PTSD and subthreshold PTSD generalized to three degrees of differentiation (i.e., GS3–GS1). Ellipses around the rightmost data points highlight response slopes from CS+ to GS3 that are more gradual and linear in PTSD and subthreshold PTSD but more steep than linear in trauma control subjects.
Group effects are further detailed in Figure 1, which displays gradients relative to hypothetical linear declines (dotted lines) with which to visualize gradient steepness. As shown in Figure 1, gradients for trauma control subjects decline in a more precipitous than linear fashion, whereas gradients for PTSD subjects decline along a more gradual, linear slope, reflecting higher levels of behavioral generalization in PTSD. Figure 1 also displays the number of GS to which generalization extended, with trauma control subjects generalizing perceived risk only to the closest CS+ approximation (GS3) and generalization in subjects with PTSD and subthreshold PTSD extending to the third approximation (GS1). These group differences demonstrate that those with PTSD, relative to trauma control subjects, manifest less steep generalization gradients, with fear responses extending to safety cues with less danger cue similarity.
fMRI Results
Several functional regions of interest, defined using a CS+ contrast compared with a V-shaped conditioned safety cue (vCS−) contrast, fell along either positive or negative generalization gradients during the generalization test (see Table S3 in the online data supplement). Positive gradients reflect strongest responding to CS+, with decreases as rings differentiate from CS+. Negative gradients reflect strongest responding to vCS−, with decreases as rings differentiate from vCS−.
Group effects.
Functional regions of interest displaying both generalization gradients and group (PTSD, versus subthreshold PTSD versus trauma control subjects; or PTSD versus trauma control subjects)-by-stimulus type interactions are shown in Table 1 and include the dorsomedial prefrontal cortex, the left and right anterior insula, the dorsolateral prefrontal cortex (Brodmann’s area 9 [BA9]), and the left and right caudate body (positive gradients); and the left ventral hippocampus/amygdala and the left ventral hippocampus (negative gradients). Blood-oxygen-level-dependent (BOLD) signal in these functional regions of interest were next plotted across stimulus type for each group. As shown in Figure 2, group differences in the degree of linear deviation in positive gradients are remarkably consistent across functional regions of interest. Specifically, positive generalization gradients in trauma control subjects are substantially steeper than linear gradients and are characterized by quadratic declines as the presented stimulus deviates from CS+. By contrast, comparable neural gradients in PTSD subjects, and to some degree in subthreshold PTSD subjects, deviate little from the linear and fall along more gradual linear declines compared with trauma control subjects. This visual assessment is supported statistically by significant group (PTSD versus trauma control subjects)-by-stimulus type quadratic trends in all six positive gradient functional regions of interest with significant multivariate group (PTSD versus trauma control subjects)-by-stimulus type interactions (Table 1), which were driven by stronger quadratic declines in slopes in trauma control subjects relative to PTSD subjects. That is, PTSD patients display less steep (less quadratic) neural gradients of generalization, indicative of overgeneralization, in the dorsomedial prefrontal cortex, right anterior insula, left anterior insula, right BA9, right caudate body, and left caudate body. This was also found in the right inferior parietal lobule, an area without significant multivariate group-by-stimulus type interactions. No significant group-by-stimulus type interactions were found in functional regions of interest falling along positive gradients when defining the group as subthreshold PTSD versus trauma control subjects. Furthermore, as shown in Figure 2, positive neural gradients of generalization in PTSD often extend as far as two to three CS+ approximations (i.e., GS2 or GS1), while generalization in subthreshold PTSD and trauma control subjects consistently extends only to the closest approximation (i.e., GS3). These neural findings demonstrate that subjects with PTSD require less danger cue similarity to trigger fear-related brain processes and provide further evidence of overgeneralization in PTSD.
TABLE 1.
Group Effects in Functional Regions of Interest Instantiating Positive or Negative Generalization Gradients in a Study of Neural Substrates of Overgeneralized Conditioned Feara
| Group by Generalization Gradient | ||||||||
|---|---|---|---|---|---|---|---|---|
| PTSD, Subthreshold PTSD, and Trauma Control Subjects | PTSD and Trauma Control Subjects | Subthreshold PTSD and Trauma Control Subjects | PTSD and Subthreshold PTSD Subjects | |||||
| Functional Region of Interest | Wilks’s Lambda | Quadratic Component of Gradient | Wilks’s Lambda | Quadratic Component of Gradient | Wilks’s Lambda | Quadratic Component of Gradient | Wilks’s Lambda | Quadratic Component of Gradient |
| Positive gradients | ||||||||
| Dorsomedial prefrontal cortex | 2.62** | 4.77** | 2.76* | 9.55** | 2.01 | 2.00 | 329* | 2.88 |
| Right anterior insula | 2.74** | 8.98*** | 6.62*** | 27.26*** | 0.90 | 1.92 | 274* | 6.81** |
| Left anterior insula | 1.21 | 4.24* | 3.85** | 16.42*** | 0.28 | 0.67 | 127 | 3.22 |
| Right dorsolateral prefrontal cortex (Brodmann’s area 9) | 3.81* | 4.55* | 4.73** | 10.93** | 1.17 | 1.97 | 241 | 2.36 |
| Right caudate body | 1.21 | 2.32 | 2.67* | 4.90* | 0.46 | 0.42 | 088 | 2.00 |
| Left caudate body | 0.79 | 2 07 | 2.74* | 5.68* | 0.55 | 0 57 | 052 | 136 |
| Right inferior parietal lobule | 0.80 | 1.94 | 1.51 | 4.40* | 0.24 | 0.14 | 0.98 | 2.08 |
| Negative gradients | ||||||||
| Left ventral hippocampus/amygdala | 1.29 | 5.27** | 2.96* | 12.63*** | 1.55 | 6.34* | 0.09 | 0.01 |
| Left ventral hippocampus | 1.15 | 2.11 | 2.42* | 7.44** | 1.22 | 0.89 | 042 | 0.90 |
| Right ventral hippocampus | 1.51 | 2.90 | 2.22 | 4.43* | 1.72 | 4.21* | 097 | 1.04 |
| Right caudate head | 0.74 | 2.82 | 2.16 | 4.49* | 1.70 | 0.39 | 137 | 3.54 |
| Ventromedial prefrontal cortex | 1.91 | 0.78 | 1.35 | 1.71 | 2.84* | 0.40 | 089 | 0.37 |
Positive gradients are slopes with the strongest brain activations to a conditioned danger cue (CS+) and have gradual decreases as rings differentiate from CS+. Negative gradients are slopes with the strongest brain activations to a V-shaped conditioned safety cue (vCS−) and have gradual decreases as rings differentiate from vCS−. PTSD = posttraumatic stress disorder.
p≤0.05.
p≤0.01.
p≤0.001.
FIGURE 2.
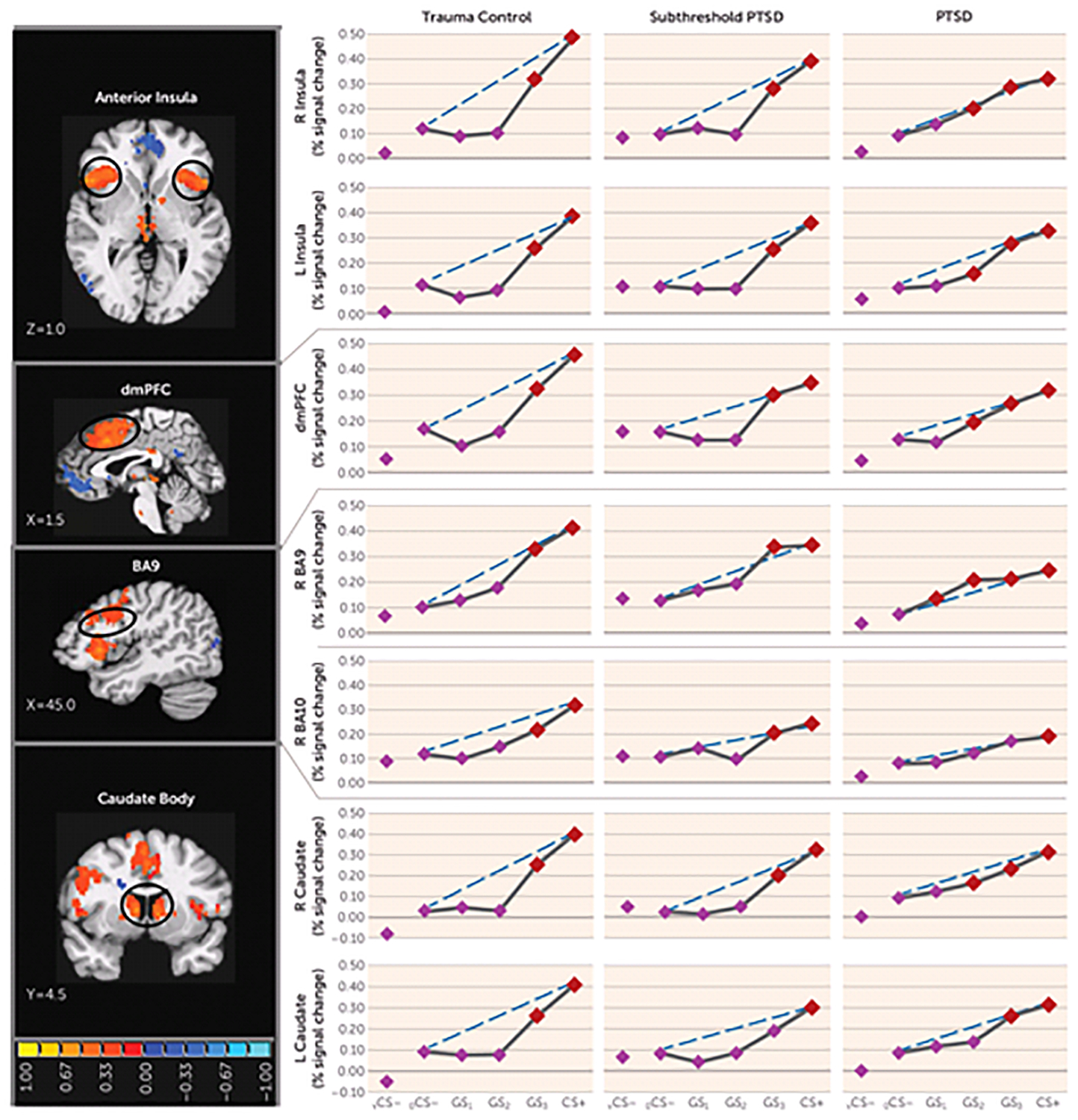
Group Differences in Positive Neural Gradients of Generalization in a Study of Neural Substrates of Overgeneralized Conditioned Feara
a Brain activations reflect functional regions of interest responding more strongly to a conditioned danger cue (CS+) compared with a V-shaped conditioned safety cue (vCS−) (orange activations) for which the shape of positive generalization gradients depended on group status. Dotted lines indicate hypothetical linear decreases in responding from CS+ to a ring-shaped conditioned safety cue (oCS−) with which to visualize the deviation of gradients from linearity in each group. Subjects with posttraumatic stress disorder (PTSD) (relative to trauma control subjects) displayed markedly less deviation from linearity (i.e., fewer quadratic declines) in all functional regions of interest, indicating a tendency toward less steep generalization gradients (or stronger generalization) in veterans with PTSD. This assertion was further tested with planned comparisons contrasting levels of reactivity to oCS− compared with CS+ and the three classes of generalization stimuli (GS3, GS2, and GS1) to identify the point on the continuum of similarity at which activations in functional regions of interest cease to generalize for each group. Red data points signify stimulus types eliciting brain responses stronger than oCS− after applying Hochberg’s adjustment for multiple comparisons at the group level for each functional region of interest. As stipulated by the Hochberg procedure (23), specific criterion p values were a function of p values generated by each set of comparisons and ranged from p≤0.0125 to p≤0.05. L=left; R=right; dmPFC=dorsomedial prefrontal cortex; BA=Brodmann’s area. Coordinates are based on the left-posterior-inferior (LPI) system.
We also found group effects for negative neural gradients, with PTSD versus trauma control subject-by-stimulus type interactions in the left ventral hippocampus/amygdala and in the left ventral hippocampus (Table 1). As shown in Figure 3, significant group differences in negative gradient shape were driven by BOLD increases from CS+ to GS to CS− that tended to be quadratic and more steep than linear in trauma control subjects but more linear (or less steep than linear) in PTSD subjects. Indeed, group (PTSD versus trauma control subjects)-by-stimulus type quadratic trends indicated that quadratic components of negative gradients were significantly stronger in trauma control subjects relative to PTSD subjects in both the left ventral hippocampus/amygdala and the left ventral hippocampus (see Table S3 in the online data supplement). This finding was also made in the left ventral hippocampus (p=0.01), right ventral hippocampus (p=0.04), and right caudate head (p=0.04) (Table 1), three areas without significant multivariate group-by-stimulus type interactions. Contrary to predictions, no group effects were found in the ventromedial prefrontal cortex. Because such functional regions of interest all responded strongest to safe stimuli (to vCS− and to a ring-shaped conditioned safety cue [oCS−]), activations at these loci are thought to reflect safety-related processes (e.g., pattern separation of GS from CS+ [ventral hippocampus] [11], behavioral inhibition [caudate head] [24], and fear inhibition [ventromedial prefrontal cortex] [17]). Thus, less steep increases in responses from CS+ to GS to CS− in the ventral hippocampus and caudate head, found among those with PTSD, suggest that presented stimuli require more differentiation from CS+ before safety-related processes come online in those with PTSD compared with those without PTSD. This assertion was further supported by follow-up comparisons of reactivity to CS+ compared against each of the other stimuli, indicating elevated safety-related brain processes in the ventral hippocampus and caudate head to GS with as little as one degree of CS+ differentiation (i.e., GS3) in trauma control subjects but with three or more degrees of differentiation in PTSD subjects, indicating that more CS+ dissimilarity is needed before safety processes come online in PTSD.
FIGURE 3.
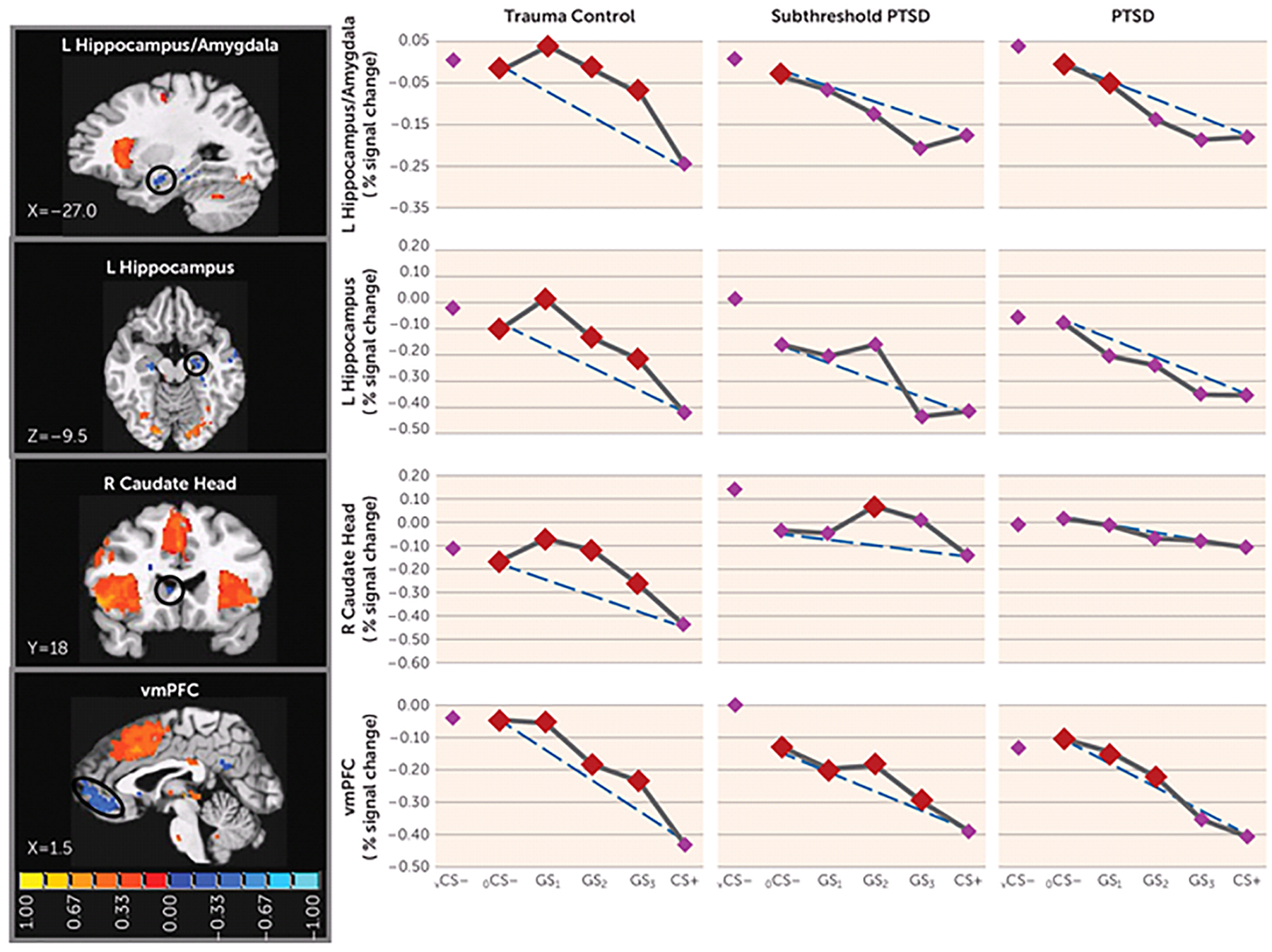
Group Differences in Negative Neural Gradients of Generalization in a Study of Neural Substrates of Overgeneralized Conditioned Feara
a Brain activations reflect functional regions of interest responding more strongly to a V-shaped conditioned safety cue (vCS−) compared with a conditioned danger cue (CS+) (blue activations) for which the shape of negative generalization gradients depended on group status. Dotted lines indicate hypothetical linear decreases in responding from a ring-shaped conditioned safety cue (oCS−) to CS+ with which to visualize the deviation of gradients from linearity in each group. To identify the point on the continuum of similarity at which safety-related brain responses come online in each group, planned comparisons contrasted responses to CS+ versus oCS−, GS1, GS2, and GS3. Red data points signify stimulus types eliciting brain responses stronger than CS+ after applying Hochberg’s adjustment for multiple comparisons at the group level for each functional region of interest. As stipulated by the Hochberg procedure (23), specific criterion p values were a function of p values generated by each set of comparisons and ranged from p≤0.0125 to p≤0.05. L=left; R=right; vmPFC=ventromedial prefrontal cortex. Coordinates are based on the left-posterior-inferior (LPI) system.
PTSD Symptom Severity and Strength of Generalization
Across all participants (N=61), CAPS scores were correlated with the steepness of behavioral generalization gradients as well as with neural gradients of generalization in the eight functional regions of interest with significant multivariate group-by-stimulus type interactions (Table 1). As has been done previously, strength of generalization in behavioral and neural measures was quantified using a linear deviation score (25): a single number capturing the steepness of generalization gradients, with larger values indicating more shallow gradients reflective of stronger generalization (see the data supplement). Although total CAPS scores did not significantly correlate with strength of behavioral generalization (r=0.15, p=0.24), after applying Hochberg’s correction for multiple comparisons (23) to the eight correlations between CAPS score and activations in functional regions of interest (criterion p≤0.007), CAPS scores were correlated with levels of generalization in the right anterior insula (Figure 4; r=0.34, p=0.007), the left ventral hippocampus/amygdala (Figure 4; r=0.36, p=0.004), and fell short of significance in the right ventral hippocampus (r=0.24, p=0.07), BA9 (r=0.24, p=0.06), and dorsomedial prefrontal cortex (r=0.21, p=0.11). The extent of generalization in these functional regions of interest thus represents promising brain markers of severity of PTSD symptoms.
FIGURE 4.
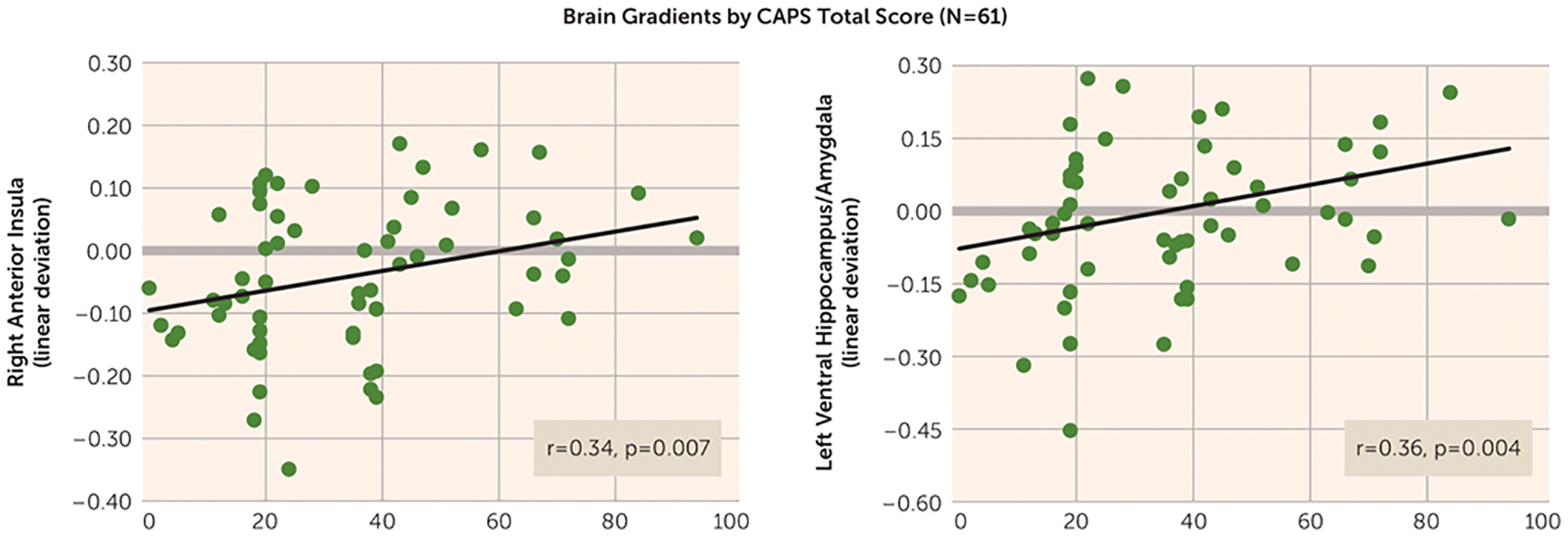
Scatterplots Displaying Significant Bivariate Relations Across All Subjects (N=61) Between Total Scores on the Clinician-Administered PTSD Scale (CAPS) and the Steepness of Positive and Negative Neural Gradients of Generalization in a Study of Neural Substrates of Overgeneralized Conditioned Feara
a Bivariate relation between CAPS scores and the steepness of both positive neural gradients of generalization in the right anterior insula and negative neural gradients of generalization in the left ventral hippocampus/amygdala. The y-axis shows a measure of gradient steepness reflecting the degree to which generalization gradients deviated from linearity. Larger linear deviation scores reflect more shallow downward slopes from the conditioned danger cue (CS+) to the conditioned safety cue (CS−) in the case of positive gradients and more shallow upward slopes from CS+ to CS− in the case of negative gradients. Of note, linear deviation was reverse scored for negative gradients such that larger scores consistently indicated greater generalization whether the generalization gradient was negative or positive. As shown, increasing levels of generalization at both these neural loci are associated with increasing levels of PTSD symptom severity as measured by the CAPS.
Connectivity results.
Psychophysiological interaction findings are fully detailed in Table S4 in the online data supplement. Consistent with predictions, psychophysiological interaction analyses with ventral hippocampus functional regions of interest as seeds revealed greater generalization-related coupling (i.e., connectivity during “all rings” > vCS−) in PTSD relative to trauma control subjects in brain areas associated with fear excitation: the right amygdala (seed: right ventral hippocampus), the left anterior insula (seeds: left ventral hippocampus/amygdala, and right ventral hippocampus), the dorsomedial prefrontal cortex (seed: right ventral hippocampus), and the right inferior parietal lobule (seeds: left ventral hippocampus/amygdala, left ventral hippocampus, right ventral hippocampus). Contrary to predictions, greater generalization-related coupling in PTSD relative to trauma control subjects was also found between the ventral hippocampus functional region of interest seeds and brain areas associated with fear inhibition: the ventromedial prefrontal cortex (seeds: left ventral hippocampus/amygdala, and right ventral hippocampus) and precuneus (seed: left ventral hippocampus). This finding could be due to GS eliciting more activity in fear-excitation areas in PTSD, requiring greater hippocampal activation of fear-inhibition processes.
Discussion
The present findings demonstrate overgeneralized fear to safe stimuli resembling conditioned danger cues in PTSD and elucidate brain substrates of this abnormality. Specifically, behavioral gradients of generalized conditioned fear in trauma control subjects formed steep quadratic declines, the gradient shape repeatedly found in intact animals (3) and healthy humans (4). By contrast, individuals with PTSD and to some degree those with subthreshold PTSD displayed behavioral gradients characterized by more gradual linear declines, reminiscent of gradient shapes found in panic (26) or generalized anxiety disorder (25) and indicating heightened persistence (generalization) of fear as presented stimuli differentiate from CS+. Neural results consistently mirrored this behavioral pattern, with most functional regions of interest displaying steeper, quadratic generalization gradients in trauma control subjects but more gradual linear gradients in PTSD subjects and, to some degree, in subthreshold PTSD individuals, indicative of overgeneralization. This pattern of group differences was found in brain areas coding for both positive (left anterior insula, right anterior insula, dorsomedial prefrontal cortex, dorsolateral prefrontal cortex [BA9], left and right caudate, right inferior parietal lobule) and negative generalization gradients (left ventral hippocampus/amygdala, and right caudate head).
Many of the aforementioned neural substrates of overgeneralization in PTSD have been previously shown to subserve human fear generalization (anterior insula [11, 16, 18–20], ventromedial prefrontal cortex [11, 18–20], ventral hippocampus [11, 20], dorsomedial prefrontal cortex [11, 18, 19], inferior parietal lobule [11], dorsolateral prefrontal cortex [11]), and many are constituents of a proposed neurobiology of generalization (11). Central to the model is discrimination of CS+ from resembling stimuli via hippocampally mediated pattern separation. When faced with a new stimulus event (i.e., a generalization stimulus) that resembles a past event stored in memory (i.e., CS+), the hippocampus is thought to perform a same-different determination between cortical representations of current and past events (27, 28). With decreasing representational overlap, the hippocampus increasingly differentiates cortical representations of current (GS) and past events (CS+) through pattern separation (9). When the past event is fear related, such pattern separation culminates in hippocampal activation of brain regions associated with fear inhibition (e.g., the ventromedial prefrontal cortex) (11). As in past generalization work (11), the present findings document ventral hippocampus activations falling along negative gradients, with the strongest responses to stimuli with the least schematic match to CS+ (i.e., vCS−, oCS−), or in other words, to stimuli most likely to elicit pattern separation. Furthermore, past and current hippocampal activations are least strong to CS+ and gradually strengthen with increasing CS+ differentiation (i.e., as pattern separation becomes more appropriate). Importantly, these negative generalization gradients in the ventral hippocampus were steep or quadratic in trauma control subjects but were gradual or linear in PTSD and subthreshold PTSD subjects, with significant ventral hippocampus increases (relative to CS+) requiring at least three degrees of CS+ differentiation in PTSD and subthreshold PTSD subjects (GS1–GS3) but only one degree in trauma control subjects (GS3). Such results suggest deficits in hippocampally mediated pattern separation in PTSD through which subjects with PTSD or subthreshold PTSD require more CS+ dissimilarity before generalization is blocked by such pattern separation. Further supporting the relationship between PTSD and ventral hippocampus gradient shape was the correlation between CAPS scores and steepness of ventral hippocampus gradients, implicating levels of generalization in the ventral hippocampus as a promising brain marker of PTSD symptom severity.
The less steep ventral hippocampus gradients in PTSD described above were expected to be accompanied by less steep ventromedial prefrontal cortex gradients in PTSD, given the proposed positive relationship between hippocampally mediated pattern separation and activations in the ventromedial prefrontal cortex (11). Although negative gradients in the ventral hippocampus were mirrored by similarly shaped negative gradients in the ventromedial prefrontal cortex, no group differences in the shape of the ventromedial prefrontal cortex emerged. Furthermore, functional connectivity between the ventral hippocampus and the ventromedial prefrontal cortex differed across groups but in a direction opposite to what was predicted. PTSD showed stronger coupling of the ventral hippocampus and ventromedial prefrontal cortex during GS compared with vCS−, suggesting that hippocampally mediated pattern separation actually activated fear inhibition mediated by the ventromedial prefrontal cortex during GS (relative to vCS−) to a greater extent in subjects with PTSD, which should culminate in less fear to GS in PTSD. However, this connectivity finding is consistent with overgeneralization in PTSD if it is interpreted as being driven by overly strong fear reactivity to GS in PTSD, which then necessitates more connectivity between the ventral hippocampus and ventromedial prefrontal cortex to (perhaps unsuccessfully) attempt inhibition of the heightened fear to GS.
In addition to hippocampal activation of areas of fear inhibition in the brain (ventromedial prefrontal cortex) commensurate with the dissimilarity of a given generalization stimulus from CS+, our generalization model proposes separate hippocampal areas (i.e., the CA3 subregion) that activate fear-excitation areas commensurate with the degree of similarity (29). That is, greater schematic matches between a given generalization stimulus and the previously encountered CS+ should increase the likelihood of hippocampally mediated pattern completion (30), resulting in activation of the total pattern of brain activity subserving CS+, including fear-excitation brain areas. Although no observed hippocampal activation showed positive gradients reflective of pattern completion, GS with stronger schematic matches to CS+ resulted in stronger activation of such brain areas associated with fear excitation as the left and right anterior insula (13, 14), dorsomedial prefrontal cortex (13–15), and inferior parietal lobule (14). Importantly, generalization gradients in these fear-excitation areas were consistently less steep in PTSD subjects relative to trauma control subjects, implicating these regions as neural substrates of PTSD-related overgeneralization. Further supporting this assertion were the observed correlations between PTSD symptom severity and gradient steepness in the right anterior insula and approaching significance in the dorsomedial prefrontal cortex. Finally, psychophysiological interaction results indicated a ventral hippocampus contribution to group effects in these and other fear-excitation brain loci, with PTSD subjects showing greater functional connectivity than trauma control subjects between the ventral hippocampus and anterior insula, dorsomedial prefrontal cortex, inferior parietal lobule, and amygdala during GS compared with vCS−. Such results provide some evidence for the predicted increases in hippocampal connectivity to fear-related brain areas during stimuli resembling CS+.
Our results also link PTSD-related overgeneralization to brain regions not included in the model: the dorsolateral prefrontal cortex (BA9) and caudate nucleus. Because lateral portions of BA9 have been shown to code for cognitive control of emotion (31), BA9 activations may reflect subjects’ attempts to cognitively down-regulate fear evoked by CS+ or GS. Thus, greater generalization instantiated in BA9 among those with PTSD may indicate a more persistent need for cognitive control of fear as the presented stimulus differentiates from CS+. The caudate body has been found to subserve motivated behavior including anxiety-driven avoidance (32, 33). Although behavioral avoidance of shock was not possible in this study, subjects may have felt the impulse to avoid when exposed to CS+ or resembling GS. Thus, group effects in the caudate body may reflect greater generalization of the impulse to avoid among those with PTSD.
One unexpected finding was the absence of a correlation between PTSD symptom severity and behavioral generalization. Although this correlation was in the expected direction, it was not significant. Thus, while both PTSD and subthreshold PTSD groups showed heightened behavioral generalization, when measuring PTSD symptoms continuously, the relation between PTSD symptoms and generalization was not significant. One possibility for this occurrence is that our study was sufficiently powered to identify group differences in behavioral generalization but was underpowered for assessing relations between continuous symptom severity and behavioral generalization.
Treatment Implications
The link between PTSD and overgeneralization prescribes a therapeutic focus on reducing fear to benign stimulus events resembling features of the traumatic encounter, in addition to the actual features of the trauma. Specifically, exposure treatments might focus on reducing fear reactivity to both stimuli associated with the trauma and stimuli approximating those associated with the trauma. This could be done through in vivo systematic desensitization using a hierarchy of feared stimuli, with exposures to stimuli resembling the feared stimulus added at each level of the hierarchy. In addition, patients could undergo discrimination training, whereby they learn to differentiate trauma cues indicative of genuine danger from benign cues with inconsequential resemblance to the trauma.
For patients with refractory symptoms, this “generalization-focused” exposure could potentially be enhanced pharmacologically in at least one of two ways. First, studies in humans and lower mammals suggest that conditioning-dependent retuning of sensory representations of the conditioned danger cue toward resembling stimuli leads to overgeneralization by rendering perceptual discrimination of the danger cue from its approximations more difficult (34, 35). This conditioning-dependent effect has been tightly linked to the cholinergic system (35, 36). Consequently, medications with anticholinergic properties (e.g., scopolamine) given just prior to sessions of generalization-focused exposure therapy may enhance the therapeutic efficacy of this exposure treatment by facilitating improved sensory discrimination of feared stimuli from their approximations during discrimination training.
Second, findings in lower mammals demonstrate that pretraining administration of D-cycloserine, a partial agonist at the N-methyl-D-aspartate receptor, reduces generalization of Pavlovian fear by enhancing an organism’s ability to discriminate conditioned danger cues from resembling conditioned safety cues (37, 38). Given findings that D-cycloserine strengthens acquisition of aversive conditioning (39), D-cycloserine may enhance conditioned discrimination by strengthening the accuracy of learning, with a resulting decrease in generalization errors. Thus, D-cycloserine has the potential to strengthen the corrective learning acquired during generalization-focused exposure therapy (i.e., reduced generalization) by both enhancing fear reduction to benign stimuli resembling feared stimuli and strengthening discrimination learning. Although these novel pharmacologically enhanced exposure treatments are, at this point, speculative, the feasibility of these treatment approaches is supported by the fact that both scopolamine and D-cycloserine are safe for human use.
In conclusion, these results represent the first demonstration of overgeneralized fear conditioning in PTSD using generalization gradients, the gold standard for systematic assessments of stimulus generalization. Patterns of overgeneralization in PTSD were highly consistent across behavioral measures and a host of generalization-coding neural activations (the left and right anterior insula, dorsomedial prefrontal cortex, right dorsolateral prefrontal cortex, left ventral hippocampus, left and right caudate body, right caudate head). Specifically, behavioral and neural gradients in PTSD subjects relative to trauma control subjects were uniformly characterized by more gradual linear generalization gradients indicative of greater persistence of fear as the presented stimulus differentiated from CS+. Furthermore, PTSD symptom severity was positively correlated with strength of generalization at two of these neural loci (the right anterior insula and left ventral hippocampus). The present results validate the applied translational paradigm for behavioral and neural assessments of trauma-related abnormalities in conditioned fear generalization and support the development of novel interventions for PTSD that aim to reduce levels of generalized conditioned fear. Longitudinal work is needed to determine whether PTSD-related abnormalities in generalization predate the onset of PTSD and contribute toward its development or reflect ongoing disease processes of the disorder.
Supplementary Material
Acknowledgments
Supported by NIMH grant MH-080130 to Dr. Lissek and by grant PT-074550 from the Congressionally Directed Medical Research Program and the Department of Defense to Dr. Sponheim.
Footnotes
Publisher's Disclaimer: Disclosures
Publisher's Disclaimer: Presented at the annual convention of the Society of Biological Psychiatry, Atlanta, May 2016; at the annual convention of the Anxiety and Depression Association of America, Miami, April 2015; at the annual convention of the Association for Behavioral and Cognitive Therapies, Philadelphia, November 2014; at the annual meeting of the Anxiety and Depression Association of America, Chicago, March 2014; and at the annual conference of the Society for Psychophysiological Research, Florence, Italy, October 2013.
The authors report no financial relationships with commercial interests.
References
- 1.Pavlov IP: Conditioned Reflexes. New York, Oxford University Press, 1927 [Google Scholar]
- 2.Cahill SP, Foa EB: Psychological theories of PTSD, in Handbook of PTSD: Science and Practice. Edited by Friedman MJ, Keane TM, Resick PA. New York, Guilford, 2007, pp 55–77 [Google Scholar]
- 3.Armony JL, Servan-Schreiber D, Romanski LM, et al. : Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex 1997; 7:157–165 [DOI] [PubMed] [Google Scholar]
- 4.Lissek S, Biggs AL, Rabin SJ, et al. : Generalization of conditioned fear potentiated startle in humans: experimental validation and clinical relevance. Behav Res Ther 2008; 46:678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norrholm SD, Jovanovic T, Briscione MA, et al. : Generalization of fear-potentiated startle in the presence of auditory cues: a parametric analysis. Front Behav Neurosci 2014; 8:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon PR, Moore JW: Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol 1975; 89:1192–1203 [DOI] [PubMed] [Google Scholar]
- 7.Wild JM, Blampied NM: Hippocampal lesions and stimulus generalization in rats. Physiol Behav 1972; 9:505–511 [DOI] [PubMed] [Google Scholar]
- 8.Bucci DJ, Saddoris MP, Burwell RD: Contextual fear discrimination is impaired by damage to the post rhinal or perirhinal cortex. Behav Neurosci 2002; 116:479–488 [PubMed] [Google Scholar]
- 9.O’Reilly RC, Rudy JW: Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev 2001; 108:311–345 [DOI] [PubMed] [Google Scholar]
- 10.Bakker A, Kirwan CB, Miller M, et al. : Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 2008; 319: 1640–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lissek S, Bradford DE, Alvarez RP, et al. : Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci 2014; 9:1134–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackintosh NJ: The Psychology of Animal Learning. New York, Academic Press, 1974 [Google Scholar]
- 13.Sehlmeyer C, Schöning S, Zwitserlood P, et al. : Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 2009; 4:e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullana MA, Harrison BJ, Soriano-Mas C, et al. : Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry 2016; 21:500–508 [DOI] [PubMed] [Google Scholar]
- 15.Etkin A, Egner T, Kalisch R: Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunsmoor JE, Prince SE, Murty VP, et al. : Neurobehavioral mechanisms of human fear generalization. Neuroimage 2011; 55:1878–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torrents-Rodas D, Fullana MA, Bonillo A, et al. : No effect of trait anxiety on differential fear conditioning or fear generalization. Biol Psychol 2013; 92:185–190 [DOI] [PubMed] [Google Scholar]
- 18.Greenberg T, Carlson JM, Cha J, et al. : Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety 2013; 30:242–250 [DOI] [PubMed] [Google Scholar]
- 19.Greenberg T, Carlson JM, Cha J, et al. : Neural reactivity tracks fear generalization gradients. Biol Psychol 2013; 92:2–8 [DOI] [PubMed] [Google Scholar]
- 20.Onat S, Büchel C: The neuronal basis of fear generalization in humans. Nat Neurosci 2015; 18:1811–1818 [DOI] [PubMed] [Google Scholar]
- 21.Blake DD, Weathers FW, Nagy LM, et al. : The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8:75–90 [DOI] [PubMed] [Google Scholar]
- 22.Weathers FW, Keane TM, Davidson JR: Clinician-Administered PTSD Scale: a review of the first ten years of research. Depress Anxiety 2001; 13:132–156 [DOI] [PubMed] [Google Scholar]
- 23.Hochberg Y: A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 75:800–802 [Google Scholar]
- 24.Peterson BS, Skudlarski P, Anderson AW, et al. : A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry 1998; 55:326–333 [DOI] [PubMed] [Google Scholar]
- 25.Lissek S, Kaczkurkin AN, Rabin S, et al. : Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 2014; 75:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lissek S, Rabin S, Heller RE, et al. : Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry 2010; 167:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otto T, Eichenbaum H: Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus 1992; 2:323–334 [DOI] [PubMed] [Google Scholar]
- 28.Sander D, Grandjean D, Scherer KR: A systems approach to appraisal mechanisms in emotion. Neural Netw 2005; 18:317–352 [DOI] [PubMed] [Google Scholar]
- 29.Lissek S: Toward an account of clinical anxiety predicated on basic, neutrally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress Anxiety 2012; 29:257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakazawa K, McHugh TJ, Wilson MA, et al. : NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 2004; 5: 361–372 [DOI] [PubMed] [Google Scholar]
- 31.Ochsner KN, Silvers JA, Buhle JT: Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251:E1–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado MR, Jou RL, Ledoux JE, et al. : Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci 2009; 3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen JD, Davison CS: Effects of caudate lesions on signaled and non-signaled Sidman avoidance in the rat. Behav Biol 1973; 8:239–250 [DOI] [PubMed] [Google Scholar]
- 34.Scheich H, Simonis C, Ohl F, et al. : Learning Related Plasticity of Gerbil Auditory Cortex: Feature Maps Versus Meaning Maps. London, Kluwer Academic, 1992 [Google Scholar]
- 35.Thiel CM, Bentley P, Dolan RJ: Effects of cholinergic enhancement on conditioning-related responses in human auditory cortex. Eur J Neurosci 2002; 16:2199–2206 [DOI] [PubMed] [Google Scholar]
- 36.Weinberger NM: Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem 2007; 14: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Land C, Riccio DC: d-Cycloserine: effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiol Learn Mem 1999; 72:158–168 [DOI] [PubMed] [Google Scholar]
- 38.Thompson LT, Disterhoft JF: Age- and dose-dependent facilitation of associative eyeblink conditioning by D-cycloserine in rabbits. Behav Neurosci 1997; 111:1303–1312 [DOI] [PubMed] [Google Scholar]
- 39.Monahan JB, Handelmann GE, Hood WF, et al. : D-cycloserine, a positive modulator of the N-methyl-D-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav 1989; 34:649–653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


