Abstract
Background:
Rats exposed to water avoidance stress (WAS) show increased urinary frequency, increased somatosensory nociceptive reflex responses, as well as altered brain responses to bladder distension, analogous to similar observations made in patients with urologic chronic pelvic pain syndrome (UCPPS). Exercise has been proposed as a potential treatment option for patients with chronic urinary frequency and urgency. We examined the effects of exercise on urinary voiding parameters and functional brain activation during bladder distension in rats exposed to WAS.
Methods:
Adult, female Wistar Kyoto rats were exposed to 10 days of WAS and thereafter randomized to either voluntary exercise for 3 weeks or sedentary groups. Voiding parameters were assessed at baseline, post-WAS, and weekly for 3 weeks. Thereafter, cerebral blood flow (CBF) mapping was performed during isotonic bladder distension (20 cm H2O) after intravenous bolus injection of [14C]-iodoantipyrine. Regional CBF was quantified in autoradiographs of brain slices and analyzed in 3-D reconstructed brains by statistical parametric mapping. Functional connectivity was examined between regions of the micturition circuit through interregional correlation analysis.
Results:
WAS exposure in sedentary animals (WAS/no-EX) increased voiding frequency and decreased urinary volumes per void. Exercise exposure in WAS animals (WAS/EX) resulted in a progressive decline in voiding frequency back to the baseline, as well as increased urinary volumes per void. Within the micturition circuit, WAS/EX compared to WAS/no-EX demonstrated a significantly lower rCBF response to passive bladder distension in Barrington’s nucleus that is part of the spinobulbospinal voiding reflex, as well as in the periaqueductal gray (PAG) which modulates this reflex. Greater rCBF was noted in WAS/EX animals broadly across corticolimbic structures, including the cingulate, medial prefrontal cortex (prelimbic, infralimbic areas), insula, amygdala, and hypothalamus, which provide a ‘top-down’ decision point where micturition could be inhibited or triggered. WAS/EX showed a significantly greater positive brain functional connectivities compared to WAS/no-EX animals within regions of the extended reflex loop (PAG, Barrington’s nucleus, intermediodorsal thalamic nucleus, pons), as well as within regions of the corticolimbic decision-making loop of the micturition circuit, with a strikingly negative correlation between these pathways. Urinary frequency was positively correlated with rCBF in the pons, and negatively correlated with rCBF in the cingulate cortex.
Conclusion:
Our results suggest that chronic voluntary exercise may decrease urinary frequency at two points of control in the micturition circuit. During the urine storage phase, it may diminish the influence of the reflex micturition circuit itself, and/or it may increase corticolimbic control of voiding. Exercise may be an effective adjunct therapeutic intervention for modifying the urinary symptoms in patients with UCPPS.
Keywords: Psychological stress, Exercise, Interstitial cystitis, Bladder pain syndrome, Functional brain mapping, Micturition circuit
1. Introduction
Based on epidemiological evidence, chronic emotional stress plays a role in the exacerbation and possibly the development of functional lower urinary tract disorders [1–5]. From a clinical perspective, these disorders can be viewed as a spectrum of bladder hypersensitivity syndromes sharing the common symptom of urinary frequency and urgency, with patients with urologic chronic pelvic pain syndrome (UCPPS) additionally experiencing pain.
An animal model based on chronic exposure to water avoidance stress (WAS), a form of psychological stress in rats, reproduces aspects of interstitial cystitis/bladder pain syndrome (IC/BPS), a subtype of UCPPS. WAS induces urinary frequency, bladder hyperalgesia, tactile hindpaw allodynia and suprapubic hyperalgesia that persist up to 1 month following removal of the stressor [6, 7]. When examined during bladder distension, stressed compared to control animals show increased functional brain activation broadly across the supraspinal micturition circuit [8].
The underlying mechanisms of IC/BPS are not well understood, and evaluation of current therapeutic interventions have not identified any generally effective treatments. The effects of aerobic exercise on this functional disorder is just beginning to be examined. Several studies examining urinary function in patients with a variety of diagnoses indirectly suggest that exercise and/or motor training may improve symptoms. Physical activity has been shown to have beneficial effects in individuals suffering from chronic pain, and patients with IC often use exercise as a therapeutic intervention [9, 10].
The effects of running exercise on micturition have not been extensively studied. Using the WAS model, we now examine in stressed rats, the effects three weeks of voluntary aerobic exercise has on urinary voiding parameters. Uniquely, we for the first time examine the effects exercise training has on functional brain responses of the micturition circuit during bladder filling. We hypothesize that exercise will ameliorate urinary symptoms and attenuate the stress-associated hyperactivation previously noted in the WAS model in micturition centers of the brain, including Barrington’s nucleus and the periaqueductal gray (PAG) [8].
2. Methods
2.1. Overview
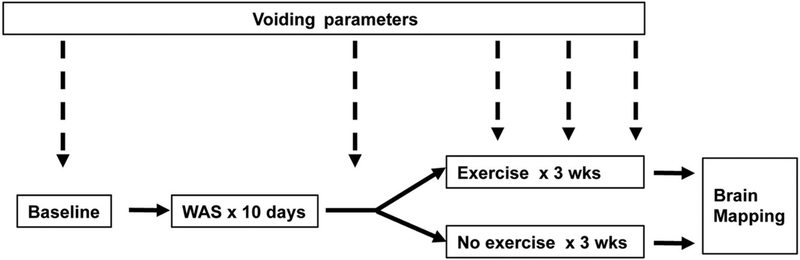
The experimental design is depicted in Fig. 1. In brief, rats underwent measurement of urinary voiding parameters in metabolic cages at baseline and following 10 days of WAS. Thereafter, animals were given three weeks access in their home cages to voluntary running wheels (‘exercise’) or remained without access to these (‘no-exercise’). During these three weeks, animals received further assessment of urinary voiding parameters at weekly intervals. On completion of the exercise or no-exercise phase, rats were sedated, and functional brain mapping was performed during bladder distension.
Fig. 1.
Experimental design. Adult, female Wistar rats received measurement of urinary voiding parameters first at baseline, then following 10 days of water avoidance stress (WAS), and at weekly intervals thereafter for three weeks. During these three weeks, animals either received access to voluntary running wheels or remained sedentary, followed by functional brain mapping during passive bladder distension.
2.2. Animals
Twenty-six adult, female Wistar Kyoto rats aged 11–12 weeks (200–300 g) were purchased from a commercial vendor (Charles River, Wilmington, MA, USA). This particular strain was utilized due to their genetic predisposition for anxiety [11]. We only studied female rats as IC/BPS occurs with greater frequency in women [12]. We did not control for estrous cycle during these experiments. Experimental procedures were approved by the University of Southern California’s Institutional Animal Care and Use Committee (IACUC) (protocol #20651) and were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals [13].
We allowed an adjustment period of one to two weeks between the animals’ arrival and the start of experimental procedures. Rats were housed under standard vivaria conditions (lights off from 6 p.m. to 6 a.m.), with dry bedding and ad libitum access to standard lab chow (Laboratory Rodent Diet 5001, Constant Nutrition, Purina, ON, Canada) and water (internal reverse osmosis system).
2.3. Chronic water avoidance stress protocol
After collection of baseline voiding parameters (see below), all of the animals were exposed to chronic WAS as previously described [6, 7, 14]. Briefly, the rat was placed on a standing cube (5.7 × 5.7 × 10.7 cm3) in the middle of a Plexiglas cage (25 × 50 × 25 cm3). The container was filled with room temperature water 1 cm below the top of the cube. The rat was placed atop of the cube for 1 h every day for 10 consecutive days. The protocol was performed between the hours of 8:00a.m. and 12:00 p.m. to minimize circadian effects.
2.4. Exercise protocol
Animals were then randomized into either exercise (n = 12) or no-exercise groups (n = 14). During the daytime (6 a.m.–6 p.m.), animals in the exercise and no-exercise arms were separately housed in assigned groups of two to reduce the stress of social isolation [15]. At night (6 p.m.–6 a.m.), exercised animals were placed individually in cages with access to a voluntary running wheel connected to a sensor to count revolutions and speed (Scurry Activity Wheel, model 80859S, 35.6 cm diameter, 1.6 mm rungs, 6.4 mm interrung gap, Lafayette Instrument, Lafayette, IN, USA). In order to control for the possible effects of single housing, sedentary animals were also placed individually into a similar cage at night, but without access to a running wheel. Exercise occurred Monday-Saturday, with a day of rest and dual housing for all animals on Sunday. Exercise data are described using mean ± standard errors.
2.5. Assessment of voiding parameters
All animals underwent metabolic cage assessment at baseline (pre-WAS), post-WAS, and at the end of weeks 1, 2, and 3 thereafter prior to termination. Rats were placed individually in metabolic cages (Model 3600M024, Techniplast West Chester, PA, USA) with ad libitum access to water. After 30 min of behavioral accommodation, urine output was quantified in real time over a 12-hour period (6 p.m.–6 a.m.) using a data acquisition system (MP150; Biopac Systems Inc. Goleta, CA, USA). Total volume of urine, number of urinations, water consumption, and fecal pellets were measured, with volume per void being calculated.
2.6. Statistical analysis of voiding parameters
Metabolic cage variables are described using mean ± standard errors. Animals were separated based on the tertile cut points of the total amount of exercise: no exercise (n = 14), low exercise (n = 4), moderate exercise (n = 4), and high exercise (n = 4). Kruskal-Wallis test was used to test differences in non-normally distributed continuous variables between/among groups and subgroups in the setting of univariate and stratified analyses. Multivariable mixed modeling was performed (SAS, Version 9.4; SAS Institute Inc., Cary, NC, USA) to identify the effects/influences of main interested features (e.g. amount of exercise, time points) on outcomes (e.g. urinary frequency, void volume), while controlling for the covariate of water consumption. Tukey-Kramer method was used to adjust for the multiple/pairwise comparisons based on least square means. All p-values reported were 2-sided and p < 0.05 was considered statistically significant.
2.7. Functional brain mapping
After 3 weeks of either the exercise (n = 10) or no-exercise (n = 12) protocol, the animals underwent assessment of cerebral blood flow mapping as a response to physiologic bladder distention stimulus. This represents a subset of the animals assessed in the metabolic cages which included additionally two subjects per group as part of an initial pilot study with animals that were not subjected to brain mapping. Animals were anesthetized with isoflurane (1–2% in the gas mixture of 30% oxygen and 70% nitrogen) and a percutaneous venous catheter was placed in the right external jugular vein. Forty mg/mL of α-chloralose (Sigma Aldrich, St. Louis, MO, USA) was dissolved in 20% β-cyclodextrin (Sigma Aldrich, St. Louis, MO, USA) and administered as a bolus (40 mg/kg, i.v.). To allow the α-chloralose to take effect, isoflurane anesthesia was maintained at 1% for 30 min and then discontinued. A PE-50 catheter was inserted into the bladder through the urethra. The bladder catheter was connected to an infusion pump (KD Scientific Inc., Holliston, MA, USA) and a pressure sensor (MP150, Biopac Systems Inc., Goleta, CA, USA) via a 3-way connector. Two hours after discontinuation of the isoflurane, WAS rats (WAS/EX, WAS/no-EX) were connected via their venous catheter to a cannula extension and a mechanical infusion pump. Light sedation was maintained with α-chloralose (15 mg/kg/h., i. v.). We chose light sedation using α-chloralose because of its favorable profile in maintaining cerebral function intact [16] as compared to isoflurane [17, 18] or urethane [19]. At the same time, α-chloralose when given at a low dose allows assessment of bladder urodynamics and the micturition reflex in rats [8, 20].
Rats received an isotonic bladder distension to 20 cmH20. [14C]-iodoantipyrine (100 μCi/kg, American Radiolabelled Chemicals, Inc., St. Louis, MO, USA) was infused intravenously as a bolus at 45 s following bladder distension and in the absence of a visceromotor response. Radiotracer administration was immediately followed by infusion of an euthanasia solution (pentobarbital 50 mg/mL, 3 M KCl), which resulted in cardiac arrest within 10 s, a rapid fall of arterial blood pressure, termination of brain perfusion, and death [21]. This 10 s time window provided the temporal resolution during which the distribution of rCBF-related tissue radioactivity was mapped.
2.7.1. Autoradiography
Brains were flash frozen and serially sectioned (60 coronal 20-μm slices, 300-μm interslice distance). Autoradiographic images along with [14C] standards (Amersham Biosci., Little Chalfont, UK) were digitized and CBF-related tissue radioactivity was measured [22–24]. The [14C]-iodoantipyrine method has an estimated spatial resolution of 100 μm [25].
2.7.2. Statistical parametric mapping
3-D brains were reconstructed and spatially normalized to a template. To ensure that only voxels mapping cerebral tissue were included in the analysis, voxels for each brain failing to reach a specified threshold (70% of the mean voxel value) were masked out to eliminate the background and ventricular spaces without masking gray or white matter. Global differences in the absolute amount of radiotracer delivered to the brain were adjusted by the statistical parametric mapping software (SPM, version 8) for each animal by scaling the voxel intensities so that the mean intensity for each brain was the same (proportional scaling). Changes in rCBF were analyzed by SPM [26], with voxel-wise t-tests as per our prior methods [27]. A threshold for significance was set at p < 0.05 at the voxel level, with cluster-extent based thresholding (>100 contiguous significant voxels) commonly used in neuroimaging studies. The minimum cluster criterion was applied to avoid basing our results on significance at a single or small number of suprathreshold voxels. In addition, we examined the false discovery rate, which controls the expected proportion of false positives among suprathreshold voxels. Brain regions were identified using coronal, sagittal and transverse views according to the rat brain atlas [28]. In addition, using SPM, we performed a correlation between urinary frequency at the end of week 3 and rCBF across the brain (p < 0.05 for >100 contiguous voxels) for the combined WAS/EX and WAS/No-EX groups. Correlations of urinary frequency with rCBF were depicted graphically for the cingulate cortex and pons (WAS/EX, n = 10; WAS/No-EX, n = 12).
2.7.3. Functional connectivity analysis
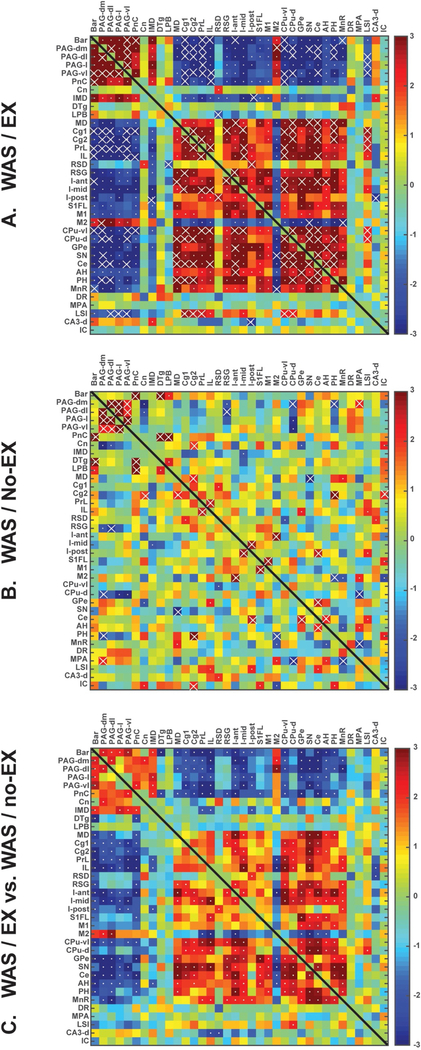
A total of 36 bilateral anatomic region of interests (ROIs, see legend Fig. 7) were selected within the micturition circuit [29, 30], including regions within both its reflex loop, including subdivisions of the PAG [31], as well as its decision making loop, with afferent projections to Barrington’s nucleus (also referred to as the pontine micturition center, PMC or ‘M-region’) as defined in the rat by Valentino et al. [32]. Regions were drawn manually in MRIcro (version 1.40, http://cnl.web.arizona.edu/mricro.htm) over the template brain according to the rat brain atlas [28]. Mean optical density of each functional ROI was extracted for each animal using the Marsbar toolbox for SPM (version 0.42, http://marsbar.sourceforge.net/).
Fig. 7.
Functional brain connectivity within the extended micturition circuit during passive bladder distention. Interregional correlation matrix shows functional connectivity patterns of (A) WAS/EX and (B) WAS/no-EX. Z scores of Pearson’s correlation coefficients are color-coded with significant correlations marked with a white dot. Significant correlations (p < 0.05) determined by the more conservative jackknife procedure are marked with white crosses. (C) The matrix of Fisher’s Z-statistics represents differences in Pearson’s correlation coefficients (r) between the WAS/EX and the WAS/no-EX group. Positive Z-values indicate greater r in the WAS/EX group, while negative Z-values smaller r. Significant between-group differences (p < 0.05) are marked with white dots. Abbreviations as appearing along the figures’ axes: Bar, Barrington’s n; PAG-dm, Periaqueductal gray, dorsomedial; PAG-dl, Periaqueductal gray, dorsolateral; PAG-l, Periaqueductal gray, lateral; PAG-vl, Periaqueductal gray, ventrolateral; PnC, Pons, reticular n., caudal; Cn, Cuneiform n; IMD, Intermediodorsal n. (thalamus); DTg, Dorsal Tegmental area; LPB, Lateral parabrachial area; MD, Mediodorsal n. (thalamus); Cg1, Cingulate, dorsal; Cg2, Cingulate, ventral; PrL, Prelimbic cortex; IL, Infralimbic cortex; RSD, Retrosplenial cortex, dysgranular; RSG, Retrosplenial cortex, granular; I-ant, Insula, anterior; I-mid, Insula, midl; I-post, Insula, posteriorl; S1FL, Primary somatosensory cx, forelimb; M1, Primary motor cortex; M2, Secondary motor cortex; CPu-vl, Striatum, ventrolateral; CPu-d, Striatum, dorsal; GPe, Globus pallidus, external; SN, Substantia nigra; Ce, Central n. (amygdala); AH, Anterior hypothalamus; PH, Posterior hypothalamus; MnR, Median raphe; DR., Dorsal raphe; MPA, Medial preoptic area; LSI, Lateral septal n., intermediate; CA3-d, CA3, dorsal hippocampus; IC, Inferior colliculus.
We applied interregional correlation analysis to investigate functional connectivity as previously described [33]. This is a well-established method, which has been applied to analyze rodent brain mapping data of multiple modalities, including autoradiographic deoxyglucose uptake [34–36], autoradiographic CBF [37], cytochrome oxydase histochemistry [38–40], and fMRI [41]. In these studies, correlations are calculated in an inter-subject manner, i.e., across subjects within a group.
Pearson’s correlation coefficients between each pair of ROIs were calculated across subjects within a group in Matlab (version R2015a, Mathworks, Inc., Natick, MA, USA) to construct a correlation matrix for each treatment group. The sequence of ROIs was arranged such that subregions of a brain structure were listed together. Pearson’s coefficients (r) were then transformed into Zscores using the Fisher transformation [42]: Correlation matrices were plotted as heatmaps in Matlab. To control Type I error caused by the large number of correlations computed, we implemented a jackknife procedure following Barrett et al. [36] For a group of n subjects, n iterations were performed in which one subject was dropped sequentially and the correlation matrix recalculated with the remaining n−1 subjects. A correlation was considered `reliably’ significant only if it was significantly different from zero (p < 0.05) in all iterations. Statistical significance of between-group differences in correlation coefficients was evaluated using the Fisher’s Z-transform test (p < 0.05).
3. Results
3.1. Exercise
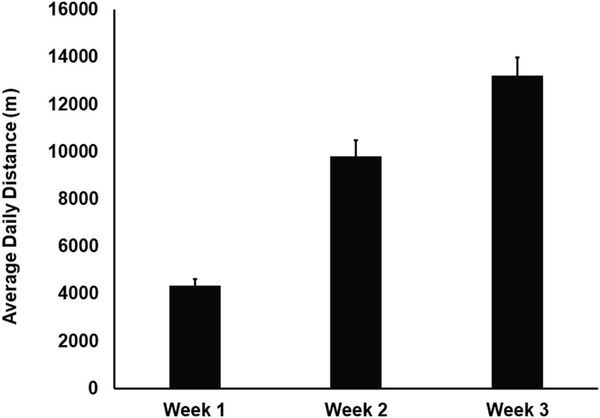
Fig. 2 shows progressive increases in daily average distance run by the WAS/EX animals during weeks 1, 2 and 3 of exposure to the voluntary running wheels. A final average distance per day of 13,211 ± 771 m (average ± standard error) was achieved during week 3, comparable to reports by others in the Wistar Kyoto strain [43, 44].
Fig. 2.
Distance run in voluntary running wheels. Depicted are the daily average distance (in meters ± standard error) run by WAS-EX animals (n = 12) during weeks 1, 2 and 3.
3.2. Voiding parameters in the metabolic cage
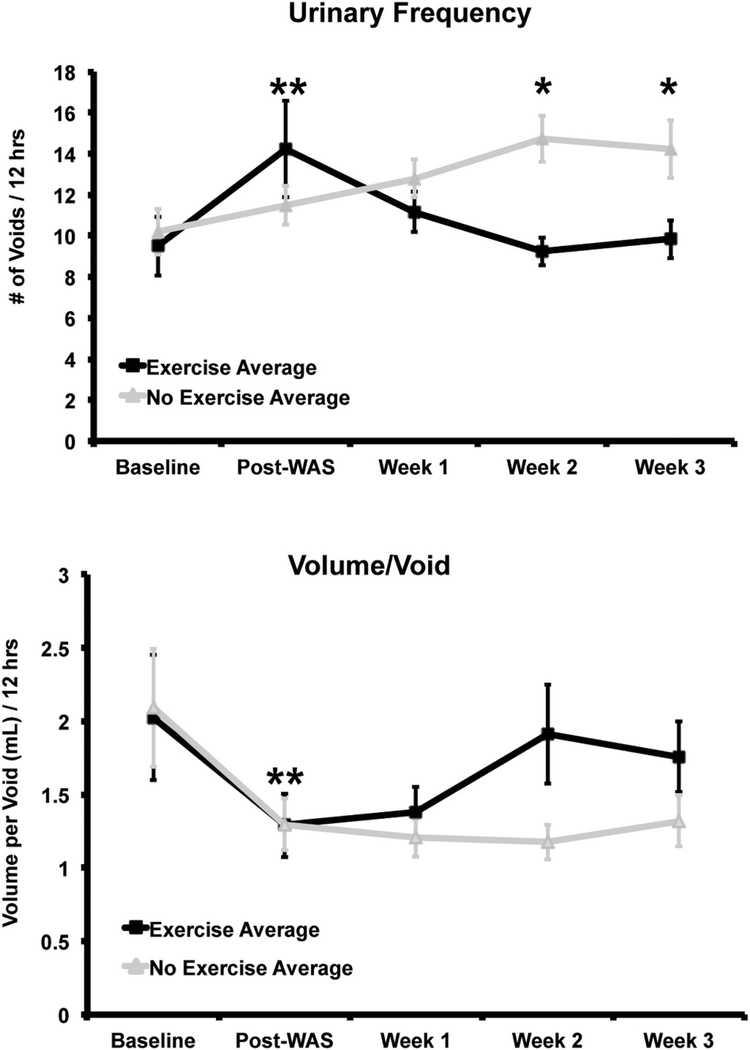
There were no significant group differences in urinary frequency and volume per void at baseline (Sedentary group: 10.2 ± 1.1 voids/ 12 h, 2.1 ± 0.4 mL/void; Exercise group: 9.5 ± 1.4 voids/12 h, 2.1 ± 0.4 mL/void) on univariate analysis. Water avoidance stress (WAS) increased urinary frequency and decreased volume per void compared to baseline (Fig. 3, Sedentary group: 11.5 ± 0.9 voids/12 h, 1.3 ± 0.2 mL/void; Exercise group: 14.3 ± 2.4 voids/12 h; 1.3 ± 0.2 mL/void). Increases in urinary frequency compared to baseline were significant in the exercise group on univariate analysis (p = 0.03), and significant with both sedentary and exercise groups on multivariable mixed modeling (p = 0.009). Decreases in volume per void were significant in the sedentary group on univariate analysis (p = 0.02) and significant in both groups on multivariable mixed modeling (p = 0.02), with no significant group difference.
Fig. 3.
Urinary frequency and volume per void by group over time (average ± standard error). Water avoidance stress (WAS) increased urinary frequency and decreased volume per void (average ± standard error). Significant changes from baseline (**= p < 0.05) were noted for both groups in the frequency variable, and for the sedentary group in the volume/void variable, with no significant group difference. With voluntary exercise, the exercise group (WAS/EX, n = 12) showed a decline in urinary frequency and increase in volume per void back to baseline compared to the no-exercise group (WAS/no-EX, n = 14). Significant group differences for the frequency variable were noted at weeks 2 and 3 (*=p < 0.02 for both weeks), which was confirmed with multivariable mixed modeling (p = 0.02). For the volume/void variable, there was a trend towards significance (p = 0.08) at week 3, with significant group differences demonstrated on multivariable mixed modeling (p = 0.02). Data presented is reproduced from Sanford et al. [82].
After being initiated on the voluntary running wheel, animals in the exercise group compared to those in the no-exercise group showed a progressive decline in voiding frequency back to the baseline (Week 1: Sedentary 12.8 ± 0.9 voids/12 h vs. Exercise 11.2 ± 0.9 voids/12 h, p = 0.2; Week 2: Sedentary 14.7 ± 1.1 voids/12 h vs. Exercise 9.3 ± 1.2 voids/12 h, p = 0.02; Week 3: Sedentary 14.2 ± 1.4 voids/ 12 h vs. Exercise 9.8 ± 0.9 voids/12 h, p = 0.02). At the same time, the exercise compared to the no-exercise group showed a progressive increase in urinary volumes per void which showed a trend towards significance on univariate analysis at week 3 (Week 1: Sedentary 1.2 ± 0.1 mL/void vs. Exercise 1.4 ± 0.2 mL/void, p = 0.2; Week 2: Sedentary 1.2 ± 0.1 mL/void vs. Exercise 1.9 ± 0.3 mL/void,p = 0.4; Week 3: Sedentary 1.3 ± 0.2 mL/void vs. Exercise 1.8 ± 0.2 mL/void, p = 0.08). Group differences in frequency of voiding and volume/void were both significant on multivariable mixed modeling (p = 0.02). There was no difference between groups in water consumption, urinary volume, or fecal pellets at any time point. Data has been reported as from our concurrent publication Sanford et al. [82].
3.3. Statistical parametric mapping: between group t-test
Representative results of the whole brain mapping are depicted in Fig. 4 and Table 1, with exercise-related changes in rCBF as related to the micturition circuit summarized in Fig. 5.
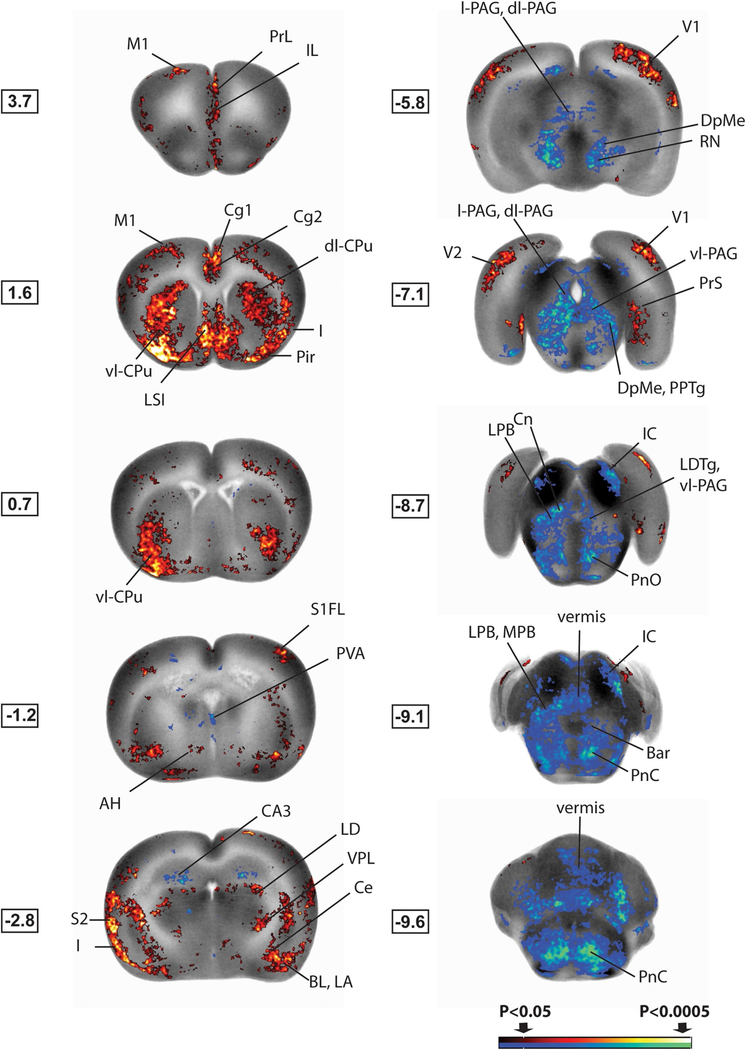
Fig. 4.
Brain regions showing significant exercise-related group differences in cerebral activation during bladder distension. Shown are statistical parametric maps showing significant difference in brain activation in sedated WAS/EX rats compared to WAS/no-EX rats during bladder filling. Changes in regional cerebral blood flow related tissue radioactivity are depicted on representative coronal sections (anterior–posterior coordinates relative to the bregma) of the template brain. Color-coded overlays show statistically significant group differences, with red and blue colors showing positive and negative changes, respectively (p < 0.05 for clusters of >100 contiguous significant voxels). Coordinates relative to bregma (in millimeters) are shown to the left of each slice. Abbreviations are based on the rat brain atlas [28] with modifications. Acb n, accumbens; AH, anterior hypothalamus; Bar, Barrington’s n; BL, basolateral amygdala; CA3, hippocampus, CA3; Ce, central n. of the amygdala; Cg1, dorsal anterior cingulate cx; Cg2, ventral anterior cingulate cx; Cn, cuneiform n; dl-PAG, dorsolateral periaqueductal gray; dl-CPu, dorsolateral striatum; I, insula; IC, inferior colliculus; IL, infralimbic cx; IMD, intermediodorsal thalamic n; LA, lateral amygdala; LPB, lateral parabrachial n; LD, lateral dorsal thalamic n; LDTg, lateral dorsal tegmental area; LPB, lateral parabrachial n; l-PAG, lateral periaqueductal gray; LSI, lateral septal n., intermediate part; M1, primary motor cx; MPB, medial parabrachial n; PBN, parabrachial n; Pir, piriform cortex; PnC, PnO, pontine reticular n., caudal, oral part; PPTg, pedunculopontine tegmental n; PrL, prelimbic cx; PrS, presubiculum; PVA, paraventricular n. anterior (thalamus); RN, red n; S1FL, primary somatosens. cx forelimb area; S2, secondary somatosens. cx; Sim, simple cerebellar lobule; V1, primary visual cx; V2, secondary visual cx; vl-CPu, ventrolateral striatum; vl-PAG, ventrolateral periaqueductal gray; VPL, ventral post. lateral thalamic n. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Summary of statistically significant exercise-related group differences in cerebral activation during bladder distension (20 cm H2O). Significant increases ‘ + ‘ or decreases ‘-’ in regional cerebral blood flow are noted (Student’s t-test, p < 0.05, cluster >100 contiguous significant voxels, n = 10–12 per group) in the left and the right hemisphere (L/R). or indicates significant group differences was lost after correction for false discovery rate at the cluster level. Abbreviations are based on the rat brain atlas [28] with modifications.
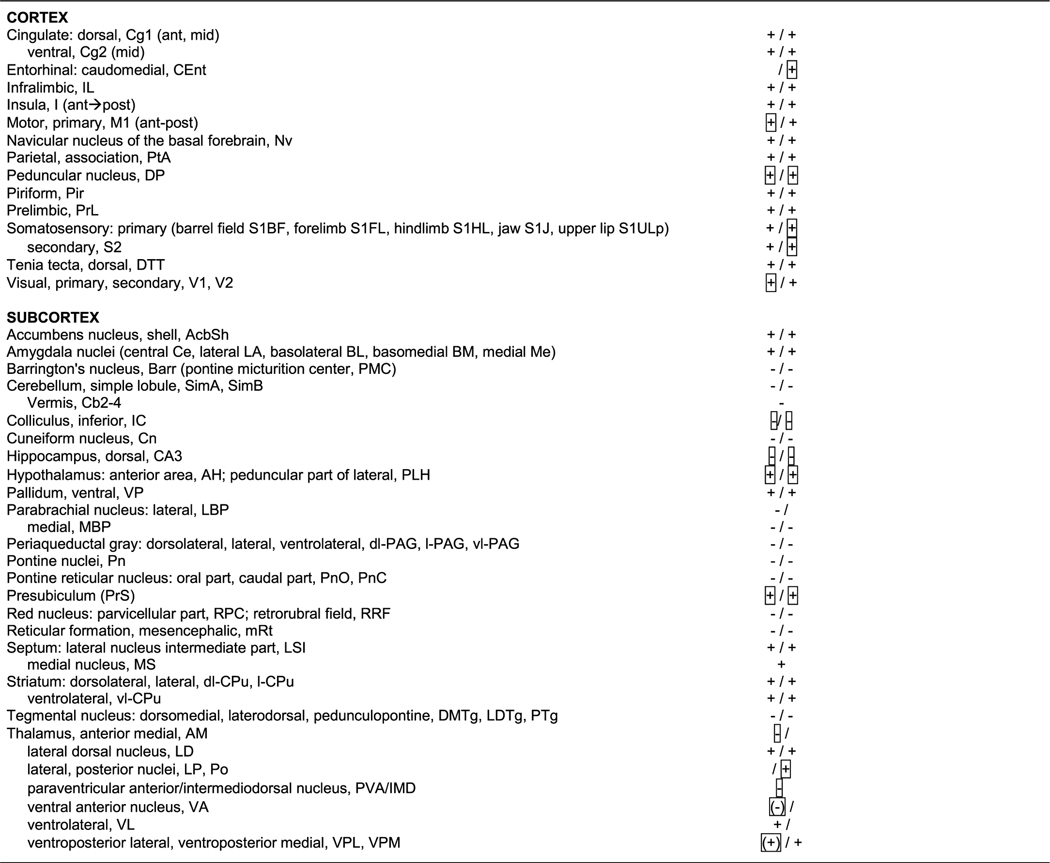 |
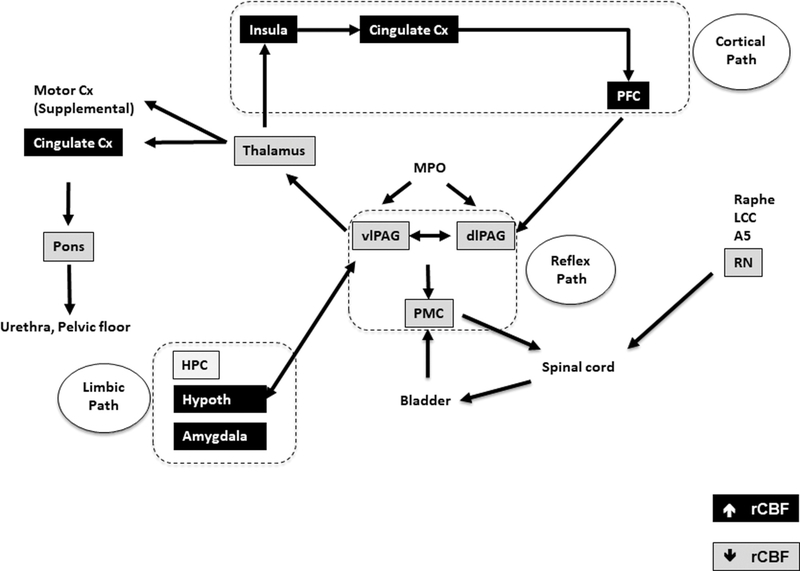
Fig. 5.
Summary of the significant changes in regional cerebral blood flow (rCBF) of WAS/EX vs. WAS/no-EX rats during passive bladder filling as related to a simplified model of the micturition circuit, and its cortical, limbic and reflex loops. Data are summarized from the SPM analysis shown in Fig. 4, with black shading indicating significant increases in rCBF and gray shading indicating significant decreases in rCBF. Abbreviations: A5 (A5 noradrenaline cells), Cx (cortex), dl-PAG, (dorsolateral periaqueductal gray), HPC (hippocampus), Hypoth (hypothalamus), LCC (locus coeruleus complex), MPO (medial preoptic nucleus), PFC (prefrontal cortex), vl-PAG (ventrolateral periaqueductal gray), PMC (pontine micturition center, Barrington nucleus complex), RN (red nucleus), rCBF (regional cerebral blood flow). Adapted from Griffiths [29], DeGroat et al. [30] and Zare et al. [31], with lines between boxes delineating known major neuronal pathways connecting each region.
3.3.1. Micturition circuit
Exercised animals compared to non-exercised controls showed significantly greater rCBF during passive bladder distension in cortical and limbic regions of the micturition circuit, including the dorsal cingulate, insula, medial prefrontal cortex (prelimbic, infralimbic), amygdala (central, lateral, basal, medial nuclei), and to a lesser extent the presubiculum and hypothalamus (anterior, lateral-peduncular) (p < 0.05, with >100 contiguous significant voxels). A presence of left–right symmetry was noted, with clusters of significant voxels typically contained within the boundaries of known anatomical structures. These are not statistically captured by the SPM software but increase the confidence of clusters found significant by the SPM algorithm. Significantly lower rCBF in exercised compared to non-exercised WAS animals was noted in the PAG (dorsolateral, lateral, ventrolateral), Barrington’s nucleus, parabrachial nucleus (lateral, medial), lateral dorsal tegmental area, cuneiform nucleus and pons (pons, pontine reticular nucleus caudal, pontine reticular nucleus oral), as well as in the pedunculopontine tegmental nucleus. No significant change was noted in the hypothalamic medial preoptic nucleus. Most rCBF changes were significant after correction for false discovery rate at the cluster level, except as indicated in Table 1.
3.3.2. Nociceptive circuit
Exercised WAS animals compared to non-exercised controls showed significantly greater rCBF during passive bladder distension in regions of the nociceptive circuit, many of whose regions overlap with those of the micturition circuit. These included the cingulate (anterior to mid), insula (anterior to posterior), medial prefrontal cortex (prelimbic, infralimbic), amygdala (central, lateral, basal, medial nuclei). In addition, increased rCBF was noted in the sensory thalamus (ventroposter-olateral/ventroposteromedial nuclei, bilateral lateral dorsal nuclei) and in primary somatosensory cortex (barrel field, forelimb, hindlimb, jaw, upper lip) and secondary somatosensory cortex.
3.3.3. Motor circuit
WAS/EX animals compared to WAS/no-EX animals showed greater rCBF during passive bladder distension in many of the regions associated with the motor circuit and functional changes in response to motor training, including primary motor cortex (anterior-posterior), the striatum (dorsolateral, lateral, ventrolateral), and ventrolateral thalamic nucleus, with decreased rCBF noted in the red nucleus, cerebellar vermis (Cb2–4) and lateral cerebellum (simple lobules A, B).
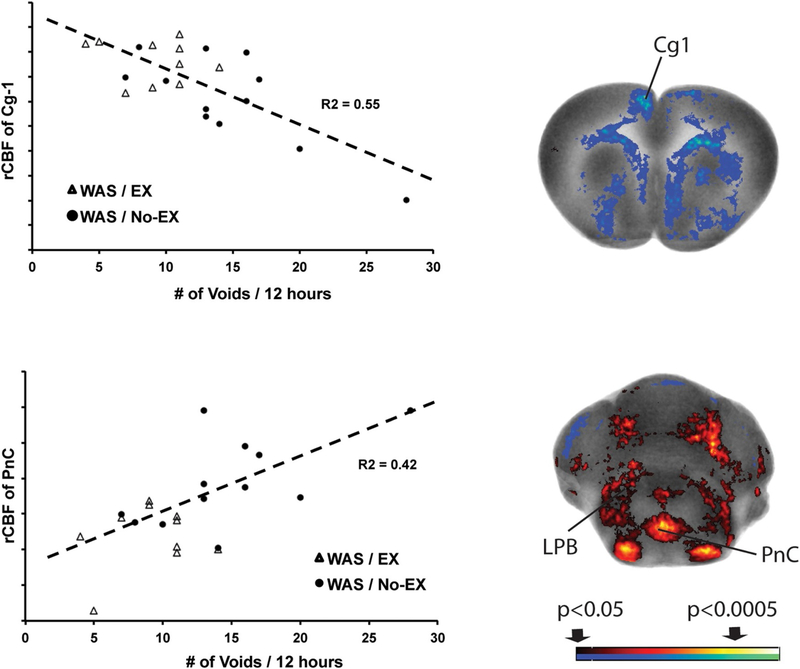
3.3.4. Correlation of urinary frequency with functional brain responses
A decrease in urinary frequency was significantly correlated with an increase in rCBF in dorsal cingulate cortex (Fig. 6), medial prefrontal areas (including prelimbic cortex), anterior secondary motor cortex, as well as with the amygdala (central, basolateral) and hypothalamus (anterior area)(data not shown). A significant positive correlation of urinary frequency was noted with rCBF in the pontine reticular nucleus and lateral parabrachial area (Fig. 6). No significant correlation with urinary frequency was noted with rCBF in the PAG or in Barrington’s nucleus.
Fig. 6.
Correlation of urinary frequency and rCBF. Significant correlations between the urinary frequency at the end of the third week of exercise and rCBF across the combined WAS/ EX and WAS/No-EX groups (n = 10–12 per group). The right column shows representative coronal sections at the level of the dorsal cingulate cortex region (+2.3 mm anterior to bregma) and the caudal portion of the pontine reticular nucleus (−9.7 mm posterior to bregma) that shows correlation of rCBF. Red and blue colors showing significant positive and negative correlations (p < 0.05 for clusters of >100 contiguous significant voxels). The left column graphically depicts the correlation of urinary frequency with rCBF of the dorsal cingulate cortex and the pons. Abbreviations: Cg1, dorsal cingulate; LPB, lateral parabrachial nucleus; PnC, pontine reticular nucleus, caudal. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.5. Brain functional connectivity
Fig. 7 shows the pair-wise correlation matrices of the WAS/EX and WAS/no-EX groups, as well as statistical significance between-group differences in correlation coefficients. In the WAS/EX group (Fig. 7A) a total of 314 significant correlations between differing ROIs were identified of which 175 were positive (27.8% of total ROI pairs) and 139 negative (22.1% of total ROI pairs). Strong, positive intra-regional correlations were noted within regions of the reflex micturition circuit (Barrington’s nucleus, PAG, caudal pons) and associated intermediodorsal thalamic nucleus. Separately, strongly positive inter-regional correlations were noted within cortical regions of the decision-making part of the micturition circuit, as well as associated within and between limbic/paralimbic, sensory and motor regions. This included medial prefrontal cortex (prelimbic, infralimbic) and cingulate cortex (dorsal, ventral), the insula (anterior→posterior), limbic regions constituted by the hippocampus (dorsal CA3), hypothalamus (anterior, posterior), amygdala (central nucleus), and raphe (median nucleus), and also included primary somatosensory cortex (forelimb, S1FL), as well as motor regions (primary motor cortex, external globus pallidus, dorsal striatum, substantia nigra). Though intra-regional correlation was positive within the reflex loop, as well as within the cortical decision-making loop of the micturition circuit, a significant negative functional correlation was noted between these two loops, as well as between the regions of the reflex loop and regions within limbic/paralimbic, sensory, and motor areas. Especially prominent was the negative correlation between the reflex loop and medial prefrontal cortical (cingulate, prelimbic, infralimbic) and insular regions, which remained significant after applying the jackknife procedure (p < 0.05).
The pair-wise correlation matrix of the WAS/no-EX group showed fewer inter-regional correlations (Fig. 7B). A total of 52 significant correlations between differing ROIs were identified, of which 34 were positive (5.4% of total ROI pairs) and 18 were negative (2.9% of total ROI pairs). A positive correlation was noted between subregions of the PAG and between Barrington’s nucleus and the pons, and parabrachial nucleus. Absent were the significant interregional correlation between regions of the decision-making loop of the micturition circuit. Also absent was the significant negative functional correlations between regions of the reflex micturition circuit and the cortical regions of the decision-making loop, or between regions of the reflex micturition circuit and those of limbic/paralimbic, as well as motor areas.
Profound group differences between the WAS/EX and WAS/no-EX groups were noted in functional brain connectivity (Fig. 7C). Most important, WAS/EX compared to WAS/no-EX showed significantly more positive correlation coefficients (p < 0.05) within the reflex micturition circuit (Barrington’s nucleus, PAG, pontine reticular formation caudal) and associated intermediodorsal thalamic n., as well as the cuneiform nucleus, and anterior secondary motor cortex (supplementary motor cortex). A significantly negative correlation was noted between the reflex loop (Barrington’s nucleus, PAG, pontine reticular formation caudal) and the decision making loop, comprised of higher cortical (dorsal and ventral cingulate, prelimbic, infralimbic) regions, and associated limbic/paralimbic regions (central nucleus of the amygdala, median raphe, dorsal raphe, anterior and posterior hypothalamus), sensory regions (anterior→posterior insula, primary somatosensory cortex of the forelimb, retrosplenial granular cortex), and motor regions (ventrolateral striatum, globus pallidus externa, substantia nigra).
WAS/EX compared to WAS/no-EX groups showed significantly greater correlations coefficients (p < 0.05) within limbic regions (amygdala, hypothalamus, median raphe, insula) and within motor regions (primary motor cortex, striatum, globus pallidus externa, substantia nigra). Furthermore, significantly greater correlations were noted between limbic regions (insula, amygdala, hypothalamus, raphe) connecting, either to prefrontal/midline cortex (cingulate, prelimbic, granular retrosplenial) or to select motor regions (primary motor cortex, striatum, globus pallidus externa, substantia nigra).
4. Discussion
WAS exposure in sedentary animals (WAS/no-EX) increased voiding frequency and decreased urinary volumes per void. Exercise exposure in WAS animals (WAS/EX) resulted in a progressive decline in voiding frequency back to the baseline, while at the same time demonstrating increased urinary volumes per void. These results are consistent with a recent report that voluntary wheel running in mice attenuates urinary frequency and bladder hypersensitivity in animals exposed to early life stress [45].
Exercised compared to nonexercised WAS animals demonstrated widespread significant differences in their functional brain response to passive bladder distension. A prominent group difference was the relative deactivation of areas of micturition control in the pons in WAS/EX. This included Barrington’s nucleus (also referred to as the pontine micturition center, PMC or ‘M-region’). The PMC has been classically defined as a supraspinal on-off switch that regulates the storage and elimination of urine [46]. A region that maintains direct projections to the PMC is the periaqueductal gray (PAG) [32, 47, 48]. In our study, rCBF within the PAG was prominently decreased in WAS/EX compared to WAS/no-EX animals in the ventrolateral PAG (vl-PAG) column and lateral/dorsal PAG (l-PAG/d-PAG) sectors. The vl-PAG and L-PAG show opposing autonomic functions, with the vl-PAG having a parasympathetic function and receiving direct parasympathetic input from the lumbosacral cord and bladder [48–50], and the L-PAG/d-PAG having a sympathetic function and receiving input from the cerebral cortex, amygdala and hypothalamus [51, 52]. In addition, WAS/EX compared to WAS/no-EX showed also a lesser rCBF response in an area of the pontomesencephalic central gray bounded by the cuneiform nucleus and parabrachial nucleus, [32], as well as the laterodorsal tegmental nucleus. Regions within the lateral parabrachial nucleus may overlap with those of the ‘L-region’, a relatively diffuse area of the pons located ventral and lateral to the PMC, and which has been proposed to serve as a pontine urinary storage center [53]. While we did not observe a significant correlation of urinary frequency with rCBF in the PAG or PMC, we did observe a significant positive correlation with rCBF in the pontine reticular nucleus, as well as lateral aspects of the parabrachial nucleus, regions that have been reported to modulate micturition [54]. Taken together, our results suggest that exercise exposure diminished functional brain activation in the pons, in particular in the vl-PAG and PMC, which constitute a part of the parasympathetic reflex micturition circuit, and which is felt to be functionally distinct from the sympathetic decision-making part of the micturition circuit [31]. We do not believe that these results are due to non-specific stress effects of voluntary running, an activity rodents appear to enjoy as they progressively engage in increasing amounts of it. Though we did not measure corticosterone levels, work by others has shown that voluntary running does not elicit a stress hormone response in rats [55–57]. In addition, we provided group housing to all animals during the daytime hours, to minimize any possible stress effects from individual housing during overnight exposure to the running wheels.
The effects of exercise on the PAG-PMC have previously not been extensively studied. Timofeeva et al. demonstrated that acute treadmill running in normal rats leads to c-Fos mRNA expression in Barrington’s nucleus and the PAG [58], with activation of these cells having been proposed to inhibit micturition [59]. Nelson et al. examining the response to chronic voluntary wheel running, noted an attenuation of dendritic branching in the PAG of rats exposed to running wheels, suggesting exercise-related structural changes in this central region of bladder control [60, 61]. In addition, Ko et al. examining a rat model of urethrolysis-induced urinary incontinence, noted that chronic treadmill exercise suppressed neuronal activation in the PMC and vl-PAG in response to repeated bladder distension and emptying as measured by diminished c-Fos expression in these brain regions [62]. The findings that chronic exercise diminished dendritic complexity and attenuated neuronal activation within the reflex micturition circuit are consistent with our findings in this circuit of diminished functional activation during bladder distension.
The decision to void is determined also by higher cortical and limbic processes that represent an integration of sensory input, social cues, as well as the subject’s emotional state. Relevant to this is that exercised compared to nonexercised WAS animals demonstrated significant increases in rCBF within the decision-making loop of the micturition circuit, comprised of the cingulate and medial prefrontal cortex (prelimbic, infralimbic areas), as well the insula which may play a role in bladder sensation [63, 64], and more indirectly the limbic/paralimbic regions, including the amygdala (central, lateral, basolateral, basomedial, medial nuclei), hippocampus (dorsal, ventral), and hypothalamus (anterior, lateral-peduncular). These regions provide a ‘top-down’ decision point whether micturition should be inhibited or triggered [29, 30], and have been shown by others to be activated by passive bladder distension in the rat [65], as well as in human subjects [46, 66, 67]. Our findings suggest the possibility that chronic exercise training may decrease urinary frequency at two points of control in the micturition circuit. During the urine storage phase, it may diminish responsivity of the reflex micturition circuit itself, and/or it may increase cortical control of voiding, in particular the medial prefrontal cortex and cingulate which are proposed to maintain the tonic suppression of the voiding reflex during the storage phase [68]. Our findings showed a significant negative correlation between measures of urinary frequency and rCBF in the cingulate cortex of the medial prefrontal area, suggesting that higher rCBF was associated with a decrease in urinary frequency.
Significant differences in functional connectivity were noted between groups, suggesting that indeed there could be a differential effect of exercise on the extended reflex pathway, as well as on the decision-making loop of the micturition circuit. Regarding the former, exercised WAS animals showed a significant positive functional connectivity within regions of the reflex micturition pathway, namely the PAG and several of the brain regions with which it has direct structural connections, including the PMC, as well as the intermediodorsal thalamus. Also included here were positive functional connections to anterior secondary motor cortex, which in rats may serve some of the functions of supplemental motor cortex in humans [69–71], a cortical region associated with pelvic floor and gluteal muscle activation. A striking finding in WAS/EX compared to WAS/no-EX was the significantly more negative correlation noted between the reflex loop and the decision-making loop, comprised of higher cortical regions, associated limbic/paralimbic regions, sensory regions, and motor regions. This negative correlation was striking between the reflex loop and the medial prefrontal cortex for which a role has been proposed in the inhibition and facilitation of the micturition reflex [72]. This suggests the possibility that exercise training may ‘uncouple’ the PAG and Barrington’s nucleus from sympathetic input from the extended decision-making pathway of the micturition circuit. Regarding ‘top down’ influences on the micturition circuit, here we found exercised compared to nonexercised WAS animals to show a significantly greater positive functional connectivity within limbic regions, within motor regions, as well as between limbic regions and connections to prefrontal/midline cortex and to motor regions—possibly reflecting a strengthening of the decision-making loop.
The mechanisms for improving bladder dysfunction through activity-based training remain unknown. Potential mechanisms, include the strengthening of pelvic floor muscles, resulting in greater control in voiding. Alternatively, exercise could elicit adaptive changes to afferent neurons in the bladder or to neural networks in the dorsal horn of the lumbosacral spinal cord, either at the level of receptors and/or cell bodies [73, 74]. In principle, exercise may within the PAG enhance GABAergic tone [52, 75] or decrease glutamatergic tone [76, 77] to diminish functional activation of this node, with the result of depressing reflex voiding frequency. Alternatively, exercise is well known to modulate dopaminergic circuits within the brain, leading to a sensitized dopamine release [78] and increased inhibitory effects mediated by dopamine on the PMC [79, 80]. In addition, it is well established that aerobic exercise conditioning can alter autonomic balance (increasing parasympathetic tone and decreasing sympathetic activity), and such shifts could affect the micturition reflex, as well as the decision-making loop of the micturition circuit [81].
In summary, WAS exposure in sedentary animals (WAS/no-EX) increased voiding frequency and decreased urinary volumes per void. Exercise exposure in WAS animals (WAS/EX) resulted in a progressive decline in voiding frequency back to the baseline, while at the same time demonstrating increased urinary volumes per void. Exercised animals compared to sedentary animals showed a significantly lower rCBF response to passive bladder distension in the PMC that is part of the spinobulbospinal voiding reflex, as well as in the PAG, which modulates this reflex. Greater rCBF was noted in WAS/EX compared to WAS/no-EX animals in the forebrain circuit, including the cingulate, medial prefrontal cortex (prelimbic, infralimbic areas), and in the insula and limbic/paralimbic regions, which provide a ‘top-down’ decision point whether micturition should be inhibited or triggered. Exercise was associated with a significantly greater negative correlation of regions within the reflex pathway with those within the corticolimbic decision-making loop of the micturition circuit. Urinary frequency was positively correlated with rCBF in the pons, and negatively correlated with rCBF in cingulate cortex. Our results suggest the possibility that exercise may facilitate a depression of voiding frequency by either diminishing the influence the reflex pathway has and/or strengthening the influence the corticolimbic decision-making pathway has on the micturition circuit that is engaged during bladder filling. Clearly the hypothesis that exercise training elicits a differential effect on the reflex pathway and/or on higher cortical/limbic regions of the micturition circuit would need to be further tested in the future with electrophysiologic recordings. While our study does not address the effects of exercise in normal, non-stressed rats, it does suggest that exercise may be an effective adjunct therapeutic intervention for modifying the symptoms of urinary frequency and urgency in stressed animals, a finding which would need to be confirmed in clinical patients suffering from functional lower urinary tract disorders and/or chronic pelvic pain syndrome. Furthermore, it remains unclear if different types of exercise might accentuate the effects motor training has on bladder function. Future work should compare different exercise types, including aerobic training, Kegel exercises, or skilled motor training. In addition, further clarification is needed regarding different exercise parameters, including frequency of training per week, duration per session, duration over weeks, as well as exercise intensity.
Acknowledgments
Funding
Supported by a MAPP Research Network grant (U01 DK082370) from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), and a grant # W81XWH18-1-0666 from the US Department of Defense grant (Army, CDMRP). We would like to thank Dr. Jie Cai, statistician in the USC Dept. of Urology for his help with the multivariate statistical analysis.
Footnotes
Declaration of Competing Interest
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.physbeh.2019.112796.
References
- [1].Macaulay AJ, Stern RS, Holmes DM, Stanton SL, Micturition and the mind: psychological factors in the aetiology and treatment of urinary symptoms in women, Br. Med. J 294 (1987) 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B, Stress and symptoms in patients with interstitial cystitis: a life stress model, Urology 57 (2001) 422–427. [DOI] [PubMed] [Google Scholar]
- [3].Mayer EA, Naliboff BD, Chang L, V Coutinho SV, Stress and irritable bowel syndrome, Am. J. Physiol. Gastrointest. Liver Physiol 280 (2001) G519–G524. [DOI] [PubMed] [Google Scholar]
- [4].Lim JR, Bak CW, Lee JB, Comparison of anxiety between patients with mixed incontinence and those with stress urinary incontinence, Scand. J. Urol. Nephrol 41 (2007) 403–406. [DOI] [PubMed] [Google Scholar]
- [5].Rogers R, Bachmann G, Jumadilova Z, Sun F, Morrow JD, Guan Z, et al. , Efficacy of tolterodine on overactive bladder symptoms and sexual and emotional quality of life in sexually active women, Int. Urogynecol. J. Pelvic Floor Dysfunct 19 (2008) 1551–1557. [DOI] [PubMed] [Google Scholar]
- [6].Lee UJ, Ackerman AL, Wu A, Zhang R, Leung J, Bradesi S, et al. , Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia, Physiol. Behav 139 (2015) 541–548. [DOI] [PubMed] [Google Scholar]
- [7].Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, et al. , The effects of acute and chronic psychological stress on bladder function in a rodent model, Urology 78 (2011) 967 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, et al. , Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study, PLoS ONE 12 (2017) e0182976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].O’Hare PG 3rd, Hoffmann AR, Allen P, Gordon B, Salin L, Whitmore K, Interstitial cystitis patients’ use and rating of complementary and alternative medicine therapies, Int. Urogynecol. J 24 (2013) 977–982. [DOI] [PubMed] [Google Scholar]
- [10].Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH, Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews, Cochrane Database Syst. Rev 4 (2017) CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pare WP, The performance of WKY rats on three tests of emotional behavior, Physiol. Behav 51 (1992) 1051–1056. [DOI] [PubMed] [Google Scholar]
- [12].Clemens JQ, Meenan RT, Rosetti MC, Gao SY, Calhoun EA, Prevalence and incidence of interstitial cystitis in a managed care population, J. Urol 173 (2005) 98–102 discussion. [DOI] [PubMed] [Google Scholar]
- [13].National-Research-Council-(US)-Committee-for-the-Update-of-the-Guide-for-theCare-and-Use-of-Laboratory-Animals, Guide for the Care and Use of Laboratory Animals, eight ed., National Academies Press, Washington, DC, 2011. [Google Scholar]
- [14].Ackerman AL, Jellison FC, Lee UJ, Bradesi S, Rodriguez LV, Glt1 glutamate receptor mediates the establishment and perpetuation of chronic visceral pain in an animal model of stress-induced bladder hyperalgesia, Am. J. Physiol. Renal. Physiol 310 (2015) F628–FF36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Raz S, Ameliorative effects of brief daily periods of social interaction on isolation-induced behavioral and hormonal alterations, Physiol. Behav 116-117 (2013) 13–22. [DOI] [PubMed] [Google Scholar]
- [16].Baek K, Shim WH, Jeong J, Radhakrishnan H, Rosen BR, Boas D, et al. , Layer-specific interhemispheric functional connectivity in the somatosensory cortex of rats: resting state electrophysiology and fMRI studies, Brain Struct. Funct 221 (2016) 2801–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kalthoff D, Po C, Wiedermann D, Hoehn M, Reliability and spatial specificity of rat brain sensorimotor functional connectivity networks are superior under sedation compared with general anesthesia, NMR Biomed. 26 (2013) 638–650. [DOI] [PubMed] [Google Scholar]
- [18].Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD, Time-dependent effects of isoflurane and dexmedetomidine on functional connectivity, spectral characteristics, and spatial distribution of spontaneous bold fluctuations, NMR Biomed. 27 (2014) 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martin C, Martindale J, Berwick J, Mayhew J, Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat, Neuroimage 32 (2006) 33–48. [DOI] [PubMed] [Google Scholar]
- [20].Zeng J, Pan C, Jiang C, Lindstrom S, Cause of residual urine in bladder outlet obstruction: an experimental study in the rat, J. Urol 188 (2012) 1027–1032. [DOI] [PubMed] [Google Scholar]
- [21].Holschneider DP, Maarek JM, Harimoto J, Yang J, Scremin OU, An implantable bolus infusion pump for use in freely moving, nontethered rats, Am. J. Physiol. Heart Circ. Physiol 283 (2002) H1713–H1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goldman H, Sapirstein LA, Brain blood flow in the conscious and anesthetized rat, Am. J. Physiol 224 (1973) 122–126. [DOI] [PubMed] [Google Scholar]
- [23].Patlak CS, Blasberg RG, Fenstermacher JD, An evaluation of errors in the determination of blood flow by the indicator fractionation and tissue equilibration (Kety) methods, J. Cereb. Blood Flow Metab 4 (1984) 47–60. [DOI] [PubMed] [Google Scholar]
- [24].Sakurada O, Kennedy C, Jehle J, Brown JD, Carbin GL, Sokoloff L, Measurement of local cerebral blood flow with iodo [14C] antipyrine, Am. J. Physiol 234 (1978) H59–H66. [DOI] [PubMed] [Google Scholar]
- [25].Stumpf WE, F Solomon H, Autoradiography and Correlative Imaging, Academic Press, New York, 1995. [Google Scholar]
- [26].Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE (Eds.), Statistical Parametric Mapping: The Analysis of Functional Brain Images, Elsevier, Academic Press, New York, 2007. [Google Scholar]
- [27].Nguyen PT, Holschneider DP, Maarek JM, Yang J, Mandelkern MA, Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats, Neuroimage 23 (2004) 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Paxinos G, Watson C, The Rat Brain in Stereotactic Coordinates, sixth ed., Elsevier Academic Press, New York, 2007. [Google Scholar]
- [29].Griffiths D, Neural control of micturition in humans: a working model, Nat. Rev. Urol 12 (2015) 695–705. [DOI] [PubMed] [Google Scholar]
- [30].de Groat WC, Griffiths D, Yoshimura N, Neural control of the lower urinary tract, Compr. Physiol 5 (2015) 327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zare A, Jahanshahi A, Rahnama’i MS, Schipper S, van Koeveringe GA, The role of the periaqueductal gray matter in lower urinary tract function, Mol. Neurobiol 56 (2019) 920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G, Evidence for widespread afferents to Barrington’s nucleus, a brainstem region rich in corticotropin-releasing hormone neurons, Neuroscience 62 (1994) 125–143. [DOI] [PubMed] [Google Scholar]
- [33].Wang Z, Pang RD, Hernandez M, Ocampo MA, Holschneider DP, Anxiolytic-like effect of pregabalin on unconditioned fear in the rat: an autoradiographic brain perfusion mapping and functional connectivity study, Neuroimage 59 (2012) 4168–4188. [DOI] [PubMed] [Google Scholar]
- [34].Soncrant TT, Horwitz B, Holloway HW, Rapoport SI, The pattern of functional coupling of brain regions in the awake rat, Brain Res. 369 (1986) 1–11. [DOI] [PubMed] [Google Scholar]
- [35].Nair HP, Gonzalez-Lima F, Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal, and ventral tegmental regions, J. Neurosci 19 (1999) 8646–8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Barrett D, Shumake J, Jones D, Gonzalez-Lima F, Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response, J. Neurosci 23 (2003) 5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Z, Bradesi S, Charles JR, Pang RD, Maarek J-MI, Mayer EA, et al. , Functional brain activation during retrieval of visceral pain-conditioned passive avoidance in the rat, Pain 152 (2011) 2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shumake J, Conejo-Jimenez N, Gonzalez-Pardo H, Gonzalez-Lima F, Brain differences in newborn rats predisposed to helpless and depressive behavior, Brain Res. 1030 (2004) 267–276. [DOI] [PubMed] [Google Scholar]
- [39].Padilla E, Shumake J, Barrett DW, Sheridan EC, Gonzalez-Lima F, Mesolimbic effects of the antidepressant fluoxetine in Holtzman rats, a genetic strain with increased vulnerability to stress, Brain Res. 1387 (2011) 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fidalgo C, Conejo NM, Gonzalez-Pardo H, L Arias J, Cortico-limbic-striatal contribution after response and reversal learning: a metabolic mapping study, Brain Res. 1368 (2011) 143–150. [DOI] [PubMed] [Google Scholar]
- [41].Schwarz AJ, Gozzi A, Reese T, Heidbreder CA, Bifone A, Pharmacological modulation of functional connectivity: the correlation structure underlying the phMRI response to D-amphetamine modified by selective dopamine D3 receptor antagonist SB277011A, Magn. Reson. Imaging 25 (2007) 811–820. [DOI] [PubMed] [Google Scholar]
- [42].Fisher RA, On the ‘probable error’ of a coefficient of correlation deduced from a small sample, Metron 1 (1921) 3–32. [Google Scholar]
- [43].Shyu BC, Andersson SA, Thoren P, Spontaneous running in wheels. A microprocessor assisted method for measuring physiological parameters during exercise in rodents, Acta Physiol. Scand 121 (1984) 103–109. [DOI] [PubMed] [Google Scholar]
- [44].Kingwell BA, Arnold PJ, Jennings GL, Dart AM, The effects of voluntary running on cardiac mass and aortic compliance in Wistar-Kyoto and spontaneously hypertensive rats, J. Hypertens 16 (1998) 181–185. [DOI] [PubMed] [Google Scholar]
- [45].Pierce AN, Eller-Smith OC, Christianson JA, Voluntary wheel running attenuates urinary bladder hypersensitivity and dysfunction following neonatal maternal separation in female mice, Neurourol. Urodyn 37 (2018) 1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kavia RB, Dasgupta R, Fowler CJ, Functional imaging and the central control of the bladder, J. Comp. Neurol 493 (2005) 27–32. [DOI] [PubMed] [Google Scholar]
- [47].Blok BF, Holstege G, Direct projections from the periaqueductal gray to the pontine micturition center (M-region). An anterograde and retrograde tracing study in the cat, Neurosci. Lett 166 (1994) 93–96. [DOI] [PubMed] [Google Scholar]
- [48].Ding YQ, Zheng HX, Gong LW, Lu Y, Zhao H, Qin BZ, Direct projections from the lumbosacral spinal cord to Barrington’s nucleus in the rat: a special reference to micturition reflex, J. Comp. Neurol 389 (1997) 149–160. [DOI] [PubMed] [Google Scholar]
- [49].Klop EM, Mouton LJ, Kuipers R, Holstege G, Neurons in the lateral sacral cord of the cat project to periaqueductal grey, but not to thalamus, Eur. J. Neurosci 21 (2005) 2159–2166. [DOI] [PubMed] [Google Scholar]
- [50].Kuipers R, Klop EM, Neurons in the guinea pig (Cavia porcellus) lateral lumbosacral spinal cord project to the central part of the lateral periaqueductal gray matter, Brain Res. 1101 (2006) 43–50. [DOI] [PubMed] [Google Scholar]
- [51].Benarroch EE, Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation, Neurology 70 (2008) 1991–1995. [DOI] [PubMed] [Google Scholar]
- [52].Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D, Neuroimaging of the periaqueductal gray: state of the field, Neuroimage 60 (2012) 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Blok BF, Holstege G, Two pontine micturition centers in the cat are not interconnected directly: implications for the central organization of micturition, J. Comp. Neurol 403 (1999) 209–218. [DOI] [PubMed] [Google Scholar]
- [54].Chen SC, Chu PY, Hsieh TH, Li YT, Peng CW, Feasibility of deep brain stimulation for controlling the lower urinary tract functions: an animal study, Clin. Neurophysiol 128 (2017) 2438–2449. [DOI] [PubMed] [Google Scholar]
- [55].Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, et al. , Forced, not voluntary, exercise effectively induces neuroprotection in stroke, Acta Neuropathol. 115 (2008) 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ke Z, Yip SP, Li L, Zheng XX, Tong KY, The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model, PLoS ONE 6 (2011) e16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Griesbach GS, Tio DL, Vincelli J, McArthur DL, Taylor AN, Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury, J. Neurotrauma 29 (2012) 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Timofeeva E, Huang Q, Richard D, Effects of treadmill running on brain activation and the corticotropin-releasing hormone system, Neuroendocrinology 77 (2003) 388–405. [DOI] [PubMed] [Google Scholar]
- [59].Pavcovich LA, Valentino RJ, Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus, Neurosci. Lett 196 (1995) 185–188. [DOI] [PubMed] [Google Scholar]
- [60].Nelson AJ, Juraska JM, Musch TI, Iwamoto GA, Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas, J. Appl. Physiol 99 (2005) 2312–2322. [DOI] [PubMed] [Google Scholar]
- [61].Nelson AJ, Juraska JM, Ragan BG, Iwamoto GA, Effects of exercise training on dendritic morphology in the cardiorespiratory and locomotor centers of the mature rat brain, J. Appl. Physiol 108 (2010) 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ko IG, Kim SE, Kim CJ, Jung JH, Lee SJ, Kim DH, et al. , Effect of treadmill exercise on leak-point pressure and neuronal activation in brain of rats with stress urinary incontinence, Int. Neurourol. J 14 (2010) 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Griffiths D, Tadic SD, Schaefer W, Resnick NM, Cerebral control of the bladder in normal and urge-incontinent women, Neuroimage 37 (2007) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tadic SD, Griffiths D, Schaefer W, Resnick NM, Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence, Neuroimage 39 (2008) 1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, et al. , Brain switch for reflex micturition control detected by FMRI in rats, J. Neurophysiol 102 (2009) 2719–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Deutsch G, Deshpande H, Frolich MA, Lai HH, Ness TJ, Bladder distension increases blood flow in pain related brain structures in subjects with interstitial cystitis, J. Urol 196 (2016) 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kuhtz-Buschbeck JP, Gilster R, van der Horst C, Hamann M, Wolff S, Jansen O, Control of bladder sensations: an fMRI study of brain activity and effective connectivity, Neuroimage 47 (2009) 18–27. [DOI] [PubMed] [Google Scholar]
- [68].Nishijima S, Sugaya K, Kadekawa K, Ashitomi K, Yamamoto H, Effect of chemical stimulation of the medial frontal lobe on the micturition reflex in rats, J. Urol 187 (2012) 1116–1120. [DOI] [PubMed] [Google Scholar]
- [69].Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, et al. , The organization of the rat motor cortex: a microstimulation mapping study, Brain Res. 396 (1986) 77–96. [DOI] [PubMed] [Google Scholar]
- [70].Rouiller EM, Moret V, Liang F, Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area, Somatosens Mot. Res 10 (1993) 269–289. [DOI] [PubMed] [Google Scholar]
- [71].Sievert CF, Neafsey EJ, A chronic unit study of the sensory properties of neurons in the forelimb areas of rat sensorimotor cortex, Brain Res. 381 (1986) 15–23. [DOI] [PubMed] [Google Scholar]
- [72].Sugaya K, Nishijima S, Kadekawa K, Ashitomi K, Ueda T, Yamamoto H, Intravenous or local injections of flavoxate in the rostral pontine reticular formation inhibit urinary frequency induced by activation of medial frontal lobe neurons in rats, J. Urol 192 (2014) 1278–1285. [DOI] [PubMed] [Google Scholar]
- [73].Hubscher CH, Montgomery LR, Fell JD, Armstrong JE, Poudyal P, Herrity AN, et al. , Effects of exercise training on urinary tract function after spinal cord injury, Am. J. Physiol. Renal. Physiol 310 (2016) F1258–F1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ward PJ, Herrity AN, Smith RR, Willhite A, Harrison BJ, Petruska JC, et al. , Novel multi-system functional gains via task specific training in spinal cord injured male rats, J. Neurotrauma 31 (2014) 819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kitta T, Matsumoto M, Tanaka H, Mitsui T, Yoshioka M, Nonomura K, GABAergic mechanism mediated via D receptors in the rat periaqueductal gray participates in the micturition reflex: an in vivo microdialysis study, Eur. J. Neurosci 27 (2008) 3216–3225. [DOI] [PubMed] [Google Scholar]
- [76].Zare A, Jahanshahi A, Meriaux C, Steinbusch HW, van Koeveringe GA, Glutamatergic cells in the periaqueductal gray matter mediate sensory inputs after bladder stimulation in freely moving rats, Int. J. Urol 25 (2018) 621–626. [DOI] [PubMed] [Google Scholar]
- [77].Matsuura S, Downie JW, Allen GV, Micturition evoked by glutamate microinjection in the ventrolateral periaqueductal gray is mediated through Barrington’s nucleus in the rat, Neuroscience 101 (2000) 1053–1061. [DOI] [PubMed] [Google Scholar]
- [78].Greenwood BN, The role of dopamine in overcoming aversion with exercise, Brain Res. 1713 (2019) 102–108. [DOI] [PubMed] [Google Scholar]
- [79].Ogawa T, Seki S, Masuda H, Igawa Y, Nishizawa O, Kuno S, et al. , Dopaminergic mechanisms controlling urethral function in rats, Neurourol. Urodyn 25 (2006) 480–489. [DOI] [PubMed] [Google Scholar]
- [80].Seki S, Igawa Y, Kaidoh K, Ishizuka O, Nishizawa O, Andersson KE, Role of dopamine D1 and D2 receptors in the micturition reflex in conscious rats, Neurourol. Urodyn 20 (2001) 105–113. [DOI] [PubMed] [Google Scholar]
- [81].E Billman G, Aerobic exercise conditioning: a nonpharmacological antiarrhythmic intervention, J. Appl. Physiol 92 (1985) 446–454 2002. [DOI] [PubMed] [Google Scholar]
- [82].Sanford MT, Yeh JC, Mao JJ, Guo Y, Wang Z, Zhang R, et al. , Voluntary exercise improves voiding function and bladder hyperalgesia in an animal model of stress-induced visceral hypersensitivity: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study, Neurourol. Urodyn (2019) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]