Abstract
Objective:
Visfatin is an adipokine secreted mainly by adipose tissue and has been implicated in obesity. It also mimics the effects of insulin and its expression is hormonally regulated by hormones. Serum visfatin concentrations were evaluated in Saudi women of different body weights to determine its relationships with sex hormones and obesity-induced insulin resistance (IR) in women in Saudi Arabia.
Methods:
In this cross-sectional study, 83 healthy Saudi women of different body weights were recruited between 2014 and 2016, from King Abdulaziz University staff and students. They were divided into three groups according to their body mass indexes (BMIs). Anthropometric measurements were recorded for all of the participants. Blood samples were collected to assess the biochemical variables, including glucose, insulin, lipid profile, visfatin, sex hormone-binding globulin (SHBG), and sex hormones levels.
Results:
Obese women exhibited significantly higher blood pressure (BP), glucose, insulin, IR, lipid profile, and visfatin levels than overweight and lean women. However, lean women had significantly higher high-density lipoprotein-cholesterol (HDL)-C, estradiol (E2), luteinizing hormone (LH), and SHBG levels than overweight and obese women. Positive correlations were observed between visfatin levels and waist and hip circumferences, BMI, diastolic BP, systolic BP (SBP) insulin, IR, and LDL-C levels (P < 0.001 – P < 0.05). Negative correlations were observed between visfatin levels and HDL-C, SHBG, LH, and E2 levels (P < 0.001 – P < 0.05).
Conclusions:
The results of this study revealed that E2 and SHBG concentrations were decreased in obese women, while visfatin levels were increased in obese women with high IR levels. This suggests that visfatin levels and sex hormones interact synergistically with obesity with regard to the IR risk in obese women.
Keywords: Insulin resistance, obesity, Saudi women, sex hormones, sex hormone-binding globulin, visfatin
Introduction
Obesity is becoming a serious global issue due to its negative impact on health and its contributions to mortality and morbidity. It was estimated that more than 1.9 billion people were overweight in 2016, with 650 million meeting the criteria for obesity.[1] Previous studies from Middle Eastern countries, including Saudi Arabia, have indicated that in both adults and children, obesity has reached an alarming level.[2-5] Over the last few decades, Saudi Arabia has become more Westernized, and, at this point in time, it has some of the highest obesity and overweight prevalence rates.[6] It was reported that 44% of the Saudi females were found to be obese and 71% were reported to be overweight.[7] Moreover, it is well-known that obesity is associated with several sex steroid hormone abnormalities. In females, the levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone, and estradiol (E2) are lower in obese females when compared to lean females.[8] The associations between excess body fat, especially abdominal fat, and irregular menstrual cycles have also been proven in previous studies.[9] In addition, it has been reported that the relationships between obesity and multiple metabolic abnormalities may result of insulin resistance (IR) or hyperinsulinemia, which may lead to further alterations involving estrogen, androgens, their carrier proteins, and sex hormone-binding globulin (SHBG).[10-13]
Today, adipose tissue is recognized as an endocrine and paracrine organ that releases various adipokines, including leptin, resistin, and adiponectin.[14] Visfatin is a protein that is expressed mainly in the visceral adipose tissue, and its secretion is upregulated in obese humans and animals.[15] It acts as an autocrine, paracrine, and endocrine mediator. In addition, visfatin can participate in the regulation of several physiological functions, such as cell proliferation and glucose metabolism.[16] Moreover, visfatin levels can affect lipid homeostasis in ways similar to those of insulin and triglyceride (TG) metabolism.[15] However, the factors that regulate visfatin levels and visfatin mechanisms of action have not been fully elucidated. As obesity is associated with higher levels of hormones and IR, it may serve as a link between visfatin levels and sex hormones. However, there is a lack of consensus on visfatin levels and their relationships to sex hormones. Therefore, the aim of the current study was to evaluate serum visfatin concentrations in Saudi women of different body weights during the follicular phase of the menstrual cycle, to elucidate its relation to sex hormones, SHBG, and obesity-induced IR in women.
Subjects and Methods
Subjects
In this cross-sectional study, a total of 83 healthy Saudi women were recruited from King Abdulaziz University’s staff and students, Saudi Arabia, for the period from January 2014 to December 2016. Raosoft sample size calculator was used to calculate the sample size. Raosoft depends on four factors in determining sample size: Confidence level, margin of error, the population, and the expected response distribution.[17] According to Raosoft’s method, the minimum sample size was 73. However, 83 samples were collected, which was a comparable size to that in similar larger studies. The blood samples of all participants were collected at King Abdulaziz University Clinic (Female campus). Inclusion criteria were Saudi females between the age of 18 and 30 years, no signs of endocrine disorders, and fasting between 12 to 14 h. The exclusion criteria were the presence of any chronic diseases, treatment with any medication, pregnancy, and irregular menstrual cycle.
Written informed consent has been obtained from all volunteers before their inclusion. This study was approved was by the research committee of the biomedical ethics unit at the Faculty of Medicine, KAU (Reference No 450-16). The medical histories of the study population were obtained using a detailed questionnaire and physical examination.
The participants were divided into the following three groups according to their body mass indexes (BMIs): 35 obese women (42%) (29.0 ± 4.9 years old), 15 overweight women (18%) (23.6 ± 3.4 years old), and 33 lean women (39.76%) (22.87 ± 2.64 years old).
Anthropometric measurements
Anthropometric measurements, including body weight, height, and waist and hip circumferences, were measured. Weights and heights were recorded in light clothing without shoes. Body weight was taken to the nearest 0.1 kg and height was taken to the nearest 0.1 cm. Waist circumference was measured midway between the costal margins and the iliac crest, and the hip circumference was measured around the widest portion of the buttocks. BMI values were calculated by dividing the person’s weight in kilograms by height in meters square, and the waist-to-hip ratio (WHR) was calculated by dividing the waist circumference by the hip circumference in centimeters. Blood pressure (BP) was measured in millimeters of mercury (mmHg) using an automatic sphygmomanometer (OMRON Healthcare Europe B.V., Hoofddorp, The Netherlands).
Biochemical analysis
Ten milliliter of blood was drawn from each participant after an overnight fast between the 2nd and the 3rd day of her menstrual cycle. Serum samples were carefully collected and stored at -40°C until analysis. Glucose and lipid profiles, including the TGs, total cholesterol (TC), very low-density lipoprotein (VLDL-C), low-density lipoprotein (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) concentrations were measured using enzymatic colorimetric assays (Siemens Healthcare Diagnostics INC., Tarrytown, NY, USA). Serum insulin levels were determined with an electrochemiluminescence immunoassay (ECLIA) using an immunoassay analyzer (Elecsys 1010/2010 and the E 170 module for Modular Analytics; Roche Diagnostics, Risch-Rotkreuz, Switzerland). Degree of IR was calculated according to the Homeostasis Model Assessment based on the following formula: HOMRA-IR = fasting insulin level (µU/ml) × fasting glucose level (mmol−1)/22.5.[18] FSH, LH, progesterone, and 17-ß E2 levels were also measured with an ECLIA using an immunoassay analyzer (Elecsys 1010/2010 and E 170 module for Modular Analytics; Roche Diagnostics). Enzyme-linked immunosorbent assay kits were used to determine SHBG serum concentrations (R&D Systems, Minneapolis, MN, USA) and visfatin serum concentrations (BioVision Inc., Mountain View, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS Version 21.0 statistics software package (IBM Corp., Armonk, NY, USA). The comparison between groups was made using a one-way ANOVA followed by a post hoc test. The correlation levels between visfatin and the study parameters were assessed using the Spearman rank correlation analysis. P < 0.05 were considered statistically significant.
Results
As shown in Table 1, the anthropometric and biochemical variables from all groups were compared to that of the lean group. No significant difference was observed between the age, WHR, FSH levels, and progesterone levels.
Table 1.
Mean values and standard deviations of the anthropometric measurements and biochemical parameters of the lean, overweight, and obese women who participated in this study
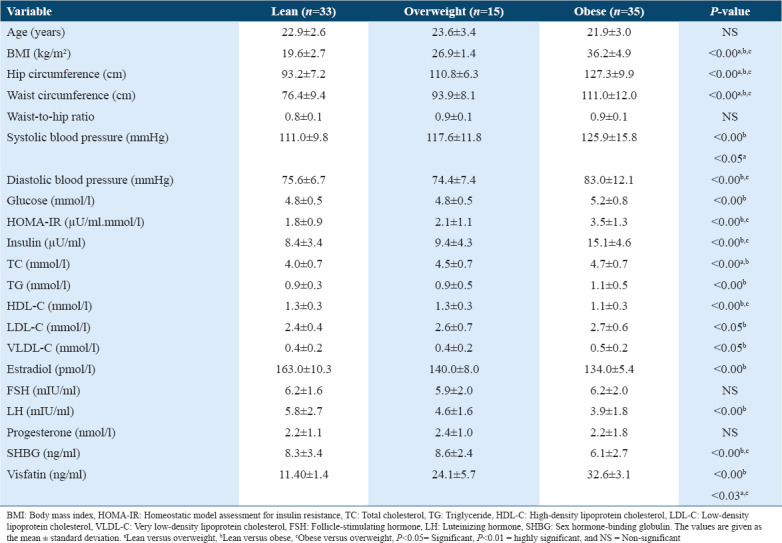
With regard to the anthropometric measurements, BMI, hip, and waist circumferences were significantly different between all groups (P < 0.001). In addition, significant differences were also observed in the mean SBP among all groups (P < 0.001–P< 0.05). The obese groups showed significantly higher SBP than those in the overweight and lean groups 125.9 ± 15.80 mmHg versus 117.6 ± 11.8 mmHg and 111.0 ± 9.8 mmHg, respectively (P < 0.001–P < 0.05). Moreover, diastolic BP (DBP) was significantly different among all groups. Mean DBP was significantly higher in the obese group when compared to the lean and overweight groups (83.0 ± 12.1 mmHg versus 74.4 ± 7.4 mmHg and 75.6 ± 6.7 mmHg, respectively, P < 0.001). Mean visfatin, insulin, IR, glucose, LDL-C, TC, VLDL-C, and TG levels were also significantly higher in the obese group when compared to the lean and overweight groups (P < 0.001). However, the mean E2, SHBG, LH, and HDL-C levels were significantly higher in the lean group than in the overweight and obese groups (P < 0.01–P < 0.05).
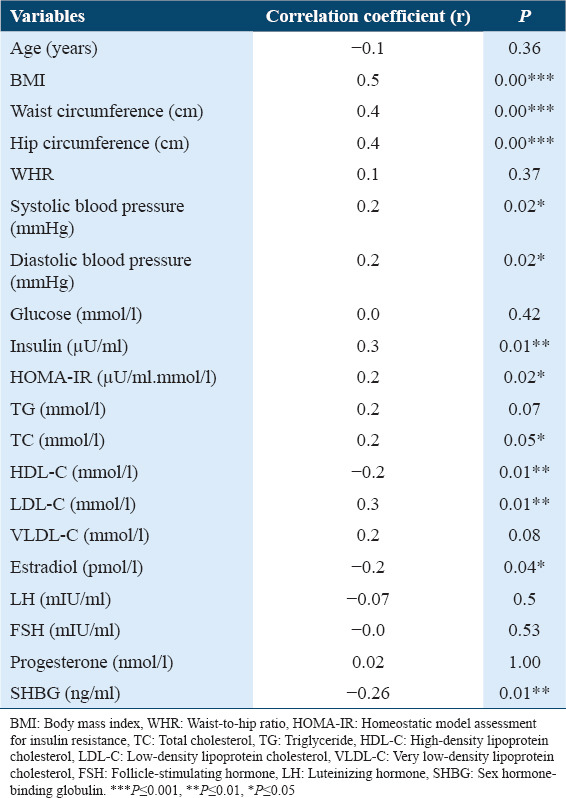
Serum visfatin level was positively correlated with waist and hip circumferences, BMI, DBP, SBP, insulin, IR, and LDL-C values (P < 0.01–P< 0.05), and it was significantly negatively correlated with HDL-C, E2, and SHBG values (P < 0.05) [Table 2].
Table 2.
Correlation between serum visfatin concentrations and all of the variables
Discussion
Visfatin is an adipokine that possesses strong insulin mimicking effects, and it has previously been reported to be associated with obesity.[19] As far as the authors are aware, this is the first report to evaluate the relationship between visfatin and the sex hormones during the early follicular phase in Saudi women of different body weights. In this study, the authors also aimed to explore the relation between visfatin levels and obesity-induced IR in women. The results of this study revealed that there were significant increases in anthropometric measurements and TG, LDL-C, TC, and BP levels, but significant decreases in HDL-C levels of the obese women when compared to those of the lean and overweight women. Moreover, the results revealed that visfatin, fasting glucose, insulin, and IR values were higher in obese women when compared to lean and overweight women. However, serum concentrations of E2, LH, and SHBG were higher in lean women when compared to obese women. Many studies have confirmed that obesity causes the release of visfatin from adipocytes.[19,20] It has also been reported that obesity is related to chronic inflammation and the increased production of cytokines, which are key factors that lead to hyperinsulinemia and IR.[21] Moreover, sex hormone synthesis and the inhibition of SHBG synthesis are stimulated by the insulin levels; thus, the influence of multiple hormonal and metabolic factors is biologically related.[22] It is well known that SHBG concentration is affected by body fat distribution [23] and the accumulation of abdominal visceral fat has been reported as a possible cause of IR and metabolic syndrome. Recently, it has been reported that subjects with central obesity have low SHBG concentrations.[23,24] Therefore, our findings suggest that the increase in the visceral fat of the obese women may have resulted in the considerable increase in visfatin levels, while the imbalances in SHBG and sex hormones might have been due IR. Since it was reported previously that visfatin is produced mainly by adipocytes, it is expected that visfatin levels increase with the increase of body fat.[25] However, the factors that regulate visfatin production and mechanism of action have not yet been fully understood.
The results also showed positive correlations between the BMI and the IR and serum visfatin concentrations. In contrast, visfatin level was negatively correlated with SHBG and E2. The results also proved that there was a positive correlation between visfatin concentrations and IR, and this correlation was in agreement with the results of previous research studies.[20,25-26] Moreover, in the present study, we expected relationships between circulating visfatin concentrations and BMI or other anthropometric measurements. However, the correlation between visfatin concentration and BMI was inconsistent.[20-28] In agreement with our results, previous reports have shown that circulating visfatin level was positively correlated with LH, TG, and insulin levels and IR and that it was negatively correlated with SHBG levels in obese women with polycystic ovary syndrome.[25,29] Interestingly, Wyskida et al.[30] recently reported that no correlation was observed between visfatin levels and sex hormone levels in normal-weight women; this may have been due to their normal insulin levels, and thus, they did not have IR. In general, the excessive storage of fat is now considered to be the lost link between the mechanisms of obesity that stimulates IR and the secretion of several adipocytokines released by the adipose tissue.[15,22,31] Nevertheless, it is still not apparent whether the induction of visfatin release is in response to compensation for IR for a specific tissue or as a result of the secretion of inflammatory markers from macrophages in the adipose tissue.[32] The results of our study suggested that due to the high visfatin levels in obese women, E2 and SHBG levels interacted synergistically with obesity on the IR risk of obese women.
Conclusion
The results clearly showed that visfatin levels and IR were higher in obese women than lean and overweight women. On the other hand, E2, LH, and SHBG levels were significantly decreased in obese women. Thus, increased circulating visfatin concentrations in obese women may be one of the compensatory mechanisms at the early stage of the development of an imbalance in sex hormones.
Limitations and recommendations
The limitation in this research was the small number of participants. However, the results of the study were similar to larger studies.[26] To elucidate the exact effects of visfatin on sex hormones, further research is needed during the different phases of the menstrual cycle in women.
Funding Statement
The research was funded by King Abdulaziz City for Science and Technology (KACST) in Riyadh, KSA (grant no. P-S-35-249).
Acknowledgments
The authors would like to express their gratitude to KACST in Riyadh, KSA, for their financial support (reference no. P-S-35-249).
References
- 1.World Health Organization. Obesity and Overweight. [[Last accessed on 2018 Feb 16]]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight .
- 2.Gunaid AA. Obesity, overweight and underweight among adults in an urban community in Yemen. East Mediterr Health J. 2012;18:1187–93. doi: 10.26719/2012.18.12.1187. [DOI] [PubMed] [Google Scholar]
- 3.Kilpi F, Webber L, Musaigner A, Aitsi-Selmi A, Marsh T, Rtveladze K, et al. Alarming predictions for obesity and non-communicable diseases in the Middle East. Public Health Nutr. 2014;17:1078–86. doi: 10.1017/S1368980013000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M Alqarni SS. A review of prevalence of obesity in Saudi Arabia. J Obes Eat Disord. 2016;2:2. [Google Scholar]
- 5.Habib SS. Body mass index and body fat percentage in assessment of obesity prevalence in Saudi Adults. Biomed Environ Sci. 2013;26:94–9. doi: 10.3967/0895-3988.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.DeNicola E, Aburizaiza OS, Siddique A, Khwaja H, Carpenter DO. Obesity and public health in the Kingdom of Saudi Arabia. Rev Environ Health. 2015;30:191–205. doi: 10.1515/reveh-2015-0008. [DOI] [PubMed] [Google Scholar]
- 7.ALNohair S. Obesity in gulf countries. Int J Health Sci (Qassim) 2014;8:79–83. doi: 10.12816/0006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Sairam MR. Sex hormone imbalances and adipose tissue dysfunction impacting on metabolic syndrome;a paradigm for the discovery of novel adipokines. Horm Mol Biol Clin Investig. 2014;17:89–97. doi: 10.1515/hmbci-2014-0002. [DOI] [PubMed] [Google Scholar]
- 9.Rafique M, Nuzhat A. Role of obesity in female infertility and assisted reproductive technology (ART) outcomes. Saudi J Obesity. 2016;4:75–9. [Google Scholar]
- 10.Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7:14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, et al. Polycystic ovary syndrome, insulin resistance, and obesity:Navigating the pathophysiologic labyrinth. Int J Reprod Med. 2014;2014:719050. doi: 10.1155/2014/719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Xian T, Jia X, Zhang L, Liu L, Man F, et al. A cross-sectional study on the associations of insulin resistance with sex hormone, abnormal lipid metabolism in T2DM and IGT patients. Medicine (Baltimore) 2017;96:e7378. doi: 10.1097/MD.0000000000007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki H, Kushiyama A, Sakoda H, Fujishiro M, Yamamotoya T, Nakatsu Y, et al. Protective effect of sex hormone-binding globulin against metabolic syndrome In vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflamm. 2018;2018:3062319. doi: 10.1155/2018/3062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davutoglu M, Ozkaya M, Guler E, Garipardic M, Gursoy H, Karabiber H, et al. Plasma visfatin concentrations in childhood obesity:Relationships with insulin resistance and anthropometric indices. Swiss Med Wkly. 2009;139:22–7. doi: 10.4414/smw.2009.12400. [DOI] [PubMed] [Google Scholar]
- 15.Nourbakhsh M, Nourbakhsh M, Gholinejad Z, Razzaghy-Azar M. Visfatin in obese children and adolescents and its association with insulin resistance and metabolic syndrome. Scand J Clin Lab Inv. 2015;75:183–8. doi: 10.3109/00365513.2014.1003594. [DOI] [PubMed] [Google Scholar]
- 16.Liang Z, Wu Y, Xu J, Fang Q, Chen D. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. J Diabetes Invest. 2016;7:247–52. doi: 10.1111/jdi.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raosoft Sample Size Calculator. Raosoft Inc. 2004. [[Last accessed on 2019 Aug 25]]. Available from: http://www.raosoft.com/samplesize.html .
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment:Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Martínez Larrad MT, Anchuelo AC, Pérez CF, Barba MP, Redondo YL, Ríos MS, et al. Obesity and cardiovascular risk:Variations in visfatin gene can modify the obesity associated cardiovascular risk Results from the Segovia population based-study. Spain. PLoS One. 2016;11:e0153976. doi: 10.1371/journal.pone.0153976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li RZ, Ma XY, Hu XF, Kang SX, Chen SK, Cianflone K, et al. Elevated visfatin levels in obese children are related to proinflammatory factors. J Pediatr Endocrinol Metab. 2013;26:111–8. doi: 10.1515/jpem-2012-0237. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo F, Catellani C, Sartori C, Lazzeroni P, Amarr S, Street ME. Obesity, insulin resistance, and colorectal cancer:Could miRNA dysregulation play a role? Int J Mol Sci. 2019;20:2922. doi: 10.3390/ijms20122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson RE, Rock CL, Kerr J, Natarajan L, Marshall SJ, Pakiz B, et al. Metabolism and breast cancer risk:Frontiers in research and practice. J Acad Nutr Diet. 2013;113:288–96. doi: 10.1016/j.jand.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford TN, Arikawa AY, Kurzer MS, Schmitz KH, Phipps WR. Cross-sectional study of factors influencing sex hormone-binding globulin concentrations in normally cycling premenopausal women. Fertil Steril. 2015;104:1544–51. doi: 10.1016/j.fertnstert.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azrad M, Gower BA, Hunter GR, Nagy TR. Intra-abdominal adipose tissue is independently associated with sex-hormone binding globulin in premenopausal women. Obesity (Silver Spring) 2012;20:1012–5. doi: 10.1038/oby.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taşkesen D, Kirel B, Us T. Serum visfatin levels, adiposity and glucose metabolism in obese adolescents. J Clin Res Pediatr Endocrinol. 2012;4:76–81. doi: 10.4274/Jcrpe.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olszanecka-Glinianowicz M, Kocełak P, Nylec M, Chudek J, Zahorska-Markiewicz B. Circulating visfatin level and visfatin/insulin ratio in obese women with metabolic syndrome. Arch Med Sci. 2012;8:214–8. doi: 10.5114/aoms.2012.28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma V, Yao-Borengasser A, Rasouli N, Bodles AM, Phanavanh B, Lee MJ, et al. Human visfatin expression:Relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab. 2007;92:666–72. doi: 10.1210/jc.2006-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CC, Li TC, Li CI, Liu CS, Lin WY, Wu MT, et al. The relationship between visfatin levels and anthropometric and metabolic parameters:Association with cholesterol levels in women. Metabolism. 2007;56:1216–20. doi: 10.1016/j.metabol.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Panidis D, Farmakiotis D, Rousso D, Katsikis I, Delkos D, Piouka A, et al. Plasma visfatin levels in normal weight women with polycystic ovary syndrome. Eur J Intern Med. 2008;19:406–12. doi: 10.1016/j.ejim.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Wyskida K, Franik G, Wikarek T, Owczarek A, Delroba A, Chudek J, et al. The levels of adipokines in relation to hormonal changes during the menstrual cycle in young, normal-weight women. Endocr Connect. 2017;6:892–900. doi: 10.1530/EC-17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung UJ, Choi MS. Obesity and its metabolic complications:The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naz R, Tauqeer S, Bibi Y, Ayub M. Level of visfatin in obese and diabetic Balb/c mice. Pak J Physiol. 2017;13:36–8. [Google Scholar]


