Abstract
Objective:
Skin cancers are relatively uncommon malignancies worldwide, but there is a progressive increase in the incidence over the last few decades.
Methods:
We have studied the clinical and histopathological features of malignant skin tumors received in the department of pathology of our tertiary care institute over a period of 3 years and 8 months. A detailed analysis of clinical, gross, and microscopic findings was performed, followed by clinicopathological correlation.
Results:
One hundred and one specimens of skin tumors were received during this period, of which 37 (36.27%) were malignant tumors. Keratinocytic tumors were most common (81.08%) followed by appendageal tumors (10.81%). Squamous cell carcinoma (SCC) was the most frequent malignancy followed by basal cell carcinoma (BCC). Malignant melanoma, hidradenocarcinoma, malignant proliferating trichilemmal tumor (MPTT), sebaceous carcinoma, and fibrosarcomatous dermatofibrosarcoma were also observed. Variants such as hybrid verrucous SCC, basosquamous carcinoma, infiltrating BCC, and MPTT with spindle SCC were also found. Malignant skin tumors were most frequent in the seventh decade (40.54%). Males and females were almost equally affected. Overall, head and neck region was the most common site for malignant skin tumors.
Conclusion:
The vast diversity of skin tumors produces difficulty in diagnosis. Any lesion, for which the diagnosis is uncertain, based on the history and clinical examination, should be biopsied for histopathological examination to rule out malignancy.
Keywords: Appendageal tumors, basal cell carcinoma, keratinocytic, malignant skin tumors, squamous cell carcinoma
Introduction
The incidence of skin cancer is increasing exponentially around the world.[1] Three main types of skin cancer are basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and malignant melanoma. BCC and SCC, in combination, are referred to as non-melanoma skin cancers (NMSC).
The worldwide incidence of both malignant melanoma and NMSC varies widely. Number of new cases of malignant melanoma worldwide in 2018 was 287,723, whereas NMSC (1,042,056) was almost 3.6 times higher than melanoma.[2] Europe (50.1%) showed the highest incidence of malignant melanoma, followed by North America (27.7%), Asia (7.6%), Latin America and the Caribbean (6.3%), Oceania (6%), and Africa (2.3%). North America showed the highest incidence of NMSC, with an incidence of 46.3%, followed by Europe (30.5%), Asia (7.4%), Oceania (6.8%), Latin America and the Caribbean (6.6%), and Africa (2.3%).[2] Asia showed an almost similar incidence of both NMSC and malignant melanoma.
The incidence of both malignant melanoma and NMSC is on the rise, with an annual increase in malignant melanoma of 0.6% among adults over 50 years.[3] In India, the estimated age-standardized incidence rate of NMSC is 0.82/100,000 population and melanoma is 0.24/100,000 population.[2]
Most of the NMSC has low mortality and a high cure rate when detected early.[4] Hence, there is a growing interest in NMSC worldwide. They are showing an increasing incidence and is a massive burden to the health care systems. BCC is the most common skin cancer worldwide, but various studies from India have reported SCC as the most prevalent skin malignancy.[3,5,6]
Malignant melanomas are the most lethal cancers of the skin that occurs mainly in fair-skinned people.[7,8] From a clinical and public health point of view, they are the most important group of skin cancers. Although less common than the familiar basal and squamous cell tumors of the skin, they are much more frequently fatal, due to their intrinsic tendency of lymphatic and hematogenic metastasis. Early detection of malignant melanoma remains the key factor in lowering mortality.[9]
Skin adnexal tumors are a vast and varied group which exhibits morphological differentiation towards one of the different types of adnexal epithelium present in normal skin, i.e., pilosebaceous, eccrine, and apocrine. They may display more than one line of differentiation (hybrid/composite tumors), resulting in difficulty to classify these neoplasms precisely.[10] Hence, the diagnosis relies on histological evaluation[11] and they are usually classified according to the predominant morphological component. Mesenchymal tumors are often a diagnostic challenge due to considerable morphologic overlap among the various tumor types.
The histopathological study is a valuable means for the diagnosis of malignant tumors of the skin and they also help distinguish benign tumors as well as tumor-like lesions.[12] Our study aims at the analysis of histopathological and clinical features of different malignant tumors. We have also correlated the clinical data with the histopathological findings.
Materials and Methods
We have studied the clinical and histopathological features of malignant skin tumors received in the department of pathology of our tertiary care institute after procuring a clearance from the ethical committee and involved patients’ written consent were taken. The study duration is of 3 years and 8 months. Malignant skin tumors of the genitalia were excluded from the study.
This study included excision specimens and incisional biopsy specimens. Detailed gross examination of the specimens was done, including external surface – size, shape, appearance, borders, overlying skin, consistency, and cut surface – color and appearance. Tissue was further processed and stained by hematoxylin and eosin. Immunohistochemistry was done for lesions with diagnostic difficulty.
The tumors were reported, as per the World Health Organisation Classification of Skin tumors 2018.[13] The data were compiled and analyzed for various parameters such as age, gender, clinical features, site, gross findings, and histopathological features. In addition, a clinicopathological correlation was also performed. The obtained parameters were evaluated using descriptive statistical analysis and presented in terms of percentage.
Results
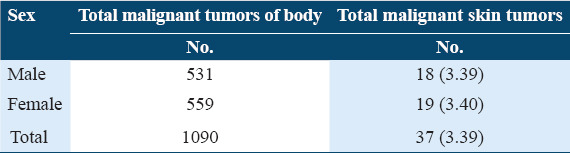
A total of 102 specimens of skin tumors were received during this period, of which 37 (36.27%) were malignant tumors. Malignant skin tumors constituted 3.39% of total malignant tumors of the body.
Category-wise distribution of malignant skin tumors [Table 1] showed that keratinocytic tumors were most common (81.08%) followed by appendageal tumors (10.81%). SCC was the most frequent malignancy followed by BCC.
Table 1.
Distribution of different types of malignant skin tumors according to histopathological diagnosis (as per the World Health Organisation Classification of Skin Tumors-2018)
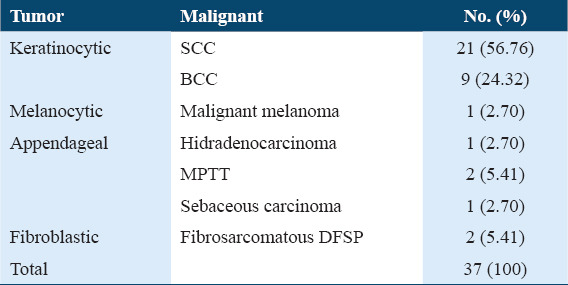
Malignant skin tumors were most frequent in the seventh decade (40.54%) [Table 2].
Table 2.
Age-wise distribution of malignant skin tumors
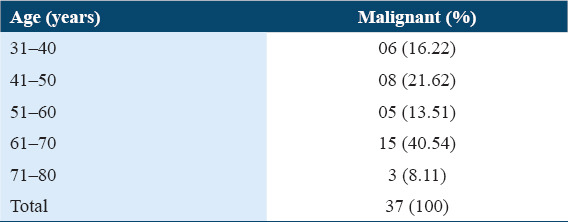
Slight female predominance was observed [Table 3]. SCC was more common in males, while BCC was more common in females.
Table 3.
Percentage distribution of malignant tumors of skin with respect to malignant tumors of body
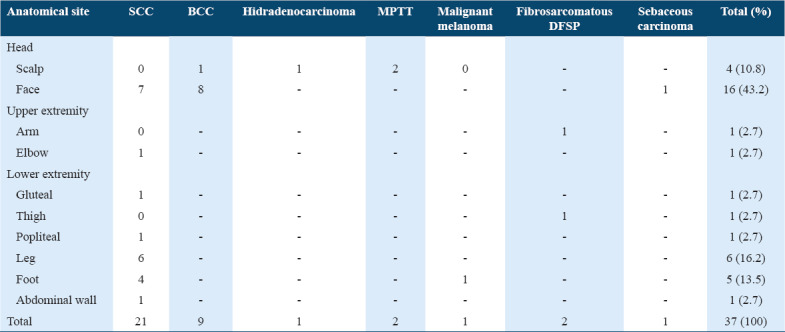
Overall, head and neck region was the most common site for malignant skin tumors (54%), as shown in Table 4. BCC was most frequently found on face, while SCC was most frequently found on the lower extremity.
Table 4.
Anatomical distribution of malignant skin tumors
SCC
Twenty-one cases of SCC were observed. The peak frequency of SCC was seen in the seventh decade (33.33%). It was seen in 14 males (66.67%) and seven females (33.33%), with male to female ratio of 2: 1. The most common site was lower extremity (12 cases). Majority of the cases had an ulceroproliferative growth (57.14%) [Figure 1a], while six cases had fungating growth (28.57%) and three cases had nodular growth (14.29%). Tumor size ranged between 0.3 cm and 8 cm. On histopathological examination, 17 lesions (80.95%) were diagnosed as well-differentiated SCC and three lesions (14.29%) as moderately differentiated SCC. All cases showed an infiltrating tumor arising from epidermis composed of round to polyhedral cells with pleomorphic hyperchromatic nuclei and eosinophilic cytoplasm arranged in sheets and clusters. Individual cell keratinization, keratin pearls, and mitotic figures were noted [Figure 1b]. One case, diagnosed as hybrid verrucous SCC, showed skin with an exophytic tumor showing papillomatosis, hyperkeratosis, and parakeratosis. The rete pegs had a bulbous appearance and were composed of large round to polyhedral cells, with mild nuclear pleomorphism and were seen infiltrating the deeper dermis in broad, blunt cellular tongues. In areas, the tumor cells showed moderate nuclear pleomorphism invading the dermis in uneven, sharply pointed, and jagged manner.
Figure 1.
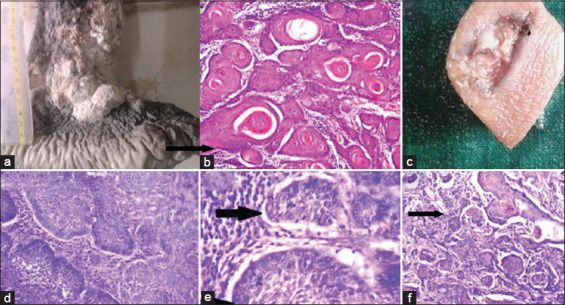
(a) Gross photograph of squamous cell carcinoma (SCC) showing large ulceroproliferative growth over the right leg. (b) Well-differentiated SCC – photomicrograph showing tumor cells with keratin pearls (black arrow) (hematoxylin and eosin [H and E], × 100). (c) Gross appearance of basal cell carcinoma showing ulcerative growth. (d and e) photomicrograph showing clefting artifact (black arrow) and peripheral palisading of tumor cells (arrow head) (H and E, × 400). (f) Basosquamous carcinoma – photomicrograph showing areas of squamous differentiation (black arrow) (H and E, × 100)
BCC
Nine cases of BCC were found in the present study, two of which were reported as variants, i.e., basosquamous carcinoma and infiltrating BCC. The peak frequency was seen in the seventh decade (44.44%) with female preponderance (3.5:1). Head and neck region was the most common site (100%), of which 8 lesions (88.89%) were located on the face. Ulcerative growth (66.67%) [Figure 1c] was the most common presentation. The size ranged from 0.5 cm to 3.5 cm. Histologically, sections showed a tumor composed of round to oval basaloid cells having uniform hyperchromatic nuclei and scanty cytoplasm arranged in sheets and clusters with prominent peripheral palisading and clefting artifact of the stroma [Figure 1d and e]. In infiltrating BCC, areas of irregular tumor nests were seen infiltrating the deeper tissue surrounded by desmoplastic stroma. In basosquamous carcinoma, the tumor cells were seen arising from the basal cell layer of skin and at places, showing squamous differentiation [Figure 1f].
Malignant melanoma
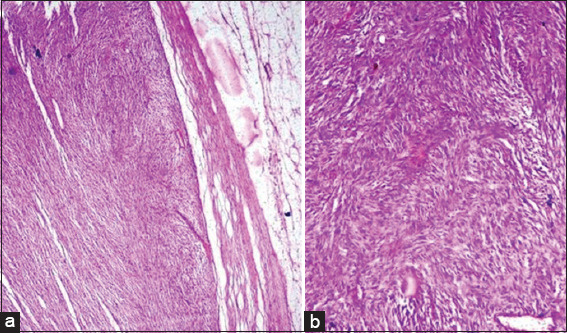
In the present study, one case of malignant melanoma was seen in a 70-year-old female who presented with a 6 cm ulceroproliferative growth over the left great toe and 4 cm inguinal lymphadenopathy. Histologically, sections showed tissue covered by skin with acanthosis, surface ulceration, and the base of ulcer showed a tumor composed of round to polygonal cells having pleomorphic hyperchromatic to vesicular nuclei with focally prominent eosinophilic nucleoli and eosinophilic cytoplasm arranged in nests, clusters, and cords [Figure 2a and b] Many of the tumor cells showed abundant melanin pigment in the cytoplasm. This lesion was Clark level IV, i.e., invasion into reticular dermis and the Breslow thickness was 1.5 mm.
Figure 2.
Photomicrograph of malignant melanoma (a) tumor cells arranged in nests and cords (Hematoxylin and eosin [H and E], × 100). (b) Tumor cells showing pleomorphic nuclei with prominent nucleoli and intracytoplasmic melanin pigment (H and E, × 400). Photomicrograph of hidradenocarcinoma (c) tumor cells (white arrow) with fibrovascular collagenous stroma (black arrow) (H and E, × 40). (d) Tumor cells arranged in sheets and areas of tumor necrosis (black arrow) (H and E, × 40)
Hidradenocarcinoma
A 10 cm nodular scalp lesion in 70 years female. Microscopy showed skin with a dermal tumor composed of atypical cells with pleomorphic nuclei. Intracytoplasmic vacuolation and lumen formation were seen with prominent ductal differentiation. Tumor lobules were surrounded by fibrovascular collagenous stroma. Areas of tumor necrosis, infiltration into the surrounding dermis, and few mitotic figures were noted [Figure 2c and d].
Malignant proliferating trichilemmal tumor (MPTT)
Two cases of this entity were observed, both in females and on the scalp. Of the two cases, one was MPTT with spindle SCC. Gross appearance showed skin covered multilobulated mass [Figure 3a]. On histology, epidermis was proliferating and infiltrating the deeper tissue to form a tumor composed of lobules of round to polyhedral cells with markedly pleomorphic hyperchromatic nuclei and moderate amount of eosinophilic cytoplasm showing peripheral palisading at places. In areas, the tumor cells showed a trichilemmal type of keratinization [Figure 3b]. In MPTT with spindle SCC, microscopy showed lobules of round to polyhedral cells with markedly pleomorphic hyperchromatic nuclei and moderate amount of eosinophilic cytoplasm showing peripheral palisading at places. The lobules were separated by spindle cells having pleomorphic hyperchromatic nuclei and eosinophilic cytoplasm [Figure 3c and d]. Numerous tumor giant cells were also noted. Immunohistochemistry showed positivity for cytokeratin and vimentin in the spindle cells [Figure 3e and f].
Figure 3.
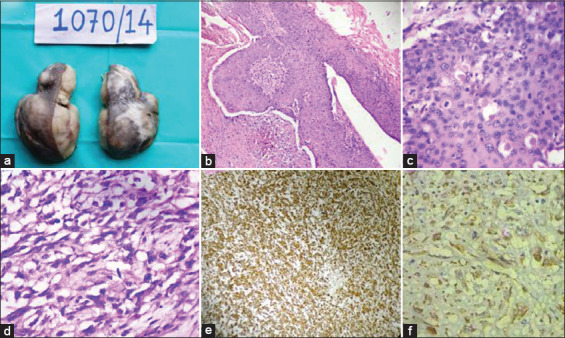
(a) Malignant proliferating trichilemmal tumor (MPTT)- Gross appearance showing skin covered multilobulated mass. (b and c) Photomicrograph of malignant squamous epithelium showing abrupt (trichilemmal) keratinization (Hematoxylin and eosin [H and E], b-× 100 and c-× 400). (d) MPTT – Photomicrograph showing malignant spindle cells with marked pleomorphism (H and E, × 400). (e and f) MPTT – Immunohistochemistry showing positivity for vimentin and cytokeratins, e-× 100, f-× 400)
Sebaceous carcinoma
One case of this entity was seen in a 70-year-old male who presented with a 1.7 cm nodular growth over the left eyebrow. Microscopically, the dermis showed an infiltrative tumor composed of round to polygonal cells having round to oval pleomorphic vesicular nuclei with prominent nucleoli and scant to moderate amount of fine eosinophilic vacuolated cytoplasm arranged in irregular lobules separated by thin fibrocollagenous septae. Few scattered mitotic figures were seen. The lobules showed variable sebaceous differentiation.
Malignant fibroblastic tumor – Fibrosarcomatous dermatofibrosarcoma protuberans (Fibrosarcomatous DFSP)
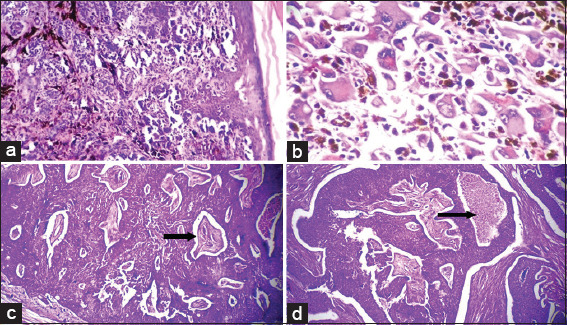
Two cases were studied, one was male, and the other was a female. Both lesions were present over the extremities. Grossly, both lesions were single and nodular, measuring 4 cm in one case and 9.5 cm in other cases. Histologically, both the lesions showed an ill-defined tumor composed of spindle-shaped cells having elongated tapered end nuclei and eosinophilic cytoplasm arranged in storiform pattern and fascicles [Figure 4a]. In addition, both the lesions showed increased cellularity with cells arranged in a herringbone pattern and increased mitosis (8–10/10 hpf) [Figure 4b].
Figure 4.
(a) Photomicrograph of fibrosarcomatous dermatofibrosarcoma protuberans (DFSP) showing densely packed spindle cells in the dermis (hematoxylin and eosin [H and E], × 40). (b) Photomicrograph of fibrosarcomatous DFSP showing tumor cells arranged in intersecting fascicles and bundles and storiform pattern (H and E, × 100)
Discussion
Thirty-seven types of malignant tumors were observed in the present study. These were SCC (56.76%), BCC (24.32%), malignant melanoma (2.70%), hidradenocarcinoma (2.70%), MPTT (5.41%), sebaceous carcinoma (2.70%), and fibrosarcomatous DFSP (5.41%).
Percentage of malignant skin tumors with respect to total malignancies of the body was compared with similar reports from other areas of India. As per the population-based cancer registry, it ranged from 0.98% to 2.52%. The frequency observed was highest in Thiruvananthapuram (2.52%), followed by Barshi (2.45%), Bhopal (2.18%), Chennai (1.68%), Ahmedabad (1.63%), Kolkata (1.61%), Aurangabad (1.48%), Delhi (1.28%), Mumbai (1.23%), Nagpur (1.19%), Bangalore (0.99%), and Pune (0.98%).[14] The percentage in all these cities was lower than the present study (3.39%).
In all the studies, the most common age group was more than the 5th decade[14] analogous to our study. In the present study, male:female ratio was 1:1.05. As per the cancer registry 2012–2016,[14] malignant skin tumors were slightly more common in females from Aurangabad, with NSMC being the more common type.[14] Whereas, cancer registry 2009–2011[15] as well as 2005–2008 showed male predominance in Aurangabad.[16] Male predominance was noted at all the other places except Pune, which showed no sex predilection as per cancer registry 2012–2016.[14] This may be due to certain risk factors for males such as increased outdoor activity, prolonged exposure to sun rays, trauma, and occupation (farmers).[17]
SCC
The maximum number of SCC was found in the seventh decade, similar to the findings by Laishram et al.[18] It was seen in 14 males (66.67%) and seven females (33.33%). There was a male preponderance in the present study which is consistent with observations of a few other studies.[18-21] This can be attributed to the cumulative effect of solar energy on the male population.[21]
In the present study, SCC was most commonly found over the lower extremity which is consistent with observations Chalya et al.[20] On histopathological examination, majority were well-differentiated SCC (n = 17, 80.95%). Laishram et al.[18] in their study of 40 cases found 65% cases as well-differentiated SCC.
We found a single case of hybrid verrucous carcinoma (VC) presenting as an exophytic growth. They are a mixed tumor and showed the features of VC as well as classic SCC. In
predominant pattern is of VC with only focal areas of SCC. The awareness of this entity is necessary and a vigilant search for areas with SCC should be performed.[22] Hybrid carcinomas should be staged and managed as conventional SCC because they show excellent prognosis following complete surgical removal in the early stages contrary to classical VC.[23]
BCC
The frequency of BCC with respect to malignancies of skin was lower in the study done by Adinarayan and Krishnamurthy.[19] and Chalya et al.,[20] whereas it was much higher in the study by Soomro et al.[17]
In the present study, BCC was most commonly found in the seventh decade (44.44%). Laishram et al.[18] also found maximum cases of BCC in the seventh decade (30%) followed by the fifth decade (26.67%). Furthermore, in the study of Adinarayan and Krishnamurthy,[19] maximum cases of BCC were encountered in the seventh and eighth decade.
There was a female preponderance of BCC in the present study which is similar to the observations of other studies Laishram et al., Souza et al., Kumar et al., and Saldanha et al.[18,24-26] However, few other groups Adinarayan and Krishnamurthy, Chalya et al., Nandyal and Puranik, and Malhotra et al.[19-21,27] found male preponderance. Nandyal et al.[21] proposed that the varying sex distribution of BCC noted in different geographical areas depends on skin color, lifestyle variations, climate, sun exposure, and habits. In most of the Indian literature, BCC shows a male predominance, whereas, in western literature, there is a female predominance.
In the present study, all the cases of BCC were found in the head and neck region, a finding noted by all of the above studies. Two variants, infiltrating and metatypical (basosquamous), were found in the present study. Saldanha et al.[26] found three cases of basosquamous carcinoma. The infiltrating variant is important in that the margins at the time of surgery may be frequently underestimated. Due to the cord-like arrangement of this variant, there is a morphological overlap with the tumor pattern seen in microcystic adnexal carcinoma (sclerosing sweat duct carcinoma), desmoplastic SCC, and desmoplastic trichoepithelioma. The metatypical variant is important in that it may merge with sebaceous carcinoma as lipid vacuoles or ducts may be focally apparent. Furthermore, prognosis wise metatypical and infiltrative variants have the highest recurrence rates[28] and hence, poor prognosis.
Nodular or solid type was the predominant form in the present study, as observed by other studies Adinarayan and Krishnamurthy, Saldanha et al., Malhotra et al.[19,26,27] Souza et al.,[24] and Laishram et al.[18] also showed solid (nodular) type to be most common (72%).
Malignant melanoma
The percentage frequency of melanoma (2.70%) was less than that observed in other studies. The seventh decade was the presenting age group similar to findings in studies by other studies Gundalli et al., Laishram et al., Chalya et al.,[7,18,20] Katalinic et al.,[29] and Mukhopadhyay et al.[30] reported melanoma most commonly in the age group of 60–79 years. Talley and Harrison et al.[31] (63.9%) and Gundalli et al.[7] (66.7%) found more cases in females. The site in the present study was great toe, i.e.,, extremity. Few studies Gundalli et al., Mukhopadhyay et al., Sampat and Sirsat, and Budharaja et al.[7,30,32,33] found that extremity was the most common site with 82%, 80%, 80%, and 44.44% cases, respectively. Breslow thickness of tumor – it is the single most important factor in predicting survival of patients. The thickness of tumor is measured from granular layer to the deepest tumor cell. In the present study, the Breslow thickness of tumor was 1.5 mm. Melanomas with Breslow thickness <0.76 mm are thin melanomas and generally have an excellent prognosis.[7] Clark’s level of invasion has a prognostic and descriptive value. The survival rates lower as the stage progresses. The tumor studied in the present study was of Clark level IV.
Hidradenocarcinoma
Asati et al.[34] also reported a case of hidradenocarcinoma on the scalp of an elderly female. Microscopy revealed a tumor in the dermis consisting of interconnected nodules as well as differentiated ducts, the neoplastic cells showing mild pleomorphism of nuclei, mitotic figures, and abundant pale cytoplasm. Clefts, sclerotic stroma, and foci of necrosis were also seen.
MPTT
In the present study, MPTT of the scalp was observed in elderly females. These findings were consistent with findings of other studies Asati et al., Siddha et al., Trabelsi et al., Garg et al., and Goyal et al.[35-38] A male patient with MPTT was observed in the study by Yotsuyanagi et al.[39] In Rao et al.[40] study, the patient with MPTT presented at a very young age (26 years).
The malignant spindle cell transformation in the present study was similar to a study by Alvarez-Quinones et al.[41] In their study, they reported a case of spindle cell carcinoma developed in a proliferating trichilemmal tumor on the scalp of an 84-year-old woman. Two years later, the patient was well, and neither recurrence nor metastases were observed in our study. Similar to the other adnexal tumors, on immunohistochemistry MPTT expresses cytokeratin 5/6 immunostaining. Normal immunoreactivity pattern for CD 34 noticed in tumors of outer root sheath is lost in MPTT, a feature of undifferentiation.[40] MPTT is a very rare trichilemmal tumor. Moreover, MPTT with spindle cell SCC is very uncommon. It is important to be aware of this entity because it has a tendency to recur and metastasize more frequently than SCC.
Sebaceous carcinoma
Radhika et al.[42] studied two cases of sebaceous carcinoma, both in the age group was 60–67. Vani et al.[43] studied four cases of sebaceous carcinoma. The histopathological findings in the present study were similar to the study by Radhika et al.[42] in which the tumor had infiltrative zones with pleomorphic cell populations of clear and solid cells. In the study by Sharma et al.,[44] sebaceous carcinoma was the only malignant tumor observed and constituted 19.64% (11/56). Most of them were meibomian gland carcinomas occurring in people above 50 years of age with a maximum number of cases, that is, four observed in females above 70 years of age.
Fibrosarcomatous DFSP
Few other studies Angouridakis et al., Achouri et al., and Prabhu et al.[45-47] observed a similar case of fibrosarcomatous transformation. In the study by Angouridakis et al.,[45] a 28 years female presented with the lesion on the face. Achouri et al.[46] study studied a series of nine cases of fibrosarcomatous DFSP, five male and four female. The lesions were located on the abdominal wall (three cases), the upper limb (two cases), the back (two cases), the lower limb (one case), and the chest wall (one case). Prabhu et al.[47] found fibrosarcomatous variant of DFSP in a 46-year-old male who presented with an erythematous swelling on his upper back. Fibrosarcomatous DFSP exhibits more aggressive behavior than DFSP; hence, awareness of this entity is important.
Conclusion
Malignant tumors were most frequent in the seventh decade (40.54%) and frequency was almost equal in males and females, with head and neck region (54.05%) being the most common site involved. SCC (56.76%) was the most common malignant tumor followed by BCC. Both SCC and BCC were most commonly seen in the seventh decade. SCC showed male preponderance while BCC was more common in females. The lower extremity (legs) was the most common site for SCC, while the face was the most common site for BCC. The vast diversity of skin tumors produces difficulty in diagnosis. The ability to properly diagnose and treat the tumors is a vital skill for all clinicians. To conclude, any lesion, for which the diagnosis is uncertain, based on the history and clinical examination, should be biopsied for histopathological examination to rule out malignancy.
Consent
Patient’s informed consent was taken for publishing the case in a scientific journal.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2014;810:120–40. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization International Agency for Research on Cancer GLOBOCAN 2018. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. 2020. [[Last accessed on 2020 Mar 20]]. Available from: https://www.gco.iarc.fr/today/online-analysis-map .
- 3.Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miolo N, Rodrigues RF, Silva ER, Piati PK, Campagnolo OA, Marques LF. Skin cancer incidence in rural workers at a reference hospital in western Paraná. An Bras Dermatol. 2019;94:157–63. doi: 10.1590/abd1806-4841.20197335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhumidi A. Acantholytic squamous cell carcinoma arising in a nevus sebaceous:A case report. Int J Health Sci (Qassim) 2013;7:343–6. doi: 10.12816/0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzolibani AA, Al Shobaili HA, Robaee AA, Khan A, Haque IU, Rao NS, et al. Clinical and histopathologic characteristics of skin malignancies in Qassim region, Saudi Arabia. Int J Health Sci (Qassim) 2013;7:61–5. doi: 10.12816/0006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gundalli SM, Kolekar R, Kolekar A, Pai K. Clinical and histopathological characteristics of malignant melanoma of the skin in tertiary care centre. Int J Sci Res. 2012;3:2519–23. [Google Scholar]
- 8.Ali SA, Naaz I. Biochemical aspects of mammalian melanocytes and the emerging role of melanocyte stem cells in dermatological therapies. Int J Health Sci (Qassim) 2018;12:69–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma:Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005–11. [PubMed] [Google Scholar]
- 10.Venugopal S, Madhu CP, Kamath BA. Malignant adnexal tumors:A rare case of cutaneous malignancy. Int Surg J. 2017;4:1786–88. [Google Scholar]
- 11.Alshehri AA, Al-Khowailed MS, Alnuaymah FM, Alharbi AS, Alromaihi MS, Alghofaili RS, et al. Knowledge, attitude, and practice toward evidence-based medicine among hospital physicians in Qassim region, Saudi Arabia. Int J Health Sci (Qassim) 2018;12:9–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Kindley KJ, Jackson JD, Sisson WT, Brodell RT. Improving dermatology clinical efficiency in academic medical centers. Int J Health Sci (Qassim) 2015;9:351–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Elder DE, Massi D, Scolyer RA, Willemze R. WHO Classification of Skin Tumours. 4th ed. France: IARC; 2018. [Google Scholar]
- 14.National Cancer Registry Programme. Three-year Report of Population Based Cancer Registries 2012-2016. New Delhi, India: Indian Council of Medical Research 2012-2016; 2012. [Google Scholar]
- 15.National Cancer Registry Programme. Three-year Report of Population Based Cancer Registries 2009-2011. New Delhi, India: Indian Council of Medical Research 2009-2011; 2009. [Google Scholar]
- 16.National Cancer Registry Programme. Three-year Report of Population Based Cancer Registries 2005-2008. New Delhi, India: Indian Council of Medical Research 2005-2008; 2005. [Google Scholar]
- 17.Soomro FR, Bajaj DR, Pathan GM, Abbasi P, Hussain J, Abbasi SA. Cutaneous malignant tumors:A profile of ten years at LINAR, Larkana-Pakistan. J Pak Assoc Dermatol. 2010;20:133–6. [Google Scholar]
- 18.Laishram RS, Banerjee A, Punyabati P, Sharma LD. Pattern of skin malignancies in Manipur, India:A 5-year histopathological review. J Pak Assoc Dermatol. 2010;20:128–32. [Google Scholar]
- 19.Adinarayan M, Krishnamurthy SP. Clinicopathological evaluation of nonmelanoma skin cancer. Indian J Dermatol. 2011;56:670–2. doi: 10.4103/0019-5154.91826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalya PL, Gilyoma JM, Kanumba ES, Mawala B, Masalu N, Kahima KJ, et al. Dermatological malignancies at a university teaching hospital in North-Western Tanzania:A retrospective review of 154 cases. Tanzan J Health Res. 2012;14:1–7. doi: 10.4314/thrb.v14i1.3. [DOI] [PubMed] [Google Scholar]
- 21.Nandyal S, Puranik RB. Study of demographic profile of skin tumors in a tertiary care hospital. Int J Curr Res Rev. 2014;6:24–8. [Google Scholar]
- 22.Costache M, Desa LT, Mitrache LE, Pătraşcu OM, Dumitru A, Costache D, et al. Cutaneous verrucous carcinoma-report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383–8. [PubMed] [Google Scholar]
- 23.Santoro A, Pannone G, Contaldo M, Sanguedolce F, Esposito V, Serpico R, et al. A troubling diagnosis of verrucous squamous cell carcinoma (“the bad kind”of keratosis) and the need of clinical and pathological correlations:A review of the literature with a case report. J Skin Cancer. 2011;2011:370605. doi: 10.1155/2011/370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souza CF, Thome EP, Menegotto PF, Schmitt JV, Shibue JR, Tarlé RG. Topography of basal cell carcinoma and their correlations with gender, age and histologic pattern:A retrospective study of 1042 lesions. An Bras Dermatol. 2011;86:272–7. doi: 10.1590/s0365-05962011000200010. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Mahajan BB, Kaur S, Yadav A, Singh N, Singh A. A study of basal cell carcinoma in South Asians for risk factor and clinicopathological characterization:A hospital based study. J Skin Cancer. 2014;2014:173582. doi: 10.1155/2014/173582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saldanha P, Shanthala PR, Upadhaya K. Cutaneous basal cell carcinoma:A morphological spectrum. Arch Med Health Sci. 2015;3:24–8. [Google Scholar]
- 27.Malhotra P, Singh A, Ramesh V. Basal cell carcinoma in the North Indian population:Clinicopathologic review and immunohistochemical analysis. Indian J Dermatol Venereol Leprol. 2011;77:328–30. doi: 10.4103/0378-6323.79710. [DOI] [PubMed] [Google Scholar]
- 28.Calonje E, Brenn T, Lazar A, Mckee PH Tumors of the surface epithelium. Mckee's Pathology of the Skin with Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012. pp. 1076–149. [Google Scholar]
- 29.Katalinic A, Kunze U, Schafer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany:Distribution, clinical subtypes, tumour stages and localization. Br J Dermatol. 2003;149:1200–6. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay S, Ghosh S, Siddhartha D, Mitra PK. A clinicopathological study of malignant melanoma with special reference to atypical presentation. Indian J Pathol Microbiol. 2008;51:485–8. doi: 10.4103/0377-4929.43736. [DOI] [PubMed] [Google Scholar]
- 31.Talley LI, Soong SJ, Harrison RA. Clinical outcomes of localized melanoma of the foot:A case control study. University of Alabama, University of Sydney. J Clin Epidemiol. 1998;51:853–57. doi: 10.1016/s0895-4356(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 32.Sampat MB, Sirsat MV. Malignant melanoma of the skin and mucous membranes in Indians. Indian J Cancer. 1966;6:228–53. [PubMed] [Google Scholar]
- 33.Budharaja SN, Pillai VC, Periyanagam WJ, Kaushik SP, Bedi BM. Malignant neoplasms of skin in Pondicherry-a study of 102 cases. Indian J Cancer. 1972:284–95. [PubMed] [Google Scholar]
- 34.Asati DP, Brahmachari S, Kudligi C, Gupta C. Hidradenocarcinoma:A rare sweat gland neoplasm presenting as small turban tumor of the scalp. Indian J Dermatol. 2015;60:418. doi: 10.4103/0019-5154.160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddha M, Budrukkar A, Shet T, Deshpande M, Basu A, Patil N, et al. Malignant pilar tumor of the scalp:A case report and review of literature. J Can Res Ther. 2007;3:240–3. doi: 10.4103/0973-1482.39001. [DOI] [PubMed] [Google Scholar]
- 36.Trabelsi A, Stita W, Gharbi O, Kanani N, Sriha B, Korbi S. Malignant proliferating trichilemmal tumor of the scalp:A case report. Dermatol Online J. 2008;14:11. [PubMed] [Google Scholar]
- 37.Garg PK, Dangi A, Khurana N, Hadke NS. Malignant proliferating trichilemmal cyst:A case report with review of literature. Malaysian J Pathol. 2009;31:71–6. [PubMed] [Google Scholar]
- 38.Goyal S, Jain BB, Jana S, Bhattacharya SK. Malignant proliferating trichilemmal tumor. Indian J Dermatol. 2012;57:50–2. doi: 10.4103/0019-5154.92679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yotsuyanagi T, Urushidate S, Yokoi K, Sawada Y. A malignant proliferating trichilemmal tumor simulating a squamous cell carcinoma. Eur J Plast Surg. 1997;20:320–2. [Google Scholar]
- 40.Rao S, Ramakrishnan R, Kamakshi D, Chakravarthi S, Sundaram S, Prathiba D. Malignant proliferating trichilemmal tumour presenting early in life:An uncommon feature. J Cutan Aesthet Surg. 2011;4:51–5. doi: 10.4103/0974-2077.79196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Quinones M, Garijo MF, Fernández F, Val-Bernal JF. Malignant aneuploid spindle-cell transformation in a proliferating trichilemmal tumour. Acta Derm Venereol. 1993;73:444–6. doi: 10.2340/0001555573444446. [DOI] [PubMed] [Google Scholar]
- 42.Radhika K, Phaneendra BV, Rukmangadha N, Reddy MK. A study of biopsy confirmed skin adnexal tumours:Experience at a tertiary care teaching hospital. J Clin Sci Res. 2013;2:132–8. [Google Scholar]
- 43.Vani D, Ashwini N, Sandhya M, Dayananda TR, Bharathi M. A 5 year histopathological study of skin adnexal tumors at a tertiary care hospital. IOSR J Dent Med Sci. 2015;14:1–5. [Google Scholar]
- 44.Sharma A, Paricharak DG, Nigam JS, Rewri S, Soni PB, Omhare A, et al. Histopathological study of skin adnexal tumours-institutional study in South India. J Skin Cancer. 2014;2014:543756. doi: 10.1155/2014/543756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angouridakis N, Kafas P, Jerjes W, Triaridis S, Upile T, Karkavelas G, et al. Dermatofibrosarcoma protuberans with fibrosarcomatous transformation of the head and neck. Head Neck Oncol. 2011;4:5. doi: 10.1186/1758-3284-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achouri L, Triki A, Bouzaiene H, Chemleli M, Laamouri B, Slimen M, et al. Transformed dermatofibrosarcoma protuberans:A series of nine cases and literature review. J Dermatol Dermatol Surg. 2016;20:1–4. [Google Scholar]
- 47.Prabhu R, Kumar N, Sadhu S, Shenoy R. Fibrosarcomatous dermatofibrosarcoma protuberans:A case report of an aggressive soft tissue sarcoma. J Clin Exp Res. 2013;1:71–3. [Google Scholar]


