Abstract
Caffeine is the most widely consumed psychoactive substance in the world. However, there is controversy about whether becoming addicted to caffeine is possible and a lack of well-established animal models to examine caffeine consumption. The present study sought to establish a model of caffeine consumption in Wistar rats, identify different rat populations based on caffeine preference, and determine whether extended voluntary caffeine consumption produces compulsive-like caffeine intake and withdrawal symptoms.
Male Wistar rats were used throughout the experiment. The optimal concentration of caffeine to maximize caffeine consumption and caffeine preference was determined. Rats were then given continuous access to caffeine, followed by intermittent access. Rats were tested for signs of withdrawal-like behavior by measuring mechanical nociception and irritability-like behavior. Rats were further examined for compulsive-like caffeine consumption using quinine adulteration.
Dose-response testing indicated an optimal caffeine concentration of 0.3 mg/mL. During intermittent access to caffeine, the rats did not escalate their caffeine intake and instead exhibited a decrease in intake over sessions. Three groups of rats were identified based on caffeine preference (high, medium, and low) across continuous and intermittent access. These three groups of rats matched low (1 cup), medium (2 cups), and high (4 cups) levels of daily coffee consumption in humans. Caffeine-consuming rats did not exhibit differences in mechanical nociception or irritability-like behavior compared with controls. In high caffeine-preferring rats but not in medium or low caffeine-preferring rats, compulsive-like caffeine consumption was observed.
The present study established a rodent model of caffeine consumption that resulted in large individual differences in caffeine intake, similar to humans. Compulsive-like caffeine consumption in high caffeine-preferring rats and differences in caffeine preference between groups suggest that caffeine may result in compulsive-like intake in a subpopulation of subjects. Further testing is necessary to determine the factors that contribute to differences in caffeine preference and compulsive-like intake.
Keywords: Caffeine use disorder, Compulsivity, Dependence, Substance abuse, Two-bottle choice
1. Introduction
Caffeine is the most widely consumed drug in the world. It is regularly consumed in mild amounts for its anxiolytic and mood-boosting effects. It is generally regarded as safe, with barely any restrictions worldwide on purchase and consumption compared with other psychoactive substances (Nehlig et al., 1992; Temple et al., 2017; Nieber, 2017; Richards and Smith, 2016). Over 85% of the United States population consumes at least one caffeinated beverage per day, and the average individual consumes approximately 2 or more caffeinated beverages per day (Mitchell et al., 2014; Jain et al., 2019; Juliano et al., 2012).
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, does not recognize caffeine among substance use disorders (SUDs). Although abstinence from chronic caffeine consumption can produce withdrawal, unclear is whether it is also associated with uncontrollable drug use and drug use despite adverse consequences (American Psychiatric Association, 2013; Meredith et al., 2013). In humans, high levels of regular caffeine use can lead to withdrawal symptoms, such as anxiety, headaches, fatigue, and irritability (American Psychiatric Association, 2013; Mitchell et al., 2014; Juliano and Griffiths, 2004; Stringer and Watson, 1987; Griffiths and Chausmer, 2000; Heckman et al., 2010; Jain et al., 2019). Although caffeine users can present withdrawal symptoms, studies have reported inconsistent evidence of compulsive caffeine consumption (i.e., consuming despite harmful consequences), despite a growing number of cases that meet these criteria (Strain et al., 1994; Hughes et al., 1998; Juliano et al., 2012; Richards and Smith, 2015). Unknown is whether caffeine is addictive and results in dependence or compulsive-like intake.
Few studies have evaluated the effects of caffeine and caffeine dependence in humans and animal models. Many previous clinical studies focused on specific aspects of the effects of caffeine on overall health and not caffeine addiction per se. A notable common pattern among these previous studies is that individual humans consume different levels of caffeine (Seal et al., 2017; Kolahdouzan and Hamadeh, 2017; Mitchell et al., 2014). Given differences in the amount of caffeine that is consumed among the human population, caffeine dependence and compulsive-like caffeine intake might only be seen in individuals who regularly drink high levels of caffeine, but this distinction has not yet been well explored.
Preclinical animal models are widely used to study addictive behaviors (O’Dell et al., 2004; Gilpin et al., 2008a; Gilpin et al., 2008b; Vendruscolo and Roberts, 2014; Wade et al., 2015; Carnicella et al., 2014; Park et al., 2015; Edwards et al., 2012; Avegno and Gilpin, 2019). Caffeine dependence and withdrawal have been reported in studies that utilized animal models of involuntary/forced consumption (Nehlig, 1999). Mixed results have been reported in studies that behaviorally modeled voluntary caffeine intake in rats. Intravenous caffeine self-administration did not result in consistent levels of caffeine intake (Atkinson and Enslen, 1976). However, rats learned to prefer a flavor that was associated with oral caffeine consumption (Fedorchak et al., 2002), suggesting that caffeine may be voluntarily consumed orally in rats at proper doses and in appropriate models of drinking.
The present study established a voluntary model of oral caffeine consumption in Wistar rats. We identified distinct rat populations based on caffeine preference and determined whether extended voluntary caffeine consumption in the two-bottle choice model produces compulsive-like caffeine drinking and withdrawal symptoms.
2. Methods
2.1. Subjects
Adult male Wistar rats (n = 36; 60 days old, ~250 g at the start of the study) were used for all of the experiments. The animals were single housed and maintained on a 12 h/12 h light/dark cycle with ad libitum access to food and water throughout the experiment. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by The Scripps Research Institute Institutional Animal Care and Use Committee.
2.2. Experimental design
The rats (n = 24) received continuous and intermittent access to a caffeine solution in a 24 h two-bottle choice paradigm. The remaining control rats (n = 12) were experimentally naive and received no caffeine throughout the study. The rats were first tested to establish a caffeine dose-response curve. Following dose-response testing, the rats then received continuous access to caffeine for 8 days. After continuous access, the rats received intermittent access (every other day) to caffeine for 10 total sessions over 3 weeks. During intermittent access, all of the rats were tested for irritability-like behavior with the bottle-brush test and pain sensitivity with the von Frey test 24 h after the last access to caffeine. The rats were then returned to continuous access for 8 days and then tested for compulsive-like caffeine intake with the quinine adulteration test. See Fig. 1 for a diagram of the experimental design.
Fig. 1.
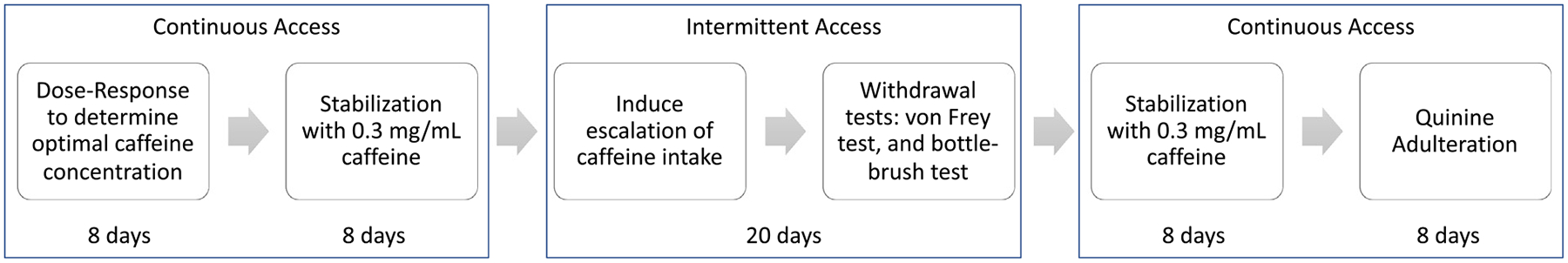
Rats (n = 24) received continuous and intermittent access to a caffeine solution in a 24 h two-bottle choice paradigm. The remaining control rats (n = 12) were experimentally naive and received no caffeine throughout the experiment. The rats were first tested to establish a caffeine dose-response curve using four different concentrations of caffeine (0.07, 0.14, 0.3, and 0.7 mg/mL). The optimal concentration (0.3 mg/mL), based on the dose response, was used for the subsequent experiments. After the dose-response test, the rats received continuous access to caffeine for 8 days. After continuous access, the rats received intermittent access (every other day) to caffeine for 10 total sessions over 3 weeks. During intermittent access, all of the rats were tested for irritability-like behavior and pain sensitivity 24 h after the last access to caffeine. The rats were then returned to continuous access to caffeine for 8 days and then tested for compulsive-like caffeine intake with the quinine adulteration test.
2.3. Drugs
Research-grade caffeine (Sigma Aldrich, St. Louis, MO, USA) was dissolved in water. The rats were given access to the caffeine solution in a voluntary two-bottle choice paradigm. The bitter tastant quinine hydrochloride dihydrate (Sigma Aldrich, St. Louis, MO, USA) was used for the quinine adulteration test.
2.4. Dose-response test
We first sought to determine which concentration of caffeine (0.07, 0.14, 0.3, and 0.7 mg/mL) was most preferred by the rats while maximizing caffeine consumption. Each concentration was tested for 2 days. The positions of the caffeine and water bottles were alternated every day to avoid possible side preference. Caffeine and water intake was recorded by weighing each bottle daily at the end of the rats’ light cycle. The 0.3 mg/mL concentration resulted in the highest total caffeine consumption and was the most preferred. Therefore, this concentration was used for the subsequent phases of the experiment.
2.5. Continuous and intermittent access two-bottle choice of caffeine and water
A model of continuous and intermittent access to caffeine in a two-bottle choice procedure was used. This model was similar to previous studies of alcohol drinking (George et al., 2012; Simms et al., 2008; Wise, 1973; Kimbrough et al., 2017b). The rats were presented with 24 h access to two bottles throughout the experiment, one containing water and the other containing caffeine. The bottle positions were switched daily to avoid possible side preference. Daily water and caffeine intake was recorded by weighing the bottles each day at the end of the light cycle. The rats were first given continuous access to 0.3 mg/mL caffeine and water for 8 days. Following continuous access, the rats were given intermittent access to caffeine, in which caffeine and water days alternated with water-only days for a total of 10 caffeine days over 3 weeks. We sought to determine whether the escalation of caffeine intake would occur. On days when the rats did not receive caffeine (i.e., water-only days), empty bottles were placed in the open slot where the caffeine bottle would be.
2.6. Determination of separate populations of caffeine preference
Average preference across the continuous and intermittent access periods was calculated for each rat. The rats were then split into three categories based on preference: high preference (> 70% preference for caffeine over water), medium preference (30–70% preference for caffeine over water), and low preference (< 30% preference for caffeine over water).
2.7. Measurement of mechanical nociception during caffeine withdrawal
The von Frey test was used to measure mechanical nociception in rats (Kononoff et al., 2018a; Kallupi et al., 2018). The test was performed 24 h after the rats’ last access to caffeine. We used an automated von Frey device (Dynamic Plantar Aesthesiometer, Ugo Basile) to measure touch sensitivity on the plantar surface of the rats’ hind paws. The rats were placed in individual chambers on top of an elevated wire grid floor and were given 5 min to acclimate to the apparatus before the procedure began. A thin 0.5 mm von Frey filament was attached to the automated machine and placed under the wire grid. The filament was applied perpendicularly to the plantar surface of the rat’s hind paw. The filament was applied with gradually increasing force (maximum force = 40 g) until a reflex reaction occurred. The Dynamic Plantar Aesthesiometer automatically recorded the paw withdrawal latency and force of the filament that was applied at the time of paw withdrawal. Each rat underwent six trials (three trials for the left hind paw and three trials for the right hind paw). The chambers were cleaned with ethanol between each session.
2.8. Measurement of irritability-like behavior during caffeine withdrawal
We used the bottle-brush test to test for irritability-like behavior 24 h after the rats’ last access to caffeine. The test was based on previous studies (Kononoff et al., 2018b; Kimbrough et al., 2017a). The bottle-brush test uses a bottle brush to measure aggressive and defensive responses. Increases in irritability-like behavior in the bottle-brush test have been observed during alcohol, oxycodone, and nicotine withdrawal (Sidhu et al., 2018; Somkuwar et al., 2017; Kimbrough et al., 2017a; Xue et al., 2018; Kallupi et al., 2018; Kimbrough et al., 2020; Kimbrough et al., 2020a). The test began at the start of the rats’ dark cycle and was conducted under dim red light. The session consisted of 10 trials in a clean plastic cage (26.67 cm × 48.26 cm × 20.32 cm; Ancare, Bellmore, NY, USA) with fresh bedding. Each trial began with the rat positioned at the back of the cage. The bottle-brush was inserted into the cage from the front, rotating toward the rat’s whiskers for approximately 3 s. The rotating brush was then returned to the front of the cage where it hung vertically for approximately 2 s before it was removed entirely from the cage. During this time, three observers recorded aggressive and defensive responses in each trial. Total responses over all 10 trials per session per rat were then summed, and an average across all observers was calculated for each rat. The following aggressive responses were recorded: sniffing the brush, biting the brush, boxing the brush, following the brush, and exploring the brush (i.e., manipulating the brush without biting or boxing). Although sniffing and exploring the brush are not aggressive behaviors per se, they consistently occurred during bouts of other aggressive behaviors (e.g. biting, boxing, and following) and thus were included in the list of aggressive behaviors. The following defensive responses were recorded: escaping from the brush, digging, jumping, climbing, vocalization, and grooming.
2.9. Quinine adulteration test
After receiving intermittent access, the rats were given continuous access for 8 days to restabilize caffeine intake before beginning the quinine adulteration test. Quinine (5, 10, 25, and 50 mg/L) was added to the 0.3 mg/mL caffeine solution. This method has been used in previous studies to examine compulsive-like alcohol drinking despite aversive consequences and results in a reduction of the preference for preferred solutions (Kimbrough et al., 2017b; Vendruscolo et al., 2012; Seif et al., 2013). Beginning at the 5 mg/L quinine concentration, each concentration was tested in ascending order for 2 days. The bottle positions of water and adulterated caffeine solution were switched every day to avoid possible side preference.
2.10. Determination of the Human Equivalent Dose of caffeine
We calculated the Human Equivalent Dose (HED; in mg/kg) of caffeine that was consumed by rats in each preference group (low, medium, and high). Caffeine consumption at the 0.3 mg/mL dose during the dose-response test was used as a baseline value of caffeine consumption in each group. The dose conversion from rats to humans (i.e., the HED) was estimated based on body surface area, which is associated with body weight (Nair and Jacob, 2016). To compare our rat HED results to actual human caffeine dose averages, we estimated an approximate daily intake of caffeine in mg/kg for high, medium, and low caffeine drinking humans. We calculated the mg of caffeine consumed for high caffeine drinkers based on a study indicating that the 90th percentile of US caffeine consumers takes 380 mg of caffeine daily (Mitchell et al., 2014). We then calculated the medium caffeine drinkers intake to be 50% of the high caffeine drinkers intake (190 mg) and low caffeine drinkers to be 25% of the high caffeine drinkers intake (95 mg). This equates to approximately 4 cups of coffee for high drinkers, 2 cups for medium drinkers, and 1 cup for low drinkers for a cup of coffee with an average of 95 mg of caffeine. We then calculated intake (in mg/kg) in humans based on an average body weight of 80 kg for each dose (high, medium and low).
2.11. Statistical analysis
Caffeine preference was calculated as caffeine consumption (in mL) divided by total intake (caffeine + water; in mL). The caffeine intake results are presented as intake (in mg/kg) and preference for caffeine over water. These data are expressed as 2-day averages. The initial dose-response data before splitting the groups into high, medium, and low preference were analyzed using one-way repeated-measures analysis of variance ANOVA, with caffeine concentration as the within-subjects factor. The data on caffeine intake during the different phases of the study (dose-response, continuous access, and intermittent access), with the groups split into high, medium, and low preference, were analyzed using two-way repeated-measures ANOVA, with group as the between-subjects factor and concentration and days as the within-subjects factors. Caffeine intake in the quinine adulteration test was examined using one-way repeated-measures ANOVA for each group, with quinine concentration as the within-subjects factor. The data from the bottle-brush test and the von Frey test were analyzed using one-way ANOVA, with group as the between-subjects factor. Significant main effects in the ANOVAs were followed by the Student-Newman-Keuls (SNK) post hoc test. The data were analyzed using Statistica software (Tibco). Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Dose-response test
For the initial caffeine dose-response test, the one-way repeated-measures ANOVA revealed a significant main effect of caffeine concentration on caffeine consumption (F3,69 = 49.70, p < 0.0005). The SNK post hoc test indicated that caffeine consumption at the 0.07 and 0.7 mg/mL caffeine concentrations was significantly lower compared with the 0.14 and 0.3 mg/mL concentrations. Caffeine consumption at 0.3 mg/mL caffeine was significantly higher than caffeine consumption at the 0.14 mg/mL concentration (Fig. 2A).
Fig. 2.
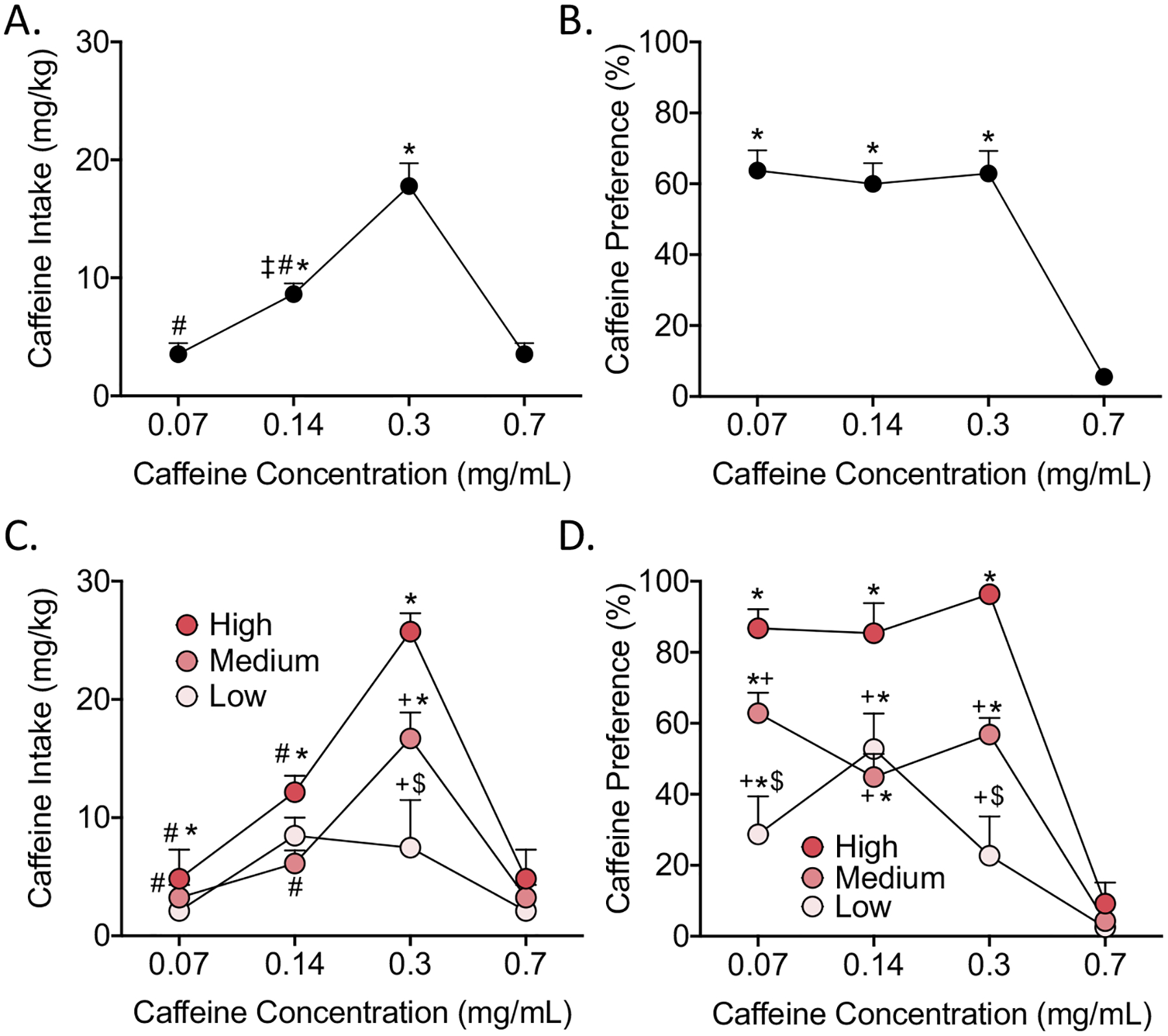
Dose-response test. To determine an optimal concentration for voluntary caffeine consumption, the rats were given 24 h continuous access to caffeine in a two-bottle choice procedure (caffeine solution and water) with four different caffeine concentrations (0.07, 0.14, 0.3, and 0.7 mg/mL) for 2 days per concentration. (A) Caffeine intake, expressed as mg/kg and (B) Percent preference for caffeine over water. (C) Caffeine intake in the high preference group (bright pink), medium preference group (pink), and low preference group (light pink), expressed as mg/kg. (D) Percent preference for caffeine over water in the high preference group (bright pink), medium preference group (pink), and low preference group (light pink). The data are expressed as 2-day averages for each concentration. *p < 0.05, compared with 0.7 mg/mL within group; ‡p < 0.05, compared with 0.07 mg/mL within group; #p < 0.05, compared with 0.3 mg/mL within group; +p < 0.05, compared with high preference group; $p < 0.05, compared with medium preference group.
The one-way repeated-measures ANOVA revealed a significant main effect of caffeine concentration on the preference for caffeine (F3,69 = 49.63, p < 0.0005). The SNK post hoc indicated that caffeine preference at the 0.7 mg/mL caffeine concentration was significantly lower compared with all of the other caffeine concentrations (Fig. 2B).
We then analyzed caffeine consumption (in mg/kg) based on separate groups of caffeine preference (high, medium, and low). The two-way repeated-measures ANOVA revealed significant main effects of group (F2,21 = 5.38, p < 0.05) and concentration (F3,63 = 59.34, p < 0.0005) and a significant group × concentration interaction (F6,63 = 7.02, p < 0.0005). The SNK post hoc test indicated that caffeine consumption in the high preference group at the 0.07 and 0.7 mg/mL caffeine concentrations was significantly lower than at the 0.14 and 0.3 mg/mL doses. Additionally, in the high preference group, caffeine consumption (in mg/kg) at the 0.3 mg/mL caffeine concentration was significantly higher than caffeine consumption at the 0.14 mg/mL concentration. In the medium preference group, caffeine consumption (in mg/kg) at the 0.3 mg/mL caffeine concentration was significantly higher than all of the other concentrations. In the low preference group, caffeine consumption (in mg/kg) at the 0.7 mg/mL caffeine concentration was significantly lower than caffeine consumption at the 0.14 mg/mL concentration. Comparisons among groups showed that the high preference group consumed significantly more caffeine (in mg/kg) compared with the medium and low preference groups at the 0.7 and 0.3 mg/mL caffeine concentration. The medium preference group consumed significantly more caffeine (in mg/kg) than the low preference group at the 0.3 mg/mL concentration (Fig. 2C).
The two-way repeated-measures ANOVA revealed significant main effects of group (F2,21 = 29.09, p < 0.0005) and concentration (F3,63 = 62.28, p < 0.0005) on caffeine preference and a significant group × concentration interaction (F6,63 = 6.92, p < 0.0005). The SNK post hoc test indicated that caffeine preference in the high and medium preference groups at the 0.7 mg/mL caffeine concentration was significantly lower than all of the other concentrations. In the low preference group, caffeine preference at the 0.07 and 0.14 mg/mL caffeine concentration was significantly higher than at the 0.7 mg/mL concentration. Caffeine preference was significantly lower in the low and medium preference groups at the 0.07, 0.14, and 0.3 mg/mL caffeine concentration compared with the high preference group. Caffeine preference in the low preference group was significantly lower than in the medium preference group at the 0.07 and 0.3 mg/mL caffeine concentrations (Fig. 2D).
3.2. Continuous and intermittent access to caffeine
One-way repeated-measures ANOVA was used to analyze caffeine preference across the continuous and intermittent access periods, with days (expressed as 2-day averages) as the within-subjects factor. The one-way ANOVA revealed a significant main effect of day on caffeine preference (F8,184 = 5.73, p < 0.0005). The SNK post hoc test indicated that caffeine preference on Day 1 was significantly higher than on Days 16–28 (Fig. 3A).
Fig. 3.
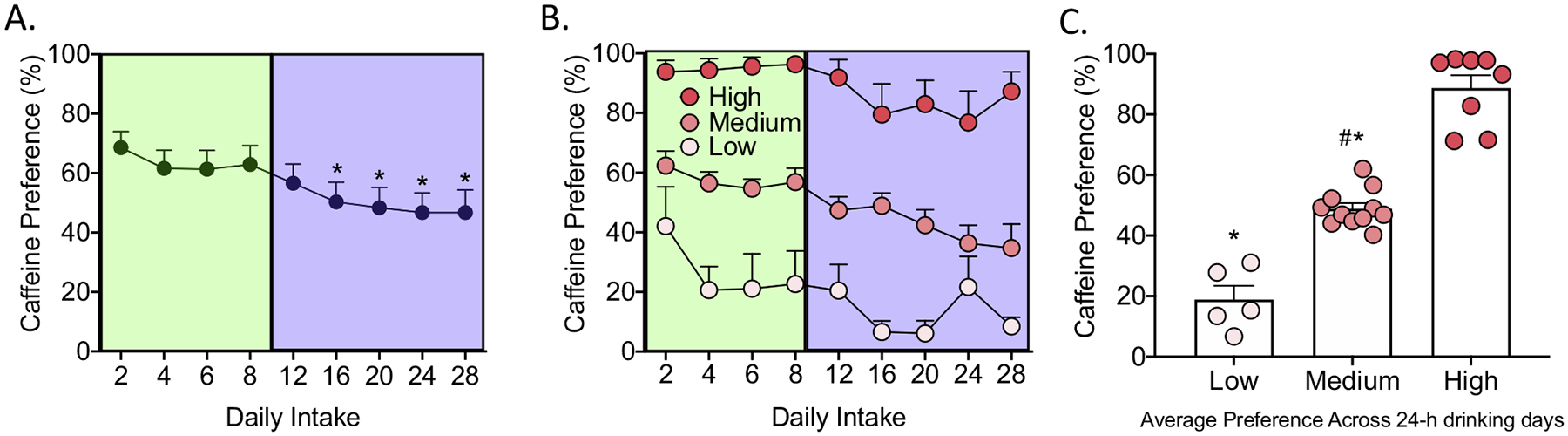
Caffeine preference during 24 h continuous and intermittent access to caffeine. (A) Percent caffeine preference across continuous (green) and intermittent (purple) drinking days. Caffeine preference significantly decreased compared with the initial 2-day average during continuous access on days 16–28, shortly after the beginning of intermittent access. The data are expressed as 2-day averages for each data point. *p < 0.05, compared with day 2. (B) Percent caffeine preference in the high preference group (dark pink), medium preference group (pink), and low preference group (light pink) across continuous (green) and intermittent (purple) drinking days. The data are expressed as 2-day averages for each data point. (C) Average caffeine preference in the high, medium, and low preference groups across the entire continuous and intermittent periods. The medium and low preference groups exhibited significantly lower preference for caffeine (*p < 0.05). The medium preference group exhibited significantly higher preference for caffeine than the low preference group (#p < 0.05).
Two-way repeated-measures ANOVA was also used to analyze caffeine preference, with group (high, medium, and low preference) as the between-subjects factor and days (expressed as 2-day averages) during continuous and intermittent access to caffeine as the within-subjects factor. The two-way repeated-measures ANOVA revealed significant effects of group (F2,21 = 94.87, p < 0.0005) and day (F8,168 = 5.16, p < 0.0005) on caffeine preference but no significant group × day interaction (Fig. 3B).
3.3. Determination of separate populations of rats based on caffeine preference
The average preference of individual rats across the continuous and intermittent access periods was determined and used to split the rats into high, medium, and low preference. One-way repeated-measures ANOVA was used to analyze average caffeine preference in the three different groups across the continuous and intermittent access periods. The one-way ANOVA revealed a significant main effect of group on caffeine preference (F2,21 = 94.94, p < 0.0005). The SNK post hoc test indicated that caffeine preference in the high preference group was significantly higher than in the medium and low preference groups. Caffeine preference in the medium preference group was significantly higher than in the low preference group (Fig. 3C).
3.4. Behavioral testing
In the von Frey test, the one-way repeated measures ANOVA revealed no effect of group on the paw withdrawal latency (F3,32 = 1.57, p > 0.05) or the force that was required to elicit a withdrawal response (F3,32 = 1.05, p > 0.05; Fig. 4A, B).
Fig. 4.
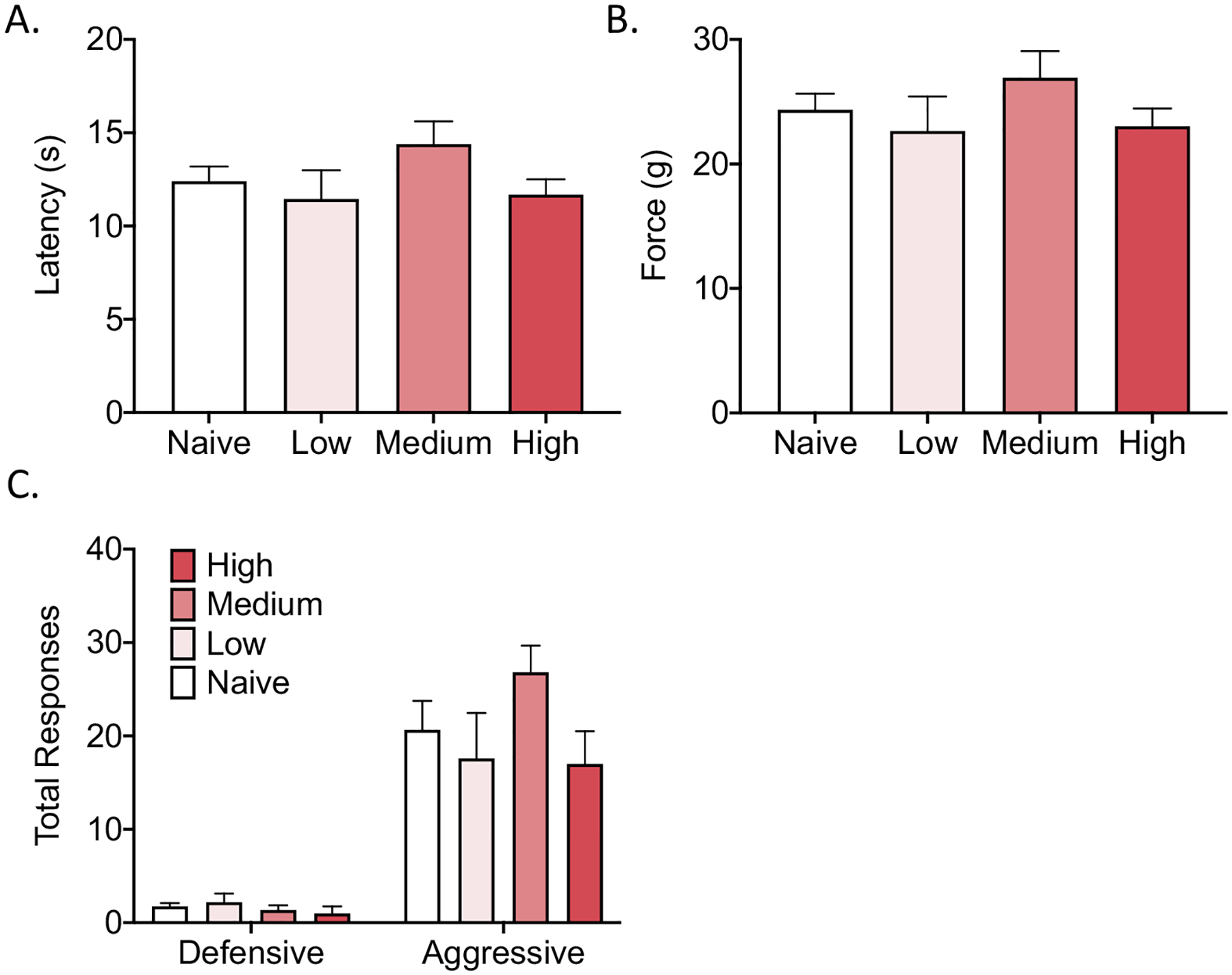
Behavioral testing 24 h after the last access to caffeine. (A, B) No significant difference in the paw withdrawal latency (A) or force required to elicit a paw withdrawal response (B) was found between groups in the von Frey test. (D) No significant differences in the number of defensive or aggressive responses were found between groups in the bottle-brush test.
In the bottle-brush test, the one-way repeated-measures ANOVA revealed no effect of group on aggressive responses (F3,32 = 1.79, p > 0.05) or defensive responses (F3,32 = 0.63, p > 0.05; Fig. 4C).
3.5. Quinine adulteration of caffeine solution
The one-way ANOVA revealed a significant effect of quinine concentration on caffeine preference (F4,92 = 7.87, p < 0.0005). The SNK post hoc test indicated that caffeine preference significantly decreased compared with baseline at the 0.025 and 0.05 g/L quinine concentrations (Fig. 5A).
Fig. 5.
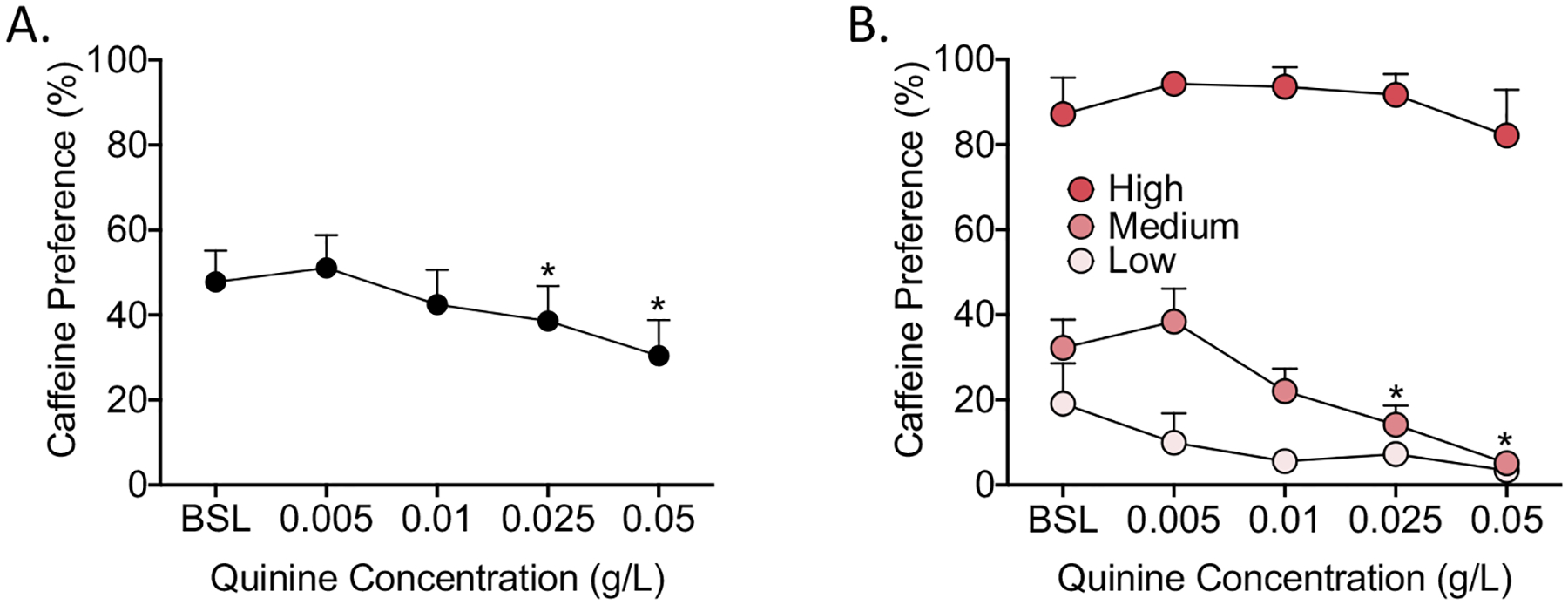
Compulsive-like caffeine intake in the quinine adulteration test. Four different quinine concentrations (0.005, 0.01, 0.025, and 0.05 g/L) were added to the caffeine solution and tested for 2 days at each concentration. (A) Caffeine preference was significantly lower than baseline at the 0.025 and 0.05 g/L quinine concentrations. (B) Caffeine preference was significantly lower at the 0.025 and 0.05 g/L quinine concentrations in the medium preference group (pink). No significant differences in caffeine preference from baseline were observed in the high preference group (bright pink) or low preference group (light pink). The data are expressed as 2-day averages for each concentration. *p < 0.05, compared with the rats’ own baseline.
The two-way repeated-measures ANOVA revealed significant main effects of group (F2,21 = 80.99, p < 0.0005) and quinine concentration (F4,84 = 5.86, p < 0.0005) on caffeine preference and a significant group × quinine concentration interaction (F8,84 = 2.30, p < 0.05). The SNK post hoc test indicated that the high preference group and low preference group did not exhibit a significant decrease in caffeine preference at any quinine concentration tested compared with their baseline preference. The medium preference group exhibited a significant decrease in caffeine preference compared with baseline at the 0.025 and 0.05 g/L quinine concentrations (Fig. 5B).
3.6. Human Equivalent Dose of caffeine
The HED of caffeine consumption was 4.15 mg/kg in the high preference group, 2.69 mg/kg in the medium preference group, and 1.21 mg/kg in the low preference group. These HEDs were comparable to estimated doses for high, medium, and low caffeine consumption in humans (Fig. 6).
Fig. 6.
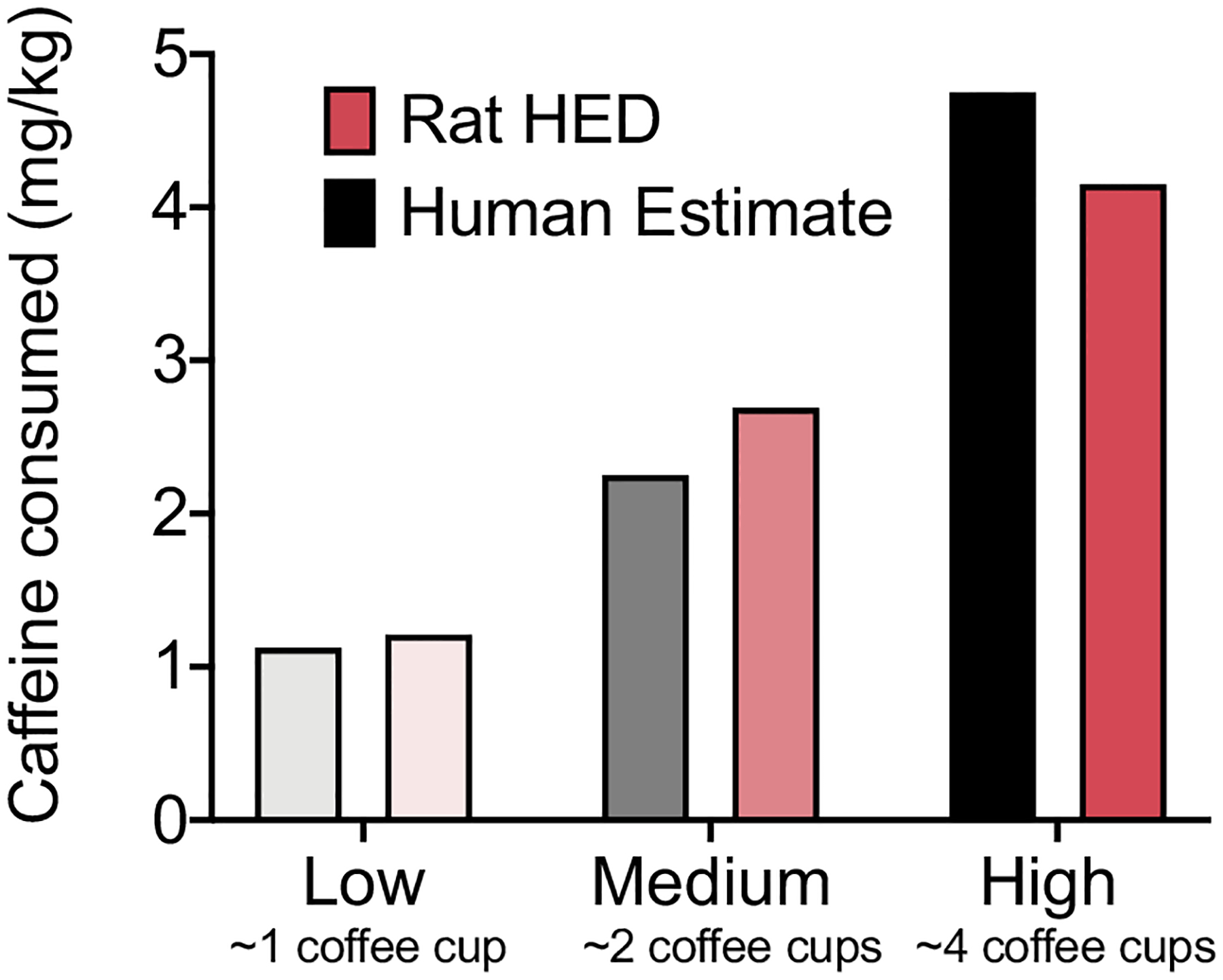
Human Equivalent Dose in rats (pink) compared with estimated caffeine consumption in human caffeine drinkers (gray). A Human Equivalent Dose was calculated for each group (high, medium, and low preference) based on the amount of caffeine consumed. An estimated dose for humans who drink similar levels of caffeine was calculated for comparison. For each group, the rat HED was comparable to the estimated dose of human consumption.
4. Discussion
The present study sought to establish a rat model of voluntary oral caffeine consumption using the two-bottle choice paradigm, identify different groups based on caffeine preference, and determine whether chronic caffeine consumption leads to compulsive-like caffeine intake or behavioral signs of withdrawal. We first established an optimal concentration of caffeine (0.3 mg/mL) to maximize caffeine preference and total caffeine consumption by testing intake at several doses of caffeine. We found that caffeine consumption did not escalate with intermittent access and instead decreased slightly. Separate populations of rats (high, medium, and low caffeine preference) were identified based on caffeine preference throughout the experiment. The rats exhibited no behavioral signs of withdrawal in all of the caffeine preference groups, but the high preference group exhibited signs of compulsive-like caffeine intake in the quinine adulteration test. When we calculated the HED of caffeine intake in each group of rats (high, medium, and low preference), the amount of intake resembled estimated amounts of caffeine intake in humans. Overall, these data establish a preclinical model of voluntary caffeine drinking that can distinguish different groups of rats based on caffeine preference that resemble populations of human caffeine drinkers.
In the dose-response test, caffeine preference and total caffeine intake were evaluated to determine the optimal oral caffeine dose. The 0.3 mg/mL caffeine concentration was the optimal concentration for further testing in the two-bottle choice paradigm. This concentration was highly preferred and led to the largest amount of caffeine consumption. This concentration of caffeine was very similar to the amount of caffeine (0.38 mg/mL) that is contained in an average cup of coffee that is consumed by humans (Temple et al., 2017). Our dose-response data are consistent with a previous study that reported that rats form a flavor preference for lower concentrations of caffeine (0.25 and 0.125 mg/mL) but form aversions to higher concentrations (0.5 and 0.75 mg/mL; Fedorchak et al., 2002). Other studies have employed forced caffeine exposure to assess subsequent free-choice caffeine consumption. Preference has been shown to depend on the concentration of caffeine (Newland and Brown, 1992; Vitiello and Woods, 1975). The present findings and previous studies suggest that rats prefer caffeine at concentrations that are similar to those that are consumed by humans in a standard cup of coffee.
Previous rodent studies that examined nicotine, alcohol, and cocaine intake have demonstrated that intermittent access schedules result in the escalation of drug intake, a hallmark of drug dependence (O’dell and Koob, 2007; Cohen et al., 2012; Melendez, 2011; Kimbrough et al., 2017b; Kawa et al., 2016). Withdrawal symptoms, such as irritability-like behavior, pain sensitivity, and anxiety-like behavior, have been shown to occur within 8–72 h of the last drug exposure in many preclinical models of substance us disorders (Cohen et al., 2012; Melendez, 2011; Kimbrough et al., 2017b; Kawa et al., 2016). Caffeine administration using methods of involuntary or forced consumption in animal models has been shown to produce dependence and withdrawal symptoms (Nehlig, 1999). Forced oral caffeine administration at high concentrations (1 g/L) for 20 days induced aversions to a flavor that was paired with caffeine withdrawal in rodents (Dingle et al., 2008), suggesting caffeine dependence can be induced by oral administration. However, in the present study, intermittent access did not result in the escalation of intake compared with continuous access; instead, intermittent access results in a slight decrease in intake. Additionally, the rats did not exhibit behavioral signs of withdrawal 24 h after the last access to caffeine. Altogether, these data suggest that voluntary caffeine drinking in an intermittent access two-bottle choice procedure does not result in caffeine dependence and does not produce the escalation of intake (i.e., two key behaviors that are observed after chronic intermittent access to cocaine, nicotine, and alcohol; O’dell and Koob, 2007; Melendez, 2011; Kimbrough et al., 2017b; Kawa et al., 2016; Cohen et al., 2012; Simms et al., 2008; Ahmed et al., 2002). Drug tolerance is a key component of escalated drug use and the fact that rats did not escalate caffeine intake during continuous or intermittent access could in part be explained by a lack of tolerance development to caffeine. Caffeine consumption does not cause a high level of tolerance in the central nervous system, a drastic difference compared with other stimulants such as cocaine. Tolerance to cocaine involves dopamine signaling in the nucleus accumbens (Hammer et al., 1997), whereas caffeine has been shown to cause dopamine release in the prefrontal cortex, but not the nucleus accumbens (Nehlig, 1999; Acquas et al., 2002).
Examinations of caffeine preference in individual rats during the extended period of two-bottle choice revealed three distinct populations of rats that could be divided into high, medium, and low preference groups. The high preference group maintained high and consistent caffeine preference throughout the experiment. The low preference group exhibited a similar behavioral pattern as the high preference group, with low preference throughout the continuous and intermittent access periods. The medium preference group exhibited a modest decrease in caffeine preference from the continuous access period to the intermittent access period. In humans, similar populations of caffeine drinkers have been distinguished (Goncalves et al., 2017; Barnung et al., 2018; Mitchell et al., 2014; Kuang et al., 2018; Cornelis, 2019). Interestingly, the amount of caffeine that was consumed in the different preference groups in the present study were similar to amounts of caffeine that are consumed in different groups of humans who drink caffeinated beverages. The HED of caffeine (in mg/kg) that was consumed by rats in the present study was 4.15 mg/kg in the high preference group, 2.69 mg/kg in the medium preference group, and 1.21 mg/kg in the low preference group. These HED doses in rats are comparable to estimated human doses of 4.2 mg/kg for high-preferring drinkers, 2.1 mg/kg for medium-preferring drinkers, and 1.1 mg/kg for low-preferring drinkers (see Fig. 6; Mitchell et al., 2014; Nair and Jacob, 2016). The similarity of caffeine intake in rats in the present study to caffeine intake in humans supports the use of the present model of caffeine self-administration in future preclinical studies that explore voluntary caffeine consumption and genetic determinants of caffeine preference.
We tested compulsive-like caffeine intake using the quinine adulteration test. The high preference group but not the low or medium preference groups exhibited persistent caffeine preference even at high concentrations of quinine. The low preference group did not exhibit a significant decrease in preference as the quinine concentration increased. This is likely attributable to a floor effect. The 0.05 g/L concentration of quinine that high caffeine drinking rats showed a persistent preference for caffeine without reduction is the same concentration of quinine that resulted in a significant reduction of alcohol intake in alcohol dependent rats (Vendruscolo et al., 2012), but not alcohol dependent rats with a prior binge drinking history (Kimbrough et al., 2017b). This suggests that although the rats did not escalate their caffeine intake or exhibit withdrawal symptoms, rats that consistently preferred higher concentrations of caffeine exhibited compulsive-like caffeine drinking.
A limitation of the present study is that it only examined male subjects. In animal models of several drugs of abuse, such as alcohol, cocaine, and oxycodone, females have been shown to take more drug than males (Priddy et al., 2017; Algallal et al., 2019; Kimbrough et al., 2020a). Further, sex differences have been reported regarding the behavioral effects of caffeine (Sallaberry et al., 2018; Hughes et al., 2014). Thus, it will be critical in the future to identify if sex differences in voluntary caffeine intake (escalation, preference breakdown, response to quinine, etc.) exist in this model.
In summary, the present study established a model of voluntary oral caffeine consumption, and identified 0.3 mg/mL as the most appropriate concentration in this model. Intermittent access to caffeine did not result in the escalation of caffeine intake. We identified three distinct populations of rats (high, medium, and low preference) based on caffeine preference that mirrored intake in humans. We found evidence of compulsive-like caffeine intake in the high preference group. The present model of voluntary oral caffeine consumption recapitulates caffeine preferences that are observed in humans, suggesting its utility for studying the neurobiological and pharmacological effects of caffeine. The significant difference in caffeine preference that was observed between the high, medium, and low groups suggests potential genetic differences that result in different rewarding or aversive effects of caffeine. We did not observe the escalation of caffeine intake with intermittent access, which contrasts with other common drugs of abuse. However, compulsive-like caffeine intake in the high preference group suggests that compulsive-like behavior may develop after chronic caffeine use in individuals with a high preference for caffeine. Caffeine intake has been shown to modulate the consumption of alcohol and nicotine (Fritz et al., 2016; Rezvani et al., 2013), for example, caffeinated alcoholic beverages can lead to increased alcohol intake (Takahashi et al., 2015), suggesting complex interactions with these drugs that warrant further investigation.
Acknowledgements
The authors would like to thank Michael Arends for his diligent work in proofreading the manuscript.
This work was supported by National Institutes of Health grants DA044451, DA043799, and AA027301. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
The authors declare no conflicts of interest.
References
- Acquas E, Tanda G, Di Chiara G, 2002. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 27, 182–193. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A, 2002. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat. Neurosci 5, 625–626. [DOI] [PubMed] [Google Scholar]
- Algallal H, Allain F, Ndiaye NA, Samaha AN, 2019. Sex differences in cocaine self-administration behaviour under long access versus intermittent access conditions. Addict. Biol e12809. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th edition American Psychiatric Press, Washington, DC. [Google Scholar]
- Atkinson J, Enslen M, 1976. Self-administration of caffeine by the rat. Arzneimittelforschung 26, 2059–2061. [PubMed] [Google Scholar]
- Avegno EM, Gilpin NW, 2019. Inducing alcohol dependence in rats using chronic intermittent exposure to alcohol vapor. Bio Protoc 9, e3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnung R,B, Nøst T,H, Ulven SM, Skeie G & K SO 2018. Coffee consumption and whole-blood gene expression in the Norwegian Women and Cancer Post-Genome Cohort. Nutrients, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S, 2014. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O, 2012. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37, 2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC, 2019. The impact of caffeine and coffee on human health. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle RN, Dreumont-Boudreau SE, Lolordo VM, 2008. Caffeine dependence in rats: effects of exposure duration and concentration. Physiol. Behav 95, 252–257. [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF, 2012. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology 62, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorchak PM, Mesita J, Plater SA, Brougham K, 2002. Caffeine-reinforced conditioned flavor preferences in rats. Behav. Neurosci 116, 334–346. [PubMed] [Google Scholar]
- Fritz BM, Quoilin C, Kasten CR, Smoker M & Boehm SL 2ND 2016. Concomitant caffeine increases binge consumption of ethanol in adolescent and adult mice, but produces additive motor stimulation only in adolescent animals. Alcohol. Clin. Exp. Res, 40, 1351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF, 2012. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc. Natl. Acad. Sci. U. S. A 109, 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF, 2008a. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res 32, 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF, 2008b. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 10.1002/0471142301.ns0929s44. (Chapter 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves LS, Painelli VS, Yamaguchi G, Oliveira LF, Saunders B, Da, S.I.L.V.A. P,R, Maciel E, Artioli GG, Roschel H, Gualano B, 2017. Dispelling the myth that habitual caffeine consumption influences the performance response to acute caffeine supplementation. J Appl Physiol (1985) 123, 213–220. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Chausmer AL, 2000. Caffeine as a model drug of dependence: recent developments in understanding caffeine withdrawal, the caffeine dependence syndrome, and caffeine negative reinforcement. Nihon Shinkei Seishin Yakurigaku Zasshi 20, 223–231. [PubMed] [Google Scholar]
- Hammer RP JR., Egilmez Y, Emmett-Oglesby MW, 1997. Neural mechanisms of tolerance to the effects of cocaine. Behav. Brain Res 84, 225–239. [DOI] [PubMed] [Google Scholar]
- Heckman MA, Weil J, Gonzalez De Mejia E, 2010. Caffeine (1,3,7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci 75, R77–R87. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Oliveto AH, Liguori A, Carpenter J, Howard T, 1998. Endorsement of DSM-IV dependence criteria among caffeine users. Drug Alcohol Depend. 52, 99–107. [DOI] [PubMed] [Google Scholar]
- Hughes RN, Hancock NJ, Henwood GA, Rapley SA, 2014. Evidence for anxiolytic effects of acute caffeine on anxiety-related behavior in male and female rats tested with and without bright light. Behav. Brain Res 271, 7–15. [DOI] [PubMed] [Google Scholar]
- Jain S, Srivastava AS, Verma RP, Maggu G, 2019. Caffeine addiction: need for awareness and research and regulatory measures. Asian J. Psychiatr 41, 73–75. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Griffiths RR, 2004. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology 176, 1–29. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Evatt DP, Richards BD, Griffiths RR, 2012. Characterization of individuals seeking treatment for caffeine dependence. Psychol. Addict. Behav 26, 948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Xue S, Zhou B, Janda KD, George O, 2018. An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse. Sci. Adv 4, eaat4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE, 2016. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233, 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, De Guglielmo G, Kononoff J, Kallupi M, Zorrilla EP, George O, 2017a. CRF1 receptor-dependent increases in irritability-like behavior during abstinence from chronic intermittent ethanol vapor exposure. Alcohol. Clin. Exp. Res 41, 1886–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kim S, Cole M, Brennan M, George O, 2017b. Intermittent access to ethanol drinking facilitates the transition to excessive drinking after chronic intermittent ethanol vapor exposure. Alcohol. Clin. Exp. Res 41, 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, Conlisk D, Momper JD, DE Guglielmo G & George O 2020a. Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology, (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Lurie D, Collazo A, Kreifeldt M, Sidhu H, Macedo GC, D’esposito M, Contet C, George O, 2020. Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proc. Natl. Acad. Sci. U. S. A 117 (4), 2149–2159. 10.1073/pnas.1909915117. 31937658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahdouzan M, Hamadeh MJ, 2017. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther 23, 272–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononoff J, Kallupi M, Kimbrough A, Conlisk D, De Guglielmo G, George O, 2018a. Systemic and intra-habenular activation of the orphan G protein-coupled receptor GPR139 decreases compulsive-like alcohol drinking and hyperalgesia in alcohol-dependent rats. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononoff J, Melas PA, Kallupi M, De Guglielmo G, Kimbrough A, Scherma M, Fadda P, Kandel DB, Kandel ER, George O, 2018b. Adolescent cannabinoid exposure induces irritability-like behavior and cocaine cross-sensitization without affecting the escalation of cocaine self-administration in adulthood. Sci. Rep 8, 13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang A, Erlund I, Herder C, Westerhuis JA, Tuomilehto J, Cornelis MC, 2018. Lipidomic response to coffee consumption. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, 2011. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol. Clin. Exp. Res 35, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Juliano LM, Hughes JR, Griffiths RR, 2013. Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res 3, 114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ, 2014. Beverage caffeine intakes in the U.S. Food Chem. Toxicol 63, 136–142. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, 1999. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav. Rev 23, 563–576. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G, 1992. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Brain Res. Rev 17, 139–170. [DOI] [PubMed] [Google Scholar]
- Newland MC, Brown K, 1992. Oral caffeine consumption by rats: the role of flavor history, concentration, concurrent food, and an adenosine agonist. Pharmacol. Biochem. Behav 42, 651–659. [DOI] [PubMed] [Google Scholar]
- Nieber K, 2017. The impact of coffee on health. Planta Med. 83, 1256–1263. [DOI] [PubMed] [Google Scholar]
- O’dell LE, Koob GF, 2007. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol. Biochem. Behav 86, 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’dell LE, Roberts AJ, Smith RT, Koob GF, 2004. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res 28, 1676–1682. [DOI] [PubMed] [Google Scholar]
- Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF, 2015. Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addict. Biol 20, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF, 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Sexton HG, Johnson J, Wells C, Gordon K, Levin ED, 2013. Effects of caffeine on alcohol consumption and nicotine self-administration in rats. Alcohol. Clin. Exp. Res 37, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G, Smith A, 2015. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J. Psychopharmacol 29, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G, Smith AP, 2016. A review of energy drinks and mental health, with a focus on stress, anxiety, and depression. J Caffeine Res 6, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallaberry C, Ardais AP, Rocha A, Borges MF, Fioreze GT, Mioranzza S, Nunes F, Pagnussat N, Botton PHS, Porciuncula LO, 2018. Sex differences in the effects of pre- and postnatal caffeine exposure on behavior and synaptic proteins in pubescent rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 81, 416–425. [DOI] [PubMed] [Google Scholar]
- Seal AD, Bardis CN, Gavrieli A, Grigorakis P, Adams JD, Arnaoutis G, Yannakoulia M, Kavouras SA, 2017. Coffee with high but not low caffeine content augments fluid and electrolyte excretion at rest. Front Nutr 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci 16, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Kreifeldt M, Contet C, 2018. Affective disturbances during withdrawal from chronic intermittent ethanol inhalation in C57BL/6J and DBA/2J male mice. Alcohol. Clin. Exp. Res 42 (7), 1281–1290 29687895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE, 2008. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol. Clin. Exp. Res 32, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD, 2017. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 84, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Mumford GK, Silverman K, Griffiths RR, 1994. Caffeine dependence syndrome. Evidence from case histories and experimental evaluations. JAMA 272, 1043–1048. [PubMed] [Google Scholar]
- Stringer KA, Watson WA, 1987. Caffeine withdrawal symptoms. Am. J. Emerg. Med 5, 469. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Vendruscolo LF, Takahashi RN, 2015. Binge-like ingestion of a combination of an energy drink and alcohol leads to cognitive deficits and motivational changes. Pharmacol. Biochem. Behav 136, 82–86. [DOI] [PubMed] [Google Scholar]
- Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA, 2017. The safety of ingested caffeine: a comprehensive review. Front Psychiatry 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ, 2014. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW JR., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci 32, 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV, Woods SC, 1975. Caffeine: preferential consumption by rats. Pharmacol. Biochem. Behav 3, 147–149. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, 2015. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29, 203–210. [DOI] [PubMed] [Google Scholar]
- Xue S, Kallupi M, Zhou B, Smith LC, Miranda PO, George O, Janda KD, 2018. An enzymatic advance in nicotine cessation therapy. Chem Commun (Camb) 54, 1686–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]


