Abstract
Rationale
Over the last decade, oxycodone has become one of the most widely abused drugs in the USA. Oxycodone use disorder (OUD) is a serious health problem that has prompted a need to develop animal models of OUD that have both face and predictive validity. Oxycodone use in humans is more prevalent in women and leads to pronounced hyperalgesia and irritability during withdrawal. However, unclear is whether current animal models of oxycodone self-administration recapitulate these characteristics in humans.
Objectives
We assessed the face validity of a model of extended-access oxycodone self-administration in rats by examining the escalation of oxycodone intake and behavioral symptoms of withdrawal, including irritability-like behavior and mechanical nociception, in male and female Wistar rats.
Results
Both male and female rats escalated their oxycodone intake over fourteen 12-h self-administration sessions. After escalation, female rats administered more drug than male rats. No differences in plasma oxycodone levels were identified, but males had a significantly higher level of oxycodone in the brain at 30 min. Extended access to oxycodone significantly decreased aggressive-like behavior and increased defensive-like behaviors when tested immediately after a 12-h self-administration session, followed by a rebound increase in aggressive-like behavior 12 h into withdrawal. Tests of mechanical nociception thresholds during withdrawal indicated pronounced hyperalgesia. No sex differences in irritability-like behavior or pain sensitivity were observed.
Conclusions
The present study demonstrated the face validity of the extended access model of oxycodone self-administration by identifying sex differences in the escalation of oxycodone intake and pronounced changes in pain and affective states.
Keywords: Oxycodone, von Frey test, Irritability, Pain, Self-administration, Opioid, Hyperalgesia, Aggression, Defensive, Sex differences
Introduction
Opioids, such as oxycodone, are commonly used as pain relievers and primarily function as μ-opioid receptor agonists (Narita et al. 2008; Lalovic et al. 2006). Oxycodone is one of the most widely abused prescription drugs worldwide, especially in the USA (Manchikanti et al. 2010; Manchikanti and Singh 2008; Substance Abuse and Mental Health Services Administration 2013). The prevalence of oxycodone use and oxycodone-related deaths has risen dramatically in the USA over the last decade (Kolodny et al. 2015; Compton et al. 2016). To combat the rising rate of oxycodone addiction and determine viable treatment options, oxycodone use disorder (OUD) needs to be investigated in animal models that have strong translational validity.
Significant sex differences in the use of opioid pain relievers have been observed in humans. Although the number of deaths from opioid overdose is greater in males than in females (Scholl et al. 2018), deaths have increased much more rapidly in women than in men over the last two decades (Centers for Disease Control and Prevention 2017). This coincides with evidence that women abuse opioids at a higher rate than men (Substance Abuse and Mental Health Services Administration 2014). Women may be more sensitive to pain than men (Riley et al. 1998) and are more likely to use opioids to cope with negative affect (Mchugh et al. 2013), which may be a major factor in their higher rate of opioid abuse.
Drug dependence and substance use disorder are thought to be driven by negative affect, leading to the escalation of drug intake and withdrawal symptoms (Koob et al. 2004; Koob and Volkow 2010). In humans, opioid withdrawal can result in hyperalgesia and irritability, among other symptoms (Carcoba et al. 2011; Compton et al. 2003; Wesson and Ling 2003; Gowing et al. 2017; Amato et al. 2013; Rieb et al. 2016).
Other opioids, such as heroin and morphine, have been extensively studied using animal models of self-administration (Wade et al. 2017; Schmeichel et al. 2015; Steidl et al. 2015; Lucantonio et al. 2015; de Guglielmo et al. 2015), but animal models of oxycodone self-administration have been relatively understudied. Injection models of oxycodone have been shown to induce withdrawal symptoms in mice (Enga et al. 2016). Animal models of extended-access oxycodone self-administration have recently been developed (Zhang et al. 2017; Yuferov et al. 2018; Zhang et al. 2018; Zhang et al. 2014; Wade et al. 2015; You et al. 2017; Nguyen et al. 2019; Kallupi et al. 2020; de Guglielmo et al. 2020). Extended-access oxycodone self-administration has been shown to induce compulsive-like intake and the escalation of intake (Zhang et al. 2014; Wade et al. 2015), but behavioral withdrawal symptoms that are associated with OUD have not been thoroughly explored in these models (de Guglielmo et al. 2020).
The present study tested the face validity of an extended-access model of oxycodone self-administration in rats by assessing irritability-like behavior and hyperalgesia immediately after oxycodone use and during withdrawal. Irritability and hyperalgesia are central features of withdrawal from drug dependence in humans, together with greater aggression and frustration (Miczek et al. 2015; Cardoso et al. 2006; Winward et al. 2014; Baars et al. 2013; Lubman et al. 1983; Carcoba et al. 2011; Compton et al. 2003; Wesson and Ling 2003; Gowing et al. 2017; Amato et al. 2013; Rieb et al. 2016). Assessments of these characteristics may be key to demonstrating the translational validity of a given model of substance use disorders. Irritability has been difficult to characterize in rodents. The bottle-brush test has been used to measure components of aggressive and defensive responses that have been suggested to measure irritability-like behavior (Riittinen et al. 1986). Previous studies indicated that impoverished rearing conditions (Riittinen et al. 1986), age (Lagerspetz and Portin 1968), and drug use can affect irritability-like behavior. In addiction studies, irritability-like behavior in the bottle-brush test has been shown to increase during withdrawal from alcohol, nicotine, and oxycodone (Sidhu et al. 2018; Somkuwar et al. 2017; Kimbrough et al. 2017; Xue et al. 2018; Kallupi et al. 2018; Kimbrough et al. 2020; de Guglielmo et al. 2020) and may be altered by other drugs of abuse. Additionally, hyperalgesia has been shown to be elevated during withdrawal from alcohol, nicotine, oxycodone, and heroin (Hamouda et al. 2018; Bagdas et al. 2018; Jackson et al. 2018; Kononoff et al. 2018a; Edwards et al. 2012; Cohen et al. 2015; Park et al. 2015; de Guglielmo et al. 2017a; Kallupi et al. 2018; de Guglielmo et al. 2020). We hypothesized that the extended-access model of oxycodone self-administration would result in the escalation of oxycodone intake and the emergence of hyperalgesia and irritability-like behavior during withdrawal.
Materials and methods
Animals
Gonadally intact adult male and female Wistar rats (Charles River, Wilmington, MA, USA), 60 days old at the beginning of the experiments, were used. For oxycodone self-administration and behavioral testing, n = 10 males and n = 9 females were used. For measurements of plasma oxycodone levels, n = 4 males and 4 females were used. For measurements of brain oxycodone levels, n = 5 males and n = 6 females were used. The rats were same-sex group-housed, two per cage, in a temperature-controlled (22°C) vivarium on a 12 h/12 h light/dark cycle (lights on at 8:00 P.M.) with ad libitum access to food and water. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by The Scripps Research Institute and University of California San Diego Institutional Animal Care and Use Committees.
Experimental design
Male and female rats were tested for baseline mechanical nociception and irritability-like behavior and then allowed to self-administer oxycodone for 14 days (5 days/week, Monday–Friday). On the penultimate day of self-administration, mechanical nociception was measured immediately after the 12-h self-administration session and 12 h after the self-administration session (i.e., 12 h of withdrawal). On the last day of self-administration, irritability-like behavior was measured immediately after the 12-h self-administration session and 12 h into withdrawal (Fig. 1).
Fig. 1.
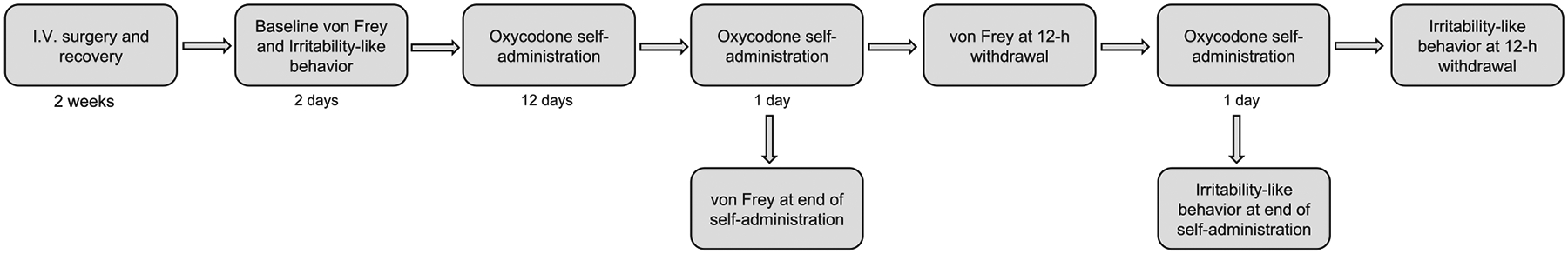
Experimental design. Male and female rats were tested for baseline mechanical nociception and irritability-like behavior and then allowed to self-administer oxycodone for 14 days. On the penultimate day of self-administration, mechanical nociception was measured immediately after the 12 h self-administration session and 12 h after the self-administration session (i.e., 12 h of withdrawal). On the last day of self-administration, irritability-like behavior was measured immediately after the 12-h self-administration session and 12 h after the 12-h self-administration session (i.e., 12 h of withdrawal)
Drugs
Oxycodone (Sigma Aldrich, St. Louis, MO, USA) was dis-solved in 0.9% sodium chloride (Hospira, Lake Forest, IL, USA) and administered at a dose of 150 μg/0.1 ml/kg.
Intravenous catheterization
The animals were anesthetized by inhalation with a mixture of isoflurane, and intravenous catheters were aseptically inserted in the right jugular vein using a modified version of a procedure that was described previously (Caine and Koob 1993; de Guglielmo et al. 2013; de Guglielmo et al. 2017b). The vein was punctured with a 22-gauge needle, and the tubing was inserted and secured inside the vein by tying the vein with suture thread. The catheter assembly consisted of an 18-cm length of Micro-Renathane tubing (0.05842 cm inner diameter, 0.09398 cm outer diameter; Braintree Scientific, Braintree, MA, USA) that was attached to a guide cannula (Plastics One, Roanoke, VA, USA). The guide cannula was bent at a near right angle, embedded in dental acrylic, and anchored with a mesh (1 mm thick, 2 cm square). The catheter exited through a small incision on the back, and the base was sealed with a small plastic cap and metal cover cap. This design helped keep the catheter base sterile and protected. The catheters were flushed daily with heparinized saline (10 U/ml heparin sodium; American Pharmaceutical Partners, Schaumburg, IL, USA) in 0.9% bacteriostatic sodium chloride (Hospira, Lake Forest, IL, USA) that contained 52.4 mg/0.2 ml of the antibiotic cefazolin.
Oxycodone self-administration
Each self-administration session was initiated by the extension of two retractable levers into the operant chamber (29 cm × 24 cm × 19.5 cm; Med Associates, St. Albans, VT, USA). Self-administration sessions occurred in 12-h daily sessions, starting at the beginning of the dark phase of the light/dark cycle, for 14 total sessions (5 sessions/week, Monday–Friday). Responses on the right active lever were reinforced on a fixed-ratio 1 schedule by intravenous oxycodone (150 μg/0.1 ml/infusion) administration that was infused over 6 s, followed by a 20-s timeout (TO20 s) period that was signaled by the illumination of a cue light above the active lever. Responses on the left inactive lever were recorded but had no scheduled consequences.
Mechanical nociceptive von Frey testing
Mechanical nociception, reflected by hind paw withdrawal thresholds, was determined by an observer who was blind to the experimental condition using von Frey filaments. The test days did not occur immediately before or after the 2-day break (i.e., Saturday and Sunday) in self-administration. The test was performed similarly to previous studies (Kononoff et al. 2018a; Kallupi et al. 2018). The test began after 10 min of habituation to the testing environment. A series of von Frey filaments was applied from below the wire mesh to the central region of the plantar surface of the left hind paw in ascending order of force. The filament was applied until buckling of the hair occurred, and the filament remained in place for 2 s. Rapid withdrawal of the hind paw was considered a positive response. The stimulus was incrementally increased until a positive response was observed and then decreased until a negative response was observed to determine a pattern of responses to apply to previously described statistical methods (Dixon 1980). Once the threshold was determined for the left hind paw, the same testing procedure was applied to the right hind paw after 5 min. The 50% paw withdrawal threshold was determined by the formula Xf + kδ, where Xf is the last von Frey filament applied, k is the Dixon value that corresponded to the response pattern, and δ is the mean difference between stimuli. Paw withdrawal thresholds were determined for rats before self-administration initiation (baseline), on the penultimate day of self-administration immediately after the 12-h self-administration session, and 12 h after the self-administration session (12 h of withdrawal).
Irritability-like behavior
To test irritability-like behavior, we used the bottle-brush test, based on the methods of Riittinen et al. (1986) and Lagerspetz and Portin (1968) and modified slightly for rats (Kononoff et al. 2018b; Kimbrough et al. 2017). Irritability-like behavior was tested before self-administration initiation (baseline), on the last day of self-administration immediately after the 12-h self-administration session, and 12 h after the last self-administration session (12 h of withdrawal). The test days did not occur immediately before or after the 2-day break in self-administration (i.e., Saturday and Sunday). Irritability-like behavior was examined by measuring aggressive and defensive responses in the bottle-brush test.
Irritability-like behavior sessions were conducted in a randomized order for each animal. Testing consisted of 10 trials per rat in plastic cages (26.67 cm × 48.26 cm × 20.32 cm; Ancare, Bellmore, NY, USA) with fresh bedding. During each trial, the rat started at the back of the cage. A bottle-brush was rotated toward the animal’s whiskers (from the front of the cage) by an experimenter who was blind to treatment. The brush was rotated around the whiskers for approximately 1 s. The brush was then rotated back to the front of the cage where it was allowed to hang vertically for approximately 2 s, during which behavioral responses were recorded. A 10-s intertrial interval was used. Three observers who were blind to treatment scored the behaviors in real time. The average correlation of all observers for all measurements in the present study was R = 0.85 ± 0.02, indicating a high level of consistency between observers.
For each rat, separate sums of aggressive and defensive responses across all trials were determined for each observer. Aggressive and defensive response scores for each rat were then calculated by averaging the observers’ sums. This average was then used to calculate a group mean and SEM. The following were scored as aggressive responses: smelling the target, biting the target (during the initial phase of rotating the brush forward and back to the starting position), boxing the target, following the target, exploring the target (using paws or the mouth to manipulate the brush without biting or boxing), mounting the target, and delayed biting (during the 2 s that the brush hung at the starting position). The following were scored as defensive responses: escaping from the target, digging, burying, defecation, jumping, climbing, vocalization, and grooming. Grooming and digging were additionally recorded during the 10-s intertrial intervals.
Determination of oxycodone levels in plasma and brain
To characterize sex differences in oxycodone pharmacokinetics in rats, an additional cohort of male and female rats had intravenous catheters implanted as described above. The rats were intravenously injected with a single 1 mg/kg dose of oxycodone, and blood was collected before the injection and 5, 15, 30, and 120 min after the injection (n = 4 males, n = 4 females). Brains were collected separately 30 min after the intravenous injection of 1 mg/kg oxycodone (n = 5 males, n = 6 females). The quantitative determination of plasma and brain oxycodone levels was performed using high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). Oxycodone was precipitated from 20 μl of plasma with 60 μl of 500 ng/ml internal standard (ISTD; oxycodone-D6) in acetonitrile. Brain tissues were ho-mogenized and filtered, and 20 μl of the supernatant was injected directly into a C-18 reverse-phase HPLC column (MacMod Ace-5, 2.1 × 150 mm). The LC mobile phase consisted of HPLC-grade water with 0.1% formic acid (elute A) and acetonitrile with 0.1% formic acid (elute B), which was eluted with a gradient program of 0.0–0.25 min of 95% B at a flow rate of 0.4 ml/min, 1.0–3.0 min of 95% B at a flow rate of 0.6 ml/min, and 3.1–6.0 min of 5% B at a flow rate of 0.3 ml/min. MS/MS detection was performed in positive electrospray ionization mode at mass transitions of 316 → 241 m/z (oxycodone) and 322 → 247 m/z (ISTD). The method had a dynamic range of 9.8–313 ng/ml. For analytes in plasma and brain, calibration standards were used to generate a curve using a linear regression algorithm to plot the peak area ratio vs. concentration with 1/x weighting over the full dynamic range of analyte concentrations.
Statistical analysis
The results are expressed as mean ± SEM. Oxycodone self-administration in male and female rats was analyzed together using repeated-measures analysis of variance (ANOVA), with sex as the between-subjects factor and day of self-administration as the within-subjects factor. For each animal, an average baseline value was calculated using the first 3 days of self-administration. Based on our observation of sex differences in daily self-administration, we analyzed the average rewards that were received in each 4-h bin of the last 3 days of self-administration using separate within-subjects repeated-measures ANOVAs for each sex. For irritability-like behavior and von Frey testing, we first analyzed the data using repeated-measures ANOVA, with sex as the between-subjects factor and drug state (baseline, immediately after the 12-h self-administration session, and 12-h into withdrawal) as the within-subjects factor. We found no sex differences in irritability-like behavior or mechanical nociception; therefore, we combined both sexes into a single group and analyzed each dataset using repeated-measures ANOVA, with drug state (baseline, immediately after the 12-h self-administration session, and 12-h into withdrawal) as the within-subjects factor. Plasma oxycodone levels were analyzed using repeated-measures ANOVA, with sex as the between-subjects factor and time post-infusion as the within-subjects factor. Brain oxycodone levels were analyzed using t tests. The ANOVAs were followed by the Student-Newman-Keuls (SNK) post hoc test when appropriate. Differences were considered significant at p < 0.05. All of the data were analyzed using Statistica 13 software (StatSoft, Palo Alto, CA, USA).
Results
Oxycodone self-administration
After 14 self-administration sessions, both male and female rats significantly escalated their oxycodone intake above their level of intake on day 1 of self-administration. The repeated-measures ANOVA revealed a significant day × sex interaction (F13,221 = 2.13, p < 0.05). The SNK post hoc test showed that males exhibited a significant increase in oxycodone intake on days 11–14 compared with their own intake on day 1. Females exhibited a significant increase in oxycodone intake on days 8–14 compared with their own intake on day 1. Females also exhibited a significant increase in oxycodone intake compared with males on days 11 and 13 (Fig. 2a).
Fig. 2.
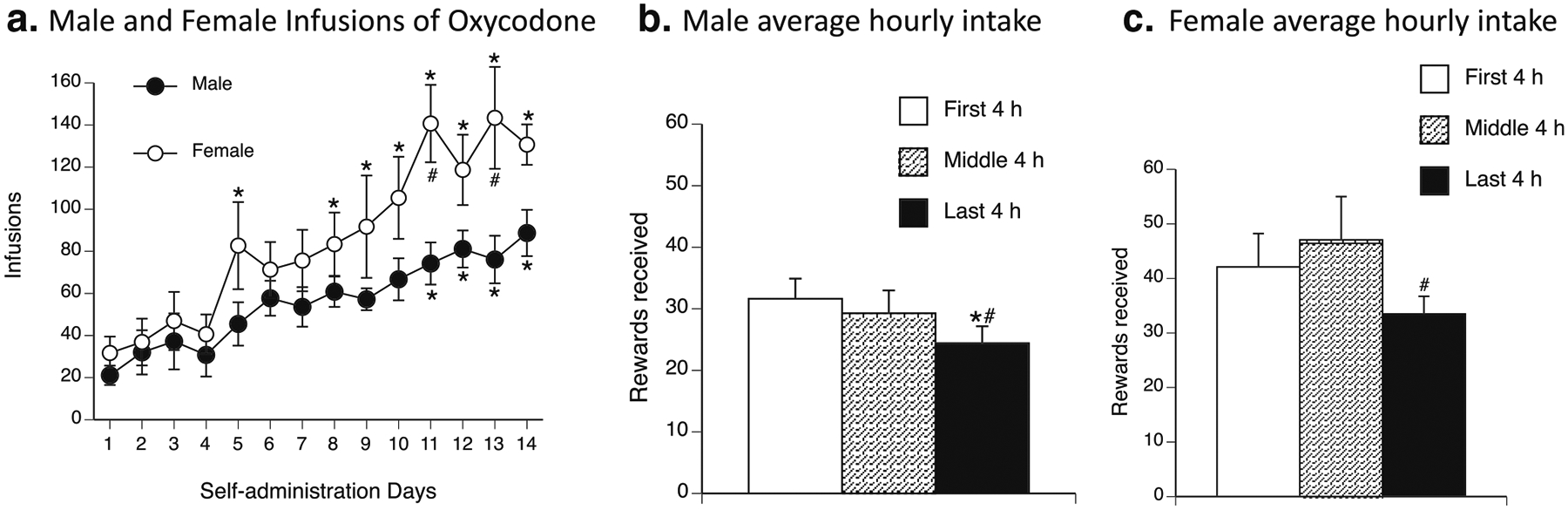
Escalation of oxycodone self-administration. a Oxycodone infusions in male and female rats over the course of oxycodone self-administration. Female rats (white circles) exhibited a significant increase in infusions of oxycodone on days 8–14 compared with day 1. Male rats (black circles) exhibited a significant increase in infusions of oxycodone on days 11–14 compared with day 1. Female rats also exhibited a significant increase in infusions of oxycodone compared with male rats on days 11 and 13. *p < 0.05, significant difference in lever presses for oxycodone compared with day 1; #p < 0.05, significant difference in lever presses for oxycodone between males and females. b, c Average oxycodone intake in 4-h bins during the last 3 days of self-administration. b Male rats self-administered significantly less oxycodone during the last 4 h of self-administration compared with the first 4 h and middle 4 h of self-administration. c Female rats self-administered significantly less oxycodone during the last 4 h of self-administration compared with the middle 4 h of self-administration. *p < 0.05, significant difference in oxycodone rewards received between the last 4 h and first 4 h of self-administration; #p < 0.05, significant difference in oxycodone rewards received between the last 4 h and middle 4 h of self-administration
We split the average intake of the last three self-administration sessions into 4-h bins (first 4 h, middle 4 h, and last 4 h) for male rats. The repeated-measures ANOVA revealed a significant effect of time bin (F2,18 = 6.26, p < 0.05). The SNK post hoc test showed that males took significantly less oxycodone during the last 4 h (24.40 ± 2.76 rewards received) of the session compared with the first 4 h (31.63 ± 3.27 rewards received) and middle 4 h (29.27 ± 3.72 rewards received; Fig. 2b).
We split the average intake of the last three self-administration sessions into 4-h bins (first 4 h, middle 4 h, and last 4 h) for female rats. The repeated-measures ANOVA revealed a significant effect of time bin (F2,16 = 4.37, p < 0.05). The SNK post hoc test showed that females took significantly less oxycodone during the last 4 h (33.48 ± 3.24 rewards received) of the session compared with the middle 4 h (47.07 ± 7.94 rewards received; Fig. 2c).
Mechanical nociception in the von Frey test
Prior to the initiation of self-administration and on the penultimate day of self-administration, the rats were tested for paw withdrawal thresholds using von Frey filaments. We found no effect of sex on paw withdrawal thresholds, although a trend suggested a potential sex difference during withdrawal. Therefore, both sexes were combined for the analyses (baseline: 28.5 ± 4.7 g for males vs. 38.8 ± 6.8 g for females; after 12-h self-administration: 24.5 ± 4.5 g for males vs. 24.5 ± 5.3 g for females; 12-h withdrawal: 15.3 ± 1.2 g for males vs. 9.0 ± 2.2 g for females). The repeated-measures ANOVA of paw withdrawal thresholds revealed a significant effect of drug state (F2,34 = 17.6, p < 0.0005). The SNK post hoc test showed that paw withdrawal thresholds at baseline (33.62 ± 4.19 g) were significantly higher than thresholds immediately after the 12-h self-administration session (24.50 ± 3.39 g) and 12 h after the self-administration session (i.e., 12 h of withdrawal; 12.12 ± 1.43 g). Paw withdrawal thresholds immediately after the 12-h self-administration session were also significantly higher than thresholds 12 h after the 12-h self-administration session (i.e., 12 h of withdrawal; Fig. 3a).
Fig. 3.

a Paw withdrawal thresholds measured by von Frey filaments. The rats exhibited significantly lower paw withdrawal thresholds 12 h into withdrawal (black bar) compared with baseline (white bar) and compared with immediately after the 12-h self-administration session (hashed bar). The rats also exhibited significantly lower paw withdrawal thresholds immediately after the 12-h self-administration session compared with baseline. b Irritability-like behavior in the bottle-brush test. The rats exhibited a significantly higher number of defensive responses immediately after the 12-h self-administration session (12-h self-administration) (hashed bars) compared with baseline (white bars) and 12 h after the 12-h self-administration session (12-h withdrawal) (black bars). The rats exhibited a significantly lower number of aggressive responses immediately after the 12-h self-administration session compared with baseline and compared with 12 h of withdrawal. *p < 0.05, vs. baseline; #p < 0.05 vs. 12-h withdrawal
Irritability-like behavior
Prior to the initiation of self-administration and on the last day of self-administration, the rats were tested for irritability-like behavior using the bottle-brush test. We found no sex differences in either aggressive or defensive responses and thus combined both sexes for the analyses: aggressive responses (baseline: 9.2 ± 1.7 for males vs. 5.4 ± 1.6 for females; after 12-h self-administration: 3.6 ± 1.1 for males vs. 1.9 ± 0.6 for females; 12-h withdrawal: 7.4 ± 1.2 for males vs. 8.5 ± 2.1 for females), defensive responses (baseline: 6.5 ± 0.9 for males vs. 8.8 ± 1.7 for females; after 12-h self-administration: 10.7 ± 0.7 for males vs. 10.6 ± 0.9 for females; 12-h withdrawal: 7.0 ± 0.7 for males vs. 8.8 ± 1.2 for females). The repeated-measures ANOVA of aggressive responses revealed a significant effect of drug state (F2,36 = 7.12, p < 0.005). The SNK post hoc test showed that the number of aggressive responses was significantly lower immediately after the 12-h self-administration session (2.79 ± 0.69) compared with baseline (7.40 ± 1.25) and compared with 12 h after the 12-h self-administration session (i.e., 12 h of withdrawal; 8.04 ± 1.13; Fig. 3b).
The repeated-measures ANOVA of defensive responses revealed a significant effect of drug state (F2,36 = 6.83, p < 0.005). The SNK post hoc test showed that the number of defensive responses was significantly higher immediately after the 12-h self-administration session (10.67 ± 0.55) compared with baseline (7.60 ± 0.95) and compared with 12 h after the 12-h self-administration session (7.86 ± 0.68; Fig. 3b). For an individual breakdown of each behavior, see Table 1.
Table 1.
Individual irritability-like behaviors
| Baseline | 12-h self-administration | 12-h withdrawal | |||||
|---|---|---|---|---|---|---|---|
| Behavior | Repeated-measures ANOVA | Mean ± SEM | SNK post hoc test | Mean ± SEM | SNK post hoc test | Mean ± SEM | SNK post hoc test |
| Escape |
F2,36 = 23.09 p < 0.0005 |
4.35 ± 0.50 |
p < 0.05, vs. SA p < 0.0005, vs. WD |
5.40 ± 0.33 |
p < 0.05, vs. BSL p < 0.0005, vs. WD |
2.28 ± 0.27 |
p < 0.0005, vs. BSL p < 0.0005, vs. WD |
| Digging | n.p. | 0.05 ± 0.05 | n.a. | 0.02 ± 0.02 | n.a. | 0.00 ± 0.00 | n.a. |
| Jumping |
F2,36 = 3.82 p < 0.05 |
0.81 ± 0.34 | p <0.05, vs. WD | 0.40 ±0.21 | n.s. | 0.05 ± 0.05 | p < 0.05, vs. BSL |
| Climbing |
F2,36 = 18.75 p < 0.0005 |
1.88 ± 0.27 |
p < 0.0005, vs. SA p < 0.0005, vs. WD |
4.39 ± 0.41 | p < 0.0005, vs. BSL | 3.84 ± 0.42 | p < 0.0005, vs. BSL |
| Defecation | n.p. | 0.11 ±0.07 | n.a. | 0.00 ± 0.00 | n.a. | 0.12 ±0.12 | n.a. |
| Vocalization |
F2,36 = 3.34 p < 0.05 |
0.30 ± 0.23 | p <0.05, vs. WD | 0.19 ±0.12 | n.s. | 1.09 ± 0.45 | p <0.05, vs. WD |
| Grooming | n.s. | 0.11 ± 0.11 | n.a. | 0.26 ±0.16 | n.a. | 0.47 ±0.16 | n.a. |
| Total defensive |
F2,36 = 6.83 p < 0.005 |
7.60 ± 0.95 | p < 0.05, vs. SA | 10.67 ± 0.55 | p < 0.05, vs. BSL p < 0.005, vs. WD | 7.86 ± 0.68 | p < 0.005, vs. SA |
| Smelling |
F2,36 = 3.50 p < 0.05 |
1.53 ± 0.39 | n.s. | 0.63 ±0.19 | p < 0.05, vs. WD | 2.00 ± 0.43 | p < 0.05, vs. SA |
| Biting | n.s. | 0.02 ± 0.02 | n.a. | 0.04 ± 0.04 | n.a. | 0.14 ±0.06 | n.a. |
| Delayed biting | n.s. | 0.05 ± 0.04 | n.a. | 0.02 ± 0.02 | n.a. | 0.18 ±0.09 | n.a. |
| Boxing |
F2,36 = 6.57 p < 0.005 |
2.02 ± 0.39 | p < 0.05, vs. SA | 0.54 ±0.15 |
p < 0.05, vs. BSL p < 0.05, vs. WD |
2.19 ±0.52 | p < 0.05, vs. SA |
| Following |
F2,36 = 3.33 p < 0.05 |
2.40 ± 0.55 | n.s. | 0.86 ± 0.28 | p < 0.05, vs. WD | 2.32 ± 0.48 | p < 0.05, vs. SA |
| Exploration | n.s. | 1.39 ± 0.24 | n.a. | 0.70 ±0.21 | n.a. | 1.21 ±0.22 | n.a. |
| Total aggressive |
F2,36 = 7.12 p < 0.005 |
7.40 ± 1.25 | p < 0.005, vs. SA | 2.79 ± 0.69 |
p < 0.005, vs. BSL p < 0.005, vs. WD |
8.04 ±1.13 | p < 0.005, vs. SA |
Within-subjects repeated-measures ANOVAs were performed for each behavior. Significant effects in the within-subjects repeated-measures ANOVAwere followed by the SNK post hoc test. The F and p values are shown if the effects in the ANOVA were significant
n.s. non-significant; n.p. tests not performed because of data with no variance in one or more groups (e.g., all values of 0 for a behavior in one group); n.a. post hoc test not performed because no significant effect was found in the ANOVA; BSL baseline; SA self-administration; WD withdrawal
Plasma and brain oxycodone concentrations
The repeated-measures ANOVA of plasma oxycodone levels revealed a significant effect of time post-oxycodone infusion (F3,18 = 27.93, p < 0.005) but no effect of sex and no time × sex interaction (Fig. 4a). The t test revealed that male rats had significantly higher brain oxycodone levels 30 min post-infusion than female rats (10.81 ± 1.37 ng/ml for males vs. 6.66 ± 1.07 ng/ml for females; t9 = 2.42, p < 0.05; Fig. 4b).
Fig. 4.

Plasma and brain oxycodone levels in male and female rats after 1 mg/kg intravenous infusion of oxycodone. a Plasma oxycodone concentration in male (black circles) and female (white circles) rats over time. No differences were found between sexes. b Brain oxycodone concentrations 30 min after the intravenous infusion of 1 mg/kg oxycodone in male (black bar) and female (white bar) rats. Males exhibited a significantly higher level of oxycodone in the brain compared with females. *p < 0.05
Discussion
The present study assessed the escalation of oxycodone self-administration and examined behavioral measures that are associated with withdrawal in male and female Wistar rats. The extended-access model of oxycodone self-administration led to the escalation of oxycodone intake in both male and female rats. Female rats self-administered more drug after escalation occurred, indicating sex differences in oxycodone use. We also found that after the escalation of oxycodone intake occurred, irritability-like behavior was altered immediately after the 12-h self-administration session. Compared with baseline and the 12-h withdrawal time points, the rats exhibited an increase in defensive responses and a decrease in aggressive responses. Hyperalgesia was altered by the state of drug use (i.e., during withdrawal and immediately after the 12-h self-administration session). After the escalation of oxycodone intake occurred, the rats exhibited a slight increase in pain sensitivity immediately after the 12-h self-administration session compared with baseline pain sensitivity. At 12 h of withdrawal from oxycodone self-administration, the rats exhibited pronounced hyperalgesia compared with both baseline and immediately after the 12-h self-administration session. No sex differences in irritability-like behavior or pain sensitivity were observed, suggesting that sex differences in oxycodone intake may derive from other sources of motivation. We found no sex differences in plasma oxycodone levels, but males exhibited an increase in brain oxycodone levels at 30 min compared with females, which may play a role in differences in intake.
Our findings demonstrated that the present extended-access model of oxycodone self-administration in rats leads to the reliable escalation of drug use and alterations of behavioral measures of withdrawal. Female rats self-administered more oxycodone than male rats. Interestingly, in a short-access model of oxycodone self-administration, female rats also self-administered more oxycodone at higher doses than males, although no sex differences in brain or plasma levels of oxycodone were found with a low-dose infusion of oxycodone (Mavrikaki et al. 2017). Similar to Mavrikaki et al. (2017) who used a low-dose infusion of oxycodone, we found no sex differences in plasma oxycodone levels after a high-dose infusion of oxycodone. However, unlike Mavrikaki et al. (2017), who found no sex differences in brain oxycodone levels, we found a significant elevation of brain oxycodone levels in male rats compared with female rats at 30 min. Our data suggest that the higher level of oxycodone self-administration that was observed in females may reflect behavioral compensation for lower brain oxycodone levels in females, which could be attributable to sex-related differences in the distribution characteristics of oxycodone. These data are consistent with human findings, in which the abuse of, and craving for, prescription pain medications and opioids are more frequent in women (Back et al. 2011; Substance Abuse and Mental Health Services Administration 2014). Our findings are also consistent with pharmacokinetic data in humans that show lower oxycodone levels in females (Kaiko et al. 1996; Andreassen et al. 2011; Elder et al. 2014). Additionally, although the total number of opioid-related deaths is greater in males than in females (Scholl et al. 2018), deaths have also risen significantly more in women than in men over the past 10 years in the USA (Choo et al. 2014).
Opioid dependence in humans and rodents has been found to be associated with an increase in aggression, presumably because of bouts of withdrawal (Kantak and Miczek 1986; Tidey and Miczek 1992a; Tidey and Miczek 1992b; Mckernan et al. 2015; Moore et al. 2011). Interestingly, in the present study, we observed a significant decrease in aggressive responses immediately after the 12-h self-administration session compared with baseline and after 12 h of withdrawal, which could be attributable to sedative effects of oxycodone use.
The increase in hyperalgesia that was observed after 12 h of withdrawal is similar to the increase in pain levels that has been observed during opioid withdrawal in humans (Gowing et al. 2017; Rieb et al. 2016; Carcoba et al. 2011; Compton et al. 2003). Interestingly, immediately after the 12-h oxycodone self-administration session, we found that the rats exhibited a slight increase in the level of pain (i.e., lower thresholds). This result may seem counterintuitive because opioids are usually used for pain relief (Olesen et al. 2010), but similar effects have been observed in both mice and humans. In a mouse model of pain, a 10-day course of morphine treatment prolonged the duration of pain (Grace et al. 2016). Human studies have shown that opioid use can result in an increase in pain, which has been termed opioid-induced hyperalgesia (Angst and Clark 2006; Chu et al. 2008). Additionally, the present findings are consistent with other models of opioid self-administration, such as heroin, in which hyperalgesia was observed during withdrawal from heroin dependence (Edwards et al. 2012; Park et al. 2015).
Altogether, the present study found that extended access to oxycodone self-administration produced the robust escalation of oxycodone self-administration in both male and female Wistar rats, and female rats reached slightly higher levels of intake that may be explained by differences in levels of oxycodone that reach the brain. Extended access to oxycodone self-administration also led to robust changes in irritability-like behavior and hyperalgesia during oxycodone use and withdrawal. No sex differences in irritability-like behavior or pain sensitivity were observed, suggesting that sex differences in oxycodone intake may derive from other sources of motivation. These findings demonstrate that the extended-access model of oxycodone self-administration is valid for future studies that seek to identify novel therapeutic targets for OUD, such as dopamine D3 receptors (You et al. 2017; You et al. 2018; Kumar et al. 2016), and elucidate the neurobiological basis of OUD.
Acknowledgments
The authors thank Michael Arends for proofreading the manuscript.
Funding information This work was supported by National Institutes of Health grants DA044451, DA043799, AA007456, and AA027301, the Sigrid Juselius Foundation, and the Pearson Center for Alcoholism and Addiction Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
References
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M (2013) Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev 2:CD003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen TN, Klepstad P, Davies A, Bjordal K, Lundstrom S, Kaasa S, Dale O (2011) Influences on the pharmacokinetics of oxycodone: a multicentre cross-sectional study in 439 adult cancer patients. Eur J Clin Pharmacol 67:493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Clark JD (2006) Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 104:570–587 [DOI] [PubMed] [Google Scholar]
- Baars MY, Muller MJ, Gallhofer B, Netter P (2013) Relapse (number of detoxifications) in abstinent male alcohol-depenFnt patients as related to personality traits and types of tolerance to frustration. Neuropsychobiology 67:241–248 [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W (2011) Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 37:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Alkhlaif Y, Jackson A, Carroll FI, Ditre JW, Damaj MI (2018) New insights on the effects of varenicline on nicotine reward, withdrawal and hyperalgesia in mice. Neuropharmacology 138:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260:1814–1816 [DOI] [PubMed] [Google Scholar]
- Carcoba LM, Contreras AE, Cepeda-Benito A, Meagher MW (2011) Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J Addict Dis 30:258–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso JM, Barbosa A, Ismail F, Pombo S (2006) NETER alcoholic typology (NAT). Alcohol Alcohol 41:133–139 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2017) Multiple cause of death files, 1999–2016. wonder.cdc.gov/mcd-icd10.html. Accessed 13 Feb 2020
- Choo EK, Douriez C, Green T (2014) Gender and prescription opioid misuse in the emergency department. Acad Emerg Med 21:1493–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D (2008) Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 24:479–496 [DOI] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF, George O (2015) Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol 20:56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Athanasos P, Elashoff D (2003) Withdrawal hyperalgesia after acute opioid physical dependence in nonaddicted humans: a preliminary study. J Pain 4:511–519 [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT (2016) Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 374:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Cippitelli A, Somaini L, Gerra G, Li H, Stopponi S, Ubaldi M, Kallupi M, Ciccocioppo R (2013) Pregabalin reduces cocaine self-administration and relapse to cocaine seeking in the rat. Addict Biol 18:644–653 [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, de Luca MA, Kallupi M, Li HW, Niswender K, Giordano A, Senzacqua M, Somaini L, Cippitelli A, Gaitanaris G, Demopulos G, Damadzic R, Tapocik J, Heilig M, Ciccocioppo R (2015) PPARgamma activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsycho-pharmacology 40:927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Cole MD, George O (2017a) Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration. Psychopharmacology 234:2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Matzeu A, Kononoff J, Mattioni J, Martin-Fardon R, George O (2017b) Cebranopadol blocks the escalation of cocaine intake and conditioned reinstatement of cocaine seeking in rats. J Pharmacol Exp Ther 362:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O (2020) Dopamine D3 receptor antagonism reverses the escalation of oxycodone self-administration and decreases withdrawal-induced hyperalgesia and irritability-like behavior in oxycodone-dependent heterogeneous stock rats. Front Behav Neurosci 13:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462 [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF (2012) Development of mechanical hyper-sensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology 62:1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder NM, Atayee RS, Best BM, Ma JD (2014) Observations of urinary oxycodone and metabolite distributions in pain patients. J Anal Toxicol 38:129–134 [DOI] [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI, Beardsley PM (2016) Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. Eur J Pharmacol 789:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM (2017) Opioid antagonists with minimal sedation for opioid withdrawal. Cochrane Database Syst Rev 5: CD002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR (2016) Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 113:E3441–E3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda AK, Jackson A, Bagdas D, Imad Damaj M (2018) Reversal of nicotine withdrawal signs through positive allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors in male mice. Nicotine Tob Res 20:903–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Papke RL, Damaj MI (2018) Pharmacological modulation of the alpha7 nicotinic acetylcholine receptor in a mouse model of mecamylamine-precipitated nicotine withdrawal. Psycho-pharmacology 235:1897–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD (1996) Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther 59:52–61 [DOI] [PubMed] [Google Scholar]
- Kallupi M, Xue S, Zhou B, Janda KD, George O (2018) An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse. Sci Adv 4:eaat4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Carrette LLG, Kononoff J, Solberg Woods LC, Palmer AA, Schweitzer P, George O, De Guglielmo G (2020) Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA-GABA transmission in highly addicted rats. Proc Natl Acad Sci U S A 117:2140–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Miczek KA (1986) Aggression during morphine withdrawal: effects of method of withdrawal, fighting experience, and social role. Psychopharmacology 90:451–456 [DOI] [PubMed] [Google Scholar]
- Kimbrough A, de Guglielmo G, Kononoff J, Kallupi M, Zorrilla EP, George O (2017) CRF1 receptor-dependent increases in irritability-like behavior during abstinence from chronic intermittent ethanol vapor exposure. Alcohol Clin Exp Res 41:1886–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Lurie D, Collazo A, Kreifeldt M, Sidhu H, Macedo GC, D’esposito M, Contet C, George O (2020) Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proc Natl Acad Sci U S A 117:2149–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC (2015) The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 36:559–574 [DOI] [PubMed] [Google Scholar]
- Kononoff J, Kallupi M, Kimbrough A, Conlisk D, de Guglielmo G, George O (2018a) Systemic and intra-Habenular activation of the orphan G protein-coupled receptor GPR139 decreases compulsive-like alcohol drinking and hyperalgesia in alcohol-dependent rats. eNeuro 5(3). 10.1523/ENEURO.0153-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononoff J, Melas PA, Kallupi M, de Guglielmo G, Kimbrough A, Scherma M, Fadda P, Kandel DB, Kandel ER, George O (2018b) Adolescent cannabinoid exposure induces irritability-like behavior and cocaine cross-sensitization without affecting the escalation of cocaine self-administration in adulthood. Sci Rep 8:13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’dell LE, Parsons LH, Sanna PP (2004) Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27:739–749 [DOI] [PubMed] [Google Scholar]
- Kumar V, Bonifazi A, Ellenberger MP, Keck TM, Pommier E, Rais R, Slusher BS, Gardner E, You ZB, Xi ZX, Newman AH (2016) Highly selective dopamine D3 receptor (D3R) antagonists and partial agonists based on eticlopride and the D3R crystal structure: new leads for opioid dependence treatment. J Med Chem 59:7634–7650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerspetz K, Portin R (1968) Simulation of cues eliciting aggressive responses in mice at two age levels. J Genet Psychol 113:53–63 [DOI] [PubMed] [Google Scholar]
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (2006) Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 79:461–479 [DOI] [PubMed] [Google Scholar]
- Lubman A, Emrick C, Mosimann WF, Freedman R (1983) Altered mood and norepinephrine metabolism following withdrawal from alcohol. Drug Alcohol Depend 12:3–13 [DOI] [PubMed] [Google Scholar]
- Lucantonio F, Kambhampati S, Haney RZ, Atalayer D, Rowland NE, Shaham Y, Schoenbaum G (2015) Effects of prior cocaine versus morphine or heroin self-administration on extinction learning driven by overexpectation versus omission of reward. Biol Psychiatry 77: 912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Singh A (2008) Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 11:S63–S88 [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Ailinani H, Pampati V (2010) Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 13:401–435 [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E (2017) Oxycodone self-administration in male and female rats. Psychopharmacology 234:977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchugh RK, Devito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, Connery HS, Weiss RD (2013) Gender differences in a clinical trial for prescription opioid dependence. J Subst Abus Treat 45:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckernan LC, Nash MR, Gottdiener WH, Anderson SE, Lambert WE, Carr ER (2015) Further evidence of self-medication: personality factors influencing drug choice in substance use disorders. Psychodyn Psychiatry 43:243–275 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Debold JF, Hwa LS, Newman EL, de Almeida RM (2015) Alcohol and violence: neuropeptidergic modulation of monoamine systems. Ann N Y Acad Sci 1349:96–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BC, Easton CJ, Mcmahon TJ (2011) Drug abuse and intimate partner violence: a comparative study of opioid-dependent fathers. Am J Orthop 81:218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nakamura A, Ozaki M, Imai S, Miyoshi K, Suzuki M, Suzuki T (2008) Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity to morphine. Neuropsychopharmacology 33: 1097–1112 [DOI] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Hwang CS, Vandewater SA, Janda KD, Cole M, Taffe MA (2019) Delta(9)-tetrahydrocannabinol attenuates oxycodone self-administration under extended access conditions. Neuropharmacology 151:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen AE, Staahl C, Arendt-Nielsen L, Drewes AM (2010) Different effects of morphine and oxycodone in experimentally evoked hyperalgesia: a human translational study. Br J Clin Pharmacol 70: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF (2015) Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addict Biol 20: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieb LM, Norman WV, Martin RE, Berkowitz J, Wood E, Mcneil R, Milloy MJ (2016) Withdrawal-associated injury site pain (WISP): a descriptive case series of an opioid cessation phenomenon. Pain 157:2865–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riittinen ML, Lindroos F, Kimanen A, Pieninkeroinen E, Pieninkeroinen I, Sippola J, Veilahti J, Bergstrom M, Johansson G (1986) Impoverished rearing conditions increase stress-induced irritability in mice. Dev Psychobiol 19:105–111 [DOI] [PubMed] [Google Scholar]
- Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB (1998) Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 74:181–187 [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, Williams JP, Karlsson C, Pitcairn C, Heilig M, Koob GF, Vendruscolo LF (2015) Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology 40:1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G (2018) Drug and opioid-involved overdose death - United States, 2013–2017. MMWR Morb Mortal Wkly Rep 67:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Kreifeldt M, Contet C (2018) Affective disturbances during withdrawal from chronic intermittent ethanol inhalation in C57BL/6J and DBA/2J male mice. Alcohol Clin Exp Res 42:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD (2017) Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 84:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S, Myal S, Wise RA (2015) Supplemental morphine infusion into the posterior ventral tegmentum extends the satiating effects of self-administered intravenous heroin. Pharmacol Biochem Behav 134: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2013) Results from the 2012 National Survey on drug use and health: summary of National Findings. In: ADMINISTRATION, S. A. A. M. H. S (ed) NSDUH Series H-46; HHS, Rockville [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2014) The TEDS report: gender differences in primary substance of abuse across age groups. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, Rockville, MD [Google Scholar]
- Tidey JW, Miczek KA (1992a) Heightened aggressive behavior during morphine withdrawal: effects of d-amphetamine. Psychopharmacology 107:297–302 [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA (1992b) Morphine withdrawal aggression: modification with D1 and D2 receptor agonists. Psychopharmacology 108:177–184 [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF (2015) Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Kallupi M, Hernandez DO, Breysse E, de Guglielmo G, Crawford E, Koob GF, Schweitzer P, Baunez C, George O (2017) High-frequency stimulation of the subthalamic nucleus blocks compulsive-like re-escalation of heroin taking in rats. Neuropsychopharmacology 42:1850–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W (2003) The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs 35:253–259 [DOI] [PubMed] [Google Scholar]
- Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA (2014) Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol Clin Exp Res 38:1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Kallupi M, Zhou B, Smith LC, Miranda PO, George O, Janda KD (2018) An enzymatic advance in nicotine cessation therapy. Chem Commun (Camb) 54:1686–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Gao JT, Bi GH, He Y, Boateng C, Cao J, Gardner EL, Newman AH, Xi ZX (2017) The novel dopamine D3 receptor antagonists/partial agonists CAB2–015 and BAK4–54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology 126: 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, Newman AH (2018) Dopamine D3R antagonist VK4–116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology 44:1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Zhang Y, Liang Y, Zhao C, Randesi M, Kreek MJ (2018) Oxycodone self-administration induces alterations in expression of integrin, semaphorin and ephrin genes in the mouse striatum. Front Psychiatry 9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, Ott J, Kreek MJ (2014) Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology 231:1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C, Kreek MJ (2017) Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology 234:2259–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Y, Randesi M, Yuferov V, Zhao C, Kreek MJ (2018) Chronic oxycodone self-administration altered reward-related genes in the ventral and dorsal striatum of C57BL/6J mice: an RNA-seq analysis. Neuroscience 393:333–349 [DOI] [PubMed] [Google Scholar]


