ABSTRACT
Background
An adequate maternal iron supply is crucial for maternal red blood cell (RBC) expansion, placental and fetal growth, and fetal brain development. Obese women may be at risk for poor iron status in pregnancy due to proinflammatory-driven overexpression of hepcidin leading to decreased iron bioavailability.
Objective
The objective of this study was to determine the impact of prepregnancy (PP) obesity on third-trimester maternal iron utilization.
Design
Using the stable isotope 57Fe, we measured iron utilization in the third trimester in PP obese [BMI (in kg/m2): ≥30] and nonobese (BMI: 18.5–29.9) women. We also assessed iron status, hepcidin, inflammation, erythropoietin, dietary iron intake, and gestational weight gain. Descriptive and inferential statistical tests (e.g., Student t test, Pearson correlation) were used for data analysis.
Results
Fifty pregnant women (21 PP obese, 29 PP nonobese) were included. Mean age was 27.6 ± 6.8 y and mean gestational age at time of 57Fe administration was 32.7 ± 0.7 wk. Anemia (hemoglobin <11 g/dL for non-black and <10.2 g/dL for black women) affected 38% of women (43% PP obese compared with 35% PP nonobese; P = 0.55). Women with PP obesity had significantly higher C-reactive protein (8.5 compared with 3.4 mg/L, P = 0.0007) and total body iron corrected for inflammation (6.0 compared with 4.3 mg/kg, P = 0.04) compared with the nonobese women. There was no difference in serum hepcidin or iron utilization between the PP BMI groups.
Conclusion
This is the first study to assess the impact of PP obesity on maternal iron utilization. We found no difference in iron utilization in the third trimester of pregnancy in women with and without PP obesity. Despite higher frequency of anemia, women with PP obesity had less depleted body iron stores, suggesting some degree of iron sequestration. This finding should be followed up and extended to understand effects on fetal iron bioavailability.
Keywords: iron utilization, iron status, pregnancy, obesity, hepcidin, stable isotope
Introduction
Iron is fundamental to cellular metabolism, oxygen transport, and cell growth and differentiation (1). In pregnancy, an adequate maternal iron supply is crucial for maternal red blood cell (RBC) expansion, placental and fetal growth, and fetal brain development (2). To meet the demands of pregnancy, the ability of a woman's body to utilize its iron stores and absorb iron from the diet is markedly enhanced (2). Yet, maternal iron deficiency (ID) remains a significant health problem in the United States, with national data indicating that 29.5% of pregnant women have ID when evaluated during the third trimester of pregnancy (3). This is an alarming statistic given that ID in pregnancy is associated with several adverse health effects, including spontaneous preterm birth (4), low birth weight (5), and long-term neurocognitive defects in infants (6) and mortality in mothers and infants (4).
Obesity is also a significant health problem among pregnant women in the United States, with recent statistics indicating that approximately 36% of US women entering pregnancy are obese (BMI ≥30) (7). Maternal prepregnancy (PP) obesity is associated with several maternal and neonatal disorders, including pre-eclampsia, gestational diabetes mellitus, and large-for-gestational-age infants, which may require a caesarean section delivery (8). Infants born to obese mothers also have an increased risk of metabolic dysfunction and are more likely to develop obesity themselves later in life (8). The underlying mechanisms that link maternal obesity to adverse perinatal and child health outcomes remain poorly understood, but a likely candidate is the heightened proinflammatory milieu common to obesity (9). Maternal systemic inflammation can significantly compromise maternal nutritional status and impair nutrient transfer to the fetus (10, 11). Impaired maternal–fetal nutrient transfer may thereby explain the causal link between maternal PP obesity and adverse perinatal and child health outcomes.
Several recent studies suggest a link between obesity and ID. In nonpregnant adults, obesity is associated with lower iron status and higher risk of ID (12). Studies have shown that the relation between obesity and ID may be related to adipose-derived inflammation (13) and its effect on the main homeostatic regulator of systemic iron metabolism, hepcidin (14). Hepcidin is a peptide hormone produced mainly by the liver that serves as a negative regulator of the ferroportin-1 iron exporter, facilitating its degradation and consequently blocking release of iron from cells, notably enterocytes, hepatocytes, and macrophages (14, 15). Hepcidin concentrations are dictated simultaneously by iron stores, erythropoietic activity, and inflammation (16). There is evidence that hepcidin is overexpressed in people with obesity (with control for iron status), positively associated with systemic inflammation (17), and negatively associated with dietary iron absorption (18). Moreover, when obese individuals with ID lose weight, levels of markers of systemic inflammation and hepcidin simultaneously decrease, and iron absorption and iron status improve (18, 19).
In the second and third trimesters of relatively healthy pregnancies, maternal hepcidin is largely suppressed (20). This suppression is likely a compensatory mechanism to allow for enhanced maternal iron absorption in order to fulfill increasing maternal and fetal iron needs as the pregnancy progresses (20). However, several recent studies have reported that hepcidin is upregulated in women with PP obesity and gestational obesity (21–23). These results suggest that iron bioavailability may be impaired in pregnant women with excess adiposity, yet no studies have specifically examined the effect of PP obesity on maternal iron bioavailability. Such data promise to inform the need for clinical interventions, public health programs, and nutritional recommendations to optimize iron bioavailability in a growing population of pregnant women.
We aimed to examine the impact of PP obesity on maternal utilization of an orally administered stable isotope (57Fe) ingested as ferrous sulfate. We also examined the relations between maternal iron utilization, hepcidin, iron status–related indices, hemoglobin, systemic inflammation, erythropoietin, gestational weight gain, and dietary iron intake. We hypothesized that PP obesity would be associated with increased systemic inflammation and elevated hepcidin that would negatively affect maternal iron utilization in the third trimester of pregnancy.
Subjects and Methods
Fifty-two women seeking prenatal care at the University of Illinois at Chicago (UIC) Center for Women's Health were recruited in their third trimester (29–33 wk of gestation) between 2014 and 2017. Criteria for study eligibility included the following: singleton pregnancy; naturally conceived pregnancy; 17–45 y of age; PP BMI ≥18.5 (based on measured height recorded in the medical record and self-reported preconception weight; there was no upper limit for BMI); <34 wk of gestation; sufficient fluency in English to provide consent and complete the study; and ability to independently provide consent. Criteria for study exclusion included the following: live birth or another pregnancy (including ectopic and molar pregnancies) in the previous 12 months; preeclampsia; gestational diabetes mellitus or previously diagnosed type 1 or type 2 diabetes; autoimmune disorder; current or previous premature rupture of membranes or chorioamnionitis; previous spontaneous premature birth; current bacterial or viral infection; current steroid or anti-inflammatory treatment; history of bariatric surgery; malabsorptive condition (e.g., celiac disease); current hyperemesis; hematologic disorder (e.g., sickle cell anemia or trait, hemochromatosis); current tobacco use; alcohol consumption or illicit drug use; and current use of medications that decrease nutrient absorption (e.g., proton pump inhibitors). All women provided written informed consent. Study procedures and materials were approved by the UIC Institutional Review Board (2015-0353).
Stable isotope preparation
The stable isotope 57Fe was purchased at >95% enrichment (Trace Sciences International). The isotope was prepared as a sterile, pyrogen free, ferrous sulfate solution according to previously published methods (24–28). The isotopic composition of the ferrous sulfate tracer solution was verified via inductively coupled plasma MS and tested for sterility (Baylor College of Medicine). The target dosage of 57Fe was 8.4 mg and was ingested along with 2 mL raspberry syrup (Humco) containing 0.391% ascorbic acid to enhance palatability (24, 29).
Research visits
Data collection occurred at the UIC Clinical Research Center. Women attended a baseline research visit at 32–34 wk of gestation. Women were asked to refrain from all vitamins, minerals, and supplements for 48 h, and fast for 90 min prior to arrival. At the visit, weight and height were measured using a calibrated digital scale and fixed stadiometer and antecubital venous blood was obtained. Subsequently, the 57Fe ferrous sulfate solution was provided orally to each participant. Women remained fasting for 2 h, except for water, after administration of the isotope solution. Women also completed a variety of surveys, provided data regarding their recent dietary intake, and consumed a standardized meal (containing: 482 kcal, 35% of calories from fat, 9.5% of calories from protein, 55.5% calories from carbohydrate, 2.6 mg dietary iron, and 4.7 g of dietary fiber) prior to discharge. Women returned to UIC 2 wk after ingesting the isotope (34–36 wk of gestation) for collection of venous blood, body weight measurement, and dietary assessment.
Laboratory procedures
Hemoglobin (Hb) was measured from whole blood with the Hemocue point-of-care monitor (Abbott), and Hb <11.0 g/dL was indicative of anemia in nonblack women and adjusted downward by 0.8 g/dL to 10.2 g/dL for black women per Institute of Medicine guidelines (30). Serum iron, transferrin saturation (TSAT), and ferritin were measured at a local commercial laboratory (Quest Diagnostics). Serum ferritin (<12 μg/L) was used to define depleted iron stores and ID (29). Serum soluble transferrin receptor (sTFR) was measured from serum in duplicate using an immunoassay kit (R&D Systems). Our laboratory's intra-assay CV for this assay is 5.3%. Total body iron (TBI) was calculated by using sTFR and serum ferritin measurements and using the equation devised by Cook et al. (31). Serum hepcidin was measured using a competitive immunoassay (Intrinsic LifeSciences). The lower level of detection for this assay was 5.0 ng/mL (32). Serum erythropoietin (EPO) was measured in duplicate using an immunoassay kit (R&D Systems). Our laboratory's intra-assay CV for this assay was 2.6%. Serum IL-6 was measured in duplicate using an immunoassay kit (R&D Systems). Our lab's intra-assay CV for this assay was 5.7%. Serum high-sensitivity C-reactive protein (hs-CRP) was measured by a local commercial laboratory (Quest Diagnostics).
Iron biomarker adjustment for inflammation
Ferritin, sTFR and TBI were adjusted for individuals with hs-CRP >5.0 mg/L using the correction factor methods developed by the BRINDA (Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia) project (33–35).
Isolation of iron from blood samples and determination of iron utilization
Iron was isolated from whole blood following previously published methods via anion exchange chromatography (24, 29). Iron utilization was determined by using the iron isotope ratios obtained via thermal ionization MS as previously described (28, 29). The enrichment of the isotope in RBCs was measured as the degree to which the natural abundance ratio was increased as a result of the stable isotope (57Fe). The total circulating body iron pool was calculated from the participant weight (in kilograms) and hemoglobin at baseline, using an estimated blood volume of 70 mL/kg for pregnancy and an estimated concentration of iron in Hb of 3.47 g/kg (36). The total 57Fe incorporated was calculated using the RBC enrichment, the total circulating Fe, the 57Fe dose, and the natural abundance of the isotope (57Fe = 0.0214). The final value for 57Fe incorporation into RBCs was based on the assumption that 80% of absorbed iron is incorporated into erythrocytes (37, 38).
Other measures
Gestational weight gain was determined as the average weekly rate of weight gain between the first weight in pregnancy recorded in the medical record and the weight at the research visit at week 34–36 of gestation divided by the number of weeks between the measures (39).
Dietary intake data were collected at each research visit (at 32–24 and 34–36 wk of gestation) via multiple-pass 24-h diet recall using Nutrition Data System for Research software (39, 40). The nutrient data from the 2 recalls were averaged, and the data pertaining to the total (food plus supplements) and food-based dietary iron are reported.
Participants also completed surveys related to their medical and reproductive history, sociodemographics (e.g., age, race/ethnicity, education), and lifestyle factors (e.g., smoking).
Statistical analysis
Data were analyzed with SAS v9.4 (SAS Institute). Variables were tested for normality using the Shapiro–Wilk goodness-of-fit test. Variables that were not normally distributed (adjusted serum ferritin, hs-CRP, adjusted sTFR, 57Fe utilization, IL-6, and total dietary iron per 1000 calories) were transformed using a natural logarithm prior to statistical analysis. Differences between groups were analyzed with the Student's t test or the Wilcoxon 2-sample test for nonparametric data. Differences in categorical variables were assessed with the Pearson's chi-square or Fisher's exact test. Pearson correlation coefficients were used to examine linear relations among iron status indices, 57Fe utilization, inflammatory markers, hepcidin, erythropoietin, PP BMI, gestational weight gain, and dietary iron intake per 1000 calories were measured in each of the PP BMI groups. A separate liner regression model with PP BMI on the x axis and observed (nontransformed) maternal iron utilization on the y axis was conducted and the corresponding scatterplot visualized to examine individual estimates.
We then performed multivariable regression modeling of maternal iron utilization with the following covariates: PP obesity, maternal age, education, race/ethnicity (non-Hispanic black compared with other), marital status (single compared with married/cohabitating), gravida (first pregnancy compared with second or more), and dietary iron intake per 1000 calories. We also tested the regression model with weighted covariates. Lastly, given the evidence suggesting maternal hepcidin may be significantly elevated in women with severe PP obesity, (41, 42) we explored differences in iron status–related indices, Hb, hepcidin, and iron utilization in women with severe PP obesity (i.e., BMI ≥35.0) compared with women who were not severely obese (BMI: <35.0) using the Student's t test or the Wilcoxon rank-sum test. Statistical significance was set at P < 0.05.
Results
Fifty of 52 pregnant women (21 PP obese and 29 nonobese) were included in the analysis. One obese woman was lost to follow-up. The other woman (obese) was excluded, given that her iron utilization percentage was >2 SDs above that of the other women. The characteristics of the study participants are shown in Table 1. The mean gestational age at baseline was 32.7 ± 0.7 wk. Half of the sample were non-Hispanic black women with a mean age of 26.6 ± 6.7 y. Just over half of the women were receiving Women, Infants, and Children (WIC) benefits and 38% were receiving Supplemental Nutrition Assistance Program (SNAP) benefits. Nonobese women had a significantly higher rate of weekly gestational weight gain than women with PP obesity (0.5 ± 0.9 compared with 0.3 ± 3.0, P = 0.03). There were no statistically significant differences in race/ethnicity, educational attainment, employment, receipt of government nutritional benefits (WIC/SNAP), age, gravida, or dietary iron intake/1000 kcal by PP BMI group (P > 0.05).
TABLE 1.
Characteristics of pregnant women overall and by prepregnancy BMI category1
| Prepregnancy BMI category | ||||
|---|---|---|---|---|
| Variable | Prepregnancy nonobese (n = 29) | Prepregnancy obese (n = 21) | Overall (n = 50) | P value |
| Race, % (n) | 0.11 | |||
| Non-Hispanic black | 38 (11) | 67 (11) | 50 (25) | |
| Hispanic | 28 (8) | 23 (5) | 26 (13) | |
| Non-Hispanic white | 28 (8) | 5 (1) | 18 (9) | |
| Other/unknown | 6 (2) | 5 (1) | 6 (3) | |
| Relationship status, % (n) | 0.01 | |||
| Single not living with significant other | 17 (5) | 62 (13) | 36 (18) | |
| Single but living with significant other | 48 (14) | 24 (5) | 38 (19) | |
| Married | 35 (10) | 14 (3) | 26 (13) | |
| Employment, % (n) | 0.78 | |||
| Employed | 62 (18) | 52 (11) | 58 (29) | |
| Unemployed | 24 (7) | 29 (6) | 26 (13) | |
| Other (homemaker/student) | 14 (4) | 19 (4) | 16 (8) | |
| Education, % (n) | 0.05 | |||
| High school or less | 28 (8) | 33 (7) | 30 (15) | |
| Some college | 28 (8) | 52 (11) | 38 (19) | |
| College and beyond | 44 (13) | 15 (3) | 32 (16) | |
| Receiving WIC, % (n) | 0.13 | |||
| Yes | 45 (13) | 67 (14) | 54 (27) | |
| Receiving SNAP, % (n) | 0.99 | |||
| Yes | 38 (11) | 38 (8) | 38 (19) | |
| Maternal age, y | 27.6 ± 6.8 | 25.2 ± 6.3 | 26.6 ± 6.7 | 0.21 |
| Gravida | 2 ± 1.2 | 2.6 ± 1.6 | 2.2 ± 1.4 | 0.16 |
| Gestational age at baseline, wk | 32.6 ± 0.8 | 32.8 ± 0.7 | 32.7 ± 0.7 | 0.33 |
| Maternal prepregnancy BMI, kg/m2 (self-report) | 23.1 ± 2.7 | 35.1 ± 5.0 | 28.1 ± 7.1 | <0.0001 |
| Maternal BMI at baseline, kg/m2 | 27.0 ± 6.7 | 37.8 ± 4.5 | 32.2 ± 7.6 | <0.0001 |
| Rate of weight gain, kg/wk | 0.5 ± 0.9 | 0.3 ± 2 | 0.3 ± 1.5 | 0.03 |
| Total dietary iron, mg/1000 kcal, median (IQR) | 23.3 (11.9) | 19.9 (13.2) | 20.3 (14.4) | 0.19 |
| Food iron, mg/1000 kcal | 7.6 ± 3.0 | 6.6 ± 1.8 | 7.2 ± 2.6 | 0.15 |
Values are means ± SDs, medians (IQRs), or % (n) unless otherwise indicated. The Student's t test was used for normally distributed variables, the Wilcoxon rank-sum for nonnormally distributed continuous variables, and the chi-square and Fisher's exact test for categoric variables to analyze differences between the groups. All comparisons were nonsignificant at P > 0.05. SNAP, Supplemental Nutrition Assistance Program; WG, weeks of gestation; WIC, Women Infants and Children Supplemental Nutrition Program.
Third-trimester iron status–related and inflammatory indicators are shown in Table 2. Anemia affected 38% of women, with no difference in women with or without PP obesity (43 compared with 35%, P = 0.55). Hepcidin was detectable in all of the women included in our study, and there was no difference by PP BMI group (P > 0.05). Women with PP obesity had significantly higher mean hs-CRP (8.5 compared with 3.4 mg/L, P = 0.0007) and TBI corrected for inflammation (6.0 compared with 4.3 mg/kg, P = 0.04) compared with the nonobese women. There was no significant difference in corrected serum ferritin, iron utilization, Hb, serum iron, TSAT, corrected sTFR, IL-6, or EPO between the PP BMI groups (P > 0.05).
TABLE 2.
Iron and inflammatory indicators in the third trimester of pregnancy overall and by prepregnancy BMI category1
| Prepregnancy BMI category | ||||
|---|---|---|---|---|
| Variable | Prepregnancy nonobese (n = 29) | Prepregnancy obese (n = 21) | Total (n = 50) | P value |
| Hb, g/dL | 11.0 ± 1.2 | 10.6 ± 1.4 | 10.8 ± 1.3 | 0.31 |
| Anemia,2 %, (n) | 35 (10) | 43 (9) | 56 (19) | 0.55 |
| Adjusted serum ferritin,3 μg/L, median (IQR) | 9.0 (9.0) | 14.2 (17.8) | 10.3 (11.9) | 0.12 |
| Adjusted serum ferritin <12 μg/L, %, (n) | 59 (17) | 48 (10) | 46 (23) | 0.44 |
| Serum iron, μg/dL | 63.1 ± 21.9 | 59.2 ± 19 | 61.5 ± 20.6 | 0.52 |
| Adjusted sTFR,3 nmol/L, median (IQR) | 29.5 (11.0) | 25.0 (8.8) | 28.3 (11.5) | 0.23 |
| Transferrin saturation, % | 12.9 ± 5.4 | 13.5 ± 5.4 | 13.2 ± 5.4 | 0.70 |
| Serum hepcidin, ng/mL | 20.5 ± 8.9 | 22.9 ± 8.0 | 21.5 ± 8.5 | 0.31 |
| Serum hs-CRP, mg/L, median (IQR) | 3.4 (3.4) | 8.5 (6.8) | 4.6 (6.6) | 0.0007 |
| Serum IL-6, pg/mL, median (IQR) | 1.5 (1.3) | 1.9 (1.8) | 1.7 (1.4) | 0.55 |
| Serum erythropoietin, mIU/mL | 30.8 ± 19.7 | 32.5 ± 12.3 | 31.5 ± 16.9 | 0.71 |
| Adjusted total body iron,3 mg/kg | 4.3 ± 2.6 | 6.0 ± 3.1 | 4.9 ± 2.9 | 0.04 |
| 57Fe utilization, %, median (IQR) | 11.2 (8.3) | 7.7 (8.9) | 9.8 (9.9) | 0.23 |
Values are means ± SDs, medians (IQRs), or % (n) unless otherwise indicated. The Student's t test was used for normally distributed variables, the Wilcoxon rank-sum test for nonnormally distributed continuous variables, and the chi-square test for categoric variables to analyze differences between the groups. All comparisons were nonsignificant at P > 0.05. Hb, hemoglobin; hs-CRP, high sensitivity C-reactive protein; sTFR, serum soluble transferrin receptor.
Hb <11.0 g/dL was used to indicate anemia in nonblack women and adjusted downward by 0.8 g/dL to 10.2 g/dL for black women per Institute of Medicine guidelines (30).
Adjusted using the BRINDA (Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia) correction factor methods (33–35).
Figure 1 shows results of the linear modeling examining individual nontransformed iron utilization values by PP BMI; the model was not significant (R2 = 0.03, P = 0.27). Selected Pearson correlation coefficients examining associations between clinical, iron, and inflammatory parameters by PP BMI group are shown in Table 3. Iron utilization (log transformed) in PP nonobese women was not linearly associated with PP BMI or any of the iron-related parameters. PP BMI was positively associated with log hs-CRP (r = 0.48, P = 0.01, data not shown) but not with hepcidin, log IL-6, or any other iron-related parameter. We also observed that log hs-CRP was positively associated with EPO (r = 0.40, P = 0.03, data not shown). Total dietary iron intake per 1000 calories and gestational weight gain were not significantly associated with the iron or inflammatory markers tested (P > 0.05, data not shown). Iron utilization (log transformed) in PP obese women was not significantly correlated with PP BMI (P > 0.05). However, log iron utilization was inversely associated with serum hepcidin (r = −0.48, P = 0.03). PP BMI was positively associated with log IL-6 (r = 0.48, P = 0.03), but not with hepcidin or any other iron-related parameters. Total dietary iron intake per 1000 kcal was positively associated with TSAT (r = 0.55, P = 0.0096, data not shown). Gestational weight gain was not related to any of the variables tested (data not shown).
FIGURE 1.
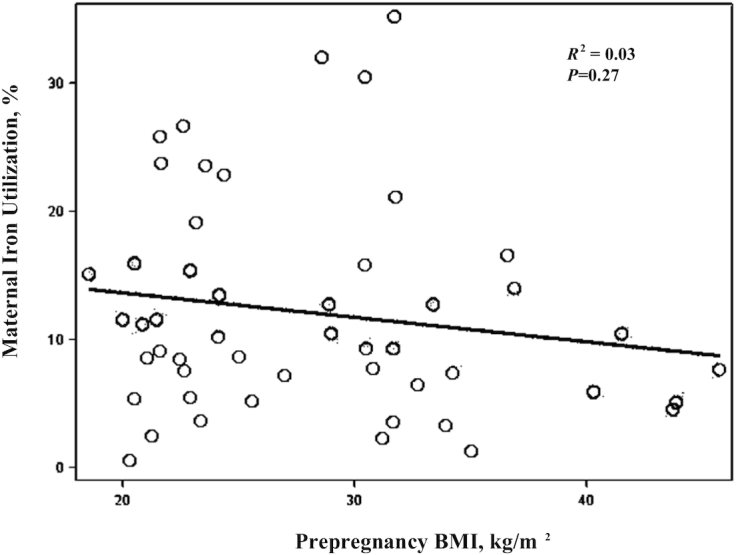
Maternal third-trimester iron utilization percentage by prepregnancy BMI (kg/m2). Scatter plot of prepregnancy BMI on the x-axis and observed (nontransformed) maternal iron utilization percentage on the y-axis, n = 50. The black line is the regression fit estimate.
TABLE 3.
Selected Pearson correlation coefficients between prepregnancy BMI and third-trimester iron and inflammatory indicators by prepregnancy BMI category1
| Prepregnancy nonobese (n = 29) | Prepregnancy obese (n = 21) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | Log 57Fe utilization, % | Adjusted log ferritin,2 μg/L | Adjusted log sTFR,2 nmol/L | Hepcidin, ng/mL | Log IL-6, pg/mL | EPO, mIU/mL | BMI | Log 57Fe Utilization, % | Adjusted log ferritin,2μg/L | Adjusted log sTFR,2, nmol/L | Hepcidin, ng/mL | Log IL-6, pg/mL | EPO, mIU/mL | |
| BMI | 0.18 | −0.06 | −0.26 | 0.24 | 0.09 | −0.003 | −0.23 | 0.25 | −0.31 | −0.26 | 0.48* | −0.09 | ||
| Log 57Fe utilization, % | 0.18 | −0.01 | 0.10 | −0.11 | 0.02 | 0.13 | −0.23 | −0.44* | 0.08 | −0.48* | −0.29 | 0.17 | ||
| Adjusted log ferritin,2 μg/L | −0.06 | −0.01 | −0.30 | 0.34 | −0.19 | −0.34 | 0.25 | −0.44* | −0.20 | 0.35 | 0.13 | −0.31 | ||
| Adjusted log sTFR,2 nmol/L | −0.26 | 0.10 | −0.30 | −0.01 | 0.15 | 0.68* | −0.31 | 0.08 | −0.20 | 0.08 | −0.19 | 0.45* | ||
| Hepcidin, ng/mL | 0.24 | −0.11 | 0.34 | −0.01 | 0.02 | −0.02 | −0.26 | −0.48* | 0.35 | 0.08 | 0.03 | −0.23 | ||
| Log IL-6, pg/mL | 0.09 | 0.02 | −0.19 | 0.15 | 0.02 | 0.06 | 0.48* | −0.29 | 0.13 | −0.19 | 0.03 | 0.05 | ||
| EPO, mIU/mL | −0.003 | 0.13 | −0.34 | 0.68* | −0.02 | 0.06 | −0.09 | 0.17 | −0.31 | 0.45* | −0.23 | 0.05 | ||
Values presented are r, coefficients of correlation, *P < 0.05. BMI, prepregnancy body mass index; EPO, serum erythropoietin; hs-CRP, serum high-sensitivity C-reactive protein; sTFR, log-transformed serum soluble transferrin receptor.
Adjusted using the BRINDA (Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia) correction factor methods (33–35).
Multivariate models of maternal iron utilization
Neither multivariable model (weighted with entropy balancing or unweighted) was highly predictive of maternal iron utilization (R2 = 0.18 compared with 0.33 for weighted regression); however, the models did support the negative effect of PP obesity on maternal iron utilization, as did being non-Hispanic black, single, or having lower dietary iron intake (Table 4).
TABLE 4.
Predictive multiple linear regression models of log-transformed maternal 57Fe utilization (%) in the third trimester1
| Variable | β (SE) | P value |
|---|---|---|
| Weighted (R2 = 0.37) | ||
| Prepregnancy obese (BMI: ≥30.0) | −0.01 (0.26) | 0.96 |
| Age, y | 0.01 (0.02) | 0.72 |
| Some college vs. high school or less | 0.46 (0.40) | 0.26 |
| College and beyond vs. high school or less | 0.97 (0.50) | 0.06 |
| non-Hispanic black vs. other | 0.15 (0.36) | 0.87 |
| Single vs. married/cohabitating | −0.10 (0.27) | 0.45 |
| Gravida ≥2 | 0.58 (0.28) | 0.05 |
| Total dietary iron, mg/1000 kcal | 0.002 (0.004) | 0.63 |
| Unweighted (R2 = 0.25) | ||
| Prepregnancy obese (BMI: ≥30.0) | −0.11 (0.28) | 0.71 |
| Age, y | 0.20 (0.03) | 0.93 |
| Some college vs. high school or less | 0.35 (0.29) | 0.29 |
| College and beyond vs. high school or less | 0.28 (0.41) | 0.51 |
| Non-Hispanic black vs. other | 0.04 (0.25) | 0.86 |
| Single vs. married/cohabitating | −0.41 (0.31) | 0.22 |
| Gravida ≥2 | 0.46 (0.26) | 0.10 |
| Total dietary iron, mg/1000 kcal | 0.002 (0.003) | 0.49 |
Values are βs (SEs), are unstandardized regression coefficient and standard error unless otherwise indicated.
Post hoc exploratory analysis examining the impact of severe PP obesity on maternal iron status–related indices, hepcidin, and iron utilization
In the women with severe PP obesity (BMI ≥35.0, n = 7), there was no statistically significant difference in the prevalence of anemia, serum hepcidin, hs-CRP, iron utilization, or any iron-related marker compared with women who were not severely obese (BMI <35.0) (data not shown).
Discussion
To our knowledge, this is the first study designed to assess the impact of PP maternal obesity on utilization of oral ferrous sulfate in the third trimester of human pregnancy. There is increasing evidence that obesity is associated with ID in nonpregnant and pregnant populations (12, 13, 17, 22, 42). Additionally, many investigators have hypothesize that adipose-derived inflammation increases hepcidin, which in turn limits iron bioavailability (18). Our findings indicated that dietary absorption of a 57Fe-labeled ferrous sulfate solution in the third trimester of a singleton pregnancy was not significantly different in women with and compared with those without PP obesity. Moreover, PP BMI was not associated with iron utilization. Our result of no linear association between iron utilization and PP BMI is in line with a previous report examining third-trimester nonheme iron utilization in a small group of pregnant women (n = 18) with varying degrees of PP BMI (29). However, our results are in contrast with those of 2 studies of nonpregnant obese adults (n = 62 and n = 92, respectively) reporting that increasing BMI is inversely associated with iron utilization from nonheme iron sources (18, 43). It is possible that the relatively small sample size in each of our PP BMI groups, and limited range of hepcidin and iron status, contributed to the nonsignificant findings.
Several studies have reported that maternal obesity is associated with elevated maternal hepcidin (21–23). We saw no evidence of such an association in our study, a finding consistent with an investigation of pregnant adolescents (44) and a study of pregnant normal-weight and obese women (42). However, in studies reported by Cao and Fleming (44) and Flynn et al (42), hepcidin concentrations were higher in women with severe obesity (BMI ≥35.0). Considering this previously reported finding, we explored if women with BMI ≥35.0 had higher serum hepcidin and lower iron utilization compared with women without severe obesity. We found no difference in these parameters. In our group of predominately non-Hispanic black women, we observed that (irrespective of PP BMI) serum hepcidin was higher than previously reported for women in the third trimester of pregnancy (29, 42). However, our values were consistent with the cohort of pregnant adolescents mentioned above, who were predominately non-Hispanic black (44). Two specific single-nucleotide polymorphisms of the transmembrane serine protease 6 (TMPRSS6) gene encoding the protein matripase-2, a protein that may regulate hepcidin, (45) have been observed at a higher frequency in black individuals (46, 47). This suggests a possible genetic explanation for the higher concentrations of maternal hepcidin observed in our participants and in the study completed by Cao and Fleming (44), a hypothesis that should be pursued in future studies given higher rates of maternal ID reported in US non-Hispanic black women (3).
We observed that serum hepcidin was inversely associated with maternal iron utilization, a finding consistent with a previous report from Young and colleagues (29) (although the association in our study was only statistically significant in the women with PP obesity). The inverse relationship between serum hepcidin and maternal iron utilization is consistent with hepcidin's role in controlling the flow of iron from the small intestine by binding of the ferroportin-1 iron exporter, which leads to iron degradation (1).
In this study, we observed a nonsignificant relation between serum hepcidin, IL-6, hs-CRP, and EPO in both PP BMI groups. Nonsignificant associations between IL-6 and hepcidin in pregnant women have been reported previously (44). Other investigators have suggested that the level of inflammation observed in obese pregnancy may not be sufficient to override signals from body iron stores and erythropoiesis to impact hepcidin production (44). In both groups of women, however, we did show that serum ferritin was positively correlated with hepcidin, suggesting that maternal body iron stores were playing a role in regulating hepcidin. Erythroferrone, an erythroid-derived protein that increases with erythropoiesis (48), and estradiol (49), which is significantly increased in the third trimester of pregnancy (50), may play significant roles in regulating maternal hepcidin and iron homeostasis in pregnancy. However, their roles in maternal iron regulation in human pregnancy remains largely understudied (51). Some investigators also suggest that there is a yet unknown maternal, placental or fetal factor responsible for regulating and suppressing hepcidin in pregnancy (51).
Previous studies in nonpregnant adults and pregnant women have indicated that obesity is associated with decreased serum ferritin and elevated sTFR compared with normal-weight and nonobese controls (19, 42, 52). In the aforementioned adolescent cohort, maternal iron status did not differ by PP BMI category at midgestation or delivery (44). Unexpectedly, we observed higher TBI corrected for inflammation in the women with PP obesity, indicating that in these obese women body iron stores were less depleted than those of the nonobese women. This finding may help to explain the somewhat lower iron utilization in the women with PP obesity. Moreover, the obese women had lower Hb and a greater percentage of the women in this group had anemia. Taken together, these findings suggest mild anemia of inflammation, with iron sequestering in body stores.
Strengths of this study included the use of a stable isotope to directly measure maternal iron utilization; measurement of many factors known to influence iron absorption (i.e., hepcidin, iron status, inflammation, erythropoiesis, and recent dietary intake); and involvement of participants from a range of socioeconomic backgrounds. However, this study also had several limitations. Because we used an oral iron isotope, we were not able to directly assess iron incorporation into RBCs but instead assumed 80% incorporation across subjects (18). The use of a single isotope may have biased blood volume and RBC incorporation assumptions. We also did not measure maternal blood volume directly and relied on self-reported maternal PP body weight, which may have biased our calculations (18). It may also have been fruitful to examine iron utilization in the context of a food matrix, as well as heme iron utilization. Our relatively small convenience sample may have contributed to the nonsignificant findings and certainly precluded us from meaningful subgroup analyses by PP BMI category and limits generalizability of results. Lastly, the iron isotope dosing and blood draws were not performed at a standardized time across the study participants. Given hepcidin's diurnal variation, increasing as the day progresses (53), interpretation of iron utilization, serum hepcidin, and markers of iron status may have been affected. A standard time for iron isotope dosing and blood sampling should be instituted in future iron utilization studies.
To conclude, despite evidence indicating that being obese reduces dietary iron utilization, and is associated with poorer iron stores in nonpregnant adults (18, 43), iron utilization from an oral ferrous sulfate solution in the third trimester of pregnancy was similar in women with and without PP obesity, and iron stores were more sufficient in those with PP obesity. This latter finding was surprising given that the women with PP obesity had a higher prevalence of anemia, suggesting to some extent that iron was sequestering in their stores. This finding should be followed up in a larger cohort of ethnically/racially diverse women and extended to understand how this iron phenotype affects fetal iron bioavailability.
Acknowledgments
The authors would like to thank Drs. Steven Abrams (University of Texas at Austin) and Zhensheng Chen (Baylor College of Medicine) for assistance preparing the isotope solution. The authors would also like to thank Amy Haara and Dr. Gloria Elam for their assistance with clinical recruitment (University of Illinois at Chicago).
The authors’ responsibilities were as follows—MDK and LTH: designed the study; MDK, LTH, KOO, VD, RR, LW, NH, BL, LP, AM and BH: conducted the study; HP and AS: performed the statistical analysis; EK, MDK and LT-H: wrote the manuscript; CEF and KOO: provided valuable knowledge and scientific consultation throughout the study; and all authors: critically reviewed and read and approved the final manuscript.
Notes
Supported by the Robert Wood Johnson Foundation Nurse Faculty Scholars Program (grant 72117), the Center for Health Equity Research (CHER) Chicago (grant U54MD012523; subaward 088917), and the University of Illinois College of Nursing Dean's Award, University of Illinois Department of Medicine . The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author disclosures: The authors report no conflicts of interest.
Data described in the manuscript, code book, and analytic code will be made available upon request in de-identified form.
Abbreviations used: EPO, erythropoietin; Hb, hemoglobin; hs-CRP, high-sensitivity C-reactive protein; ID, iron deficiency; PP, prepregnancy; RBC, red blood cell; SNAP, Supplemental Nutrition Assistance Program; sTFR, serum soluble transferrin receptor; TBI, total body iron; TSAT, transferrin saturation; UIC, University of Illinois at Chicago; WIC, Women, Infants and Children.
References
- 1. Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–42. [DOI] [PubMed] [Google Scholar]
- 2. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:257S–64S. [DOI] [PubMed] [Google Scholar]
- 3. Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 4. Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280S–4S. [DOI] [PubMed] [Google Scholar]
- 5. Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78:773–81. [DOI] [PubMed] [Google Scholar]
- 6. Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA. Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta. 2011;32:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30:441–6. [DOI] [PubMed] [Google Scholar]
- 10. King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–91. [DOI] [PubMed] [Google Scholar]
- 11. Sen S, Iyer C, Meydani SN. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol. 2014;34:105–11. [DOI] [PubMed] [Google Scholar]
- 12. Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 2015;16:1081–93. [DOI] [PubMed] [Google Scholar]
- 13. Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Amor IB et al.. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–96. [DOI] [PubMed] [Google Scholar]
- 14. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. [DOI] [PubMed] [Google Scholar]
- 15. Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darshan D, Anderson GJ. Interacting signals in the control of hepcidin expression. Biometals. 2009;22:77–87. [DOI] [PubMed] [Google Scholar]
- 17. Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, Holterman AX, Braunschweig C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring). 2010;18:1449–56. [DOI] [PubMed] [Google Scholar]
- 18. Cepeda-Lopez AC, Melse-Boonstra A, Zimmermann MB, Herter-Aeberli I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am J Clin Nutr. 2015;102:1389–97. [DOI] [PubMed] [Google Scholar]
- 19. Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Holterman AX, Galvani C, Ayloo S, Vitello J, Braunschweig C. Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity (Silver Spring). 2010;18:2010–6. [DOI] [PubMed] [Google Scholar]
- 20. Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85:345–52. [DOI] [PubMed] [Google Scholar]
- 21. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link?. J Perinatol. 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, McArdle HJ. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015;39:571–8. [DOI] [PubMed] [Google Scholar]
- 23. Jones AD, Zhao G, Jiang YP, Zhou M, Xu G, Kaciroti N, Zhang Z, Lozoff B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr. 2016;70:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, O'Brien KO. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89:533–8. [DOI] [PubMed] [Google Scholar]
- 25. Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr. 1994;71:411–24. [DOI] [PubMed] [Google Scholar]
- 26. O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77:924–30. [DOI] [PubMed] [Google Scholar]
- 27. Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69:509–15. [DOI] [PubMed] [Google Scholar]
- 29. Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr. 2010;140:2162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Earl RO, Woteki CE, Calloway DH, Institute of Medicine (U.S.). Committee on the Prevention Detection and Management of Iron Deficiency Anemia among U.S. Children and Women of Childbearing Age., Institute of Medicine (U.S.). Food and Nutrition Board . Iron deficiency anemia: recommended guidelines for the prevention, detection, and management among U.S. children and women of childbearing age. Washington (DC): National Academies Press; 1993. [PubMed] [Google Scholar]
- 31. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 32. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–7. [DOI] [PubMed] [Google Scholar]
- 33. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ et al.. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mei Z, Namaste SM, Serdula M, Suchdev PS, Rohner F, Flores-Ayala R, Addo OY, Raiten DJ. Adjusting total body iron for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:383S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rohner F, Namaste SM, Larson LM, Addo OY, Mei Z, Suchdev PS, Williams AM, Sakr Ashour FA, Rawat R, Raiten DJ et al.. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:372S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fomon SJ, Ziegler EE, Rogers RR, Nelson SE, Edwards BB, Guy DG, Erve JC, Janghorbani M. Iron absorption from infant foods. Pediatr Res. 1989;26:250–4. [DOI] [PubMed] [Google Scholar]
- 37. Barrett JF, Whittaker PG, Fenwick JD, Williams JG, Lind T. Comparison of stable isotopes and radioisotopes in the measurement of iron absorption in healthy women. Clin Sci (Lond). 1994;87:91–5. [DOI] [PubMed] [Google Scholar]
- 38. Whittaker PG, Barrett JF, Lind T. The erythrocyte incorporation of absorbed non-haem iron in pregnant women. Br J Nutr. 2001;86:323–9. [DOI] [PubMed] [Google Scholar]
- 39. Huang A, Ji Z, Zhao W, Hu H, Yang Q, Chen D. Rate of gestational weight gain and preterm birth in relation to prepregnancy body mass indices and trimester: a follow-up study in China. Reprod Health. 2016;13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harnack L, Stevens M, Van Heel N, Schakel S, Dwyer JT, Himes J. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls. J Food Compos Anal. 2008;21:S78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reproductive sciencesci. 2016;23:613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flynn AC, Begum S, White SL, Dalrymple K, Gill C, Alwan NA, Kiely M, Latunde-Dada G, Bell R, Briley AL et al.. Relationships between maternal obesity and maternal and neonatal iron status. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond). 2008;32:1098–104. [DOI] [PubMed] [Google Scholar]
- 44. Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev. 2016;74:421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K et al.. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gichohi-Wainaina WN, Towers GW, Swinkels DW, Zimmermann MB, Feskens EJ, Melse-Boonstra A. Erratum to: Inter-ethnic differences in genetic variants within the transmembrane protease, serine 6 (TMPRSS6) gene associated with iron status indicators: a systematic review with meta-analyses. Genes Nutr. 2015;10:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McLaren CE, McLachlan S, Garner CP, Vulpe CD, Gordeuk VR, Eckfeldt JH, Adams PC, Acton RT, Murray JA, Leiendecker-Foster C et al.. Associations between single nucleotide polymorphisms in iron-related genes and iron status in multiethnic populations. PLoS One. 2012;7:e38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lehtihet M, Bonde Y, Beckman L, Berinder K, Hoybye C, Rudling M, Sloan JH, Konrad RJ, Angelin B. Circulating hepcidin-25 is reduced by endogenous estrogen in humans. PLoS One. 2016;11:e0148802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso HA, Idahl A, Lehtinen M, Surcel HM, Fortner RT. Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy Childbirth. 2016;16:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, Armitage AE, Drakesmith H, Moore SE, Prentice AM. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the gambia. J Nutr. 2017;147:1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes. 2007;31:1412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schaap CC, Hendriks JC, Kortman GA, Klaver SM, Kroot JJ, Laarakkers CM, Wiegerinck ET, Tjalsma H, Janssen MC, Swinkels DW. Diurnal rhythm rather than dietary iron mediates daily hepcidin variations. Clin Chem. 2013;59:527–35. [DOI] [PubMed] [Google Scholar]


