ABSTRACT
Background
In animal models cis-palmitoleic acid (9-hexadecenoic acid; 16:1n–7c), a lipokine, improves insulin sensitivity, inflammation, and lipoprotein profiles; in humans trans-palmitoleic acid (16:1n–7t) has been associated with lower incidence of type 2 diabetes. The response to dose-escalation of supplements containing cis- and trans-palmitoleic acid has not been evaluated.
Objectives
We examined dose-escalation effects of oral supplementation with seabuckthorn oil and seabuckthorn oil augmented in 16:1n–7t on serum phospholipid fatty acids (PLFAs).
Methods
Thirteen participants (7 women and 6 men; age 48 ± 16 y, BMI 30.4 ± 3.7 kg/m2) participated in a randomized, double-blind, crossover, dose-escalation trial of unmodified seabuckthorn oils relatively high in 16:1n–7c (380, 760, and 1520 mg 16:1n–7c/d) and seabuckthorn oils augmented in 16:1n–7t (120, 240, and 480 mg 16:1n–7t/d). Each of the 3 escalation doses was provided for 3 wk, with a 4-wk washout period between the 2 supplements. At the end of each dose period, fasting blood samples were used to determine the primary outcomes (serum concentrations of the PLFAs 16:1n–7t and 16:1n–7c) and the secondary outcomes (glucose homeostasis, serum lipids, and clinical measures). Trends across doses were evaluated using linear regression.
Results
Compared with baseline, supplementation with seabuckthorn oil augmented in 16:1n–7t increased phospholipid 16:1n–7t by 26.6% at the highest dose (P = 0.0343). Supplementation with unmodified seabuckthorn oil resulted in a positive trend across the dose-escalations (P-trend = 0.0199). No significant effects of either supplement were identified on blood glucose, insulin, lipids, or other clinical measures, although this dosing study was not powered to detect such effects. No carryover or adverse effects were observed.
Conclusions
Supplementation with seabuckthorn oil augmented in 16:1n–7t and unmodified seabuckthorn oil moderately increased concentrations of their corresponding PLFAs in metabolically healthy adults, supporting the use of supplementation with these fatty acids to test potential clinical effects in humans.
This trial was registered at clinicaltrials.gov as NCT02311790.
Keywords: seabuckthorn oil, cis-palmitoleic acid, trans-palmitoleic acid, dose-escalation, phospholipid fatty acids, glucose, insulin, cholesterol, triglyceride
Introduction
Growing research indicates that the conventional chemistry categorization of fatty acids (e.g., SFA, MUFA, PUFA) belies the biological complexity of potential health effects of individual fatty acids of varying structure and chain length, including those present at relatively low concentrations (1). Among these, palmitoleic acid (9-hexadecenoic acid; 16:1n–7), which exists in both cis (16:1n–7c) and trans (16:1n–7t) isomeric forms, is of particular interest. In in vivo animal and human observational studies, supplementation with 16:1n–7c improves insulin sensitivity (2–5), decreases inflammation and dyslipidemia (6), and slows the development of atherosclerotic lesions (5). Because 16:1n–7c is a relatively specific downstream product of hepatic de novo lipogenesis, it may reflect the conversion of excess dietary starch, sugar, and protein into fatty acids. It has been hypothesized that exogenous administration of 16:1n–7c participates in a negative feedback loop to suppress hepatic de novo lipogenesis (2, 7). Other postulated mechanisms include stimulation of β-cell insulin secretion, elevation of hepatic fatty acid oxidation, and acceleration of macrophage differentiation into an anti-inflammatory phenotype (4).
Yet, these promising results in animal models have been challenging to test in humans, in part because of limited evidence on blood dose-response effects of 16:1n–7c supplements. Dietary sources are rare, including seabuckthorn oil and macadamia nuts (8). Because these foods are not commonly consumed, blood concentrations of 16:1n–7c largely reflect endogenous synthesis. Positive associations have been reported between higher blood concentrations and lower metabolic risk (9, 10). Thus, these observational studies of endogenously produced 16:1n–7c do not provide inference on the potential health effects of exogenously provided 16:1n–7c.
The trans isomer of palmitoleic acid, 16:1n–7t, naturally present in dairy fat, is a metabolic product of bacterial synthesis in the rumen of animals (9–11). In observational studies, higher blood concentrations of 16:1n–7t have been associated with greater insulin sensitivity and lower incidence of type 2 diabetes (T2D) (10–14), suggesting a potential role of this isomer in metabolic health. Yet, like 16:1n–7c, these results in observational studies have been challenging to confirm in intervention studies owing to limited data on the dose-response effect of 16:1n–7t supplementation on blood concentrations.
Thus, the dearth of information on the dose-response effect of oral supplementation with 16:1n–7c or 16:1n–7t on blood concentrations has limited the design and implementation of interventional studies. To address this gap in knowledge we investigated, in a randomized, double-blind, dose-escalation trial, the effects of unmodified seabuckthorn oil and seabuckthorn oil augmented in 16:1n–7t on serum phospholipid fatty acid (PLFA) profiles in generally healthy adults. We hypothesized that supplementation with unmodified seabuckthorn oil and seabuckthorn oil augmented in 16:1n–7t would result in a dose-dependent increase in their respective PLFA concentrations.
Methods
Study population
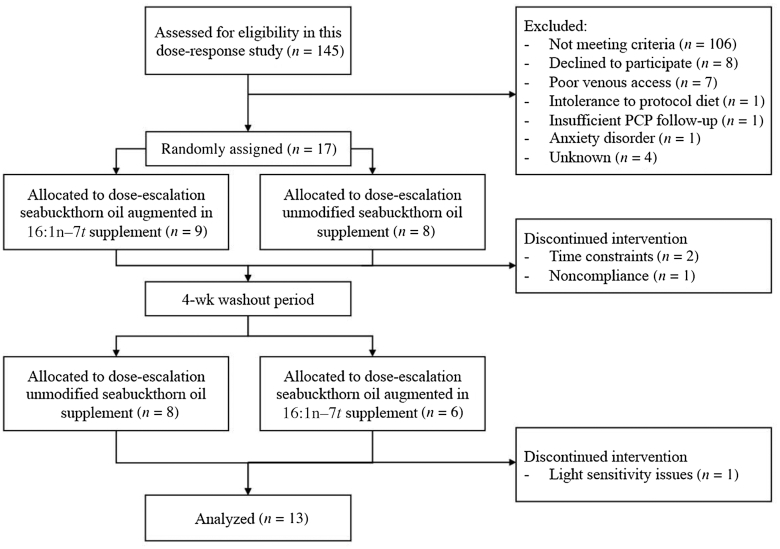
Study subjects (n = 13; 7 women and 6 men) were recruited from the greater Boston area using advertisements and the Human Nutrition Research Center on Aging at Tufts (HNRCA) volunteer database (Figure 1). Inclusion criteria were age 18–70 y; BMI (in kg/m2) 25–40; fasting plasma glucose ≤120 mg/dL; normotensive (blood pressure <140/90 mm Hg) with or without medications; and normal thyroid (with or without medication for ≥6 mo), kidney, liver, and gastrointestinal clinical blood measures. Exclusion criteria were use of supplements containing fish oil or other lipid supplements within 3 mo of randomization; habitual consumption of ≥3 combined servings/d of cheese, full-fat milk, or full-fat yogurt (the major dietary source of 16:1n–7t); alcohol intake >7 alcoholic beverages/wk; inability or difficulty in taking pills; diagnosed type 1 or type 2 diabetes and/or taking glucose-lowering medications; use of medication known to affect lipid metabolism; and hormone replacement therapy. This study (NCT02311790) was conducted in accordance with the Declaration of Helsinki guidelines, all procedures were approved by the Institutional Review Board of Tufts University/Tufts Medical Center, and written informed consent was obtained from the study participants. The study was conducted between January 2016 and March 2017.
FIGURE 1.
Flow diagram of the metabolically healthy adults supplemented with seabuckthorn oil augmented in 16:1n–7t and unmodified seabuckthorn oil. PCP, primary care provider.
Recruitment and screening
Participants who responded to the study advertisements or were recruited from the HNRCA volunteer database were screened for eligibility. The participants were provided with information about the study design and procedures, and for those who were interested in this research, a prescreening telephone interview was administrated to assess potential eligibility. Participants who appeared to meet the inclusion criteria were scheduled for a prescreening in-person appointment to collect a fasting (12-h) blood sample. Based on the predetermined criteria, the qualified participant was invited for a subsequent screening visit to conduct a full health screen, obtain a blood sample, and assess their dietary habits with specific emphasis on the habitual consumption of full-fat dairy products. A total of 145 participants were screened for eligibility and 17 participants were enrolled into the study (Figure 1). Four of the participants did not complete the study because of the following reasons: time constraints (n = 2), noncompliance with study procedures (n = 1), and light sensitivity issues (n = 1).
Study design and interventions
We conducted a randomized, double-blind, crossover, dose-escalation study over 22 wk, including 2 randomly assigned supplementation periods consisting of escalating doses of either unmodified seabuckthorn oil (380, 760, and 1520 mg 16:1n–7c/d) or seabuckthorn oil augmented in 16:1n–7t (120, 240, and 480 mg 16:1n–7t/d). Each dose was provided for 3 wk, with a 4-wk washout phase between the 2 supplements (Supplemental Figure 1). The random assignment sequence for each participant was generated according to a block design and assigned at the time of study consent. The study statistician generated the random allocation sequence. Enrollment and assignment were conducted by the study coordinator. The 2 supplements were labeled A and B, and neither were aware of their identity. The appearance and characteristics of the 2 supplements were identical. Serum concentrations of the PLFAs 16:1n–7t and 16:1n–7c were the primary outcomes for this dose-escalation study, and the other fatty acids were exploratory outcomes. All participants, investigators, and laboratory personnel were blinded to the random assignment. For each of the 2 supplementation periods, participants were instructed to consume 2 capsules/d during the first 3 wk, 4 capsules/d during the second 3 wk, and 8 capsules/d during the third 3 wk, evenly divided between morning and evening meals. Compliance was evaluated using pill counts.
Unmodified seabuckthorn oil and seabuckthorn oil augmented in 16:1n–7t supplements
The seabuckthorn oil augmented in 16:1n–7t was developed and provided for this trial free of charge by Unilever, Netherlands. Both oils were derived from fresh seabuckthorn berries. The 16:1n–7c oil was unmodified seabuckthorn oil, containing 35.5% 16:1n–7c by weight and no detectable 16:1n–7t. Each seabuckthorn oil supplement capsule (500 mg) contained 178 mg 16:1n–7c and no detectable 16:1n–7t. The seabuckthorn oil augmented in 16:1n–7t was produced by partial hydrogenation of naturally occurring seabuckthorn oil. The goal was to calibrate the process to augment the amount of 16:1n–7t produced and minimize the formation of other trans fatty acids. Owing to technical limitations the resulting product contained 18:1t others (12%), 16:1n–7t (9.6%), 16:1n–9t (5.7%), 18:1n–7t (2.78%), 18:1n–9t (1.93%), and 18:2t (0.36%) (Table 1). Each seabuckthorn oil augmented in 16:1n–7t supplement capsule (500 mg) contained 48 mg 16:1n–7t and 113 mg 16:1n–7c. The total PUFA content of the seabuckthorn oil augmented in 16:1n–7t was lower than that of the unmodified seabuckthorn oil.
TABLE 1.
Detectable FAs in seabuckthorn oil augmented in 16:1n–7t and unmodified seabuckthorn oil supplements1
| Seabuckthorn oil augmented in 16:1n–7t | Unmodified seabuckthorn oil | |||
|---|---|---|---|---|
| FAs | mg/500 mg capsule | % FA | mg/500 mg capsule | % FA |
| SFA total | 182 | 36.3 | 168 | 33.5 |
| 16:0 | 168 | 33.5 | 160 | 31.9 |
| MUFA total | 314 | 62.9 | 301 | 60.2 |
| cis total | 154 | 30.9 | 301 | 60.2 |
| 16:1n–7c | 113 | 22.5 | 178 | 35.5 |
| 16:1n–9c | 12.5 | 2.50 | ND | ND |
| 18:1n–7c | 22.5 | 4.5 | 38.6 | 7.71 |
| 18:1n–9c | 6.85 | 1.37 | 84.5 | 16.9 |
| 24:1n–9c | 0.1 | 0.02 | 0.05 | 0.01 |
| trans total | 160 | 32.0 | ND | ND |
| 16:1n–7t | 48.0 | 9.6 | ND | ND |
| 16:1n–9t | 28.5 | 5.7 | ND | ND |
| 18:1n–7t | 13.9 | 2.78 | ND | ND |
| 18:1n–9t | 9.65 | 1.93 | ND | ND |
| 18:1t, others | 59.9 | 12.0 | ND | ND |
| PUFA total | 3.85 | 0.77 | 31.7 | 6.34 |
| cis total | 2.05 | 0.41 | 31.7 | 6.34 |
| 18:2n–6c | 1.2 | 0.24 | 26.0 | 5.20 |
| 20:2n–6c | 0.4 | 0.08 | 0.05 | 0.01 |
| 20:4n–6c | 0.05 | 0.01 | ND | ND |
| 18:3n–3c | 0.15 | 0.03 | 5.65 | 1.13 |
| 18:4n–3c | 0.25 | 0.05 | ND | ND |
| trans 18:2t | 1.80 | 0.36 | ND | ND |
1Values are milligrams or percentages of FA. The unit of each supplement is mg/500-mg capsule. FA labeled as ND indicates a concentration <0.01%. FA, fatty acid; ND, not detectable.
Serum PLFA measurements
Overnight fasting blood was drawn from the antecubital vein at the beginning (baseline) and end of each 3-wk dosing period. Serum was immediately separated by centrifugation at 1100 × g for 10 min at 4°C and stored at −80°C until analyses. All samples were analyzed in a single batch at the conclusion of the trial, using an established GC method (15). Briefly, lipids were extracted from serum using the Folch method (16) after addition of an internal standard (1,2 diheptadecanoyl-glycero-3-phosphocholine). The serum phospholipid (PL) fraction was isolated using solid-phase chromatography (aminopropyl columns), saponified, and methylated as previously described (15). The resulting FAMEs were analyzed using an Autosystem XL GC (Perkin Elmer) equipped with a 100 m × 0.25 mm capillary column (HP INNOWax, Agilent Technologies). Fatty acid peaks were identified by comparison with authenticated standards (NuCheck Prep) and expressed as mol% and μmol/L. The intra-assay CVs were 5.4% for 16:1n–7c, 6.8% for 16:1n–7t, 0.5%–4.3% for other fatty acids present at concentrations >5 mol%, 1.8%–7.1% for fatty acids present at concentrations between 1 and 5 mol%, and 2.8%–11.1% for fatty acids present at concentrations <1 mol%.
Clinical laboratory measures
Fasting blood samples were used to determine the secondary outcomes, including glucose homeostasis, lipids, and clinical measures. As safety measures, plasma total cholesterol, HDL-cholesterol, and triglyceride (TG) concentrations and serum glucose concentrations were measured using an AU400e automated analyzer (Beckman Coulter; assay CV <3%) with enzymatic reagents (Beckman-Coulter). LDL-cholesterol concentrations were calculated using the Friedewald formula (17). Serum insulin concentrations were determined by RIA (EMD Millipore). VLDL-cholesterol [TG (mmol/L)/2.2] concentrations, non-HDL-cholesterol (total cholesterol minus HDL-cholesterol) concentrations, and HOMA-IR [glucose (mmol/L) × insulin (mU/L)/22.5] (18) were calculated. Hematology and clinical chemistry measures were determined using a Horiba ABX Pentra 60c+ Hematology Analyzer (HORIBA Instruments Inc.) and AU480 Clinical Chemistry Analyzer (Beckman Coulter Inc.), respectively.
Power calculation
In the absence of available 16:1n–7t supplementation data, sample size calculations were based on estimates of circulating fatty acid concentrations. The lowest dose for 16:1n–7t corresponded to the mean and the lowest dose of 16:1n–7c corresponded to the intermediate circulating concentrations. We took this approach because, compared with the concentration of 16:1n–7t, 16:1n–7c is relatively high and it can be synthesized endogenously. Based on observed associations between consumption of whole-fat dairy foods and PL 16:1n–7t concentrations, and the average content of 16:1n–7t in dairy foods, we hypothesized a potential 33%–50% increase in serum PL 16:1n–7t concentrations at the lowest dose, a 66%–100% increase at the middle dose, and a 100%–150% increase at the highest dose. Based on paired t testing, 90% power, and 2-sided α = 0.05, a sample size of 11 subjects was calculated to detect a 33% increase between baseline and the lowest-dose group. For serum PL 16:1n–7c, we anticipated a potential increase in concentrations of 20%–30% at the lowest dose, 40%–50% at the middle dose, and 60%–70% at the highest dose. Performing sample size calculations as above, a sample size of 11 subjects was calculated to detect a 40% increase between baseline and the middle-dose group. Assuming a potential dropout rate of 15%–30% for a 22-wk dietary supplement study, we enrolled 17 participants to ensure that ≥12 participants completed the study (Figure 1).
Statistical analysis
All data were tested for normality using the D'Agostino–Pearson normality test with α = 0.05, before statistical analysis (Prism 8, GraphPad Software). A paired-sample t test was conducted for the power calculation. Statistical significance for the primary outcome (serum PLFAs) and for the secondary outcomes (glucose homeostasis, serum lipids, and clinical measures) in both seabuckthorn oil augmented in 16:1n–7t and unmodified seabuckthorn oil across the 4 time points (baseline, phase 1, phase 2, phase 3) within each supplementation period was tested using a mixed-effects model ANOVA, and Tukey's test was conducted only when P values for ANOVA were <0.05 (Prism 8, GraphPad Software). Carryover effects across supplementation periods were assessed by Student's t test. The primary dose comparisons for PL 16:1n–7t and 16:1n–7c were baseline compared with 120 mg/d, and baseline compared with 760 mg/d, respectively, and the remaining dose comparisons were secondary dose comparisons. Labelled data with different superscripts indicate statistical differences from each other, and no additional adjustment was applied to individual PLFAs for multiple comparisons.
In a secondary analysis, we evaluated trends in changes in PL 16:1n–7t and 16:1n–7c across the dose-escalation by modeling the fatty acid dose as a continuous dependent variable in a linear regression model with time as a continuous linear predictor. Correlation of repeated measures within subject was accounted for by including a random effect in the model. For all tests, P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participants
The mean ± SD age of the participants was 48 ± 16 y, 54% were women, and mean ± SD BMI was 30.4 ± 3.7 (Table 2). Consistent with the general US population, waist circumference measures were higher than optimal, especially in women. On the basis of pill counts, compliance was high: 98.9%.
TABLE 2.
Demographic profile of study participants1
| Variables | Values (n = 13) |
|---|---|
| Age, y | 48 ± 16 |
| Women | 7 (53.9%) |
| Height, m | 167 ± 7.1 |
| Weight, kg | 85.7 ± 15.6 |
| BMI, kg/m2 | 30.4 ± 3.7 |
| Waist circumference,2 cm | 98 ± 13 |
| Women | 93 ± 15 |
| Men | 102 ± 8 |
| Hip circumference,2 cm | 106 ± 8 |
| Waist-to-hip ratio2 | 0.9 ± 0.1 |
| Systolic blood pressure, mm Hg | 117 ± 13 |
| Diastolic blood pressure, mm Hg | 75 ± 10 |
Values are means ± SDs or n (%).
n = 12 owing to missing values.
Serum PLFA concentrations
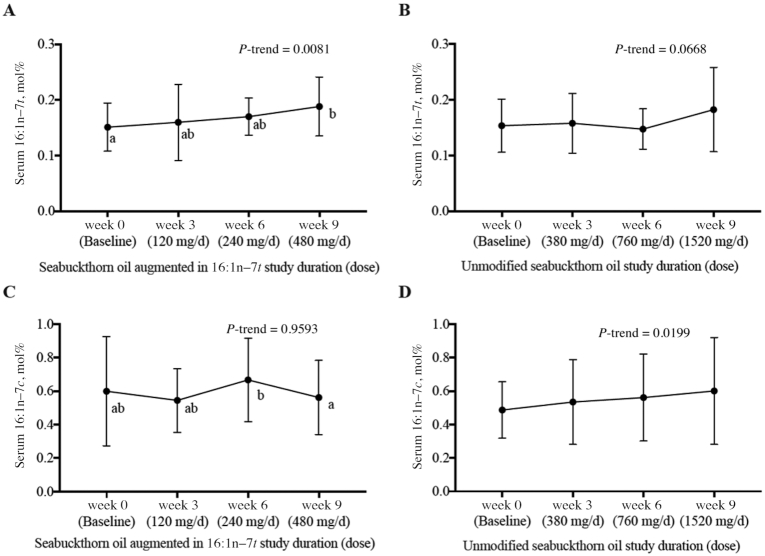
Supplementation with the seabuckthorn oil augmented in 16:1n–7t resulted in a 26% increase in PL 16:1n–7t (mol%) (Figure 2A) at the highest dose (480 mg/d) compared with baseline (P = 0.0343 by ANOVA; P-trend = 0.0081). The seabuckthorn oil augmented in 16:1n–7t did not consistently alter PL 16:1n–7c concentrations (Figure 2C). The seabuckthorn oil augmented in 16:1n–7t also increased PL 18:1n–7c by 16.3%; observed effects on other PLFAs were smaller and not dose-dependent (Table 3).
FIGURE 2.
Fasting serum phospholipid fatty acid concentrations at the end of each supplement phase in metabolically healthy adults. (A) 16:1n–7t in the seabuckthorn oil augmented in 16:1n–7t. (B) 16:1n–7t in the unmodified seabuckthorn oil. (C) 16:1n–7c in the seabuckthorn oil augmented in 16:1n–7t. (D) 16:1n–7c in the unmodified seabuckthorn oil. Values are means ± SDs in mol% (A, C: n = 13; B, D: n = 13 in weeks 0, 6, and 9; n = 12 in week 3 owing to limited sample volume). Labeled means without a common letter differ, P < 0.05.
TABLE 3.
Fasting serum phospholipid fatty acid concentrations during the dose-escalation course of the seabuckthorn oil augmented in 16:1n–7t in metabolically healthy adults1
| Fatty acid, mol% | Baseline | Week 3 (120 mg 16:1n–7t/d) | Week 6 (240 mg 16:1n–7t/d) | Week 9 (480 mg 16:1n–7t/d) | ANOVA P values |
|---|---|---|---|---|---|
| SFA | 47.5 ± 2.81 | 47.3 ± 3.76 | 47.4 ± 2.65 | 46.4 ± 1.88 | 0.358 |
| 10:0 | 0.01 ± 0.007 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.179 |
| 12:0 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.265 |
| 14:0 | 0.50 ± 0.12 | 0.45 ± 0.11 | 0.48 ± 0.11 | 0.41 ± 0.14 | 0.052 |
| 15:0 | 0.24 ± 0.05 | 0.25 ± 0.06 | 0.24 ± 0.04 | 0.23 ± 0.05 | 0.688 |
| 16:0 | 28.9 ± 2.36 | 28.8 ± 2.87 | 28.8 ± 2.07 | 28.2 ± 1.41 | 0.520 |
| 18:0 | 15.8 ± 1.79 | 15.7 ± 1.83 | 15.9 ± 1.63 | 15.5 ± 1.77 | 0.575 |
| 20:0 | 0.22 ± 0.16 | 0.24 ± 0.18 | 0.23 ± 0.13 | 0.22 ± 0.17 | 0.668 |
| 22:0 | 1.03 ± 0.16 | 1.06 ± 0.23 | 0.99 ± 0.18 | 1.02 ± 0.19 | 0.496 |
| 24:0 | 0.76 ± 0.12 | 0.81 ± 0.20 | 0.73 ± 0.11 | 0.78 ± 0.14 | 0.215 |
| MUFA | 11.7 ± 1.67 | 11.8 ± 1.60 | 12.3 ± 1.50 | 11.7 ± 1.85 | 0.405 |
| cis | |||||
| 14:1n–5 | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.107 |
| 16:1n–9 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.11 ± 0.04 | 0.995 |
| 16:1n–7 | 0.60 ± 0.33a,b | 0.54 ± 0.19a,b | 0.67 ± 0.25b | 0.56 ± 0.22a | 0.045 |
| 18:1n–9 | 8.61 ± 1.43 | 8.62 ± 1.46 | 9.00 ± 1.20 | 8.35 ± 1.64 | 0.395 |
| 18:1n–7 | 1.23 ± 0.16a | 1.31 ± 0.19a,b | 1.35 ± 0.23b | 1.43 ± 0.26b | 0.001 |
| 20:1n–9 | 0.11 ± 0.03 | 0.12 ± 0.03 | 0.11 ± 0.02 | 0.11 ± 0.03 | 0.178 |
| 22:1n–9 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.501 |
| 24:1n–9 | 1.03 ± 0.30 | 1.04 ± 0.19 | 0.99 ± 0.12 | 1.11 ± 0.21 | 0.465 |
| trans | |||||
| 16:1n–9 | 0.03 ± 0.009 | 0.03 ± 0.025 | 0.03 ± 0.009 | 0.03 ± 0.011 | 0.442 |
| 16:1n–7 | 0.15 ± 0.04a | 0.16 ± 0.07a,b | 0.17 ± 0.03a,b | 0.19 ± 0.05b | 0.034 |
| 18:1n–10–12 | 0.03 ± 0.02 | 0.03 ± 0.03 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.688 |
| 18:1n–9 | 0.15 ± 0.05 | 0.16 ± 0.06 | 0.14 ± 0.03 | 0.15 ± 0.05 | 0.832 |
| 18:1n–7 | 0.11 ± 0.04 | 0.13 ± 0.07 | 0.13 ± 0.02 | 0.11 ± 0.04 | 0.413 |
| n–6 PUFA | 35.9 ± 2.60 | 35.8 ± 3.30 | 35.2 ± 3.15 | 36.6 ± 2.64 | 0.297 |
| cis | |||||
| 18:2n–6 | 22.6 ± 2.59 | 22.4 ± 2.86 | 22.0 ± 3.17 | 22.3 ± 2.57 | 0.743 |
| 18:3n–6 | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.11 ± 0.08 | 0.08 ± 0.05 | 0.179 |
| 20:2n–6 | 0.28 ± 0.05 | 0.27 ± 0.04 | 0.27 ± 0.05 | 0.26 ± 0.05 | 0.624 |
| 20:3n–6 | 2.68 ± 0.71 | 2.55 ± 0.57 | 2.62 ± 0.73 | 2.45 ± 0.71 | 0.556 |
| 20:4n–6 | 9.69 ± 1.56a | 9.91 ± 2.66a,b | 9.7 ± 1.79a | 10.9 ± 2.08b | 0.014 |
| 22:2n–6 | 0.02 ± 0.008 | 0.02 ± 0.005 | 0.02 ± 0.007 | 0.02 ± 0.008 | 0.567 |
| 22:4n–6 | 0.31 ± 0.07 | 0.30 ± 0.08 | 0.32 ± 0.09 | 0.32 ± 0.08 | 0.519 |
| 22:5n–6 | 0.23 ± 0.04 | 0.22 ± 0.07 | 0.23 ± 0.08 | 0.24 ± 0.09 | 0.501 |
| trans | |||||
| 18:2n–6 | 0.16 ± 0.10 | 0.17 ± 0.15 | 0.16 ± 0.10 | 0.11 ± 0.10 | 0.505 |
| 18:2-CLA | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.352 |
| n–3 PUFA | 4.19 ± 0.67 | 4.43 ± 1.38 | 4.41 ± 1.15 | 4.72 ± 0.75 | 0.501 |
| 18:3n–3 | 0.22 ± 0.07 | 0.22 ± 0.09 | 0.21 ± 0.08 | 0.19 ± 0.06 | 0.619 |
| 18:4n–3 | 0.10 ± 0.07 | 0.11 ± 0.07 | 0.10 ± 0.07 | 0.08 ± 0.05 | 0.457 |
| 20:3n–3 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.084 |
| 20:5n–3 | 0.71 ± 0.3 | 0.70 ± 0.40 | 0.75 ± 0.40 | 0.67 ± 0.30 | 0.084 |
| 22:5n–3 | 0.68 ± 0.13 | 0.70 ± 0.21 | 0.72 ± 0.22 | 0.74 ± 0.10 | 0.716 |
| 22:6n–3 | 2.45 ± 0.69 | 2.66 ± 0.91 | 2.60 ± 0.68 | 3.00 ± 0.63 | 0.168 |
n = 13. Values are means ± SDs. Findings were analyzed by a mixed-effects model ANOVA followed by Tukey's test. Labeled means in a row without a common letter differ, P < 0.05. CLA, conjugated linoleic acid.
There was no significant effect of supplementation with unmodified seabuckthorn oil on mean PL 16:1n–7c concentrations (0.49% to 0.53% to 0.56% to 0.60% for doses of 380, 760, and 1520 mg 16:1n–7c/d, respectively) (Table 4); however, there was a positive trend across increasing doses (P-trend: 0.0199) (Figure 2D). The unmodified seabuckthorn oil increased concentrations of PL 18:1n–7c by 10.2% at the middle dose (760 mg/d), with inconsistent and non-dose-dependent effects on the other PLFAs.
TABLE 4.
Fasting serum phospholipid fatty acid concentrations during the dose-escalation course of the unmodified seabuckthorn oil supplement in metabolically healthy adults1
| Fatty acid, mol% | Baseline2 | Week 33 (380 mg 16:1n–7c/d) | Week 62 (760 mg 16:1n–7c/d) | Week 92 (1520 mg 16:1n–7c/d) | ANOVA P values |
|---|---|---|---|---|---|
| SFA | 47.4 ± 2.81b | 47.5 ± 2.72b | 44.9 ± 1.75a | 46.3 ± 2.18a,b | 0.008 |
| 10:0 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.691 |
| 12:0 | 0.04 ± 0.03 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.373 |
| 14:0 | 0.39 ± 0.12 | 0.44 ± 0.12 | 0.35 ± 0.09 | 0.43 ± 0.15 | 0.103 |
| 15:0 | 0.24 ± 0.04 | 0.24 ± 0.06 | 0.21 ± 0.03 | 0.23 ± 0.06 | 0.099 |
| 16:0 | 28.8 ± 2.41 | 29.0 ± 2.38 | 27.8 ± 1.96 | 28.4 ± 1.84 | 0.273 |
| 18:0 | 15.7 ± 1.66b | 15.8 ± 1.96b | 14.6 ± 1.93a | 15.3 ± 2.0a,b | 0.004 |
| 20:0 | 0.35 ± 0.16b | 0.23 ± 0.16a | 0.24 ± 0.15a | 0.24 ± 0.14a | 0.004 |
| 22:0 | 1.05 ± 0.16 | 1.03 ± 0.21 | 0.92 ± 0.17 | 0.98 ± 0.16 | 0.056 |
| 24:0 | 0.78 ± 0.16 | 0.80 ± 0.19 | 0.72 ± 0.14 | 0.74 ± 0.15 | 0.171 |
| MUFA | 11.0 ± 1.11a | 11.4 ± 1.52a | 12.3 ± 1.47b | 11.8 ± 1.72a,b | 0.003 |
| cis | |||||
| 14:1n–5 | 0.01 ± 0.004 | 0.01 ± 0.002 | 0.01 ± 0.003 | 0.01 ± 0.005 | 0.676 |
| 16:1n–9 | 0.11 ± 0.03 | 0.11 ± 0.04 | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.094 |
| 16:1n–7 | 0.49 ± 0.17 | 0.53 ± 0.25 | 0.56 ± 0.26 | 0.60 ± 0.32 | 0.159 |
| 18:1n–9 | 7.86 ± 0.97a | 8.25 ± 1.49a | 9.05 ± 1.18b | 8.50 ± 1.29a,b | 0.004 |
| 18:1n–7 | 1.28 ± 0.22a | 1.29 ± 0.17a,b | 1.39 ± 0.24b | 1.41 ± 0.23a,b | 0.033 |
| 20:1n–9 | 0.11 ± 0.03 | 0.11 ± 0.03 | 0.11 ± 0.02 | 0.12 ± 0.03 | 0.603 |
| 22:1n–9 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.130 |
| 24:1n–9 | 1.09 ± 0.35 | 1.08 ± 0.33 | 1.06 ± 0.20 | 1.04 ± 0.17 | 0.837 |
| trans | |||||
| 16:1n–9 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.115 |
| 16:1n–7 | 0.15 ± 0.05 | 0.16 ± 0.05 | 0.15 ± 0.04 | 0.18 ± 0.08 | 0.323 |
| 18:1n–10–12 | 0.02 ± 0.02 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.236 |
| 18:1n–9 | 0.14 ± 0.05 | 0.15 ± 0.05 | 0.14 ± 0.06 | 0.17 ± 0.07 | 0.341 |
| 18:1n–7 | 0.11 ± 0.04 | 0.12 ± 0.05 | 0.10 ± 0.04 | 0.14 ± 0.07 | 0.079 |
| n–6 PUFA | 36.5 ± 2.79 | 35.9 ± 2.74 | 37.3 ± 2.15 | 36.6 ± 2.51 | 0.373 |
| cis | |||||
| 18:2n–6 | 22.6 ± 2.63 | 22.3 ± 3.42 | 23.3 ± 2.36 | 22.8 ± 2.14 | 0.633 |
| 18:3n–6 | 0.08 ± 0.04 | 0.11 ± 0.07 | 0.09 ± 0.04 | 0.10 ± 0.05 | 0.299 |
| 20:2n–6 | 0.26 ± 0.05 | 0.28 ± 0.05 | 0.27 ± 0.06 | 0.27 ± 0.05 | 0.694 |
| 20:3n–6 | 2.64 ± 0.71 | 2.60 ± 0.76 | 2.57 ± 0.84 | 2.56 ± 0.68 | 0.904 |
| 20:4n–6 | 10.3 ± 2.45 | 10.2 ± 2.51 | 10.6 ± 1.87 | 10.4 ± 2.00 | 0.714 |
| 22:2n–6 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.669 |
| 22:4n–6 | 0.33 ± 0.12 | 0.32 ± 0.08 | 0.33 ± 0.07 | 0.33 ± 0.09 | 0.697 |
| 22:5n–6 | 0.22 ± 0.07 | 0.23 ± 0.05 | 0.22 ± 0.06 | 0.23 ± 0.07 | 0.748 |
| trans | |||||
| 18:2n–6 | 0.13 ± 0.12 | 0.15 ± 0.09 | 0.07 ± 0.05 | 0.13 ± 0.12 | 0.106 |
| 18:2-CLA | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.466 |
| n–3 PUFA | 4.59 ± 1.03 | 4.50 ± 1.04 | 4.93 ± 0.78 | 4.54 ± 0.63 | 0.456 |
| 18:3n–3 | 0.17 ± 0.06 | 0.19 ± 0.07 | 0.19 ± 0.08 | 0.19 ± 0.05 | 0.461 |
| 18:4n–3 | 0.09 ± 0.07 | 0.09 ± 0.03 | 0.08 ± 0.04 | 0.08 ± 0.06 | 0.646 |
| 20:3n–3 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.897 |
| 20:5n–3 | 0.79 ± 0.30 | 0.78 ± 0.34 | 0.88 ± 0.50 | 0.71 ± 0.25 | 0.633 |
| 22:5n–3 | 0.76 ± 0.24 | 0.70 ± 0.14 | 0.78 ± 0.13 | 0.72 ± 0.17 | 0.353 |
| 22:6n–3 | 2.74 ± 0.84 | 2.69 ± 0.75 | 2.96 ± 0.58 | 2.80 ± 0.71 | 0.554 |
Values are means ± SDs. Findings were analyzed by a mixed-effects model ANOVA followed by Tukey's test. Labeled means in a row without a common letter differ, P < 0.05. CLA, conjugated linoleic acid.
n = 13.
n = 12 owing to limited sample volume in week 3.
No significant carryover effect (P > 0.05) was observed in fasting PLFAs between randomization periods for either the seabuckthorn oil augmented in 16:1n–7t or the unmodified seabuckthorn oil.
We evaluated PLFA measures in absolute concentrations (μmol/L), which are jointly influenced by fatty acid composition as well as absolute concentrations in the fasting state. Supplementation with the oil augmented in 16:1n–7t produced dose-dependent increases in PL 16:1n–7t concentrations, 15% at the highest dose, compared with baseline (P = 0.0471) (Supplemental Table 1). Change in serum PL 18:3n–6c was not consistent or dose-dependent. Supplementation with the unmodified seabuckthorn oil did not significantly alter PL 16:1n–7c concentrations (by ANOVA, P = 0.27; P-trend = 0.06) and produced inconsistent and non-dose-dependent changes in other fatty acids (Supplemental Table 2).
Safety outcomes
No significant effects of either supplement were observed in serum glucose, serum insulin, and lipid concentrations (Supplemental Figure 2). Likewise, no significant effect of the supplements was observed in clinical chemistry measures, with 1 exception: a 4% increase in serum lymphocytes (P = 0.0298) after the highest 16:1n–7t supplement dose (480 mg/d) (Supplemental Tables 3 and 4). All the markers were within their normal ranges, and no carryover effect on the basis of random assignment sequence was observed. No adverse events were reported by any of the participants.
Discussion
Dietary 16:1n–7c and 16:1n–7t are fatty acid isomers that occur naturally in foods at relatively low concentrations. Supplementation with 16:1n–7c in experimental animal models inhibits hepatic de novo lipogenesis and improves insulin sensitivity, inflammation, and plasma lipid profiles (19). Experimental studies of 16:1n–7t in humans have not, to our knowledge, been reported. In this randomized dose-escalation intervention trial, supplementation with seabuckthorn oil augmented in 16:1n–7t modestly increased PLFA concentrations. Supplementation with unmodified seabuckthorn oil had smaller effects that were only statistically significant in a secondary analysis of the trend across escalating doses. Based on the clinical chemistry measures and subject reports, no adverse effects or supplement intolerance were identified.
These findings build upon and expand the prior literature by assessing the dose–response relations of unmodified seabuckthorn oil or seabuckthorn oil augmented in 16:1n–7t with PLFA concentrations. An understanding of these relations is a critical foundation for pursuing a more thorough evaluation of biological effects of these 16:1n–7 isomers in human studies.
Few prior trials have assessed potential effects of 16:1n–7c in humans. Among 60 adults with dyslipidemia and mildly elevated high-sensitivity C-reactive protein (hs-CRP) concentrations, 220 mg/d 16:1n–7c for 30 d, compared with placebo, was reported to reduce concentrations of hs-CRP, TG, and LDL cholesterol by 44%, 15%, and 8%, respectively, and increased HDL cholesterol by 5% (5). This dose was below the lower dose used in the current study. These findings could have been confounded, however, by the placebo formulation, which was not inert but contained 1000 mg medium-chain saturated fats, predominantly caprylic acid and capric acid. Among 20 patients with ulcerative colitis, provision of 720 mg/d 16:1n–7c for 8 wk, compared with placebo, was reported to reduce hs-CRP concentrations by 25% and to increase erythrocyte sedimentation rate levels by 29%, with accompanying upregulation of hepatocyte nuclear factor 4 gamma (HNF4γ) and hepatocyte nuclear factor 4 alpha (HNF4α) gene expression and downregulation of interleukin 6 (IL6) gene expression (20). The dose was in the mid-range of that used in the current study.
With regard to 16:1n–7t, no published human trials were identified. This is partly due to the lack of currently available dietary supplements containing 16:1n–7t. To our knowledge, 16:1n–7t has never before been developed nor administered as a dietary supplement. In our study, partial hydrogenation of seabuckthorn oil increased the 16:1n–7t level from undetectable to 9.6%, 16:1n–9t from undetectable to 5.7%, and total 18:1t from undetectable to 16.7%. These increases were at the expense of 16:1n–7c, which declined from 35.5% to 22.5%; 18:1n–7c, which declined from 7.7% to 4.5%; and 18:1n–9c, which declined from 16.9% to 1.4%. This amount of enrichment of 16:1n–7t allowed for a dose-escalation of 0 to 480 mg/d. At the highest dose, we observed a 15.0% increase in PL 16:1n–7t concentrations, achieving a mean concentration of 0.19 mol%, although some participants achieved much higher values (≤0.27%). In observational cohorts, those with PL 16:1n–7t concentrations at the 90th percentile, between 0.10% and 0.26%, were at significantly lower risk of T2D (11, 14).
16:1n–7t is naturally present in very small quantities in dairy products. Most prior reports assessing trans fat in dairy products have focused on trans-18 isomers. One report found that total trans-16 isomers represented a range of between 0.20% and 0.65% of total milk fatty acids (14). Of these, one-third (33%) were 16:1n–7t, or ∼0.066%–0.21% of total milk fatty acids. Assuming the value may be as high as 0.5%, our highest dose of 480 mg/d could correspond to about 96 g dairy fat, or ∼12 glasses of full-fat milk per day. However, this estimated correspondence has considerable uncertainty, because the quality and precision of these prior dairy compositional data are not high. Thus, amounts of 16:1n–7t in dairy foods and in the diet are not well established, and the present dosing study represents an advance in determining the circulating fatty acid response to 16:1n–7t supplementation doses at and possibly above dietary ranges.
Naturally occurring seabuckthorn oil contains ∼35.5% 16:1n–7c. This allowed for a dose-escalation from 0 to 1520 mg/d, considerably higher than doses used in 2 prior human trials (220 mg/d and 760 mg/d) (11, 14). Also present in the seabuckthorn oil were 7.7% 18:1n–7c, 16.9% 18:1n–9c, and no detectable 16:1n–9c, 16:1n–7t, 16:1n–9t, or 18:1t. After 9 wk escalating supplementation with the unmodified seabuckthorn oil, only a modest trend in mean PL 16:1n–7c concentrations was seen. PL 18:1n–7c also modestly increased, consistent with the seabuckthorn oil content of this fatty acid. As a percentage of baseline concentrations, at the highest supplement dose, PL 18:1n–7c increased by 10.2% and PL 16:1n–7c by 22.4%. This suggests that the absence of statistical significance using ANOVA for the latter could relate to larger variation in background PL 16:1n–7c concentrations, perhaps related to varying hepatic de novo lipogenesis or other determinants. Our results highlight the need for further investigation of the pharmacokinetics and dose–response relations of 16:1n–7c supplementation.
In in vitro studies, 16:1n–7c upregulates gene expression of ELOVL5, the gene encoding the enzyme fatty acid elongase 5, which plays an important role in the metabolism of long-chain unsaturated fatty acids (21, 22). In the present study, PL 18:1n–7c, the product of the reaction for elongation of 16:1n–7c, was significantly increased after the participants received both supplements, albeit not in a dose-dependent manner. Similar increases in PL 18:1n–7t were not observed in response to either supplement, consistent with an absence of significant de novo synthesis of 16:1n–7t (23).
Our investigation has several strengths. We used a standard dose-escalation design, with randomization of the cis and trans study periods as well as an intervening washout period, to diminish potential carryover effects. A broad range of doses was utilized, including doses of 16:1n–7c higher than those in the few prior trials. Pill count suggested a high level of compliance with the study protocol.
Potential limitations include the variation in background PL 16:1n–7c concentrations among participants, which may have limited the statistical power to identify a significant response in this fatty acid using ANOVA. In addition, no adjustment was applied to account for multiple testing across fatty acids in order to minimize type II error, but there is an increased risk of type I error.
The serum phospholipid fraction was selected for analysis because the majority of available data for circulating fatty acids and cardiovascular disease risk/diabetes risk is for this lipid fraction. Although it is unlikely, the possibility cannot be ruled out that the results may have been different had another lipid fraction or RBCs been chosen.
Owing to the technical limitations of partial hydrogenation and existing natural substrates, we could not directly compare the effects of 16:1n–7t and 16:1n–7c on the basis of a one-to-one molar dose-escalation. However, we demonstrated the feasibility of assessing the PLFA response to the preparations available. Because whole full-fat dairy products contain 16:1n–7t, a change in dietary intake during the intervention period could have confounded the study results. To evaluate this possibility, concentrations of PL 15:0 and conjugated linoleic acid (18:2-CLA) (24, 25), 2 biomarkers of full-fat dairy intake, were monitored: no significant changes were observed over the 9-wk supplementation period.
In conclusion, supplementation with a seabuckthorn oil augmented in 16:1n–7t increased its corresponding PLFA in a dose-dependent manner. Supplementation with unmodified seabuckthorn oil also modestly increased its corresponding PLFA, although this observation was statistically significant as a trend only. No adverse effects were apparent. Given the evidence linking palmitoleic acid and metabolic health, these findings provide important dose-response information on 16:1n–7 isomers to inform the design of future interventional trials in humans.
Supplementary Material
Acknowledgments
We acknowledge the assistance of the Metabolic Research Unit and Nutrition Evaluation Laboratory. We are grateful to Peter Zock at Unilever for developing and donating free of charge the unmodified seabuckthorn oil and seabuckthorn oil augmented in 16:1n–7t supplements for this trial. The authors’ responsibilities were as follows—NKH: performed the data analysis, interpretation, and wrote the initial draft of the manuscript; NRM, AHL, and DM: designed the research; PS: performed the statistical analysis; NRM, AHL, JMG, and DM: conducted the research; NKH, AHL, and DM: had primary responsibility for the final content of the manuscript; and all authors: contributed to the critical review of the manuscript and read and approved the final manuscript.
Notes
Supported by National Heart, Lung, and Blood Institute of the NIH grant 2R01HL085710 (to DM), the Harvard TH Chan School of Public Health (to DM), and USDA-Agricultural Research Service agreement no. 58-1950-4-401 (to AHL).
Author disclosures: DM reports personal fees from GOED, Nutrition Impact, Bunge, Indigo Agriculture, Amarin, Acasti Pharma, Cleveland Clinic Foundation, America's Test Kitchen, and Danone; being a scientific advisory board member for DayTwo, Elysium Health, Filtricine, and Omada Health; and chapter royalties from UpToDate; all outside of the submitted work. Tufts University holds patents US8889739 and US9987243 (unlicensed), listing DM as a co-inventor, for use of trans-palmitoleic acid to prevent and treat insulin resistance, type 2 diabetes, and related conditions, as well as reduce metabolic risk factors. All other authors report no conflicts of interest.
The funding organizations and Unilever were not involved in the study design, implementation, analysis, manuscript drafting, or decision to submit the manuscript for publication. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the NIH, USDA, Harvard University, or Tufts University.
Supplemental Tables 1–4 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
The data (in de-identified form, if human data) used in the article, code book, and analytic code will be made available to editors upon request either before or after publication for checking.
Abbreviations used: HNF, hepatocyte nuclear factor; HNF4α, hepatocyte nuclear factor 4 alpha; HNF4γ, hepatocyte nuclear factor 4 gamma; HNRCA, Human Nutrition Research Center on Aging at Tufts; hs-CRP, high-sensitivity C-reactive protein; IL6, interleukin 6; PCP, primary care provider; PL, phospholipid; PLFA, phospholipid fatty acid; TG, triglyceride; T2D, type 2 diabetes.
References
- 1. Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. 2019;16(10):581–601. [DOI] [PubMed] [Google Scholar]
- 2. Frigolet ME, Gutiérrez-Aguilar R. The role of the novel lipokine palmitoleic acid in health and disease. Adv Nutr. 2017;8:173S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene Jet al.. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duckett SK, Volpi-Lagreca G, Alende M, Long NM. Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep. Diabetes Metab Syndr Obes. 2014;7:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang ZH, Pryor M, Noguchi A, Sampson M, Johnson B, Pryor M, Donkor K, Amar M, Remaley AT. Dietary palmitoleic acid attenuates atherosclerosis progression and hyperlipidemia in low-density lipoprotein receptor-deficient mice. Mol Nutr Food Res. 2019;63:1900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernstein AM, Roizen MF, Martinez L. Purified palmitoleic acid for the reduction of high-sensitivity C-reactive protein and serum lipids: a double-blinded, randomized, placebo controlled study. J Clin Lipidol. 2014;8:612–7. [DOI] [PubMed] [Google Scholar]
- 7. Yang ZH, Miyahara H, Hatanaka A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay mice with genetic type 2 diabetes. Lipids Health Dis. 2011;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maguire LS, O'Sullivan SM, Galvin K, O'Connor TP, O'Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–8. [DOI] [PubMed] [Google Scholar]
- 9. Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr. 2010;91:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr. 2010;92:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. trans-Palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010;153:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Wong Ket al.. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15:e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirahatake KM, Slavin JL, Maki KC, Adams SH. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions. Metabolism. 2014;63:618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walker ME, Matthan NR, Goldbaum A, Meng H, Lamon-Fava S, Lakshman S, Jang S, Molokin A, Solano-Aguilar G, Urban JF Jret al.. Dietary patterns influence epicardial adipose tissue fatty acid composition and inflammatory gene expression in the Ossabaw pig. J Nutr Biochem. 2019;70:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 19. Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bueno-Hernández N, Sixtos-Alonso MS, Milke García MDP, Yamamoto-Furusho JK. Effect of cis-palmitoleic acid supplementation on inflammation and expression of HNF4γ, HNF4α and IL6 in patients with ulcerative colitis. Minerva Gastroenterol Dietol. 2017;63:257–63. [DOI] [PubMed] [Google Scholar]
- 21. Shi HB, Du Y, Zhang CH, Sun C, He YL, Wu YH, Liu JX, Luo J, Loor JJ. Fatty acid elongase 5 (ELOVL5) alters the synthesis of long-chain unsaturated fatty acids in goat mammary epithelial cells. J Dairy Sci. 2018;101:4586–94. [DOI] [PubMed] [Google Scholar]
- 22. Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res. 2009;50:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mozaffarian D, Kabagambe EK, Johnson CO, Lemaitre RN, Manichaikul A, Sun Q, Foy M, Wang L, Wiener H, Irvin MRet al.. Genetic loci associated with circulating phospholipid trans fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. Am J Clin Nutr. 2015;101:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hennessy AA, Ross PR, Fitzgerald GF, Stanton C. Sources and bioactive properties of conjugated dietary fatty acids. Lipids. 2016;51:377–97. [DOI] [PubMed] [Google Scholar]
- 25. Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998;68:291–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.