ABSTRACT
Background
Many cancer patients initiate dietary supplement use after cancer diagnosis. How dietary supplement use contributes to the total nutrient intake among cancer survivors as compared with individuals without cancer needs to be determined.
Objectives
We aimed to evaluate nutrient intakes from dietary supplements among cancer survivors in relation to their total nutrient intake and compare those with individuals without cancer.
Methods
We evaluated the prevalence, dose, and reason for using dietary supplements among 2772 adult cancer survivors and 31,310 individuals without cancer who participated in the NHANES 2003–2016.
Results
Cancer survivors reported a higher prevalence of any (70.4% vs. 51.2%) and multivitamin/mineral (48.9% vs. 36.6%) supplement use and supplement use of 11 individual vitamins and 8 minerals than individuals without cancer. Overall, cancer survivors had significantly higher amounts of nutrient intake from supplements but lower nutrient intakes from foods for the majority of the nutrients. Compared with individuals without cancer, cancer survivors had a higher percentage of individuals with inadequate intake (total nutrient intake <Estimated Average Requirement or Adequate Intake) for folate, vitamin B-6, niacin, calcium, copper, and phosphorus, due to lower intakes of these nutrients from foods. Cancer survivors also had a higher proportion of individuals with excess intake (total nutrient intake ≥Tolerable Upper Intake Level) for vitamin D, vitamin B-6, niacin, calcium, magnesium, and zinc, contributed by higher intakes of these nutrients from dietary supplements. Nearly half (46.1%) used dietary supplements on their own without consulting health care providers.
Conclusions
Cancer survivors reported a higher prevalence and dose of dietary supplement use but lower amounts of nutrient intake from foods than individuals without cancer. The inadequate nutrient intake from foods and the short-term and long-term health impact of dietary supplement use, especially at high doses, need to be further evaluated among cancer survivors.
Keywords: dietary supplement use, cancer survivors, nutrients, inadequate intake, excess intake
Introduction
Patients with cancer often initiate dietary changes after cancer diagnosis and treatment, including the use of dietary supplements (1). It has been estimated that 15–50% of cancer patients initiate new supplement use after diagnosis (2, 3). The nutrition guidelines recommend that cancer survivors achieve adequate nutrition through a healthy balanced diet rather than relying on supplements (4). However, cancer survivors may still choose to take dietary supplements for improving their nutritional intake or overall health, and some may initiate dietary supplement use without consulting their health care providers (5, 6). Given that large doses of supplement use may interact with cancer treatments and potentially result in long-term health risks (7, 8), it is important to quantify the dose of dietary supplement use among cancer survivors, and assess how supplement use contributes to the total nutrient intake of cancer survivors. In addition, factors associated with dietary supplement use among cancer survivors need to be further evaluated.
Although a high prevalence of dietary supplement use (50–85%) has been reported in prior cohorts of breast, prostate, and colorectal cancer survivors (2, 9–11), few studies have assessed dietary supplement use among cancer survivors who participated in the recent cycles of the NHANES (6, 9). In particular, whether cancer survivors also use dietary supplements at a higher dose than individuals without a history of cancer diagnosis (individuals without cancer) has not been previously determined. In a representative sample of US adults who participated in the recent cycles of NHANES (2003–2016), we examined and compared the prevalence and dose of dietary supplement use among cancer survivors and individuals without cancer. In addition, we assessed whether supplement use contributed to a lower prevalence of inadequate nutrient intake and/or a higher prevalence of excess nutrient intake in cancer survivors compared with individuals without cancer. We further assessed factors associated with dietary supplement use and reasons for dietary supplement use among cancer survivors. Our findings will inform evidence-based guidelines for improving the nutritional intake among cancer survivors.
Methods
Study design and population
We used data from adults aged ≥20 y who participated in the 7 cycles (2003–2004 through 2015–2016) of the NHANES, a nationally representative, cross-sectional survey conducted by the National Center for Health Statistics (NCHS) of the CDC. The NHANES assesses information on health and nutritional status of noninstitutionalized civilian subjects residing in the United States. This survey program was approved by the research ethics review board of NCHS, and all participants provided written informed consent. Participants were classified as cancer survivors if they responded “yes” to the interview question: “Have you ever been told by a doctor or other health professional that you had a cancer or malignancy of any kind?” Individuals who responded “no” to this question from the same NHANES cycles (2003–2016) were included as individuals without cancer. We excluded pregnant or lactating women (n = 369) and individuals who reported a diagnosis of nonmelanoma skin cancer (n = 547). This resulted in 3092 cancer survivors and 35,143 individuals without cancer. Of these, all cancer survivors and 35,106 individuals without cancer provided complete information on dietary supplement use in the previous 30 d, among whom 2772 (89.7%) cancer survivors and 31,310 (89.2%) individuals without cancer who also had ≥1 valid 24-h diet recall were included as the study population of this analysis (Supplemental Figure 1).
Prevalence and dose of dietary supplement use
The NHANES participants were asked whether they used any dietary supplements in the past 30 d during an in-house interview. For those who reported supplement use, they were asked about the product name and the frequency (e.g., how many times in a day), duration (e.g., how many days in the past 30 d), and serving form (e.g., capsules, tablets, pills, soft-gels, drops, or other forms) of supplement use. For each nutrient, the daily dose was calculated by combining the frequency (e.g., the number of capsules taken in each day) with the product information on ingredient (e.g., vitamin D, calcium), amount of ingredient per serving (e.g., 0–1,000,000), and ingredient unit (e.g., International Units, milligrams). Nutrient intake from each product was summed to estimate the total daily dose of each supplemental nutrient for each individual (12). Detailed information on the methods used to estimate the dose of supplement use is provided in the supplemental material (Supplemental Methods).
Nutrient intake from foods
Dietary intake of nutrients from foods was assessed using 24-h dietary recalls conducted by trained interviewers. From 1999 to 2002, one 24-h diet recall was conducted in-person in the Mobile Examination Center (MEC); from 2003 to 2016, a second recall was added by telephone interview ∼3–10 d after the first recall. Participants with ≥1 valid 24-h dietary recall were included in the analysis to estimate nutrient intake from foods. Using the Automated Multiple Pass Method, all foods and beverages consumed during the previous day were recorded. A standard set of measuring guides was used to help the respondent report the volume and dimension of the food items consumed. Food intakes were coded and nutrient values were determined using the USDA Food and Nutrient Database for Dietary Studies (FNDDS), versions 1.0–5.0.
Reasons for taking dietary supplements
For 5 NHANES survey cycles, 2007–2008 to 2015–2016, NHANES participants were asked whether they took dietary supplements based on their own decision or advised by a doctor. Respondents were further asked to provide reasons for taking supplements for ≥1 reasons such as whether they took supplements “to improve my overall health,” “to maintain health,” “for bone health,” or other reasons. The percentage of cancer survivors and individuals without cancer using dietary supplements was estimated for each of the reasons asked in the Dietary Supplement Questionnaire (DSQ). For a participant who used multiple products, if he/she indicated the same reason (e.g., reason A) for using different products, he/she was considered to be using dietary supplements for reason A only once.
Demographic and lifestyle factors
Demographic and lifestyle factors including age, sex, race/ethnicity, education, income, smoking, alcohol intake, and physical activity were collected during household interviews. Body weight, and height were obtained during physical examinations at the MEC. Smokers were defined as participants who reported smoking ≥100 cigarettes during their lifetime, with former smokers defined as participants who reported smoking ≥100 cigarettes but not currently smoking. Drinkers were defined as participants who drank ≥12 alcohol drinks in any given year. Moderate versus heavy drinkers were defined as participants who consumed <1 versus ≥1 drink/d for women and <2 versus ≥2 drinks/d for men. Participants who had ≥150 min of moderate-to-vigorous physical activities/wk were classified as being physically active, and physically inactive otherwise (13). Diet quality was assessed using the Healthy Eating Index–2015, which measures the adherence to the 2015 Dietary Guidelines for Americans (14). A higher score corresponds to a healthier diet. For cancer survivors, time since diagnosis was calculated as the interval from the year of cancer diagnosis to the year that they completed the DSQ that assesses dietary supplement use.
Statistical analysis
We estimated the prevalence of any supplement use, multivitamin/mineral (MVM) supplement use, and the supplement use of 24 individual nutrients, including 12 vitamins, 8 minerals, and 4 nonvitamin, nonmineral supplements among cancer survivors and compared the prevalence with that in individuals without cancer using the chi-square test. MVM supplement use was defined as using supplements for ≥3 vitamins with or without minerals (9, 15). We then estimated and compared the mean daily dose of supplement use between cancer survivors and individuals without cancer using ANOVA. We further estimated and compared the mean nutrient intake from foods between the 2 groups. To estimate the total nutrient intake from foods and supplements combined, we used the recommended “shrink then add” method (16). We first used the National Cancer Institute method to estimate the usual nutrient intake from foods, and then added nutrient intake from supplements to the estimated usual intake from foods (Supplemental Methods) (16–19). We then estimated the proportions of cancer survivors and individuals without cancer with inadequate or excess nutrient intake. Inadequate nutrient intake was defined as amounts of total nutrient intake below the Estimated Average Requirements (EARs) or Adequate Intakes (AIs) according to the DRIs (20). Excess nutrient intake was defined as amounts of total nutrient intake above the Tolerable Upper Intake Levels (ULs) defined in the DRIs (Supplemental Table 1) (20). The proportion of inadequate or excess nutrient intake was estimated by comparing the total nutrient intake to the EAR or AI (for inadequate intake) or to the UL (for excess intake), and the proportion was compared between the cancer survivors and individuals without cancer using the chi-square test. We adjusted the P values for multiple comparisons using the Benjamini and Hochberg procedure (21). We additionally adjusted for age, sex, and race/ethnicity for the above comparisons as supplemental analyses. In addition, we assessed whether dietary supplement use among cancer survivors was associated with demographic, lifestyle factors, and cancer-related characteristics using ANOVA for continuous variables and the chi-square test for categorical variables. Multivariable logistic regression models were used to estimate the ORs and 95% CIs for factors associated with dietary supplement use. Last, we assessed and compared the reasons for dietary supplement use among cancer survivors and individuals without cancer who participated in 5 cycles of NHANES (2007–2016).
We accounted for the complex survey design in all analyses, including examination sample weights, which adjusted survey nonresponses and day of the week (22). Balanced repeated replication (BRR) weights were generated from dietary day 1 sample weights using Fay's BRR method and used in estimating variances (23, 24). All analyses were 2-sided and significance was considered at the α level of 0.05. A cutoff of false discovery rate of 10% was used to determine significance. SAS version 9.4 (SAS Institute, Inc.), was used for all analysis.
Results
Characteristics of cancer survivors and individuals without cancer from a nationally representative sample of US adults are described in Table 1. Cancers of the breast, prostate, colorectum, and lung were the primary diagnoses, accounting for ∼40% of all cancer types. The mean time since diagnosis was ∼11 y. Approximately one-third of the cancer survivors had been diagnosed with cancer within 5 y of completing the questionnaire assessing dietary supplement use. Compared with individuals without cancer, cancer survivors were older, more likely to be female, non-Hispanic whites, former smokers, nondrinkers, and physically inactive, and had a higher level of income and better diet quality.
TABLE 1.
Characteristics of adult cancer survivors and individuals without cancer, NHANES 2003–20161
| Characteristics | Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | P |
|---|---|---|---|
| Age, y | 62.3 ± 0.38 | 45.5 ± 0.22 | <0.01 |
| Age groups, n (%) | <0.01 | ||
| 20–39 y | 196 (8.51) | 11,532 (39.5) | |
| 40–49 y | 224 (11.8) | 5634 (20.9) | |
| 50–59 y | 353 (18.1) | 4895 (18.4) | |
| 60–69 y | 666 (24.1) | 4824 (12.0) | |
| 70–79 y | 782 (24.2) | 2797 (6.14) | |
| 80–85 y | 551 (13.4) | 1628 (3.04) | |
| Sex, n (%) | <0.01 | ||
| Male | 1262 (40.0) | 15,388 (49.1) | |
| Female | 1510 (60.0) | 15,922 (50.9) | |
| Race/ethnicity, n (%) | <0.01 | ||
| Non-Hispanic white | 1844 (84.7) | 13,297 (67.1) | |
| Non-Hispanic black | 450 (6.69) | 6855 (11.8) | |
| Hispanic | 351 (4.89) | 8326 (14.2) | |
| Other | 127 (3.76) | 2832 (6.94) | |
| Education, n (%) | 0.33 | ||
| Less than high school | 690 (15.8) | 8238 (17.1) | |
| High school graduate/GED or equivalent | 649 (22.6) | 7296 (23.5) | |
| Some college | 783 (31.9) | 8984 (31.5) | |
| College graduate or above | 648 (29.7) | 6764 (27.9) | |
| Family income to poverty ratio,2n (%) | 0.02 | ||
| <1.30 | 895 (23.2) | 11,639 (26.2) | |
| 1.30–2.99 | 880 (28.3) | 9091 (27.3) | |
| 3.00–4.99 | 495 (22.1) | 5617 (22.9) | |
| ≥5.00 | 502 (26.4) | 4963 (23.6) | |
| Smoking,3n (%) | <0.01 | ||
| Nonsmokers | 1212 (43.4) | 17,239 (54.9) | |
| Former smokers | 1104 (39.0) | 7241 (23.2) | |
| Current smokers | 454 (17.6) | 6813 (21.9) | |
| Alcohol4 (drinks/wk), n (%) | <0.01 | ||
| Nondrinkers | 1320 (42.7) | 11,621 (32.5) | |
| Moderate drinkers | 1202 (50.5) | 15,551 (58.9) | |
| Heavy drinkers | 132 (6.82) | 2121 (8.61) | |
| Physical activity,5n (%) | <0.01 | ||
| Active | 1296 (50.8) | 17,351 (59.9) | |
| Inactive | 1476 (49.2) | 13,952 (40.1) | |
| HEI-20156 | 60.0 ± 0.32 | 56.9 ± 0.17 | <0.01 |
| HEI-2015 quartile,6n (%) | <0.01 | ||
| Q1 (0–50.6) | 195 (14.5) | 3900 (25.1) | |
| Q2 (50.7–56.3) | 312 (20.4) | 4295 (24.7) | |
| Q3 (56.4–62.4) | 441 (27.9) | 4765 (25.2) | |
| Q4 (62.5–100) | 643 (37.2) | 5016 (25.0) | |
| BMI,7 kg/m2 | 29.1 ± 0.17 | 28.8 ± 0.08 | 0.24 |
| Weight status, n (%) | 0.43 | ||
| Underweight (BMI <18.5) | 43 (1.62) | 479 (1.59) | |
| Normal weight (BMI = 18.5–24.9) | 716 (27.3) | 8606 (29.3) | |
| Overweight (BMI = 25.0–29.9) | 966 (34.0) | 10,287 (33.1) | |
| Obese (BMI ≥30.0) | 1000 (37.2) | 11,584 (36.0) | |
| Primary diagnosis, n (%) | |||
| Breast cancer | 475 (17.2) | – | |
| Prostate cancer | 470 (10.7) | – | |
| Colon cancer | 246 (7.04) | – | |
| Lung cancer | 69 (2.49) | – | |
| Other cancer | 1512 (62.5) | – | |
| Time since diagnosis, y | 11.4 ± 0.29 | – | |
| Time since diagnosis, n (%) | |||
| 0–4.99 y | 924 (31.1) | – | |
| 5.00–9.99 y | 637 (23.0) | – | |
| ≥10.0 y | 1211 (45.8) | – |
Values are means ± SEss or number of observations (n) and percentages (%). All values were adjusted for survey weights. GED, General Education Development; HEI, Healthy Eating Index; MVPA, moderate-to-vigorous physical activity.
Represents the ratio of family income, the federal poverty threshold, adjusting for household size. For reference, the federal threshold in 2014 for a family of 4 was $23,850/y. A family of 4 earning $44,123/y would have a ratio of 1.85.
Smokers were defined as individuals who reported smoking ≥100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking.
Moderate drinkers were defined as individuals who reported drinking alcohol ≤1 drink/d for women and ≤2 drinks/d for men.
Minutes per week of MVPA was calculated by summarizing minutes, with vigorous physical activities weighted at 2 min for each minute of vigorous physical activity. Participants were classified as physically active if MVPA they met or exceeded 150 min/wk, according to the CDC Physical Activity Guidelines for Americans (13).
HEI-2015 measures adherence to the 2015 Dietary Guidelines for Americans, with higher scores corresponding to higher adherence (14). HEI-2015 scores were categorized based on quartiles of HEI-2015 among individuals without cancer.
BMI was calculated by dividing weight in kilograms by height in meters squared. Participants were classified as underweight (BMI <18.5), normal weight (BMI = 18.5–24.9), overweight (BMI = 25–29.9), or obese (BMI ≥30).
Cancer survivors reported a significantly higher prevalence of any dietary supplement use (70.4% vs 51.2%), MVM supplement use (48.9% vs 36.6%), and supplement use for 11 individual vitamins and 8 minerals than individuals without cancer (Table 2). The 5 most frequently used supplements for individual vitamins or minerals were vitamin D, vitamin C, calcium, vitamin E, and vitamin B-12. Cancer survivors also reported a significantly higher prevalence of using nonvitamin, nonmineral supplements, such as lutein or zeaxanthin, and EPA or DHA. Male cancer survivors reported a significantly higher prevalence of lycopene supplement use than male individuals without cancer. After adjusting for age, sex, and race/ethnicity, the prevalence of dietary supplement use remained significantly higher among cancer survivors for use of any supplements, MVM supplements, and supplements of vitamins D, C, and B-6, folic acid, niacin, retinol, calcium, and lycopene (Supplemental Table 2).
TABLE 2.
Prevalence of dietary supplement use in adult cancer survivors and individuals without cancer in NHANES 2003–20161
| Weighted % (95% CI) | |||
|---|---|---|---|
| Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | P | |
| Any dietary supplements | 70.4 (68.0–72.9) | 51.2 (50.1–52.4) | <0.012 |
| Multivitamins/minerals | 48.9 (46.3–51.6) | 36.6 (35.5–37.6) | <0.012 |
| Vitamins | |||
| Vitamin D (μg/d) | 55.8 (53.2–58.4) | 38.3 (37.3–39.3) | <0.012 |
| Vitamin C (mg/d) | 49.9 (47.1–52.7) | 38.3 (37.3–39.3) | <0.012 |
| Vitamin E (mg/d) | 47.3 (44.4–50.2) | 35.4 (34.5–36.3) | <0.012 |
| Vitamin B-12 (μg/d) | 47.3 (44.8–49.8) | 36.0 (35.0–37.0) | <0.012 |
| Folic acid (DFE, μg/d) | 45.6 (43.0–48.2) | 34.5 (33.5–35.5) | <0.012 |
| Vitamin B-6 (mg/d) | 45.5 (42.9–48.0) | 34.7 (33.7–35.7) | <0.012 |
| Niacin (mg/d) | 44.2 (41.6–46.8) | 33.4 (32.4–34.4) | <0.012 |
| Riboflavin (mg/d) | 42.7 (40.2–45.2) | 32.4 (31.5–33.4) | <0.012 |
| Thiamin (mg/d) | 42.6 (40.0–45.1) | 32.4 (31.4–33.4) | <0.012 |
| Vitamin A (RAE, μg/d) | 43.3 (40.7–45.8) | 33.2 (32.3–34.1) | <0.012 |
| β-carotene (RAE, μg/d) | 43.2 (40.6–45.8) | 33.1 (32.2–34.0) | <0.012 |
| Retinol (RAE, μg/d) | 40.1 (37.5–42.6) | 29.7 (28.8–30.5) | <0.012 |
| Vitamin K (μg/d) | 33.7 (31.3–36.1) | 24.1 (23.3–24.9) | <0.012 |
| Choline (mg/d) | 5.98 (4.61–7.34) | 5.91 (5.48–6.33) | 0.92 |
| Minerals | |||
| Calcium (mg/d) | 50.0 (47.6–52.4) | 36.5 (35.5–37.4) | <0.012 |
| Zinc (mg/d) | 42.5 (40.0–45.1) | 32.2 (31.3–33.2) | <0.012 |
| Magnesium (mg/d) | 41.0 (38.7–43.4) | 30.2 (29.2–31.1) | <0.012 |
| Copper (mg/d) | 38.3 (35.6–40.9) | 28.1 (27.3–28.9) | <0.012 |
| Selenium (μg/d) | 36.8 (34.3–39.4) | 27.0 (26.1–27.9) | <0.012 |
| Potassium (mg/d) | 30.2 (27.8–32.7) | 21.0 (20.2–21.7) | <0.012 |
| Phosphorus (mg/d) | 26.9 (24.4–29.3) | 17.5 (16.8–18.2) | <0.012 |
| Iron (mg/d) | 22.7 (20.4–25.1) | 20.8 (20.0–21.6) | 0.12 |
| Other nutrients | |||
| Lycopene (μg/d) | 31.3 (28.0–34.6) | 18.9 (18.0–19.7) | <0.012 |
| Lutein or zeaxanthin (μg/d) | 24.2 (21.9–26.5) | 14.7 (14.1–15.4) | <0.012 |
| EPA or DHA (mg/d) | 15.4 (13.2–17.6) | 9.30 (8.56–10.0) | <0.012 |
| Fiber (g/d) | 3.58 (2.49–4.67) | 2.69 (2.36–3.03) | 0.12 |
Values are prevalences (95% CIs). The percentages (95% CIs) were adjusted for survey weights of NHANES. DFE, Dietary Folate Equivalents; RAE, Retinol Activity Equivalents.
Significant difference in the prevalence of dietary supplement uses between cancer survivors and individuals without cancer after multiple comparison adjustments using the Benjamini-Hochberg procedure. The difference was considered statistically significant when the Benjamini-Hochberg–adjusted P value was less than the false discovery rate of 0.10 (21).
Compared with individuals without cancer, cancer survivors had higher amounts of total nutrient intake for most nutrients as well as nutrient intake from dietary supplements for nearly all nutrients. In contrast, cancer survivors had a lower nutrient intake from foods for most nutrients (Table 3). After adjustments for age, sex, and race/ethnicity, cancer survivors and individuals without cancer had similar total nutrient intake for most nutrients but still had lower intake for niacin, folate, magnesium, phosphorus, and fiber from foods (Supplemental Table 3).
TABLE 3.
Nutrient intakes from foods and supplements among adult cancer survivors and individuals without cancer in the United States, NHANES 2003–20161
| Total nutrient intake | Nutrient intake from foods | Nutrient intake from dietary supplements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | Mean difference2 | P | Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | Mean difference2 | P | Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | Mean difference2 | P | |
| Vitamins | ||||||||||||
| Vitamin A, RAE, μg/d | 1045 ± 25.6 | 900 ± 8.56 | 145 ± 24.4 | <0.013 | 671 ± 12.4 | 637 ± 5.21 | 33.1 ± 13.0 | 0.013 | 373 ± 19.3 | 263 ± 6.03 | 110 ± 18.7 | <0.013 |
| β-carotene, RAE, μg/d | 295 ± 12.6 | 249 ± 4.57 | 45.5 ± 12.0 | <0.013 | 196 ± 6.62 | 181 ± 2.95 | 14.9 ± 6.17 | 0.023 | 98.5 ± 9.50 | 66.9 ± 2.55 | 29.8 ± 9.42 | <0.013 |
| Retinol, RAE, μg/d | 729 ± 16.7 | 628 ± 5.97 | 101 ± 16.5 | <0.013 | 453 ± 7.89 | 434 ± 4.14 | 19.3 ± 8.53 | 0.033 | 277 ± 13.1 | 196 ± 4.57 | 80.7 ± 12.7 | <0.013 |
| Vitamin C, mg/d | 249 ± 12.6 | 196 ± 4.57 | 53.0 ± 12.4 | <0.013 | 83.4 ± 1.58 | 84.5 ± 0.98 | −1.20 ± 1.59 | 0.45 | 161 ± 11.8 | 112 ± 4.67 | 48.2 ± 11.3 | <0.013 |
| Vitamin D, μg/d | 25.2 ± 1.66 | 15.7 ± 0.52 | 9.40 ± 1.84 | <0.013 | 4.90 ± 0.13 | 4.71 ± 0.06 | 0.20 ± 0.13 | 0.14 | 17.5 ± 1.57 | 9.14 ± 0.45 | 8.36 ± 1.69 | <0.013 |
| Vitamin E, mg/d | 50.6 ± 3.19 | 35.9 ± 1.06 | 14.7 ± 3.00 | <0.013 | 7.70 ± 0.12 | 8.16 ± 0.10 | −0.50 ± 0.15 | <0.013 | 42.8 ± 3.20 | 27.7 ± 1.13 | 15.2 ± 3.97 | <0.013 |
| Thiamin, mg/d | 9.15 ± 1.06 | 6.02 ± 0.24 | 3.13 ± 1.03 | <0.013 | 1.54 ± 0.04 | 1.65 ± 0.07 | −0.11 ± 0.07 | 0.15 | 7.10 ± 0.95 | 4.41 ± 0.23 | 2.69 ± 0.94 | <0.013 |
| Riboflavin, mg/d | 7.24 ± 0.71 | 5.78 ± 0.20 | 1.44 ± 0.67 | 0.043 | 2.13 ± 0.04 | 2.19 ± 0.03 | −0.07 ± 0.04 | 0.023 | 4.77 ± 0.52 | 3.55 ± 0.18 | 1.22 ± 0.52 | 0.023 |
| Niacin, mg/d | 46.4 ± 2.99 | 38.1 ± 0.58 | 8.30 ± 3.01 | <0.013 | 22.9 ± 0.24 | 25.8 ± 0.14 | −2.90 ± 0.26 | <0.013 | 22.3 ± 2.57 | 12.5 ± 0.56 | 9.88 ± 2.62 | <0.013 |
| Vitamin B-6, mg/d | 8.83 ± 0.61 | 6.96 ± 0.22 | 1.90 ± 0.67 | <0.013 | 1.91 ± 0.01 | 2.10 ± 0.01 | −0.20 ± 0.01 | <0.013 | 6.77 ± 0.55 | 4.96 ± 0.22 | 1.81 ± 0.60 | <0.013 |
| Folate, DFE, μg/d | 880 ± 22.8 | 803 ± 7.97 | 77.8 ± 23.2 | <0.013 | 500 ± 5.81 | 541 ± 3.97 | −41.2 ± 6.39 | <0.013 | 375 ± 20.0 | 263 ± 6.04 | 112 ± 20.6 | <0.013 |
| Vitamin B-12, μg/d | 83.3 ± 9.23 | 59.2 ± 3.55 | 24.1 ± 9.76 | 0.023 | 5.00 ± 0.08 | 5.25 ± 0.09 | −0.29 ± 0.13 | 0.12 | 74.7 ± 7.06 | 53.2 ± 3.54 | 21.6 ± 7.76 | <0.013 |
| Choline, mg/d | 331 ± 5.64 | 349 ± 2.80 | −17.3 ± 6.21 | <0.013 | 318 ± 4.42 | 337 ± 1.69 | −19.7 ± 4.55 | <0.013 | 10.5 ± 2.52 | 10.2 ± 1.69 | 0.28 ± 3.13 | 0.93 |
| Vitamin K, μg/d | 125 ± 8.27 | 113 ± 1.37 | 12.2 ± 8.21 | 0.14 | 104 ± 2.48 | 106 ± 1.28 | −2.00 ± 2.51 | 0.43 | 23.1 ± 9.13 | 7.74 ± 0.22 | 15.3 ± 9.09 | 0.09 |
| Minerals | ||||||||||||
| Calcium, mg/d | 1196 ± 22.9 | 1126 ± 8.32 | 70.0 ± 23.0 | <0.013 | 892 ± 11.8 | 960 ± 6.04 | −68.4 ± 12.3 | <0.013 | 298 ± 15.5 | 163 ± 3.95 | 135 ± 15.1 | <0.013 |
| Selenium, μg/d | 130 ± 2.79 | 138 ± 5.00 | −8.70 ± 5.80 | 0.14 | 102 ± 1.40 | 115 ± 0.54 | −12.6 ± 1.35 | <0.013 | 26.1 ± 1.85 | 26.1 ± 7.48 | −0.04 ± 7.69 | 0.99 |
| Magnesium, mg/d | 347 ± 7.34 | 341 ± 3.09 | 5.40 ± 7.22 | 0.46 | 280 ± 3.08 | 299 ± 1.79 | −19.8 ± 3.07 | <0.013 | 65.6 ± 5.84 | 41.3 ± 1.68 | 24.2 ± 5.93 | <0.013 |
| Potassium, mg/d | 2631 ± 28.6 | 2719 ± 13.0 | −87.5 ± 28.4 | <0.013 | 2604 ± 28.1 | 2699 ± 12.7 | −94.5 ± 28.1 | <0.013 | 27.4 ± 1.79 | 19.9 ± 1.00 | 7.52 ± 2.12 | <0.013 |
| Phosphorus, mg/d | 1272 ± 15.4 | 1394 ± 6.76 | −122 ± 15.3 | <0.013 | 1251 ± 14.8 | 1380 ± 6.53 | −129 ± 15.0 | <0.013 | 20.8 ± 3.22 | 13.8 ± 0.80 | 6.99 ± 3.25 | 0.033 |
| Iron, mg/d | 18.8 ± 0.40 | 19.0 ± 0.14 | −0.20 ± 0.41 | 0.63 | 14.4 ± 0.17 | 15.3 ± 0.12 | −0.90 ± 0.18 | <0.013 | 4.42 ± 0.31 | 3.70 ± 0.09 | 0.72 ± 0.32 | 0.033 |
| Zinc, mg/d | 19.1 ± 0.52 | 17.2 ± 0.17 | 1.96 ± 0.53 | <0.013 | 10.9 ± 0.17 | 11.9 ± 0.10 | −1.00 ± 0.19 | <0.013 | 8.04 ± 0.44 | 5.32 ± 0.14 | 2.72 ± 0.45 | <0.013 |
| Copper, mg/d | 1.82 ± 0.05 | 1.73 ± 0.03 | 0.10 ± 0.05 | 0.053 | 1.21 ± 0.01 | 1.29 ± 0.01 | −0.01 ± 0.01 | <0.013 | 0.59 ± 0.26 | 0.43 ± 0.10 | 0.15 ± 0.27 | <0.013 |
| Other nutrients | ||||||||||||
| Lutein + zeaxanthin, μg/d | 1756 ± 60.6 | 1653 ± 33.2 | 103 ± 64.3 | 0.11 | 1503 ± 44.7 | 1453 ± 23.4 | 50.0 ± 41.1 | 0.23 | 251 ± 37.1 | 205 ± 22.3 | 46.5 ± 41.0 | 0.26 |
| Lycopene, μg/d | 4670 ± 72.8 | 5390 ± 26.9 | −720 ± 75.4 | <0.013 | 4480 ± 48.2 | 5280 ± 24.8 | −800 ± 53.2 | <0.013 | 203 ± 62.0 | 124 ± 12.7 | 79.2 ± 63.2 | 0.21 |
| Fiber, g/d | 16.2 ± 0.26 | 16.9 ± 0.14 | −0.70 ± 0.25 | <0.013 | 15.9 ± 0.24 | 16.7 ± 0.13 | −0.80 ± 0.23 | <0.013 | 0.23 ± 0.06 | 0.18 ± 0.02 | 0.06 ± 0.06 | 0.38 |
| EPA or DHA, mg/d | 172 ± 9.85 | 153 ± 3.46 | 19.1 ± 9.87 | 0.063 | 110 ± 5.43 | 112 ± 2.74 | −2.00 ± 4.55 | 0.66 | 57.5 ± 7.32 | 40.3 ± 2.37 | 17.3 ± 7.03 | 0.023 |
Values are means ± SEs. The means and SEs were adjusted for survey weights of NHANES. We applied the National Cancer Institute method to estimate usual nutrient intake from foods and then add in the nutrient intake from supplements (the shrink-then-add method) (16). The total nutrient intake was calculated as the sum of intake from both foods and supplements. DFE, Dietary Folate Equivalents; RAE, Retinol Activity Equivalents.
The mean difference was calculated as the difference in mean intake between cancer survivors and individuals without cancer. A positive mean difference corresponds to a higher mean intake in cancer survivors than individuals without cancer, and a negative mean difference corresponds to a lower mean intake in cancer survivors than individuals without cancer.
Significant difference in nutrient intake amount between cancer survivors and individuals without cancer after multiple comparison adjustments using the Benjamini-Hochberg procedure. The difference was considered statistically significant when the Benjamini-Hochberg–adjusted P value was less than the false discovery rate of 0.10 (21).
Cancer survivors had a lower prevalence of inadequate nutrient intake (total nutrient intake < EAR or AI) for vitamins A, C, D, and E and iron than did individuals without cancer. This was largely contributed by a higher intake of these nutrients from dietary supplements among cancer survivors as compared with individuals without cancer (Table 3 and Table 4). However, compared with individuals without cancer, cancer survivors still had a higher proportion of individuals with inadequate intake for folate, vitamin B-6, niacin, calcium, copper, and phosphorus, which was attributable to a lower intake of these nutrients from foods among cancer survivors. In addition, compared with individuals without cancer, cancer survivors had a higher proportion of individuals with excess nutrient intake (total nutrient intake ≥ UL) for vitamin D, vitamin B-6, niacin, calcium, magnesium, and zinc, although the prevalence of individuals with excess nutrient intake was small, ranging from 0.10% to 9.49% in cancer survivors and from 0.10% to 6.26% in individuals without cancer. After adjustments for age, sex, and race/ethnicity, cancer survivors still had a lower prevalence of inadequate intake for vitamin D but a higher prevalence of excess intake for calcium (Supplemental Table 4).
TABLE 4.
Percentage of cancer survivors and individuals without cancer with inadequate (intake < EAR) or excessive (≥UL) nutrient intake, NHANES 2003–20161
| Percentage with inadequate nutrient intake (<EAR), weighted % (95% CI) | Percentage with excess nutrient intake (≥UL), weighted % (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total nutrient intake | Nutrient intake from foods | Total nutrient intake | Nutrient intake from foods | |||||||||
| Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | P | Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | P | Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | P | Cancer survivors (n = 2772) | Individuals without cancer (n = 31,310) | P | |
| Vitamin A2 | 23.9 (21.6–26.2) | 33.1 (31.9–34.3) | <0.013 | 39.3 (36.1–42.5) | 45.6 (44.2–47.0) | <0.013 | 0.60 (0.25–0.95) | 0.49 (0.33–0.67) | 0.61 | 0 | 0 | |
| Vitamin C | 23.6 (21.7–25.5) | 29.7 (28.6–30.8) | <0.013 | 44.4 (41.8–47.0) | 44.7 (43.2–46.2) | 0.82 | 1.00 (0.49–1.50) | 0.76 (0.61–0.92) | 0.44 | 0 | 0 | |
| Vitamin D | 46.6 (43.6–49.6) | 64.5 (63.3–65.7) | <0.013 | 94.4 (93.3–95.5) | 95.1 (94.5–95.7) | 0.19 | 3.62 (2.44–4.76) | 2.08 (1.66–2.54) | 0.023 | 0 | 0 | |
| Vitamin E4 | 50.4 (47.4–53.5) | 58.9 (57.7–60.1) | <0.013 | 89.9 (88.4–91.4) | 87.1 (86.0–88.2) | <0.013 | 0.10 (0–0.29) | 0.20 (0.11–0.24) | 0.37 | 0 | 0 | |
| Folate5 | 9.66 (8.69–10.7) | 8.19 (7.58–8.82) | 0.013 | 16.6 (15.0–18.2) | 12.4 (11.5–13.3) | <0.013 | 4.76 (3.65–5.95) | 3.32 (2.98–3.62) | 0.02 | 0 | 0 | |
| Vitamin B-6 | 11.2 (10.1–12.4) | 7.10 (6.44–7.76) | <0.013 | 19.9 (18.0–21.8) | 11.3 (10.3–12.3) | <0.013 | 2.76 (1.95–3.65) | 1.34 (1.08–1.52) | <0.013 | 0 | 0 | |
| Niacin5 | 2.10 (1.69–2.51) | 1.16 (0.95–1.45) | <0.013 | 3.60 (2.90–4.30) | 1.80 (1.47–2.13) | <0.013 | 9.49 (7.86–11.1) | 6.26 (5.79–6.73) | <0.013 | – | – | |
| Choline6 | 92.3 (90.8–93.8) | 91.4 (90.6–92.2) | 0.17 | 93.7 (92.4–95.0) | 92.1 (91.3–92.9) | <0.013 | 0 | 0.10 (0.01–0.19) | 0.043 | 0 | 0 | |
| Vitamin B-127 | 2.71 (2.21–3.19) | 2.59 (2.23–2.97) | 0.68 | 4.78 (3.94–5.66) | 4.04 (3.46–4.54) | 0.043 | – | – | – | – | ||
| Thiamin7 | 5.73 (4.86–6.54) | 4.78 (4.28–5.28) | 0.023 | 9.44 (8.11–10.7) | 7.02 (6.32–7.68) | <0.013 | – | – | – | – | ||
| Riboflavin7 | 1.99 (1.66–2.34) | 2.30 (2.02–2.58) | 0.09 | 3.23 (2.71–3.69) | 3.17 (2.82–3.58) | 0.99 | – | – | – | – | ||
| Vitamin K6, 7 | 49.6 (46.1–53.1) | 52.8 (51.0–54.6) | 0.08 | 57.5 (53.9–61.1) | 58.0 (56.3–59.7) | 0.79 | – | – | – | – | ||
| Calcium | 38.1 (35.5–40.7) | 31.6 (30.3–32.9) | <0.013 | 58.1 (55.5–60.7) | 42.6 (41.3–43.9) | <0.013 | 8.79 (7.41–10.2) | 3.83 (3.46–4.14) | <0.013 | 0.50 (0.34–0.66) | 0.40 (0.33–0.47) | 0.21 |
| Copper | 5.45 (4.68–6.32) | 4.46 (3.98–5.02) | 0.013 | 8.25 (7.21–9.39) | 6.00 (5.33–6.67) | <0.013 | 0.21 (0–0.49) | 0.21 (0.13–0.27) | 0.82 | 0 | 0 | |
| Iron | 1.52 (1.19–1.81) | 2.31 (2.03–2.57) | <0.013 | 2.07 (1.69–2.51) | 3.06 (2.76–3.44) | <0.013 | 2.77 (2.05–3.55) | 2.29 (2.09–2.51) | 0.20 | 0 | 0 | |
| Magnesium8 | 47.8 (45.2–50.4) | 46.7 (45.2–48.2) | 0.41 | 62.4 (60.0–64.9) | 55.9 (54.5–57.3) | <0.013 | 4.31 (3.21–5.42) | 2.59 (2.25–2.94) | <0.013 | – | – | |
| Phosphorus | 2.15 (1.64–2.56) | 1.21 (0.99–1.41) | <0.013 | 2.38 (1.92–2.88) | 1.32 (1.08–1.52) | <0.013 | 0 | 0 | 0 | 0 | ||
| Selenium | 1.02 (0.69–1.31) | 0.51 (0.35–0.65) | <0.013 | 1.55 (1.08–1.92) | 0.70 (0.52–0.88) | <0.013 | 1.32 (0.66–1.94) | 0.79 (0.64–0.96) | 0.15 | 0 | 0 | |
| Zinc | 11.0 (9.47–12.5) | 10.1 (9.26–10.9) | 0.25 | 18.3 (15.9–20.7) | 14.5 (13.4–15.6) | <0.013 | 5.56 (4.46–6.74) | 3.50 (3.21–3.83) | <0.013 | 0 | 0 | |
| Potassium6, 7 | 67.3 (64.8–69.8) | 66.3 (65.2–67.4) | 0.43 | 68.6 (66.2–71.1) | 67.2 (66.1–68.3) | 0.26 | – | – | – | – | ||
Values are percentages (95% CIs). The percentages (95% CIs) were adjusted for survey weights of NHANES. We applied the National Cancer Institute method to estimate usual nutrient intake from foods and then add in the nutrient intake from supplements. The total nutrient intake was calculated as the sum of intake from both foods and supplements. AI, Adequate Intake; EAR, Estimated Average Requirement; UL, Tolerable Upper Intake Level.
UL for vitamin A applies to preformed vitamin A only (37).
Significant difference in percentage with inadequate or excess nutrient intake between cancer survivors and individuals without cancer after multiple comparison adjustments using the Benjamini-Hochberg procedure. The difference was considered statistically significant when the Benjamini-Hochberg–adjusted P value was less than the false discovery rate of 0.10 (21).
Vitamin E assessed as α-tocopherol; UL for vitamin E applies to any form of supplemental α-tocopherol (37).
ULs for niacin and folate apply to synthetic forms obtained from supplements, fortified foods, or a combination of the 2 (37).
No EAR for choline, vitamin K, and potassium. The percentages estimated were percentages of intake less than the AI (37).
No UL for vitamin B-12, thiamin, riboflavin, vitamin K, and potassium (37).
UL for magnesium represents intake from a pharmacological agent only and does not include intake from food and water (37).
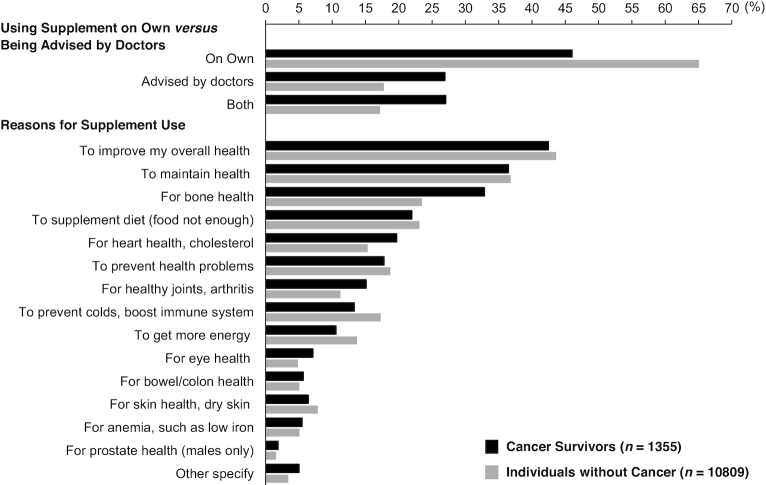
Among cancer survivors who used dietary supplements, ∼46% reported taking supplements on their own, 27% being advised by doctor and 27% for both (Figure 1). Among individuals without cancer, the percentage of those taking supplements on their own, being advised by their doctor, or both was 65%, 18%, and 17%, respectively. The top 5 reasons for taking supplements were to improve overall health, to maintain health, to support bone health, to supplement the diet, and for heart health among both cancer survivors and individuals without cancer.
FIGURE 1.
Reasons for taking dietary supplements among cancer survivors and individuals without cancer in the United States, NHANES 2007–2016. Values are percentages, adjusted for survey weights of NHANES.
Compared with those not using dietary supplements, cancer survivors who used dietary supplements were older and more likely to be women and non-Hispanic whites; had higher levels of education, income, and better diet quality; and were less likely to be current smokers, physically inactive, and overweight/obese (Supplemental Table 5 and Supplemental Figure 2). Breast cancer survivors reported the highest prevalence of dietary supplement use among survivors of all cancer types.
Discussion
In a nationally representative sample of the noninstitutionalized US adult population, a higher prevalence of dietary supplement use was reported among cancer survivors compared with individuals without a history of cancer. Overall, cancer survivors had significantly high amounts of nutrient intake from supplements but significantly lower nutrient intakes from foods for the majority of the nutrients. The higher proportions of cancer survivors with inadequate intakes for folate, vitamin B-6, niacin, calcium, copper, and phosphorus were due to lower intakes of these nutrients from foods, whereas the higher proportions of cancer survivors with excess intakes for vitamin D, vitamin B-6, niacin, calcium, magnesium, and zinc were contributed by higher intakes of these nutrients from supplements. Nearly half of the cancer survivors took dietary supplements on their own without consulting their health care providers.
Prior cohorts of survivors with breast, prostate, and colorectal cancers reported a prevalence of dietary supplement in the range of 50–85% (2, 9–11). Our analysis among cancer survivors who participated in a national representative sample of US adults supported the high prevalence of dietary supplement use among cancer survivors—70% of the cancer survivors in the United States reported taking dietary supplements in the past 30 d. Our analysis further revealed that, compared with individuals without a history of cancer diagnosis, cancer survivors reported a higher prevalence and dose for dietary supplement use for nearly all nutrients being examined. While dietary supplement use did contribute to a lower prevalence of the cancer survivors with an inadequate intake for a few nutrients, nearly half of the cancer survivors still had an inadequate nutrient intake for vitamin D and vitamin E, more than one-third of the cancer survivors had an inadequate intake for calcium, and ∼20% had inadequate intake for vitamin A and vitamin C. Importantly, cancer survivors had low intakes of most of these nutrients from foods. We previously reported that their amounts of nutrient intake from foods were far below the recommended intake for several nutrients (e.g., vitamin D, vitamin E, potassium, fiber, and calcium) (25). The consequence of these “shortfall nutrients” on health outcomes of cancer survivors has not been well studied, although there are some suggestions that serum markers of low vitamin D status are associated with poor survival. For example, a meta-analysis of 6 prospective cohort studies of 6092 breast cancer survivors found that low circulating concentrations of 25-hydroxyvitamin D [25(OH)D] were associated with increased risk of breast cancer–specific and all-cause mortality (26). Such findings have been further supported by a recent study of 1666 breast cancer survivors that reported worse overall survival among women in the lowest tertile of 25(OH)D concentrations compared with those in the highest tertile (27). The suboptimal nutrient intake among cancer survivors could be due to the long-lasting impact of cancer and its therapies on intake, and likely an unmet need, given that a recent survey revealed that nutrition care is severely lacking in the current delivery model of outpatient oncology in the United States (28). Future efforts are required to identify effective strategies to integrate nutrition into oncology care for improving the nutritional intake of cancer survivors.
It is important to note that the use of dietary supplements also contributed to a higher prevalence of excess nutrient intake in cancer survivors for a few nutrients, including niacin, calcium, zinc, magnesium, vitamin D, and vitamin B-6. The overall percentage of the cancer survivors with amounts of nutrient intake exceeding the UL was low for most nutrients (i.e., <5%). However, >5% of the cancer survivors had an excess intake of niacin, calcium, and zinc. Our analysis suggested that the excess intake of these nutrients is due to a higher intake of these nutrients from supplements, not from foods. The potential harm of high doses of dietary supplement use has been reported by randomized controlled trials and prospective cohort studies. For example, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study and the Beta-Carotene and Retinol Efficacy Trial (CARET) found that supplemental use of β-carotene (20 or 30 mg/d) increased the risk of lung cancer risk among smokers (29, 30). The Selenium and Vitamin E Cancer Prevention Trial (SELECT) reported that supplement use of vitamin E (400 IU/d) increased the risk of prostate cancer among men (31). From observational studies, the Cancer Prevention Study (CPS) II Nutrition Cohort study found that high doses of supplemental calcium intake (≥1000 mg/d) were associated with an increased risk of all-cause mortality in men, whereas lower doses (<1000 mg/d) or calcium intake from foods were not associated with mortality (32). We recently reported that, among the general US adult population who participated in the NHANES 1999–2010, high calcium intake from supplements (≥1000 mg/d) but not foods was associated with an increased cancer mortality (8). There were also some suggestions that vitamin D supplement use at ≥10 μg/d among individuals with no signs of vitamin D insufficiency [serum 25(OH)D concentrations ≥50 nmol/L] was associated with an increased risk of all-cause mortality and cancer mortality. These findings call for future studies to evaluate the short-term and long-term health impact of dietary supplement use among cancer survivors, especially supplement use at high doses.
Nearly half of the cancer survivors used dietary supplements on their own without consulting their health care providers, which may reflect the lack of communication between cancer survivors and their health care providers about dietary supplement use during clinical visits. A previous study reported that only 2% of the 1081 cancer survivors obtained advice on dietary supplement use from a registered dietitian (33). As expected, the main reasons for cancer survivors to use dietary supplements were to improve and maintain overall health and to supplement the diet (5), and supplement use among cancer survivors was positively associated with high levels of education and income and an overall healthy lifestyle (5, 34).
Although our study has numerous strengths (e.g., a population-based study with a large sample size and collection of data using validated measures), there are some limitations that need to be considered. First, cancer diagnosis was based on self-report and may be associated with misclassification. Second, cancer stage and treatment may affect survivors’ choices of dietary supplement use. Such information was not captured by NHANES and we were not able to assess how cancer stage and treatment may impact dietary supplement use among cancer survivors. In addition, NHANES does not provide a sufficient number of cancer survivors to be evaluated by specific cancer types. Thus, we did not assess whether dietary supplement use varied by cancer types. Despite NHANES being a nationally representative sample of noninstitutionalized civilians in the United States, cancer survivors who participated in NHANES do not directly represent the cancer survivors in the general population due to the sampling strategies of NHANES. However, the prevalence of some common cancers estimated from NHANES and from the national program of cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) program was largely similar. For example, the estimated prevalence from NHANES 2013–2016 and SEER 2016 was 2.66% and 2.89% for female breast cancer, 0.56% and 0.61% for colorectal cancer, and 0.20% and 0.22% for lung cancer, respectively (35). Third, the prevalence and dose of dietary supplement use were based on self-report and are thus subject to recall bias. However, the NHANES documented that the ingredient and dosage information was obtained from the bottles and nutrition facts labels >75% of the time when the interviews were conducted about dietary supplement use (36), which reduces the measurement error due to recall bias. The self-reported dietary intake is also subject to measurement error. The NHANES incorporated one or two 24-h diet recalls per person, which do not capture long-term intake due to large day-to-day variations in food intake. To improve the estimation of usual intake, we applied the National Cancer Institute method to reduce measurement errors associated with dietary intake estimated using diet recalls. Fourth, because of the cross-sectional nature of the NHANES, we were not able to assess whether cancer survivors initiated the dietary supplement use before or after cancer diagnosis and whether the dietary supplement use persisted into later years. Last, the limited sample size does not facilitate evaluating trends in dietary supplement use among cancer survivors over various cycles of NHANES.
Despite these limitations, our study provided a comprehensive assessment on the prevalence and dose of dietary supplement use in cancer survivors in comparison to individuals without cancer from a nationally representative sample of the US population. We found that cancer survivors used dietary supplements at a higher frequency and dose than individuals without cancer but had an overall lower intake of nutrients from foods. Although dietary supplement use contributed to a lower prevalence of cancer survivors with an inadequate intake for a few nutrients, it also contributed to a higher prevalence of excess intake for other nutrients. Nearly half of the cancer survivors used dietary supplements without consulting their health care providers. These findings underscore the need for health care providers to assess dietary supplement use among cancer survivors and call for strategies to integrate nutrition into oncology care for reducing inadequate nutrient intake among cancer survivors.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—FFZ and MD: designed this research; MD, HL, FC, MR, and ZS: performed statistical analysis; MD: wrote the original draft; FFZ, JBB, GR, and EB: critically reviewed and revised the manuscript; FFZ: has primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This study was supported by NIH/National Institute on Minority Health and Health Disparities (NIMHD) (1R01MD011501; to FFZ).
Author disclosures: JBB reports personal fees from AdvoCare International, personal fees from Pfizer Consumer Healthcare, and personal fees from Pharmavite, outside the submitted work. The other authors report no conflicts of interest. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental Figures 1 and 2, Supplemental Methods, and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Data described in the manuscript, code book, and analytic code will be made available upon request.
Abbreviations used: AI, Adequate Intake; BRR, balanced repeated replication; DSQ, Dietary Supplement Questionnaire; EAR, Estimated Average Requirement; MEC, Mobile Examination Center; MVM, multivitamin/mineral; NCHS, National Center for Health Statistics; SEER, Surveillance, Epidemiology, and End Results; UL, Tolerable Upper Intake Level; 25(OH)D, 25-hydroxyvitamin D.
References
- 1. Satia JA, Walsh JF, Pruthi RS. Health behavior changes in white and African American prostate cancer survivors. Cancer Nurs. 2009;32(2):107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–73. [DOI] [PubMed] [Google Scholar]
- 3. Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103(3):323–8. [DOI] [PubMed] [Google Scholar]
- 4. Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M et al.. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 5. Bours MJ, Beijer S, Winkels RM, van Duijnhoven FJ, Mols F, Breedveld-Peters JJ, Kampman E, Weijenberg MP, van de Poll-Franse LV. Dietary changes and dietary supplement use, and underlying motives for these habits reported by colorectal cancer survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) registry. Br J Nutr. 2015;114(2):286–96. [DOI] [PubMed] [Google Scholar]
- 6. Rock CL, Newman VA, Neuhouser ML, Major J, Barnett MJ. Antioxidant supplement use in cancer survivors and the general population. J Nutr. 2004;134(11):3194s–5s. [DOI] [PubMed] [Google Scholar]
- 7. American Cancer Society Medical and Editorial Content Team. Risks and Side Effects of Dietary Supplements: American Cancer Society 2015 [updated 31 Mar 2015], [Accessed 9 Dec., 2018][Internet]. Available from: https://www.cancer.org/treatment/treatments-and-side-effects/complementary-and-alternative-medicine/dietary-supplements/risks-and-side-effects.html. [Google Scholar]
- 8. Chen F, Du M, Blumberg JB, Ho Chui KK, Ruan M, Rogers G, Shan Z, Zeng L, Zhang FF. Association among dietary supplement use, nutrient intake, and mortality among U.S. adults: a cohort study. Ann Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rock CL. Multivitamin-multimineral supplements: who uses them?. Am J Clin Nutr. 2007;85(1):277S–9S. [DOI] [PubMed] [Google Scholar]
- 10. Miller PE, Vasey JJ, Short PF, Hartman TJ. Dietary supplement use in adult cancer survivors. Oncol Nurs Forum. 2009;36(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller P, Demark-Wahnefried W, Snyder DC, Sloane R, Morey MC, Cohen H, Kranz S, Mitchell DC, Hartman TJ. Dietary supplement use among elderly, long-term cancer survivors. J Cancer Surviv. 2008;2(3):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Prevention and Control.NHANES comprehensive data list. [cited 18 Dec., 2017] [Internet].Available from: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component = Dietary&CycleBeginYear = 2009. [Google Scholar]
- 13. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Washington (DC): US Department of Health and Human Services; 2018; [Accessed 18 Feb., 2019][Internet]. Available from: https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. [Google Scholar]
- 14. US Department of Agriculture. Healthy Eating Index (HEI). 2019; [Accessed 25 Oct., 2019][Internet]. Available from: https://www.fns.usda.gov/resource/healthy-eating-index-hei. [Google Scholar]
- 15. Kwan ML, Greenlee H, Lee VS, Castillo A, Gunderson EP, Habel LA, Kushi LH, Sweeney C, Tam EK, Caan BJ. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: the Life After Cancer Epidemiology Study. Breast Cancer Res Treat. 2011;130(1):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, Cowan AE, Jun S, Eicher-Miller HA, Guenther PM, Bhadra A, Thomas PR et al.. Best practices for dietary supplement assessment and estimation of total usual nutrient intakes in population-level research and monitoring. J Nutr. 2019;149(2):181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freedman LS, Midthune D, Carroll RJ, Krebs-Smith S, Subar AF, Troiano RP, Dodd K, Schatzkin A, Bingham SA, Ferrari P et al.. Adjustments to improve the estimation of usual dietary intake distributions in the population. J Nutr. 2004;134(7):1836–43. [DOI] [PubMed] [Google Scholar]
- 18. Herrick KA, Rossen LM, Parsons R, Dodd KW. Estimating usual dietary in take from national health and nut rition examination survey data using the national cancer institute method. Vital Health Stat 2 Data Eval Meth Res. 2018;; 2(178):1–63. [PubMed] [Google Scholar]
- 19. Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune D, Tooze JA, Krebs-Smith SM. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106(10):1640–50. [DOI] [PubMed] [Google Scholar]
- 20. Otten JJ, Hellwig JP, Meyers LD. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 21. Benjamini YH, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Ser A. 1995;57(1):289–300. [Google Scholar]
- 22. Centers for Disease Control and Prevention; National Center for Health Statistics. The National Health and Nutrition Examination Survey (NHANES) Methods and Analytic Guidelines 2018[Accessed 27 Nov., 2018] [Internet]. Available from: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx#analytic-guidelines. [Google Scholar]
- 23. SAS. The SURVEYFREQ Procedure 2019[Accessed 12 Jan., 2020] [Internet]. Available from: https://documentation.sas.com/?docsetId = statug&docsetTarget = statug_surveyfreq_details28.htm&docsetVersion = 15.1&locale = en. [Google Scholar]
- 24. National Cancer Institute. User's guide for Analysis of Usual Intakes for Use with Versions 2.1 of the MIXTRAN, DISTRIB, and INDIVINT SAS Macros, [accessed 27 May, 2019] Available from: https://epi.grants.cancer.gov/diet/usualintakes/Users_Guide_v2.1.pdf. [Google Scholar]
- 25. Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: results from a national survey. Cancer. 2015;121(23):4212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014;110(11):2772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, Hong CC, McCann SE, Tang L, Davis W, Liu S et al.. Association of serum level of vitamin D at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol. 2017;3(3):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trujillo EB, Claghorn K, Dixon SW, Hill EB, Braun A, Lipinski E, Platek ME, Vergo MT, Spees C. Inadequate nutrition coverage in outpatient cancer centers: results of a national survey. J Oncol. 2019;2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330(15):1029–35. [DOI] [PubMed] [Google Scholar]
- 30. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH et al.. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–5. [DOI] [PubMed] [Google Scholar]
- 31. Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM et al.. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang B, Campbell PT, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, Flanders WD, McCullough ML. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr. 2016;103(3):886–94. [DOI] [PubMed] [Google Scholar]
- 33. Pouchieu C, Fassier P, Druesne-Pecollo N, Zelek L, Bachmann P, Touillaud M, Bairati I, Hercberg S, Galan P, Cohen P et al.. Dietary supplement use among cancer survivors of the NutriNet-Sante cohort study. Br J Nutr. 2015;113(8):1319–29. [DOI] [PubMed] [Google Scholar]
- 34. Ferrucci LM, McCorkle R, Smith T, Stein KD, Cartmel B. Factors related to the use of dietary supplements by cancer survivors. J Altern Complement Med. 2009;15(6):673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta (GA): American Cancer Society; 2016; [Accessed 19 Feb., 2020] [Internet]. Available from: https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. [Google Scholar]
- 36. Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.