ABSTRACT
Background
Severe iodine deficiency during pregnancy can cause intellectual disability, presumably through inadequate placental transfer of maternal thyroid hormone to the fetus. The association between mild-to-moderate iodine deficiency and child neurodevelopmental problems is not well understood.
Objectives
We investigated the association of maternal iodine status during pregnancy with child attention-deficit hyperactivity disorder (ADHD) and autistic traits.
Methods
This was a collaborative study of 3 population-based birth cohorts: Generation R (n = 1634), INfancia y Medio Ambiente (n = 1293), and the Avon Longitudinal Study of Parents and Children (n = 2619). Exclusion criteria were multiple fetuses, fertility treatment, thyroid-interfering medication use, and pre-existing thyroid disease. The mean age of assessment in the cohorts was between 4.4 and 7.7 y for ADHD symptoms and 4.5 and 7.6 y for autistic traits. We studied the association of the urinary iodine-to-creatinine ratio (UI/Creat) <150 μg/g—in all mother–child pairs, and in those with a urinary-iodine measurement at ≤18 weeks and ≤14 weeks of gestation—with the risk of ADHD or a high autistic-trait score (≥93rd percentile cutoff), using logistic regression. The cohort-specific effect estimates were combined by random-effects meta-analyses. We also investigated whether UI/Creat modified the associations of maternal free thyroxine (FT4) or thyroid-stimulating hormone concentrations with ADHD or autistic traits.
Results
UI/Creat <150 μg/g was not associated with ADHD (OR: 1.2; 95% CI: 0.7, 2.2; P = 0.56) or with a high autistic-trait score (OR: 0.8; 95% CI: 0.6, 1.1; P = 0.22). UI/Creat <150 μg/g in early pregnancy (i.e., ≤18 weeks or ≤14 weeks of gestation) was not associated with a higher risk of behavioral problems. The association between a higher FT4 and a greater risk of ADHD (OR: 1.3; 95% CI: 1.0, 1.6; P = 0.017) was not modified by iodine status.
Conclusions
There is no consistent evidence to support an association of mild-to-moderate iodine deficiency during pregnancy with child ADHD or autistic traits.
Keywords: iodine, deficiency, pregnancy, nutrition, behavior problems, ALSPAC, INMA, Generation R
Introduction
Attention-deficit hyperactivity disorder (ADHD)—characterized by symptoms of inattention, impulsivity, and/or hyperactivity—and Autism Spectrum Disorder (ASD)—characterized by difficulties with social interaction, communication, and restricted and repetitive behavior—are co-occurring neurodevelopmental disorders (1–5). The prevalence of ADHD has been estimated to be 5.9%–7.1% in childhood and adolescence (6) and globally, ∼1 in 130 individuals had ASD in 2010 (7). The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) requires an age of onset of symptoms before 12 y of age for the diagnosis of ADHD. For an ASD diagnosis, symptoms must be present in “early childhood” (8). The etiology of these 2 neurodevelopmental disorders is yet to be elucidated, but it is assumed that there is an (overlapping) heritable component to these conditions (9).
Given the neurobiological origin of these disorders, research has focused on investigating whether the maternal supply of thyroid hormone to the fetus is associated with childhood ADHD and ASD. Thyroid hormone regulates neuronal proliferation, differentiation, migration, synapse formation, and myelination in the fetal brain (10, 11) and during early pregnancy the fetus acquires thyroid hormone solely from the mother (12). Epidemiological studies do not consistently show an association between maternal thyroid function and childhood ADHD (13–20). In our previously conducted meta-analysis of individual participant data, we reported no consistent evidence linking maternal thyroid-stimulating hormone (TSH) and free thyroxine (FT4) concentrations with child ADHD (21). Maternal hypothyroidism and overt hyperthyroidism have been associated with a greater risk of diagnosed ASD (15, 20), and a low maternal FT4 concentration measured in the first 18 wk of pregnancy has been associated with a greater risk of autistic traits (22). In a previous study, we also reported a suggestive association of both hypothyroxinemia, characterized by low FT4 and normal TSH, and high FT4 with a greater risk of autistic traits within the clinical range (23). It is unclear whether iodine deficiency underpins the association between mild thyroid dysfunction and these neurodevelopmental disorders.
Iodine deficiency in pregnant populations, which is defined by the WHO as a median urinary iodine concentration (UIC) <150 μg/L, is common (24, 25). Severe iodine deficiency during pregnancy has been associated with severe health outcomes including goiter, abortion, stillbirths, and intellectual disability in the offspring (26). Mild-to-moderate iodine deficiency—which has been defined in pregnant populations as a median UIC between 50 and 150 μg/L (27)—before conception and during pregnancy has been associated with neurodevelopmental outcomes, including lower child intelligence quotient (IQ) scores (28–30). A study suggested that maternal iodine status may affect child outcomes in a dose-dependent manner, but the authors could not test whether the effects of iodine availability for the developing brain were related to impaired maternal thyroid function in pregnancy (30). Investigating such underlying mechanisms may elucidate which subgroups of pregnant women may be at a high risk of giving birth to children with neurobehavioral problems.
Given the important role of iodine for thyroid hormone production and fetal brain development, maternal iodine deficiency during a critical developmental window may potentially increase the risk of neurodevelopmental disorders in the offspring (31). Studies on the association between maternal iodine status during pregnancy and ADHD or ASD are rare. A small study performed in Italy (n = 27) showed that 68.7% of children (11 out of 16) born to mildly-to-moderately iodine-deficient mothers—more than half of whom also suffered from hypothyroxinemia—were diagnosed with ADHD, whereas none of the children born to mothers originating from an iodine-sufficient area were diagnosed with ADHD (32). In a larger Norwegian cohort, maternal iodine intake <200 μg/d (which is lower than currently recommended in pregnancy) (33) as reported by a questionnaire at week 22 of gestation was also associated with higher ADHD symptoms but not with ADHD diagnosis (34). However, in that same cohort, the use of iodine-containing supplements was not associated with a lower risk of ADHD or a lower symptom score. In fact, children born to mothers with low iodine intake and who initiated iodine supplementation in the first trimester of pregnancy had a higher risk of ADHD (34). To the best of our knowledge, maternal iodine status has not been studied in relation to childhood ASD or autistic traits in large, prospective cohort studies. Against this background we carefully posit that iodine deficiency is related to a higher likelihood of ADHD or ASD. This hypothesis implies a threshold, i.e., nonlinear relation, because we have no evidence that, if sufficient, iodine is more protective at higher concentrations.
The primary aim of this study was to investigate the association of maternal iodine status during pregnancy with child ADHD and autistic traits. A second aim was to examine whether maternal iodine status modifies the association between maternal thyroid function and neurobehavioral outcomes.
Methods
Study design and population
The study was embedded in 3 population-based birth cohorts: Generation R (Netherlands) (35), the INfancia y Medio Ambiente Project (INMA) (Spain: Valencia, Sabadell, and Gipuzkoa) (36), and the Avon Longitudinal Study of Parents and Children (ALSPAC) (United Kingdom) (37, 38). Briefly, in Generation R, 9778 mothers from Rotterdam, Netherlands with a delivery date between April 2002 and January 2006 were enrolled. The INMA Project consists of 7 birth cohorts in Spain, of which 3 were included in the current research: Valencia (n = 855), Sabadell (n = 657), and Gipuzkoa (n = 638). Pregnant women from these 3 regions were enrolled from November 2003 until June 2005, July 2004 until July 2006, and April 2006 until January 2008, respectively. In ALSPAC, pregnant women resident in Avon, United Kingdom with expected delivery dates between April 1991 and December 1992 were invited to take part in the study. The initial number of pregnancies enrolled was 14,541, of which 13,998 children were alive at 1 y of age. The ALSPAC website contains all the data that are available, which can be accessed via a searchable data dictionary and variable search tool (39). Inclusion criteria for the current study were data availability of measures of urinary iodine and creatinine during pregnancy and an assessment of ADHD symptoms and/or autistic traits in childhood. Exclusion criteria were multiple fetuses, fertility treatment, thyroid-interfering medication use, and pre-existing thyroid disease (Figure 1). Women with undiagnosed thyroid disorder were not excluded. Ethical approval was obtained before recruitment from a number of bodies: the Medical Ethical Committee of the Erasmus Medical Center (Generation R), Ethical Committee of the Municipal Institute of Medical Investigation and the Ethical Committees of the hospitals involved in the study (INMA), and the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees; approval by parents or guardians of the children was given via a signed informed-consent form. The current study did not follow a prespecified registered protocol.
FIGURE 1.
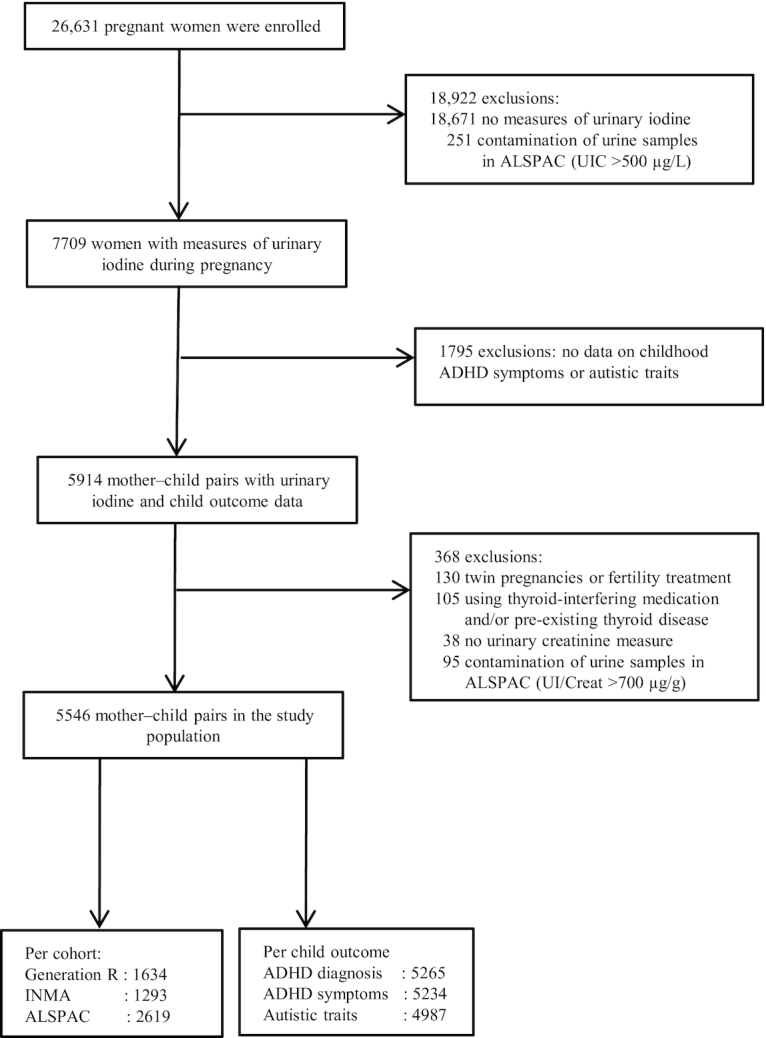
Flowchart of the study population. ADHD, attention-deficit hyperactivity disorder; ALSPAC, Avon Longitudinal Study of Parents and Children; INMA, INfancia y Medio Ambiente; UIC, urinary iodine concentration; UI/Creat, urinary iodine-to-creatinine ratio.
Maternal iodine status
UIC and creatinine were measured in spot-urine samples stored at −20°C after collection. UIC was measured in 3 different laboratories using different assays. Detailed information on the measurement methods is described elsewhere (29). To take into account hydration status, we used the iodine-to-creatinine ratio (UI/Creat) as a measure of iodine status. Owing to the possible use of iodine-containing test strips in ALSPAC, contamination of some urine samples in ALSPAC was suspected (40); hence, in this cohort only, women with a UIC >500 μg/L and/or a UI/Creat >700 μg/g were excluded from the analyses (Figure 1). These cutoffs were based on previous work in ALSPAC and from other studies of pregnant women in the United Kingdom (30, 41, 42).
Maternal thyroid function
In a previous study, we investigated whether maternal thyroid function was associated with child ADHD (21) and autistic traits (23). For the second aim of the current study—to test whether iodine modifies the association between thyroid function and neurodevelopmental outcomes—we used previously measured TSH and FT4 in maternal serum samples and created interaction terms with UI/Creat. In Generation R, serum samples were centrifuged and stored at −80°C after collection at a mean ± SD gestational age of 13.2 ± 1.8 wk. FT4 and TSH were measured using the Vitros ECi Immunodiagnostic System (Ortho Clinical Diagnostics) (43). Thyroid peroxidase antibodies (TPOAbs) were also measured using the Phadia 250 immunoassay analyzer (Phadia AB) and the manufacturer cutoff for TPOAb positivity was a thyroid peroxidase (TPO) titer ≥60 IU/mL. In INMA, serum samples were stored at −80°C after collection at a mean ± SD gestational age of 13.2 ± 1.4 wk. FT4 and TSH were measured using a solid-phase, time-resolved sandwich fluoro-immunoassay (AutoDEL-FIA, PerkinElmer Life and Analytical Sciences, Wallac Oy) and a lanthanide metal europium label (44). TPOAbs were not measured. In ALSPAC, serum samples were collected at a mean ± SD gestational age of 10.3 ± 2.7 wk and stored at −20°C. FT4, TSH, and TPOAb measurements were performed using the Abbott Architect i2000 (17). The manufacturer cutoff for TPOAb positivity was a TPO titer ≥6 IU/mL.
ADHD symptoms
In Generation R, ADHD symptoms were rated by parents at a mean ± SD age of 5.8 ± 0.2 y using the DSM-oriented scale Attention-Deficit/Hyperactivity of the Child Behavioral Checklist for ages 1.5–5 y (CBCL1½–5) (45). This scale consists of 6 questions on a 3-point Likert scale, the sum score constituting the total ADHD symptom rating. The CBCL1½–5 was chosen at the time of follow-up, because the majority of children were expected to be younger than 6 y old at assessment and the CBCL1½–5 was collected at 2 earlier time points (i.e., 18 mo and 3 y) and thus chosen for continuity reasons. All subscales of the CBCL1½–5 showed Cronbach's αs ranging from 0.60 to 0.89, and are the same for 5-y-old children and children older than 5 y (46). Next, positive screens [i.e., children who scored in the top 15 percentiles of the CBCL1½–5 total problem score and/or in the top 2% of the syndrome scale scores; scores above the 97th percentile are in the clinical range (45)] were invited for further assessment with the Diagnostic Interview Schedule for Children—Young Child version (DISC-YC) (47). This DSM-IV-based interview was used to establish an ADHD diagnosis and was conducted with parents or caregivers by trained research assistants at a mean ± SD age of 6.6 ± 0.4 y. More detailed information on the procedures and the DISC-YC assessment is described elsewhere (48).
In INMA, ADHD symptoms were assessed by teachers by means of the ADHD criteria of the DSM fourth edition (DSM-IV) (49) at a mean ± SD age of 5.9 ± 0.3 y in Valencia, 4.4 ± 0.3 y in Sabadell, and 4.4 ± 0.2 y in Gipuzkoa. The DSM-IV consists of questions on 9 inattention symptoms and 9 hyperactivity-impulsivity symptoms on a 4-point Likert scale. The sum score of these 18 questions constituted the total symptom score. Based on the symptom criteria of the DSM-IV, ADHD was diagnosed when the child had ≥6 inattention and/or hyperactivity-impulsivity symptoms.
In ALSPAC, inattention and hyperactivity symptoms were assessed through a parental semistructured interview as part of the Development and Well-Being Assessment (DAWBA) at a mean ± SD age of 7.7 ± 0.1 y (50). The total symptom score consisted of the sum of the inattention and hyperactivity-impulsivity symptoms. In addition, teachers completed the DAWBA questionnaire for half of all children (51). Data from the interview and/or questionnaire were used to assign an ADHD diagnosis following the DSM-IV symptom criteria.
Autistic traits
Autistic traits in children were measured by assessing the number of symptoms common to ASD. In Generation R, parents completed the Social Responsiveness Scale (SRS) questionnaire at a mean ± SD child age of 5.9 ± 0.2 y (52). We used the short version with 18 items, including 4-point Likert-scale questions on social cognition, social communication, and stereotypical behavior. The correlation between the full SRS score and the shortened SRS version is 0.93–0.99, as shown in 3 different studies (53). The complete 18-item version of the SRS is provided elsewhere (54).
In INMA, autistic traits were assessed using the Childhood Autism Spectrum Test, which was administered to the parents by a psychologist at a mean ± SD child age of 5.8 ± 0.2, 4.5 ± 0.2, and 4.5 ± 0.1 y in the regions of Valencia, Sabadell, and Gipuzkoa, respectively (55). The sum score of 31 items, which could be answered with only 2 response options, yielded the total sum score.
In ALSPAC, autistic traits were assessed using the Social Communication Disorder Checklist by parents at a mean ± SD child age of 7.6 ± 0.1 y (56). This questionnaire with a total of 12 items on a 3-point Likert scale covered questions on social reciprocity, nonverbal skills, pragmatic language usage, and functional impairment. The ratings of these 12 items were summed to obtain a total score.
Covariates
Covariates were chosen based on prior knowledge and a directed acyclic graph (Supplemental Figure 1), and available for all cohorts. Information on maternal age, parity (0, 1, ≥2), prepregnancy BMI, smoking during pregnancy (never, smoked in the beginning or until pregnancy confirmed, continued smoking), ethnicity/country of birth (cohort-specific categories), and maternal educational level (low, middle, high) was collected through questionnaires during pregnancy. Gestational age at urine and blood sampling was defined using ultrasound and/or last menstrual period. Information on sex of the child was obtained from community midwives, obstetricians, hospital registries, clinical records, or questionnaires. Child age was obtained at the time of the ascertainment of ADHD symptoms and autistic traits. All further analyses were adjusted for the mentioned covariates.
Statistical analyses
We imputed missing values of the covariates (0%–11.3% missing; see Table 1) by chained equations and generated 25 imputed data sets (57). Because our study population differed from those mother–child pairs who were lost to follow-up (Supplemental Table 1), we used inverse probability weighting (58). First, we predicted the probability of participation in the study with the characteristics of all participants at recruitment, and then applied the inverse of this probability as weights in all analyses.
TABLE 1.
Population characteristics1
| Generation R (n = 1634) | INMA (n = 1293) | ALSPAC (n = 2619) | ||||
|---|---|---|---|---|---|---|
| n | Values | n | Values | n | Values | |
| ADHD,2 % | 1588 | 3.5 | 1066 | 5.0 | 2611 | 1.7 |
| Autistic traits ≥93rd percentile, % | 1291 | 7.4 | 1111 | 8.5 | 2585 | 8.0 |
| Iodine status, all women | ||||||
| UI/Creat, μg/g | 1634 | 212 (153–291) | 1293 | 168 (110–255) | 2619 | 131 (88–203) |
| UI/Creat <150 μg/g, % | 1634 | 23.4 | 1293 | 43.1 | 2619 | 58.7 |
| Gestational age, wk | 1634 | 16.1 (15.0–17.3) | 1293 | 20.6 (19.5–21.8) | 2619 | 13.0 (9.2–17.0) |
| Iodine status at ≤18 wk | ||||||
| UI/Creat, μg/g | 1555 | 211 (141–309) | 1161 | 154 (97–259) | 2403 | 125 (85–198) |
| UI/Creat <150 μg/g, % | 1555 | 28.0 | 1161 | 48.5 | 2403 | 61.0 |
| Gestational age, wk | 1555 | 12.9 (12.1–14.4) | 1161 | 12.9 (12.3–13.7) | 2403 | 12.0 (9.0–15.0) |
| Iodine status at ≤14 wk | ||||||
| UI/Creat, μg/g | 1082 | 210 (141–303) | 952 | 157 (99–265) | 1530 | 111 (75–165) |
| UI/Creat <150 μg/g, % | 1082 | 28.0 | 952 | 47.5 | 1530 | 69.7 |
| Gestational age, wk | 1082 | 12.4 (11.6–13.1) | 952 | 12.7 (12.1–13.3) | 1530 | 10.0 (8.0–12.0) |
| Maternal thyroid function | ||||||
| TSH, mIU/L | 1451 | 1.32 (0.81–2.01) | 1251 | 1.25 (0.84–1.80) | 965 | 0.98 (0.64–1.40) |
| FT4, pmol/L | 1459 | 14.5 (13.0–16.4) | 1253 | 10.6 (9.7–11.6) | 970 | 16.2 (14.9–17.6) |
| TPOAb positivity, % | 1470 | 5.4 | NA | 973 | 12.7 | |
| Gestational age, wk | 1460 | 13.2 ± 1.8 | 1252 | 13.2 ± 1.4 | 979 | 10.3 ± 2.7 |
| Female sex, % | 1634 | 50.1 | 1292 | 49.8 | 2619 | 50.7 |
| Educational level,3 % | 1580 | 1289 | 2570 | |||
| Low | 6.5 | 21.0 | 18.7 | |||
| Middle | 39.7 | 41.1 | 62.8 | |||
| High | 53.9 | 37.6 | 18.5 | |||
| Maternal ethnicity/country of birth, % | 1633 | 1291 | 2562 | |||
| Majority4 | 56.8 | 93.5 | 98.6 | |||
| Minority5 | 43.2 | 6.5 | 1.4 | |||
| Maternal age, y | 1634 | 30.8 ± 4.6 | 1281 | 31.6 ± 3.9 | 2619 | 28.7 ± 4.4 |
| Parity, % | 1634 | 1291 | 2545 | |||
| 0 | 60.0 | 56.2 | 47.6 | |||
| 1 | 28.7 | 37.1 | 34.0 | |||
| ≥2 | 11.3 | 6.6 | 18.5 | |||
| Smoking during pregnancy, % | 1490 | 1293 | 2586 | |||
| Never | 76.6 | 69.9 | 84.4 | |||
| In the beginning of pregnancy | 10.0 | 13.2 | 3.7 | |||
| Continued | 13.4 | 16.9 | 11.9 | |||
| Prepregnancy BMI, kg/m2 | 1450 | 22.6 (20.8–25.1) | 1293 | 22.5 (20.8–25.0) | 2417 | 22.2 (20.5–24.4) |
Values are means ± SDs, medians (IQRs), or percentages. Values are shown without multiple imputation (percentages of missing data: 0.0%, 0.1%, and 0.0% for child sex; 3.3%, 0.3%, and 1.9% for maternal education; 0.1%, 0.2%, and 2.1% for maternal ethnicity/country of birth; 0.1%, 0.9%, and 2.2% for maternal age; 0.0%, 0.2%, and 2.8% for parity; 8.8%, 1.3%, and 1.3% for smoking; and 11.3%, 0.0%, and 7.7% for prepregnancy BMI in Generation R, INMA, and ALSPAC, respectively). ADHD, attention-deficit hyperactivity disorder; ALSPAC, Avon Longitudinal Study of Parents and Children; FT4, free thyroxine; INMA, INfancia y Medio Ambiente; NA, not available; TPOAb, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; UI/Creat, urinary iodine-to-creatinine ratio.
ADHD diagnosis was established by interview but not confirmed by medical-record data.
Generation R: low = no education or primary; middle = secondary phase 1 and 2; high = higher phase 1 and 2; INMA: low = no education, unfinished primary, or primary; middle = secondary; high = university degree; ALSPAC: low = no qualification, certificate of secondary education, or vocational; middle = O level or A level; high = a degree.
Defined as Dutch (Generation R), Spanish (INMA), or white (ALSPAC).
Defined as non-Dutch (Generation R), non-Spanish (INMA), or nonwhite (ALSPAC).
A proportion of women had multiple measurements of UIC and creatinine throughout pregnancy (Supplemental Table 2). To have a measure of average fetal iodine availability during the course of pregnancy, we calculated a geometric mean of the UI/Creat values for these women, which is a measure that is less susceptible to outliers than the arithmetic mean. A geometric mean was also calculated to have a measure of average gestational age at the time of measurement. The continuous UI/Creat measures were transformed by the natural logarithm to achieve a normal distribution. We grouped women into 2 groups: those with a UI/Creat <150 μg/g or a UI/Creat ≥150 μg/g. The former cutoff relates to iodine deficiency based on the WHO median UIC classification (33), and when adjusted for creatinine has been used previously (28–30, 59, 60).
We studied the associations of UI/Creat <150 μg/g and UI/Creat on a continuous scale with ADHD or a high autistic-trait score, the latter defined as a score ≥93rd percentile, using multivariable logistic regression in each cohort separately. The reference group consisted of women with a UI/Creat ≥150 μg/g. The 93rd-percentile cutoff was derived from a Dutch norm sample as a cutoff score to define children with problem behavior using the DSM-oriented scales of the CBCL (61). In the absence of a normative sample in INMA and ALSPAC, we also used the 93rd-percentile cutoff scores in these 2 cohorts. We did not use a cutoff that defines autistic traits within the clinical range, as we have used previously (23), because of the low prevalence of children with such a score. The cohort-specific estimates were combined using random-effects meta-analysis (termed “pooled analysis” in this article). Statistical heterogeneity was explored and quantified using the Cochran Q test and the I2 statistic (62). Because the fetus is largely dependent on the thyroidal state of the mother during early pregnancy (63), we wanted to investigate whether there is a particularly high risk of neurobehavioral outcomes in childhood in the offspring born to women with (mild-to-moderate) iodine deficiency in early pregnancy. Therefore, we repeated the analysis in those mother–child pairs, in which the mothers had ≥1 measure of urinary iodine at ≤18 weeks of gestation and in those with ≥1 measure at ≤14 weeks of gestation. The pregnancy period of ≤14 wk was chosen because our previous study indicated that low iodine status within this time window, but not thereafter, was associated with low child verbal IQ (29). For women with 2 available measures of urinary iodine and creatinine in early pregnancy (≤18 wk: Generation R, n = 0; INMA, n = 0; ALSPAC, n = 306; ≤14 wk: Generation R, n = 0; INMA, n = 0; ALSPAC, n = 27), a geometric mean of the 2 UI/Creat values and of the gestational age at the time of measurement was calculated.
We conducted several sensitivity analyses supporting the primary aim of the current study. First, we repeated all analyses using the UIC as an indicator of iodine status instead of UI/Creat. For these UIC analyses we re-added mother–child pairs that were excluded from the UI/Creat analyses due to missing creatinine data (n = 38 mother–child pairs from INMA) or those that were excluded due to possible contamination of urine samples (i.e., UI/Creat >700 μg/g; n = 95 mother–child pairs from ALSPAC). Although correcting UIC for creatinine takes into account the hydration status and better reflects the 24-h iodine excretion than UIC alone (64), the median UIC is recommended by the WHO to assess the iodine status of a population (33). Second, considering that the distribution of ADHD symptoms and autistic traits in a population is on a continuous spectrum, we also investigated the association of UI/Creat, either <150 μg/g or on a continuous scale, with ADHD symptoms and autistic traits as count scores using negative binomial regression models. The symptom scores were not comparable between cohorts because they did not share a common metric and therefore the associations were analyzed and presented by cohort.
Next, we studied whether the associations of maternal FT4 and TSH with ADHD and a high autistic-trait score differed depending on the iodine status of the mother. First, FT4 and TSH concentrations were logarithmically transformed to approach normality. To take into account the varying assays, cohort-specific SD scores were calculated with a mean of 0 and an SD of 1. These SD scores were based on the data of TPOAb-negative women or all women if TPOAb status was unknown (i.e., in INMA). FT4 and TSH SD scores outside the mean ± 4 SD range were considered as outliers and excluded from further analyses. The associations of FT4 SD scores and TSH SD scores with ADHD and a high-autistic trait score were assessed using multivariable logistic regression per cohort. The cohort-specific effect estimates were combined in random-effects meta-analyses (65). The time of thyroid function measurements coincided in a high proportion of women with the time of the first available measurement of UI/Creat. We therefore used the latter to stratify these associations into 2 groups of mother–child pairs: those in which the mother had a UI/Creat <150 μg/g and those that had a UI/Creat value ≥150 μg/g. Interaction of FT4 or TSH SD scores with UI/Creat in relation to ADHD and autistic traits was also formally tested per cohort by adding a product interaction term in the cohort-specific models. As a sensitivity analysis, we examined whether excluding TPOAb-positive women changed the association of maternal thyroid function with ADHD and autistic traits. All statistical analyses were performed in STATA version 15.0 (StataCorp.). Values were considered statistically significant at P < 0.05.
Results
A total of 5546 mother–child pairs were included (Figure 1). The iodine status of the 3 cohorts differed; the median UI/Creat in pregnancy was 212 μg/g in Generation R, 168 μg/g in INMA, and 131 μg/g in ALSPAC (Table 1). The median UIC was 178 μg/L [adequate intake, i.e., median UIC in the range 150–249 μg/L (33)], 134 μg/L [inadequate intake, i.e., median UIC <150 μg/L (33)], and 98 μg/L (inadequate intake) in Generation R, INMA, and ALSPAC, respectively. A total of 1290 (78.9%), 929 (71.8%), and 412 (15.7%) women had 2–4 repeated measurements of UI/Creat in Generation R, INMA, and ALSPAC, respectively (Supplemental Table 2). Women with repeated measures in INMA and ALSPAC differed in several characteristics from those that only provided a single urine sample. This may reflect the fact that repeated measures are conditional to early study inclusion. Moreover, the concentration of the first UI/Creat sample of women with repeated measurements in ALSPAC was lower than that of later measurements, and also lower than that of women with only a single measurement, possibly reflecting gestational changes.
ADHD
Children born to women with a UI/Creat <150 μg/g during pregnancy (i.e., “iodine deficiency”) were not at greater risk of ADHD in the pooled analysis than those born to women with UI/Creat ≥150 μg/g (OR: 1.2; 95% CI: 0.7, 2.2; P = 0.56; I2 = 66.5%; P for heterogeneity = 0.051) (Figure 2). In Generation R, UI/Creat <150 μg/g was associated with a 2.0-fold higher risk of ADHD (95% CI: 1.2, 3.5; P = 0.014) (Figure 2). Our random-effects meta-analysis also shows no association of UI/Creat <150 μg/g in the gestational age period of ≤18 wk or ≤14 wk with ADHD (Figure 2). When UI/Creat was analyzed continuously, there was no association between UI/Creat and ADHD. Again, only in Generation R a 1-unit increase in the natural logarithm of UI/Creat was associated with a 60% lower relative risk of ADHD (OR: 0.4; 95% CI: 0.2, 0.7; P < 0.001; Supplemental Figure 2). UIC was not associated with ADHD (Supplemental Figures 3 and 4). Similarly to UI/Creat, lower UIC was associated with a higher risk of ADHD in the Generation R cohort only (Supplemental Figures 3 and 4). UI/Creat, modeled either categorically or on a continuous scale, was not associated with ADHD symptoms on a continuous scale in any of the 3 cohorts (Supplemental Tables 3 and 4, respectively).
FIGURE 2.
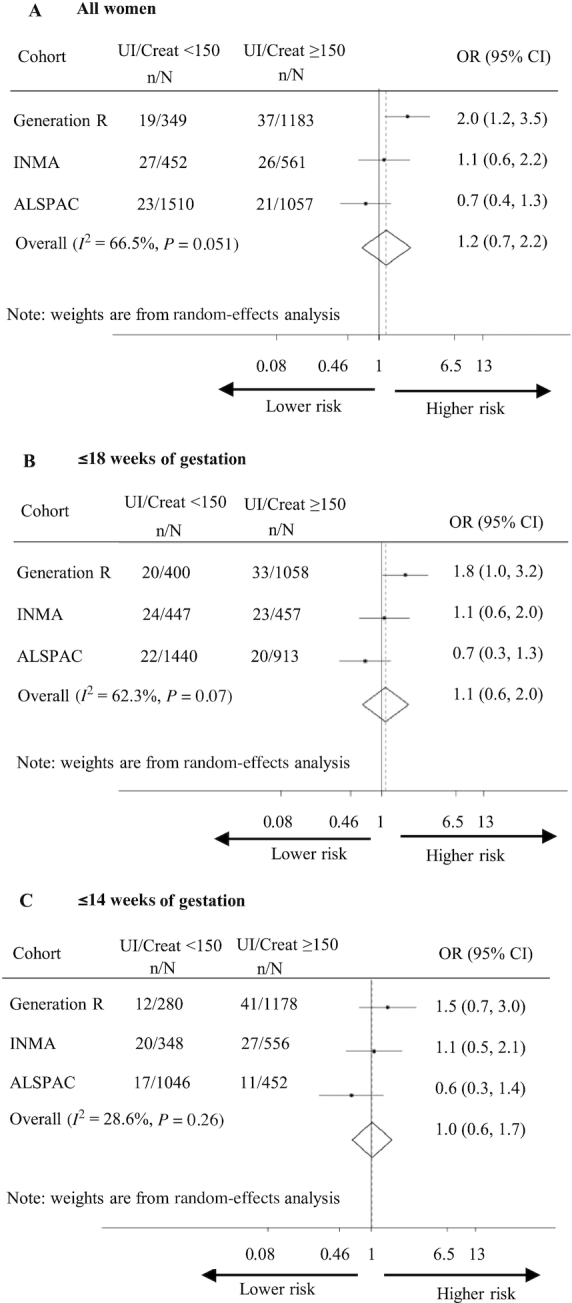
Association of maternal UI/Creat <150 μg/g with child ADHD. Associations depicted as OR (dot) with 95% CI per cohort and overall associations as estimated by random-effects meta-analysis (diamond) in (A) all mother–child pairs, (B) those with ≥1 measure of UI/Creat at ≤18 weeks of gestation, and (C) those with ≥1 measure of UI/Creat at ≤14 weeks of gestation. Analyses adjusted for maternal age, parity, prepregnancy BMI, smoking during pregnancy, ethnicity/country of birth, maternal educational level, gestational age at urine sampling, child sex, child age, and subcohort in INMA. n = children with ADHD, N = children without ADHD. ADHD, attention-deficit hyperactivity disorder; ALSPAC, Avon Longitudinal Study of Parents and Children; INMA, INfancia y Medio Ambiente; UI/Creat, urinary iodine-to-creatinine ratio.
A high autistic-trait score
Children born to women with UI/Creat <150 μg/g during pregnancy were not at greater risk of a high autistic-trait score (OR: 0.8; 95% CI: 0.6, 1.1; P = 0.22; I2 = 30.4%; P for heterogeneity = 0.24) in the pooled analysis than those born to women with UI/Creat ≥150 μg/g (Figure 3). In the Generation R cohort only, UI/Creat <150 μg/g was associated with a 50% lower relative risk of a high autistic-trait score (OR: 0.5; 95% CI: 0.3, 1.0; P = 0.035) (Figure 3). Further pooled analyses in those with a urinary iodine assessment in the gestational age period of ≤18 wk or ≤14 wk also showed no association between UI/Creat <150 μg/g and a high autistic-trait score (Figure 3). Next, we performed an analysis of continuously modeled UI/Creat concentrations; a 1-unit increase in the natural logarithm of UI/Creat was associated with a 1.2-fold higher risk of a high autistic-trait score (95% CI: 1.0, 1.5; P = 0.044; I2 = 0.0%; P for heterogeneity = 0.63) (Supplemental Figure 5). The latter effect estimates were similar when this association was investigated in the 2 early time periods during pregnancy (Supplemental Figure 5). UIC, modeled either categorically or continuously, was not associated with a high autistic-trait score in any of the cohorts (Supplemental Figures 6 and 7, respectively). UI/Creat, modeled either as <150 μg/g or on a continuous scale, was not associated with autistic traits on a continuous scale in any of the 3 cohorts (Supplemental Tables 3 and 4, respectively).
FIGURE 3.

Association of maternal UI/Creat <150 μg/g with a high child autistic-trait score ≥93rd percentile. Associations depicted as OR (dot) with 95% CI per cohort and overall associations as estimated by random-effects meta-analysis (diamond) in (A) all mother–child pairs, (B) those with ≥1 measure of UI/Creat at ≤18 weeks of gestation, and (C) those with ≥1 measure of UI/Creat at ≤14 weeks of gestation. Analyses adjusted for maternal age, parity, prepregnancy BMI, smoking during pregnancy, ethnicity/country of birth, maternal educational level, gestational age at urine sampling, child sex, child age, and subcohort in INMA. n = children with a score >93rd percentile, N = children with a score <93rd percentile. ALSPAC, Avon Longitudinal Study of Parents and Children; INMA, INfancia y Medio Ambiente; UI/Creat, urinary iodine-to-creatinine ratio.
Maternal thyroid function and child ADHD and autistic traits
Neither FT4 nor TSH concentrations nor TPOAb positivity rates differed between women with UI/Creat <150 μg/g or ≥150 μg/g (Supplemental Table 5). A 1-unit increase in the FT4 SD score was associated with a 1.3-fold higher risk of ADHD (95% CI: 1.0, 1.6; P = 0.017; I2 = 0.0%; P for heterogeneity = 0.93) (Table 2). This association was not modified by UI/Creat (P for interaction = 0.70, 0.40, 0.96, in Generation R, INMA, and ALSPAC, respectively). TSH was not associated with ADHD (OR: 0.8; 95% CI: 0.7, 1.0; P = 0.11; I2 = 0.0%; P for heterogeneity = 0.63) (Table 2). This association was not modified by UI/Creat (P for interaction = 0.57, 0.54, and 0.09 in Generation R, INMA, and ALSPAC, respectively).
TABLE 2.
Association of FT4 and TSH with ADHD in all mother–child pairs and stratified by groups of UI/Creat1
| ADHD2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| FT4 | TSH | ||||||||
| Subgroup | Cohort | n/N3 | OR (95% CI) | P | I 2 (P)4 | n/N3 | OR (95% CI) | P | I 2 (P)5 |
| All mother–child pairs | Pooled | 117/3295 | 1.3 (1.0, 1.6) | 0.017 | 0.0% (0.93) | 114/3266 | 0.8 (0.7, 1.0) | 0.11 | 0.0% (0.63) |
| Generation R | 51/1362 | 1.3 (0.9, 1.7) | 0.13 | 0.70 | 50/1359 | 0.8 (0.6, 1.0) | 0.08 | 0.57 | |
| INMA | 53/979 | 1.2 (0.9, 1.7) | 0.16 | 0.40 | 52/970 | 0.9 (0.6, 1.3) | 0.57 | 0.54 | |
| ALSPAC | 13/954 | 1.4 (0.8, 2.4) | 0.22 | 0.96 | 12/937 | 1.0 (0.5, 2.1) | 0.93 | 0.09 | |
| UI/Creat <150 μg/g | Pooled | 57/1497 | 1.3 (1.0, 1.7) | 0.08 | 0.0% (0.80) | 56/1481 | 0.8 (0.6, 1.1) | 0.15 | 0.0% (0.75) |
| Generation R | 21/381 | 1.5 (0.9, 2.4) | 0.12 | NA | 21/379 | 0.8 (0.5, 1.3) | 0.43 | NA | |
| INMA | 28/477 | 1.3 (0.8, 1.9) | 0.31 | NA | 27/472 | 0.7 (0.4, 1.2) | 0.15 | NA | |
| ALSPAC | 8/639 | 1.1 (0.5, 2.3) | 0.80 | NA | 8/630 | 0.9 (0.4, 2.2) | 0.89 | NA | |
| UI/Creat ≥150 μg/g | Pooled | 60/1798 | 1.4 (0.9, 2.2) | 0.13 | 39.4% (0.19) | 58/1785 | 0.8 (0.6, 1.1) | 0.22 | 7.9% (0.34) |
| Generation R | 30/981 | 1.1 (0.7, 1.7) | 0.59 | NA | 29/980 | 0.7 (0.4, 1.0) | 0.049 | NA | |
| INMA | 25/502 | 1.4 (0.9, 2.2) | 0.17 | NA | 25/498 | 1.0 (0.6, 1.6) | 0.98 | NA | |
| ALSPAC | 5/315 | 3.8 (1.1, 13.1) | 0.038 | NA | <5/307 | 1.5 (0.2, 10.3) | 0.68 | NA | |
The pooled estimate represents the overall effect estimates (OR with 95% CI) calculated with a random-effects meta-analysis. ADHD, attention-deficit hyperactivity disorder; ALSPAC, Avon Longitudinal Study of Parents and Children; FT4, free thyroxine; INMA, INfancia y Medio Ambiente; NA, not applicable; TSH, thyroid-stimulating hormone; UI/Creat, urinary iodine-to-creatinine ratio.
ADHD diagnosis was established by interview but not confirmed by medical-record data.
n represents the number of children with ADHD; N represents the number of children without ADHD.
Values represent quantification of statistical heterogeneity using the I2 statistic (P for heterogeneity of the Cochran Q test) or represent the cohort-specific P for interaction between the FT4 SD score and UI/Creat in relation to ADHD.
Values represent quantification of statistical heterogeneity using the I2 statistic (P for heterogeneity of the Cochran Q test) or represent the cohort-specific P for interaction between the TSH SD score and UI/Creat in relation to ADHD.
FT4 was not associated with a high autistic-trait score (OR: 1.1; 95% CI: 0.9, 1.2; P = 0.27; I2 = 0.0%; P for heterogeneity = 0.27) (Table 3). This association was not modified by UI/Creat (P for interaction 0.48, 0.82, and 0.11 in Generation R, INMA, and ALSPAC, respectively). TSH was not associated with a high autistic-trait score (OR: 0.9; 95% CI: 0.8, 1.1; P = 0.46; I2 = 6.2%; P for heterogeneity = 0.34) (Table 3). A statistically significant effect modification by UI/Creat was only seen in INMA (P for interaction = 0.007), showing that higher TSH is associated with higher risk of a high autistic-trait score when the mother has UI/Creat <150 μg/g (OR: 1.7; 95% CI: 1.0, 2.8; P = 0.049) (Table 3). However, when we combined the 3 cohorts using a random-effects meta-analysis, this association was not apparent (Table 3). Excluding TPOAb-positive women from Generation R and ALSPAC (information on TPOAb status was only available in these 2 cohorts) yielded similar results (data not shown).
TABLE 3.
Association of FT4 and TSH with a high autistic-trait score ≥93rd percentile in all mother–child pairs and stratified by groups of UI/Creat1
| High autistic-trait score ≥93rd percentile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| FT4 | TSH | ||||||||
| Subgroup | Cohort | n/N2 | OR (95% CI) | P | I 2 (P)3 | n/N2 | OR (95% CI) | P | I 2 (P)4 |
| All mother–child pairs | Pooled | 255/2920 | 1.1 (0.9, 1.2) | 0.27 | 0.0% (0.27) | 210/2441 | 0.9 (0.8, 1.1) | 0.46 | 6.2% (0.34) |
| Generation R | 85/1062 | 1.1 (0.9, 1.3) | 0.69 | 0.48 | 84/1056 | 1.0 (0.8, 1.3) | 0.81 | 0.33 | |
| INMA | 88/985 | 1.1 (0.9, 1.4) | 0.50 | 0.82 | 46/528 | 1.0 (0.8, 1.3) | 0.98 | 0.007 | |
| ALSPAC | 82/873 | 1.1 (0.9, 1.4) | 0.45 | 0.11 | 80/857 | 0.8 (0.6, 1.0) | 0.10 | 0.45 | |
| UI/Creat <150 μg/g | Pooled | 121/1330 | 0.9 (0.7, 1.1) | 0.42 | 0.0% (0.98) | 124/1396 | 1.1 (0.8, 1.6) | 0.61 | 53.8% (0.11) |
| Generation R | 21/299 | 1.0 (0.6, 1.6) | 0.86 | NA | 21/297 | 1.0 (0.6, 1.7) | 0.97 | NA | |
| INMA | 42/452 | 0.9 (0.6, 1.4) | 0.64 | NA | 46/528 | 1.7 (1.0, 2.8) | 0.049 | NA | |
| ALSPAC | 58/579 | 0.9 (0.7, 1.2) | 0.51 | NA | 57/571 | 0.9 (0.7, 1.2) | 0.45 | NA | |
| UI/Creat ≥150 μg/g | Pooled | 134/1590 | 1.2 (1.0, 1.5) | 0.06 | 13.6% (0.31) | 132/1573 | 0.8 (0.7, 1.0) | 0.12 | 18.1% (0.29) |
| Generation R | 64/763 | 1.1 (0.8, 1.4) | 0.55 | NA | 63/759 | 1.0 (0.8, 1.3) | 0.85 | NA | |
| INMA | 46/533 | 1.2 (0.9, 1.7) | 0.26 | NA | 46/528 | 0.8 (0.5, 1.1) | 0.15 | NA | |
| ALSPAC | 24/294 | 1.6 (1.0, 2.5) | 0.032 | NA | 23/286 | 0.7 (0.4, 1.1) | 0.08 | NA | |
The pooled estimate represents the overall effect estimates (OR with 95% CI) calculated with a random-effects meta-analysis. ALSPAC, Avon Longitudinal Study of Parents and Children; FT4, free thyroxine; INMA, INfancia y Medio Ambiente; NA, not applicable; TSH, thyroid-stimulating hormone; UI/Creat, urinary iodine-to-creatinine ratio.
n represents the number of children with a high autistic-trait score ≥93rd percentile; N represents the number of children with an autistic trait score <93rd percentile.
Values represent quantification of statistical heterogeneity using the I2 statistic (P for heterogeneity of the Cochran Q test) or represent the cohort-specific P for interaction between the FT4 SD score and UI/Creat in relation to a high autistic-trait score.
Values represent quantification of statistical heterogeneity using the I2 statistic (P for heterogeneity of the Cochran Q test) or represent the cohort-specific P for interaction between the TSH SD score and UI/Creat in relation to a high autistic-trait score.
Discussion
This meta-analysis of individual-participant data from 3 large cohorts showed no consistent evidence to support an association of maternal iodine status with child ADHD or autistic traits in the general population. The association of maternal FT4 with child ADHD was not affected by the iodine status of the mother.
This study was performed against the background of mild-to-moderate iodine deficiency being a common problem among pregnant women (24) that has been associated with lower IQ scores (28–30), suboptimal reading accuracy and comprehension (30), poorer spelling (66, 67), reduced receptive and expressive language skills (68), worse executive function (69), poorer fine motor skills (70), internalizing and externalizing problems (70), and higher ADHD symptom scores (34). Separate studies within Generation R or INMA reported no evidence for an association between UIC and language comprehension at the age of 6 y (59) or cognitive and psychomotor development measured at 1 y of age (71, 72). The current meta-analysis of individual-participant data from 3 different studies also finds no support for an association between maternal iodine status and child ADHD or autistic traits.
There may be several explanations as to why no association was observed in this study. Firstly, although use of urinary iodine concentration is recommended to determine population iodine status, it is only a crude proxy for individual iodine status owing to large day-to-day variability (73, 74). Although it is assumed that a low excretion of iodine reflects a low recent iodine intake, it is uncertain how well this reflects the ability of a person to utilize the available iodine supply for thyroid hormone synthesis, or whether this reflects an iodine-depleted thyroid. Second, it is suggested that iodine deficiency before preconception and in early pregnancy may constitute a risk factor for neurodevelopmental problems (28–30). Hence, optimal iodine intake needs to be achieved in early pregnancy, and preferably before conception to anticipate the increased need for thyroid hormone production during pregnancy (75, 76). On the assumption that the urine collection may have occurred too late in pregnancy, we also investigated the association of iodine status in early pregnancy with neurodevelopmental problems (i.e., ≤18 and ≤14 wk), but maternal iodine status in these early time-windows was also not associated with child ADHD or autistic traits. Third, the clinical relevance of our outcome measures may be debated. Not all 3 cohorts obtained clinical diagnoses of ADHD and ASD, which may have led to (nondifferential) outcome misclassification. Against this, the questionnaires were valid quantitative measures of ADHD symptoms or autistic traits and have been extensively used in epidemiological studies.
Interestingly, only in the Generation R cohort, which is an overall iodine-sufficient population, was “iodine deficiency” associated with a higher risk of ADHD. These associations in the Generation R cohort only may seem counter-intuitive, because at population level, iodine deficiency in this population is relatively less severe and certainly less common than in the INMA or ALSPAC populations. The Netherlands has a well implemented iodine fortification program (77). The proportion of households consuming iodized salt is estimated to be 60%–70%, which is relatively high compared with Spain and the UK [16% and 2%, respectively (78)]. As such, an association between maternal iodine deficiency and child neurobehavioral problems might be less likely in Generation R than in INMA or ALSPAC. However, it has previously been suggested that iodine-deficient women with a more sporadic iodine supply may have a more efficient thyroidal uptake of iodine (79) and the strength of the association between iodine deficiency and child neurodevelopmental outcomes need not depend on the degree of iodine sufficiency in the population. Racial differences may also contribute to heterogeneity in results across cohorts. The Generation R cohort consists of a multiethnic population, whereas in the INMA and ALSPAC cohorts there is less ethnic variability. Whether genetic variation modifies the association between maternal iodine status and child neurobehavioral problems remains to be investigated.
The association between higher UI/Creat and a higher risk of autistic traits was unexpected. If not a chance finding, then this may be explained by the fact that more-than-adequate or excessive iodine intake in an iodine-replete population has previously been linked to maternal hypothyroidism and hypothyroxinemia (80); both of these have also been associated with a higher risk of ASD or autistic traits (15, 22). However, we did not identify differences in FT4 or TSH concentrations, or the TPOAb-positivity rates between the “iodine-deficient” group (i.e., UI/Creat <150 μg/g) and the “iodine-sufficient” group (i.e., UI/Creat ≥150 μg/g). Because iodine and thyroid measures were both taken in pregnancy, there is a possible lag time between low iodine status and impaired thyroid function.
The present study shows that the maternal FT4 concentration during pregnancy was associated with child ADHD, but maternal iodine status did not seem to underpin this association. First, the association between higher FT4 and child ADHD did not reach statistical significance in our previous analysis (21), which suggests that conditioning on iodine concentrations may have introduced a selection effect. Second, the cohort-specific analysis showed that, solely in INMA, a higher TSH was associated with a high child autistic-trait score in “iodine-deficient” mothers only. Iodine deficiency may induce TPOAb positivity (80), and the presence of these antibodies could potentially lead to impaired thyroid function, including higher TSH. Children born to TPOAb-positive mothers may be at a higher risk of ASD (81). Unfortunately, we could not investigate whether TPOAb positivity could explain why there was effect modification in the association between TSH and autistic traits in INMA, because TPOAb titers were not determined in this cohort.
We have performed random-effects meta-analyses because we assumed that differences in effect estimates across cohorts are not due to chance only. Despite having used individual-participant data to harmonize the analysis across cohorts, some degree of heterogeneity is inevitable. We previously discussed different factors that could contribute to heterogeneity in the results across cohorts, including the differing ages at assessment, types of evaluators (i.e., parents or teachers), and methodologies (21, 23). We explored and quantified the statistical heterogeneity. A high percentage of I2 (i.e., ≥75%) typically indicates that studies are highly heterogeneous and in the absence of strict criteria, it is up to the meta-analyst to decide whether the meta-analysis is meaningful or if it is better to present the cohort-specific effect estimates only (82). In the present study, several meta-analyses showed moderate statistical heterogeneity (i.e., I2 ∼50%). The only meta-analysis with a high I2 of 77.7% was that of the association of maternal UI/Creat with child autistic traits in the subgroup with ≥1 measure of UI/Creat in the first 14 wk of pregnancy. This finding should therefore be interpreted with caution.
This study enabled us to investigate the association of maternal iodine status during pregnancy with child neurobehavioral problems in a large population-based sample and to examine the heterogeneity of results across cohorts. This study has several potential limitations. Firstly, although the sample size was large enough to evaluate the iodine status of the population from 1–4 spot urine samples, this is insufficient for assessing individual iodine status (73, 83). Second, there is variability between urinary iodine measurements undertaken in different laboratories (84); however, the 3 laboratories that measured samples from these cohorts used certified reference materials to ensure accurate measurements. Next, the ascertainment of ADHD and autistic traits was performed at different ages by different instruments and evaluators, which may have introduced “noise” and heterogeneity. Furthermore, we had no medical-record data to confirm ADHD or ASD diagnosis or data on therapeutic drug use by the children in the study. Lastly, this meta-analysis was not conducted in the context of a systematic review. Ideally, meta-analyses of individual participant data should be performed by a systematic review that searches for both published and unpublished studies (85).
To conclude, no consistent evidence for an association of maternal iodine status with child ADHD and autistic traits was found across cohorts with differing iodine status.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The authors’ responsibilities were as follows—DL: performed the statistical analysis and wrote the paper; DL, SCB, MG, TIMK, MD, MPR, RPP, and HT: contributed to the study design; SCB, MG, TIMK, MD, MPR, RPP, EF, JMI, SL, MM, JS, and HT: revised the manuscript; HT: had the primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the European Union's Horizon 2020 research and innovation program under grant agreement 634453 (to DL, MG, SCB, TIMK, MD, MPR, JS, RPP, and HT). The Generation R study is conducted by the Erasmus Medical Center in close collaboration with the Faculty of Social Sciences of the Erasmus University Rotterdam; the Municipal Health Service Rotterdam area, Rotterdam; and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. The Generation R Study is supported by the Erasmus Medical Center, Rotterdam; the Erasmus University Rotterdam; the Netherlands Organization for Health Research and Development (ZonMw); the Netherlands Organization for Scientific Research; and the Ministry of Health, Welfare and Sport. A grant from the Sophia Children's Hospital Research Funds supports the neurodevelopmental work on thyroid; RPP is supported by a ZonMw VIDI grant, project number 1717331. HT is supported by a ZonMw VICI grant with personal grant number 016.VICI.170.200. The INMA study was funded by UE grants FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5-1; Spain: Instituto de Salud Carlos III grants Red INMA G03/176; CB06/02/0041; FIS-FEDER PI041436, PI05/1079, PI06/0867, PI081151, PI09/00090, PI11/01007, PI11/02591, PI11/02038, PI13/1944, PI13/2032, PI14/00891, PI14/01687, and PI16/1288; Miguel Servet-FEDER CP11/00178, CP15/00025, CPII16/00051, and MS13/00054; and Miguel Servet-FSE MS15/0025 and MSII16/0051; the Alicia Koplowitz Foundation 2017; Generalitat Valenciana: FISABIO grants UGP-15-230, UGP-15-244, and UGP-15-249; Generalitat de Catalunya-CIRIT grant 1999SGR 00241; Fundació La marató de TV3 grant 090430; Department of Health of the Basque Government grants 2005111093 and 2009111069; and Provincial Government of Gipuzkoa grants DFG06/004 and DFG08/001. The Avon Longitudinal Study of Parents and Children (ALSPAC) is supported by the UK Medical Research Council and Wellcome Trust grant 102215/2/13/2, and by the University of Bristol who provide core support for ALSPAC. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). The existing iodine measurements in ALSPAC were funded from 1) the NUTRIMENTHE project, which received a research grant from the European Community's 7th Framework Programme (FP7/2008–2013) under grant agreement 212652; and 2) a Ph.D. studentship that was funded by Wassen International and the Waterloo Foundation (2009–2012).
Author disclosures: The authors report no conflicts of interest.
This publication is the work of the authors and HT will serve as guarantor for the contents of this article.
Supplemental Figures 1–7 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: ADHD, attention-deficit hyperactivity disorder; ALSPAC, Avon Longitudinal Study of Parents and Children; ASD, Autism Spectrum Disorder; CBCL1½–5, Child Behavioral Checklist for ages 1.5–5 y; DAWBA, Development and Well-Being Assessment; DISC-YC, Diagnostic Interview Schedule for Children—Young Child version; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders fourth edition; FT4, free thyroxine; INMA, INfancia y Medio Ambiente; IQ, intelligence quotient; SRS, Social Responsiveness Scale; TPO, thyroid peroxidase; TPOAb, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; UIC, urinary iodine concentration; UI/Creat, urinary iodine-to-creatinine ratio.
References
- 1. Holtmann M, Bölte S, Poustka F. Attention deficit hyperactivity disorder symptoms in pervasive developmental disorders: association with autistic behavior domains and coexisting psychopathology. Psychopathology. 2007;40:172–7. [DOI] [PubMed] [Google Scholar]
- 2. Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–61. [DOI] [PubMed] [Google Scholar]
- 3. Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome?. J Atten Disord. 2009;13:117–26. [DOI] [PubMed] [Google Scholar]
- 4. Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–9. [DOI] [PubMed] [Google Scholar]
- 5. Jang J, Matson JL, Williams LW, Tureck K, Goldin RL, Cervantes PE. Rates of comorbid symptoms in children with ASD, ADHD, and comorbid ASD and ADHD. Res Dev Disabil. 2013;34:2369–78. [DOI] [PubMed] [Google Scholar]
- 6. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601–13. [DOI] [PubMed] [Google Scholar]
- 8. American Psychiatric Association. Neurodevelopmental disorders. In: Diagnostic and statistical manual of mental disorders. 5th ed Arlington (VA): American Psychiatric Association; 2013. [Google Scholar]
- 9. Rommelse NNJ, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19:281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavado-Autric R, Ausó E, García-Velasco JV, del Carmen Arufe M, Escobar del Rey F, Berbel P, Morreale de Escobar G. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111:1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122. [DOI] [PubMed] [Google Scholar]
- 12. Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. [DOI] [PubMed] [Google Scholar]
- 13. Päkkilä F, Männistö T, Pouta A, Hartikainen A-L, Ruokonen A, Surcel H-M, Bloigu A, Vääräsmäki M, Järvelin M-R, Moilanen I et al.. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab. 2014;99:E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VWV, Visser TJ, Visser W, de Muinck Keizer-Schrama SMPF, Hooijkaas H, Steegers EAP, Hofman A et al.. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the Generation R study. Pediatr Res. 2011;69:454–9. [DOI] [PubMed] [Google Scholar]
- 15. Andersen S, Laurberg P, Wu C, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121:1365–74. [DOI] [PubMed] [Google Scholar]
- 16. Modesto T, Tiemeier H, Peeters RP, Jaddoe VWV, Hofman A, Verhulst FC, Ghassabian A. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr. 2015;169:838–45. [DOI] [PubMed] [Google Scholar]
- 17. Fetene DM, Betts KS, Alati R. Maternal prenatal thyroid function and offspring ADHD: findings from the ALSPAC cohort. J Nerv Ment Dis. 2018;206:859–64. [DOI] [PubMed] [Google Scholar]
- 18. Oostenbroek MHW, Kersten RHJ, Tros B, Kunst AE, Vrijkotte TGM, Finken MJJ. Maternal hypothyroxinaemia in early pregnancy and problem behavior in 5-year-old offspring. Psychoneuroendocrinology. 2017;81:29–35. [DOI] [PubMed] [Google Scholar]
- 19. Chevrier J, Harley KG, Kogut K, Holland N, Johnson C, Eskenazi B. Maternal thyroid function during the second half of pregnancy and child neurodevelopment at 6, 12, 24, and 60 months of age. J Thyroid Res. 2011:426427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersen SL, Andersen S, Vestergaard P, Olsen J. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: a Danish nationwide case-cohort study. Thyroid. 2018;28:537–46. [DOI] [PubMed] [Google Scholar]
- 21. Levie D, Korevaar T, Mulder TA, Bath SC, Dineva M, Lopez-Espinosa M-J, Basterrechea M, Santa Marina L, Rebagliato M, Sunyer J et al.. Maternal thyroid function in early pregnancy and child attention-deficit hyperactivity disorder: an individual-participant meta-analysis. Thyroid. 2019;29:1316–26. [DOI] [PubMed] [Google Scholar]
- 22. Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VWV, Hofman A, de Rijke YB, Verhulst FC, Tiemeier H. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol. 2013;74:733–42. [DOI] [PubMed] [Google Scholar]
- 23. Levie D, Korevaar TIM, Bath SC, Dalmau-Bueno A, Murcia M, Espada M, Dineva M, Ibarluzea JM, Sunyer J, Tiemeier H et al.. Thyroid function in early pregnancy, child IQ, and autistic traits: a meta-analysis of individual participant data. J Clin Endocrinol Metab. 2018;103:2967–79. [DOI] [PubMed] [Google Scholar]
- 24. Iodine Global Network. Global scorecard of iodine nutrition 2017. [Internet] Zurich (Switzerland): Iodine Global Network;2017; [cited 2018 May 8]. Available from: http://www.ign.org/cm_data/IGN_Global_Map_PW_30May2017_1.pdf. [Google Scholar]
- 25. Gizak M, Rogers L, Gorstein J, Zimmermann M, Andersson M. Global iodine status in school-age children, women of reproductive age, and pregnant women in 2017. Poster presented at Nutrition 2018, the American Society for Nutrition annual conference, June 9–12, 2018, Boston, MA, USA. [Google Scholar]
- 26. Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008;372:1251–62. [DOI] [PubMed] [Google Scholar]
- 27. Zimmermann MB. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: a review. Thyroid. 2007;17:829–35. [DOI] [PubMed] [Google Scholar]
- 28. Robinson SM, Crozier SR, Miles EA, Gale CR, Calder PC, Cooper C, Inskip HM, Godfrey KM. Preconception maternal iodine status is positively associated with IQ but not with measures of executive function in childhood. J Nutr. 2018;148:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levie D, Korevaar TIM, Bath SC, Murcia M, Dineva M, Llop S, Espada M, van Herwaarden AE, de Rijke YB, Ibarluzea JM et al.. Association of maternal iodine status with child IQ: a meta-analysis of individual-participant data. J Clin Endocrinol Metab. 2019;104:5957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;382:331–7. [DOI] [PubMed] [Google Scholar]
- 31. Velasco I, Bath SC, Rayman MP. Iodine as essential nutrient during the first 1000 days of life. Nutrients. 2018;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G, Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisà A et al.. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–60. [DOI] [PubMed] [Google Scholar]
- 33. WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–11. [DOI] [PubMed] [Google Scholar]
- 34. Abel MH, Ystrom E, Caspersen IH, Meltzer HM, Aase H, Torheim LE, Askeland RB, Reichborn-Kjennerud T, Brantsæter AL. Maternal iodine intake and offspring attention-deficit/hyperactivity disorder: results from a large prospective cohort study. Nutrients. 2017;9:1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, de Jongste JC, Klaver CCW, van der Lugt A, Mackenbach JP et al.. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guxens M, Ballester F, Espada M, Fernández MF, Grimalt JO, Ibarluzea J, Olea N, Rebagliato M, Tardón A, Torrent M et al.. Cohort profile: the INMA—INfancia y Medio Ambiente—(Environment and Childhood) Project. Int J Epidemiol. 2012;41:930–40. [DOI] [PubMed] [Google Scholar]
- 37. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A et al.. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ALSPAC Executive. Explore data and samples. [Internet] Bristol (UK): University of Bristol; [cited 2018 Mar 12]. Available from: http://www.bris.ac.uk/alspac/researchers/our-data/. [Google Scholar]
- 40. Pearce EN, Lazarus JH, Smyth PP, He X, Smith DF, Pino S, Braverman LE. Urine test strips as a source of iodine contamination. Thyroid. 2009;19:919. [DOI] [PubMed] [Google Scholar]
- 41. Bath SC, Pop VJM, Furmidge-Owen VL, Broeren MAC, Rayman MP. Thyroglobulin as a functional biomarker of iodine status in a cohort study of pregnant women in the United Kingdom. Thyroid. 2017;27:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearce EN, Lazarus JH, Smyth PPA, He X, Dall'Amico D, Parkes AB, Burns R, Smith DF, Maina A, Bestwick JP et al.. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95:3207–15. [DOI] [PubMed] [Google Scholar]
- 43. Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SMPF, Jaddoe VVW, Hofman A, Hooijkaas H, Steegers EAP, Tiemeier H et al.. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R Study. J Clin Endocrinol Metab. 2012;97:646–52. [DOI] [PubMed] [Google Scholar]
- 44. Rebagliato M, Murcia M, Espada M, Álvarez-Pedrerol M, Bolúmar F, Vioque J, Basterrechea M, Blarduni E, Ramón R, Guxens M et al.. Iodine intake and maternal thyroid function during pregnancy. Epidemiology. 2010;21:62–9. [DOI] [PubMed] [Google Scholar]
- 45. Achenbach TM, Rescorla LA. ASEBA preschool forms & profiles. Burlington (VT): University of Vermont, Research Center for Children, Youth and Families; 2000. [Google Scholar]
- 46. Basten M, Tiemeier H, Althoff RR, van de Schoot R, Jaddoe VWV, Hofman A, Hudziak JJ, Verhulst FC, van der Ende J. The stability of problem behavior across the preschool years: an empirical approach in the general population. J Abnorm Child Psychol. 2016;44:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fisher P, Lucas C. Diagnostic Interview Schedule for Children (DISC-IV)—Young Child. New York: Columbia University; 2006. [Google Scholar]
- 48. Rijlaarsdam J, Stevens GWJM, van der Ende J, Hofman A, Jaddoe VWV, Verhulst FC, Tiemeier H. Prevalence of DSM-IV disorders in a population-based sample of 5- to 8-year-old children: the impact of impairment criteria. Eur Child Adolesc Psychiatry. 2015;24:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR). Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- 50. Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–55. [PubMed] [Google Scholar]
- 51. Wolke D, Waylen A, Samara M, Steer C, Goodman R, Ford T, Lamberts K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br J Psychiatry. 2009;195:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Constantino JN, Gruber CP. Social Responsiveness Scale (SRS): manual. Los Angeles (CA): Western Psychological Services; 2005. [Google Scholar]
- 53. Blanken LME, Mous SE, Ghassabian A, Muetzel RL, Schoemaker NK, El Marroun H, van der Lugt A, Jaddoe VWV, Hofman A, Verhulst FC et al.. Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am J Psychiatry. 2015;172:479–86. [DOI] [PubMed] [Google Scholar]
- 54. Vinkhuyzen AAE, Eyles DW, Burne THJ, Blanken LME, Kruithof CJ, Verhulst F, Jaddoe VW, Tiemeier H, McGrath JJ. Gestational vitamin D deficiency and autism-related traits: the Generation R study. Mol Psychiatry. 2016;23:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams J, Scott F, Stott C, Allison C, Bolton P, Baron-Cohen S, Brayne C. The CAST (Childhood Asperger Syndrome Test): test accuracy. Autism. 2005;9:45–68. [DOI] [PubMed] [Google Scholar]
- 56. Skuse DH, Mandy WPL, Scourfield J. Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005;187:568–72. [DOI] [PubMed] [Google Scholar]
- 57. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weisskopf MG, Sparrow D, Hu H, Power MC. Biased exposure–health effect estimates from selection in cohort studies: are environmental studies at particular risk?. Environ Health Perspect. 2015;123:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ghassabian A, Steenweg-de Graaff J, Peeters RP, Ross HA, Jaddoe VW, Hofman A, Verhulst FC, White T, Tiemeier H. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:e005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Murcia M, Espada M, Julvez J, Llop S, Lopez-Espinosa M-J, Vioque J, Basterrechea M, Riaño I, González L, Alvarez-Pedrerol M et al.. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: the INMA Mother and Child Cohort Study. J Epidemiol Community Health. 2018;72:216–22. [DOI] [PubMed] [Google Scholar]
- 61. Tick NT, van der Ende J, Koot HM, Verhulst FC. 14-year changes in emotional and behavioral problems of very young Dutch children. J Am Acad Child Adolesc Psychiatry. 2007;46:1333–40. [DOI] [PubMed] [Google Scholar]
- 62. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 63. Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med. 1991;324:532–6. [DOI] [PubMed] [Google Scholar]
- 64. Knudsen N, Christiansen E, Brandt-Christensen M, Nygaard B, Perrild H. Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. Eur J Clin Nutr. 2000;54:361–3. [DOI] [PubMed] [Google Scholar]
- 65. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 66. Hynes KL, Otahal P, Burgess JR, Oddy WH, Hay I. Reduced educational outcomes persist into adolescence following mild iodine deficiency in utero, despite adequacy in childhood: 15-year follow-up of the Gestational Iodine Cohort investigating auditory processing speed and working memory. Nutrients. 2017;9:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the Gestational Iodine Cohort. J Clin Endocrinol Metab. 2013;98:1954–62. [DOI] [PubMed] [Google Scholar]
- 68. Markhus MW, Dahl L, Moe V, Abel MH, Brantsæter AL, Øyen J, Meltzer HM, Stormark KM, Graff IE, Smith L et al.. Maternal iodine status is associated with offspring language skills in infancy and toddlerhood. Nutrients. 2018;10:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van Mil NH, Tiemeier H, Bongers-Schokking JJ, Ghassabian A, Hofman A, Hooijkaas H, Jaddoe VWV, de Muinck Keizer-Schrama SM, Steegers EAP, Visser TJ et al.. Low urinary iodine excretion during early pregnancy is associated with alterations in executive functioning in children. J Nutr. 2012;142:2167–74. [DOI] [PubMed] [Google Scholar]
- 70. Abel MH, Caspersen IH, Meltzer HM, Haugen M, Brandlistuen RE, Aase H, Alexander J, Torheim LE, Brantsæter A-L. Suboptimal maternal iodine intake is associated with impaired child neurodevelopment at 3 years of age in the Norwegian Mother and Child Cohort Study. J Nutr. 2017;147:1314–24. [DOI] [PubMed] [Google Scholar]
- 71. Murcia M, Rebagliato M, Iñiguez C, Lopez-Espinosa M-J, Estarlich M, Plaza B, Barona-Vilar C, Espada M, Vioque J, Ballester F. Effect of iodine supplementation during pregnancy on infant neurodevelopment at 1 year of age. Am J Epidemiol. 2011;173:804–12. [DOI] [PubMed] [Google Scholar]
- 72. Rebagliato M, Murcia M, Alvarez-Pedrerol M, Espada M, Fernández-Somoano A, Lertxundi N, Navarrete-Muñoz E-M, Forns J, Aranbarri A, Llop S et al.. Iodine supplementation during pregnancy and infant neuropsychological development. INMA Mother and Child Cohort Study. Am J Epidemiol. 2013;177:944–53. [DOI] [PubMed] [Google Scholar]
- 73. König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141:2049–54. [DOI] [PubMed] [Google Scholar]
- 74. Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53:401–7. [DOI] [PubMed] [Google Scholar]
- 75. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman W, Laurberg P, Lazarus JH, Mandel SJ et al.. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315–89. [DOI] [PubMed] [Google Scholar]
- 76. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Verkaik-Kloosterman J, Buurma-Rethans EJM, Dekkers ALM, van Rossum CTM. Decreased, but still sufficient, iodine intake of children and adults in the Netherlands. Br J Nutr. 2017;117:1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Andersson M, De Benoist B, Darnton-Hill I, Delange F. Iodine deficiency in Europe: a continuing public health problem. Geneva: World Health Organization; 2007. [Google Scholar]
- 79. Elnagar B, Eltom A, Wide L, Gebre-Medhin M, Karlsson FA. Iodine status, thyroid function and pregnancy: study of Swedish and Sudanese women. Eur J Clin Nutr. 1998;52:351–5. [DOI] [PubMed] [Google Scholar]
- 80. Shi X, Han C, Li C, Mao J, Wang W, Xie X, Li C, Xu B, Meng T, Du J et al.. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J Clin Endocrinol Metab. 2015;100:1630–8. [DOI] [PubMed] [Google Scholar]
- 81. Brown AS, Surcel H-M, Hinkka-Yli-Salomäki S, Cheslack-Postava K, Bao Y, Sourander A. Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Melsen WG, Bootsma MCJ, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–9. [DOI] [PubMed] [Google Scholar]
- 83. Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99:813–8. [DOI] [PubMed] [Google Scholar]
- 84. Ittermann T, Johner S, Below H, Leiterer M, Thamm M, Remer T, Völzke H. Interlaboratory variability of urinary iodine measurements. Clin Chem Lab Med. 2018;56:441–7. [DOI] [PubMed] [Google Scholar]
- 85. Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


