Abstract
Gram-negative bacteria possess an asymmetric outer membrane (OM) composed primarily of lipopolysaccharides (LPSs) on the outer leaflet and phospholipids (PLs) on the inner leaflet. The loss of this asymmetry due to mutations in the LPS biosynthesis or transport pathways causes the externalization of PLs to the outer leaflet of the OM and leads to OM permeability defects. Here, we used metabolic labeling to detect a compromised OM in intact bacteria. Phosphatidylcholine synthase expression in Escherichia coli allowed for the incorporation of exogenous propargylcholine into phosphatidyl(propargyl)choline and exogenous 1-azidoethyl-choline (AECho) into phosphatidyl(azidoethyl)choline (AEPC), as confirmed by LC/MS analyses. A fluorescent copper-free click reagent poorly labeled AEPC in intact wild-type cells but readily labeled AEPC from lysed cells. Fluorescence microscopy and flow cytometry analyses confirmed the absence of significant AEPC labeling from intact wild-type E. coli strains and revealed significant AEPC labeling in an E. coli LPS transport mutant (lptD4213) and an LPS biosynthesis mutant (E. coli lpxC101). Our results suggest that metabolic PL labeling with AECho is a promising tool for detecting a compromised bacterial OM, revealing aberrant PL externalization, and identifying or characterizing novel cell-active inhibitors of LPS biosynthesis or transport.
Keywords: bioorthogonal, click chemistry, flow cytometry, lipid biochemistry, mass spectrometry, microscopy, membrane lipids, biosynthesis, phosphatidylcholine
The widespread emergence of antibiotic resistance in pathogenic Gram-negative bacteria is a rapidly growing threat and represents one of today’s greatest public health problems (1, 2). The discovery of new antibiotics for Gram-negative bacteria is challenging, partly due to the action of various multidrug efflux pumps and the Gram-negative-specific outer membrane (OM) (3–5). The OM is composed of an inner leaflet of phospholipids (PLs) and an outer leaflet of lipopolysaccharides (LPSs; also known as endotoxins) (6). This strict asymmetry is important for the OM to function as a permeability barrier, protecting the cells from the immune system and hindering many toxic compounds from entering the cell (4, 7). If compounds manage to penetrate through the OM, they also need to cross the PL bilayer that makes up the inner membrane (IM) to reach a cytoplasmic target. Consequently, these compounds need to cross two membranes with very different properties.
The critical OM asymmetry results from the externalization of LPSs from the IM directly to the outer leaflet of the OM by the Lpt pathway (8) and from the removal of any PLs that reach the outer leaflet of the OM by internalization (e.g., via the Mla pathway) or degradation (e.g., by PldA and PagP) (9–12). Previous studies have found that Escherichia coli strains with mutations in genes required for LPS biosynthesis or transport, such as lpxC (previously known as envA) (13) or lptD (also known as ostA or imp) (8), have permeability defects in their OM compared with their wild-type counterparts (14, 15). A similar hyperpermeable phenotype can be observed in wild-type Gram-negative bacteria when treated with cationic OM permeabilizers, such as polymyxins, or compounds that inhibit key enzymes in the LPS biosynthesis or transport pathways (16). The inhibition of the LPS transport or biosynthesis pathway leads to lower levels of LPSs on the outer leaflet and concomitant higher levels of externalized PLs. The formation of PL bilayer patches in the OM leads to enhanced permeation of large and/or lipophilic compounds (4). Thus, an assay for specifically detecting Gram-negative bacterial PLs would identify compounds that disrupt OM asymmetry, causing an OM permeability defect (9, 17, 18).
Currently available assays for identifying a compromised OM by detecting bacterial PLs are indirect, low-throughput, and labor-intensive. One assay quantifies the level of OM palmitoylated lipid A that is formed by PagP-dependent palmitoyl transfer from PLs to LPSs in the outer leaflet of the OM. This methodology requires radiolabeling, acid hydrolysis, organic extraction, and TLC of the lipid A anchor of LPSs (9, 19). Different radioactive compounds, such as [32P]phosphoric acid or [3H]glycerol, have been used to label PLs followed by organic extraction and liquid scintillation counting to determine indirectly the amount of PLs (20, 21). A more direct assay utilizes the treatment of intact bacteria by exogenous phospholipase C to hydrolyze externalized PLs, followed by organic extraction, separation by TLC, and analysis by iodine deposition and image analysis (20, 22, 23). More recently, a cinnamycin-conjugated fluorophore that is known to bind to curved patches of ethanolamine lipids was utilized to measure OM defects in E. coli lplT mutants, although the interaction is noncovalent and the specificity for PE and/or lysophosphatidylethanolamine on the outer leaflet of the OM was not evaluated (24). In mammalian systems, PS externalization is a hallmark of apoptosis, and various methods for visualizing the externalization have been extensively used (25). One method for quantifying externalized PS makes use of the cell-impermeant N-hydroxy-sulfosuccinimido-biotin that reacts with surface-exposed PS (and other primary amines). However, this methodology is a resource-intensive destructive endpoint assay that utilizes LC/MS (26). Another methodology pioneered in mammalian systems is the selective labeling of cellular PC by the metabolic incorporation of propargylcholine (PCho) into phosphatidyl(propargyl)choline (PPC), which can then be fluorescently labeled using biorthogonal click chemistry to visualize PPC from intact cells (27).
Because metabolic labeling has been used to incorporate synthetic analogs into different bacterial biomolecules, for example, LPSs (28–32), we chose to investigate whether metabolic labels could be incorporated into E. coli PLs and whether they would be labeled by fluorescent click reagents in cells with intact OM asymmetry or in cells with a compromised OM. The most common PLs in the E. coli membrane are PE (∼80%), PG (∼15%), and cardiolipin (∼5%) (33). Unfortunately, the head groups of these PLs are not suitable for metabolic labeling because their biosynthetic pathways do not present a clear opportunity for the introduction of an analog (supplemental Fig. S1).
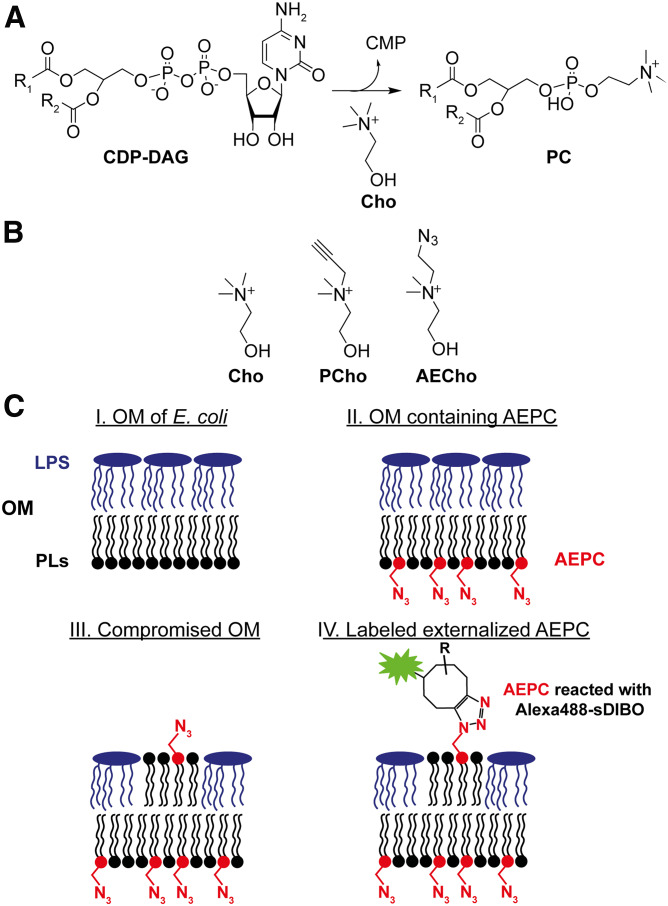
While the naturally occurring E. coli lipids are not suitable, approximately 15% of all bacterial species produce PC (e.g., pathogens such as Pseudomonas aeruginosa and Legionella pneumophila) (34). Two PC biosynthetic pathways are found in bacteria: the PL N-methyltransferase pathway and the phosphatidylcholine synthase (Pcs) pathway (supplemental Fig. S1) (34–36). While PC is synthesized starting from PE in the PL N-methyltransferase pathway (supplemental Fig. S1A), the Pcs enzyme catalyzes the reaction of choline (Cho) with CDP-diacylglycerol (DAG) to form PC (Fig. 1A). The mammalian PC biosynthetic pathway (the Kennedy pathway) uses an unrelated third pathway where Cho is phosphorylated and then activated to CDP-Cho, which further reacts with DAG to form PC (supplemental Fig. S1B) (37, 38). Two analogs of Cho, PCho and 1-azidoethyl-choline (AECho) (Fig. 1B), have been used to metabolically label PC in mammalian cells (27, 39, 40). Both analogs react with an azide or alkyne after incorporation into PC to allow further detection. More recently, PCho has been also used to label PC in plants (41). Although Cho analogs can be incorporated into PC in eukaryotic cells, there was no certainty that the bacterial Pcs pathway would accept Cho analogs.
Fig. 1.
Principle of PC labeling in bacteria to detect PLs. A: Critical step of PC biosynthesis via Pcs in bacteria (34) suitable for metabolic labeling. B: Chemical structures of Cho, PCho, and AECho. C: Principle of metabolic labeling with AECho: I, intact OM of E. coli with PLs on the inner leaflet and LPS on the outer leaflet; II, AEPC is in the OM after cells are incubated with AECho; III, compromised OM (genetic mutation or chemical inhibition of LPS pathways) have PLs on the outer leaflet; and IV, exposed AEPC can be labeled with fluorescent alkyne reagents to allow fluorescent detection. Note that the LPS is represented without core sugars and O-antigens for simplicity.
Here, we present for the first time the successful metabolic labeling of PC with AECho to visualize PLs in intact E. coli with a bioorthogonal fluorescent method (Fig. 1C). Exogenously supplied AECho was incorporated by Pcs into PC to form phosphatidyl(azidoethyl)choline (AEPC) (Fig. 1C; II). When the OM asymmetry and permeability were compromised by mutation, PLs (including AEPC) were exposed (Fig. 1C; III). The azide moiety in the head group was then reacted with a fluorescent alkyne reagent in a click reaction (Fig. 1C; IV) to enable the fluorescent detection of AEPC on the OM.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and materials
All bacterial strains and plasmids used in this study are described in Table 1. The strains were grown in lysogeny broth (LB) containing, per liter, 10 g tryptone, 5 g yeast extract, 5 g NaCl (pH 7.5 adjusted with 6 M HCl or 6 M NaOH, as needed) or M9 minimal medium containing, per liter, 0.3% KH2PO4, 0.6% Na2HPO4, 0.5% NaCl, 0.1% NH4Cl, 2 mM MgSO4, 0.1 mM CaCl2, 0.2% casamino acids, 50 µg ml−1 l-tryptophan, 1 mM thiamine hydrochloride, and 0.4% sterile-filtered glycerol as indicated. M9 was used for all labeling experiments because LB contains Cho at sufficient levels to produce PC when Pcs is expressed (data not shown). Labeling experiments were performed in 14 ml Falcon round-bottom tubes (Corning) when not otherwise mentioned. Where noted, kanamycin (50 µg ml−1) and/or an inducer (0.2% l-rhamnose) were added to the growth medium. M9 with maltose containing, per liter, 0.3% KH2PO4, 0.6% Na2HPO4, 0.5% NaCl, 0.1% NH4Cl, 2 mM MgSO4, 0.1 mM CaCl2, and 0.2% sterile-filtered maltose was used for washing or flow cytometry medium. Cho was purchased from Sigma-Aldrich, and Pcho was purchased from Aobious; AECho was synthesized as described previously (40). AlexaFluor488-DIBO alkyne (Alexa488-DIBO) and AlexaFluor488-sDIBO alkyne (Alexa488-sDIBO) were obtained from Thermo Fisher Scientific. PG, PE, PC, and polar lipid extract (PLE) standards were obtained from Avanti Polar Lipids.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or Plasmid | Genotype/Description | Source |
| E. cloni 10G | Chemically competent E. coli K-12 cloning strain | Lucigen |
| BW25113 | F- λ- Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) rph-1Δ(rhaD-rhaB)568 hsdR514, CP009273.1 | Coli Genetic Stock Center (CGSC) 7636 |
| MC4100 | [araD139]B/r, Δ(argF-lac)169, λ-, e14-, flhD5301, Δ(fruK-yeiR)725(fruA25), relA1, rpsL150(strR), rbsR22, Δ(fimB-fimE)632(::IS1), deoC1 | CGSC 6152 |
| lptD4213 | NR698, lptD4213 (imp4213) mutant of MC4100 | Ruiz et al. (56) |
| D21 | F-, proA23, lac-28, tsx-81, trp-30, his-51, rpsL173(strR), ampCp-1 | CGSC 5158 |
| lpxC101 | D22, lpxC101 mutant of D21 | CGSC 5163 |
| pUC57-ATMA | L. pneumophila pcsA (pcs), AmpR | This study |
| N-His pRham | rhaPBAD (rhamnose-inducible) N-term. 6×His tag, KanR | Lucigen |
| No-His pRham | rhaPBAD (rhamnose-inducible) with no His tag, KanR | This study |
| pRham-pcs | rhaPBAD pcs, rhamnose-inducible, no His tag | This study |
Construction of the pRham-pcs plasmid and transformation into E. coli
The pcsA gene from Legionella pneumophila subsp. pneumophila str. Philadelphia 1 (lpg1584; gene ID 19833149) (42, 43) was synthesized and provided in plasmid pUC57-ATMA (Genewiz). The pUC57-ATMA plasmid was transformed into E. cloni 10G chemically competent cells on LB agar supplemented with 30 μg ml−1 carbenicillin. A set of primers (5′-CAT CAT CAC CAC CAT CAC AAT CCG ATT AAG CCG CCG TTC-3′ and 5′-GTG GCG GCC GCT CTA TTA ATC TTT ATT ATT GGC GGT AAT-3′) was designed to amplify the pcs gene from pUC57-ATMA with overhanging sequences that matched the sequences at the ends of the N-His pRham vector (Lucigen). Because the N-His pcs insert was found to be toxic (data not shown), a version of the pRham vector without a His-tag (No-His pRham vector) was generated with a new pair of primers (5′-TAA TAG AGC GGC CGC CAC-3′ and 5′-CAT ATG TAT ATC TCC TTC TTA TAG TTA AAC-3′). New pcs gene inserts were synthesized so that their overlapping ends were compatible with those of the No-His pRham vector (5′-GAA GGA GAT ATA CAT ATG AAT CCG ATT AAG CCG CCG TTC-3′ and 5′-GTG GCG GCC GCT CTA TTA ATC TTT ATT ATT GGC GGT AAT-3′). The pRham transformation cultures were plated on LB agar supplemented with 50 μg ml−1 kanamycin and incubated overnight at 37°C. Plasmids containing an insert of the expected size were confirmed by sequencing (Elim Biopharmaceuticals). No-His pRham-pcs was transformed via electroporation to into E. coli K-12 (BW25113), E. coli lptD4213 and its parent strain MC4100, and E. coli lpxC101 and its parent strain E. coli D21 (Table 1).
Preparation of total lipid extracts from E. coli strains grown with Cho, Pcho, or AECho
Overnight cultures of E. coli and the corresponding strain containing the pcs plasmid (Table 1) grown in M9 and kanamycin were inoculated into fresh M9 containing rhamnose and kanamycin or rhamnose, kanamycin, and an appropriate amount of a sterile aqueous stock solution of Cho, PCho, or AECho to a starting OD600 of 0.05. The cultures were grown at 37°C at 225 rpm to stationary phase (∼5 h; OD600: ∼1.8). Cells were harvested by centrifugation at 3,950 g for 20 min and washed once with 20 ml PBS (pH 7.4). Cell pellets were stored at −20°C until needed. For the total lipid extraction, the cell pellets were resuspended in a glass centrifuge tube in 2 ml PBS, and a single-phase Bligh-Dyer mixture was prepared by adding 5 ml methanol and 2.5 ml chloroform (44, 45). Lipids were extracted by rocking the suspension for 1 h at room temperature. The supernatant was converted to a two-phase Bligh-Dyer solution by adding 2.5 ml PBS and 2.5 ml chloroform. After centrifugation at 1,000 g for 20 min at room temperature to separate the two phases, the lower phase (predominantly chloroform) was isolated with a glass pipette and dried down under a stream of nitrogen. The samples were stored at −20°C until needed and used for TLC and/or LC/MS/MS analysis of the PL species.
TLC of total lipid extracts
Total lipid extracts were dissolved in chloroform-methanol (4:1; v/v) and spotted onto TLC silica gel 60 F254 plates (Millipore Sigma). Chloroform-methanol-water (65:35:8; v/v/v) was used as solvent system and was allowed to equilibrate in the TLC chamber at least 1 h before usage. TLC plates were developed by spraying them with 10% ethanolic H2SO4 solution and charring with a heat gun for about 2 min.
Normal-phase LC/MS/MS analysis of total lipid extracts from E. coli
LC/MS/MS experiments were performed on a SCIEX 4000 QTRAP mass spectrometer with Turbo V ion source coupled to an Agilent 1100 LC fitted with glass and steel capillaries and normal-phase solvent-safe pump seals. The instrument vendors were consulted prior to running these normal-phase LC/MS conditions to ensure the instruments were compatible. To avoid solvent exposure, the instruments were well-ventilated. Chromatographic conditions and MS settings were adapted from previous methods (46, 47). All PLs (PG, PE, PC, PPC, and AEPC) were scanned for the 11 most abundant acyl chain combinations (supplemental Table S3) (33, 48). Analyst Software (SCIEX) was used for data acquisition. A detailed description of the LC/MS/MS analysis can be found in the supplemental Methods.
Sucrose density gradient separation of IM and OM
E. coli BW25113 and BW25113 pcs were grown as described above. The IM and OM were separated as described previously (49). The fractions were collected in 1 ml steps and were used to identify the IM via the NADH assay as described previously (50) and the OM by LPS gel as described previously (51, 52) (supplemental Figs. S2, S3). A detailed description of the sucrose gradient separation can be found in the supplemental Methods.
Fluorescent labeling of E. coli strains (lptD4213 and lpxC101 mutants) grown with AECho
A bacterial overnight culture was inoculated into M9 containing an appropriate volume of a sterile aqueous stock solution of AECho or the same volume of sterile water to a starting OD600 between 0.002 and 0.005. The cultures were incubated at 37°C at 225 rpm for 16 h (to stationary phase), pelleted at 11,600 g for 2 min, and washed three times with M9 + maltose. For the click reaction, the pellets were resuspended in the M9 + maltose medium, and Alexa488-sDIBO (or Alexa488-DIBO, 50 mM stock solution in DMSO) was added to a final concentration of 0.25 mM. All samples were incubated for 3 h (1 h for Alexa488-DIBO) at 37°C at 225 rpm in the dark. After the click reaction, the cells were pelleted and washed three times with M9 + maltose. The cell pellets were prepared for fluorescence microscopy, flow cytometry, or total lipid extraction followed by TLC analysis.
Fluorescence microscopy imaging of click-labeled bacteria
E. coli samples were grown in M9 and click-labeled as described above. A 3 µl aliquot of the live, fluorescently labeled E. coli samples, diluted in M9 + maltose, was deposited onto a thin, 1.2% agar pad that was prepared on a glass microscope slide. The cells were imaged using a Nikon Eclipse Ti inverted microscope with a Nikon halogen illuminator (D-LH/LC), a Sola light engine from Lumencor, and a Clara Interline CCD camera from Andor. A Nikon CFI Plan Apo LambdaDM 100× oil objective lens (1.45 numerical aperture) was used for phase-contrast and fluorescent imaging. Images for green fluorescence were taken by using the FITC-5050A-NTE-ZERO filter set (Semrock). Images were captured by using Nikon Elements software and exported for figure preparation in ImageJ (53).
Flow cytometry analysis of click-labeled bacteria
E. coli samples were grown in M9 and click-labeled as described above. The washed pellets were resuspended in M9 + maltose and analyzed on an Attune NxT flow cytometer (Thermo Fisher Scientific) using the FITC channel to read AlexaFluor488 with an excitation wavelength of 488 nm (blue laser) and emission wavelength of 530 nm. Approximately 100,000 cells were analyzed for each sample.
Statistics of flow cytometry analysis
Error bars in all figures represent means ± SDs. Mean values were compared via two-way ANOVA using Tukey’s multiple-comparison tests to determine statistical significance, which is indicated in the figures as ns, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, or ****P ≤ 0.0001.
Plasmolysis of BW25113 pcs grown with AEPC and treated with Alexa488-DIBO
For plasmolysis, samples of E. coli BW25113 pcs were grown in M9 with 500 µM AECho and fluorescently labeled with Alexa488-DIBO as described above. After imaging the nonplasmolyzed cells for fluorescence, the samples were pelleted at 11,600 g for 2 min, resuspended in 100 µl plasmolysis solution containing 15% sucrose, 25 mM HEPES (pH 7.4), and 20 mM sodium azide and deposited on an agar pad containing 15% sucrose and 1.2% agarose freshly prepared on a glass microscope slide and directly imaged (54, 55). For membrane staining of E. coli K-12, N-3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl)-pyridinium dibromide (FM 4-64; Invitrogen; 500 µg/ml in DMSO) was added to the plasmolyzed sample to a final concentration of 1% (v/v) before depositing a 3 µl aliquot on an agar pad.
RESULTS
Confirmation of Pcs functionality for PC production
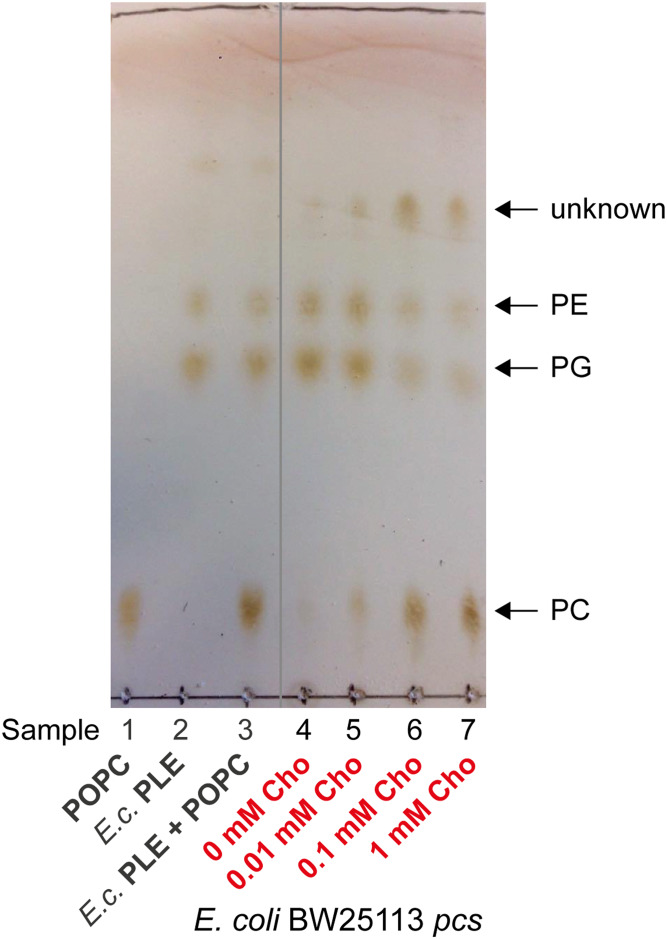
Because E. coli does not naturally produce PC, the pcs gene of L. pneumophila was inserted into E. coli K-12 to synthesize PC as described previously (42). To confirm that the plasmid was functional, BW25113 pcs was grown to stationary phase in M9 containing different concentrations of Cho (0, 0.01, 0.1, and 1 mM), followed by lipid isolation by Bligh-Dyer extraction (44, 45). The total lipid extracts were separated by TLC and visualized by charring (Fig. 2). In all BW25113 pcs samples grown with Cho, PC was observed in addition to PE and PG (Fig. 2; samples 5–7). PC was not detected in the control E. coli PLE standard (Fig. 2; sample 2) or in the BW25113 pcs sample grown without Cho (Fig. 2; sample 4), which showed only PE and PG.
Fig. 2.
TLC of E. coli BW25113 pcs lipid extracts confirms PC production. BW25113 pcs was grown in M9 supplemented with 0, 0.01, 0.1, or 1 mM Cho. Rhamnose (0.2%) was used in the medium to induce Pcs expression. Total lipids were extracted and separated with chloroform-methanol-water (65:35:8; v/v/v) on a TLC plate and developed by charring. The data shown are representative of two independent experiments. Samples: 1, POPC (16:0/18:1); 2, E. coli PLE standard; 3, mixture of POPC and PLE standards (1:1); and 4–7, total lipid extracts of E. coli BW25113 pcs grown with 0, 0.01, 0.1, and 1 mM Cho, respectively.
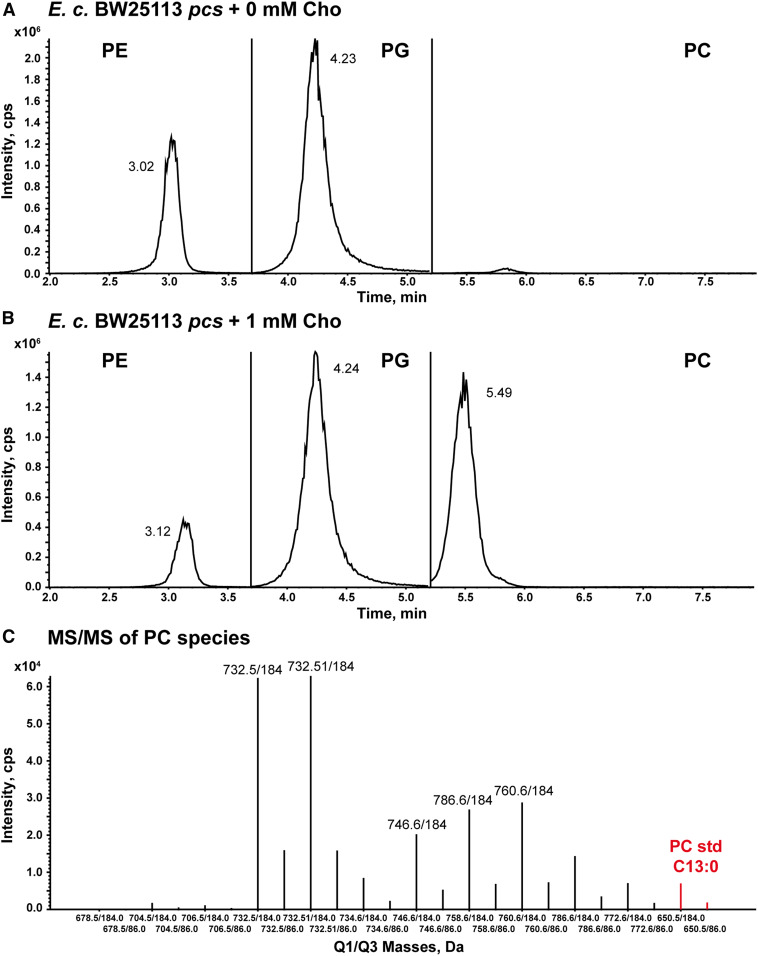
To confirm PC production, the total lipid extracts of BW25113 pcs samples grown to stationary phase with or without 1 mM Cho were analyzed by LC/MS/MS in the negative-ion mode to detect PE and PG and in the positive-ion mode to detect PC (Fig. 3) (46, 47). For each lipid species, the 11 most abundant combinations of acyl chains found in E. coli PLs were identified by multiple reaction monitoring (MRM; supplemental Table S3) (33, 48). The isobaric ions of PC with equal sums of the two acyl chain lengths and number of double bonds (e.g., chain lengths C18:1/C14:0 and C16:1/C16:0) have identical m/z and cannot be distinguished with the MRM transitions used. An LC/MS peak for PC was only observed for the sample grown with Cho (Fig. 3B), while peaks for PE and PG were present in both BW25113 pcs samples grown with Cho (Fig. 3B) and without Cho (Fig. 3A). The results obtained by TLC and LC/MS/MS analysis confirmed that the Pcs plasmid was functional, and PC was produced upon Cho supplementation as expected.
Fig. 3.
Normal-phase LC/MS/MS analysis of the total lipid extract of E. coli BW25113 pcs grown in the presence of Cho. Chromatograms from the LC/MS/MS analysis of total lipids from E. coli BW25113 pcs grown in M9 without Cho (A) and supplemented with 1 mM Cho (B). The extracted ion currents of the MRM transitions for each PL class were summed together (PC, PE, or PG). The PL class-specific MRM methods were applied to the regions delineated by the vertical lines. PE and PG were detected in the negative-ion mode, while PC was detected in the positive-ion mode. Rhamnose (0.2%) was used in the medium to induce Pcs expression. C: MRM line plots corresponding to the 11 targeted PC species detected in the sample grown with 1 mM Cho. The PC standard (C13:0/C13:0) was added to all samples as an internal standard. The two MRM transitions monitored the release of phosphorylcholine (m/z 184) and Cho (m/z 86) from each PC species. The data shown are representative of two independent experiments.
Confirmation of PC in both IM and OM by sucrose gradient separation
PLs can be generally present in both leaflets of the IM as well as in the inner leaflet of the OM; thus, it was important to confirm that PC is distributed in both membranes. BW25113 and BW25113 pcs were grown to stationary phase in M9 in the presence of Cho. The cells were lysed and the membranes separated via sucrose gradient separation as described previously (49). For each strain, 12 fractions were collected and analyzed by an NADH oxidase assay (50) to identify fractions containing IM and SDS PAGE gel (51, 52) to identify fractions containing OM. The results of the NADH oxidase assay (supplemental Fig. S2) indicated that the IM in both strains was present predominantly in fractions 1–4, so they were combined as the IM fraction. In the image of the LPS gel (supplemental Fig. S3), fractions 9–12 in both strains (supplemental Fig. S3A, B; lanes 12–15) showed the highest abundance of rough LPS, so they were combined as OM fraction. The results observed are in agreement with the data described in the literature (49, 50).
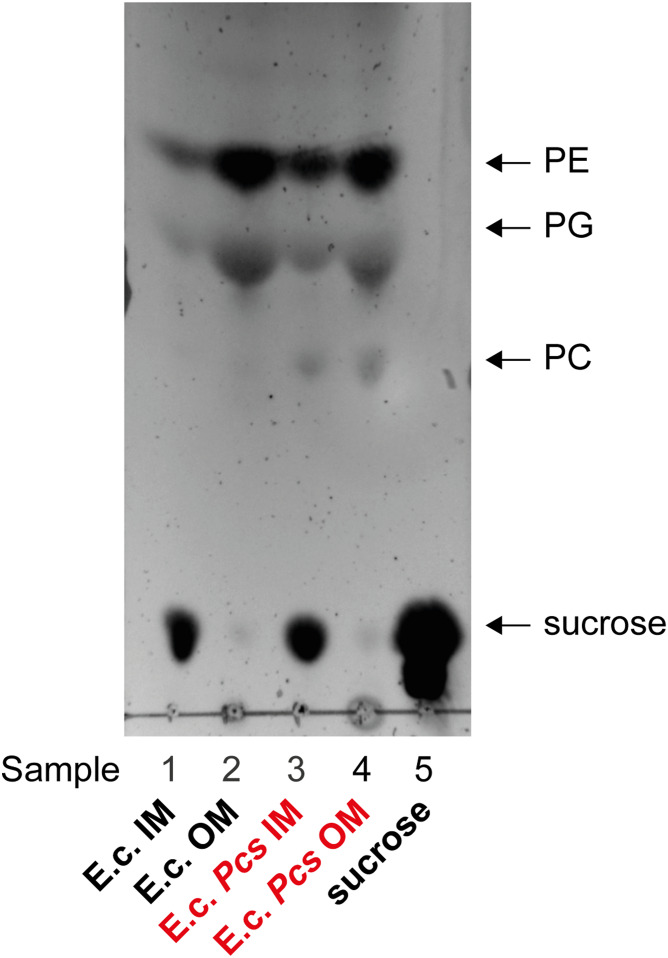
The total lipids of the IM and OM fractions were isolated by Bligh-Dyer extraction (44, 45). The sucrose fractions were used as part of the aqueous phase. The total lipid extracts were separated via TLC and visualized by charring (Fig. 4). The Cho grown BW25113 pcs sample showed spots for PE, PG, and PC in the IM and OM (Fig. 4; samples 3 and 4), respectively, while the control Cho grown BW25113 had only spots for PE and PG (Fig. 4; samples 1 and 2) in both membranes. Sucrose was still present in the IM fractions (Fig. 4; samples 1, 3, and 5) despite Bligh-Dyer extraction and washing the organic phase three times. The IM and OM fractions of both strains were also analyzed by LC/MS/MS (supplemental Fig. S5) for PC and confirmed the presence of PE, PG, and PC in both membranes of Cho grown BW25113 pcs.
Fig. 4.
TLC of E. coli BW25113 and E. coli BW25113 pcs lipid extracts after sucrose gradient separation confirms that PC is present in the IM and OM. Both strains were grown in M9 with 100 µM Cho and 0.2% rhamnose. The cells were harvested. The IM and OM of the lysate were separated via sucrose gradient separation with 2.2 M (bottom) to 0.77 M (top) sucrose concentrations and ultracentrifugation. The fractions were taken in 1 ml steps from the top. Total lipids were extracted from the fractions and separated with chloroform-methanol-water (65:35:8; v/v/v) on a TLC plate and developed by charring. The data shown are representative of two independent experiments. Samples: 1, E. coli K-12 fractions 1–4 (IM); 2, E. coli K-12 fractions 9–12 (OM); 3, E. coli K-12 pcs fractions 1–4 (IM); 4, E. coli K-12 pcs fractions 9–12 (OM); and 5, sucrose standard.
Confirmation of PCho and AECho incorporation by E. coli pcs
Two requirements for using PC to detect exposed PLs were fulfilled with the confirmation that PC is produced and present in both membranes. The next steps were to evaluate whether E. coli can uptake Cho analogs such as PCho or AECho and incorporate them into PC using Pcs (27, 39) (Fig. 1B). To test this, BW25113 pcs was grown to stationary phase in M9 supplemented with or without 1 mM PCho, followed by lipid isolation by Bligh-Dyer extraction. The total lipid extracts were first separated by TLC, and then the plate was developed by charring. However, PPC was not detected on the TLC plate (data not shown), indicating that either PPC was not produced in sufficient levels to char or that it comigrated with an existing PL under the TLC conditions. Therefore, the total lipid extracts were analyzed for PPC by LC/MS/MS analysis by adapting the method used for PC. The sample grown with PCho showed a peak corresponding to PPC (supplemental Fig. S6B), while the sample grown without PCho did not (supplemental Fig. S6A). Both samples showed peaks for PE and PG (supplemental Fig. S7C), as expected. The retention time of PPC was shifted significantly from PC and overlapped with PG. Thus, the LC/MS/MS analyses of 11 species each for PE, PG, and PPC could not be combined into one LC run as had been done for PC.
Because AECho has the advantage of reacting with fluorescent strained alkynes in a single step without the use of cytotoxic copper, AECho was also tested to determine whether it could be taken up by E. coli and incorporated into PC using Pcs. Following the same procedures used for PCho, the sample grown with AECho showed an LC/MS/MS peak corresponding to AEPC, while the sample grown without AECho did not (Fig. 5A, B). Both samples showed peaks for PE and PG (supplemental Fig. S7A, B), as expected. As was seen for PPC, the AEPC retention time overlapped with PG and, thus, had to be analyzed independently from PE and PG. These results showed that E. coli was capable of taking up PCho and AECho and that Pcs was able to incorporate them to produce PPC and AEPC, respectively.
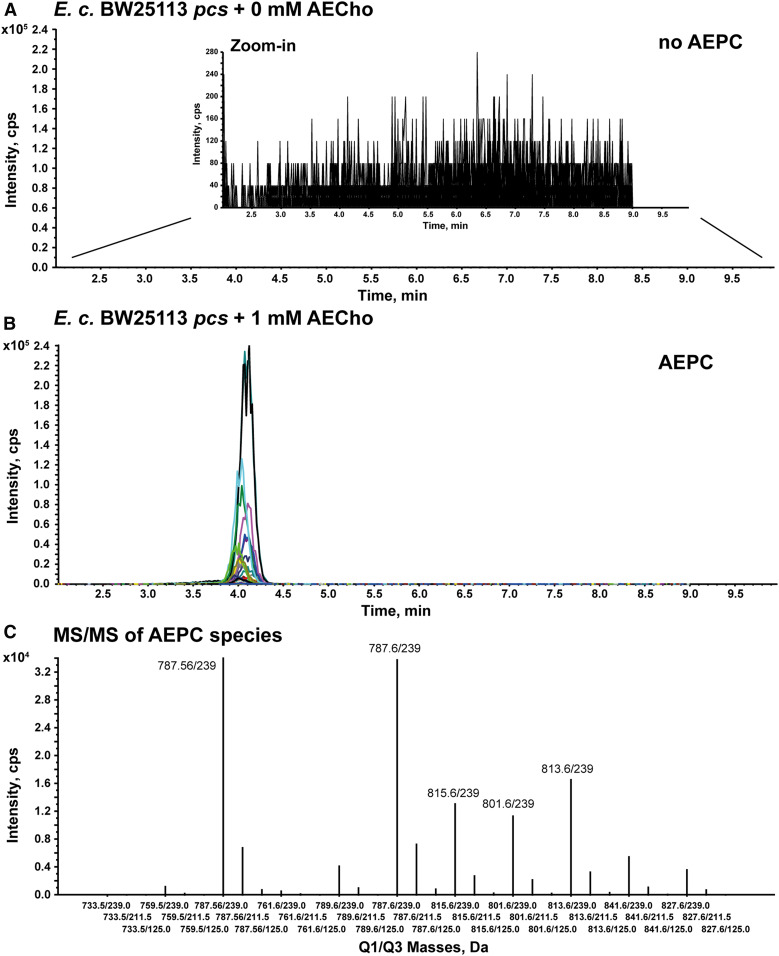
Fig. 5.
LC/MS/MS analysis of total lipids obtained from E. coli BW25113 pcs grown in the presence of AECho confirms the production of AEPC. Chromatograms from the LC/MS/MS analysis of total lipids from E. coli BW25113 pcs grown in M9 without AECho (A) and supplemented with 1 mM AECho (B). A zoom-in was used for panel A to highlight the absence of AEPC signal in the absence of AECho. The extracted ion currents for the AEPC MRM transitions are each plotted in a different color. The elution times overlap for all AEPC MRM transitions. Rhamnose (0.2%) was used in the medium to induce Pcs expression. C: MRM line plot of the various AEPC peaks observed in panel B showing the relative abundance of the parent ions and consistent relative yields for the transitions to m/z 239, m/z 125, and m/z 211 (supplemental Fig. S4D) for all species. The data shown are representative of two independent experiments.
Confirmation of the reactivity of AEPC in lysates
After showing that Pcs can incorporate AECho into AEPC, the reactivity of bacterial AEPC with a fluorescent alkyne reagent needed to be confirmed. To test this, BW25113 pcs was grown for 16 h with or without 500 µM Cho or 500 µM AECho followed by cell lysis. The cell lysates were treated for 0 h (time zero control) or 1 h with the green fluorescent Alexa488-DIBO. This commercially available strained cyclooctyne reacts selectively with azides via copper-free click reaction and allows fluorescent click labeling with a single step. After the reaction, the total lipids were isolated by Bligh-Dyer extraction and separated via TLC. The TLC plate was visualized for green fluorescence before charring (supplemental Fig. S8A, B).
A green fluorescent spot corresponding to Alexa488-DIBO clicked to AEPC was observed only for the BW25113 pcs sample grown with AECho and treated with Alexa488-DIBO for 1 h (supplemental Fig. S8B; sample 7). This spot ran slightly higher than the Alexa488-DIBO reagent itself (supplemental Fig. S8B; sample 2). All E. coli samples showed spots by charring for PE and PG (supplemental Fig. S8A; samples 3–7), while the samples grown with Cho also showed a spot for PC (supplemental Fig. S8A; samples 4 and 5) as expected. The results suggest that the additional spot observed for the BW25113 pcs sample grown with AECho corresponds to AEPC reacted with the fluorescent click reagent and, thus, that the azide moiety in the head group of AEPC can react with Alexa488-DIBO.
Fluorescent labeling of AEPC in E. coli lptD4213
After confirming that bacterial AEPC in E. coli lysate can react with a fluorescent strained alkyne, the last step was to test whether AEPC can be detected in live, intact E. coli cells from an isogenic pair: wild-type and a mutant that has increased PLs on the outer leaflet of the OM. E. coli lptD4213 is a well-characterized mutant that is hyperpermeable to a range of compounds (15, 56). This strain has a defect in LptD, the OM component of the Lpt system, which is responsible for LPS transport from the outer leaflet of the IM to the outer leaflet of the OM (8). Due to impaired LptD function, the mutant has less LPS on the outer leaflet of the OM and consequently more surface-exposed PLs compared with wild-type E. coli (21).
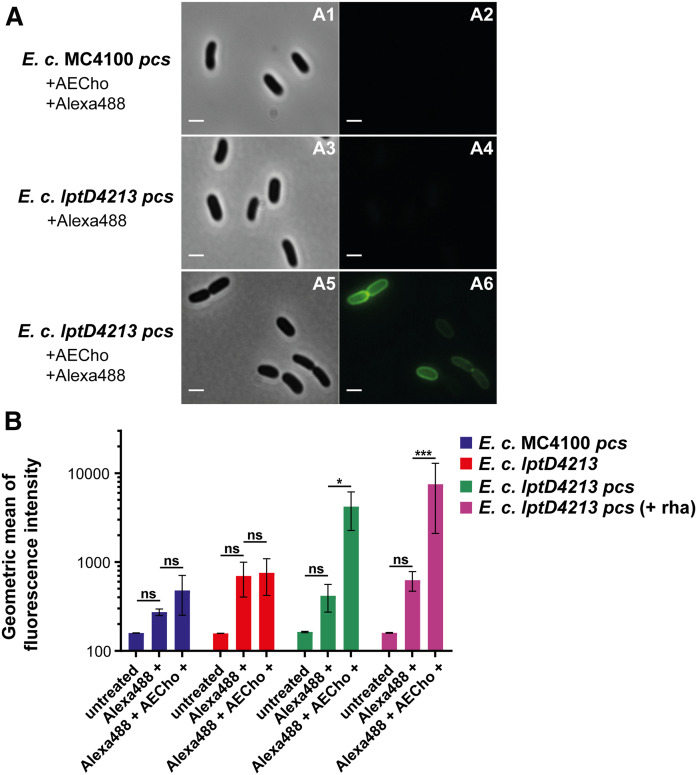
To evaluate AEPC labeling, lptD4213 pcs and its parent MC4100 pcs were grown for 16 h with or without 500 µM AECho and treated with or without the green fluorescent Alexa488-sDIBO. This commercially available strained cyclooctyne, which replaced the discontinued Alexa488-DIBO, also reacts selectively with azides via copper-free click reaction and allows fluorescent click labeling with a single step. The cells were analyzed by bright-field and fluorescence microscopy. The AECho-grown lptD4213 pcs strain, treated with Alexa488-sDIBO, was fluorescent (Fig. 6A; A2). The key control samples, lptD4213 pcs treated only with Alexa488-sDIBO (Fig. 6A; A4) and the intact AECho-grown parent MC4100 pcs treated with Alexa488-sDIBO (Fig. 6A; A2), were not fluorescent.
Fig. 6.
Microscopic images and flow cytometry analysis of E. coli MC4100 pcs and E. coli lptD4213 pcs grown with AECho. A: E. coli MC4100 pcs (parent) grown with AECho and treated with Alexa488-sDIBO (A1, A2) and E. coli lptD4213 pcs treated with Alexa488-sDIBO (A3, A4) and grown with AECho and treated with Alexa488-sDIBO (A5, A6). Brightfield images (A1, A3, A5) and corresponding fluorescence images detecting green fluorescence of AlexaFluor488 (A2, A4, A6). The bar represents 3 µm. B: Comparison of the geometric means of the fluorescence intensities of E. coli MC4100 pcs (blue), E. coli lptD4213 (red), E. coli lptD4213 pcs (green), and E. coli lptD4213 pcs inducing Pcs production (purple). Bacteria were untreated, treated with Alexa488-sDIBO only, or incubated with AECho followed by treatment with Alexa488-sDIBO. The cells were incubated overnight with 500 µM AECho. l-Rhamnose (0.2%) was used to induce Pcs production except for the samples with the green bar. Experiments were performed in biological triplicates. Error bars represent the SD from the mean. The statistical significance was determined via two-way ANOVA (*P ≤ 0.05, ***P ≤ 0.001).
Fluorescent flow cytometry was used to quantitate the qualitative microscopy results. MC4100 pcs, lptD4213 without the pcs plasmid, and lptD4213 pcs (± rhamnose) were grown for 16 h with or without 500 µM AECho and treated with or without Alexa488-sDIBO. The cells were analyzed by flow cytometry for fluorescence. The lptD4213 pcs cells grown with AECho and rhamnose to induce Pcs production followed by the treatment with Alexa488-sDIBO were fluorescent, while the same strain treated only with Alexa488-sDIBO showed no significant fluorescence (Fig. 6B). No fluorescent labeling was detected in lptD4213 without the Pcs plasmid or in MC4100 pcs after rhamnose-induced Pcs expression, incubation with AECho, and treatment with Alexa488-sDIBO (Fig. 6B). The AECho-grown lptD4213 pcs (− rhamnose) cells, treated with Alexa488-sDIBO, were fluorescent (Fig. 6B) despite the lack of Pcs induction, which suggested a leaky expression of Pcs. To confirm the leaky expression of Pcs in the absence of rhamnose, MC4100 pcs and lptD4213 pcs were grown with and without rhamnose induction and Cho supplementation. The total lipids were separated by TLC (supplemental Fig. S9). A spot corresponding to PC was detected for MC4100 pcs and lptD4213 pcs grown in the presence of Cho independent of rhamnose supplementation (supplemental Fig. S9; samples 4, 5, 8, and 9).
To ensure that the fluorescent flow cytometry signal observed was due to the fluorescent reagent reacting with AEPC, MC4100 pcs and lptD4213 pcs were grown for 16 h with or without 500 µM Cho or 500 µM AECho, followed by the treatment of all samples with the green fluorescent Alexa488-DIBO. The total lipids were isolated by Bligh-Dyer extraction and separated via TLC. Parallel TLC plates were visualized by charring (supplemental Fig. S10A) or green fluorescence (supplemental Fig. S10B). A fluorescent spot corresponding to Alexa488-DIBO clicked to AEPC was only observed for the lptD4213 pcs sample grown with AECho (supplemental Fig. S10B; sample 6). All samples showed spots for PE and PG (supplemental Fig. S10A; samples 1–6), while the samples grown with Cho also showed a spot for PC (supplemental Fig. S10A; samples 2 and 4).
The results obtained by microscopy and flow cytometry suggest that the AEPC from the lptD4213 mutant can be detected after treatment with AECho and Alexa488-sDIBO. The TLC showed AEPC click-labeled with Alexa488-DIBO, suggesting that the signal corresponds to AEPC crosslinked to the fluorescent click reagent.
Evaluation of the Alexa click-labeled AEPC localization in plasmolyzed E. coli lptD4213
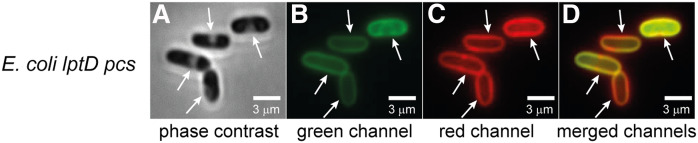
The green fluorescence observed for lptD4213 pcs should be localized on the OM if AEPC is externalized from the inner leaflet to the outer leaflet of the OM. However, to evaluate whether the click reagent also reaches the periplasm of the hyperpermeable lptD4213 pcs strain, the click-labeled lptD4213 pcs was imaged after osmotic shock treatment with a high-sucrose solution (plasmolysis). During plasmolysis, the cytoplasm shrank, whereas the OM retained its shape, leading to an increased separation of the IM and OM (Fig. 7A; arrows). The expanded periplasm facilitated the differential localization of fluorophores to the cytoplasm, IM, periplasm, and OM (57). In Alexa488-DIBO-labeled AECho-grown lptD4213 pcs cells stained with the membrane dye FM 4-64, the green fluorescent Alexa488 (Fig. 7B) and the red fluorescent FM 4-64 (Fig. 7C, D) were colocalized to the OM. This is consistent with previous reports that FM 4-64 preferentially stains the OM and not the IM of intact wild-type E. coli (52, 54). While the OM showed a consistent green fluorescent signal, there was a green fluorescent signal from the IM in at least one cell (Fig. 7B), suggesting that the Alexa488-DIBO could permeate across the OM to reach the periplasmic face of the IM in the permeable lptD4213 pcs.
Fig. 7.
Localization of the click-labeled AEPC by plasmolysis. E. coli lptD4213 pcs was grown with 0.5 mM AECho, labeled with Alexa488-DIBO, and stained with FM 4-64 membrane dye. The cells were then plasmolyzed and immediately visualized on a Nikon Eclipse Ti inverted microscope using phase contrast (A) and fluorescence (B, C). The green fluorescent Alexa488 signal (B) localized primarily to the periphery of cells (white arrows). The membranes of E. coli lptD4213 pcs cells stained with FM 4-64 (C) colocalized with the Alexa488 (D; overlay of B and C). The bar represents 3 µm.
Dose- and time-dependence of AEPC labeling in E. coli lptD4213
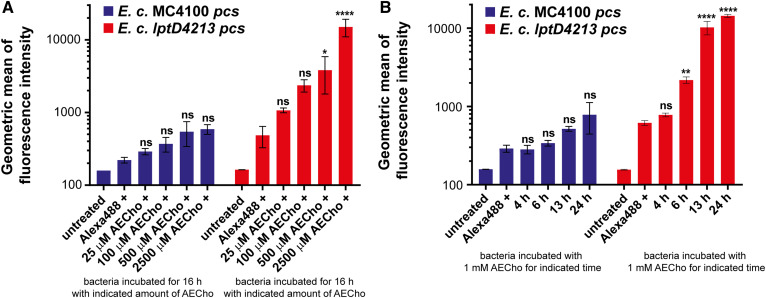
For the development of a fluorescence-based assay for AEPC detection, it was important to determine how much AECho was needed and how long cells need to be incubated with AECho to see significant fluorescent labeling. To test the amount of AECho needed, lptD4213 pcs and MC4100 pcs were grown for 16 h in M9 supplemented with different concentrations of AECho (0, 0.025, 0.1, 0.5, and 2.5 mM) followed by treatment with Alexa488-sDIBO. The cells were analyzed by flow cytometry for fluorescence. Significant fluorescent labeling was observed in lptD4213 pcs when grown with higher AECho concentrations (Fig. 8A; 0.5 and 2.5 mM), while all lower AECho concentrations (0, 0.025, and 0.1 mM) showed no significant labeling. The parent strain MC4100 pcs showed no significant fluorescent labeling for any AECho concentration used.
Fig. 8.
Flow cytometry analysis of E. coli MC4100 pcs and E. coli lptD4213 pcs grown with AECho and treated with Alexa488-sDIBO. A: Comparison of the geometric means of the fluorescence intensities of MC4100 pcs (blue) and lptD4213 pcs (red) samples grown for 16 h with different concentrations of AECho (0–2.5 mM AECho) followed by treatment with Alexa488-sDIBO. B: Comparison of the geometric means of the fluorescence intensities of MC4100 pcs (blue) and lptD4213 pcs (red) samples grown in the presence of 1 mM AECho for different periods of time (4–24 h) followed by treatment with Alexa488-sDIBO. l-Rhamnose (0.2%) was used to induce Pcs production. Experiments were performed in biological triplicates. Error bars represent the SD from the mean. The statistical significance represents the comparison with the sample without AECho supplementation as determined via two-way ANOVA (*P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001).
To test the incubation time needed for sufficient labeling, lptD4213 pcs and MC4100 pcs were grown in M9 with 1 mM AECho for different periods of time (4, 6, 13, and 24 h) followed by treatment with Alexa488-sDIBO. The cells were analyzed by flow cytometry for fluorescence. Cells grown for 6, 13, or 24 h showed significant fluorescent labeling, while cells grown for only 4 h with AECho did not show significant fluorescent labeling (Fig. 8B). The parent strain MC4100 pcs showed no significant fluorescent labeling for any incubation time tested. Our results suggest a concentration- and time-dependence of AECho labeling in lptD4213 pcs. At least 0.5 mM AECho for a 16 h incubation or at least a 6 h incubation with 1 mM AECho is needed to see significant fluorescent labeling in lptD4213 pcs, while the parent strain did not show significant fluorescent labeling.
Fluorescent labeling of AEPC in E. coli lpxC101
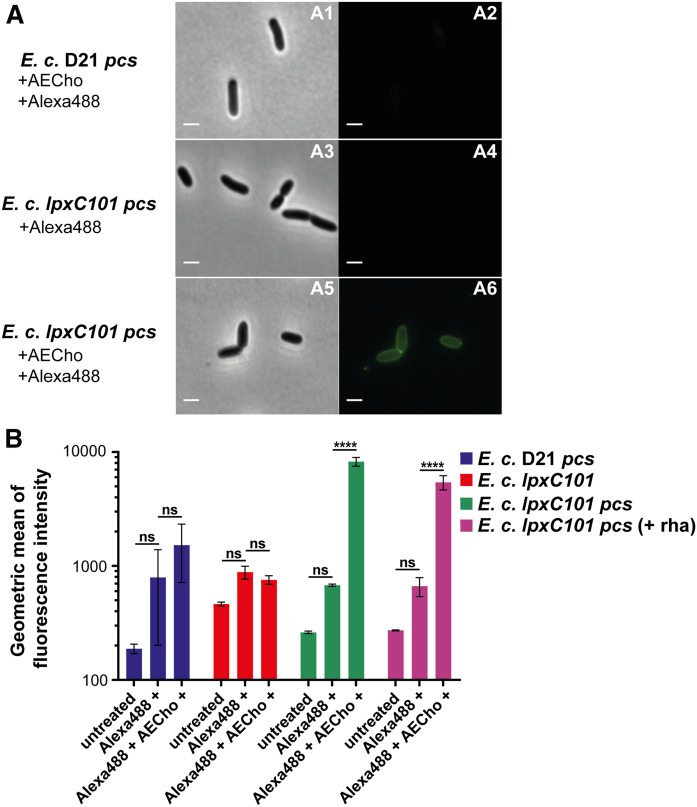
After showing that AECho can be used to detect AEPC in E. coli lptD4213 pcs, a second well-characterized isogenic strain pair was chosen to test whether AEPC could also be detected in the mutant, E. coli lpxC101. This mutant has a defect in LPS biosynthesis instead of in LPS transport and is hyperpermeable to a range of compounds, similar to lptD4213 (13, 14). The mutation is in lpxC, which encodes the enzyme responsible for the first committed step of the Raetz Pathway for lipid A biosynthesis (58). E. coli lpxC101 has reduced LPS on the outer leaflet of the OM and, therefore, increased surface-exposed PLs compared with wild-type E. coli (13, 14).
To test whether lpxC101 shows an increased labeling of AEPC, lpxC101 pcs and its parent D21 pcs were grown for 16 h with or without 500 µM AECho and treated with or without Alexa488-sDIBO. The cells were analyzed by bright-field and fluorescence microscopy. lpxC101 pcs grown in the presence of AECho and treated with Alexa488-sDIBO was fluorescent (Fig. 9A; A6). The control samples, lpxC101 pcs grown without AECho but treated with Alexa488-sDIBO (Fig. 9A; A4) and the parent D21 pcs grown with AECho and treated with Alexa488-sDIBO (Fig. 9A; A2), showed no fluorescent labeling. These results are in agreement with the results obtained for lptD4213 pcs.
Fig. 9.
Microscopic images and flow cytometry analysis of E. coli D21 pcs and E. coli lpxC101 pcs grown with AECho. A: Microscopic images of E. coli D21 pcs grown with AECho and treated with Alexa488-sDIBO (A1, A2) and E. coli lpxC101 pcs treated with Alexa488-sDIBO (A3, A4) and grown with AECho and treated with Alexa488-sDIBO (A5, A6). Brightfield images (A1, A3, A5) and corresponding fluorescence images detecting green fluorescence of AlexaFluor488 (A2, A4, A6). The bar represents 3 µm. B: Comparison of the geometric means of the fluorescence intensities of E. coli D21 pcs (blue), E. coli lpxC101 (red), E. coli lpxC101 pcs (green), and E. coli lpxC101 pcs with induced Pcs production (purple). Bacteria were untreated, treated with Alexa488-sDIBO only, or incubated with AECho followed by treatment with Alexa488-sDIBO. The cells were incubated overnight with 500 µM AECho. l-Rhamnose (0.2%) was used to induce Pcs production where indicated. Experiments were performed in biological triplicates. Error bars represent the SD from the mean. The statistical significance was determined via two-way ANOVA (****P ≤ 0.0001).
Fluorescent flow cytometry was used to quantitate the labeling. Two strains, lpxC101 without the Pcs plasmid and lpxC101 pcs (± rhamnose), were grown for 16 h with or without 500 µM AECho and treated with or without Alexa488-sDIBO. The cells were analyzed by flow cytometry for fluorescence. The lpxC101 pcs cells grown with AECho and rhamnose to induce Pcs expression followed by the treatment with Alexa488-sDIBO were fluorescent, while the same strain treated only with Alexa488-sDIBO showed no significant fluorescence (Fig. 9B). No fluorescent labeling was detected in lpxC101 without the Pcs plasmid or D21 pcs after rhamnose-induced Pcs expression, incubation with AECho, and treatment with Alexa488-sDIBO (Fig. 9B). The lpxC101 pcs (Pcs production not induced) cells grown with AECho and treated with Alexa488-sDIBO were also fluorescent (Fig. 9B), as detected for lptD4213 pcs (see Fig. 6B), which again confirmed the leaky expression of Pcs (see also supplemental Fig. S9). The results obtained by microscopy and flow cytometry suggest that AEPC can be detected via metabolic labeling with AECho for the lpxC101 mutant, as seen in the lptD4213 mutant.
DISCUSSION
Gram-negative OM asymmetry requires that LPS localizes exclusively on the outer leaflet and that PLs localize preferentially to the inner leaflet. This asymmetry is critical for maintaining the OM permeability barrier. PLs are externalized in higher levels onto the outer leaflet in bacteria with genetic mutations in the LPS biosynthesis/transport pathways as well as bacteria treated with compounds inhibiting key enzymes in either of the two pathways (4, 14–16). Thus, an assay to detect PLs on the OM may hold promise for identifying mutants with a perturbed OM permeability barrier due to OM asymmetry defects as well as compounds that can interact with and/or enter the cell to compromise OM asymmetry (17, 18).
To develop an assay for detecting the loss of bacterial OM asymmetry, we pursued metabolic labeling of PLs to enable a fluorescent PL detection assay with intact bacteria. Because the natural PLs in E. coli are not amenable to metabolic labeling, we explored metabolic labeling of PC by introducing Pcs into E. coli. Results confirmed previous results that the Pcs enzyme from L. pneumophila produces PC in E. coli upon Cho supplementation (42) and further demonstrated that PC is found in both the IM and OM. We then showed for the first time that E. coli could take up the Cho analogs PCho and AECho and that bacterial Pcs could utilize these analogs to form PPC and AEPC, respectively. Most importantly, we showed that two E. coli mutants (lptD4213 and lpxC101) with defects in the LPS biosynthesis or transport pathway possessed AEPC that could be fluorescently labeled, while the corresponding isogenic parent strains did not show significant fluorescent labeling.
Several important controls demonstrated that the fluorescent labeling of these mutant strains was due to covalent click labeling between Alexa488-DIBO and AEPC. First, there was no significant fluorescent labeling of the E. coli mutants (lptD4213 and lpxC101) without both Pcs expression and AECho supplementation, confirming that covalent click chemistry was required to fluorescently label the bacteria with Alexa488-DIBO. Second, the formation of the covalent click product AEPC-Alexa488-DIBO from intact cells was confirmed by TLC. Third, microscopy of plasmolyzed cells indicated that green fluorescent labeling was present on the OM and to a lesser extent the IM, suggesting that fluorescent labeling derived primarily from click labeling AEPC. Although Alexa488-DIBO does not readily permeate across the OM of E. coli K-12 under the conditions tested, it is possible that other bacterial species, mutants, or conditions might be more permeable to this reagent. In these cases, bulkier click reagents such as DIBO-biotin-streptavidin (59) or DIBO-PEG4-bismannose-SS-biotin (60) could be evaluated.
Metabolic labeling and fluorescent detection of AEPC provides an efficient method for measuring the loss of OM asymmetry in live, intact cells without the need for cell lysis or other purification steps. This methodology is different from the various existing methods used to directly or indirectly detect externalized PLs (9, 19–26). Current methods are predominantly end-point assays that require cell lysis after labeling. Furthermore, existing quantitative assays require some sort of extraction or purification step followed by TLC or liquid scintillation counting to determine indirectly the amount of externalized PLs. These methods are therefore highly labor-intensive and time-consuming.
While all of our data were obtained in E. coli pcs strains, it is important to point out that Cho is not an essential nutrient for E. coli growth. The only known E. coli metabolic pathway in which Cho is utilized is the osmoregulatory Cho-glycine betaine pathway (61). Thus, supplementing E. coli with AECho should not have a major impact on Cho metabolism, as long as the cells are not osmotically stressed. In order for Cho and its analogs to be incorporated, they need to enter the cytoplasm, where PL biosynthesis takes place. The absence of a transporter has been reported as problematic for the metabolic labeling of LPS with a 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) analog in some strains (31). Therefore, identifying the AECho transport mechanism(s) would allow the determination of which bacteria might be able to uptake AECho and be metabolically labeled. Two Cho transport systems have been identified in E. coli. BetT has been described as the high-affinity transport protein for Cho, while ProU is characterized as the low-affinity uptake system (62). Preliminary results obtained with an E. coli ΔbetT mutant (63) suggest that at least PCho might be also transported by BetT (data not shown). However, further studies are required to evaluate the impact of BetT and ProU on the uptake of AECho to fully understand whether the absence of AECho uptake systems may limit the utility in other strains. In addition, because millimolar concentrations of AECho were necessary to see efficient PC labeling in lptD4213 pcs, the identification of the transport mechanism might reduce the concentration/time needed for optimal labeling, much like the identification of the Kdo uptake receptor NanT allowed enhanced LPS metabolic labeling with a Kdo analog (31). As PC is produced in 15% of all bacteria, including pathogens such as P. aeruginosa or L. pneumophila (34), those strains that naturally express Pcs should be able to synthesize AEPC if AECho can be readily taken up by the cells. As such, strains with Pcs and naturally occurring PC may serve as even more efficient host strains for the metabolic labeling assay than E. coli.
While we envisioned PL metabolic labeling primarily as an assay for detecting the loss of OM asymmetry and PL exposure, the labeling system could also have other uses. For example, natural PL transport between the IM and OM is critical for both providing PLs to the OM and removing PLs from the outer leaflet of the OM. In E. coli and other organisms, the Mla system has been reported to actively transport PLs from the outer leaflet of the OM to the IM to prevent PL accumulation at the cell surface (9, 17, 18). Indeed, the ΔmlaA strain has been reported to contain more surface-exposed PLs (9), which could be directly evaluated with our new assay. In addition, our assay may provide the opportunity to probe the internalization of fluorescent AEPC from the outer leaflet of the OM to the IM.
Looking forward, the metabolic labeling of PC with AECho can be used in E. coli to assess mutant phenotypes, as seen in E. coli lptD4213 and lpxC101. Furthermore, this method could serve as a direct screen of AEPC accessibility for the identification of compounds that interfere with OM lipid asymmetry. With further optimization, the fluorescent flow cytometry method should be amenable to being scaled up to a medium- to high-throughput whole-cell screening assay for OM asymmetry, PL externalization, and OM permeability defects.
Supplementary Material
Acknowledgments
We thank Pramila Tamrakar, Adriana Jones, Jun-Rong Wei, Louis E. Metzger IV, Ramadevi Prathapam, Marcella Widya, Eric Fang, Tsuyoshi Uehara, Thomas Krucker, Laura McDowell, and Jennifer Leeds for their support and helpful discussions.
Footnotes
Abbreviations:
- AECho
- 1-azidoethyl-choline
- AEPC
- phosphatidyl(azidoethyl)choline
- Alexa488-DIBO
- AlexaFluor488-DIBO alkyne
- AlexaFluor488-sDIBO
- AlexaFluor488-sDIBO alkyne
- Cho
- choline
- DAG
- diacylglycerol
- FM 4-64
- N-3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl)-pyridinium dibromide
- Kdo
- 3-deoxy-d-manno-oct-2-ulosonic acid
- LB
- lysogeny broth
- LPS
- lipopolysaccharide
- MRM
- multiple reaction monitoring
- OM
- outer membrane
- PCho
- propargylcholine
- Pcs
- phosphatidylcholine synthase
- PL
- phospholipid
- PLE
- polar lipid extract
- PPC
- phosphatidyl(propargyl)choline
W.S.S., C.M.B.R., G.L., and D.A.S. are or were full-time employees of Novartis Institutes for BioMedical Research (NIBR). I.N. was an NIBR postdoctoral fellow. S.Y.L. was a full-time NIBR intern.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Morehead M. S., and Scarbrough C.. 2018. Emergence of global antibiotic resistance. Prim. Care. 45: 467–484. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019. Antibiotic Resistance Threats in the United States, 2019. U.S. Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- 3.Lewis K. 2013. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 12: 371–387. [DOI] [PubMed] [Google Scholar]
- 4.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67: 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikaido H., and Pages J. M.. 2012. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 36: 340–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikaido H., and Vaara M.. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munguia J., LaRock D. L., Tsunemoto H., Olson J., Cornax I., Pogliano J., and Nizet V.. 2017. The Mla pathway is critical for Pseudomonas aeruginosa resistance to outer membrane permeabilization and host innate immune clearance. J. Mol. Med. (Berl.). 95: 1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz N., Kahne D., and Silhavy T. J.. 2009. Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat. Rev. Microbiol. 7: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinverni J. C., and Silhavy T. J.. 2009. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc. Natl. Acad. Sci. USA. 106: 8009–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalebroux Z. D., Matamouros S., Whittington D., Bishop R. E., and Miller S. I.. 2014. PhoPQ regulates acidic glycerophospholipid content of the Salmonella typhimurium outer membrane. Proc. Natl. Acad. Sci. USA. 111: 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May K. L., and Silhavy T. J.. 2018. The Escherichia coli phospholipase PldA regulates outer membrane homeostasis via lipid signaling. MBio. 9: e00379-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong Z. S., Woo W. F., and Chng S. S.. 2015. Osmoporin OmpC forms a complex with MlaA to maintain outer membrane lipid asymmetry in Escherichia coli. Mol. Microbiol. 98: 1133–1146. [DOI] [PubMed] [Google Scholar]
- 13.Young K., Silver L. L., Bramhill D., Cameron P., Eveland S. S., Raetz C. R., Hyland S. A., and Anderson M. S.. 1995. The envA permeability/cell division gene of Escherichia coli encodes the second enzyme of lipid A biosynthesis. UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase. J. Biol. Chem. 270: 30384–30391. [DOI] [PubMed] [Google Scholar]
- 14.Normark S., Boman H. G., and Matsson E.. 1969. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J. Bacteriol. 97: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson B. A., Misra R., and Benson S. A.. 1989. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics. 122: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56: 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers M. J., and Trent M. S.. 2018. Phospholipid retention in the absence of asymmetry strengthens the outer membrane permeability barrier to last-resort antibiotics. Proc. Natl. Acad. Sci. USA. 115: E8518–E8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeow J., Tan K. W., Holdbrook D. A., Chong Z. S., Marzinek J. K., Bond P. J., and Chng S. S.. 2018. The architecture of the OmpC-MlaA complex sheds light on the maintenance of outer membrane lipid asymmetry in Escherichia coli. J. Biol. Chem. 293: 11325–11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutterlin H. A., Shi H., May K. L., Miguel A., Khare S., Huang K. C., and Silhavy T. J.. 2016. Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway. Proc. Natl. Acad. Sci. USA. 113: E1565–E1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamio Y., and Nikaido H.. 1976. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 15: 2561–2570. [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava R., Jiang X., and Chng S. S.. 2017. Outer membrane lipid homeostasis via retrograde phospholipid transport in Escherichia coli. Mol. Microbiol. 106: 395–408. [DOI] [PubMed] [Google Scholar]
- 22.Duckworth D. H., Bevers E. M., Verkleij A. J., Op den Kamp J. A., and van Deenen L. L.. 1974. Action of phospholipase A2 and phospholipase C on Escherichia coli. Arch. Biochem. Biophys. 165: 379–387. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Larrayoz A. F., Elhosseiny N. M., Chevrette M. G., Fu Y., Giunta P., Spallanzani R. G., Ravi K., Pier G. B., Lory S., and Maira-Litran T.. 2017. Complexity of complement resistance factors expressed by Acinetobacter baumannii needed for survival in human serum. J. Immunol. 199: 2803–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y., Bogdanov M., Lu S., Guan Z., Margolin W., Weiss J., and Zheng L.. 2018. The phospholipid-repair system LplT/Aas in Gram-negative bacteria protects the bacterial membrane envelope from host phospholipase A2 attack. J. Biol. Chem. 293: 3386–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logue S. E., Elgendy M., and Martin S. J.. 2009. Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat. Protoc. 4: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 26.Thomas C. P., Clark S. R., Hammond V. J., Aldrovandi M., Collins P. W., and O’Donnell V. B.. 2014. Identification and quantification of aminophospholipid molecular species on the surface of apoptotic and activated cells. Nat. Protoc. 9: 51–63. [DOI] [PubMed] [Google Scholar]
- 27.Jao C. Y., Roth M., Welti R., and Salic A.. 2009. Metabolic labeling and direct imaging of choline phospholipids in vivo. Proc. Natl. Acad. Sci. USA. 106: 15332–15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jewett J. C., and Bertozzi C. R.. 2010. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 39: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kocaoglu O., and Carlson E. E.. 2016. Progress and prospects for small-molecule probes of bacterial imaging. Nat. Chem. Biol. 12: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L., and Zhang Z.. 2016. Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction. Molecules. 21: E1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson I., Prathapam R., Grove K., Lapointe G., and Six D. A.. 2018. The sialic acid transporter NanT is necessary and sufficient for uptake of 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) and its azido analog in Escherichia coli. Mol. Microbiol. 110: 204–218. [DOI] [PubMed] [Google Scholar]
- 32.Di Guilmi A. M., Bonnet J., Peibetaert S., Durmort C., Gallet B., Vernet T., Gisch N., and Wong Y. S.. 2017. Specific and spatial labeling of choline-containing teichoic acids in Streptococcus pneumoniae by click chemistry. Chem. Commun. (Camb.). 53: 10572–10575. [DOI] [PubMed] [Google Scholar]
- 33.Oursel D., Loutelier-Bourhis C., Orange N., Chevalier S., Norris V., and Lange C. M.. 2007. Lipid composition of membranes of Escherichia coli by liquid chromatography/tandem mass spectrometry using negative electrospray ionization. Rapid Commun. Mass Spectrom. 21: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 34.Geiger O., Lopez-Lara I. M., and Sohlenkamp C.. 2013. Phosphatidylcholine biosynthesis and function in bacteria. Biochim. Biophys. Acta. 1831: 503–513. [DOI] [PubMed] [Google Scholar]
- 35.Aktas M., Koster S., Kizilirmak S., Casanova J. C., Betz H., Fritz C., Moser R., Yildiz O., and Narberhaus F.. 2014. Enzymatic properties and substrate specificity of a bacterial phosphatidylcholine synthase. FEBS J. 281: 3523–3541. [DOI] [PubMed] [Google Scholar]
- 36.Aktas M., Danne L., Moller P., and Narberhaus F.. 2014. Membrane lipids in Agrobacterium tumefaciens: biosynthetic pathways and importance for pathogenesis. Front Plant Sci. 5: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibellini F., and Smith T. K.. 2010. The Kennedy pathway–de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 62: 414–428. [DOI] [PubMed] [Google Scholar]
- 38.Vance J. E. 2015. Phospholipid synthesis and transport in mammalian cells. Traffic. 16: 1–18. [DOI] [PubMed] [Google Scholar]
- 39.Jao C. Y., Roth M., Welti R., and Salic A.. 2015. Biosynthetic labeling and two-color imaging of phospholipids in cells. ChemBioChem. 16: 472–476. [DOI] [PubMed] [Google Scholar]
- 40.Li C., Key J. A., Jia F., Dandapat A., Hur S., and Cairo C. W.. 2014. Practical labeling methodology for choline-derived lipids and applications in live cell fluorescence imaging. Photochem. Photobiol. 90: 686–695. [DOI] [PubMed] [Google Scholar]
- 41.Paper J. M., Mukherjee T., and Schrick K.. 2018. Bioorthogonal click chemistry for fluorescence imaging of choline phospholipids in plants. Plant Methods. 14: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogdanov M., Heacock P., Guan Z., and Dowhan W.. 2010. Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc. Natl. Acad. Sci. USA. 107: 15057–15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez-Morales F., Schobert M., Lopez-Lara I. M., and Geiger O.. 2003. Pathways for phosphatidylcholine biosynthesis in bacteria. Microbiology. 149: 3461–3471. [DOI] [PubMed] [Google Scholar]
- 44.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 45.Six D. A., Carty S. M., Guan Z., and Raetz C. R.. 2008. Purification and mutagenesis of LpxL, the lauroyltransferase of Escherichia coli lipid A biosynthesis. Biochemistry. 47: 8623–8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hvattum E., Larsen A., Uran S., Michelsen P. M., and Skotland T.. 1998. Specific detection and quantification of palmitoyl-stearoyl-phosphatidylserine in human blood using normal-phase liquid chromatography coupled with electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 716: 47–56. [DOI] [PubMed] [Google Scholar]
- 47.Uran S., Larsen A., Jacobsen P. B., and Skotland T.. 2001. Analysis of phospholipid species in human blood using normal-phase liquid chromatography coupled with electrospray ionization ion-trap tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 758: 265–275. [DOI] [PubMed] [Google Scholar]
- 48.Ariöz C., Gotzke H., Lindholm L., Eriksson J., Edwards K., Daley D. O., Barth A., and Wieslander A.. 2014. Heterologous overexpression of a monotopic glucosyltransferase (MGS) induces fatty acid remodeling in Escherichia coli membranes. Biochim. Biophys. Acta. 1838: 1862–1870. [DOI] [PubMed] [Google Scholar]
- 49.Hancock R. E., and Nikaido H.. 1978. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J. Bacteriol. 136: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osborn M. J., Gander J. E., Parisi E., and Carson J.. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 247: 3962–3972. [PubMed] [Google Scholar]
- 51.Davis M. R. Jr., and Goldberg J. B.. 2012. Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. J. Vis. Exp. 28: 3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nilsson I., Grove K., Dovala D., Uehara T., Lapointe G., and Six D. A.. 2017. Molecular characterization and verification of azido-3,8-dideoxy-d-manno-oct-2-ulosonic acid incorporation into bacterial lipopolysaccharide. J. Biol. Chem. 292: 19840–19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider C. A., Rasband W. S., and Eliceiri K. W.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewenza S., Vidal-Ingigliardi D., and Pugsley A. P.. 2006. Direct visualization of red fluorescent lipoproteins indicates conservation of the membrane sorting rules in the family Enterobacteriaceae. J. Bacteriol. 188: 3516–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paradis-Bleau C., Markovski M., Uehara T., Lupoli T. J., Walker S., Kahne D. E., and Bernhardt T. G.. 2010. Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell. 143: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz N., Wu T., Kahne D., and Silhavy T. J.. 2006. Probing the barrier function of the outer membrane with chemical conditionality. ACS Chem. Biol. 1: 385–395. [DOI] [PubMed] [Google Scholar]
- 57.Cook W. R., MacAlister T. J., and Rothfield L. I.. 1986. Compartmentalization of the periplasmic space at division sites in gram-negative bacteria. J. Bacteriol. 168: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitfield C., and Trent M. S.. 2014. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 83: 99–128. [DOI] [PubMed] [Google Scholar]
- 59.Li S., Zhu H., Wang J., Wang X., Li X., Ma C., Wen L., Yu B., Wang Y., Li J., et al. 2016. Comparative analysis of Cu (I)-catalyzed alkyne-azide cycloaddition (CuAAC) and strain-promoted alkyne-azide cycloaddition (SPAAC) in O-GlcNAc proteomics. Electrophoresis. 37: 1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monteiro R., Chafsey I., Leroy S., Chambon C., Hebraud M., Livrelli V., Pizza M., Pezzicoli A., and Desvaux M.. 2018. Differential biotin labelling of the cell envelope proteins in lipopolysaccharidic diderm bacteria: exploring the proteosurfaceome of Escherichia coli using sulfo-NHS-SS-biotin and sulfo-NHS-PEG4-bismannose-SS-biotin. J. Proteomics. 181: 16–23. [DOI] [PubMed] [Google Scholar]
- 61.Landfald B., and Strom A. R.. 1986. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J. Bacteriol. 165: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamark T., Styrvold O. B., and Strom A. R.. 1992. Efflux of choline and glycine betaine from osmoregulating cells of Escherichia coli. FEMS Microbiol. Lett. 75: 149–154. [DOI] [PubMed] [Google Scholar]
- 63.Baba T., Ara T., M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, Wanner B. L., and Mori H.. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy R. C., and Harrison K. A.. 1994. Fast atom bombardment mass spectrometry of phospholipids. Mass Spectrom. Rev. 13: 57–75. [Google Scholar]
- 65.Tan B. K., Bogdanov M., Zhao J., Dowhan W., Raetz C. R., and Guan Z.. 2012. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc. Natl. Acad. Sci. USA. 109: 16504–16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C., Tan B. K., Zhao J., and Guan Z.. 2016. In vivo and in vitro synthesis of phosphatidylglycerol by an Escherichia coli cardiolipin synthase. J. Biol. Chem. 291: 25144–25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.