Abstract
Atherogenic LDL particles are physicochemically and metabolically heterogeneous. Can bioactive lipid cargo differentiate LDL subclasses, and thus potential atherogenicity? What is the effect of statin treatment? Obese hypertriglyceridemic hypercholesterolemic males [n = 12; lipoprotein (a) <10 mg/dl] received pitavastatin calcium (4 mg/day) for 180 days in a single-phase unblinded study. The lipidomic profiles (23 lipid classes) of five LDL subclasses fractionated from baseline and post-statin plasmas were determined by LC-MS. At baseline and on statin treatment, very small dense LDL (LDL5) was preferentially enriched (up to 3-fold) in specific lysophospholipids {LPC, lysophosphatidylinositol (LPI), lysoalkylphosphatidylcholine [LPC(O)]; 9, 0.2, and 0.14 mol per mole of apoB, respectively; all P < 0.001 vs. LDL1-4}, suggesting elevated inflammatory potential per particle. In contrast, lysophosphatidylethanolamine was uniformly distributed among LDL subclasses. Statin treatment markedly reduced absolute plasma concentrations of all LDL subclasses (up to 33.5%), including LPC, LPI, and LPC(O) contents (up to −52%), consistent with reduction in cardiovascular risk. Despite such reductions, lipotoxic ceramide load per particle in LDL1-5 (1.5–3 mol per mole of apoB; 3–7 mmol per mole of PC) was either conserved or elevated. Bioactive lipids may constitute biomarkers for the cardiometabolic risk associated with specific LDL subclasses in atherogenic dyslipidemia at baseline, and with residual risk on statin therapy.
Keywords: metabolic syndrome, pitavastatin calcium, isopycnic density gradient ultracentrifugation, low density lipoprotein subclass heterogeneity, liquid chromatography electrospray ionization-tandem mass spectrometry, lipoprotein-associated phospholipase A2, lysophosphatidylcholine, ceramides, low density lipoprotein
Indisputable evidence attests to the causal role of LDL in atherosclerotic CVD (ASCVD) (1, 2). Indeed, LDL is the primary target of lipid-lowering therapies in international guidelines for the management of dyslipidemia and prevention of ASCVD (3, 4). In this context, LDL-C has proven to be an informative biomarker, both in cardiovascular risk stratification and as a measure of therapeutic response (5). The measurement of the cholesterol carried by LDL as a metric of the cardiometabolic risk associated with the totality of LDL particles in plasma provides incomplete information however (6–11). For example, the sphingolipid-rich LDL subpopulation, by virtue of its susceptibility to aggregation, is independently predictive both of ASCVD progression and cardiovascular death in CAD patients (12). Clearly then, the marked structural, physicochemical, and metabolic heterogeneity of LDL in both normolipidemic and dyslipidemic states suggests that particle subclasses with elevated atherogenic potential exist within the circulating LDL pool (13–19).
Production of LDL particles may occur via two major pathways: by extensive intravascular remodeling of hepatic VLDL precursors in a pathway involving both lipolytic (lipoprotein lipase and hepatic lipase) and lipid transfer activities, and a second pathway involving de novo hepatic secretion of LDL; the former pathway typically predominates (16–20). In hypertriglyceridemic states, and particularly in those involving insulin-resistance such as type 2 diabetes or the prediabetic state of metabolic syndrome (MetS), small dense LDLs (sdLDLs) predominate in the LDL particle profile (16, 18, 21). The dense LDL profile is intimately linked to elevated plasma levels of TG-rich VLDLs and their remnants, which act as the preferential acceptors of elevated cholesteryl ester transfer protein (CETP)-mediated cholesteryl ester (CE) transfer from HDL, with attenuated CE transfer to LDL particles (18, 20–22). Concomitantly, attenuated VLDL lipolysis together with CETP-mediated TG transfer from the VLDL pool to LDL contribute to the formation of TG-enriched LDL; these particles constitute a substrate for hepatic lipase, leading to formation of small LDL with a depleted neutral lipid core (TG and CE) content (18, 20–22).
sdLDL particles are distinguished by a low lipid/protein ratio, small particle size favoring enhanced transcytosis across the arterial endothelium, enrichment in apoCIII facilitating electrostatic binding to arterial wall proteoglycans with enhanced retention, a prolonged residence time in plasma reflecting low binding affinity for the LDL receptor as a result of an altered conformation of apoB100, an elevated affinity for LDL receptor-independent cell surface binding sites, and elevated susceptibility not only to oxidative damage, as indicated by the propensity to form hydroperoxides of CEs and phospholipids, but also to modification by glycation (13–15, 19, 21). sdLDL particles equally represent preferential transporters of lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme implicated in oxidized phospholipid hydrolysis and formation of LPC (23, 24). Finally, the relationship of particle numbers or concentrations of sdLDL to cardiovascular morbi-mortality in atherogenic dyslipidemia has been assessed in several prospective and cross-sectional epidemiological cohort studies (11, 12, 21, 25–27). Overall, the weight of evidence suggests that, despite inverse relationships to TG levels and those of buoyant LDL, elevated levels of small LDL particles appear to be associated with both atherosclerosis progression and cardiovascular events (21, 25–27).
The advent of LC-MS has facilitated the molecular dissection of the lipid moiety of lipoprotein particles (9, 12, 13, 27). Significantly, lipidomic analysis of LDL subclasses in atherogenic dyslipidemia may allow insight into questions of clinical and metabolic relevance: i) Does the lipidome of LDL subclasses reflect a continuum of lipid transport and metabolism across the LDL profile, and how does the LDL lipidome relate to that of precursor VLDL and IDL particles? ii) Alternatively, are there discontinuities across the lipidomic profiles of LDL subclasses indicative of subclass-specific lipid metabolism, particularly with respect to sdLDL? iii) Are specific LDL subclasses characterized by preferential transport of bioactive lipids, such as ceramides (Cers), other sphingolipids, lysophospholipids, or plasmalogens, whose molecular and cellular actions are relevant not only to the pathophysiology of LDL in ASCVD but also to insulin resistance? iv) Is subclass-specific remodeling revealed when the lipidome is normalized to either apoB100 (revealing overall particle composition on a molecular basis) or to PC (revealing both surface-specific and surface-core lipid interrelationships) (12, 28–33)?
Statins, or HMG-CoA reductase inhibitors, constitute first line therapy for marked reduction of LDL-associated cardiovascular risk in prediabetic MetS subjects and in type 2 diabetes, and reduce levels of all LDL subclasses, including sdLDL, by 30% or more (1, 3, 4, 22, 34, 35). Statin action modulates multiple pathways of lipid metabolism; however, these effects are not limited to inhibition of cholesterologenesis, but manifest in the lipidome of plasma lipoproteins, in which they may be clinically relevant (7, 34–40). We hypothesize that statin treatment might reduce the circulating concentrations of deleterious bioactive lipids transported in the lipidome of LDL subclasses in MetS. Indeed, this hypothesis is not inconsistent with the effect of statin treatment on the plasma lipidome in MetS and in CAD patients (7, 36, 37).
We therefore evaluated the impact of statin treatment on the cargo of 23 lipid classes in LDL subclasses [light buoyant LDL, LDL1-2; intermediate LDL, LDL3; sdLDL, LDL4-5, LDL5 representing very small dense LDL (vsdLDL)] of prediabetic MetS subjects in the context of the CAPITAIN cohort; the VLDL+IDL population was equally included in our analyses in order to provide insight into their precursor-product relation with LDL. As Lp-PLA2 activity is relevant to the inflammatory dimension of atherogenesis and is preferentially transported by sdLDL, the effect of statin treatment on its mass and activity were determined (23, 24). The CAPITAIN cohort (Chronic and Acute effects of PITAvastatIN calcium on monocyte phenotype, endothelial function, and HDL atheroprotective function in patients with MetS; clinicaltrials.gov: NCT01595828) consists of a small extensively phenotyped cohort of moderately insulin-resistant obese males with MetS and a mixed dyslipidemia at elevated cardiovascular risk (22, 37, 40, 41). This cohort received high dose pitavastatin calcium (4 mg/day) for 180 days in an open label design (supplemental Fig. S1); pitavastatin calcium was selected as a model statin given evidence-based neutrality on glucose homeostasis (42–44).
MATERIALS AND METHODS
Clinical protocol, inclusion and exclusion criteria, patient cohort, dietary counseling, and compliance
Details of the clinical protocol, cohort of MetS subjects, inclusion and exclusion criteria, dietary counseling, and compliance are detailed in the supplemental data and have been reported previously (22, 37, 40, 41, 45). The study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki and received approval from the Ethics Committee of Pitie-Salpetriere University Hospital. Written informed consent was given by each subject after the purpose and nature of the investigation had been explained. No serious adverse events were reported during the study protocol.
Healthy normolipidemic control subjects
Plasma samples from overnight-fasted healthy control nonobese male subjects (n = 10) were selected from the Baker IDI biobank as indicated earlier (22, 37). These subjects were consuming a Western-type diet commensurate with their BMI (mean 23.1 ± 2.5 kg/m2), were matched for age (49 ± 11 years), and were neither hypertensive (SBP = 119 ± 10), nor hyperglycemic (fasting blood glucose = 5.0 ± 0.7), nor dyslipidemic (supplemental Table S1); none displayed a history of CVD or type 2 diabetes. Written informed consent was given by each subject after the purpose and nature of the investigation had been explained.
Analytical and preparative methods
Analytical and preparative methods are equally detailed in the supplemental data. Preparative fractionation of apoB-containing lipoproteins (VLDL+IDL, LDL1-5 subclasses) in the native nonoxidized state was performed by isopycnic density gradient ultracentrifugation (see supplemental data) (14, 15, 22, 23, 46). It is notable that this methodology facilitates isolation of nondenatured lipoprotein subclasses, with no evidence for either lipid or protein oxidation during the procedure (47). Furthermore, analysis of the distribution of molecular species of CEs, tocopherols, and carotenoids among LDL subclasses, which are of immediate relevance to the oxidative susceptibility of these particles, confirmed their differential distributions, arguing strongly for the maintenance of native particle structure in individual LDL particle subclasses during isolation (48, 49). The total LDL fraction (d 1.019–1.063 g/ml) was isolated by sequential ultracentrifugation from the plasma of each MetS and control subject as described earlier (46).
Lipid extraction of VLDL+IDL and LDL subfractions and lipidomic analyses
The order of apoB-containing subfraction samples and total LDL fractions from MetS and control groups was randomized prior to lipid extraction and analysis; samples were analyzed in triplicate and the average values taken for subsequent statistical analyses. Total lipid extraction from a 10 μl aliquot of each subfraction was performed by a single phase chloroform:methanol (2:1) extraction, as described previously (37, 40).
Lipidomic analyses of lipid extracts of apoB-containing subfractions (VLDL+IDL, LDL1-5) at baseline [day 0 (D0)] and following statin treatment [day 180 (D180)] and from total LDL fractions were performed by HPLC followed by ESI-MS/MS using an Agilent 1200 liquid chromatography system combined with an Applied Biosystems API 4000 Q/TRAP mass spectrometer with a turbo-ion spray source (350°C) and the Analyst 1.5 data system. The methodology, instrumentation, and internal standards were identical to those used in earlier studies; indeed, the conditions for MS/MS analysis of lipoprotein lipid classes and subclasses and the parameters of the assay performance were derived from a quality control plasma pool as detailed earlier (37, 40). Technical quality control samples were included throughout the run in every 20-sample interval. The following individual lipid classes and subclasses were analyzed in each lipoprotein subfraction and quantitated: dihydroceramide (dhCer), Cer, monohexosylceramide (MHC), dihexosylceramide (DHC), trihexosylceramide (THC), monosialodihexosylganglioside (GM3), phosphatidylglycerol (PG); CE, free cholesterol (COH), SM, PC, alkylphosphatidylcholine [PC(O)], alkenylphosphatidylcholine [PC(P)], LPC, lysoalkylphosphatidylcholine [LPC(O)], PE, alkylphosphatidylethanolamine [PE(O)], alkenylphosphatidylethanolamine [PE(P)], lysophosphatidylethanolamine (LPE), PI, lysophosphatidylinositol (LPI), DAG, and triacylglycerol (TAG). The relative amounts of each molecular lipid species were calculated by expressing the peak area of each species relative to the peak area of the corresponding stable isotope or nonphysiological internal standard as described previously (37, 50). In targeted lipidomics, use of a stable isotope-labeled internal standard for each lipid species under investigation is not feasible. This approach does not provide absolute quantification but rather a close approximation of the actual concentration. Nonetheless, the precision of these measures is highly satisfactory (CV range 5–15%), and so the fold change (or percent change resulting from treatment) is accurate. A correction factor of 10 was applied to the PC(P) species to account for the lower signal response of these lipid species relative to the PE (17:0/17:0) internal standard. Background measurements obtained from blank samples were then subtracted from all other samples in each run, including quality control samples. Concentrations of total lipid classes were calculated from the sum of the individual molecular lipid species within each class. Results were expressed in picomoles of each lipid class per milliliter of plasma, in picomoles of each lipid class per picomole of apoB, and in nanomoles of each lipid class per micromole of PC.
Statistical analysis
The effect of pitavastatin calcium treatment on each parameter was determined by comparison of baseline values with corresponding values at D180 by the paired t-test. Data presented in the tables and figures are expressed as mean ± SEM for normally distributed variables and as median (minimum–maximum) for asymmetrically distributed parameters; distribution normality was assessed using the Kolmogorov-Smirnov test. In view of the small size of our male cohort of MetS subjects (n = 12), all statistical analyses were conducted initially using parametric tests and confirmed on the basis of a nonparametric test. P values were determined with the paired t-test analysis for values displaying a Gaussian distribution and with the nonparametric Wilcoxon test for those that were asymmetrically distributed. Given the phenotypic homogeneity of our subjects (22, 37) and the consistency of their response to statin treatment (see Fig. 1) (22), inter-individual variability was attenuated, typically leading to a symmetrical distribution for the majority of parameters determined. Correlation analyses of parameters in our data base were performed with the Pearson and Spearman tests as a function of the distribution of component values for a given parameter. For comparison of control subjects with MetS patients at baseline and D180, the nonpaired t-test was performed. The determination of P for trend was made by repeated measures one-way ANOVA with a posttest for linear trend using GraphPad Prism® 4 software (GraphPad Software Inc., version 4.03); for analyses of molecular lipid species, GraphPad Prism versions 8.0 and 8.1 were used.
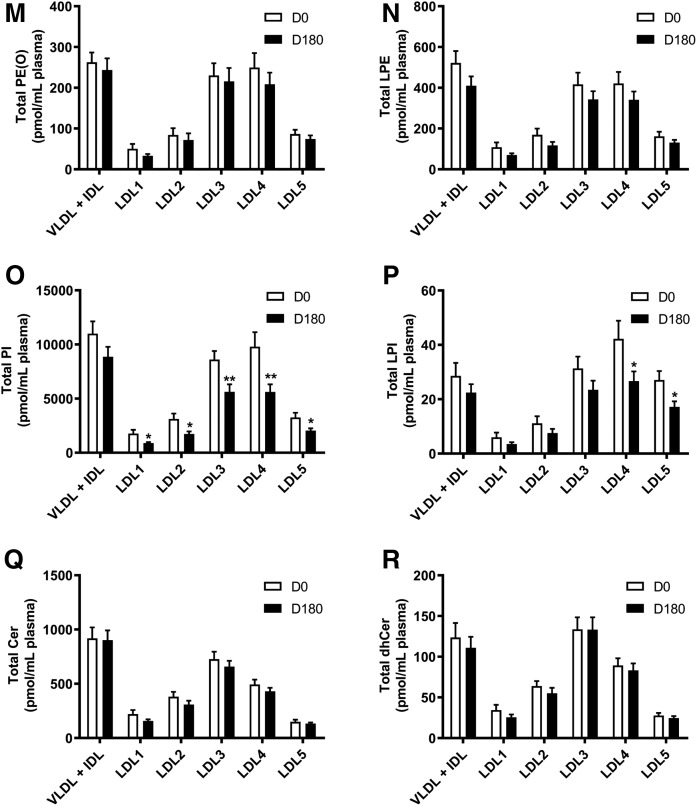
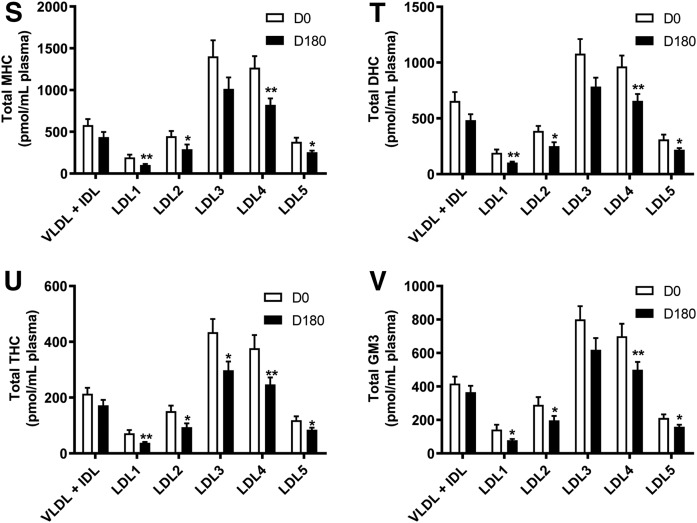
Fig. 1.
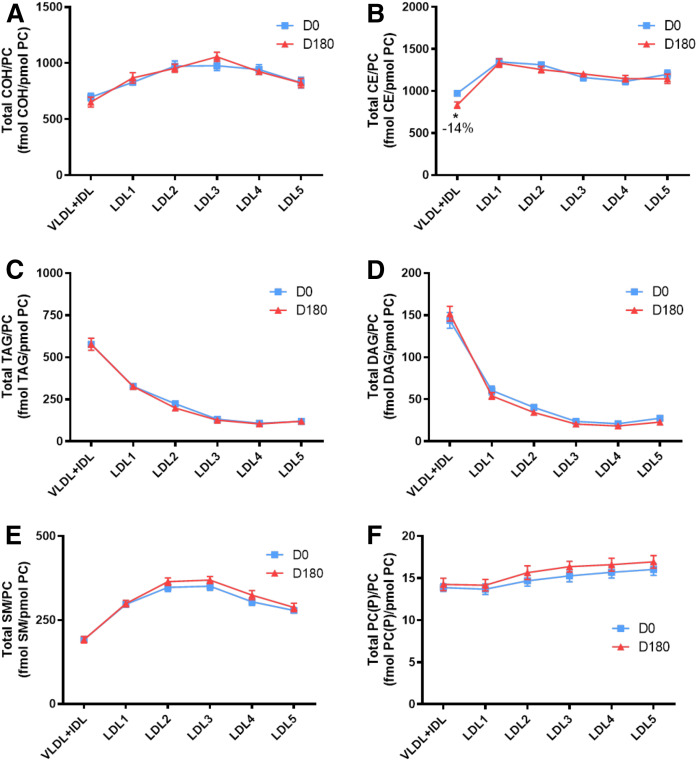
Plasma concentrations of individual lipid classes across the apoB-containing lipoprotein subfractions (VLDL+IDL, LDL1-5) at baseline (D0), and the effect of pitavastatin calcium treatment (4 mg/day) for 180 days (D180) in obese mixed dyslipidemic male MetS subjects. Values are expressed as mean ± SEM (n = 12) in picomoles of each lipid class per milliliter of plasma; absolute values are presented in supplemental Table S1. Density ranges: VLDL+IDL <1.019 g/ml, LDL1 = 1.019–1.023 g/ml, LDL2 = 1.023–1.029 g/ml, LDL3 = 1.029–1.039 g/ml, LDL4 = 1.039–1.050 g/ml, and LDL5 = 1.050–1.063 g/ml. Open columns represent baseline values; filled columns represent values after statin treatment at D180. ***P < 0.001; **0.001 < P < 0.01; and *0.01 < P < 0.05 versus D0. A–V: COH (A); CE (B); TAG (C); DAG (D); SM (E); PC (F); PC(P) (plasmalogen) (G); PC(O) (H); LPC (I); LPC(O) (J); PE (K); PE(P) (plasmalogen) (L); PE(O) (M); LPE (N); PI (O); LPI (P); Cer (Q); dhCer (R); MHC (S); DHC (T); THC (U); GM3 (V).
Using the data derived from mass spectrometric analyses of all lipid classes, the data were expressed in several ways: i) as absolute mass concentrations of each lipid class in individual lipoprotein subclasses at baseline and after statin treatment; ii) as molar concentrations of each lipid class in individual lipoprotein subfractions relative to apoB at baseline and after statin treatment, in order to reflect the size and degree of lipidation of the particle subfraction considered and to determine whether there were qualitative changes in lipoprotein lipid composition relative to apoB on statin treatment on a per particle basis; and iii) as molar concentrations of each lipid class in individual lipoprotein subfractions relative to total moles of PC at baseline and after statin treatment, in order to inform as to whether statin treatment induced changes in the molar ratios of each lipid class in relation to those of the dominant charged surface lipid, PC. For each molar ratio of lipid class/apoB or lipid class/PC, the paired t-test was used to compare the value at baseline with the corresponding value on statin treatment in the same lipoprotein subfraction; equally, comparison of lipid class/apoB and lipid class/PC values between VLDL+IDL and each LDL subfraction, and between LDL subfractions themselves, was performed separately by Bonferroni’s posttest to a two-way repeated measure ANOVA.
RESULTS
Baseline phenotypic features of MetS subjects, plasma lipid and lipoprotein profile, lipid transfer proteins, and pro-inflammatory and anti-inflammatory biomarkers: comparison with control subjects and effect of statin treatment
As outlined earlier, MetS subjects in the CAPITAIN study at baseline were obese (BMI = 31.7 ± 0.5), moderately insulin resistant (HOMA-IR = 2.7 ± 1.7), hypertriglyceridemic (fasting TG = 212.4 ± 17.1 mg/dl; 2.40 ± 0.53 mmol/l), and hypercholesterolemic (LDL-C = 153 ± 6.2 mg/dl; 3.96 ± 0.55 mmol/l; apoB = 102.0 ± 4.2 mg/dl and non-HDL-C = 185.9 ± 15.8 mg/dl; 4.81 ± 1.41 mmol/l) with low levels of lipoprotein (a) [Lp(a)] (median 8.8 mg/dl) (supplemental Table S1) (22, 37, 40). Indeed, plasma levels of total cholesterol, TGs, VLDL+IDL mass, non-HDL-C, LDL-C, and apoB at baseline in the MetS group were markedly elevated (P < 0.05–0.0001) when compared with the lipid profile in the healthy normal control group (supplemental Table S1). By contrast, both HDL-C and apoAI levels in control subjects were significantly higher relative to the MetS group both at baseline and post statin (P < 0.001).
Total plasma LDL mass (343.9 ± 7.3 mg/dl; supplemental Table S2) was 1.5-fold higher in the MetS group at baseline relative to that in a group of control male subjects (225.1 ± 10.4 mg/dl; P < 0.001) as reported earlier (22); it is however pertinent that LDL mass concentrations (as d 1.019–1.063 g/ml) in different groups of healthy normolipidemic adult males have been observed to vary over a wide range in our laboratory (225–303 mg/dl; it is noteworthy that similar spectrophotometric assays and isolation methodology were used) (22, 23, 46, 49). Such a wide range in plasma levels of LDL mass is reflected in turn in variation in the concentrations of individual LDL subclasses (supplemental Table S2). Indeed, whereas the range of concentrations of LDL1-3 in controls was below the mean value in our MetS group at baseline, the range of levels of LDL4 and LDL5 in control groups overlapped with the corresponding values in the MetS group at baseline (supplemental Table S2).
Importantly, statin treatment normalized LDL mass, LDL-C and apoB levels (225.5 ± 12.6 mg/dl, 96.1 ± 5.8 mg/dl, and 72.8 ± 5.1 mg/dl, respectively, and −31, −38 and −29%, respectively; all P < 0.0001) such that there were no longer any significant differences relative to the corresponding levels in the control subjects (supplemental Table S1) (22). Moreover, pitavastatin treatment at D180 lowered the concentrations (lipids + protein) not only of total LDL mass (241.6 mg/dl) but equally of all LDL subclasses to within the respective ranges seen among the control groups (supplemental Table S2). In addition, TG levels were lowered (−41%; P < 0.0005) to within the normal range (127.7 mg/dl; 1.44 mmol/l), although VLDL+IDL mass levels remained significantly higher in the post-statin MetS group relative to control subjects (P < 0.001) (supplemental Table S1) (4). Statin-mediated changes in lipid levels were associated with reductions in plasma CETP mass and activity (−18% and −16%, respectively; P < 0.01), and with minor elevation in plasma LCAT activity (+3%; P < 0.001) (22).
BMI was unchanged on statin treatment. As concerns systemic biomarkers of inflammation and oxidative stress, statin treatment mediated reduction in baseline plasma levels of oxidized LDL (84.0 ± 4.8 μg/dl; −34%; P < 0.01); concomitantly, an increment of 24% was observed in post-statin paraoxonase activity (n = 12; 28.1 ± 0.01 mU/μl and 34.4 ± 0.01 mU/μl serum at D0 and D180, respectively; P < 0.01). Statin treatment lowered both baseline plasma Lp-PLA2 mass and activity (223 ± 17 ng/ml, −8% and 234 ± 11 UI/l, −18%, respectively; both P < 0.001). Equally, serum amyloid A concentrations were lowered after statin treatment by 27% (baseline 26.5 ± 3 mg/l; P < 0.05). Low levels of systemic inflammation at baseline, as determined by high sensitivity CRP (1.6 ± 0.2 mg/l) and secretory PLA2 mass (data not shown), were unchanged following statin treatment. The potential impact of statin treatment on systemic inflammation was equally evaluated by assay of several pro- and anti-inflammatory cytokines, soluble cytokine receptors, growth factors, and adhesion proteins in the CAPITAIN cohort at D0 and D180 (data not shown; see supplemental data for methodological details). Findings for interleukin (IL)-6 showed no significant change from baseline on statin treatment (D0, 1.8 ± 0.3 pg/ml; D180, 2.1 ± 0.4 pg/ml); similar observations were made for IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-7, IL-8, IL-10, IL-12, IL-13, IL-15, TNF-α, IFN-γ, and monocyte chemoattractant protein-1 (MCP-1).
Plasma mass concentrations and percent weight chemical compositions of the VLDL+IDL subfraction and LDL1-5 subclasses in dyslipidemic MetS subjects at baseline and effect of statin treatment
Elevated baseline levels of the total mass of the TG-rich VLDL+IDL fraction (mean 135.8 mg/dl) in our MetS subjects were consistent with elevated concentrations of remnant cholesterol and non-HDL-C, which were between 1.6- and 2.4-fold higher (P < 0.01) than seen in control subjects as reported earlier (supplemental Table S2) (22, 46, 49). Among LDL subclasses, LDL3 and LDL4 each represented 31% of total LDL mass at baseline (22). As typical of atherogenic dyslipidemia, sdLDL4+5 represented the major LDL subclass (46.5% of total LDL mass) (supplemental Table S2). At baseline, percent TG content was almost 4-fold higher in LDL1 relative to LDL4, while percent CE content was 1.5-fold higher in the smaller LDL4 subclass. In consequence, the CE/TG ratio (5.1:1) peaked in LDL4, consistent with lipolytic loss of TG and CETP-mediated enrichment in CE (22). Interestingly, LDL5 did not follow this significant trend; rather this subclass was enriched in TG relative to LDL4, giving a lower CE/TG ratio (3.2:1) relative to LDL4 (supplemental Table S2).
On statin treatment at 180 days, plasma concentrations of all apoB-containing subfractions were reduced (LDL1-5, range −28% to −36% of total mass; VLDL+IDL, −28% of total mass), in line with reductions in lipid levels; as a consequence, the individual levels of LDL subclasses fell within the range typical of control subjects as noted above (supplemental Table S2) (22, 23, 46, 49). Qualitative changes equally occurred; whereas progressive CE enrichment (and TG depletion) with an increase in the CE/TG ratio was detected across LDL subclasses, the inverse occurred in VLDL+IDL with diminution in the CE/TG ratio, an effect highly correlated with statin-mediated reduction in CETP activity. Furthermore, the ratio of total lipid mass (COH+CE+PL+TG) to apoB mass in each subfraction was not modified by statin treatment (supplemental Table S2), indicating that average particle sizes in respective subfractions were similar before and after statin treatment.
Lipidomic analyses: plasma concentrations of 23 lipid classes in the VLDL+IDL subfraction and LDL1-5 subclasses in dyslipidemic MetS subjects at baseline
The VLDL+IDL subfraction (including IDLs as remnant particles) constituted the preferential carrier of TAGs and DAGs (95% and 60%; means 28 and 110 nmol/ml, respectively) at baseline (P < 0.001 vs. all LDL subclasses) (Fig. 1C, D; supplemental Table S3). Furthermore, the DAG content was elevated relative to TAGs, as reflected in a concentration ratio of 1:3.89, presumably the result of accumulation during TAG lipolysis. The VLDL+IDL fraction was further distinguished by transport of >53% and >50% of total PE and PG, respectively, which were enriched 3- to11-fold and 3- to 10-fold, respectively, relative to those carried in LDL1-5 (P < 0.01 vs. all LDL subclasses), and by acting as the primary transport vehicle for Cer (P < 0.05–0.01 vs. all LDL subclasses) (Fig. 1K; supplemental Table S3). Some 15–30% of the majority of the remaining major lipid classes were equally transported by this subfraction (COH, CE, SM, PC, PC(P), PC(O), LPC, LPC(O), PE(P), PE(O), PE, LPE, PI, LPI, Cer, dhCer, MHC, DHC, THC, and GM3) (Fig. 1A, B, E–V). Indeed, concentrations of COH, CE, SM, PC, PC(P), PC(O), LPC, LPC(O), PE(P), PE(O), LPE, PI, dhCer, MHC, DHC, THC, and GM3 were significantly higher in the VLDL+IDL subfraction as compared with LDL1, -2 and -5 (range of P values, <0.05–0.001). By contrast, concentrations of COH, CE, PC, PC(P), PC(O), LPC, PE(P), PE(O), LPE, PI, LPI, and dhCer were not significantly different in VLDL+IDL relative to those transported by LDL3 and -4.
At baseline, the absolute concentrations of 23 lipid classes were highest in the LDL3 and -4 subclasses relative to LDL1, -2, and -5 (range of P values from <0.05 to <0.001); indeed, lipid class concentrations transported in LDL3 and -4 accounted for 45–70% of the total for individual lipid classes, indicating that these two LDL subclasses approximated to surrogates for the total LDL fraction (Fig. 1A–V; supplemental Table S4). Minor differences in individual lipid cargos between LDL3 and -4 were nonsignificant, with the exception of Cer and dhCer, in which the LDL3 concentration was superior to that in LDL4 (P < 0.05 for both), and for LPI cargo, which was highest in LDL4 (LDL4 vs. LDL3 and LDL5, P < 0.05; vs. LDL1 and LDL2, P < 0.001) (Fig. 1P–Q).
Concentrations of cholesterol (COH and CE) at baseline in LDL3 and LDL4 ranged from 140 to 160 and 180 to 190 nmol/ml plasma/subfraction, respectively) (P < 0.001 for LDL3 and LDL4 vs. LDL1, -2, and -5 for both lipid classes) (Fig. 1A, B).
Absolute concentrations of bioactive lysophospholipids across LDL subclasses were highest for LPC (range 500–2,800 pmol per LDL fraction per milliliter of plasma), intermediate for LPE (range 100–400 pmol per LDL subfraction per milliliter of plasma), and lowest for LPI (5–40 pmol per LDL subfraction per milliliter of plasma) and LPC(O) (6–35 pmol per LDL subfraction per milliliter of plasma) (Fig. 1I, J, N, P). When expressed relative to the parent phospholipid, molar ratios of each lysophospholipid in LDL1-5 were: LPC, 0.02; LPC(O), 0.0002; LPI, 0.003; and LPE, 0.26; indeed, some 25% of PE was in the lyso form.
vsdLDL5 was distinguished from LDL1 and LDL2 by the lowest cargo of both TAG and DAG (P < 0.05 vs. LDL1-4), but equally by higher loads of lysophospholipids (LPC, LPC(O), and LPI; all P < 0.05), with the exception of LPE (Fig. 1C, D, I, J, P; supplemental Table S3). Furthermore, LDL5 carried the lowest cargo of Cer and dhCer at baseline versus LDL1-4 (<10% in both cases; P < 0.05) (Fig. 1Q, R; supplemental Table S3). Quantitatively, vsdLDL5 accounted for the transport of minor amounts (5–10% of total) of COH, CE, PC, SM, other sphingolipids, and plasmalogens and 10–15% of total lysophospholipids [LPC, LPC(O), LPE, and LPI] among all LDL subclasses (Fig. 1A-V; supplemental Table S3). Moreover, an elevated DAG/TAG ratio distinguished LDL5 from LDL1-3 (0.01 < P < 0.05) (supplemental Fig. S2A–J).
Effect of statin treatment on plasma concentrations of 23 lipid classes in the VLDL+IDL subfraction and in LDL1-5 subclasses in dyslipidemic MetS subjects
Despite consistency in confirming statin-mediated reduction in baseline VLDL+IDL mass as quantitated by absorptiometric assays (supplemental Table S2; −28%; P < 0.01), mass spectrometric data failed to achieve significance (supplemental Table S3; reductions of 28% or less across all lipid classes); the explanation may lie in the fact that the latter analyses do not allow accurate determination of absolute lipid concentrations, and equally that large inter-individual variations in VLDL+IDL levels occurred (SEM values >10% for some lipid classes) (37, 50). The VLDL+IDL fraction was distinct as the major carrier of Cer both before and after statin treatment (vs. LDL1-5; P < 0.05–0.001) (Fig. 1Q). By contrast, VLDL+IDL and LDL3 transported similar concentrations of dhCer at both baseline and D180 time points; nonetheless, VLDL+IDL dhCer cargo was higher than that of LDL1-3 and LDL5 (P < 0.05–0.001) (Fig. 1R).
Statin treatment significantly and systematically diminished plasma concentrations of the majority of lipid classes across LDL1-5 relative to baseline (up to −50%), and approximately in proportion to the statin-mediated reduction in the respective LDL subclasses (Fig. 1A–V; supplemental Table S2). Thus, individual profiles for the quantitative distribution of lipid classes across LDL1-5 subclasses post statin displayed an overall similarity to the corresponding profiles at baseline, with some exceptions (Fig. 1A–V). The major exception involved LDL subclass cargos of Cer and dhCer, whose concentrations were not significantly reduced in any LDL subclass (Fig. 1Q, R). It is noteworthy that the concentrations of dhCer and Cer transported in LDL3 were superior to those in LDL4 (each P < 0.05) (Fig. 1Q, R). Interestingly, a relatively invariant mass ratio of Cer:dhCer of ∼6:1 was observed across all apoB-containing subfractions and was not significantly changed by statin treatment (supplemental Table S3). As at baseline, vsdLDL5 was distinct in transporting higher absolute levels of lysophospholipids (LPC, LPC(O), and LPI, but not LPE) as compared with LDL1 and -2 (P < 0.05 for each), despite the fact that the mass concentration of these LDL subclasses post statin differed little (23.9, 30.7, and 29.4 mg/dl, respectively, for LDL1, -2, and -5) (Fig. 1I, J, P; supplemental Table S2).
Levels of the major lipid classes (COH, CE, TAG, and PC) were systematically lowered (range −21% to −41%, respectively) relative to baseline values within each LDL subclass (Fig. 1A–C, F; supplemental Table S3). Similar patterns were seen for DAG and SM (Fig. 1D, E; supplemental Table S3); decrements in the low plasma concentrations of DAG cargo in LDL1-5 were particularly pronounced (range −35% to −52%; P < 0.01 for LDL2-5). Although PE levels were reduced in all LDL subfractions (29–50%), significance was attained only in LDL2-4 (Fig. 1K; supplemental Table S3); LPE levels were not significantly reduced however (supplemental Table S3). Plasma levels of the major lysophospholipid, LPC, were markedly lowered by pitavastatin calcium in all LDL subfractions (−35% to −52%; P < 0.01), as were those of LPC(O) (range −32% to −49%) (Fig. 1J, L; supplemental Table S3). Furthermore, transport of PI was significantly reduced in LDL1-5 (−35% to −50%; P < 0.02), while decrement in LPI achieved significance in LDL4 and -5 only (−37%; P < 0.03) (Fig. 1O, P; supplemental Table S3). Concentrations of MHC, DHC, THC, and GM3 were significantly reduced (range −23% to −48%) by statin treatment across all LDL subfractions (Fig. 1S–V); reductions in MHC, DHC, and GM3 did not however attain significance in LDL3. Both PC and PE plasmalogen levels trended to decrease across all LDL subfractions upon statin treatment; significance against baseline was however attained only for PC(P) in LDL1 and -2, the minor LDL subfractions (P < 0.05) (Fig. 1G, L; supplemental Table S3).
Comparison of statin-mediated changes in concentrations of lipid classes reported earlier in whole plasma in MetS subjects at baseline (37) with those in the lipidome of LDL1-5 at D180 revealed consistent and significant statin-mediated reductions (range −24% to −52%) in most lipid classes across LDL subfractions [MHC, DHC, THC, GM3, SM, PC, PC(O), LPC, LPC(O), PE, PI, COH, CE, DAG, and TAG] (Fig. 1A–V; supplemental Table S3). However, while dhCer and total Cer levels were significantly reduced in total plasma (37), no change occurred in LDL1-5 (supplemental Table S3), suggesting that changes in Cer levels may have occurred independently in HDL. It is equally noteworthy that several additional lipid classes, present in low abundance and primarily ethanolamine-containing phospholipids [PE(O), PE(P), and LPE], LPI (with the exception of significant reductions in LDL4 and -5), and PC(P) (with the exception of the minor subfractions LDL1 and -2), showed nonsignificant trends to reduction in levels in both plasma and LDL1-5 on statin treatment (Fig. 1G, L–N, P; supplemental Table S3).
Baseline lipidomic profiles in the VLDL+IDL subfraction and LDL1-5 subclasses in dyslipidemic MetS subjects: normalization to moles of apoB and PC
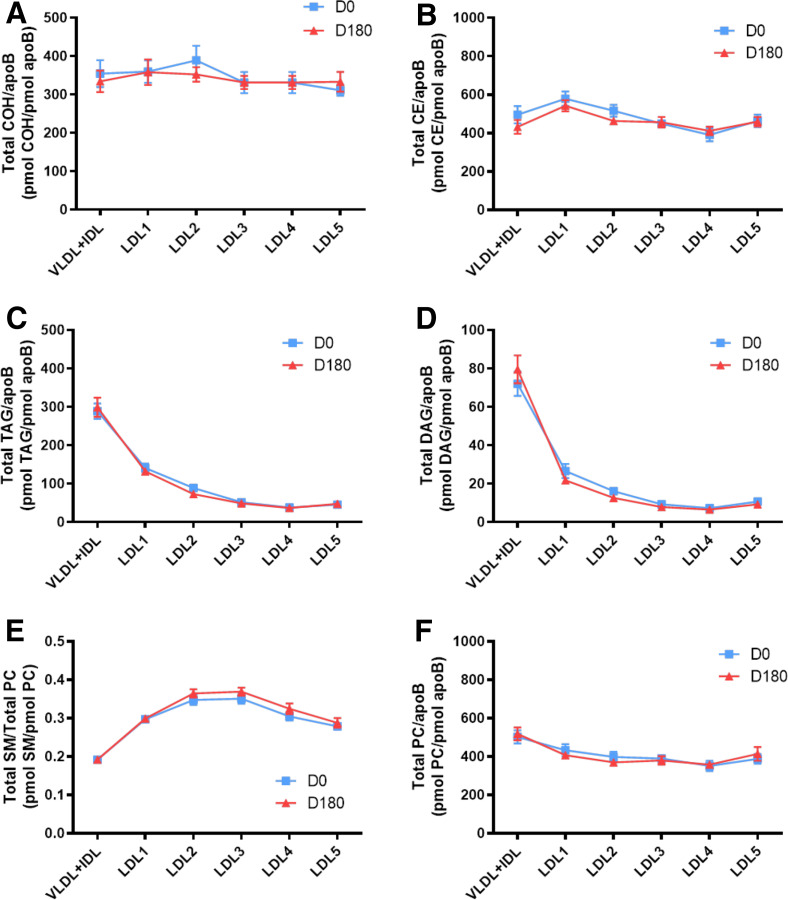
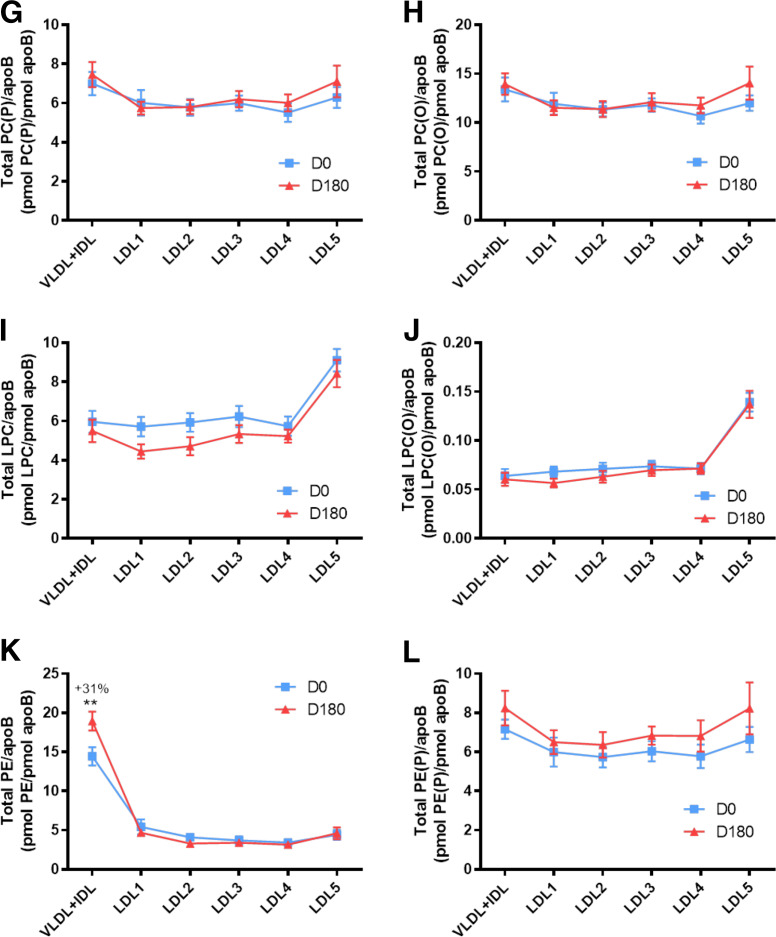
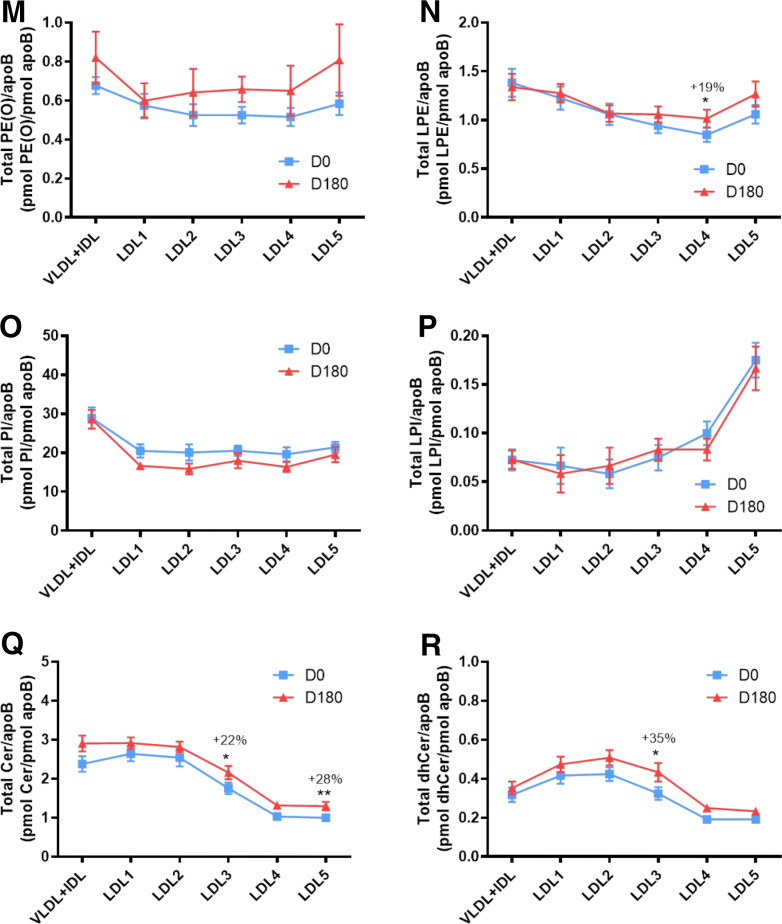
At baseline, lipidomic data for individual lipid classes in apoB-containing subfractions normalized to apoB (picomoles of lipid per picomoles of apoB) revealed five distinct baseline profiles across the 22 lipid classes (Fig. 2A–V; supplemental Fig. S2A–F): i) A predominantly monotonic profile from VLDL+IDL through LDL1-5, as exemplified by COH, CE, PC, PC(P), PC(O), PE(P), and PE(O) (Fig. 2A, B, F, G, H, L, M). ii) A profile characterized by a peak of enrichment in lipid/apoB molar ratios in VLDL+IDL as exemplified by profiles for TAG, DAG, PE, and PI, with marked discontinuities relative to lower ratios in LDL1-5 (Fig. 2C, D, K, O; supplemental Fig. S2C, E, I, O, R). iii) A profile dominated by marked lysophospholipid particle enrichment relative to apoB in vsdLDL5, indicative of marked discontinuity relative to molar ratios in VLDL+IDL and LDL1-4; these lysolipids were LPC and LPC(O) (P < 0.001 in all cases) and LPI (0.001 < P < 0.01 vs. LDL1-3 and LDL4) (Fig. 2I, J, P; supplemental Fig. S2K–N, S, T). In marked contrast, and despite the elevated proportion of LPE relative to PE across LDL subclasses (supplemental Table S3), the molar ratio of LPE/apoB was similar across all LDL subclasses, with no preferential enrichment in LDL5 (range 0.8–1.4 pmol per picomoles of apoB) (Fig. 2N); LPE therefore constitutes an internal control for the specific enrichment of LPC, LPC(O), and LPI on a per particle basis in LDL5. iv) A profile featuring a significant discontinuity in Cer/apoB and in dhCer/apoB profiles between dense LDL4-5 on the one hand, and LDL1-3 on the other; indeed molar ratios of Cer/apoB and dhCer/apoB indicated particle depletion (up to 2-fold lower) in these Cers in LDL4-5 relative to LDL1-3 and VLDL+IDL (supplemental Fig. S2X, V). v) An overall convex profile as exemplified by the sphingolipids, SM, MHC, DHC, THC, and GM3 (Fig. 2E, Q–V; supplemental Fig. S2G, H, U–AF).
Fig. 2.
Molar ratios of individual lipid classes normalized to moles of apoB in plasma VLDL+IDL, LDL1, LDL2, LDL3, LDL4, and LDL5 subfractions from obese mixed dyslipidemic male MetS subjects at baseline (D0) and after pitavastatin calcium treatment [4 mg/day for 180 days (D180)]. Values are expressed as mean ± SEM (n = 12) in picomoles of each lipid class per picomole of apoB. Percent change (%) was calculated relative to baseline values. ***P < 0.001; **0.001 < P < 0.01; and *0.01 < P < 0.05 versus D0. Density ranges: VLDL+IDL <1.019 g/ml, LDL1 = 1.019–1.023 g/ml, LDL2 = 1.023–1.029 g/ml, LDL3 = 1.029–1.039 g/ml, LDL4 = 1.039–1.050 g/ml, and LDL5 = 1.050–1.063 g/ml. Lines and square symbols in blue represent data at baseline, D0; red lines and triangular symbols represent data following 180 days of pitavastatin treatment (D180). A–V: COH (A); CE (B); TAG (C); DAG (D); SM (E); PC (F); PC(P) (plasmalogen) (G); PC(O) (H); LPC (I); LPC(O) (J); PE (K); PE(P) (plasmalogen) (L); PE(O) (M); LPE (N); PI (O); LPI (P); Cer (Q); dhCer (R); MHC (S); DHC (T); THC (U); GM3 (V).
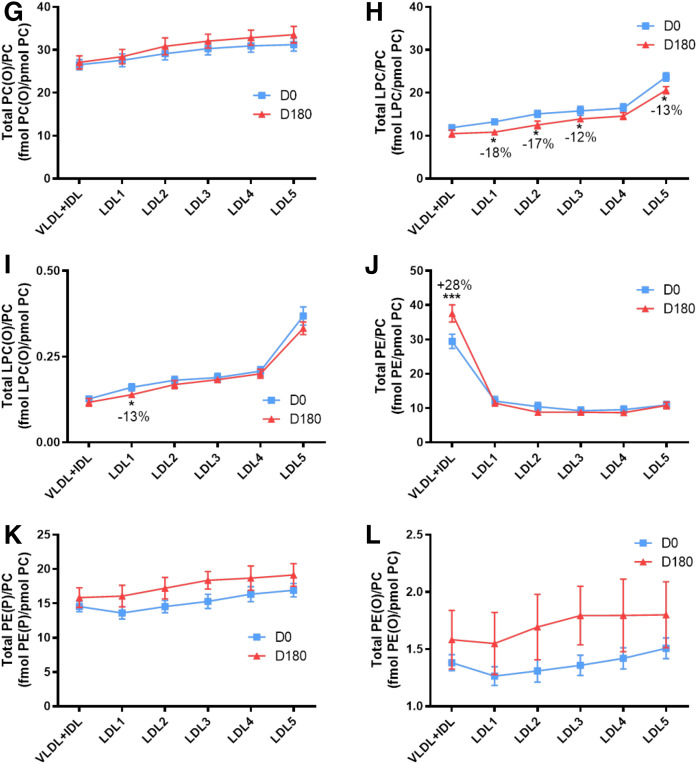
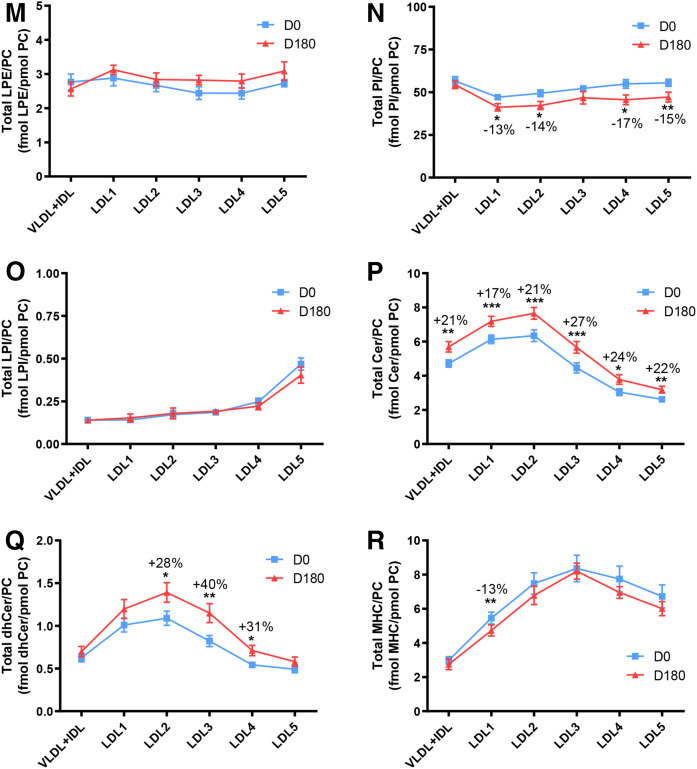
To gain insight into molecular relationships between surface-exposed polar lipids in apoB-containing subfractions at baseline, lipid profiles were normalized to picomoles of PC (Fig. 3A–U; supplemental Fig. S2AK–AL); such normalization accentuated the overall form of several lipidomic profiles, principally concerning COH (Fig. 2A vs. Fig. 3A), CE (Fig. 2B vs. Fig. 3B), and the sphingolipids, Cer, dhCer, MHC, DHC and THC (Fig. 2Q vs. Fig. 3P; Fig. 2R vs. Fig. 3Q; Figs 2S vs. Fig. 3R; Fig. 2T vs. Fig. 3S; Fig. 2U vs. Fig. 3T).
Fig. 3.
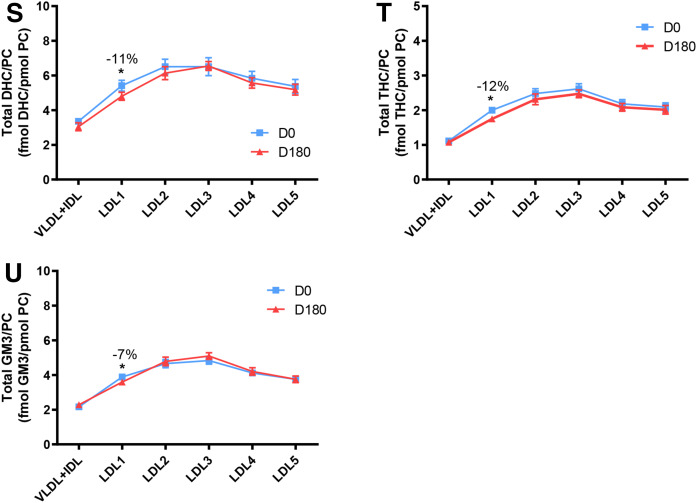
Molar ratios of individual lipid classes normalized to moles of PC in VLDL+IDL, LDL1, LDL2, LDL3, LDL4, and LDL5 subfractions from obese mixed dyslipidemic male MetS subjects at baseline (D0) and after pitavastatin calcium treatment [4 mg/day for 180 days (D180)]. Values are expressed as mean ± SEM (n = 12) in femtomoles of lipid class per picomole of PC. Percent change (%) was calculated relative to baseline values (D0). ***P < 0.001; **0.001 < P < 0.01; and *0.01;<;P < 0.05 versus D0. Density ranges: VLDL+IDL <1.019 g/ml, LDL1 = 1.019–1.023 g/ml, LDL2 = 1.023–1.029 g/ml, LDL3 = 1.029–1.039 g/ml, LDL4 = 1.039–1.050 g/ml, and LDL5 = 1.050–1.063 g/ml. A–U: COH (A); CE (B); TAG (C); DAG (D); SM (E); PC(P) (plasmalogen) (F); PC(O) (G); LPC (H); LPC(O), lysoalkylphosphtidylcholine (I); PE (J); PE(P) (plasmalogen) (K); PE(O) (L); LPE (M); PI (N); LPI (O); Cer (P); dhCer (Q); MHC (R); DHC (S); THC (T); GM3 (U).
Effect of statin treatment on the lipidomic profiles of the VLDL+IDL subfraction and LDL1-5 subclasses in dyslipidemic MetS subjects: normalization to moles of apoB or to moles of PC
Normalization to moles apoB.
Minor statin-mediated changes occurred in the profiles of the molar ratios of several lipid classes across the apoB-containing subfractions relative to baseline (typically <15% change), with conservation in large part of inter-subfraction differences for individual lipid classes seen at D0 (Fig. 1A–V; supplemental Fig. S2A–J). However, statin treatment induced marked modification in the lipid/apoB molar ratio in specific apoB-containing lipoprotein subfractions as follows: i) increase in the PE/apoB ratio by 31% in VLDL+IDL (0.001 < P < 0.01; Fig. 2K; supplemental Fig. S2O); ii) decrease in the LPE/apoB ratio by 19% in LDL4 (0.01 < P < 0.05; Fig. 2N; supplemental Fig. S2P, Q); iii) increment in the Cer/apoB ratio in both LDL3 and LDL5 (22% and 28%, respectively) (0.001 < P < 0.01; Fig. 2Q; supplemental Fig. S2U, V), and increase in the dhCer/apoB ratio by 35% in LDL3 on statin treatment (P < 0.01 < P < 0.05; Fig. 2R); iv) importantly, the elevated lipid/apoB ratios for LPC (Fig. 2I), LPC(O) (Fig. 2J), and LPI (Fig. 2P) specific to LDL5 at baseline were not altered by statin treatment (P < 0.001 for the three lipid classes and versus LDL1-4; supplemental Fig. S2K, L, N, T).
Normalization to moles of PC.
When expressed relative to PC, a polar surface lipoprotein lipid, a trend to amplification of differences between profiles for hydrophilic lipids at baseline and on statin treatment was seen as compared with profiles expressed relative to apoB. Thus, the Cer/PC ratios (Fig. 3P) were significantly increased in all subfractions relative to baseline: i.e., VLDL+IDL, LDL1, LDL2, LDL3, LDL4, and LDL5, +21% (0.01 < P < 0.01), +17% (P < 0.001), +21% (P < 0.001), +27% (P < 0.001), +24% (0.01 < P < 0.05), and +22% (0.001 < P < 0.01), respectively, indicative of largely uniform particle enrichment in Cer; the significance of inter-subfraction differences at baseline and after statin is shown in supplemental Fig. S2U and V. A second sphingolipid, dhCer, was equally enriched by statin action relative to baseline (LDL2, +28%, 0.01 < P < 0.05; LDL3, +40%, 0.001 < P < 0.01; LDL4, +31%, 0.01 < P < 0.05) with the exception of LDL1 and -5 (Fig. 3Q; supplemental Fig. S2W, X). For the lysolipid LPC (Fig. 3H), statin action depleted LDL1, LDL2, LDL3, and LDL5 (−18, −17, −12, and −13%; 0.01 < P < 0.05 for all subfractions, respectively; supplemental Fig. S2L). A trend to reduction in molar PI content relative to PC (Fig. 3N) was observed in all LDL subfractions, with significant decreases in both light and dense LDL (−13% in LDL1; −14% in LDL2, −17% in LDL4; all 0.01 < P < 0.05) and in LDL5 (−15%; P < 0.001). Finally, lipid normalization to PC revealed significant statin-mediated particle enrichment specific to the lipidome of buoyant LDL1 in LPC(O), MHC, DHC, THC, and GM3 (Fig. 3I, R–U).
Comparison of lipid class concentrations in the total LDL fraction in healthy control subjects with those of MetS subjects at baseline: normalization by statin treatment
As noted in our earlier reports and above, LC-MS analyses do not allow precise determination of absolute lipid concentrations in either plasma or lipoprotein fractions, and it is for this reason that they cannot be compared directly with lipid measurements in such samples obtained by clinical assays, as exemplified by the data in supplemental Table S1 (37, 40). Rather, LC-MS lipid quantitation allows comparison of data within a single data set obtained with the same LC-MS technology, which extends to lipid extraction, LC-MS resolution of molecularly defined lipid classes and their constituent species, and detection and quantitation of these lipids using a suitable standard(s). Thus, when comparing absolute lipid class levels in plasma determined with clinical spectrophotometric assays against those in isolated lipoprotein subfractions, multiple factors contribute to variance in absolute values. Major factors include: i) lipoprotein losses during isolation (i.e., percent recovery), and ii) the fact that clinical assays regroup lipid classes that are quantitated individually by MS; for example, both free and esterified cholesterol are estimated together in LDL-C assays, whereas they are quantitated separately by LC-MS. Indeed, earlier LC-MS analyses of plasma in MetS subjects in CAPITAIN revealed that levels of COH were not significantly elevated at baseline, whereas LDL-C assays (including both free and esterified cholesterol) revealed a 1.5-fold increment (P < 0.0001); it is also noteworthy that LDL-C assays integrate the cholesterol content of Lp(a) (37). Furthermore, phospholipids are separated into up to 18 subclasses by LC-MS, while frequently only those containing choline are measured clinically.
It was of interest, however, to compare the lipid class profile in total LDL (d 1.019–1.063 g/ml) isolated from a healthy normolipidemic control group with that in a corresponding LDL fraction from the MetS group at baseline, and subsequently with the post-statin MetS LDL fraction (supplemental Table S4). Importantly however, such a comparison provides data that represent the summed average for each lipid class among the LDL1-5 subclasses, and will not therefore reveal the more subtle differences in the relative concentration of individual lipid classes across LDL subclasses at baseline or those resulting from pitavastatin treatment.
Supplemental Table S4 shows that both TAG and DAG levels were 1.7-fold elevated in MetS at baseline versus the control group, consistent with the hypertriglyceridemia detected upon enzymatic assay of glyceride glycerol (supplemental Table S1). CE was nonsignificantly elevated in MetS (13%), with no increment in COH relative to the control group. The remaining 18 lipid classes were within 20% of levels in the control group. The only exception was a minor lipid, PG, whose level was 2-fold elevated in the MetS group (P < 0.01).
When the LDL lipid class profile of the post-statin MetS group was compared with that of the control subjects, a marked trend to normalization was observed. Levels of both COH and CE were normalized, consistent with the nonsignificant difference between LDL-C values in controls and the MetS group at baseline. The major exception concerned the supra-normal TAG levels in MetS post statin (P < 0.0004), which while decreased relative to pre-statin levels, remained elevated relative to the control levels, consistent with clinical findings in supplemental Table S1.
Lp-PLA2 activity and mass across the VLDL+IDL subfraction, LDL1-5 subclasses, and apoAI-containing lipoprotein subfractions at baseline: effect of statin treatment
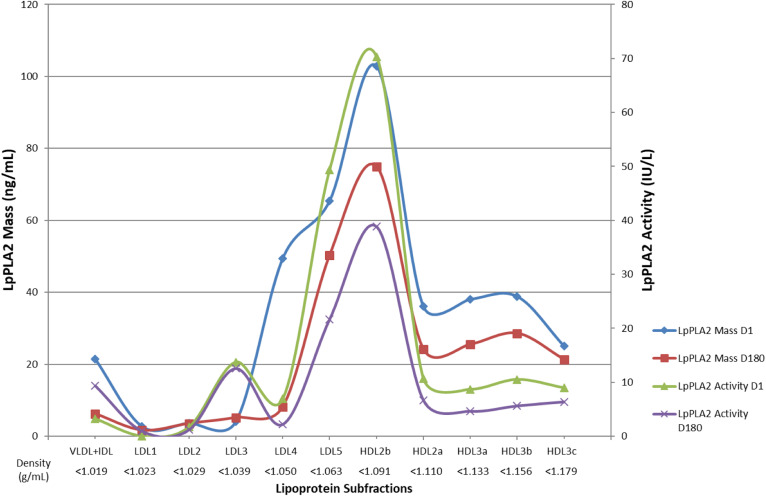
The distributions of Lp-PLA2 mass and activity among apoB-containing and apoAI-containing lipoprotein subfractions as a function of density at baseline (D0) and after 180 days of pitavastatin calcium treatment (D180) are shown in Fig. 4. Some 26% of total lipoprotein-associated Lp-PLA2 mass was carried in dense LDL4 and -5 at baseline, in contrast to VLDL+IDL, LDL1, LDL2, and LDL3, which together accounted for <10%; by comparison, HDL2b-associated Lp-PLA2 mass (23% of total plasma mass) predominated among HDL subfractions. Following statin treatment, a reduction of 37% (P < 0.05) in total lipoprotein-associated enzyme mass was observed; this reduction occurred primarily in dense LDL4 (−83% relative to baseline) with a smaller decrement in dense LDL5 (−23%). Overall, Lp-PLA2 mass associated with HDL subfractions diminished by −31%.
Fig. 4.
Lp-PLA2 activity and mass across VLDL+IDL and LDL1-5 subfractions as a function of density at baseline and effect of pitavastatin calcium treatment [4 mg/day for 180 days (D180)]. Lipoprotein subfractions were isolated from plasma as indicated in the Materials and Methods section, and LpPLA2 mass (expressed as nanograms per milliliter; n = 3; for all data points for apoB-containing lipoproteins at D0 and D180, the mean value for SEM was 6.1 IU/l) and activity (expressed as IU/l; n = 4; for all data points for apoB-containing lipoproteins at D0 and D180, the mean value for SEM was 2.8 IU/l; see the Materials and Methods). Blue line and blue diamond symbols, Lp-PLA2 mass at D0; red line and red square symbols, Lp-PLA2 mass at D180; green line and green triangular symbols, Lp-PLA2 activity at D0, and violet line and violet cross symbols, Lp-PLA2 activity at D180.
The density profile of Lp-PLA2 activity paralleled that of mass, the bulk (58%) residing in dense LDL5 and HDL2b subfractions at D0 (Fig. 4). LDL5 was the major enzyme carrier among apoB-containing lipoproteins at baseline, representing 66% of total across apoB-containing subfractions, and 24% of that of all plasma lipoprotein-associated activity. As seen for enzyme mass, HDL2b-LpPLA2 activity predominated among HDL subfractions, accounting for 34% of HDL-associated activity. Pitavastatin calcium treatment effected a −40% reduction in total lipoprotein-associated enzyme activity (P < 0.05); this decrement primarily resulted, on the one hand, from preferential reduction in dense LDL5-associated Lp-PLA2 (−56%, P < 0.03) and, on the other, from a −45% reduction in HDL2b-associated activity.
DISCUSSION
We present the first comprehensive quantitative and qualitative analysis of the lipidomic profiles of atherogenic apoB-containing lipoprotein subclasses (VLDL+IDL and five LDL particle subpopulations) in hypertriglyceridemic hypercholesterolemic prediabetic obese male subjects exhibiting the MetS; such profiles encompass 23 distinct lipid classes. We have equally evaluated the effect of pitavastatin calcium treatment for 180 days on these profiles, with a focus on bioactive lipids relevant to the pathophysiology of ASCVD.
At baseline, our novel and salient findings highlight: i) As the predominant carrier of the neutral lipids TAG and DAG, VLDL+IDL particles (d <1.019 g/ml) equally represented the primary transport vehicle not only for PE and PI but also for Cers (50% and 48%, respectively, of total in apoB-containing particles), consistent with their secretion in hepatic VLDL. Marked preferential particle enrichment of VLDL+IDL in TAG, DAG, PE, and PI was further emphasized when data were normalized to either apoB or PC. Moreover, the VLDL+IDL fraction was deficient in SM, exhibiting the lowest SM/PC ratio of all apoB-containing subclasses, indicative of elevated surface fluidity conducive to lipase activity. ii) When normalized to apoB, VLDL+IDL and LDL1-5 displayed monotonic profiles for major surface-exposed lipids implicated in maintenance of particle integrity, i.e., COH, SM, and PC, thereby implying a concerted metabolic remodeling of VLDL+IDL across LDL subclasses to LDL5. iii) Monotonic profiles equally occurred for both PC and PE plasmalogens across all apoB-containing particle subfractions when normalized to either apoB or PC; their potent scavenger activity for reactive oxygen species is consistent with a key protective role of labile lipoprotein lipids against oxidative stress. iv) While the LDL3 and -4 subclasses accounted for the major cargos of both PE and PI across LDL1-5, these bioactive lipids were distinguished by monotonic particle profiles in LDL subclasses (∼5 and ∼20 pmol/mol apoB, respectively). v) vsdLDL5 particles were preferentially enriched in three bioactive lysophospholipids, LPC, LPC(O), and LPI (as molar ratios relative to apoB or PC), revealing a marked discontinuity in particle composition relative to LDL1-4. Such preferential transport may further enhance the pro-inflammatory potential of vsdLDL5 (see Fig. 5). vi) By contrast, a fourth lysophospholipid, LPE, was uniformly distributed across LDL1-5 (1–1.5 mol per mole of apoB; 2.5–3 mol per mole of PC); in this way, LPE acted as an internal control for lysophospholipid distribution among LDL subclasses. vii) Lipotoxic Cers were deficient in the end products of the intravascular metabolism of LDL, i.e., LDL4 and -5 (∼1 mol per mole of apoB)
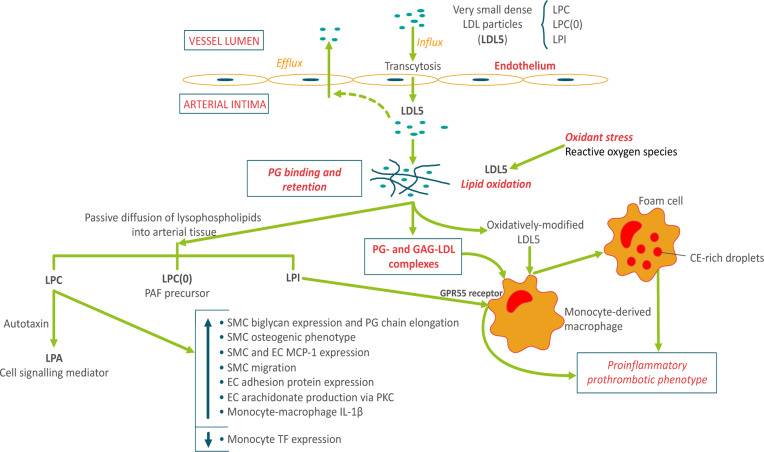
Fig. 5.
Arterial entry and retention of vsdLDL5: relevance to atherogenesis. The small particle size of vsdLDL particles (LDL5) favors enhanced arterial wall entry by endothelial transcytosis (13, 21, 51). These particles are preferentially enriched in three bioactive lysophospholipids: LPC, 9 mol per LDL5 particle; LPC(O), i.e., lyso-PAF, 1 mol per seven LDL5 particles; and LPI, 1 mol per six LDL5 particles, and equally in Lp-PLA2. sdLDLs display elevated binding affinity for proteoglycan components of the extracellular matrix, favoring enhanced intimal retention (21). Complexes of dense LDL5 particles with proteoglycans (PG) and glycosaminoglycans (GAG) are avidly taken up by human monocyte-derived macrophages (HMDMs) (52). Systemic oxidative stress is a characteristic of dyslipidemic MetS subjects (53); LDL5 lipids are highly susceptible to oxidative modification (21). Oxidative modification leads to scavenger receptor uptake in HMDMs with conversion to pro-inflammatory prothrombotic foam cells, key components of atherosclerotic plaques (1, 21). Hydrophilic lysophospholipids [LPC, LPC(O), LPI] may diffuse out from LDL5 particles into the aqueous phase in arterial tissue. LPC can exert a spectrum of biological effects via two pathways: the indirect pathway involving autotaxin-mediated hydrolysis of LPC with formation of lysophosphatidic acid (LPA), a potent cell signaling mediator acting through multiple G-coupled receptors (32, 54); and the direct pathway involving i) stimulation of biglycan expression and PG-chain elongation in vascular smooth muscle cells (VSMCs) with induction of an osteogenic phenotype in these same cells (55, 56), ii) stimulation of monocyte chemoattractant protein-1 (MCP-1) expression in both VSMCs and vascular endothelial cells (ECs) (32), iii) stimulation of VSMC migration (32), iv) induction of adhesion protein expression in ECs and VSMCs (32), v) stimulation of release of arachidonic acid from ECs via the PKC pathway (32), and vi) induction of the production of IL-1β in HMDMs (57). Together these effects of LDL5-associated LPC indicate a key role for these particles in the acute and chronic inflammatory dimensions of atherogenesis (21, 32, 55, 58). By contrast, LPC suppresses tissue factor expression in human monocytes (59). The degree to which LDL5-associated lyso-PAF may be transformed to active pro-inflammatory PAF depends largely on the potential for its acylation by HMDMs in situ (60). Finally, the LPI/GPR55 axis may initiate a wide range of cellular responses, including cytokine and chemokine secretion, cell proliferation and migration, and platelet aggregation (61, 62). Considered together, these findings indicate that the enhanced intimal entry, retention, and oxidation of lysophospholipid-laden LDL5 particles may exert a spectrum of biological actions in arterial tissue, which together favor accelerated atherogenesis in dyslipidemic individuals.
Following statin therapy, our findings highlight: i) Despite efficacious reduction of LDL1-5 subclass levels, LDL5 particles maintained preferential enrichment in LPC, LPC(O), and LPI relative to apoB and PC. ii) VLDL+IDL and LDL1-3 were principle transporters of Cer species (>95%) at baseline and post statin, with a marked discontinuity at the interface of LDL3 with LDL4 and -5 to significantly lower levels (as moles per mole of apoB). iii) Uniquely among the 23 lipid classes, plasma concentrations of Cer and dhCer were either unchanged or insignificantly decreased post statin, despite significant reductions in particle numbers (as apoB) in all apoB-containing subfractions (22). Post-statin particle enrichment in both Cer and dhCer (up to 40% in LDL3 per mole of PC) accounted for the maintenance of both Cer and dhCer levels. iv) When compared with the lipid profile in healthy control subjects, pitavastatin calcium treatment substantially normalized the key features of atherogenic dyslipidemia in MetS and attenuated biomarkers of oxidative stress and inflammation. Only TG levels in MetS remained superior to those of control subjects post statin, but nonetheless fell within the upper limit of the normal range. Concentrations of LDL subclasses fell within the range seen in control groups, while lipid class profiles were largely normalized with the exception of TAG.
Considered together, these data are of immediate relevance to the pathophysiological mechanisms operative in atherogenesis upon retention of LDL particles in the arterial intima (Fig. 5).
Metabolic and compositional continua of major and minor lipid classes across LDL particle subclasses
Consistent with the overall reduction in particle size from LDL1 to LDL5 of approximately 25 to 21 nm reported earlier in subjects of similar hypertriglyceridemic phenotype (63), the absolute decrements in molar contents of COH, CE, TAG, DAG, SM, PC, and Cer were ∼50, ∼180, ∼100, ∼10, ∼35, ∼35, and 1.5 per mole of apoB (Fig. 2A–F, Q); such decrements are comparable in large part to those reported for LDL (d 1.019–1.063 g/ml) in subjects presenting with dyslipidemic type 2 diabetes (28). Each of the bulk lipids (COH, PC, and SM) is implicated in maintenance of particle integrity (64–66). However, when expressed relative to a major surface lipid, PC, the profiles for both COH and SM became convex with significant differences between all subfractions (Fig. 3A, E), suggestive of distinct molecular lipid packing relationships between surface lipids, to which the lipid/apoB ratio was insensitive. Interestingly, both COH and SM favor tighter surface packing in the LDL surface lipid monolayer (67).
Overall monotonic profiles for molar lipid ratios to apoB and to PC occurred equally for both PC and PE plasmalogens [including the ether phospholipids PC(O) and PE(O)] (Fig. 2G, H, L, M), implying the existence of metabolic and/or physicochemical constraints for these lipids from LDL1 through to LDL5 particles (15, 64–67). The maintenance of the protection of labile lipids against oxidative stress (such as CEs and nonplasmalogen phospholipids containing polyunsaturated fatty acids) may underlie this compositional feature, as plasmalogens act as highly potent scavengers of reactive oxygen species due to their vinyl ether bond (30). Importantly, the molar ratios of both major plasmalogens [PC(P) and PE(P)] to apoB and to PC (each ∼6 and ∼15 pmol per picomole of apoB, respectively) were maintained upon statin treatment.
As low abundance lipids, both PE/apoB and PI/apoB ratios were equally invariant from LDL1-5 at 5 and at 20 pmol per picomole of apoB, respectively, in the surface monolayer of LDL particles. PI, a bioactive polar lipid, is a key anionic lipid in the LDL surface; typically, it contains polyunsaturated arachidonic acid as an eicosanoid precursor, playing critical roles in cell membranes, via interfacial binding of proteins, in regulation of protein activity at the cell interface and in cell signaling cascades (61). Whether LDL-bound PIs may act as precursors to cellular phosphoinositides is indeterminate. Overall, statin treatment reduced the bulk cargo of PE and PI transported in LDL subclasses in the range of 30–50%, with minor variation in molar lipid/apoB ratios.
Bioactive lysophospholipids are differentially distributed across LDL1-5 subclasses
A highly distinct lipid/apoB profile at baseline revealed specific lysophospholipid enrichment (up to 2-fold) in vsdLDL5, involving three lysophospholipid classes, LPC, LPC(O), and LPI (all P < 0.001 vs. LDL1-4 and VLDL+IDL) (Fig. 2I, J, P). The degree of enrichment was moderately attenuated when expressed relative to PC but remained significant (Fig. 3H, I, O). By contrast, no enrichment was found in the particle content of LPE in LDL5, despite the elevated ratio of LPE/PE (1:4 across LDL1-5) relative to those for LPC, LPC(O), and LPI [1:50–60 for LPC:PC; 1:4,000–5,000 for LPC(O); and 1:120 for LPI in LDL5], indicating that the enrichment in LPC, LPC(O), and LPI is preferential in LDL5, LPE representing a positive control for these findings. Of LPC, LPC(O), and LPI, the former was most abundant both on a particle basis (LDL5, 8 pmol per picomole apoB) and in absolute plasma concentrations in LDL5 [50-fold greater levels than LPI or LPC(O)].
As polar lipids may exchange between lipoprotein particles, it is relevant to consider the stability of the associations of bioactive polar lipids between LDL subclasses during the density gradient isolation procedure. Thus, marked consistency in the relative contents of individual polar bioactive lipids in the same LDL subclass was observed when compared between different subjects in the MetS group. Furthermore, the molar ratios of each lipid class to either apoB or PC displayed discontinuities among LDL subclasses, clearly indicating that a uniform ratio representing an equilibrium state was not established across LDL subclasses in several instances (Figs. 2, 3). Indeed, the most remarkable example of a marked discontinuity in molar ratios, and thus disequilibrium in the distribution of lipid classes between LDL subclasses, after the completion of the isolation procedure was seen in the lysophospholipid profile [LPC, LPC(O), and LPI; typically P < 0.001] in LDL5 versus LDL1-4 (Fig. 2I, J, P). Furthermore, the enrichment of the LDL5 subclass in these three lysolipids was maintained under statin treatment, thereby further validating the isolation procedure. Further evidence for the specificity of the enrichment of LDL5 in LPC, LPC(O), and LPI can be drawn from the fact that a fourth lysophospholipid, LPE, was present at similar molar ratios to apoB (range 1.0–1.5 pmol per picomole of apoB) across all LDL subclasses (supplemental Fig. S2Q), with no statistically significant difference between them (exception of LDL1 vs. LDL4; P < 0.05). The absence of enrichment of LPE in LDL5 can thus be considered as a positive control for the isolation procedure. These findings argue strongly for the stability of the associations of polar bioactive lysophospholipids with apoB in respective LDL subclasses during the isolation procedure. The same arguments apply to the VLDL+IDL fraction, which showed consistent and markedly distinct contents of several nonpolar and polar lipid classes, i.e., DAG, TAG, and PE, as compared with the LDL1-5 subclasses.
LPC enrichment of vsdLDL5, impact of statin treatment, and relevance to atherogenesis and insulin resistance
Which mechanisms might underlie such preferential enrichment in LDL5? LPC has at least four major origins in plasma: i) from the action of endothelial lipase on lipoprotein-bound PCs; ii) from the action of LCAT on PCs in HDL, the latter cleaving PC to donate the sn-2 fatty acid to COH in the process of COH esterification catalyzed by LCAT; iii) from the cleavage of dense LDL-bound oxidized phospholipids by Lp-PLA2; indeed, LPC enrichment of LDL5 occurred concomitantly with its elevated Lp-PLA2 activity (up to 40-fold relative to LDL1-4; Fig. 4); and iv) from hepatic secretion of nascent VLDL precursor particles enriched in LPC, a quantitatively significant LPC source (68). As albumin is the major transport vehicle for LPC in plasma (69% of total LPC at D0, with 12% each in LDL and HDL in the present study), it is of immediate relevance that the vsdLDL5 subfraction was not contaminated with HDL-apoAI or albumin (22). In vivo kinetic studies in hypertriglyceridemic subjects document the formation of LDL5 as the end product of the intravascular remodeling of VLDL precursors (16–19); nonetheless, it cannot be excluded that part of the LDL5 pool might result from a specific pathway involving direct secretion of LPC-enriched vsdLDL particles by the liver, particularly given the association of a genetic variant of sortilin with hepatic lipoprotein production and elevated sdLDL levels (18, 69). Alternatively, could vsdLDL act as a preferred acceptor of LPCs among LDL subfractions? Neither the SM/apoB ratio nor the SM/PC ratio provides any indication of surface lipid packing or fluidity specific to LDL5, as these ratios are within the range of other LDL subfractions (Figs. 2E, 3E). Therefore, the possibility arises that the prolonged residence time of LDL5, which is almost 2-fold that of buoyant LDL and reflects its attenuated binding affinity for the LDL receptor (14, 17, 21), favors accumulation of LPCs in this subclass (19). Such accumulation may be amplified by Lp-PLA2-mediated hydrolysis of oxidized PCs under baseline conditions, a hypothesis consistent with moderate elevation in oxidized LDL levels and with high Lp-PLA2 cargo specific to LDL5 (Fig. 4). Furthermore, small dense LDLs in atherogenic dyslipidemia are themselves highly susceptible to oxidative modification under conditions of oxidative stress (21, 48).
The biological relevance of LPC enrichment in LDL5 to the pathophysiology of atherosclerosis is of considerable interest given the elevated propensity for arterial entry and retention of these particles (Fig. 5) (6, 17, 19, 21). Indeed, despite the major cargo of LPC carried in albumin, this plasma protein does not accumulate in the arterial intima, in contrast to LDL particles (70). LPC, a markedly hydrophilic lysophospholipid, may diffuse out from subintimally retained LDL particles into the aqueous phase to exert a spectrum of pro-atherogenic and pro-inflammatory effects on endothelial cells, monocytes, macrophages, and smooth muscle cells (Fig. 5) (32). Moreover, the action of autotaxin on LPC represents a major pathway for formation of lysophosphatidic acid, a potent pro-inflammatory cell signaling molecule with action on vascular cell function (54). Furthermore, LPC acts as an effector of fatty acid-induced insulin resistance (33).
The predominant LDL3 and dense LDL4 subclasses transported approximately 2-fold greater absolute amounts of LPC in plasma as compared with LDL5. Significantly, pitavastatin markedly reduced plasma levels of LPC across LDL1-5 (−35% to −52%; LDL4 and -5, respectively; 0.01 < P < 0.05), paralleling the concomitant decrease in the concentrations of the respective LDL subclasses (Fig. 1I, J, P; supplemental Table S2). As noted above, (pitava)statin treatment attenuated both inflammation and systemic oxidative stress as indicated by reduction in levels of oxLDL, Lp-PLA2, and serum amyloid A, and increment in PON activity; reduction of Lp-PLA2 is a statin class effect (71, 72). Nonetheless, the elevated molar ratio of LPC/apoB remained stable in LDL5 under statin treatment, despite a pronounced decrement in LDL5-associated Lp-PLA2 activity (−56%), thereby suggesting that Lp-PLA2 activity is not rate limiting to the metabolic pathway responsible for preferential LPC transport in this subclass. Such marked statin-mediated reduction in LDL5-associated Lp-PLA2 activity may however attenuate its direct pro-inflammatory role in human atherosclerotic plaque tissue by attenuating in situ LPC and thence LPA production (58).
Lyso-platelet-activating factor cargo of vsdLDL5 and effect of statin treatment
The second lysophospholipid enriched in LDL5, lyso-platelet-activating factor (PAF), i.e., LPC(O), the precursor of PAF, can potentially trigger both inflammatory and thrombotic cascades. Indeed monocyte-derived macrophages and foam cells, typical components of human atherosclerotic plaques, may acylate lyso-PAF to active PAF (60). It is therefore relevant that the low plasma concentrations of LPC(O) were uniformly reduced (30–49%; P < 0.01) across LDL1-5 following statin treatment, although the higher LPC(O)/apoB ratio in LDL5 (∼1 mol per six LDL5 particles) was maintained.
LPI cargo of vsdLDL5 and arterial inflammation
Like LPC, LPI is a bioactive lipid formed by the action of phospholipase A2 on oxidized PI and may activate several intracellular signaling pathways upon binding to the ubiquitous orphan GPR55 receptor, including those underlying inflammation, obesity, and diabetes (62). As for LPC, plasma LPI levels trended to reduction in all LDL subclasses on statin treatment (−25% to −42%), but in absolute terms, were 50-fold or more lower than those of LPC. Nonetheless, the elevated LPI/apoB molar ratio at baseline was maintained in vsdLDL5 on statin treatment at 0.15–0.17 pmol per picomole of apoB (i.e., ∼1 mol per six LDL particles). Taken together, the reductions in total (plasma) LPC and LPI cargo in LDL5 under statin treatment are consistent with substantial reduction in its putative pro-inflammatory and pro-atherogenic potential in the arterial intima. However, both of these lysophospholipids may be of pathophysiological relevance under baseline dyslipidemic conditions in MetS subjects when LDL5 plasma concentration and particle numbers are elevated (Fig. 5).
Cer load in LDL subclasses is refractory to statin action
Cers, and their immediate precursors, the dhCers, were minor lipids in the plasma cargos of LDL1-5 subclasses (<750 pmol per milliliter of plasma; ∼1–3 mol and ∼0.2–0.4 pmol per picomole of apoB per particle, respectively); the lowest molar ratios occurred in dense LDL4 and -5. The overall convex form of these subclass profiles became more pronounced on expression relative to total PC, amplifying the surface Cer content in LDL1-3 to four to six molecules per 1,000 PC molecules, and to three to four molecules in LDL4-5; such amplification of Cer contents arises from the progressively diminishing PC content from LDL1 to LDL5 relative to a single constant copy of apoB (Fig. 2F). Significant discontinuity occurred in both Cer and dhCer particle profiles at the interface of LDL3 with sdLDL4 and -5. Given that the surface lipid packing of SM and PC was similar in the surface monolayer of LDL1, -4, and -5 (as indicated by similar SM/PC molar ratio), then surface lipid fluidity would not appear to account for the lower content of Cers and dhCers in the lipid surface mosaic of dense LDL4 and -5. Rather, a metabolic determinant appears probable, such as a direct pathway involving greater conversion of SM to Cer in LDL1-3 than in LDL4-5 by SMase action, for example.
In contrast to the statin-mediated normalization of LDL-C, non-HDL-C, and apoB levels (range −29% to −55% from baseline) and of LDL1-5 subfraction mass (−28% to −36%) in our MetS subjects, absolute plasma concentrations of both Cers and dhCers transported in LDL1-5 were unaltered by statin treatment. Indeed, a significant increase in molar particle ratios to both apoB and to PC (Cers, +17% to +27% in Cer/PC ratios; dhCers, up to +40% in dhCer/PC ratio) was observed across LDL subclasses. Clearly then, the content of these sphingolipids is preferentially conserved and even elevated as LDL particle numbers diminish and as the molar content of other lipid classes relative to apoB diminishes in parallel with the fall in size and molecular weight (13, 17, 28, 63). An increment in the Cer/apoB ratio was equally observed in the VLDL+IDL subfraction (+21%) at D180, indicating that statin-mediated hepatic enrichment of precursor VLDL in Cers may lead to increments in Cer/PC ratios in downstream LDL1-5 subclasses.
May additional mechanisms contribute to both the maintenance of plasma levels of Cer and dhCer in LDL subclasses, and indeed to a trend to elevation in molar particle contents of Cer and its precursor, dhCer, across LDL subclasses on statin therapy? Does statin action impact the principle pathways of Cer production by desaturase action on dhCer on the one hand, and by SMase action on SM? Statins inhibit Rab prenylation by reduction of isoprenoid production from mevalonate, the immediate product of their target enzyme; upregulation of the synthetic pathway to cellular glucosylceramides results, potentially involving enhanced production of Cer (73). Moreover, acid SMase activity in liver cells is dose-dependently regulated by mevalonate levels; lower mevalonate levels induced by statin action are associated with enhanced SMase activity (74), leading to enhanced Cer formation (74, 75). Clearly, several candidate mechanisms may contribute to our qualitative and quantitative findings on Cer and dhCer in LDL subclasses following prolonged statin treatment.
Implications of LDL Cers in glucose homeostasis, inflammation, and atherosclerosis
Cers are currently viewed not only as lipotoxic inducers of disturbed glucose homeostasis and insulin resistance in prediabetic and diabetic states but also equally as key actors in the pathophysiology of atherosclerosis (9, 29). Elevated levels of apoB-containing lipoproteins, and particularly sdLDL, have been implicated in the pathophysiology of such lipotoxicity (18). Indeed, LDL-associated Cers can induce insulin resistance both in vivo and in vitro, while depletion of Cers from LDL rendered them ineffective in antagonizing insulin action (10). Such findings imply that apoB-containing lipoproteins can donate Cers and related sphingolipids to insulin-sensitive cells, potentially via the LDL receptor pathway and/or by LDL receptor-independent mechanisms, with subsequent inhibition of intracellular insulin signaling. The LDL Cer cargo may equally induce a pro-inflammatory response in macrophages via toll-like receptor-dependent and -independent pathways (10).
Is the present finding of the maintenance of Cer and dhCer contents in the LDL subclasses of dyslipidemic MetS subjects upon statin treatment therefore relevant or not relevant to risk of conversion from a prediabetic state to type 2 diabetes? Meikle et al. (50) originally observed that levels of several plasma lipid classes in addition to Cers and dhCers were positively associated with prediabetes and diabetes, including DAG, TAG, CE, PE, PG, and PI. As noted above, all of these lipid classes, with the marked exception of Cers and dhCers, were markedly reduced on (pitava) statin therapy, suggesting that the net balance of these effects on glucose homeostasis is neutral as reported earlier (42–44).
Elevated plasma Cer levels are independently associated with major adverse cardiovascular events (9, 29). Furthermore, strong evidence for a proatherogenic role of Cers has been provided from animal models of atherosclerosis; such effects involve several mechanisms, including promotion of lipoprotein transport into the arterial wall, platelet activation, and endothelial dysfunction via uncoupling of NO signaling pathways (29, 31). Indeed, Cers can themselves act as signaling molecules, eliciting target cell responses that include differentiation, proliferation, apoptosis, and inflammation involving production of reactive oxygen species and cytokine gene expression (75). The maintenance of Cer cargo in LDL subclasses following statin therapy suggests, therefore, that these lipids may contribute to the residual cardiovascular risk associated with this therapeutic class (2–4). Moreover, preliminary data suggest that maintenance of Cer cargo in LDL is common to several HMG-CoA inhibitors (P. J. Meikle and M. J. Chapman, unpublished observations), suggesting it to be a class effect.
Limitations
Limitations in the CAPITAIN study concern the small cohort size (n = 12) and restriction of recruitment to males; these were compensated partially by selection of male subjects exhibiting a phenotypically consistent mixed dyslipidemia, which included parameters of glucose homeostasis together with multiple rigorous inclusion and exclusion criteria (see supplemental data). Volunteers displaying dyslipidemic MetS acted as their own controls in this essentially mechanistic investigation. This approach in part limited confounding effects due to differences in statin response emanating from genotypic background (76), and from potential differences in baseline phenotype which would have arisen relative to a placebo group. Moreover, key assumptions relating to the additivity of drug-placebo effects in randomized clinical trials have recently been questioned (77). We do however recognize that regression to the mean in human studies may lead to overestimation of treatment effects in the absence of a placebo control group. Further to these considerations, the high precision and reproducibility of the lipidomic analysis provided sufficient power to clearly identify lipid species and classes that were significantly altered in response to statin therapy, even with correction for multiple comparisons (37, 40, 50). In addition, conditions of blood collection, plasma separation, and storage were rigorously controlled in a Clinical Unit, on the one hand, in order to minimize any potential for oxidative lipoprotein modification and, on the other hand, to minimize potential variation in lipid and lipoprotein parameters in relation to diet, BMI, or physical activity. A statin comparator was lacking in our clinical protocol; importantly, comparable data to those presented here on interrelationships between the lipidomes of LDL subclasses in MetS at baseline and on pitavastatin calcium treatment are not yet available for another member of this therapeutic class. Our original findings now require validation in a large prospective study over a longer period in an independent cohort of mixed dyslipidemic MetS subjects to include women, preferably with a statin comparator.
CONCLUSIONS
For the first time, novel quantitative and qualitative insights into the complex lipid cargo of LDL subclasses and of their VLDL and IDL precursors in obese prediabetic dyslipidemic MetS subjects are presented, going well beyond LDL-C. Moreover, statin therapy has been used as a probe of the metabolically and structurally imposed constraints on the lipidomes of apoB-containing lipoprotein particles [to the exclusion of Lp(a)]. The marked and preferential elevation in particle content (as molar ratio to apoB or PC) of three lysophospholipids, LPC, LPC(O), and LPI, in vsdLDL5 relative to LDL1-4, was established. Such particle enrichment was refractory to statin action, highlighting not only LDL subclass-specific lipid remodeling but also potential amplification of the pro-atherogenic actions of LDL5 of long plasma residence at the arterial wall by bioactive pro-inflammatory lysophospholipids (Fig. 5). Despite the maintenance of the (molar) particle content of such lysophospholipids on statin treatment, it is critical to point out that their absolute plasma concentrations, as cargo lipids in LDL, were markedly diminished post statin. Such findings are entirely consistent with the observed action of pitavastatin calcium in reducing cardiovascular events in the REAL-CAD trial (78).
The marked exception to the efficacious statin-mediated reduction in the majority of the 23 lipid classes was represented by Cer species and dhCers. On the one hand, statin therapy did not significantly lower circulating concentrations of these two sphingolipids either across LDL subclasses or in the VLDL+IDL fraction, and, on the other hand, statin treatment induced elevation (range 10–40% relative to baseline) in both Cer and dhCer particle cargos (as molar ratios) in all LDL subclasses. The metabolic behavior of these bioactive sphingolipids is thus distinct, warranting further research both on their transport by and metabolism in both VLDL and IDL particles and in downstream LDL subclasses, and equally on their potential role in the insulin resistance and high cardiovascular risk typical of MetS.
Finally, these original findings highlight the potential therapeutic targeting of deleterious bioactive lipids in LDL particles in atherogenic dyslipidemia, as exemplified by Cers, with a view to attenuation of their putative pathogenic roles in cardiometabolic disease. Such targeting of Cers may equally allow attenuation of residual cardiometabolic risk beyond LDL-C lowering.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Mr. M. Suzuki for critical logistical support in clinical studies, Dr. P. Betting and colleagues at the Clinical Unit of SGS Aster, Paris, for patient recruitment and follow-up, and Dr. A. Carrie for apoE genotyping. The authors are indebted to Dr. N. Hounslow for critical discussion of the clinical protocol. A.O. is indebted to Kowa Research Europe, the Nouvelle Société Francaise d’Athérosclérose (NSFA), and the Association for Research on Lipoproteins and Atherogenesis (ARLA) for support.
Footnotes
Abbreviations:
- ASCVD
- atherosclerotic CVD
- CE
- cholesteryl ester
- Cer
- ceramide
- CETP
- cholesteryl ester transfer protein
- COH
- free cholesterol
- DHC
- dihexosylceramide
- dhCer
- dihydroceramide
- GM3
- monosialodihexosylganglioside
- IL
- interleukin
- Lp(a)
- lipoprotein (a)
- LPC(O)
- lysoalkylphosphatidylcholine
- LPE
- lysophosphatidylethanolamine
- LPI
- lysophosphatidylinositol
- Lp-PLA2
- lipoprotein-associated phospholipase A2
- MetS
- metabolic syndrome
- MHC
- monohexosylceramide
- PAF
- platelet-activating factor
- PC(O)
- alkylphosphatidylcholine
- PC(P)
- alkenylphosphatidylcholine
- PE(O)
- alkylphosphatidylethanolamine
- PE(P)
- alkenylphosphatidylethanolamine
- sdLDL
- small dense LDL
- TAG
- triacylglycerol
- THC
- trihexosylceramide
- vsdLDL
- very small dense LDL
All aspects of the CAPITAIN study (ClinicalTrials.gov: NCT01595828) were supported by a clinical research grant from Kowa Research Europe. Additional support was provided by Kowa Pharmaceuticals America Inc., Inserm, NSFA, and ARLA. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned funding bodies. The authors take full responsibility for the content of this manuscript. No funders or sponsors were involved in the final design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. M.J.C. has received research funding and/or modest honoraria for consultancy from Amarin, Amgen, AstraZeneca, Kowa, MSD, Sanofi, Regeneron, and Pfizer. All other authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Goldstein J. L., and Brown M. S.. 2015. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 161: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference B. A., Ginsberg H. N., Graham I., Ray K. K., Packard C. J., Bruckert E., Hegele R. A., Krauss R. M., Raal F. J., Schunkert H., et al. 2017. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38: 2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy S. M., Stone A. J., Bailey A. L., Beam C., Birtcher K. K., Blumenthal R. S., Braun L. T., de Ferranti S., Faiella-Tommasino J., Forman D. E., et al. 2019. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 73: 3168–3209. [DOI] [PubMed] [Google Scholar]
- 4.Mach F., Baigent C., Catapano A. L., Koskinas K. C., Casula M., Badimon L., Chapman M. J., De Backer G. G., Delgado V., Ference B. A., et al. ; ESC Scientific Document Group. 2020. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41: 111–188. 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 5.Langlois M. R., Chapman M. J., Cobbaert C., Mora S., Remaley A. T., Ros E., Watts G. F., Boren J., Baum H., Bruckert E., et al. ; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative. 2018. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A consensus statement from EAS and EFLM. Clin. Chem. 64: 1006–1033. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U., and Weingartner O.. 2018. Pathological phenotypes of LDL particles. Eur. Heart J. 39: 2574–2576. [DOI] [PubMed] [Google Scholar]
- 7.Ng T. W., Ooi E. M., Watts G. F., Chan D. C., Weir J. M., Meikle P. J., and Barrett P. H.. 2014. Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J. Clin. Endocrinol. Metab. 99: E2335–E2340. [DOI] [PubMed] [Google Scholar]
- 8.Hilvo M., Simolin H., Metso J., Ruuth M., Oorni K., Jauhiainen M., Laaksonen R., and Baruch A.. 2018. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis. 269: 159–165. [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen R., Ekroos K., Sysi-Aho M., Hilvo M., Vihervaara T., Kauhanen D., Suoniemi M., Hurme R., Marz W., Scharnagl H., et al. 2016. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37: 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon J., Hoy A. J., Stark R., Brown R. D., Meex R. C., Henstridge D. C., Schenk S., Meikle P. J., Horowitz J. F., Kingwell B. A., et al. 2013. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 62: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grammer T. B., Kleber M. E., März W., Silbernagel G., Wieland H., Pilz S., Tomaschitz A., Koenig W., and Scharnagl H.. 2015. Low-density lipoprotein particle diameter and mortality: the Ludwigshafen Risk and Cardiovascular Health Study. Eur. Heart J. 36: 31–38. [DOI] [PubMed] [Google Scholar]
- 12.Ruuth M., Nguyen S. D., Vihervaara T., Hilvo M., Laajala T. D., Kondadi P. K., Gistera A., Lahteenmaki H., Kittila T., Huusko J., et al. 2018. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 39: 2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman M. J., Laplaud P. M., Luc G., Forgez P., Bruckert E., Goulinet S., and Lagrange D.. 1988. Further resolution of the low density lipoprotein spectrum in normal human plasma: physicochemical characteristics of discrete subspecies separated by density gradient ultracentrifugation. J. Lipid Res. 29: 442–458. [PubMed] [Google Scholar]
- 14.Nigon F., Lesnik P., Rouis M., and Chapman M. J.. 1991. Discrete subspecies of human low-density lipoproteins are heterogeneous in their interaction with the cellular LDL receptor. J. Lipid Res. 32: 1741–1753. [PubMed] [Google Scholar]
- 15.Lund-Katz S., Laplaud P. M., Phillips M. C., and Chapman M. J.. 1998. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 37: 12867–12874. [DOI] [PubMed] [Google Scholar]
- 16.Packard C. J., Demant T., Stewart J. P., Bedford D., Caslake M. J., Schwertfeger G., Bedynek A., Shepherd J., and Seidel D.. 2000. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J. Lipid Res. 41: 305–318. [PubMed] [Google Scholar]
- 17.Berneis K. K., and Krauss R. M.. 2002. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 43: 1363–1379. [DOI] [PubMed] [Google Scholar]
- 18.Krauss R. M. 2004. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 27: 1496–1504. [DOI] [PubMed] [Google Scholar]
- 19.Diffenderfer M. R., and Schaefer E. J.. 2014. The composition and metabolism of large and small LDL. Curr. Opin. Lipidol. 25: 221–226. [DOI] [PubMed] [Google Scholar]
- 20.Chapman M. J., Le Goff W., Guerin M., and Kontush A.. 2010. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 31: 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borén J., Chapman M. J., Krauss R. M., Packard C. J., Bentzon J. F., Binder C. J., Daemen M. J., Demer L. L., Hegele R. A., Nichols S. J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. Epub ahead of print. February 13, 2020; doi:10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman M. J., Orsoni A., Robillard P., Therond P., and Giral P.. 2018. Duality of statin action on lipoprotein subpopulations in the mixed dyslipidemia of metabolic syndrome: quantity vs quality over time and implication of CETP. J. Clin. Lipidol. 12: 784–800.e4. [DOI] [PubMed] [Google Scholar]
- 23.Tselepis A. D., Dentan C., Karabina S. A., Chapman M. J., and Ninio E.. 1995. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VLDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler. Thromb. Vasc. Biol. 15: 1764–1773. [DOI] [PubMed] [Google Scholar]
- 24.Rosenson R. S., and Stafforini D. M.. 2012. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 53: 1767–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai M., Otokozawa S., Asztalos B. F., Ito Y., Nakajima K., White C. C., Cupples L. A., Wilson P. W., and Schaefer E. J.. 2010. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin. Chem. 56: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiffman D., Louie J. Z., Caulfield M. P., Nilsson P. M., Devlin J. J., and Melander O.. 2017. LDL subfractions are associated with incident cardiovascular disease in the Malmo Prevention Project Study. Atherosclerosis. 263: 287–292. [DOI] [PubMed] [Google Scholar]
- 27.Williams P. T., Zhao X. Q., Marcovina S. M., Otvos J. D., Brown R. G., and Krauss R. M.. 2014. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 233: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ståhlman M., Pham H. T., Adiels M., Mitchell T. W., Blanksby S. J., Fagerberg B., Ekroos K., and Borén J.. 2012. Clinical dyslipidaemia is associated with changes in the lipid composition and inflammatory properties of apolipoprotein-B-containing lipoproteins from women with type 2 diabetes. Diabetologia. 55: 1156–1166. [DOI] [PubMed] [Google Scholar]
- 29.Chaurasia B., and Summers S. A.. 2015. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26: 538–550. [DOI] [PubMed] [Google Scholar]
- 30.Wallner S., and Schmitz G.. 2011. Plasmalogens: the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids. 164: 573–589. [DOI] [PubMed] [Google Scholar]
- 31.Meikle P. J., and Summers S. A.. 2017. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 13: 79–91. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz G., and Ruebsaaman K.. 2010. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 208: 10–18. [DOI] [PubMed] [Google Scholar]
- 33.Han M. S., Lim Y. M., Quan W., Kim J. R., Chung K. W., Kang M., Kim S., Park S. Y., Han J. S., Park S. Y., et al. 2011. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J. Lipid Res. 52: 1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caslake M. J., Stewart G., Day S. P., Daly E., McTaggart F., Chapman M. J., Durrington P., Laggner P., Mackness M., Pears J., et al. 2003. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia. Atherosclerosis. 171: 245–253. [DOI] [PubMed] [Google Scholar]
- 35.Guerin M., Lassel T. S., Le Goff W., Farnier M., and Chapman M. J.. 2000. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler. Thromb. Vasc. Biol. 20: 189–197. [DOI] [PubMed] [Google Scholar]
- 36.Tarasov K., Ekroos K., Suoniemi M., Kauhanen D., Sylvanne T., Hurme R., Gouni-Berthold I., Berthold H. K., Kleber M. E., Laaksonen R., et al. 2014. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99: E45–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meikle P. J., Wong G., Tan R., Giral P., Robillard P., Orsoni A., Hounslow N., Magliano D. J., Shaw J. E., Curran J. E., et al. 2015. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: potential relevance to statin-associated dysglycemia. J. Lipid Res. 56: 2381–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oesterle A., Laufs U., and Liao J. K.. 2017. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paumelle R., and Staels B.. 2007. Peroxisome proliferator-activated receptors mediate pleiotropic actions of statins. Circ. Res. 100: 1394–1395. [DOI] [PubMed] [Google Scholar]
- 40.Orsoni A., Therond P., Tan R., Giral P., Robillard P., Kontush A., Meikle P. J., and Chapman M. J.. 2016. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J. Lipid Res. 57: 2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman M. J., Orsoni A., Robillard P., Hounslow N., Sponseller C. A., and Giral P.. 2014. Effect of high-dose pitavastatin on glucose homeostasis in patients at elevated risk of new-onset diabetes: insights from the CAPITAIN and PREVAIL-US studies. Curr. Med. Res. Opin. 30: 775–784. [DOI] [PubMed] [Google Scholar]
- 42.Vallejo-Vaz A. J., Seshasai S. R. K., Kurogi K., Michishita I., Nozue T., Sugiyama S., Tsimikas S., Yoshida H., and Ray K. K.. 2015. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 241: 409–418. [DOI] [PubMed] [Google Scholar]
- 43.Braun L. R., Feldpausch M. N., Czerwonka N., Weiss J., Branch K., Hang Lee R. N., Martinez-Salazar E. L., Torriani M., Sponseller C. A., Grinspoon S. K., et al. 2018. Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 103: 4176–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Medicines Authority. Livazo Consolidated SmPC. Summary of Product Characteristics. Accessed September 5, 2017, at http://www.kowapharmaceuticals.eu/assets/dl/Livazo-SmPC-28-11-17.pdf.
- 45.Alberti K. G. M. M., Zimmet P., and Shaw J.. 2006. Metabolic syndrome-a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 23: 469–480. [DOI] [PubMed] [Google Scholar]
- 46.Chapman M. J., Goldstein S., Lagrange D., and Laplaud P. M.. 1981. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 22: 339–358. [PubMed] [Google Scholar]
- 47.Zerrad-Saadi, A., P. Therond, S. Chantepie, M. Coururier, K. A. Rye, M. J. Chapman, and A. Kontush. 2009. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 29: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 48.Chancharme L., Therond P., Nigon F., Lepage S., Couturier M., and Chapman M. J.. 1999. Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler. Thromb. Vasc. Biol. 19: 810–820. [DOI] [PubMed] [Google Scholar]
- 49.Goulinet S., and Chapman M. J.. 1997. Plasma LDL and HDL subspecies are heterogeneous in particle contents of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 17: 786–796. [DOI] [PubMed] [Google Scholar]
- 50.Meikle P. J., Wong G., Barlow C. K., Weir J. M., Greeve M. A., Macintosh G. L., Almasy L., Comuzzie A. G., Mahaney A. C., Kowalczyk A., et al. 2013. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One. 8: e74341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang L., Chambliss K. L., Gao X., Yuhanna I. S., Behling-Kelly E., Bergaya S., Ahmed M., Michaely P., Luby-Phelps K., Darehshouri A., et al. 2019. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature. 569: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurt-Camejo E., Camejo G., Rosengren B., Lopez F., Wiklund O., and Bondjers G.. 1990. Differential uptake of proteoglycan-selected subfractions of low-density lipoprotein by human macrophages. J. Lipid Res. 31: 1387–1398. [PubMed] [Google Scholar]
- 53.Hansel B., Giral P., Nobecourt E., Chantepie S., Bruckert E., Chapman M. J., and Kontush A.. 2004. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89: 4963–4971. [DOI] [PubMed] [Google Scholar]
- 54.Yatomi Y., Kurano M., Ikeda H., Igarashi K., Kano K., and Aoki J.. 2018. Lysophospholipids in laboratory medicine. Proc. Jpn. Ser. B Phys. Biol. Sci. 94: 373–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang M. Y., Tsoi C., Wight T. N., and Chait A.. 2003. Lysophosphatidylcholine regulates synthesis of biglycan and the proteoglycan form of macrophage colony stimulating factor. Arterioscler. Thromb. Vasc. Biol. 23: 809–815. [DOI] [PubMed] [Google Scholar]
- 56.Vickers K. C., Castro-Chavez F., and Morrisett J. D.. 2010. Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells. Atherosclerosis. 211: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu-Wu Y., Hurt-Camejo E., and Wiklund O.. 1998. Lysophosphatidylcholine induces the production of IL-1beta by human monocytes. Atherosclerosis. 137: 351–357. [DOI] [PubMed] [Google Scholar]
- 58.Gonçalves I., Edsfeldt A., Ko N. Y., Grufman H., Berg K., Björkbacka H., Nitulescu M., Persson A., Nilsson M., Prehn C., et al. 2012. Evidence supporting a key role of Lp-PLA2-generated lyso-phosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 32: 1505–1512. [DOI] [PubMed] [Google Scholar]
- 59.Engelmann B., Zieseniss S., Brand K., Page S., Lentschat A., Ulmer A. J., and Gerlach E.. 1999. Tissue factor expression of human monocytes is suppressed by lysophosphatidylcholine. Arterioscler. Thromb. Vasc. Biol. 19: 47–53. [DOI] [PubMed] [Google Scholar]
- 60.Dentan C., Lesnik P., Chapman M. J., and Ninio E.. 1996. Phagocytic activation induces formation of platelet-activating factor in human monocyte-derived macrophages and in macrophage-foam cells. Relevance to the inflammatory reaction in atherogenesis. Eur. J. Biochem. 236: 48–55. [DOI] [PubMed] [Google Scholar]
- 61.Balla T. 2013. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93: 1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piñeiro R., and Falasca M.. 2012. Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim. Biophys. Acta. 1821: 694–705. [DOI] [PubMed] [Google Scholar]
- 63.Luc G., Gennes J. L., and Chapman M. J.. 1988. Further resolution and comparison of the heterogeneity of plasma low-density lipoproteins in human hyperlipoproteinemias: type III hyperlipoproteinemia, hypertriglyceridemia and familial hypercholesterolemia. Atherosclerosis. 71: 143–156. [DOI] [PubMed] [Google Scholar]
- 64.Segrest J. P., Jones M. K., De Loof H., and Dashti N.. 2001. Structure of apolipoprotein B-100 in low-density lipoproteins. J. Lipid Res. 42: 1346–1367. [PubMed] [Google Scholar]
- 65.McNamara J. R., Small D. M., Li Z., and Schaefer E. J.. 1996. Differences in LDL subspecies involve alterations in lipid composition and conformational changes in apolipoprotein B. J. Lipid Res. 37: 1924–1935. [PubMed] [Google Scholar]
- 66.Hevonoja T., Pentikäinen M. O., Hyvönen M. T., Kovanen P. T., and Ala-Korpela M.. 2000. Structure of low-density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochim. Biophys. Acta. 1488: 189–210. [DOI] [PubMed] [Google Scholar]
- 67.Ibdah J. A., Lund-Katz S., and Phillips M. C.. 1989. Molecular packing of high-density and low-density lipoprotein surface lipids and apolipoprotein A-I binding. Biochemistry. 28: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 68.Sekas G., Patton G. M., Lincoln E. C., and Robins S. J.. 1985. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J. Lab. Clin. Med. 105: 190–194. [PubMed] [Google Scholar]
- 69.Strong A., Ding Q., Edmondson A. C., Millar J. S., Sachs K. V., Li X., Kumaravel A., Wang M. Y., Ai D., Guo L., et al. 2012. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J. Clin. Invest. 122: 2807–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curmi P. A., Juan L., and Tedgui A.. 1990. Effect of transmural pressure on low density lipoprotein and albumin transport and distribution across the intact arterial wall. Circ. Res. 66: 1692–1702. [DOI] [PubMed] [Google Scholar]
- 71.Tsimihodimos V., Karabina S. A., Tambaki A. P., Bairaktari E., Goudevenos J. A., Chapman M. J., Elisaf M., and Tselepis A. D.. 2002. Atorvastatin preferentially reduces LDL-associated platelet-activating factor acetylhydrolase activity in dyslipidemias of type IIA and type IIB. Arterioscler. Thromb. Vasc. Biol. 22: 306–311. [DOI] [PubMed] [Google Scholar]
- 72.Albert M. A., Glynn R. J., Wolfert R. L., and Ridker P. M.. 2005. The effect of statin therapy on lipoprotein associated phospholipase A2 levels. Atherosclerosis. 182: 193–198. [DOI] [PubMed] [Google Scholar]
- 73.Binnington B., Nguyen L., Kamani M., Hossain D., Marks D. L., Budani M., and Lingwood C. A.. 2016. Inhibition of Rab prenylation by statins induces cellular glycosphingolipid remodelling. Glycobiology. 26: 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y., Xu S-C., and Duan R-D.. 2015. Mevalonate inhibits acid sphingomyelinase activity, increases sphingomyelin levels and inhibits cell proliferation of HepG2 and Caco-2 cells. Lipids Health Dis. 14: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bismuth J., Lin P., Yao Q., and Chen C.. 2008. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 196: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeGorter M. K., Tirona R. G., Schwarz U. I., Choi Y. H., Dresser G. K., Suskin N., Myers K., Zou G., Iwuchukwu O., Wei W. Q., et al. 2013. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ. Cardiovasc. Genet. 6: 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall K. T., and Loscalzo J.. 2019. Drug-placebo additivity in randomised clinical trials. Clin. Pharmacol. Ther. 106: 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taguchi I., Iimuro S., Iwata H., Takashima H., Abe M., Amiya E., Ogawa T., Ozaki Y., Sakuma I., Nakagawa Y., et al. 2018. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation. 137: 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.