Abstract
Psychological stress is a well-accepted risk factor in cancer initiation and progression. The explosive growth of psychoneuroimmunology research in the past decade has yielded an unprecedented wealth of information about the critical role of chronic stress in the immune dysfunction that influences tumor behaviors, which presents insights to mitigate distress and improve prognosis in cancer patients. Chronic stress exacerbates inflammation and causes a metabolism disorder, making it difficult for the organisms to maintain homeostasis and increasing its susceptibility to cancer. The shifted differentiation and redistribution of the immune system induced by chronic stress fail to combat cancer efficiently. Chronic stress increases the tumor-educated immune suppressive cells and impairs the cytotoxicity of cellular immunity, thereby promoting lymphatic metastasis and hematogenous metastasis. In addition, the efficacy of existing cancer therapies is undermined because chronic stress prevents the immune system from responding properly. Emerging stress-reduction measures have been administered to assist cancer patients to cope with the adverse effects of chronic stress. Here we systematically review the current molecular, cellular, physiological mechanisms about stress-mediated immune responses in the enhancement of tumor initiation and progression, remodeling of tumor microenvironment and impairment of anti-tumor treatment. We also summarize the potential clinically applicable stress-oriented strategies towards cancer and discuss briefly where important knowledge gaps remain.
Keywords: Tumor immunology, psychological distress, tumor microenvironment, biobehavioral sciences, anti-cancer therapy
Introduction
There is a growing awareness that psychological stress is common in many physical diseases and is increasingly recognized as a risk factor in cancer initiation and progression [1]. Stress can be as acute as several minutes, or as long as hours to days. The concept of stress being discussed here is defined as chronic stress associated with alternations in the hypothalamic-pituitary-adrenal (HPA) axis, activation of sympathetic nervous system (SNS) and other physiological adaptations like hormones secretion [2].
Using meta-analytic methods, Yoichi Chida and his colleges reported that stress-related psychosocial factors were related to higher cancer incidence in healthy populations based on the results of 165 studies [3]. In addition, it seems that the diagnosis of cancer and following long-term treatment put additional stress on patients [4]. In addition, stress and its induced physiological changes can in turn modulate immune function. Sympathetic nerve fibers innervate peripheral immune lymphoid tissues including thymus, spleen, lymph nodes, and bone marrow, which provides a direct pathway to regulate peripheral immune processes [5]. In addition, the immune responses can be modulated in all aspects [6,7]. These preliminary observations suggest the probable implication of chronic stress in inducing an immune dysregulation and influencing cancer behaviors. A series of studies reported that cold stress changed the immune composition of primary tumor and spleen, which contributed to tumor development. Intriguingly, the gross immune populations of naïve mice exposed to the same stressor were comparable [8,9]. This suggests the stress-immune-cancer axis has its own unique mechanism and needs further inspection. Here we provide an overview of insights from human and rodent studies highlighting the critical role of psychological stress-mediated immune dysfunction in cancer initiation, progression, metastasis, and treatment. We also focus on the clinically applicable strategies against chronic stress so as to resume the immune system and fight against tumors based on molecular, cellular, and physiological mechanisms.
Chronic stress acts on the immune system to promote tumorigenesis
The notion that a stress-mediated effect on the immune system can increase host susceptibility to cancer has been reported in many studies. The link among stress, immune system, and tumorigenesis is perceived to some extent, yet to what extent does stress-mediated immune dysregulation influence tumorigenesis is still obscure.
DNA damage
DNA damage and genomic instability are preconditions for carcinogenesis. An efficient and sufficient DNA repair system protects the organism from cancer risk.
Chronic stress causes dysregulation of the immune system, leading to a pro-inflammatory state both in the central nervous system (CNS) and periphery [10]. The excessive pro-inflammatory cytokines promote tumorigenesis by inducing DNA damage or inhibiting DNA repair through the generation of reactive oxygen species (ROS) [11] or the impairment of the DNA repair enzyme [12].
Virus-related tumors
Virus-related tumors are more affected by chronic stress. The direct association among stress, weakened cell cytotoxicity, and human papillomaviruses (HPVs)-associated tumors has been reported [13]. In a similar way, increased expression of latent Epstein-Barr virus (EBV) and a reduced cytotoxic T lymphocyte (CTL)-mediated killing have been seen in stressful events [14]. It is reasonable to believe that chronic stress blunts cellular immunity and promotes the initiation of virus-related tumors.
Intestinal barrier dysfunction and colitis-associated tumorigenesis
Chronic stress has been reported to induce barrier dysfunction in ileum and colon and initiate mucosal Inflammation, during which macromolecular permeability increases and mucus is depleted [15]. The mucosa of the stressed rats is seen with activated mast cells, increased infiltrate of neutrophils, ly6Chi macrophages, and mononuclear cells, the reinforced activity of myeloperoxidase (MPO), and more inflammation-promoting operational taxonomic units [16]. Corticotropin-releasing factor receptor subtype 1 (CRF1) signaling is a positive regulator of psychological stress-induced mast cell degranulation [17] and tumorigenesis [18]. The much less tumorigenesis in Crf1 (-/-) mice is accompanied by a lower inflammatory response, including decreased interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) expression and macrophage infiltration and increased interleukin-10 (IL-10) expression [18]. Therefore, it is plausible that chronic stress can enhance CRF1 signaling in mast cells and promote colitis-associated tumorigenesis (Figure 1).
Figure 1.
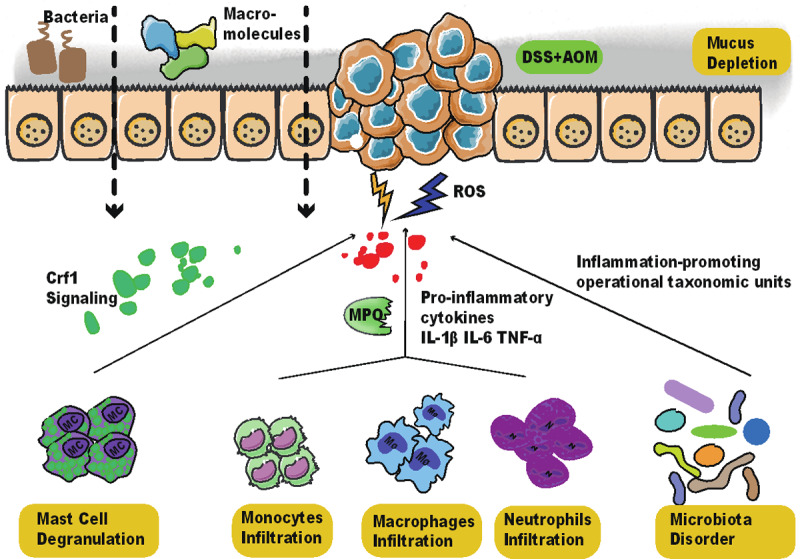
Chronic stress aggravates inflammation-mediated intestinal barrier dysfunction and promotes colitis-associated tumorigenesis. In DSS and AOM treated murine colitis model, chronic stress increases mast cell degranulation by activating CRF1 signaling. It also increases the infiltration of monocytes, neutrophils, and macrophages, which produces pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α and increases the activity of MPO. In the meantime, microbiota disorder is observed with an increase in inflammation-promoting operational taxonomic units. The inflammatory environment is observed with more ROS, leading to the increased permeability of bacteria and macromolecules and depletion of mucus. The intensified colitis in chronic stress is related to the higher incidence of colitis-associated tumorigenesis. Abbreviations: DSS, dextran sodium sulfate; AOM, azoxymethane; MPO, myeloperoxidase; CRF1, Corticotropin-releasing factor receptor subtype 1 (CRF1); IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; TNF-α, tumor necrosis factor (TNF)-α; ROS, reactive oxygen species.
Immune dysregulation in stress-induced tumor tumorigenesis
Chronic stress also exerts a negative influence on immune regulation. It suppresses the secretion of type I cytokines and decreases the infiltration of CD4+ T cells. In the meantime, stressed mice show increased infiltration of suppressive CD25+ cells both in tumors and circulation [19]. These stressed-induced modifications come together with a shorter median time of the first appearance of ultraviolet B (UVB)-induced squamous cell carcinoma (SCC) [19] and increased tumorigenesis after being exposed to the carcinogen methylcholanthrene [8]. The significance of the immune system is manifested in the observation that the tumor growth rates are similar in immune-compromised mice with or without stress exposure.
Chronic stress acts on the immune system to promote cancer progression
When irradiated animals are transferred with lymphoid cells from stressed animals, accelerated tumor growth is observed compared with those transferred with lymphoid cells from control ones [20]. This finding suggests stress-induced tumor growth can be attributed to the changes in immune cells.
Immature myelopoiesis and impairment of innate immunity
Chronic stress has been reported to induce a shifted differentiation of hematopoietic stem cells towards myeloid cell lineage [7]. In addition, it also induces higher expressions of C-X-C motif chemokine receptor 2 (CXCR2) on these expanded myeloid cells via activating β-adrenergic signaling. The increased mobilization of myeloid cells to tumor tissues promotes the progression of hepatocellular carcinoma [21].
The activation of myelopoietic development of precursor monocytes by chronic stress also increases the density of tumor-associated macrophages (TAM) in the tumor microenvironment (TME). Chronic stress can significantly increase C-C motif chemokine ligand 2 (CCL2) concentration by catecholamine exposure, and then enhance the infiltration of CD68+ and F4/80+ macrophages in ovarian carcinoma models and promote cancer growth [22]. In addition, the rising stress hormones polarize macrophages to the M2 phenotype [23]. The M2-like TAMs secrete high levels of IL-10, angiogenetic factors such as vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), and matrix metalloproteinase-9 (MMP)-9, which facilitates tumor escape [24]. It is plausible that chronic stress increases the density of TAMs, especially M2-like TAMs, to enhance tumor progression.
Chronic stress not only increases the number of splenic and circulating myeloid-derived suppressor cells (MDSCs) in tumor-bearing mice but also enhances the production of IL-10 and NO2 by splenic MDSCs, which leads to the immune suppression and enlarged tumors volume [25,26]. The mechanism may due to adrenergic activation and neuropeptide Y secretion [26].
Another group of myeloid cells that is indispensable in anti-tumor response is natural killer (NK) cells. NK cell cytotoxicity is significantly impaired in ovarian cancer patients with higher stress levels, especially in tumor-infiltrating lymphocytes (TILs) [27]. The impaired NK lysis in breast cancer patients with severe psychological stress is associated with altered surface expression of killer immunoglobulin-like receptors CD158b [28]. The association among chronic stress, abnormal cortisol rhythms and lower NK cell cytotoxic activity has been well discussed in a well-written review [29].
Stress-induced hormones like corticosteroids prevent dendritic cells (DCs) from maturation in response to inflammatory stimuli. Dexamethasone (DEX) can down-regulate surface expressions of CD86, CD40, CD54, and major histocompatibility complex (MHC) class II molecules on DCs, which causes the impaired capacity of antigen presentation. DEX-treated DCs are unable to prime T helper type 1 (Th1) cells efficiently, which leads to a significant reduction in the number of interferon-γ (IFN-γ)-producing effector cells [30] and an increase in regulatory T cell (Treg) differentiation and IL-10 production [31]. An In vivo study has confirmed that stress can induce more immature DCs which cannot be activated by tumor cells [9].
Impairment of adaptive immunity
A shift from Th1 to Th2 response in TILs also leads to the accelerated tumor progression in murine colon cancer model [32] and in patients with ovarian neoplasms [33].
When exposed to UVB light, high-anxious mice show a higher average count of SCC with more Treg infiltration [34]. β3-adrenergic receptor (B3AR) and β2-adrenergic receptor (B2AR) activation favor the recruitment of T cells within the tumor, the impairment of their cytotoxic functions, and the promotion of CD4+CD25+ Tregs differentiation [35,36]. B2AR signaling also increases cytotoxic T-lymphocyte associated protein 4 (CTLA-4) expression on Tregs as well, further switching the anti-tumor immune response to tumor-favorable response [36].
Besides, norepinephrine reduces the expressions of growth-related cytokines in resting and activated memory CD8+ T cells by B2AR activation [37]. The number of tumor-infiltrating CTLs decreases in stressful conditions [34]. Dopamine secretion is reduced under chronic stress, and dopamine depletion has been reported to decrease T cell responses and promote tumor growth in mice [38,39].
Chronic stress acts on the immune system to promote tumor metastasis
The metastatic cascade is composed of a number of sequential events that involve cell detachment from the primary tumor, invasion into surrounding tissue, intravasation migration, arrest and extravasation into distant tissues, and formation of metastasis [40]. The outcome of metastasis depends on the interactions between cancer cells and a given microenvironment. In a murine lymphoma model with chronic stress, total tumor cells secrete more MMP2 and exhibit greater migration capacity than tumor cell suspensions depleted the main infiltrating immune cell subsets [20]. These data suggest that psychological stress-mediated dysregulation in TILs contributes to enhanced tumor metastasis to a certain extent.
Mononuclear phagocyte system
It has been reported that chronic stress critically influences pre-metastatic lungs before the arrival of disseminated tumor cells by increasing the outputs of monocytes and augmenting the infiltration of macrophages into the lung. The underlying mechanism is the enhanced CCL2-CC chemokine receptor 2 (CCR2) axis: upregulated expression of CCL2 in pulmonary stromal cells and CCR2 in monocytes/macrophages [41]. In addition, the increased infiltration of CD11b+F4/80+ macrophages in primary tumor parenchyma and an M2-like macrophage differentiation promote pro-metastatic genes expression and a 30-fold increase in metastasis to distant tissues including lymph nodes and lung [42].
Inflammation
It has been reported that tress activates macrophages to express more cyclooxygenase 2 (COX2) and produce more PGE2. This elevated inflammatory signaling is a prerequisite for stress-enhanced VEGFC expression, tumor lymphatic remodeling, and tumor cell dissemination [43]. Other than lymph node metastasis, chronic stress increases the invasive potential of tumor cells into the brain by exacerbating inflammation, impairment of the blood-brain barrier, and facilitating angiogenesis of disseminated tumor cells in the brain [10,44,45]. The inflammatory environment is created by the altered neuroinflammatory profile of microglial cells and macrophages with increased levels of MMP, ROS, cytokines, chemokines, and growth factors.
Impaired NK cells
Stressed conditions have been reported to compromise NK cell cytotoxicity and reduce the resistance to the formation of metastasis of various types of cancer [46,47]. However, each study reported different aspects of NK cell impairment. The sources of impaired NK cells include the spleen, distant metastatic site, and peripheral blood. It has been reported that surgery stress reduces circulating NK cell concentrations and the expression of fatty acid synthase (FAS) ligand and CD11a [46]. Inflammatory substances like prostaglandins and stress-induced hormones, especially epinephrine, can suppress NK cell activity by activating respective membrane receptors, causing intracellular elevation of cAMP levels [47,48]. However, this mechanism may only be applied to tumors sensitive to NK cells [49].
Here we summarize the main mechanisms discussed above in stress-induced immune dysregulation in tumor progression and metastasis (Figure 2).
Figure 2.
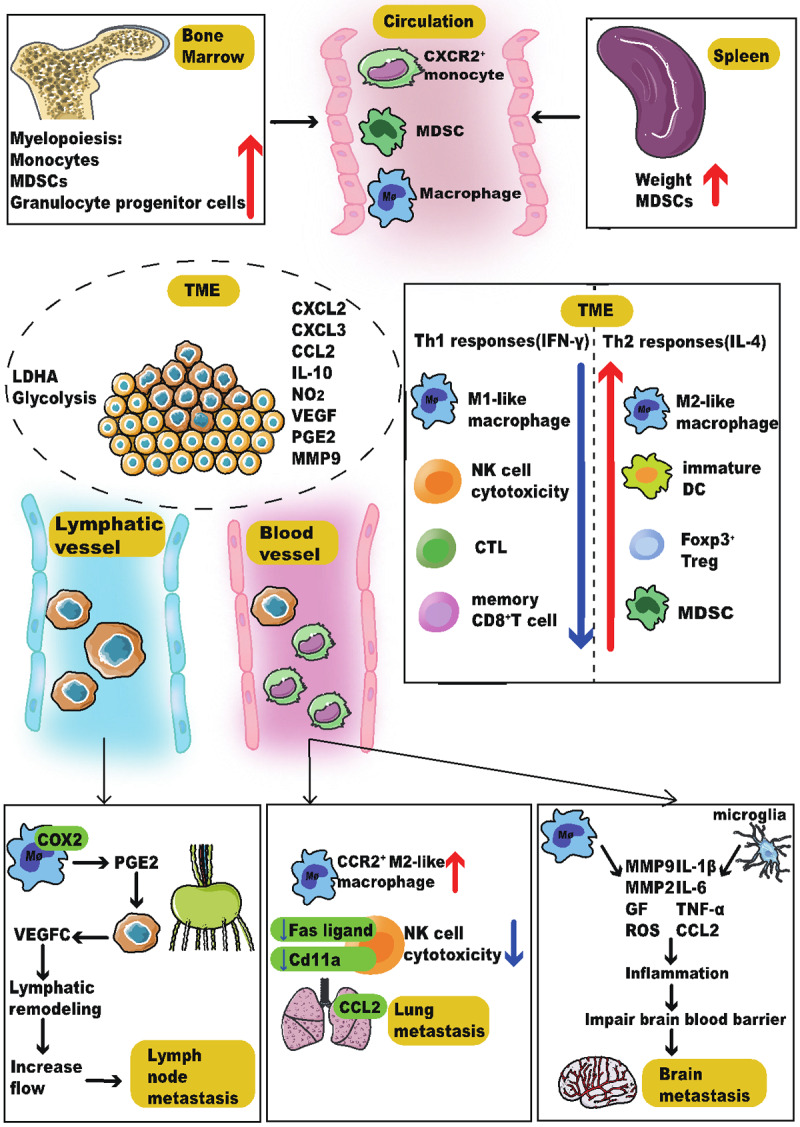
Chronic stress plays multiple roles in tumor progression and metastasis. Stress increases myelopoiesis and spleen mass. The expanded macrophages, MDSCs, and monocytes migrate into the TME due to the increased expressions of chemokines including CCL2, CXCL2, and CXCL3. Stress also alters the composition of immune cells in the TME. The number of M2-like TAM, immature DC, MDSC, and Treg increases, which produces more IL-10, NO2, VEGF, PGE2, and MMP9. At the same time, stress changes the metabolic pattern by increasing the expression of LDHA and promoting glycolysis, which creates an immunosuppressive microenvironment. In addition, stress increases the lymphatic metastasis and hematogenous metastasis. Stress activates macrophages to express more COX2 and produce more PGE2, which is a prerequisite for tumor lymphatic remodeling and tumor cell dissemination. Monocytes/macrophages have been recruited to the lungs and form a pre-metastatic niche for the subsequent arrival of disseminated tumor cells via the CCR2-CCL2 axis. Besides, the NK cell cytotoxicity has been impaired. Chronic stress also increases the secretion of the pro-inflammatory cytokines in microglia cells and macrophages, which impairs the integrity of the blood-brain barrier and increases the survival chance of disseminated tumor cells in the brain. Abbreviations: TME, tumor microenvironment; COX2, cyclooxygenase 2; MDSC, myeloid-derived suppressor cell; CCL2, C-C motif chemokine ligand 2; CXCL2, (C-X-C motif) ligand 2; CXCL3, (C-X-C motif) ligand 3; DC, dendritic cell; TAM, tumor-associated macrophage; Treg, regulatory T cell; IL-10, Interleukin-10; VEGF, vascular endothelial growth factor; PGE2, prostaglandin E2; MMP9, matrix metalloproteinase 9; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; TNF-α, tumor necrosis factor (TNF)-α; ROS, reactive oxygen species; GF, growth factor; FAS, fatty acid synthase; LDHA, lactate dehydrogenase A; COX2, cyclooxygenase 2; CCR2, CC chemokine receptor 2; NK, natural killer; CTL, cytotoxic T lymphocyte; FAS, fatty acid synthase.
Chronic stress acts on the immune system to impair anti-tumor therapy
The anti-tumor immune responses are indispensable in immunotherapy, chemotherapy, and radiation as well [50]. Any cancer treatments taking effects by boosting the immune system will be influenced by neuroimmune regulation. Researchers have conducted in-depth studies in an attempt to elucidate the underlying mechanisms, aiming to enhance the efficacy of treatments and benefit cancer patients.
Impairment of DCs
Therapeutic responses to immunogenic chemotherapy, prophylactic tumor vaccination, and programmed death receptor-1 (PD-1)-targeted immunotherapy are perturbed by chronic stress in murine transplanted tumor models [51]. Tumor-infiltrating dendritic cells in stressed mice upregulate TSC22D3 expression, which is a glucocorticoid downstream effector sufficing to induce immune suppression and weaken the tumor-inhibition effect of cancer therapy (Figure 3).
Figure 3.
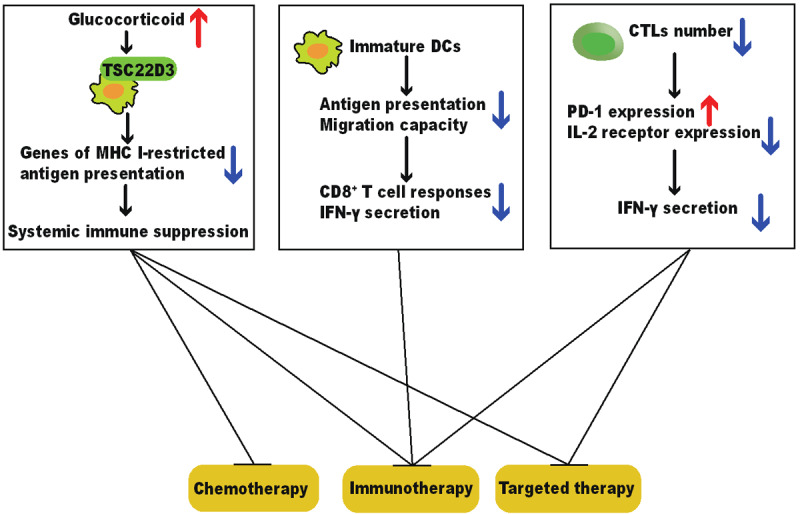
Chronic stress weakens the therapeutic responses of anti-tumor therapies. Chronic stress impairs the maturation, antigen presentation, and migration of DCs, which fails to initiate CD8+ T cell activation and IFN-γ secretion. Chronic stress also blunts CTLs by increasing PD-1 expression and decreasing IL-2 receptor expression, resulting in lower production of IFN-γ and IL-2. The stress-induced malfunction of the immune system weakens the efficacy of anti-cancer therapies and promotes tumor progression. Abbreviations: DC, dendritic cell; IFN-γ, interferon γ; CTL, cytotoxic T lymphocyte; PD-1, programmed death receptor-1; IL-2, Interleukin-2.
Similarly, it is reported that chronic stress suppresses the therapeutic efficacy of cancer vaccine of melanoma in both prophylactic and therapeutic conditions by impairing the capacity of DCs, including migration, antigen presentation, and subsequent CD8+ T cell priming [52] (Figure 3).
Malfunction of CTLs
The stress-induced malfunction of CTLs is also dependent on other mechanisms (Figure 3). Chronic stress increases the expression of PD-1 on CD8+ TILs, weakens the therapeutic efficacy of anti-PD-1 treatment, and decreases the intra-tumoral frequency of effector CD8+ T cells in tumor-bearing mice [53]. In addition, Psychological stress down-regulates the expression of interleukin-2 (IL-2) receptor in peripheral blood leukocytes [54], which may link with the observation that psychological status affects the therapeutic efficacy of IL-2 immunotherapy in renal cell cancer patients [55].
Decreased NK cells and IL-12 production in CpG-C treatment
CpG-C potently activates the immune system to produce interleukin-12 (IL-12) and type I IFNs, which has been identified as a potential treatment against brain metastases [56]. It has been reported that CpG-C-mediated IL-12 production is impaired with corticosterone administration [57]. An in vivo study has conducted experiments on three kinds of cancer models treated with CpG-C and exposed to chronic stress [58]. The extent of impairment of CpG-C efficacy is different in each disease model, and the reason may lie in the different physiological mechanisms of diseases.
Metabolism disorder in stress-immune-cancer axis
Mitochondria dysfunction and energy competition
TME is one of the key regulators in the stress-immune-cancer axis. It has been found that tumor implantation can augment the immune-suppressive effects exerted by chronic stress [25,53]. Tumor-induced intensified immune suppression may result from the intensified energy competition in the organism. A systematic review has concluded that chronic stress exposure can decrease the mitochondrial capacity of energy production and alter mitochondrial morphology [59]. Mitochondrial apoptosis signaling is enhanced by DEX administration in lymphoid cells [60]. Stress also induces mitochondrial fission in peripheral CD4+ T cells, and this morphological disorder in mitochondria causes a systemic purine metabolism disorder [61]. Since the immune activation is an energy-consuming process, the stress-induced mitochondria dysfunction and insufficient energy supply may contribute to immune suppression and tumor escape [62].
Enhanced glycolysis
Stress-induced metabolism switch may be one of the underlying reasons for immune suppression. It has been reported that chronic stress elevates lactate dehydrogenase A (LDHA) expression to enhance glycolysis [63] (Figure 2). The enhanced glycolysis contributes to the strengthened function of MDSCs and decreased infiltration of tumor-killing immune cells, which assists cancer cells to escape immune surveillance [64].
To sum up, chronic stress creates a detrimental and unfavorable environment for the immune system. This facilitates tumor progression because a weak immune system can be an easy breakthrough for cancer cells.
Strategies against chronic stress so as to resume the immune system
It has been reported that social stress alters gene expressions, yet most of the stress-induced changes in transcription are reversible after the removal of stressor [65]. This indicates the feasibility of combining stress-reduction measures with anti-cancer therapy.
Enriched environment and stress-reduction measures
Given the central role of mood regulation in the stress-immune-cancer axis, some researchers have found that the activation of the reward system results in reduced tumor weight and suppression of MDSCs [66]. Enriching the housing environment for mice also enhances the anti-tumor cytotoxicity of NK cells [67]. The enriched environment is an experimental paradigm which provides sensory, cognitive, motor, and social stimulations to the animals but does not distress them, sharing the same purposes as many stress-reduction strategies of cancer patients, including physical-based approaches, mindfulness-based stress reduction, and cognitive-behavioral stress management [68]. However, the efficacy of these approaches in clinical settings is controversial [69]. There are promising data demonstrating enhanced cellular immunity and prolonged survival time [68], yet much of the work is preliminary and limited by small sample sizes, nonstandard evaluations, and short follow-up surveys. These shortages limit the possibility of large-scale applications in clinical settings. The questions regarding “when to intervene”, “who can benefit most” and “how to intervene” are still need to be clarified in future research.
Anti-depressants
Several studies have shown the possibility to contain anti-depressants in anti-cancer treatment, aiming to ameliorate the immune suppression caused by chronic stress. Serotonin-specific reuptake inhibitors, including fluoxetine and sertraline, are frequently prescribed for the treatment of stress-associated disorders [20]. Mice injected with immune cells from stressed animals treated with fluoxetine and sertraline show decreased lymphoma growth and lower incidence of liver metastasis compared with mice injected with lymphocytes from chronically stressed mice because fluoxetine and sertraline can prevent stress-mediated impairment of NK cell cytotoxicity [20]. However, in social defeat mice, fluoxetine fails to rescue the efficacy of methotrexate (MTX) in tumor inhibition [51]. Therefore, as promising as it looks, the usage of anti-depressants prophylactically or therapeutically still needs further confirmation in clinal trials.
Blockages of stress-induced hormones
Previous analysis suggests the significant role of stress-induced hormones in altering immune function so as to assist tumor development. Therefore, hormones blockages have been studied in plenty of animal models and clinical trials. The injections of glucocorticoid receptor antagonist mifepristone (MIFE) restore the capacity of MTX to inhibit fibrosarcoma outgrowth and relieve anxious behaviors in social defeat mice. It also improves the efficacy of anti-PD-1 blockade and re-establishes the effectiveness of anti-cancer vaccination [51]. β-adrenergic receptor (BAR) blocker propranolol not only enhances cellular immunity but also improves long-term recurrence-free survival rates [46,70]. The promising combination of propranolol with anti-PD-1 blockade also shows the potential of combining BAR blockade with anti-CTLA-4 blockade [36,53]. Though some clinical practices reported no benefits from the usage of BAR blockers, yet a lot more believe that cancer patients who are taking BAR antagonists have better outcomes in terms of both progression-free survival and overall survival [70]. Further, a study reported the importance of the administration time of propranolol. Propranolol is beneficial when it is given prior to tumor formation [53]. For this reason, we need more randomized controlled trials (RCTs) to carefully evaluate the efficacy of the BAR blockade and determine the optimal administration method.
Anti-inflammatory measures
Previous analysis also establishes the association between stress-induced inflammation and cancer. The strategies to attenuate inflammation can be a possible target in future combination cancer therapy, especially for colorectal cancer (CRC) patients. Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) in CRC patients can enhance cellular immunity and improve prognosis [71,72]. In addition to NSAIDs, substances with anti-inflammatory properties, like DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL), counteract the stressed-induced adverse effects and regulate intestinal immunity by producing more anti-inflammatory cytokines like IL-10 [73]. Though these data do not provide direct proof of cancer-inhibiting effects, it is plausible to deduce the efficacy of DHA-PL, EPA-PL in colitis-associated colon cancer.
Vitamin C and metabolic transition
Metabolism is one of the key regulators in the immune system and cancer biological behaviors, yet it has not been fully studied in stressed settings. Chronic stress-induced LDHA overexpression can be restored by vitamin C [63], which combats stress-associated breast cancer.
Conclusions and perspectives
The complexity of immune system responses in enhanced tumor biological behaviors induced by chronic stress has been stated. Chronic stress creates an unfavorable environment and exerts negative influences on all aspects of immune parameters, thereby promoting tumor evasion. Stress strengthens the tumor-educated immune suppressive cells and hampers the cytotoxicity of cellular immunity. The insights from molecular, cellular, and physiological mechanisms are transformed into clinically applicable strategies, aiming to reinforce the efficacy of existing cancer therapies like chemotherapy, cancer vaccines, and immunotherapies.
Some studies have reached opposite conclusions, and the reason may due to the usage of different stressed models. The resulting hormone secretion patterns and behavioral outcomes of each model have subtle differences [74]. For example, the key regulator glucocorticoids in social defeat mice are not involved in CD4+ T-cell-mediated anxiety in mice exposed to electric shock [51,61]. Moreover, the administration time of stress is crucial, because the different sequences of stress exposure and tumor inoculation indicate different clinical settings. If the chronic stress precedes, the study focuses on the susceptibility to cancer in stressed people, while the opposite situation focuses on the influence caused by the depressing process of cancer diagnosis and treatment.
A portion of the existing research has some deficiencies due to technical restrictions and flawed experiment designs from a present point of view. Some studies neglected behavioral evaluation of the stressed mice. In a standardized protocol for imposing repeated social defeat stress on mice, approximately 30-40 percent of mice show a resilient phenotype in a large population of defeated mice [75]. The pre-existing differences in stress-responsive IL-6 concentrations affect host resilience to social stress [76]. The different perceptions of exogenous stressors can also lead to verifying consequences [77]. Therefore, it is necessary to verify the success of modeling to ensure data accuracy. Tumor cells are important members of TME and are deeply influenced by chronic stress via adrenergic signaling [1]. Some studies just simply used BAR blocker propranolol to demonstrate results, which may be interfered with other cell components of TME. The emerging methods like genetic tools offer more solid evidence in a complex circumstance. Besides, some conclusions are transferred from in vitro experiments by exogenous administrations of catecholamines, yet chronic stress comprehensively re-shapes our body and BAR agonist infusion cannot induce the same alternations as stress [78]. We need to be cautious about the differences in studies focused on hormones or chronic stress.
Most studies in this field just focused on several differentiated expressed genes in certain cell types or the transcriptional data of tissue samples, which were unable to examine the cell-to-cell variability or decipher the comprehensive modifications induced by chronic stress. It is necessary to conduct more single-cell sequencing analyses to unravel the complicated stress-immune-tumor axis. The serum metabolome of anxious mice with a morphological disorder in mitochondria and wildtype littermates are varying [61], which suggests the systematic remodeling in stressed immune. It is promising to conduct integrated genomics analysis of transcriptomics, metabolomics, and epigenomics.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81872317, 81520108024).
Disclosure of conflict of interest
None.
References
- 1.Krizanova O, Babula P, Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–428. doi: 10.1080/10253890.2016.1203415. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 4.Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, Wegscheider K, Weis J, Boehncke A, Hund B, Reuter K, Richard M, Sehner S, Sommerfeldt S, Szalai C, Wittchen HU, Koch U. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J. Clin. Oncol. 2014;32:3540–3546. doi: 10.1200/JCO.2014.56.0086. [DOI] [PubMed] [Google Scholar]
- 5.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, Repasky EA. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naïve and tumor-bearing mice. Front Immunol. 2014;5:23. doi: 10.3389/fimmu.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:309–321. doi: 10.1016/j.pnpbp.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Kiecolt-Glaser JK, Stephens RE, Lipetz PD, Speicher CE, Glaser R. Distress and DNA repair in human lymphocytes. J Behav Med. 1985;8:311–320. doi: 10.1007/BF00848366. [DOI] [PubMed] [Google Scholar]
- 13.Byrnes DM, Antoni MH, Goodkin K, Efantis-Potter J, Asthana D, Simon T, Munajj J, Ironson G, Fletcher MA. Stressful events, pessimism, natural killer cell cytotoxicity, and cytotoxic/suppressor T cells in HIV+ black women at risk for cervical cancer. Psychosom Med. 1998;60:714–722. doi: 10.1097/00006842-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 15.Söderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, Wu Q, You L, Wang Y, Lin Y, Li X, Wang Y, Bian JS, Sun D, Kong L, Birnbaumer L, Yang Y. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115:E2960–E2969. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayyadurai S, Gibson AJ, D’Costa S, Overman EL, Sommerville LJ, Poopal AC, Mackey E, Li Y, Moeser AJ. Frontline science: corticotropin-releasing factor receptor subtype 1 is a critical modulator of mast cell degranulation and stress-induced pathophysiology. J Leukoc Biol. 2017;102:1299–1312. doi: 10.1189/jlb.2HI0317-088RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Fang X, Yuan J, Sun Z, Li C, Li R, Li L, Zhu C, Wan R, Guo R, Jin L, Li S. The role of corticotropin-releasing hormone receptor 1 in the development of colitis-associated cancer in mouse model. Endocr Relat Cancer. 2014;21:639–651. doi: 10.1530/ERC-14-0239. [DOI] [PubMed] [Google Scholar]
- 19.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Rosso ME, Sterle HA, Cremaschi GA, Genaro AM. Beneficial effect of fluoxetine and sertraline on chronic stress-induced tumor growth and cell dissemination in a mouse model of lymphoma: crucial role of antitumor immunity. Front Immunol. 2018;9:1341. doi: 10.3389/fimmu.2018.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Li Y, Li ZZ, Sun J, Li JW, Wei W, Li L, Zhang C, Huang C, Yang SY, Yang J, Kong GY, Li ZF. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling. Brain Behav Immun. 2019;80:825–838. doi: 10.1016/j.bbi.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, Matsuo K, Dalton HJ, Previs RA, Jennings NB, Dorniak P, Hansen JM, Arevalo JM, Cole SW, Lutgendorf SK, Sood AK, Lopez-Berestein G. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015;6:4266–4273. doi: 10.18632/oncotarget.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H, Wang Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015;48:295–300. doi: 10.5483/BMBRep.2015.48.5.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmieder A, Michel J, Schönhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol. 2012;22:289–297. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt D, Peterlik D, Reber SO, Lechner A, Männel DN. Induction of suppressor cells and increased tumor growth following chronic psychosocial stress in male mice. PLoS One. 2016;11:e0159059. doi: 10.1371/journal.pone.0159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Tang XY, Li YX, Zhao DD, Cao QH, Wu HX, Yang HB, Hao K, Yang Y. Depression-induced neuropeptide y secretion promotes prostate cancer growth by recruiting myeloid cells. Clin Cancer Res. 2019;25:2621–2632. doi: 10.1158/1078-0432.CCR-18-2912. [DOI] [PubMed] [Google Scholar]
- 27.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Varker KA, Terrell CE, Welt M, Suleiman S, Thornton L, Andersen BL, Carson WE 3rd. Impaired natural killer cell lysis in breast cancer patients with high levels of psychological stress is associated with altered expression of killer immunoglobin-like receptors. J Surg Res. 2007;139:36–44. doi: 10.1016/j.jss.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 30.Pan J, Ju D, Wang Q, Zhang M, Xia D, Zhang L, Yu H, Cao X. Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol Lett. 2001;76:153–161. doi: 10.1016/s0165-2478(01)00183-3. [DOI] [PubMed] [Google Scholar]
- 31.Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000;30:1233–1242. doi: 10.1002/(SICI)1521-4141(200004)30:4<1233::AID-IMMU1233>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, Huang C. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. 2013;439:471–476. doi: 10.1016/j.bbrc.2013.08.101. [DOI] [PubMed] [Google Scholar]
- 33.Lutgendorf SK, Lamkin DM, DeGeest K, Anderson B, Dao M, McGinn S, Zimmerman B, Maiseri H, Sood AK, Lubaroff DM. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhabhar FS, Saul AN, Holmes TH, Daugherty C, Neri E, Tillie JM, Kusewitt D, Oberyszyn TM. High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS One. 2012;7:e33069. doi: 10.1371/journal.pone.0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiarugi P, Filippi L. beta3-adrenoreceptor and tumor microenvironment: a new hub. Oncoimmunology. 2015;4:e1026532. doi: 10.1080/2162402X.2015.1026532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, Nascimento VM, Vivanco BC, Reis VO, Keller AC, Brum PC, Basso AS. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur J Immunol. 2013;43:1001–1012. doi: 10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- 37.Slota C, Shi A, Chen G, Bevans M, Weng NP. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–179. doi: 10.1016/j.bbi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu S, Dasgupta PS, Chowdhury JR. Enhanced tumor growth in brain dopamine-depleted mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. J Neuroimmunol. 1995;60:1–8. doi: 10.1016/0165-5728(95)00044-3. [DOI] [PubMed] [Google Scholar]
- 39.Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194–199. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- 40.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol. 2018;244:49–60. doi: 10.1002/path.4988. [DOI] [PubMed] [Google Scholar]
- 42.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, Renne G, Gandini S, Pouton CW, Ferrari D, Möller A, Stacker SA, Sloan EK. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Seiler A, Sood AK, Jenewein J, Fagundes CP. Can stress promote the pathophysiology of brain metastases? A critical review of biobehavioral mechanisms. Brain Behav Immun. 2019 doi: 10.1016/j.bbi.2019.12.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 47.Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, Ben-Eliyahu S. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2014;37:207–219. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinet L, Jean C, Dietrich G, Fournié JJ, Poupot R. PGE2 inhibits natural killer and gamma delta T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 50.Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M, Fu L, Yang J, Pan Z, Yu K, He J, Morand E, Schlecht-Louf G, Krzysiek R, Zitvogel L, Kang B, Zhang Z, Leader A, Zhou P, Lanfumey L, Shi M, Kroemer G, Ma Y. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25:1428–1441. doi: 10.1038/s41591-019-0566-4. [DOI] [PubMed] [Google Scholar]
- 52.Sommershof A, Scheuermann L, Koerner J, Groettrup M. Chronic stress suppresses anti-tumor T responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav Immun. 2017;65:140–149. doi: 10.1016/j.bbi.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, Attwood K, Abrams SI, Hylander BL, Repasky EA. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8 T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639–5651. doi: 10.1158/0008-5472.CAN-17-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glaser R, Kennedy S, Lafuse WP, Bonneau RH, Speicher C, Hillhouse J, Kiecolt-Glaser JK. Psychological stress-induced modulation of interleukin 2 receptor gene expression and interleukin 2 production in peripheral blood leukocytes. Arch Gen Psychiatry. 1990;47:707–712. doi: 10.1001/archpsyc.1990.01810200015002. [DOI] [PubMed] [Google Scholar]
- 55.Messina G, Lissoni P, Bartolacelli E, Fumagalli L, Brivio F, Colombo E, Gardani GS. Efficacy of IL-2 immunotherapy in metastatic renal cell carcinoma in relation to the psychic profile as evaluated using the Rorschach test. Anticancer Res. 2007;27:2985–2988. [PubMed] [Google Scholar]
- 56.Benbenishty A, Gadrich M, Cottarelli A, Lubart A, Kain D, Amer M, Shaashua L, Glasner A, Erez N, Agalliu D, Mayo L, Ben-Eliyahu S, Blinder P. Prophylactic TLR9 stimulation reduces brain metastasis through microglia activation. PLoS Biol. 2019;17:e2006859. doi: 10.1371/journal.pbio.2006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaashua L, Rosenne E, Neeman E, Sorski L, Sominsky L, Matzner P, Page GG, Ben-Eliyahu S. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology. 2014;42:11–23. doi: 10.1016/j.psyneuen.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi B, Matzner P, Goldfarb Y, Sorski L, Shaashua L, Melamed R, Rosenne E, Page GG, Ben-Eliyahu S. Stress impairs the efficacy of immune stimulation by CpG-C: potential neuroendocrine mediating mechanisms and significance to tumor metastasis and the perioperative period. Brain Behav Immun. 2016;56:209–220. doi: 10.1016/j.bbi.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Picard M, McEwen BS. Psychological stress and mitochondria: a systematic review. Psychosom Med. 2018;80:141–153. doi: 10.1097/PSY.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 61.Fan KQ, Li YY, Wang HL, Mao XT, Guo JX, Wang F, Huang LJ, Li YN, Ma XY, Gao ZJ, Chen W, Qian DD, Xue WJ, Cao Q, Zhang L, Shen L, Zhang L, Tong C, Zhong JY, Lu W, Lu L, Ren KM, Zhong G, Wang Y, Tang M, Feng XH, Chai RJ, Jin J. Stress-induced metabolic disorder in peripheral CD4 T cells leads to anxiety-like behavior. Cell. 2019;179:864–879. e19. doi: 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. [Google Scholar]
- 63.Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, Su Q, Liu B, Yu J, Luo X, Yin L, Cheng W, An F, He B, Liang D, Wu S, Chu P, Song L, Liu X, Luo H, Xu J, Pan Y, Wang Y, Li D, Huang P, Yang Q, Zhang L, Zhou BP, Liu S, Xu G, Lam EW, Kelley KW, Liu Q. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest. 2019;129:1030–1046. doi: 10.1172/JCI121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganapathy-Kanniappan S. Linking tumor glycolysis and immune evasion in cancer: emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer. 2017;1868:212–220. doi: 10.1016/j.bbcan.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Stankiewicz AM, Goscik J, Majewska A, Swiergiel AH, Juszczak GR. The effect of acute and chronic social stress on the hippocampal transcriptome in mice. PLoS One. 2015;10:e0142195. doi: 10.1371/journal.pone.0142195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben-Shaanan TL, Schiller M, Azulay-Debby H, Korin B, Boshnak N, Koren T, Krot M, Shakya J, Rahat MA, Hakim F, Rolls A. Modulation of anti-tumor immunity by the brain’s reward system. Nat Commun. 2018;9:2723. doi: 10.1038/s41467-018-05283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song Y, Gan Y, Wang Q, Meng Z, Li G, Shen Y, Wu Y, Li P, Yao M, Gu J, Tu H. Enriching the housing environment for mice enhances their NK cell antitumor immunity via sympathetic nerve-dependent regulation of NKG2D and CCR5. Cancer Res. 2017;77:1611–1622. doi: 10.1158/0008-5472.CAN-16-2143. [DOI] [PubMed] [Google Scholar]
- 68.Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125:1417–1431. doi: 10.1002/cncr.31943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schakel L, Veldhuijzen DS, Crompvoets PI, Bosch JA, Cohen S, van Middendorp H, Joosten SA, Ottenhoff THM, Visser LG, Evers AWM. Effectiveness of stress-reducing interventions on the response to challenges to the immune system: a meta-analytic review. Psychother Psychosom. 2019;88:274–286. doi: 10.1159/000501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylander BL. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164. doi: 10.3389/fimmu.2018.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lönnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H, Lagerstedt K, Lundholm K. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immun. 2008;8:5. [PMC free article] [PubMed] [Google Scholar]
- 72.Goh CH, Leong WQ, Chew MH, Pan YS, Tony LK, Chew L, Tan IB, Toh HC, Tang CL, Fu WP, Chia WK. Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer. Anticancer Res. 2014;34:7407–7414. [PubMed] [Google Scholar]
- 73.Cao W, Wang C, Chin Y, Chen X, Gao Y, Yuan S, Xue C, Wang Y, Tang Q. DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct. 2019;10:277–288. doi: 10.1039/c8fo01404c. [DOI] [PubMed] [Google Scholar]
- 74.Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42:46–61. doi: 10.1038/npp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stewart AM, Roy S, Wong K, Gaikwad S, Chung KM, Kalueff AV. Cytokine and endocrine parameters in mouse chronic social defeat: implications for translational ‘cross-domain’ modeling of stress-related brain disorders. Behav Brain Res. 2015;276:84–91. doi: 10.1016/j.bbr.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 78.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, Zhu T, Zhang D, Wang W, Wang J, Shen B, Gao X, Shi Y, Zhang J. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One. 2013;8:e74497. doi: 10.1371/journal.pone.0074497. [DOI] [PMC free article] [PubMed] [Google Scholar]


