Abstract
Skin toxicity, especially hand-foot syndrome (HFS), is one of the most common sorafenib-induced adverse events (AEs) in hepatocellular carcinoma (HCC) patients, leading to treatment interruption and failure. Mucocutaneous inflammation may cause HFS; therefore, we investigated whether celecoxib can alleviate HFS, improve patients’ quality of life and increase survival when administered in conjunction with active therapy. Our randomized, open-label study prospectively enrolled 116 advanced HCC patients receiving sorafenib as targeted therapy from July 2015 to July 2016. All patients were randomly assigned (1:1) via a computer-generated sequence to receive sorafenib with or without celecoxib. Sorafenib-related AEs were recorded, Survival was compared between the two groups. Compared to the Sorafenib group, the SoraCele group had lower incidence rates of ≥ grade 2 and grade 3 HFS (63.8% vs 29.3%, P < 0.001; 19.0% vs 3.4%, P = 0.008, respectively), hair loss, rash and abdominal pain. Kaplan-Meier analysis revealed a lower risk of ≥ grade 2 HFS (HR, 0.384; P = 0.002) and a lower dose reduction/interruption rate (46.6% to 15.5%, P < 0.001) in the SoraCele group. Cox proportional hazards regression analysis demonstrated that celecoxib was the only independent predictive factor of developing ≥ grade 2 HFS (HR, 0.414; P = 0.004). Longer progression-free survival (PFS) was also observed in the SoraCele group (P = 0.039), although overall survival was not prolonged (P = 0.305). These results suggest that sorafenib + Celecoxib administration alleviated sorafenib-related skin toxicity. Longer PFS was achieved in clinical practice, although overall survival was not prolonged (ClinicalTrials.gov: NCT02961998).
Keywords: Hand-foot syndrome, celecoxib, sorafenib, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a common fatal malignant tumor, and even with the increase in screening during health examinations, most patients are diagnosed with HCC in advanced stages [1,2]. The overall survival rate has improved in recent decades, although the prognosis remains poor [3,4]. Up to 70% of patients are in the intermediate to terminal stages at diagnosis, limiting their treatment options and resulting in a poor median overall survival rate [5].
The oral multi-kinase small-molecule inhibitor sorafenib remains the first-line targeted therapy for unresectable, locally advanced or metastatic HCC according to the NCCN guidelines published in the last decade [6]. However, sorafenib requires long-term medication administration and causes a series of side effects, including hand-foot syndrome (HFS), limb swelling, rash, peeling, and pain. The incidence rate of HFS ranges from 21% to 51%, seriously affecting patients’ quality of life [7]. In addition, these side effects appear to be dose-related. When severe HFS occurs, the dosage of sorafenib treatment must be reduced or sorafenib treatment is interrupted, which could seriously affect survival.
HFS is also known as palmoplantar erythrodysesthesia and has long been known to be a common, dose-dependent side effect associated with some chemotherapeutical agents [8]. Despite the unknown mechanism underlying HFS, people consider it to be a type of dermatologic inflammation. A retrospective study by Gressett et al. [9] and a prospective randomized trial by Zhang et al. [10] both confirmed that combining capecitabine with celecoxib, a selective COX-2 inhibitor, can significantly reduce capecitabine-related HFS in colorectal cancer patients. Because no relevant clinical evidence has been presented regarding the use of celecoxib to prevent HFS in HCC patients, we designed this prospective randomized controlled study to explore the preventive effect of celecoxib on sorafenib-related HFS, the influence of the addition of celecoxib on HCC patients’ quality of life, and the synergistic antitumor effect of the combination of celecoxib and sorafenib in patients with HCC. This study aimed to improve treatment, quality of life and tumor control in patients with advanced HCC.
Materials and methods
Patient recruitment
The study was designed as a single-center, open-label, randomized, prospective clinical trial (Figure 1) and aimed to explore the ability of celecoxib to prevent HFS induced by sorafenib. Patients were randomly assigned (1:1) to receive either sorafenib (sorafenib group) or sorafenib plus celecoxib (SoraCoxib group). Randomization was performed via a computer-generated randomization sequence, and we calculated that the sample size of this study should be 110 patients at least to enable the detection of hazard rates with 90% power (b = 0.01) and a two-sided significance level of a = 0.05, while 116 were enrolled finally.
Figure 1.
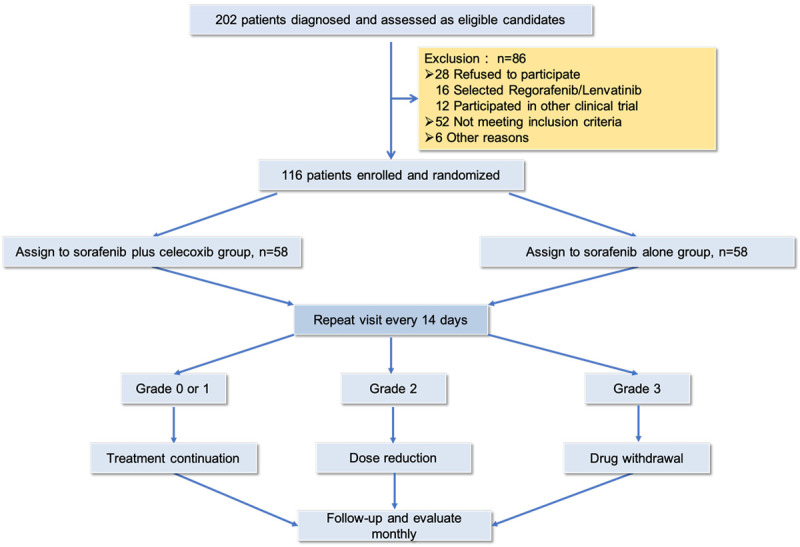
Consort flow diagram.
This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and the laws and regulations of China. This study was approved by the appropriate ethics committee of Sun Yat-sen University Cancer Center. Full written informed consent was obtained from all patients before their participation in the study. The trial was registered at Clinical Trials.gov: NCT02961998.
Patients diagnosed with unresectable or advanced HCC and treated with sorafenib at Sun Yat-Sen University Cancer Center between July 2015 and July 2016 were prospectively collected and randomly assigned to a treatment group. The inclusion/exclusion criteria are described below.
The inclusion criteria were as follows: 1. a diagnosis of HCC according to the 7th edition of the American Joint Committee on Cancer (AJCC) [11] primary liver cancer diagnosis and treatment guidelines; 2. a Karnofsky Performance Status (KPS) score ≥ 70 points; 3. age between 18 and 70 years; 4. Child-Pugh class A or B (class B patients had scores no greater than 7 points). In addition, the baseline laboratory tests had to meet the following criteria: a white blood cell (WBC) count ≥ 1.5 × 109/L, a platelet count ≥ 50 × 109/L, a hemoglobin level ≥ 80 g/L, serum aspartate transaminase (AST) and alanine transaminase (ALT) levels ≤ 2 × the upper limit of normal (ULN), a serum creatinine ≤ 1.5 × ULN, an international normalized ratio (INR) < 1.5 or a prothrombin time < the ULN + 4 seconds, an albumin level ≥ 30 g/L, and a total bilirubin level ≤ 34 mmol/L; and 5. failure of first-line therapy with surgery and radiofrequency ablation in patients with advanced HCC. 6. sorafenib treatment was consented.
The exclusion criteria were as follows: 1. child-Pugh Grade C, with massive ascites and a history of hepatic encephalopathy; 2. poor general condition or cachexia; 3. tendency to hemorrhage, including a history of peptic ulcers or gastrointestinal bleeding; and 4. other contraindications for celecoxib or allergy to celecoxib.
The patients were administered sorafenib (Nexavar Bayer Health Care AG, Leverkusen, Germany) at 400 mg twice daily as a basic treatment. The patients were randomly divided into the following two groups: the sorafenib group (treated only with sorafenib) and the SoraCoxib group (both medicines initiated at the same time). The dose of celecoxib (Pfizer Pharmaceuticals LLC, Puerto Rico) was based on a limited number of case reports, usually starting with 200 mg bid [12,13]. The dose of sorafenib in both groups was reduced when severe complications occurred.
Follow-up and statistical analyses
After the initiation of sorafenib treatment, patients were evaluated monthly. Adverse events (AEs) were recorded according to the National Cancer Institute Common Toxicity Criteria (CTCAE v3.0) [14]. Laboratory tests, including liver and kidney function tests, blood cell counts, and contrast CT or MRI scans were performed every 2 to 3 months. After the interruption of sorafenib treatment, patients were contacted by phone every 3 months to determine their survival status.
HFS is usually worse during the first 6 weeks of treatment with targeted therapy than thereafter. With sorafenib, HFS usually appears within 2 months. Once the early signs of HFS appeared, we recommended that our patients limit the use of hot water and try to cool their hands and feet, avoid activities that cause force or rubbing on the hands or feet and avoid contact with harsh chemicals. Other treatments are shown in Figure 2.
Figure 2.
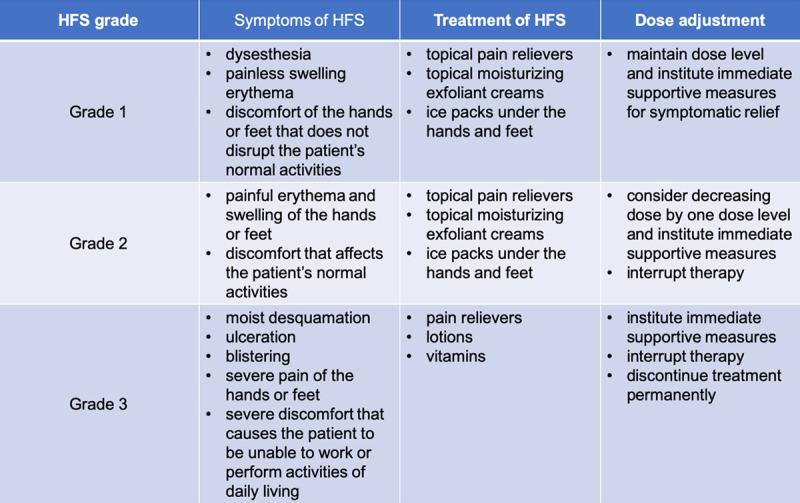
Treatment and dose adjustment strategy for different grades of hand-foot syndrome.
The baseline characteristics were examined via frequency distributions; continuous data are presented as the medians unless otherwise indicated. Kaplan-Meier analysis curves were generated to estimate the difference in the probability of developing at least grade 2 HFS in the two groups, with the endpoint calculated from the initial date of sorafenib therapy to the date of the occurrence of at least grade 2 HFS or the date of the last follow-up. Overall survival (OS) and progression-free survival (PFS) were calculated from the initial date of sorafenib therapy to the date of death from any cause or disease progression, or the date of the last follow-up.
The results were compared by student’s ttests or χ2 tests. Survival outcomes were calculated with the Kaplan-Meier method and compared by log-rank tests. Any factors that were statistically significant at P less than 0.10 in the univariate analysis were candidates for entry into a multivariable Cox proportional hazards model. All P values were 2-sided, with values less than 0.05 considered significant. The statistical package used to perform analyses was SPSS statistical software (version 23.0; SPSS Company, Chicago, IL).
Results
Baseline characteristics
From July 2015 to July 2016, 202 patients with confirmed diagnoses of advanced HCC in whom first-line therapy failed were enrolled in our clinical study, with 116 meeting our final inclusion criteria. Most were male patients (108/116, 93.1%), and the median patient age was 55.5 years (ranging from 22 to 86). At the beginning of treatment with sorafenib, every patient had adequate liver function (Child-Pugh type A) and a favorable performance status (Eastern Cooperative Oncology Group (ECOG) scores 0-1). The 116 patients were then randomly assigned to the sorafenib group (58 patients) or the SoraCele group (58 patients) via a computer-generated sequence. The two groups of patients showed similar clinicopathologic features, except that the patients in the SoraCele group were younger (Table 1).
Table 1.
Patient baseline characteristics in the two groups
| Sorafenib group (n = 58) | Sorafenib/celecoxib group (n = 58) | P value | |
|---|---|---|---|
| Age | 57.4±11.1 | 51.3±11.7 | 0.004a |
| Gender (M/F) | 52/6 | 56/2 | 0.272b |
| HBsAg | 89.1% | 100.0% | 0.083b |
| ALT | 51.6±68.5 | 72.2±101.4 | 0.229a |
| AST | 53.0±50.2 | 71.0±99.5 | 0.241a |
| TBIL | 14.3±7.1 | 15.1±5.4 | 0.509a |
| ALB | 41.0±4.4 | 41.7±4.9 | 0.551a |
| AFP (< 400/> 400) | 39/16 | 29/17 | 0.401b |
| Child-Pugh (A) | 100.0% | 100.0% | 1.000b |
| Nodules in liver | 0.349b | ||
| None | 17 (29.3%) | 14 (26.4%) | |
| 1 | 14 (24.1%) | 7 (13.2%) | |
| 2 | 2 (3.4%) | 4 (7.5%) | |
| Multiple | 25 (43.1%) | 28 (52.8%) | |
| MVI | 0.038b | ||
| None | 18 (60.0%) | 10 (33.3%) | |
| 1 | 12 (340.0%) | 20 (66.7%) | |
| PVTT | 0.639c | ||
| None | 41 (70.7%) | 33 (62.3%) | |
| 1 | 13 (22.4%) | 15 (28.3%) | |
| 2 | 4 (6.9%) | 5 (9.4%) | |
| HVTT/IVCTT | 0.587c | ||
| None | 51 (87.9%) | 46 (86.8%) | |
| HVTT | 5 (8.6%) | 3 (5.7%) | |
| IVCTT | 2 (3.4%) | 4 (7.5%) | |
| Metastasis | 0.406b | ||
| None | 37 (63.8%) | 33 (61.1%) | |
| Lung | 16 (27.6%) | 12 (22.2%) | |
| Other | 5 (8.6%) | 9 (16.7%) | |
| Previous treatment | 0.108c | ||
| None | 1 (1.7%) | 7 (12.1%) | |
| TACE | 16 (27.6%) | 16 (27.6%) | |
| Surgery | 12 (20.7%) | 13 (22.4%) | |
| TACE + Surgery | 29 (50.0%) | 22 (37.0%) | |
| Length of sorafenib treatment | 427.0 | 263.5 | 0.103d |
Values in bold indicate significance.
T-test.
χ2 test.
Continuity correction.
Mann-Whitney U test.
MVI: microvascular invasion; PVTT: portal vein tumor thrombus (1: branch portal vein; 2 trunk portal vein); HVTT: hepatic vein tumor thrombosis; IVCTT: inferior vena cava tumor thrombosis.
In accordance with the results of the previous SHARP and Asia-Pacific trials, HFS and diarrhea remained the most frequent adverse effects in patients undergoing sorafenib treatment (Table 2). Specifically, HFS occurred in 75 patients (64.7%), and 63 (54.3%) patients suffered from diarrhea. Other common adverse effects included hypertension (n = 25, 21.6%), unspecified abdominal pain (24, 20.7%), alopecia (n = 20, 17.2%), rash (n = 22, 19.0%), anorexia (n = 18, 15.5%) and weight loss (n = 15, 12.9%). Moderate to severe AEs occurred in 68 patients (58.6%), including HFS (n = 54, 46.6%), diarrhea (n = 22, 19.0%) and hypertension (n = 7, 6.0%). Dose reduction or the interruption of sorafenib either reversed or improved these AEs, and most of those recovered patients had resumed treatment with sorafenib (Table S1).
Table 2.
Sorafenib-related adverse events in previous trials and the current study
| Adverse events | SHARP (%) | Asia-Pacific (%) | Current study, number, (%) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| All | Grade 2-3 | All | Grade 2-3 | All | Grade 2-3 | |
| Overall number of incidents | 80 | 52 | 98 | 47.7 | 310 (19.1) | 19 (1.2) |
| Constitutional symptoms | ||||||
| Fatigue | 22 | 4 | 20.1 | 3.4 | 13 (11.2) | - |
| Weight loss | 9 | 2 | - | - | 15 (12.9) | - |
| Dermatologic events | - | |||||
| Alopecia | 14 | - | 24.8 | - | 20 (17.2) | - |
| Hand-foot syndrome | 21 | 8 | 45 | 10.7 | 75 (64.7) | 13 (11.2) |
| Rash | 16 | 1 | 20.1 | 0.7 | 22 (16.4) | - |
| Gastrointestinal events | ||||||
| Anorexia | 14 | < 1 | 12.8 | 0 | 18 (15.5) | - |
| Diarrhea | 39 | 8 | 7.4 | 0 | 63 (54.3) | 2 (1.7) |
| Nausea | 11 | < 1 | 11.4 | 0.7 | 2 (1.7) | - |
| Vomiting | 5 | 1 | - | - | 2 (1.7) | - |
| Voice change | 6 | - | - | - | 9 (7.8) | - |
| Hypertension | 5 | 2 | 18.8 | 2 | 24 (20.7) | 4 (3.4) |
| Liver dysfunction | < 1 | < 1 | 0.7 | - | 13 (11.2) | - |
| Abdominal pain, unspecified | 8 | 2 | - | - | 24 (20.7) | - |
| Bleeding | 7 | 1 | 2.7 | - | 10 (8.6) | - |
SHARP, Sorafenib HCC Assessment Randomized Protocol.
Sorafenib-related adverse events can be reduced by celecoxib
We compared the sorafenib-related AEs and showed that the frequencies of ≥ grade 2 and grade 3 HFS differed significantly between the SoraCele group and sorafenib group (29.3% vs 63.8%, P < 0.001, and 3.4% vs 19.0%, P = 0.008, respectively, Table 3). There was a difference in the frequency of ≥ grade 1 HFS between these two groups as well, although it was not significant (68.8% vs 72.4%, P = 0.680).
Table 3.
Adverse event frequency comparison between sorafenib with or without celecoxib
| Adverse events | Sorafenib group (n = 58) | Sorafenib/celecoxib group (n = 58) | P value |
|---|---|---|---|
| Constitutional events | |||
| Fever | 1 (1.7%) | 1 (1.7%) | 1.000c |
| Fatigue | 11 (19.0%) | 2 (3.4%) | 0.008c |
| Weight loss | 10 (17.2%) | 5 (8.5%) | 0.166c |
| Dermatologic events | |||
| Alopecia (Hair loss) | |||
| ≥ Grade 1 | 15 (25.9%) | 5 (8.6%) | 0.014b |
| ≥ Grade 2 | 2 (3.4%) | 0 (0.0%) | 0.476c |
| Hand-foot syndrome | |||
| ≥ Grade 1 | 42 (72.4%) | 33 (68.8%) | 0.680b |
| ≥ Grade 2 | 37 (63.8%) | 17 (29.3%) | < 0.001b |
| ≥ Grade 3 | 11 (19.0%) | 2 (3.4%) | 0.008b |
| Rash | |||
| ≥ Grade 1 | 16 (27.6%) | 3 (5.2%) | 0.001b |
| ≥ Grade 2 | 3 (5.2%) | 1 (1.7%) | 0.611c |
| Gastrointestinal events | |||
| Anorexia | 12 (20.7%) | 6 (10.3%) | 0.124b |
| Diarrhea | |||
| ≥ Grade 1 | 37 (63.8%) | 26 (50.0%) | 0.144b |
| ≥ Grade 2 | 11 (19.0%) | 11 (19.0%) | 1.000b |
| ≥ Grade 3 | 0 (0.0%) | 2 (3.4%) | 0.476c |
| Nausea | 0 (0.0%) | 2 (3.4%) | 0.476c |
| Vomiting | 1 (1.7%) | 1 (1.7%) | 1.000c |
| Voice change | 7 (12.1%) | 2 (3.4%) | 0.165c |
| Hypertension | |||
| ≥ Grade 1 | 16 (27.6%) | 9 (15.5%) | 0.114b |
| ≥ Grade 2 | 6 (10.3%) | 1 (1.7%) | 0.119c |
| ≥ Grade 3 | 3 (5.2%) | 1 (1.7%) | 0.611c |
| Abdominal pain, unspecified | 17 (29.3%) | 7 (12.1%) | 0.022b |
| ECOG performance status score | 0.283c | ||
| 1 | 50 (86.2%) | 55 (94.8%) | |
| 2 | 3 (5.2%) | 1 (1.7%) | |
| Dose reduction/interruption | 27 (46.6%) | 9 (15.5%) | < 0.001b |
| Bone marrow suppression | |||
| Leucopenia | 0.077c | ||
| Grade 1 | 7 (12.1%) | 7 (12.1%) | |
| Grade 2 | 3 (5.2%) | 8 (13.8%) | |
| Grade 3 | 0 (0.0) | 1 (1.7%) | |
| Thrombocytopenia | 0.108c | ||
| Grade 1 | 5 (8.6%) | 11 (19.0%) | |
| Grade 2 | 8 (13.8%) | 6 (10.3%) | |
| Grade 3 | 6 (10.3%) | 3 (5.2%) | |
| Grade 4 | 0 (0.0%) | 1 (1.7%) | |
| Liver dysfunction | |||
| ALT | 32 (55.2%) | 31 (53.4%) | 0.852b |
| AST | 41 (70.7%) | 32 (55.2%) | 0.609a |
| TBIL | 22 (37.9%) | 17 (29.3%) | 0.326a |
| ALB | 25 (43.1%) | 17 (29.3%) | 0.122b |
Values with statistical significance are in bold.
T-test.
χ2 test.
Continuity correction.
ECOG: The Eastern Cooperative Oncology Group.
In addition to the reduction in the severity of HFS in patients using sorafenib and celecoxib, we also witnessed significantly decreased rates of grade 1 hair loss (8.6% vs 25.9%, P = 0.014), grade 1 rash (5.2% vs 27.6%, P = 0.001) and unspecified abdominal pain (12.1% vs 29.3%, P = 0.022) in the SoraCele group compared to those in the control group. The incidence rates of other AEs were nearly the same in the two groups. More details can be found in Table 3.
Administration of celecoxib was the only factor that reduced the incidence of grade 2 or above HFS
We performed a Kaplan-Meier analysis to compare the probability of developing at least grade 2 HFS in the two groups and found a hazard ratio of 0.384 (P = 0.002, SoraCele group vs sorafenib group, Figure 3) and a lower dose reduction/interruption rate in the SoraCele group than in the control group (15.5% vs 46.6%, P < 0.001, Table 3). Univariate Cox proportional hazards regression analysis indicated that gender and the combined administration of celecoxib may be the factors affecting the incidence rate of grade 2 or above HFS (HR, 2.652; P = 0.012, and HR, 0.384; P = 0.002, respectively). Further multivariate analysis showed that celecoxib administration was the only factor that reduced the incidence of grade 2 or above HFS (HR, 0.414; P = 0.004) (Table 4).
Figure 3.
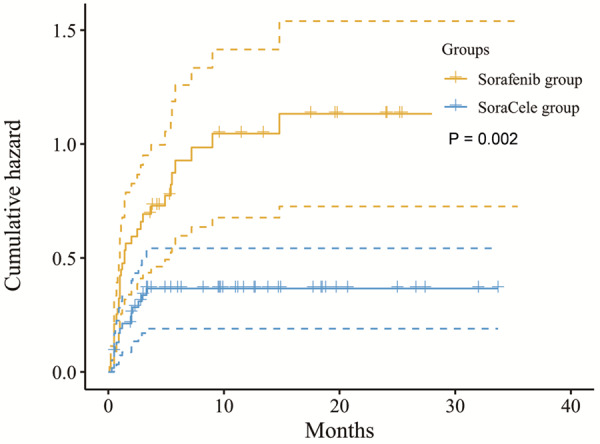
The probability of developing at least grade 2 HFS between the two groups. The data were stratified according to treatment, and compared with the sorafenib group, the SoraCele group had a significantly lower probability of developing at least grade 2 HFS, with a HR of 0.384 (P = 0.002).
Table 4.
Potential risk factors affecting the development of hand-foot syndrome determined by Cox proportional hazards regressio
| Clinical factors | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 2.652 (1.236-5.692) | 0.012 | 2.111 (0.973-4.579) | 0.059 |
| Age | 1.003 (0.980-1.027) | 0.795 | NA | |
| Length of sorafenib treatment | 1.000 (0.999-1.000) | 0.928 | NA | |
| Administration of celecoxib | 0.384 (0.212-0.696) | 0.002 | 0.414 (0.226-0.757) | 0.004 |
The addition of celecoxib resulted in a PFS benefit
The Kaplan-Meier method and log-rank test were employed to determine if patients benefited from the addition of celecoxib (Figure 4). The PFS was longer in the SoraCele group than in the sorafenib group (HR, 0.611; P = 0.039), but no prolongation of OS was detected (HR, 0.810; P = 0.393).
Figure 4.
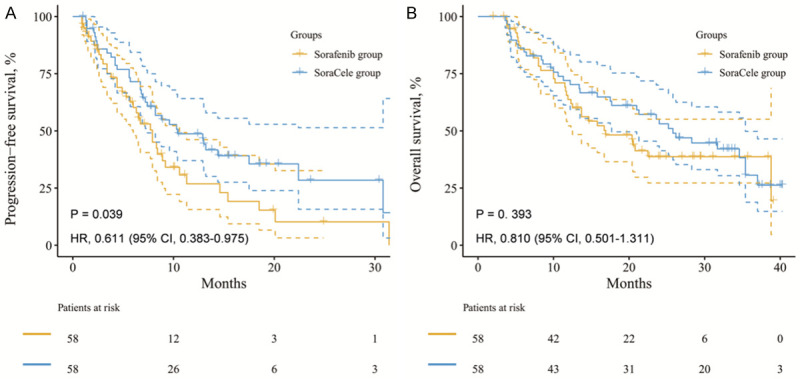
PFS was significantly longer in the SoraCele group (A. HR, 0.611; P = 0.039), but no OS benefit was detected (B. HR, 0.810; P = 0.393).
Discussion
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China, with a very poor prognosis. Its morbidity and mortality rates are among the highest for malignant tumors. Sorafenib has been recommended as the standard treatment for intermediate to advanced stage HCC in the last decade. However, sorafenib requires long-term medication administration and causes a series of side effects, which lead to treatment interruption and failure. Our prospective randomized controlled study was designed to explore whether celecoxib can help prevent and manage these complications in HCC patients.
Sorafenib is an oral multi-kinase inhibitor that targets tyrosine, and it exerts strong antiangiogenic effects and suppresses tumor proliferation in various types of solid-tumor cancers, including renal cell carcinoma [15] and thyroid cancer [16]. Although it is generally considered to be a safe option for chemotherapy [17], sorafenib-related toxicity can lead to reduced quality of life (QoL). HFS, diarrhea, abdominal pain and hypertension are the most frequently reported sorafenib-related adverse effects [18]. Our study found a slightly higher incidence of adverse effects than those reported in the SHARP and Asia-Pacific trials. More detailed AE recording and longer follow-up time are advantages of our study.
The mechanisms underlying HFS and other skin mucosal reactions remain unknown [19]. Most clinicians believe that these skin toxicities result from inflammation, which can be either directly or indirectly triggered by medications. Our present study indicated that the combination of sorafenib with celecoxib can significantly decrease the frequency of grade 2 HFS from 63.8% to 29.3% and the frequency of grade 3 HFS from 19.0% to 3.4%. Interestingly, in addition to a reduced incidence of HFS, we also observed a significant decrease in the incidences of hair loss, skin rash and abdominal pain in patients receiving celecoxib and sorafenib compared with those in patients receiving only sorafenib; this might have a substantial effect on patients’ QoL. These improvements in skin toxicity provide substantial support for the idea that COX2 inhibitors can play a vital role in regulating medication-related inflammatory cytokine production [20,21] and may improve our understanding of the mechanisms underlying HFS and other skin mucosal reactions.
The prevention and management of HFS is important in clinical practice. Until now, no effective method has been found to prevent or reverse sorafenib-related skin toxicity. Recent research has shown that 10% urea-based cream [22], hydrocolloid dressings containing ceramide [23], topical heparin-containing ointment [24], and Vitamin E 300 mg/day [25] may be effective in controlling HFS symptoms. The mainstay strategy for the management of HFS is still dose reduction or drug interruption [26]. HFS is not life threatening, and most symptoms can be managed through dose reduction or treatment failure [27]. Our Kaplan-Meier analysis demonstrated a lower probability of developing grade 2 or above HFS in the SoraCele group than in the sorafenib group, thus leading to a lower rate of dose reduction/interruption.
Risk factors for HFS induced by multi-kinase inhibitors such as sorafenib have been reported to be older age, female sex, low glomerular filtration rate (GFR) (< 30 mL/min/1.73 m2), poor performance status (ECOG performance status score of 2 or above), and metastasis [28-30]. In this study, we investigated all potential risk factors for HFS, including gender, age, length of sorafenib treatment, and the addition of celecoxib. In multivariate analysis, we found that gender may be a predictor for sorafenib-related HFS, but the combined administration of celecoxib was the only independent predictor of the development of grade 2 or above HFS. Remarkably, the length of sorafenib treatment had no relationship with the development of HFS, suggesting that the development of HFS may be related to biochemical variation between individual patients.
Sorafenib exerts antitumor activity against HCC and was demonstrated to prolong OS in the SHARP and Asia-Pacific trials [17,31]. Cyclooxygenase-2 (COX-2) inhibitor is recommended as a treatment option for several cancer types, including non-small cell lung cancer (NSCLC) [32], colorectal cancer [33], prostate cancer [34], and esophageal cancer [35]. The results of our study revealed a longer PFS in the SoraCele group than in the control group, suggesting that celecoxib may have a synergistic effect with sorafenib, stabilizing tumor progression in HCC patients. However, no prolongation of OS was observed in the long term, suggesting that the possible benefits of celecoxib are nullified by confounders and a future study should be designed to verify this finding. Moreover, we are reporting a higher PFS and OS for each group, much higher than that reported in the registered trials of sorafenib, Lenvatinib or Regorafenib or combination of anti-PD-1 therapy maybe part of explanation.
Conclusions
The results of our prospective study suggest that the combined administration of celecoxib should be recommended to decrease the incidence of adverse effects related to skin toxicity, such as HFS, hair loss, skin rash and abdominal pain, in patients treated with sorafenib. A longer PFS was observed in the SoraCele group than in the sorafenib group, although there was no prolongation of OS. Further studies identifying and validating the mechanism by which celecoxib prevents sorafenib-induced AEs are needed.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 3.Page AJ, Cosgrove DC, Philosophe B, Pawlik TM. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am. 2014;23:289–311. doi: 10.1016/j.soc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491. e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyawali B, Shimokata T, Ando M, Honda K, Ando Y. Risk of serious adverse events and fatal adverse events with sorafenib in patients with solid cancer: a meta-analysis of phase 3 randomized controlled trialsdagger. Ann Oncol. 2017;28:246–253. doi: 10.1093/annonc/mdw549. [DOI] [PubMed] [Google Scholar]
- 8.Yap YS, Kwok LL, Syn N, Chay WY, Chia JWK, Tham CK, Wong NS, Lo SK, Dent RA, Tan S, Mok ZY, Koh KX, Toh HC, Koo WH, Loh M, Ng RCH, Choo SP, Soong RCT. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: a randomized clinical trial. JAMA Oncol. 2017;3:1538–1545. doi: 10.1001/jamaoncol.2017.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract. 2006;12:131–141. doi: 10.1177/1078155206069242. [DOI] [PubMed] [Google Scholar]
- 10.Zhang RX, Wu XJ, Wan DS, Lu ZH, Kong LH, Pan ZZ, Chen G. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol. 2012;23:1348–1353. doi: 10.1093/annonc/mdr400. [DOI] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer (AJCC) Articles on AJCC 7th edition TNM staging system. Available online: https://cancerstaging.org/CSE/Physician/Pages/Articles.aspx (accessed on 24 February 2010)
- 12.Lin E, Morris JS, Ayers GD. Effect of celecoxib on capecitabine-induced hand-foot syndrome and antitumor activity. Oncology (Williston Park) 2002;16:31–37. [PubMed] [Google Scholar]
- 13.Lin EH, Curley SA, Crane CC, Feig B, Skibber J, Delcos M, Vadhan SR, Morris J, Ayers GD, Ross A, Brown T, Rodriguez-Bigas MA, Janjan N. Retrospective study of capecitabine and celecoxib in metastatic colorectal cancer: potential benefits and COX-2 as the common mediator in pain, toxicities and survival? Am J Clin Oncol. 2006;29:232–239. doi: 10.1097/01.coc.0000217818.07962.67. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Cancer therapy evaluation program (CTEP) Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 9 August, 2006)
- 15.Xu Z, Yang F, Wei D, Liu B, Chen C, Bao Y, Wu Z, Wu D, Tan H, Li J, Wang J, Liu J, Sun S, Qu L, Wang L. Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene. 2017;36:1965–1977. doi: 10.1038/onc.2016.356. [DOI] [PubMed] [Google Scholar]
- 16.Corrado A, Ferrari SM, Politti U, Mazzi V, Miccoli M, Materazzi G, Antonelli A, Ulisse S, Fallahi P, Miccoli P. Aggressive thyroid cancer: targeted therapy with sorafenib. Minerva Endocrinol. 2017;42:64–76. doi: 10.23736/S0391-1977.16.02229-X. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, Darnell A, Ríos J, Ayuso C, Bruix J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome) Ann Oncol. 2007;18:1159–1164. doi: 10.1093/annonc/mdl477. [DOI] [PubMed] [Google Scholar]
- 20.Araujo-Mino EP, Patt YZ, Murray-Krezan C, Hanson JA, Bansal P, Liem BJ, Rajput A, Fekrazad MH, Heywood G, Lee FC. Phase II trial using a combination of oxaliplatin, capecitabine, and celecoxib with concurrent radiation for newly diagnosed resectable rectal cancer. Oncologist. 2018;23:2–e5. doi: 10.1634/theoncologist.2017-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang L, Hu D, Liu W, Williams JP, Okunieff P, Ding I. Celecoxib reduces skin damage after radiation: selective reduction of chemokine and receptor mRNA expression in irradiated skin but not in irradiated mammary tumor. Am J Clin Oncol. 2003;26:S114–121. doi: 10.1097/01.COC.0000074149.95710.40. [DOI] [PubMed] [Google Scholar]
- 22.Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, Song T, Zhou W, Wang H, Yang W, Wang X, Yang Y, Shi L, Bai Y, Guo X, Ye SL. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015;33:894–900. doi: 10.1200/JCO.2013.52.9651. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara N, Nonomura N, Eto M, Kimura G, Minami H, Tokunaga S, Naito S. A randomized multicenter phase II trial on the efficacy of a hydrocolloid dressing containing ceramide with a low-friction external surface for hand-foot skin reaction caused by sorafenib in patients with renal cell carcinoma. Ann Oncol. 2014;25:472–476. doi: 10.1093/annonc/mdt541. [DOI] [PubMed] [Google Scholar]
- 24.Li JR, Yang CR, Cheng CL, Ho HC, Chiu KY, Su CK, Chen WM, Wang SS, Chen CS, Yang CK, Ou YC. Efficacy of a protocol including heparin ointment for treatment of multikinase inhibitor-induced hand-foot skin reactions. Support Care Cancer. 2013;21:907–911. doi: 10.1007/s00520-012-1693-3. [DOI] [PubMed] [Google Scholar]
- 25.Bozkurt Duman B, Kara B, Oguz Kara I, Demiryurek H, Aksungur E. Hand-foot syndrome due to sorafenib in hepatocellular carcinoma treated with vitamin E without dose modification; a preliminary clinical study. J BUON. 2011;16:759–764. [PubMed] [Google Scholar]
- 26.Marse H, Van Cutsem E, Grothey A, Valverde S. Management of adverse events and other practical considerations in patients receiving capecitabine (Xeloda) Eur J Oncol Nurs. 2004;8(Suppl 1):S16–30. doi: 10.1016/j.ejon.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787–794. doi: 10.1016/j.jaad.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 28.McLellan B, Ciardiello F, Lacouture ME, Segaert S, Van Cutsem E. Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol. 2015;26:2017–2026. doi: 10.1093/annonc/mdv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaymakcalan MD, Xie W, Albiges L, North SA, Kollmannsberger CK, Smoragiewicz M, Kroeger N, Wells JC, Rha SY, Lee JL, McKay RR, Fay AP, De Velasco G, Heng DY, Choueiri TK. Risk factors and model for predicting toxicity-related treatment discontinuation in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer. 2016;122:411–419. doi: 10.1002/cncr.29773. [DOI] [PubMed] [Google Scholar]
- 30.Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs. 2013;31:1078–1086. doi: 10.1007/s10637-013-9977-0. [DOI] [PubMed] [Google Scholar]
- 31.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Liu NB, Zhuang HQ, Zhao LJ, Yuan ZY, Wang P. Celecoxib-erlotinib combination treatment enhances radiosensitivity in A549 human lung cancer cell. Cancer Biomark. 2017;19:45–50. doi: 10.3233/CBM-160323. [DOI] [PubMed] [Google Scholar]
- 33.Alamdarsaravi M, Ghajar A, Noorbala AA, Arbabi M, Emami A, Shahei F, Mirzania M, Jafarinia M, Afarideh M, Akhondzadeh S. Efficacy and safety of celecoxib monotherapy for mild to moderate depression in patients with colorectal cancer: a randomized double-blind, placebo controlled trial. Psychiatry Res. 2017;255:59–65. doi: 10.1016/j.psychres.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Flamiatos JF, Beer TM, Graff JN, Eilers KM, Tian W, Sekhon HS, Garzotto M. Cyclooxygenase-2 (COX-2) inhibition for prostate cancer chemoprevention: double-blind randomised study of pre-prostatectomy celecoxib or placebo. BJU Int. 2017;119:709–716. doi: 10.1111/bju.13612. [DOI] [PubMed] [Google Scholar]
- 35.Cleary JM, Mamon HJ, Szymonifka J, Bueno R, Choi N, Donahue DM, Fidias PM, Gaissert HA, Jaklitsch MT, Kulke MH, Lynch TP, Mentzer SJ, Meyerhardt JA, Swanson RS, Wain J, Fuchs CS, Enzinger PC. Neoadjuvant irinotecan, cisplatin, and concurrent radiation therapy with celecoxib for patients with locally advanced esophageal cancer. BMC Cancer. 2016;16:468. doi: 10.1186/s12885-016-2485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


