Abstract
Purpose:
A phase I feasibility study to determine the accuracy of identifying seizures based on audio recordings.
Methods:
We systematically generated 166 audio clips of 30 s duration from 83 patients admitted to an epilepsy monitoring unit between 1/2015 and 12/2016, with one clip during a seizure period and one clip during a non-seizure control period for each patient. Five epileptologists performed a blinded review of the audio clips and rated whether a seizure occurred or not, and indicated the confidence level (low or high) of their rating. The accuracy of individual and consensus ratings were calculated.
Results:
The overall performance of the consensus rating between the five epileptologists showed a positive predictive value (PPV) of 0.91 and a negative predictive value (NPV) of 0.66. The performance improved when confidence was high (PPV of 0.96, NPV of 0.70). The agreement between the epileptologists was moderate with a kappa of 0.584. Hyperkinetic (PPV 0.92, NPV 0.86) and tonic-clonic (PPV and NPV 1.00) seizures were most accurately identified. Seizures with automatisms only and non-motor seizures could not be accurately identified. Specific seizure-related sounds associated with accurate identification included disordered breathing (PPV and NPV 1.00), rhythmic sounds (PPV 0.93, NPV 0.80), and ictal vocalizations (PPV 1.00, NPV 0.97).
Conclusion:
This phase I feasibility study shows that epileptologists are able to accurately identify certain seizure types from audio recordings when the seizures produce sounds. This provides guidance for the development of audio-based seizure detection devices and demonstrate which seizure types could potentially be detected.
Keywords: Seizure detection, Seizure monitor, Seizure safety, Epilepsy Audio
1. Introduction
Seizure detection devices could be invaluable tools for both people with epilepsy, their caregivers, and clinicians. For patients, having a device that could accurately detect seizures and alert caretakers would help relieve the anxiety from the helplessness that seizures can cause. It could also potentially reduce the risk of sudden unexpected death in epilepsy, particularly for those patients with nocturnal seizures who sleep without a bed partner [1]. For clinicians, seizure detection devices can be an important tool because self-reporting of seizures is unreliable [2]. Seizure detection devices provide objective tracking of seizure frequency, allowing the clinician to assess response to therapy and appropriately adjust medications.
There are multiple types of seizure detection devices ranging from movement detectors (accelerometers, bed alarms, surface EMG, video monitors), autonomic change detectors (electrodermal response, heart rate), and EEG based detectors (ambulatory EEG, implantable EEG system). The performance of these devices is variable (see reviews [3–8]) and dependent on the patient specific seizure type and characteristics. All seizure detection modalities perform well with tonic-clonic seizures. Movement detectors also perform well with seizures with a prominent motor component such as hypermotor seizures and tonic seizures. Autonomic change detectors may also be able to detect other types of focal seizures. EEG-based detectors perform well across a broad range of seizure types but are currently impractical for long-term outpatient use.
Some studies have suggested that audio sensors could be used to detect and alert for seizures [9–12] based on the fact that many seizure types are associated with characteristic sounds such the ictal cry of tonic-clonic seizures [13], stereotyped vocalizations associated with some focal seizures [14–17], and movement-related sounds associated with some motor seizures [11]. Audio has also been used to adjudicate other ambulatory seizure detection modalities such as the Neurovista detection algorithm [18] and a wrist watch accelerometer [19]. The potential disadvantage of audio-based detection is that it could miss seizures which do not produce an identifiable sound.
Several devices are available or under development that employ audio signals for seizure detection. Currently, there is one commercially available audio based seizure detection device with published performance data, the Medpage MP5, which is a multi-modal device that detects bed noises and bed movement. In one study, for tonic-clonic seizures, it had a sensitivity of 0.625, specificity of 0.904, a negative predictive value (NPV) of 0.998, but a positive predictive value (PPV) of 0.033 (with approximately 8.45 false alarms per 12 h of recording) [11]. In a newer study it performed poorly detecting only 4.3 % of all seizure types and 11.1 % of tonic-clonic seizures [20]. Alert-iT has two commercially available audio based seizure detection devices, the Companion and the Guardian, though no published performance data is available. They are both multi-modal devices that detect bed noises, along with bed movement, incontinence, and vomiting. CLB (Cornelis Louis Berghuijs) acoustic monitor is a commercially available audio-based monitoring system that has been tested on tonic and tonic-clonic seizures [12], although their automated machine learning algorithms for seizure detection are still under development. Ervitech has a device under development for detecting seizures based on respiration noises [9], but no published data on performance is available.
Although device development is underway, the effectiveness of audio-based detectors for which seizure types is unclear. The diagnostic utility of the ictal cry has been evaluated in tonic-clonic seizures, as compared to psychogenic seizures, and found to have a sensitivity of 0.85 and specificity of 1.00 [13]. A manual audio-based classification of major seizures (defined as tonic and tonic-clonic seizures greater than 30 s in duration) had a sensitivity of 0.81 and a PPV of 0.40, though this was not evaluated in a blinded fashion [12]. It remains unclear if other seizure sounds can provide accurate diagnostic information, and which seizure types could be accurately detected. In this retrospective phase I feasibility study, we assessed if seizures could be identified based on their sounds alone, and which seizure types would be most amenable to audio-based detection.
2. Materials and methods
2.1. Generation of audio clips
We systematically screened every patient admitted to the adult Stanford Hospital Adult Epilepsy Monitoring Unit (EMU) between 1/01/2015 and 12/31/2016 to generate 30 s audio clips in an unbiased manner. Video-EEGs were collected on the Nihon Kohden system and microphones were located on portable systems. We included the first seizure that was recorded during the EMU stay that fit our inclusion and exclusion criteria, and an associated non-seizure control clip which was 60 s prior to the seizure onset. Our inclusion criteria included electroclinical seizure or psychogenic non-epileptic seizure. Our exclusion criteria included electrographic or absence seizure, disruptive hospital sounds, spoken phrases that would make it obvious that a seizure was occurring (e.g., someone saying “are you okay, “event,” “seizure,” “remember the color,” etc.), poor sound quality, or any audio that contained patient identifiable information (name, DOB). Approximately 50 % of patients screened were excluded due to no audio or poor sound quality. The seizure clip was extracted during the first 30 s of the seizure, based on the electrographic onset if it was an electroclinical seizure and based on first behavioral change if it was a psychogenic seizure. The control clips used the same exclusion criteria and were screened electrographically to ensure there was no seizure activity during that time. This study was approved by the Stanford University Institutional Review Board.
2.2. Seizure clip characterization
The seizure clips were characterized by their seizure type (Table 1) based on the 2017 ILAE classification [21]. There were 12 psychogenic non-epileptic seizures included, which consisted of variable behaviors including convulsive movements, vocalizations and hypotonia. All audio clips were characterized by their sound type (Table 1) based on the presence or absence of the sound type. An audio clip could have multiple sound types present (e.g., disordered breathing and rhythmic sounds). The reference designation of the seizure type and the sound type was determined by the first author, blinded to the rater responses, and based on the review of the audio clips, full video-EEG, and clinical documentation.
Table 1.
Seizure type and ound type (total seizure clips = 83).
| Seizure Type | N | % |
|---|---|---|
| Motor | 57 | 68.7 |
| Automatism | 22 | 26.5 |
| Atonic | 2 | 2.4 |
| Clonic | 1 | 1.2 |
| Hyperkinetic | 13 | 15.7 |
| Myoclonic | 1 | 1.2 |
| Tonic | 5 | 6.0 |
| Tonic-clonic | 13 | 15.7 |
| Non-motor | 14 | 16.9 |
| Behavioral arrest | 6 | 7.2 |
| Cognitive | 5 | 6.0 |
| Emotional | 1 | 1.2 |
| Sensory | 2 | 2.4 |
| Psychogenic | 12 | 14.5 |
| Sound Type | N | % |
| Disordered breathing | 7 | 8.4 |
| Rhythmic sounds | 17 | 20.4 |
| Ictal vocalization | 30 | 36.1 |
| Interruption | 8 | 9.6 |
| Continuation of background noise | 32 | 38.6 |
2.3. Epileptologist rating
Five board-certified epileptologists rated the audio clips (PD, MGH, BALM, AAL, RSF). The audio clips were presented in a random order and for each clip the raters answered whether the clip was during a seizure or non-seizure control period and if they had low or high confidence. The raters were instructed to rate psychogenic non-epileptic seizures as seizure. The years in practice of our epileptologists ranged from 4 to 37 years with a mean of 14.8 years.
2.4. Statistical analysis
To assess the performance for each of the five epileptologists, as well as the consensus response (mode of the ratings), we calculated positive predictive value, negative predictive value, accuracy, sensitivity, and specificity. We repeated this analysis for the subgroup of clips where high confidence was indicated by the epileptologist. Agreement of responses between the five epileptologists was calculated using Fleiss’ Kappa. We also determined the accuracy based on seizure type and the sound type. Confidence intervals for the accuracy were determined using a two-sided exact binomial test using the null hypothesis that the proportion was equal to 0.5 (chance).
3. Results
Five epileptologists rated 166 audio clips, with 83 clips during seizures and 83 clips during a non-seizure control period, to determine if seizures could be accurately identified based on their sound. The seizure types that were included in this study are detailed in Table 1. The major seizure types captured were automatisms, hyperkinetic, and tonic-clonic seizures. The types of sound that where encountered during seizures are detailed in Table 1. The major sound type captured during seizures were continuation of background noise or ictal vocalization. For the non-seizure control clips, as expected, the majority of the clips (80 out of 83, 96.4 %) contained the continuation of background noise.
The performance of each rater (Table 2) showed similar results, with a high positive predictive value (PPV, mean 0.83, range 0.80 to 0.91) and a moderate negative predictive value (NPV, mean 0.65, range 0.60 to 0.69). There was moderate agreement between the raters with a kappa coefficient of 0.584. The consensus response of the five raters showed a PPV of 0.91 (95 %CI = 0.79, 0.98) and NPV of 0.66 (95 %CI = 0.57, 0.74). Performance was improved across all raters when focusing on high confidence responses (Table 2), and the consensus of the high confidence responses showed a PPV of 0.96 (95 %CI = 0.82, 1.00) and NPV 0.70 (95 %CI = 0.46, 0.88). Sensitivity and specificity for individual raters as well as the consensus response are shown in Fig. 1.
Table 2.
Performance overall and during high confidence (with 95 % CI).
| Epileptologist | PPV | NPV | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Overall | |||||
| 1 | 0.77 (0.65,0.86) | 0.67 (0.57,0.76) | 0.71 (0.64,0.78) | 0.60 (0.49,0.71) | 0.82 (0.72,0.90) |
| 2 | 0.91 (0.76,0.98) | 0.60 (0.51,0.69) | 0.66 (0.59,0.73) | 0.36 (0.26,0.47) | 0.96 (0.90,0.99) |
| 3 | 0.83 (0.71,0.92) | 0.68 (0.59,0.77) | 0.74 (0.66,0.80) | 0.59 (0.48,0.70) | 0.88 (0.79,0.94) |
| 4 | 0.87 (0.71,0.95) | 0.61 (0.51,0.69) | 0.66 (0.59,0.73) | 0.39 (0.28,0.50) | 0.94 (0.86,0.98) |
| 5 | 0.80 (0.68,0.89) | 0.69 (0.59,0.77) | 0.73 (0.65,0.79) | 0.62 (0.50,0.72) | 0.84 (0.75,0.91) |
| Consensus | 0.91 (0.79,0.98) | 0.66 (0.57,0.74) | 0.73 (0.65,0.79) | 0.51 (0.39,0.62) | 0.95 (0.88,0.99) |
| Performance during high confidence | |||||
| 1 | 0.91 (0.77,0.98) | 0.69 (0.50,0.84) | 0.81 (0.69,0.89) | 0.76 (0.61,0.88) | 0.88 (0.69,0.97) |
| 2 | 1.00 (0.79,1.00) | 0.71 (0.49,0.87) | 0.83 (0.67,0.93) | 0.70 (0.47,0.87) | 1.00 (0.80,1.00) |
| 3 | 0.91 (0.75,0.98) | 0.92 (0.62,1.00) | 0.91 (0.78,0.97) | 0.97 (0.83,1.00) | 0.79 (0.49,0.95) |
| 4 | 1.00 (0.85,1.00) | 0.74 (0.57,0.87) | 0.83 (0.71,0.92) | 0.69 (0.50,0.84) | 1.00 (0.88,1.00) |
| 5 | 0.93 (0.80,0.98) | 0.83 (0.59,0.96) | 0.90 (0.79,0.96) | 0.93 (0.80,0.98) | 0.83 (0.59,0.96) |
| Consensus | 0.96 (0.82,1.00) | 0.70 (0.46,0.88) | 0.85 (0.72,0.94) | 0.82 (0.65,0.93) | 0.93 (0.68,1.00) |
Fig. 1.
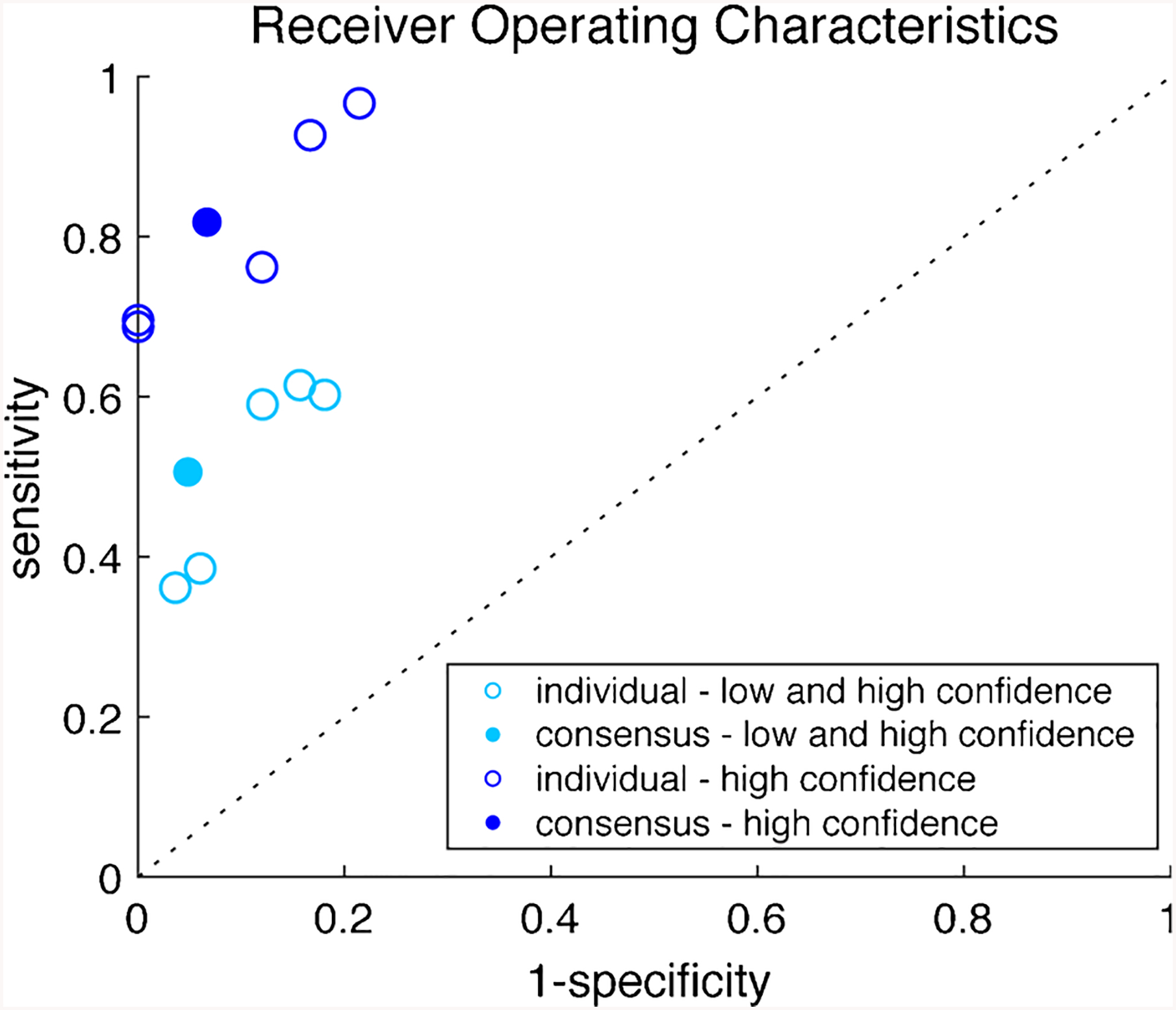
Receiver Operating Characteristics of each epileptologist and consensus response for overall performance and performance during high confidence.
High confidence responses were associated with tonic-clonic, hyperkinetic, and tonic seizure types (Table 3). Low confidence responses were associated with the sound type when there was no change in the background noise. The majority of the non-seizure control clips were rated as low confidence.
Table 3.
Characteristics of high or low confidence responses.
| Seizure Type | High Confidence N = 168 | Low Confidence N = 247 |
|---|---|---|
| Automatism (N = 110) | 20 (18.2 %) | 90 (81.8 %) |
| Atonic (N = 10) | 1 (10 %) | 9 (90 %) |
| Clonic (N = 5) | 0 (0 %) | 5 (100 %) |
| Hyperkinetic (N = 65) | 35 (53.8 %) | 30 (46.2 %) |
| Myoclonic (N = 5) | 2 (40 %) | 3 (60 %) |
| Tonic (N = 25) | 14 (56 %) | 11 (44 %) |
| Behavioral arrest (N = 30) | 10 (33.3 %) | 20 (66.7 %) |
| Cognitive (N = 25) | 9 (36 %) | 16 (64 %) |
| Emotional (N = 5) | 0 (0 %) | 5 (100 %) |
| Sensory (N = 10) | 1 (10 %) | 9 (90 %) |
| Tonic-clonic (N = 65) | 55 (84.6 %) | 10 (15.4 %) |
| Psychogenic (N = 60) | 21 (35 %) | 39 (65 %) |
| Sound Type | High Confidence N = 311 | Low Confidence N = 559 |
| Disordered breathing (N = 35) | 19 (54.3 %) | 16 (45.7 %) |
| Rhythmic sounds (N = 85) | 49 (57.6 %) | 36 (42.4 %) |
| Ictal vocalizations (N = 150) | 102 (68 %) | 48 (32 %) |
| Interruption of activity (N = 40) | 24 (60 %) | 16 (40 %) |
| Continuation of background noise (N = 560) | 117 (20.9 %) | 443 (79.1 %) |
| High Confidence | Low Confidence | |
| Control (N = 415) | 102 (24.6 %) | 313 (75.4 %) |
In order to determine which seizure types were most accurately identified by their sound, we determined the accuracy (equivalent to sensitivity) of the consensus response in each seizure subtype (Fig. 2). The seizure types that were accurately detected include hyperkinetic (accuracy = 0.85, 95 %CI = 0.55, 0.98, p = 0.02) and tonic-clonic (accuracy = 1.00, 95 %CI = 0.75, 1.00, p = 0.0002) seizures. For these seizure types, the PPV and NPV can be calculated using the associated control clips as the negative condition. Hyperkinetic seizures have a PPV of 0.92 and NPV of 0.86 and tonic-clonic seizures have a PPV and NPV of 1.00. The seizure types that could not be distinguished include automatism only, non-motor, and psychogenic seizures.
Fig. 2.
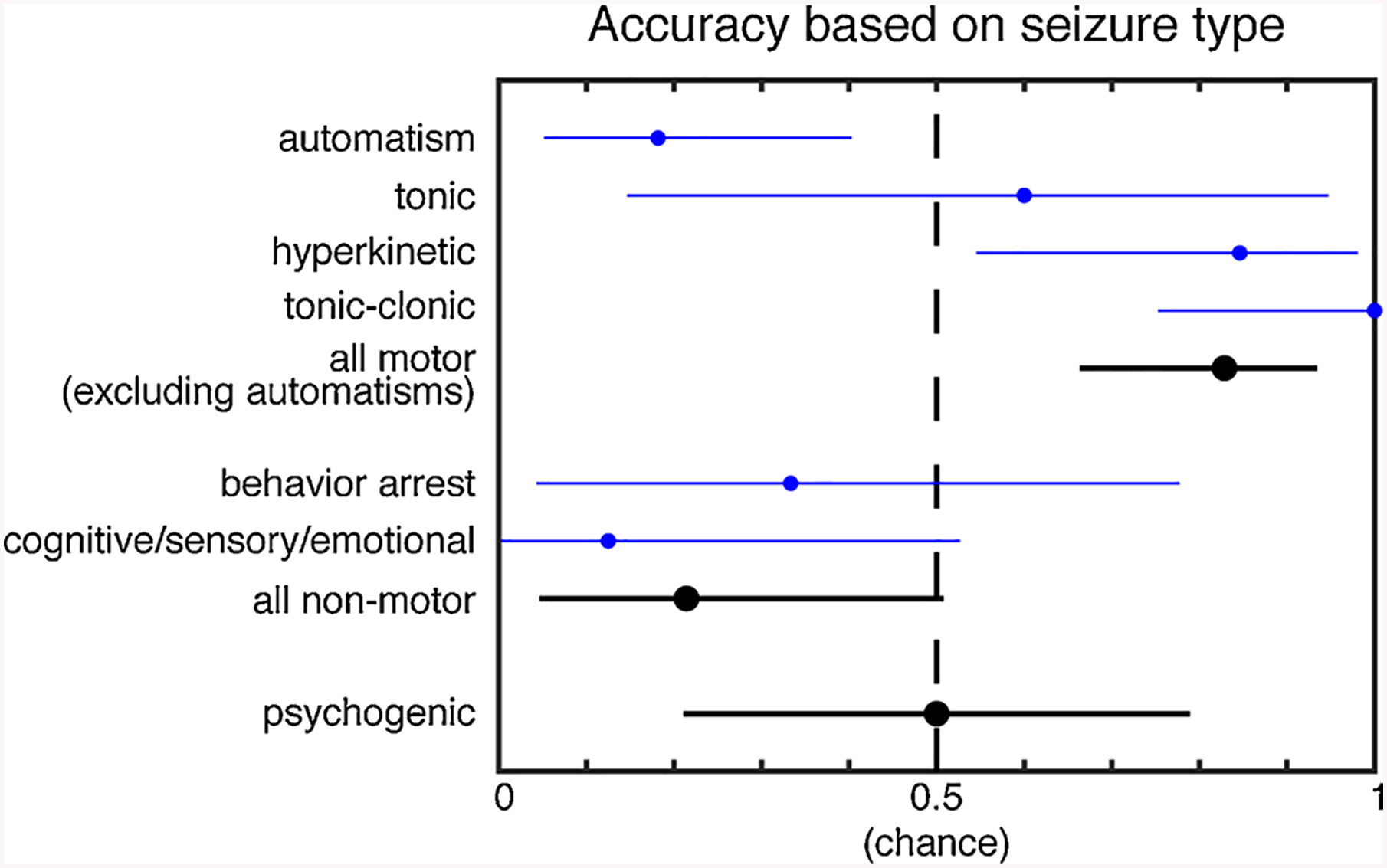
Accuracy of the consensus response based on seizure type. The accuracy and 95 % CI are plotted. All motor group includes the subtypes atonic, clonic, hyperkinetic, myoclonic, tonic, and tonic-clonic. The all non-motor group includes the subtypes behavior arrest, cognitive, emotional, and sensory. The seizure types that were accurately detected by their sound include hyperkinetic and tonic-clonic. Motor seizures overall, excluding automatisms, were accurately detected, while non-motor seizures and psychogenic seizures were not detected.
In order to determine which sound types were most accurately identified, we looked at the accuracy (equivalent to sensitivity) of the consensus response in each sound type. The types of sounds that accurately identified a seizure include disordered breathing (accuracy 1.00, 95 %CI = 0.59, 1.00, p = 0.015), rhythmic sounds (accuracy = 0.76, 95 %CI = 0.50, 0.93, p = 0.049), and ictal vocalizations (accuracy = 0.97, 95 %CI = 0.83,1, p= < 0.0001). For these sound types, the PPV and NPV can be calculated using the associated control clips as the negative condition. Disordered breathing has a PPV and NPV of 1.00, rhythmic sounds have a PPV of 0.93 and NPV of 0.80, and ictal vocalizations have a PPV of 1.00 and NPV of 0.97 (Fig. 3).
Fig. 3.
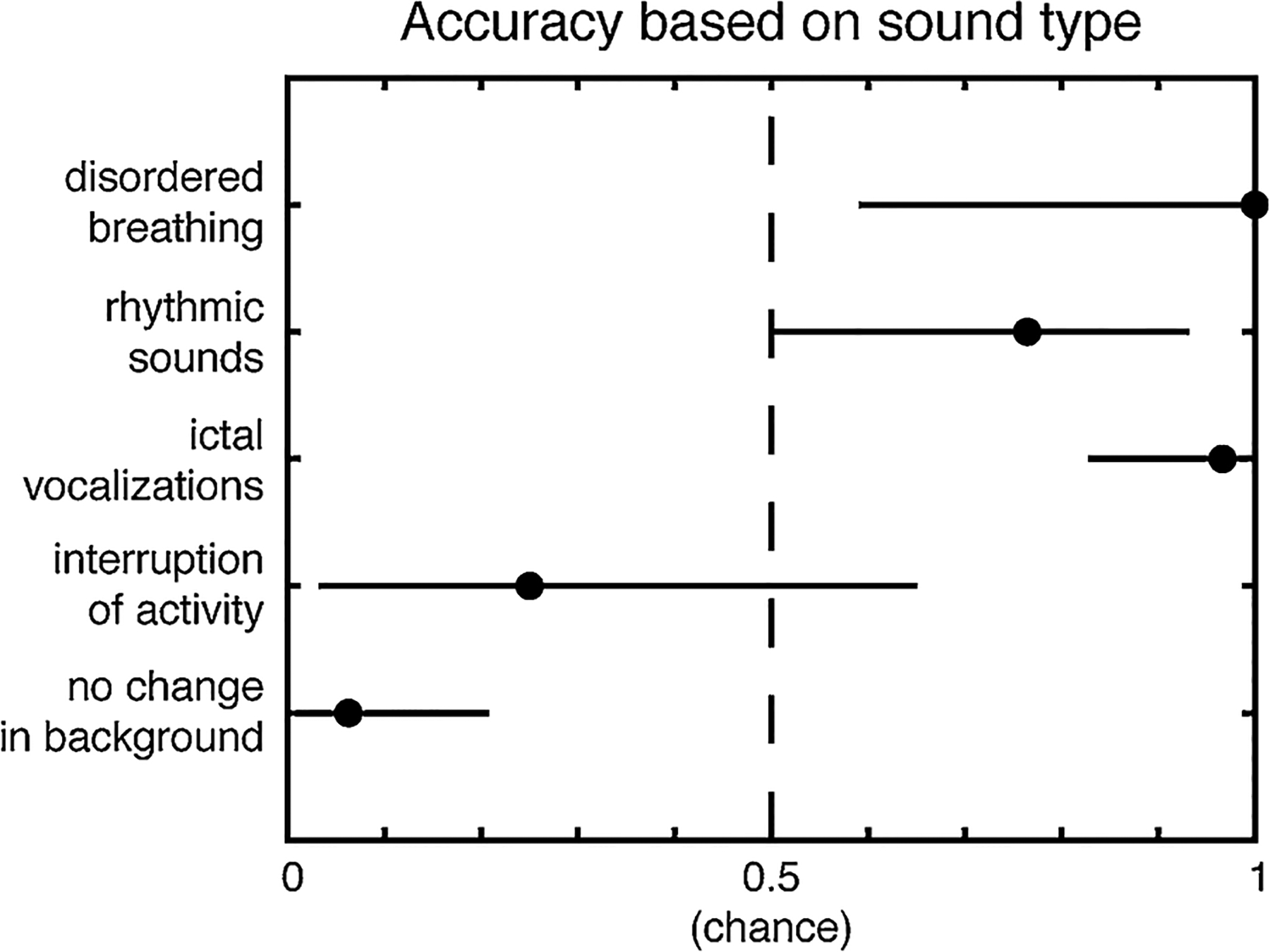
Accuracy of consensus response based on sound type. The accuracy and 95 % CI are plotted. The sound types that were accurately detected as seizures include disordered breathing, rhythmic sounds, and ictal vocalizations, while interruption of activity and no change in the background were not useful in identifying seizures.
4. Discussion
This study is a phase I feasibility study aimed at determining whether seizures can be detected by their sound with reasonable sensitivity and specificity. Our results show that epileptologists can accurately identify certain seizure types based on their sound alone, specifically hyperkinetic seizures and tonic-clonic seizures, with a respective PPV of 0.92 and 1.00, and NPV of 0.86 and 1.00. These seizure types are typically associated with disordered breathing, rhythmic sounds, and ictal vocalizations, which are the sound types associated with accurate identification of seizures. Audio review failed to accurately identify seizures with non-motor manifestations or those with automatisms only. Automatisms and behavior arrest are generally quiet, which explains the difficulty in detecting them by audio alone. If prior to the seizure the patient was doing an activity that generated audio, such as talking, there would be an interruption of that activity. However, this interruption was typically too subtle to be interpreted as seizure activity and thus was not an informative sound type in our experimental paradigm for detecting seizures. For other motor types, such as atonic, clonic, myoclonic, and tonic seizures, the number of audio clips with these seizure types were too limited to form valid conclusions. Psychogenic seizures could not be accurately identified by their sound, due to the variable behavioral semiology and therefore variable sound types. The consensus rated 50 % of the psychogenic seizure clips as seizure. Our study was not designed to distinguish between psychogenic seizure and epileptic seizures as the raters were instructed to rate psychogenic seizures as seizure. Psychogenic seizures are a potential limitation for an audio based seizure detector, but is a potential limitation for seizure detectors utilizing other non-EEG based mechanisms as well.
As the goal of our study was to assess the feasibility of using audio as a detection modality for seizures, we are unable to directly compare our results to the performance of other device modalities. Our results are from blinded human reviewers rather than a device or algorithm, and the ratio of our seizure to non-seizure clips is 1:1, but in a realistic setting the ratio would be much lower. We suspect that if a more realistic ratio was used, we would have a lower positive predictive value (as with our data set there is a 50 % chance that a positive result is truly a seizure, while in a realistic setting the probability of seizure is much lower) and a higher negative predictive value (similarly because in a realistic setting the probability of non-seizure is much higher). A truly realistic ratio would not be practical in our study because it would be too time consuming for our human reviewers to review continuous audio recordings.
Additional limitations with our data set include the EMU environment where the audio clips were obtained. Although our audio clips were not obtained from the typical environment of a person with epilepsy, the results are generalizable, as our audio clips focused on the sound of the seizure itself, rather than the environment. In order to generate audio clips that most represented the sound from a seizure rather than from the environment, we excluded any clip where a spoken phrase would make it obvious that a seizure was occurring (e.g., someone was saying “are you okay,” “seizure,” etc.). We expect the PPV to be higher if audio information from the patient or witnesses stating a seizure was happening was utilized. We excluded any audio with disruptive hospital sounds, because in the normal environment of a person with epilepsy we would not have alarms to indicate that a seizure was occurring. Thus, any seizure that caused vital sign alarms were excluded, many of which were motor seizures which caused tachycardia or significant change in the respiratory rate or pulse oximeter reading. In addition, the majority of the patients were lying or sitting in bed when the seizure occurred, thus we had no seizures that occurred while the patient was standing. The PPV could potentially be higher as audio of a patient changing from standing to another position could generate sounds that are informative that a seizure occurred. Conversely, our non-seizure control clips were taken when most patients were sedentary, and it is possible a more active patient could generate sounds that could be mistaken for seizures, potentially increasing the false alarm rate. In addition, our criteria for selecting the non-seizure clips (60 s prior to the seizure) was designed to represent an unbiased random selection of activities as seizures occur unexpectedly, however this has the potential to exclude activities which could be confused for seizures.
Our audio was obtained from clinical video-EEG systems in place at our EMU. A limitation of using audio recorded from this video-EEG monitoring systems was that the sound quality was not optimal for detecting subtle sounds. An audio-based seizure detection device would potentially be designed to record high quality audio and could therefore pick up subtle seizure sounds, such as from automatisms or tonic seizures which could not be picked up in our current study. An addition, an audio-based seizure detection device would utilize sound frequency analysis and machine-learning algorithms to detect features which the human ear may not appreciate.
Taking the above limitations into account, a rough comparison of the overall performance of our epileptologists for detecting seizures based on their sound shows that it may be comparable to other seizure detection devices, which typically have a higher performance for tonic-clonic seizures than other seizure types. Typical outcome measures reported in seizure detection device literature include sensitivity and false alarm rate [22]. Recent publications on seizure detection devices showed an EMG based device called SeizureLink with a sensitivity of 0.94 and false alarm rate of 0.67/day for detecting tonic-clonic seizures [23], a multimodal device utilizing movement and heart rate sensors called Nighwatch with a sensitivity of 0.86 and false alarm rate of 0.03/night for detecting multiple motor seizure types [24], and a multimodal wrist device utilizing movement and electrodermal sensors called Embrace with a sensitivity of 95 % and false alarm rate of 0.2/day for detecting tonic-clonic seizures [25]. Our epileptologist performed with a low sensitivity of 0.51 across multiple seizure types, however this is likely due to seizures that do not have an identifiable sound. When focusing on high confidence responses, which likely reflects seizures that do have an identifiable sound, the sensitivity improves to 0.82. In addition, when focusing on tonic-clonic seizures, our epileptologists are able to achieve a sensitivity of 1.00. Unfortunately, we are unable to calculate a true false alarm rate due to the limitations of our data set as described above. However, as our data showed 4 false positives over 83 non-seizure clips (equivalent to 1-specificity), and our clips were 30 s in duration, if we roughly extrapolated this to a 24 h period, this would suggest an unacceptably high false alarm rate.
5. Conclusion
Our phase I feasibility study shows that an epileptologist can successfully identify certain seizure types based on sound. It is possible that an audio-based seizure detection device could do so as well. This provides support to further develop audio-based seizure detection devices or to use audio-based seizure detection in conjunction with other seizure detection modalities.
Supplementary Material
Acknowledgements
Author JS acknowledges the Finding a Cure for Epilepsy and Seizures (FACES) Foundation. Author RSF acknowledges the James and Carrie Anderson Epilepsy Research Fund, the Maslah Saul MD Chair, and the Steve Chen Epilepsy Research Fund. Author BALM acknowledges Career Development Award number IK2 CX-001255-01 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R &D (CSRD) Service. Author AAL acknowledges Award Number K23-NS 104252 from the National Institutes of Health.
Funding
JS is funded by the Finding a Cure for Epilepsy and Seizures (FACES) Foundation.
Footnotes
Declaration of Competing Interest
Author DF receives salary support for consulting and clinical trial related activities performed on behalf of The Epilepsy Study Consortium, a non-profit organization. DF receives no personal income for these activities. NYU receives a fixed amount from the Epilepsy Study Consortium towards DF’s salary. Within the past year, The Epilepsy Study Consortium received payments for research services performed by DF from: Adamas, Axcella, Biogen, Crossject, Engage Pharmaceuticals, Eisai, GW Pharmaceuticals, Pfizer, SK Life Science, Takeda, Xenon, and Zynerba. DF has also served as a paid consultant for Eisai. DF has received travel support from Medtronics, Eisai and the Epilepsy Foundation. DF receives research support from the CDC, NINDS, Epilepsy Foundation, Epitel, and Neuropace. DF serves on the scientific advisory board for Receptor Life Sciences. DF holds equity interests in Neuroview Technology and Receptor Life Sciences. Author RSF has done consulting for Medtronic and has stock options in Smart-Watch, Avails Medical, Cerebral Therapeutics, Zeto, Irody, Eysz. Author PD receives research support from the NIH and NeuroPace, Inc. PD has received honoraria for educational materials from NeuroPace, Inc. and travel reimbursement from Medtronic and NeuroPace, Inc. The remaining authors have no conflicts of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.seizure.2020.03.008.
References
- [1].Ryvlin P, Ciumas C, Wisniewski I, Beniczky S. Wearable devices for sudden unexpected death in epilepsy prevention. Epilepsia 2018;59:61–6. 10.1111/epi.14054. [DOI] [PubMed] [Google Scholar]
- [2].Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology 1996;47:260–4. 10.1212/WNL.47.1.260. [DOI] [PubMed] [Google Scholar]
- [3].Ramgopal S, Thome-Souza S, Jackson M, Kadish NE, Sánchez Fernández I, Klehm J, et al. Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav 2014;37:291–307. 10.1016/j.yebeh.2014.06.023. [DOI] [PubMed] [Google Scholar]
- [4].Ulate-Campos A, Coughlin F, Gaínza-Lein M, Fernández IS, Pearl PL, Loddenkemper T. Automated seizure detection systems and their effectiveness for each type of seizure. Seizure 2016;40:88–101. 10.1016/j.seizure.2016.06.008. [DOI] [PubMed] [Google Scholar]
- [5].Aghaei-Lasboo A, Fisher RS. Methods for measuring seizure frequency and severity. Neurol Clin 2016;34:383–94. 10.1016/j.ncl.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [6].Jory C, Shankar R, Coker D, McLean B, Hanna J, Newman C. Safe and sound? A systematic literature review of seizure detection methods for personal use. Seizure 2016;36:4–15. 10.1016/j.seizure.2016.01.013. [DOI] [PubMed] [Google Scholar]
- [7].Kurada AV, Srinivasan T, Hammond S, Ulate-Campos A, Bidwell J. Seizure detection devices for use in antiseizure medication clinical trials: a systematic review. Seizure 2019;66:61–9. 10.1016/j.seizure.2019.02.007. [DOI] [PubMed] [Google Scholar]
- [8].Beniczky S, Jeppesen J. Non-electroencephalography-based seizure detection. Curr Opin Neurol 2019;32:198–204. 10.1097/WCO.0000000000000658. [DOI] [PubMed] [Google Scholar]
- [9].Corbishley P, Rodriguez-Villegas E. Breathing detection: towards a miniaturized, wearable, battery-operated monitoring system. IEEE Trans Biomed Eng 2008;55:196–204. 10.1109/TBME.2007.910679. [DOI] [PubMed] [Google Scholar]
- [10].de Bruijne GR, Sommen PCW, Aarts RM. Detection of epileptic seizures through audio classification. IFMBE Proc. 22 2009. p. 1450–4. 10.1007/978-3-540-89208-3_344. [DOI] [Google Scholar]
- [11].Carlson C, Arnedo V, Cahill M, Devinsky O. Detecting nocturnal convulsions: efficacy of the MP5 monitor. Seizure 2009;18:225–7. 10.1016/j.seizure.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [12].Arends JB, van Dorp J, van Hoek D, Kramer N, van Mierlo P, van der Vorst D, et al. Diagnostic accuracy of audio-based seizure detection in patients with severe epilepsy and an intellectual disability. Epilepsy Behav 2016;62:180–5. 10.1016/j.yebeh.2016.06.008. [DOI] [PubMed] [Google Scholar]
- [13].Elzawahry H, Do CS, Lin K, Benbadis SR. The diagnostic utility of the ictal cry. Epilepsy Behav 2010;18:306–7. 10.1016/j.yebeh.2010.04.041. [DOI] [PubMed] [Google Scholar]
- [14].Laskowitz DT, Sperling MR, French JA, O’Connor MJ. The syndrome of frontal lobe epilepsy: characteristics and surgical management. Neurology 1995;45:780–7. 10.1212/WNL.45.4.780. [DOI] [PubMed] [Google Scholar]
- [15].Janszky J Are ictal vocalisations related to the lateralisation of frontal lobe epilepsy? J Neurol Neurosurg Psychiatry 2000;69:244–7. 10.1136/jnnp.69.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Horvath RA, Fogarasi A, Schulz R, Perlaki G, Kalmar Z, Tóth V, et al. Ictal vocalizations occur more often in temporal lobe epilepsy with dominant (left-sided) epileptogenic zone. Epilepsia 2009;50:1542–6. 10.1111/j.1528-1167.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- [17].Hartl E, Knoche T, Choupina HMP, Rémi J, Vollmar C, Cunha JP, et al. Quantitative and qualitative analysis of ictal vocalization in focal epilepsy syndromes. Seizure 2018;60:178–83. 10.1016/j.seizure.2018.07.008. [DOI] [PubMed] [Google Scholar]
- [18].Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol 2013;12:563–71. 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- [19].Velez M, Fisher RS, Bartlett V, Le S. Tracking generalized tonic-clonic seizures with a wrist accelerometer linked to an online database. Seizure 2016;39:13–8. 10.1016/j.seizure.2016.04.009. [DOI] [PubMed] [Google Scholar]
- [20].Fulton S, Van Poppel K, McGregor A, Ellis M, Patters A, et al. Prospective study of 2 bed alarms for detection of nocturnal seizures. J Child Neurol 2013;28:1430–3. 10.1177/0883073812462064. [DOI] [PubMed] [Google Scholar]
- [21].Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia 2017;58:522–30. 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- [22].Beniczky S, Ryvlin P. Standards for testing and clinical validation of seizure detection devices. Epilepsia 2018;59:9–13. 10.1111/epi.14049. [DOI] [PubMed] [Google Scholar]
- [23].Beniczky S, Conradsen I, Henning O, Fabricius M, Wolf P. Automated real-time detection of tonic-clonic seizures using a wearable EMG device. Neurology 2018. 10.1212/WNL.0000000000004893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arends J, Thijs RD, Gutter T, Ungureanu C, Cluitmans P, Van Dijk J, et al. Multimodal nocturnal seizure detection in a residential care setting. Neurology 2018;91:e2010–9. 10.1212/WNL.0000000000006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Onorati F, Regalia G, Caborni C, Migliorini M, Bender D, Poh MZ, et al. Multicenter clinical assessment of improved wearable multimodal convulsive seizure detectors. Epilepsia 2017;58:1870–9. 10.1111/epi.13899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


