Abstract
Objectives
To examine the association between maternal body mass index (BMI) and gestational weight gain (GWG) and adverse birth outcomes in HIV-infected and -uninfected women.
Methods
In an urban South African community, 2921 consecutive HIV-infected and -uninfected pregnant women attending primary health care services were assessed at their first antenatal visit. A subset of HIV-infected women enrolled in a longitudinal study was assessed three times during pregnancy. All women had birth outcome data from medical records and study questionnaires. In analyses, the associations between BMI, GWG, maternal factors and adverse birth outcomes were assessed with logistic regression models.
Results
The estimated pre-pregnancy BMI median was 29 kg/m2 (IQR, 24–34) overall,29 kg/m2 (IQR, 24–34) for HIV-uninfected and 28 kg/m2 (IQR, 24–34) for HIV-infected women; HIV prevalence was 38%. In adjusted models, increased BMI in the overall cohort was positively associated with age, haemoglobin and parity at first antenatal visit. Maternal obesity was associated with increased likelihood of having high birthweight (aOR 2.54, 95% CI 1.39–4.66) and large size for gestational age (aOR 1.66, 95% CI 1.20–2.31) infants. In the subset cohort, GWG was associated with increased likelihood of spontaneous preterm delivery (aOR 4.35, 95% CI 1.55–12.21) and high birth weight (aOR 3.00, 95% CI 1.22–7.34) infants.
Conclusion
Obesity during pregnancy is prevalent in this setting and appears associated with increased risk of adverse birth outcomes in both HIV-infected and -uninfected women. Weight management interventions targeting women of child-bearing age are needed to promote healthy pregnancies and reduce adverse birth outcomes.
Keywords: Body mass index, gestational weight gain, preterm delivery, birthweight, size for gestational age
INTRODUCTION
Despite a significant reduction of maternal, neonatal and child mortality, South Africa and other sub-Saharan African (SSA) countries failed to meet the Millennium Development Goal targets in 2015 (1–3). To achieve the third Sustainable Development Goal, which aims to ‘Ensure healthy lives and promote well-being for all at all ages’ by 2030, accelerated efforts are required, especially in SSA. Poor maternal health is directly responsible for poor child health outcomes (4), one preventable risk factor that can be targeted to improve child health is obesity, which is on the rise in SSA (5, 6). A recent WHO report classed South Africa as the unhealthiest country globally, with obesity being one of the main contributing factors (7). The steady rise in obesity is partly attributed to changing lifestyle from consumption of traditional foods to a high fat and high sugar diet, and an increased sedentary lifestyle (8, 9). South Africa has the largest prevalence of obesity in SSA, especially in women of child-bearing age (18 – 44 years) (10, 11).
In pregnancy, obesity (≥30 kg/m2) in the first trimester and high GWG (per IOM guidelines for different BMI categories) have been shown to be independently associated with adverse birth outcomes (12–16). Women with either high BMI or high GWG are likely to have high birthweight (HBW, >4000 g) and large size for gestational age (LGA, >90th centile) infants (17–20). There is some evidence from South Africa to suggest that obese women tend to gain more weight during pregnancy than those with normal BMI (21, 22), suggesting an even higher risk of adverse birth outcomes compared to normal weight women. Increased BMI has also been associated with low birthweight (LBW, <2500 g), small size for gestational age (SGA, <10th centile) and preterm delivery (PTD, <37 weeks) (23–26); high GWG similarly increases risk of PTD in overweight and obese women (27). Adding complexity to the association between high BMI and adverse birth outcomes is HIV infection. This double burden poses a challenge in understanding the association between high BMI and adverse birth outcomes as HIV has also been shown to be associated with adverse birth outcomes (12, 28, 29).
Given the high prevalence of both obesity and HIV infection in women of child-bearing age in South Africa, further research to identify and quantify the association between maternal factors and overweight/obesity and high GWG and associated adverse birth outcomes in this population is needed. We take advantage of a large population-based study of HIV-infected and -uninfected women attending antenatal care (ANC) clinic in Gugulethu, Cape Town to provide further insight in this issue.
METHODS
Consecutive pregnant women seeking ANC services at the Gugulethu Community Health Centre were enrolled into a prospective cohort between April 2015 and October 2016 and followed through May 2018. Gugulethu is a peri-urban community of predominantly low socioeconomic status (30, 31). The study was reviewed and approved by the Faculty of Health Sciences Human Research Ethics Committee of the University of Cape Town and the Institutional Review Board of the University of Southampton. Written informed consent for collection of baseline and obstetric data from medical records was obtained from all participants at enrolment. For the study, consecutive women were enrolled into the overall cohort (n = 3281), with an initial questionnaire and medical record review from the first ANC visit; this cohort was followed subsequently via medical records only. A subset cohort (n = 552) of the overall cohort, those who made their first ANC visit at ≤24 weeks’ gestation and were HIV-infected, was enrolled into a prospective cohort with intensive measurements throughout pregnancy to 12 months postpartum. For the overall cohort, we excluded women with missing BMI as a result of having neither weight nor height measurements at first ANC visit (n = 316, 9.8%); and for the subset cohort we excluded women with missing GWG as a result of having one weight measurement (n = 73, 13.4%) (Figure 1). Analyses included women aged ≥18 years with pregnancy losses (stillbirth/miscarriages) and live singleton births (overall cohort n = 2921 and subset cohort n = 471). There were 91 women who had pregnancy losses in the overall cohort, with 44 in the subset cohort. Stillbirth/miscarriage are combined in analyses of BMI for the overall cohort due to small numbers. All 44 women in subset with pregnancy loss could not be included in the GWG analysis as no further weight data were available for them.
Figure 1.

Flow diagram showing the selection of women included in the overall cohort of HIV-infected and -uninfected women (A) assessed for BMI (body mass index) and the subset cohort of HIV-infected women (B) assessed for GWG (gestational weight gain)
For the overall cohort, weight and height measurements were performed by health practitioners as part of routine procedures at first ANC visit. Using standard GWG charts a correction factor on weight measured at first ANC visit was applied to estimate pre-pregnancy BMI using a method described by Santos et al (32). Briefly, using international standards for GWG in pregnancy (32), the median weight gained for each week of gestation was subtracted from the weight measured at first ANC visit based on BMI category as GWG differs according to pre-pregnancy BMI. Using this corrected weight, the estimated pre-pregnancy BMI was then calculated and categorised as underweight (<18.5), normal (18.5 – 24.9), overweight (25 – 29.9) and obese (≥30) in kg/m2). Baseline blood pressure (BP) and haemoglobin measurements were performed by nurses as part of routine care at first ANC visit. For the subset cohort, weight measurements at antenatal study visits were collected by a trained study nurse using a calibrated scale (Charder, Taichung City, Taiwan) accurate to within 0.5 kg; height measurements were taken to the nearest 0.1 cm using a stadiometer (Seca, Birmingham, United Kingdom). These measurements were used to calculate weekly maternal GWG by dividing the weight change between enrolment and last study visit by the number of weeks elapsed between the two (29), and expressed as kg/week.
For the overall cohort, GA at first ANC visit was measured by health practitioners using last menstrual period (LMP n = 1385, 47%) and symphysis-fundal height (SFH n = 92, 3%) as part of routine care. For all HIV-infected women in the subset cohort and 973 (33%) women of the remainder of the overall cohort, GA at first ANC visit was measured by ultrasonography (USS) operated by an experienced sonographer. For both cohorts, delivery GA was calculated by adding the number of weeks elapsed between enrolment and delivery dates to the GA measured at first ANC visit. Delivery GA was categorised as term (≥37) and preterm (PTD, <37) deliveries in weeks (33). Preterm PTD was separated into spontaneous and provider-initiated PTD since they have distinct causes and divergent risk factors. Spontaneous PTD was defined as onset of labour and spontaneous vaginal delivery at less than 37 weeks GA, while provider-initiated PTD was defined as delivery at less than 37 weeks after induction of labour or by caesarean section.
Infant birth weight and sex were obtained from medical records. Infant birth weight was categorised as low (<2500), normal (2500 – 4000) and high (>4000) birthweight in grams (34). Size for gestational age was obtained based on infant GA, birthweight and sex using INTERGROWTH-21st tool; and was categorised as small (<10th), appropriate (10 – 90th) and large (>90th) for GA in percentiles (35). Stillbirth was defined as delivery at ≥28 weeks and miscarriage as delivery at <28 weeks (36). Socio-demographic information was obtained from medical records for the overall cohort and additional information was obtained from face-to-face interview-administered questionnaires for the subset cohort of HIV-infected women. Socioeconomic status of the subset cohort was based on a composite score that included level of education, employment status, type of housing and whether there is a toilet, running water, electricity, fridge, telephone and television inside the house (37).
Statistical Analysis
All data were analysed using STATA version 14.0, (Stata Corporation, College Station, TX, USA). Baseline characteristics were stratified by maternal BMI and differences between groups were compared using Chi-Squared test for categorical variables and Wilcoxon rank-sum test for continuous variables. To assess potential risk factors for increased maternal BMI (in the overall cohort) and GWG (in the subset cohort), we used univariate and multivariate linear regression models. To assess the associations between adverse birth outcomes and BMI/GWG, logistic regression was used for two-level pregnancy outcome categories (livebirth and stillbirth/miscarriage) and multinomial logistic regression was used for three-level GA at delivery (spontaneous PTD, term delivery – base, provider-initiated PTD), birthweight (LBW, NBW-base, HBW) and size for GA (SGA, AGA-base, LGA) categories. Results are presented as adjusted odds ratios (aOR) and related 95% confidence intervals (CI). The adjusted multiple logistic regression models included a priori confounders such as maternal age, parity and HIV status (38). Because maternal BMI and GWG were not normally distributed, we used robust standard errors in all regression models.
RESULTS
Body mass index
Data on 2921 women in the overall cohort with live singleton births and stillbirth/miscarriage were analysed (Figure 1). The mean age was 28 years, 24% of women were primigravid and the median BMI was 29 kg/m2 (IQR, 24–34). Overall, 38% of the cohort were HIV-infected, and there were 84 (3%) underweight, 822 (28%) normal weight, 718 (25%) overweight and 1297 (44%) obese women. Of the 1297 obese women, 812 (46%) were HIV-uninfected and 485 (42%) were HIV-infected. Table 1 shows maternal baseline characteristics overall and stratified by BMI category. Overweight and obese women were likely to be older and had higher gravidity and parity than normal BMI women. Women with normal BMI were more likely to be moderately anaemic and to present late for first ANC visit compared to those who were obese.
Table 1.
Maternal baseline characteristics, overall and by maternal BMI in women with live singleton births, overall cohort
| BMI (n = 2921) | ||||||
|---|---|---|---|---|---|---|
| Total n = 2921 |
Underweight n = 84 |
Normal n = 822 |
Overweight n = 718 |
Obese n = 1297 |
Chi2 p-value |
|
| Age (years) | <0.001 | |||||
| <20 | 294 (10) | 19 (23) | 146 (18) | 70 (10) | 59 (5) | |
| 20–24 | 619 (21) | 23 (27) | 223 (27) | 156 (22) | 217 (17) | |
| 25–29 | 836 (29) | 23 (27) | 213 (26) | 217 (030) | 383 (30) | |
| 30–34 | 729 (25) | 18 (21) | 172 (21) | 173 (24) | 366 (28) | |
| ≥35 | 443 (15) | 1 (1) | 68 (8) | 102 (14) | 272 (21) | |
| Median (IQR) | 28 (23–32) | 25 (21–28) | 25 (22–30) | 28 (23–32) | 29 (25–34) | |
| Height (cm) | 0.392 | |||||
| <155 | 990 (34) | 22 (26) | 275 (33) | 233 (32) | 460 (35) | |
| 156–161 | 1086 (37) | 35 (42) | 296 (36) | 261 (36) | 494 (38) | |
| ≥162 | 820 (28) | 27 (32) | 242 (29) | 208 (29) | 343 (26) | |
| Median (IQR) | 158 (154–162) | 159 (156–163) | 158 (154–162) | 158 (154–163) | 158 (154–162) | |
| HIV status | 0.279 | |||||
| HIV-uninfected | 1779 (61) | 48 (57) | 499 (61) | 420 (59) | 812 (63) | |
| HIV-infected | 1142 (39) | 36 (43) | 323 (39) | 298 (42) | 485 (37) | |
| Haemoglobin (g/dL) | <0.001 | |||||
| Normal (≥11.0) | 1124 (38) | 28 (33) | 257 (31) | 275 (38) | 564 (43) | |
| Mild anaemia (10–10.9) | 552 (19) | 13 (15) | 173 (21) | 133 (19) | 233 (18) | |
| Moderate anaemia (7–9.9) | 490 (17) | 20 (24) | 190 (23) | 121 (17) | 159 (12) | |
| Severe anaemia (<7) | 5 (0.2) | 0 | 4 (0.5) | 0 | 1 (0.1) | |
| Median (IQR) | 11 (10–11.9) | 10.7 (9.3–11.4) | 10.7 (9.7–11.6) | 11 (10–11.9) | 11.2 (10.3–12) | |
| Blood pressure (mmHg) | 0.343 | |||||
| Normotensive (<140/90) | 2770 (95) | 82 (98) | 796 (97) | 687 (96) | 1205 (93) | |
| Hypertensive (≥140/90) | 64 (2) | 2 (2) | 14 (2) | 13 (2) | 35 (3) | |
| Median SBP (IQR) | 113 (106–122) | 106 (100–117) | 109 (102–117) | 112 (106–120) | 116 (109–125) | |
| Median DBP (IQR) | 67 (60–74) | 64 (57–73) | 65 (59–71) | 67 (60–73) | 69 (62–77) | |
| GA at first ANC visit (weeks) | <0.001 | |||||
| 1st trimester (≤13) | 789 (27) | 14 (17) | 183 (22) | 208 (29) | 384 (30) | |
| 2nd trimester (14–28) | 1706 (58) | 51 (61) | 487 (59) | 423 (59) | 745 (57) | |
| 3rd trimester (>28) | 426 (15) | 19 (23) | 152 (18) | 87 (12) | 168 (13) | |
| Median (IQR) | 19 (13–25) | 24 (17–28) | 20 (14–27) | 18 (13–25) | 18 (13–24) | |
| Gravidity (number) | <0.001 | |||||
| 1 | 707 (24) | 33 (39) | 270 (33) | 189 (26) | 215 (17) | |
| 2 | 991 (34) | 30 (36) | 290 (35) | 233 (32) | 438 (34) | |
| ≥3 | 1221 (42) | 21 (25) | 262 (32) | 295 (41) | 643 (50) | |
| Median (IQR) | 2 (2–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (2–3) | |
| Parity (number) | <0.001 | |||||
| 0 | 861 (29) | 37 (44) | 326 (40) | 231 (32) | 267 (21) | |
| 1 | 1088 (37) | 30 (36) | 294 (36) | 245 (34) | 519 (40) | |
| ≥2 | 970 (33) | 17 (20) | 202 (25) | 241 (34) | 510 (39) | |
| Median (IQR) | 1 (0–2) | 1 (0–1) | 1 (0–1) | 1 (0–2) | 1 (1–2) | |
| Prior preterm delivery# (<37 weeks GA) | 0.514 | |||||
| Yes | 224 (8) | 8 (10) | 57 (7) | 48 (7) | 111 (9) | |
Among women with a previous pregnancy
SBP - systolic blood pressure, DBP - diastolic blood pressure, GA - gestational age, ANC - antenatal care
Table 2 shows the associations between various risk factors and BMI as a continuous variable. In models adjusted for maternal characteristics, increased BMI was significantly associated with higher age, higher haemoglobin, higher parity, lower height and being HIV-uninfected. Table 3 presents results for the associations between BMI and birth outcomes using four separate models for PTD, birthweight, size for GA and stillbirth/miscarriage. Logistic regression was used for two-level pregnancy loss categories and multinomial logistic regression was used for three-level PTD, birthweight and size for GA categories. Results from the four models adjusted for maternal characteristics show that obese women were more likely to have HBW (aOR 2.54, 95% CI 1.39–4.66) and LGA (aOR 1.66, 95% CI 1.20–2.31) infants. In agreement, birthweight distribution stratified by BMI showed a dose-dependent shift to the right for obese women (Figure 2). Consequently, obese women were less likely to have LBW (aOR 0.60, 95% CI 0.44–0.82) and SGA (aOR 0.57, 95% CI 0.41–0.79) infants; they were also less likely to have spontaneous PTD (aOR 0.65, 95% CI 0.43–0.99) and had a trend towards increased likelihood of having provider-initiated PTD (aOR 1.74, 95% CI 0.94–3.21). No associations were noted between obesity and stillbirth/miscarriage (aOR 0.67, 95% CI 0.37–1.23). Associations for all outcomes assessed were similar for overweight women but did not reach statistical significance consistently. For PTD outcome, a sensitivity analysis assessing possible bias introduced by using USS dating only for the subset cohort showed no changes on the direction of the association between obesity and spontaneous/provider-initiated PTD for LMP/SFH/USS (Table S4).
Table 2.
Linear regression of maternal BMI and maternal characteristics in the overall cohort (n = 2921)
| Unadjusted Models | Adjusted Models | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Age (year) | 0.32 (0.27, 0.36) | <0.001 | 0.29 (0.23, 0.36) | <0.001 |
| Height (cm) | −0.14 (−0.24, −0.04) | 0.007 | −0.18 (−0.30, −0.05) | 0.005 |
| Hb (g/dL) | 0.85 (0.63, 1.07) | <0.001 | 0.80 (0.58, 1.03) | <0.001 |
| HIV status | ||||
| HIV-uninfected | 1.00 | 1.00 | ||
| HIV-infected | −0.66 (−1.22, −0.10) | 0.020 | −0.85 (−1.49, −0.21) | 0.009 |
| Blood pressure (mmHg) | ||||
| Normotensive | 1.00 | 1.00 | ||
| Hypertensive | 1.50 (−0.48, 3.48) | 0.139 | 1.56 (−0.64, 3.75) | 0.164 |
| GA at first ANC visit (weeks) | −0.06 (−0.10, −0.03) | <0.001 | −0.02 (−0.06, 0.02) | 0.313 |
| Parity (number) | 1.34 (1.04, 1.64) | <0.001 | 0.48 (0.07, 0.88) | 0.020 |
| Gravidity (number) | 1.18 (0.92, 1.44) | <0.001 | −0.15 (−0.75, 0.45) | 0.625 |
Hb - haemoglobin, GA - gestational age, ANC - antenatal care
All adjusted models include variables significantly different at baseline and in univariate analysis: maternal age, height, Hb, HIV status and parity. Missing data, n (%): Height n=750 (0.3), Blood pressure n=87 (0.1), Parity and Gravidity n=2 (0.0). Where data are missing on predictors, cases were excluded on the regression.
Table 3.
Association between adverse birth outcomes and BMI in the overall cohort
| N (%) N = 2921 |
Unadjusted Models | Adjusted Models | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | ||
| Delivery GA (weeks) | |||||
| Spontaneous PTD (<37 weeks GA) | 293 (10.0) | ||||
| Normal | 102 (12.4) | 1.00 | 1.00 | ||
| Underweight | 16 (19.0) | 1.71 (0.95, 3.09) | 0.076 | 1.69 (0.70, 4.11) | 0.244 |
| Overweight | 75 (10.5) | 0.87 (0.63, 1.19) | 0.381 | 0.96 (0.62, 1.49) | 0.870 |
| Obese | 100 (7.7) | 0.63 (0.47, 0.85) | 0.002 | 0.65 (0.43, 0.99) | 0.043 |
| Provider-Initiated PTD (<37 weeks GA) | 188 (6.4) | ||||
| Normal | 40 (4.9) | 1.00 | 1.00 | ||
| Underweight | 4 (4.8) | 1.09 (0.38, 3.15) | 0.874 | 1.54 (0.34, 6.95) | 0.572 |
| Overweight | 47 (6.6) | 1.39 (0.89, 2.14) | 0.144 | 1.37 (0.68, 2.76) | 0.377 |
| Obese | 97 (7.5) | 1.56 (1.07, 2.29) | 0.022 | 1.74 (0.94, 3.21) | 0.077 |
| Birth weight (g) | |||||
| Low (<2500) | 385 (13.2) | ||||
| Normal | 129 (15.7) | 1.00 | 1.00 | ||
| Underweight | 16 (19.1) | 1.29 (0.72, 2.30) | 0.394 | 1.16 (0.57, 2.37) | 0.688 |
| Overweight | 93 (13.0) | 0.84 (0.63, 1.12) | 0.232 | 0.79 (0.56, 1.12) | 0.187 |
| Obese | 147 (11.3) | 0.71 (0.55, 0.92) | 0.009 | 0.60 (0.44, 0.82) | 0.001 |
| High (>4000) | 125 (4.3) | ||||
| Normal | 20 (2.4) | 1.00 | 1.00 | ||
| Underweight | 2 (2.4) | 1.04 (0.24, 4.54) | 0.961 | 0.69 (0.09, 5.42) | 0.722 |
| Overweight | 33 (4.6) | 1.92 (0.09, 3.38) | 0.024 | 2.14 (1.09, 4.18) | 0.027 |
| Obese | 70 (5.4) | 2.19 (1.32, 3.63) | 0.002 | 2.54 (1.39, 4.66) | 0.002 |
| Size for GA (centile) | |||||
| Small (<10th) | 362 (12.3) | ||||
| Normal | 124 (15.1) | 1.00 | 1.00 | ||
| Underweight | 17 (20.2) | 1.64 (0.91, 2.94) | 0.100 | 1.63 (0.81, 3.30) | 0.173 |
| Overweight | 103 (14.4) | 1.02 (0.77, 1.36) | 0.886 | 0.98 (0.70, 1.37) | 0.903 |
| Obese | 118 (9.1) | 0.63 (0.48, 0.83) | 0.001 | 0.57 (0.41, 0.79) | 0.001 |
| Large (>90th) | 378 (12.9) | ||||
| Normal | 81 (9.9) | 1.00 | 1.00 | ||
| Underweight | 8 (9.5) | 1.18 (0.54, 2.58) | 0.681 | 1.15 (0.46, 2.87) | 0.760 |
| Overweight | 79 (11.0) | 1.20 (0.86, 1.67) | 0.285 | 1.27 (0.87, 1.87) | 0.221 |
| Obese | 210 (16.2) | 1.72 (1.31, 2.27) | <0.001 | 1.66 (1.20, 2.31) | 0.002 |
| Pregnancy outcome | |||||
| Stillbirth/Miscarriage | 91 (3.1) | ||||
| Normal | 22 (2.7) | 1.00 | 1.00 | ||
| Underweight | 2 (2.4) | 0.88 (0.20, 3.82) | 0.866 | 0.56 (0.07, 4.23) | 0.575 |
| Overweight | 31 (4.3) | 1.64 (0.94, 2.86) | 0.082 | 0.63 (0.30, 1.31) | 0.219 |
| Obese | 36 (2.8) | 1.04 (0.61, 1.78) | 0.891 | 0.67 (0.37, 1.23) | 0.192 |
OR - odds ratio, GA - gestational age, PTD - preterm delivery
Preterm model: adjusted for maternal age, HIV status, haemoglobin, parity, previous PTD
Birth weight, size for GA and birth outcome models: adjusted for maternal age, HIV status, haemoglobin and parity. Missing data, n (%): GA at delivery n=232 (7.9), Birthweight n=49 (1.7), Size for GA n=232 (7.9), Pregnancy outcome n=16 (0.6). Where data are missing on outcomes, cases were excluded on the regression. Interpretation of OR’s: Compared to normal BMI, underweight/overweight/obese BMI increases (OR>1) or decreases (OR<1) the likelihood of having spontaneous PTD/PI-PTD/LBW/HBW/SGA/LGA/Stillbirth/Miscarriage
Figure 2.
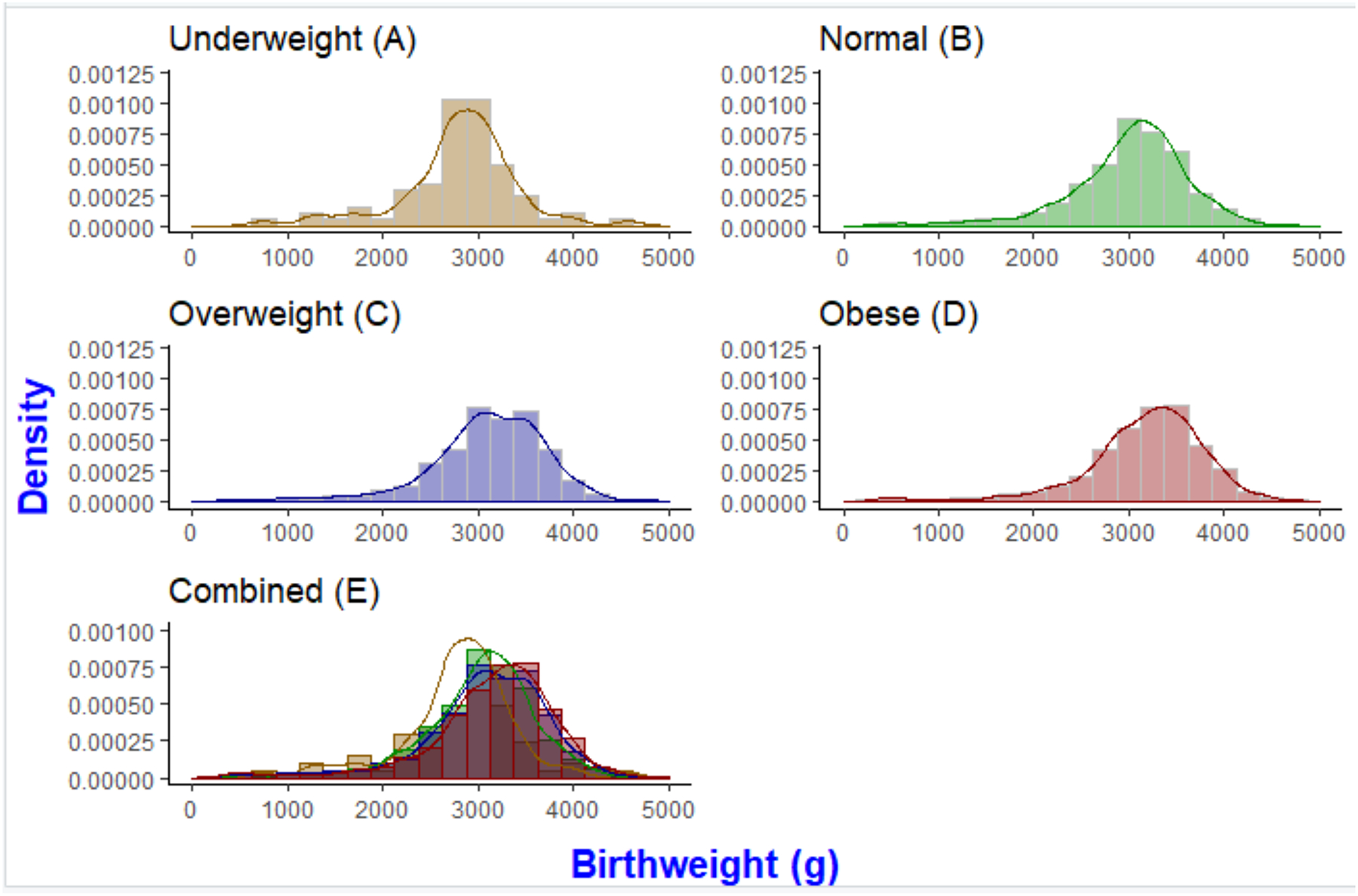
Infant birth weight distribution by underweight (A), normal (B), overweight (C), obese (D) BMI and combined BMI categories (E) in the overall cohort (n = 2921)
Gestational Weight Gain
A total of 471 HIV-infected women who came for their first ANC visit at ≤24 weeks’ gestation and were followed longitudinally in the subset cohort through to delivery and had live singleton births were included in this analysis. Comparison of BMI distribution between the subset cohort and the overall cohort (excluding the subset) showed no significant difference (p=0.275). The mean GWG was 0.27 kg/week and the median GWG was 0.25 kg/week (IQR, 0.11–0.42).
Data on the associations between various baseline risk factors and GWG as a continuous variable are shown in Table 4. The only variable significantly associated with GWG in the subset cohort was the estimated pre-pregnancy BMI, where a one-unit increase in BMI was associated with a decrease in GWG of 0.014 kg/week. Without reaching statistical significance, factors positively associated with GWG in adjusted models included haemoglobin, hypertension, being married, low socioeconomic status, alcohol use, GA at first ANC visit and CD4 cell count; factors negatively associated were age, height, ART-initiation before pregnancy, having completed high school, high socioeconomic status, parity and gravidity. Table 5 presents data for the associations between GWG and birth outcomes using three separate models for PTD, birthweight and size for GA. Adjusted models showed that GWG (kg per week) was positively associated with spontaneous PTD (aOR 4.35, 95% CI 1.55–12.21) and HBW (aOR 3.00, 95% CI 1.22–7.34). Associations with provider-initiated PTD were in the negative direction, and in the positive direction for LBW, SGA and LGA, but did not achieve statistical significance.
Table 4.
Linear regression of GWG and maternal characteristics among HIV-infected women with live singleton births, subset cohort (n= 471)
| Unadjusted Models | Adjusted Models | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Age (years) | −0.008 (−0.014, −0.002) | 0.006 | −0.007 (−0.016, 0.003) | 0.156 |
| Height (cm) | −0.001 (−0.006, 0.004) | 0.710 | −0.003 (−0.008, 0.002) | 0.221 |
| BMI (kg/m2) | −0.016 (−0.025, −0.007) | 0.001 | −0.014 (−0.026, −0.001) | 0.030 |
| Hb (g/dL) | 0.005 (−0.028, 0.038) | 0.765 | 0.029 (−0.016, 0.073) | 0.203 |
| Blood pressure (mmHg) | ||||
| Normotensive | 1.000 | 1.000 | ||
| Hypertensive | −0.178 (−0.352, −0.003) | 0.046 | 0.082 (−0.134, 0.298) | 0.457 |
| ART status | ||||
| ART-initiation during pregnancy | 1.000 | 1.000 | ||
| ART-initiation before pregnancy | −0.017 (−0.087, 0.052) | 0.626 | −0.054 (−0.156, 0.049) | 0.304 |
| Marital status | ||||
| Single | 1.000 | 1.000 | ||
| Married | −0.039 (−0.111, 0.034) | 0.293 | 0.037 (−0.052, 0.125) | 0.414 |
| Education (Grade) | ||||
| Not completed high school | 1.000 | 1.000 | ||
| Completed high school | −0.001 (−0.082, 0.079) | 0.978 | −0.046 (−0.145, 0.053) | 0.361 |
| Socioeconomic status | ||||
| Medium SES | 1.000 | 1.000 | ||
| Low SES | 0.046 (−0.037, 0.130) | 0.275 | 0.076 (−0.009, 0.161) | 0.080 |
| High SES | −0.013 (−0.103, 0.077) | 0.777 | −0.043 (−0.141, 0.056) | 0.392 |
| Alcohol use | ||||
| No alcohol use | 1.000 | 1.000 | ||
| Alcohol use | −0.013 (−0.088, 0.063) | 0.742 | 0.020 (−0.079, 0.120) | 0.689 |
| GA at first ANC visit (weeks) | 0.003 (−0.003, 0.009) | 0.350 | 0.002 (−0.006, 0.010) | 0.567 |
| Parity (number) | −0.002 (−0.005, 0.001) | 0.196 | −0.020 (−0.095, 0.055) | 0.600 |
| Gravidity (number) | −0.023 (−0.055, 0.009) | 0.159 | −0.007 (−0.069, 0.084) | 0.852 |
| CD4 cell count (cells/μl) | 0.001 (−0.001, 0.002) | 0.567 | 0.001 (−0.001, 0.002) | 0.691 |
Hb - haemoglobin, SES - Socioeconomic status, GA - gestational age, ANC - antenatal care
All adjusted models include variables significantly different at baseline and on univariate analysis: maternal age, BMI, haemoglobin, parity and CD4 cell count. Missing data, n (%): Height n=2 (0.004), BMI and Marital status n=3 (0.01), Hb n=109 (0.2), Blood pressure n=13 (0.03), Education n=1 (0.002) SES n=11 (0.02), Alcohol use, Parity and Gravidity n=5 (0.01), GA at first ANC n=14 (0.03) CD4 count n=94 (0.2). Where data are missing on predictors, cases were excluded on the regression.
Table 5.
Association between adverse birth outcomes and GWG among HIV-infected women with live singleton births in the subset cohort
| Unadjusted Models | Adjusted Models | ||||
|---|---|---|---|---|---|
| N (%) N = 471 |
OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Delivery GA (weeks) | |||||
| Term (≥37) | 416 (88.3) | 1.00 | 1.00 | ||
| Spontaneous PTD (>37) | 19 (4.0) | 1.37 (0.11, 17.13) | 0.808 | 4.35 (1.55, 12.21) | 0.005 |
| Provider-initiated PTD (>37) | 33 (7.0) | 0.92 (0.36, 2.39) | 0.869 | 0.70 (0.14, 3.55) | 0.670 |
| Birth weight (g) | |||||
| Normal (2500–4000) | 374 (79.4) | 1.00 | 1.00 | ||
| Low (<2500) | 69 (14.7) | 1.38 (0.53, 3.58) | 0.506 | 1.90 (0.70, 5.15) | 0.209 |
| High (>4000) | 19 (4.0) | 3.19 (1.29, 7.91) | 0.012 | 3.00 (1.22, 7.34) | 0.016 |
| Size for GA (centile) | |||||
| Appropriate (10–90th) | 352 (74.7) | 1.00 | 1.00 | ||
| Small (<10th) | 78 (16.6) | 1.14 (0.58, 2.24) | 0.708 | 1.15 (0.55, 2.41) | 0.706 |
| Large (>90th) | 35 (7.4) | 2.19 (0.99, 4.87) | 0.054 | 2.03 (0.87, 4.73) | 0.100 |
OR - odds ratio, GA - gestational age, PTD - preterm delivery
Preterm model adjusted for maternal age, ART status, haemoglobin, parity, previous PTD
Birth weight, size for GA and birth outcome models adjusted for maternal age, ART status, haemoglobin and parity. Missing data, n (%): GA at delivery n=3 (0.6), Birthweight n=9 (1.9), Size for GA n=6 (1.3). Where data are missing on outcomes, cases were excluded on the regression. Interpretation of OR’s: Increase in GWG increases (OR>1) or decreases (OR<1) the likelihood of having spontaneous PTD/PI-PTD/LBW/HBW/SGA/LGA compared to respective reference categories.
DISCUSSION
Using a large population-based cohort of HIV-infected and -uninfected pregnant women, we found that maternal obesity was associated with high risk of HBW and LGA infants. Further, in the subset cohort of HIV-infected women followed longitudinally through delivery, we found that high GWG was associated with increased risk of spontaneous PTD and HBW infants. In a country with both a high prevalence of obesity in women of child-bearing age (11) and a high prevalence of HIV in pregnant women (39), our findings highlight the need for weight management interventions before and during pregnancy to minimise adverse birth outcomes.
There are two methods that have been used to estimate GWG, namely, total GWG (weight gained from conception to onset of labour) and incremental GWG (rate of weight gain between two weight measurements expressed as per week, month or trimester). The limitation with using total GWG is that preconception weight is not usually available, and researchers rely on self-reported data and measured first trimester weight, the latter has been shown to be inaccurate due to under-reporting and lack of correlation with preconception weight. Alternatively, authors have estimated preconception weight by subtracting a constant of 0.5–1 kg from first trimester weight, this is also inaccurate due to high variability of weight gained between these two timepoints, which also depends on the timing of presenting for the first ANC. The use of incremental GWG, which estimates the rate of weight gain per time period between the first available measure and subsequent measures, is an alternative and generally preferred approach, and has been reported to play an important role in studying pregnancy outcomes (40) and hence was used in this study.
This is one of the few studies demonstrating a very high prevalence of obesity (42%) in HIV-infected pregnant women in sub-Saharan Africa. Historically, most studies that investigated maternal BMI and adverse birth outcomes in HIV-infected women focus on low BMI associated with chronic HIV disease, which exacerbated HIV-associated adverse birth outcomes (12, 28, 29). However with the majority of HIV-infected people using ART in keeping with recent international policy shifts, our findings reflect recent advances in weight changes in HIV-infected women where obesity is more prevalent (41–43) and affects birth outcomes.
High BMI and GWG are important causes of metabolic dysregulation. In pregnancy, uterine ‘metabolic imprinting’ related to maternal metabolic dysregulation makes the fetus susceptible to adverse birth outcomes and long-term poor health (4). Independently, HIV infection and its treatment may also affect metabolic function (44). However, there are few insights into how the combination of obesity and HIV disease may affect birth outcomes as well as women’s health over long-term. Consistent with our findings - although not consistently significant, others have reported an increased risk of provider-initiated PTD in obese women and increased risk of spontaneous PTD in women with high GWG (15, 45). The mechanisms mediating both types of PTD in women with high BMI are similar to those reported in HIV-infected women using ART – hypertension and diabetes for provider-initiated PTD and elevated inflammatory markers which mediate premature myometrial contractility, cervical dilatation and rupture of amniotic membranes for spontaneous PTD (46, 47). Although both term and PTD infants born from women with high BMI are pre-disposed to childhood obesity and cardiovascular diseases later in life (48), PTD infants are prone to additional developmental challenges including recurrent hospitalisations and neurodevelopmental challenges associated with impaired organ function (33, 49, 50).
While there are conflicting data regarding the association between high BMI and PTD, there is consensus about the association between high BMI and HBW and LGA infants (17–20). Indeed, we also found that high BMI and GWG are positively associated with HBW. Unlike previous studies which reported inadequate weight gain associated with LBW infants in HIV-infected women (12, 29), we found a strong association between GWG and HBW infants in these women. Although a study conducted in Cameroon did not find any association between GWG and birthweight (51), several other African studies have reported that high GWG reduces the risk of LBW with a parallel increase in risk of HBW, similar to what was observed in our study (12, 29, 52). These differences are possibly due to having healthier women in our cohort where all HIV-infected women were on treatment. Hyperinsulinaemia and hyperglycaemia have been implicated to promote fetal growth in the uterine environment in women with high BMI and GWG (53). Consequently, women with high BMI and GWG tend to have large babies who are permanently conditioned to experience long-term health problems related to metabolic dysregulation. Multiple studies have shown that HBW infants experience obesity and related cardio-metabolic abnormalities as early as 3 years of age and this worsens through adolescence to adulthood (54–56). Considering that female infants are genetically prone to increased weight gain regardless of maternal BMI, those born from obese mothers may face additional risks of weight-related morbidities, creating a vicious generational cycle of obesity among mothers and daughters.
Not surprisingly, we found that obese BMI is associated with reduced risk of LBW and SGA in both HIV-infected and -uninfected women. HIV-infected women with normal BMI have been reported to be at high risk of LBW/SGA (29); our results suggest that high BMI in HIV-infected women changes the direction of the relationship between HIV infection and LBW/SGA. However, in some cases obese women are likely to have LBW infants due to intrauterine growth restriction as a result of insufficient nutrients delivered to the fetus (23, 25). Although this may be plausible, especially because obese women tend to have poor micronutrient intake (57), this was not the case in our cohort as we found that increased BMI was associated with increased Hb, suggesting absence of iron-deficiency.
With the high rate of obesity in women of child-bearing age, identification of risks factors driving high BMI and related GWG in this group is critical for targeting interventions towards curbing the epidemic and associated adverse birth outcomes. In the overall cohort, maternal BMI was positively and significantly associated with age, haemoglobin and parity. In the subset cohort of HIV-infected women, risks factors that showed a trend of increased risk (not statistically significant) of GWG included haemoglobin, hypertension, being married, low socioeconomic status, alcohol use, GA at first ANC visit and CD4 cell count. These results shed light on the type of women in which weight management programs should be targeted to before and during pregnancy in low-resource settings to minimise PTD, HBW and LGA infants. We also provide evidence that risk factors of high BMI and GWG are not all in the same direction such as age and parity, reflecting the independency of these health determinants.
These findings should be interpreted in light of certain limitations. Our investigations were conducted in a cohort of women presenting for first ANC visit at varying gestations and therefore we estimated pre-pregnancy BMI using international standard developed for HIV-uninfected women. Our participants included HIV-infected women and these standards may not be completely applicable to them, however there are no GWG standards available for HIV-infected women and so whether there are differences in GWG in this population remains unclear. The limited sample size in the subset cohort may have resulted in non-significant results for the association of GWG and maternal characteristics as well some adverse birth outcomes. Finally, the use of HIV-infected women in the subset cohort may have resulted in selection bias and hence our results for GWG may not be generalisable to the general public, especially because we did not have a control group of HIV-uninfected women.
In conclusion, we found a high prevalence of obesity in HIV-infected and -uninfected women in our setting. High BMI was associated with increased risk of HBW and LGA infants; and GWG in HIV-infected women was also associated with high risk of HBW and spontaneous PTD. While it appears that high BMI reduces the risk of LBW and SGA, this may depend on the nutritional status of the women. These findings demonstrate the need to integrate weight management interventions with routine care to support women at high risk of high BMI and GWG to have healthy pregnancies.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD080385. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Statistics South Africa. Millennium Development Goals: Country report 2015. Pretoria: South Africa: Statistics South Africa; 2015:1–157. [Google Scholar]
- 2.United Nations. Lessons learned in implementing MDGs: assessing progress in Africa toward the Millennium Development Goals. Addis Ababa: Ethiopia: Economic Commission for Africa; 2015:1–112. [Google Scholar]
- 3.English M, English R, English A. Millennium Development Goals progress: a perspective from sub-Saharan Africa. Arch Dis Child 2015;100(Suppl 1):S57–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vézina LA, Nicklas TA, Baranowski T. Intergenerational effects of health issues among women of childbearing age: A review of the recent literature. Curr Nutr Rep 2018;7(4):274–85. [DOI] [PubMed] [Google Scholar]
- 5.Popkin BM. The nutrition transition in low income countries: an emerging crisis. Nutr Rev 1994;52:285–98. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Obesity. Reginal office for Africa; 2017. https://afro.who.int/health-topics/obesity (Accessed 24 March 2019). [Google Scholar]
- 7.Brief M. SA ranked as the unhealthiest nation on earth in new index. Med Brief 2019:1–2. [Google Scholar]
- 8.Agyemang C, Boatemaa S, Agyemang FG, et al. Obesity in sub-Saharan Africa. Chapter 8. SpringerLink Metab Syndr 2016:41–53. [Google Scholar]
- 9.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol 2005;35(1):93–9. [DOI] [PubMed] [Google Scholar]
- 10.Pillay-van Wyk V, Msemburi W, Laubscher R, et al. Second national burden of disease study South Africa: national and subnational mortality trends, 1997–2009. Lancet 2013;381:S113. [Google Scholar]
- 11.South African Demographic Health Survey. Key Indicators Report 2016. Statistics South Africa 2017: 1–76. [Google Scholar]
- 12.Mehta S, Manji KP, Young AM, et al. Nutritional indicators of adverse pregnancy outcomes and mother-to-child transmission of HIV among HIV-infected women. Am J Clin Nutr 2008;87(6):1639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyoke CA, Ugwu GO, Ezugwu FO, et al. Retrospective cohort study of the effects of obesity in early pregn ancy on maternal weight gain and obstetric outcomes in an obstetric population in Africa. Int J Womens Health 2013;5:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salihu HM, Lynch ON, Alio AP, Liu J. Obesity subtypes and risk of spontaneous versus medically indicated preterm births in singletons and twins. Am J Epidemiol 2008;168(1):13–20. [DOI] [PubMed] [Google Scholar]
- 15.Wise LA, Palmer JR, Heffner LJ, et al. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiol 2010;21(2):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 17.Kamanu C, Onwere S, Chigbu B, et al. Fetal macrosomia in African women: a study of 249 cases. Arch Gynaecol Obstet 2009;279(6):857–61. [DOI] [PubMed] [Google Scholar]
- 18.Liu KC, Joseph JA, Nkole TB, et al. Predictors and pregnancy outcomes associated with a newborn birth weight of 4000 g or more in Lusaka, Zambia. Int J Gynaecol Obstet 2013;122(2):150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudet L, Ferraro ZM, Wen SW, et al. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int 2014;2014:640291–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Liu E, Guo J, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One 2013;8(12):e82310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrottesley S, Pisa P, Norris S. The influence of maternal dietary patterns on body mass index and gestational weight gain in urban black South African women. Nutrients 2017;9(7):732–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruger HS. Pregnancy outcomes of overweight and normal weight women in a South African outpatient clinic. J Hum Ecol 2005;13:61–8. [Google Scholar]
- 23.Anderson SM, Naidoo RN, Ramkaran P, et al. OGG1 Ser326Cys polymorphism, HIV, obesity and air pollution exposure influences adverse birth outcome susceptibility, within South African Women. Reprod Toxicol 2018;79:8–15. [DOI] [PubMed] [Google Scholar]
- 24.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309(22):2362–70. [DOI] [PubMed] [Google Scholar]
- 25.McDonald SD, Han Z, Mulla S, et al. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010;341:c3428–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slyker JA, Patterson J, Ambler G, et al. Correlates and outcomes of preterm birth, low birth weight, and small for gestational age in HIV-exposed uninfected infants. BMC Pregnancy Childbirth 2014;14:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nohr EA, Bech BH, Vaeth M, et al. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol 2007;21(1):5–14. [DOI] [PubMed] [Google Scholar]
- 28.Villamor E, Dreyfuss ML, Baylin A, et al. Weight loss during pregnancy is associated with adverse pregnancy outcomes among HIV-1 infected women. J Nutr 2004;134(6):1424–31. [DOI] [PubMed] [Google Scholar]
- 29.Young S, Murray K, Mwesigwa J, et al. Maternal nutritional status predicts adverse birth outcomes among HIV-infected rural Ugandan women receiving combination antiretroviral therapy. PLoS One 2012;7(8):e41934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.City of Cape Town. City of Cape Town - 2011 census suburb Gugulethu. Stats SA 2011: 1–7 http://resource.capetown.gov.za/2011_Census_CT_Suburb_Nyanga_Profile.pdf (Accessed 27 February 2019). [Google Scholar]
- 31.Gadama LA. Adverse perinatal events observed in obese pregnant women in the Metro West Region. MMed Thesis, University of Cape Town 2014; 1–65. [Google Scholar]
- 32.Santos S, Eekhout I, Voerman E, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med 2018;16(1):201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Preterm birth. Switzerland, Geneva: 2018. http://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed 05 March 2019). [Google Scholar]
- 34.Ota E, Haruna M, Suzuki M, et al. Maternal body mass index and gestational weight gain and their association with perinatal outcomes in Viet Nam. Bull World Health Organ 2011;89:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villar J, Ismail LC, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384(9946):857–68. [DOI] [PubMed] [Google Scholar]
- 36.WHO. Neonatal and perinatal mortality: country, regional and global estimates. Switzerland, Geneva: 2006. https://apps.who.int/iris/handle/10665/43444 (Accessed 13 January 2020). [Google Scholar]
- 37.Myer L, Stein DJ, Grimsrud A, et al. Social determinants of psychological distress in a nationally-representative sample of South African adults. Soc Sci Med 2008;66(8):1828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oteng-Ntim E, Kopeika J, Seed P, et al. Impact of obesity on pregnancy outcome in different ethnic groups: calculating population attributable fractions. PLoS One 2013;8(1):e53749–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyun V, Brittain K, Phillips TK, et al. Prevalence and determinants of unplanned pregnancy in HIV-positive and HIV-negative pregnant women in Cape Town, South Africa: a cross-sectional study. BMJ Open 2018;8(4):e019979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilmore LA, Redman LM. Weight gain in pregnancy and application of the 2009 IOM guidelines: toward a uniform approach. Obes 2015;23(3):507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guehi C, Badjé A, Gabillard D, et al. High prevalence of being overweight and obese HIV-infected persons, before and after 24 months on early ART in the ANRS 12136 Temprano Trial. AIDS Res Ther 2016;13(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasse B, Iff M, Ledergerber B, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis 2014;1(2):ofu040–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor BS, Liang Y, Garduño LS, et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr 2014;65(2):e33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overton ET. Metabolic complications of HIV infection and its therapies. Top Antivir Med 2014;22(3):651–654. [PMC free article] [PubMed] [Google Scholar]
- 45.Silva FP, Souza RT, Cecatti JG, Passini R, Tedesco RP, Lajos GJ, et al. Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Scientific reports. 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallenstein MB, Jelliffe-Pawlowski LL, Yang W, et al. Inflammatory biomarkers and spontaneous preterm birth among obese women. J Matern Fetal Neonatal Med 2016;29(20):3317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Current HIV/AIDS Reports. 2016;13(5):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasylyeva TL, Barche A, Chennasamudram SP, et al. Obesity in prematurely born children and adolescents: follow up in pediatric clinic. Nutr J 2013;12(1):150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García-Basteiro AL, Quinto L, Macete E, et al. Infant mortality and morbidity associated with preterm and small-for-gestational-age births in Southern Mozambique: a retrospective cohort study. PLoS One 2017;12(2):e0172533–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gladstone M, White S, Kafulafula G, et al. Mortality, morbidity and developmental outcome after ultrasound-dated preterm birth in a rural sub-Saharan African setting. Arch Dis Child 2011;96(Suppl 1):A4–A100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fouelifack FY, Fouedjio JH, Fouogue JT, et al. Associations of body mass index and gestational weight gain with term pregnancy outcomes in urban Cameroon: a retrospective cohort study in a tertiary hospital. BMC Res Notes 2015;8(1):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onwuka C, Ugwu E, Onah H, et al. Patterns of gestational weight gain and its association with birthweight in Nigeria. Niger J Clin Pract 2017;20(6):754–60. [DOI] [PubMed] [Google Scholar]
- 53.Acosta O, Ramirez VI, Lager S, et al. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol 2015;212(2):227 e1–e7. [DOI] [PubMed] [Google Scholar]
- 54.Oken E, Taveras EM, Kleinman KP, et al. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196(4):322 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taveras EM, Rifas-Shiman SL, Belfort MB, et al. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatr 2009;123(4):1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li N, Liu E, Sun S, et al. Birth weight and overweight or obesity risk in children under 3 years in China. Am J Hum Biol 2014;26(3):331–6. [DOI] [PubMed] [Google Scholar]
- 57.de Luis DA, Pacheco D, Izaola O, et al. Micronutrient status in morbidly obese women before bariatric surgery. Surg Obes Relat Dis 2013;9(2):323–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


