Abstract
Objectives
Despite immune checkpoint inhibitor (ICI) approval for metastatic renal cell carcinoma (mRCC) in 2015, cytoreductive nephrectomy (CN) is guided by extrapolation from earlier classes of therapy. We evaluated survival outcomes, timing, and safety of combining CN with modern immunotherapy (IO) for mRCC.
Methods
From 96,329 renal cancer cases reported to the NCDB between 2015–2016, we analyzed 391 surgical candidates diagnosed with clear cell mRCC treated with IO±CN and no other systemic therapies. Primary outcome was overall survival (OS) stratified by the per formance of CN (CN+IO vs. IO alone). Secondary outcomes included OS stratified by the timing of CN, pathologic findings, and perioperative outcomes.
Results
Of 391 patients, 221 (56.5%) received CN+IO and 170 (43.5%) received IO only. Across a median follow-up of 14.7 months, patients who underwent CN+IO had superior OS (median NR vs. 11.6 mos.; HR 0.23, p<0.001), which was upheld on multivariable analyses. IO before CN resulted in lower pT stage, grade, tumor size, and lymphovascular invasion rates compared to upfront CN. Two of 20 patients (10%) undergoing CN post-IO achieved complete pathologic response in the primary tumor (pT0). There were no positive surgical margins, 30-day readmissions, or prolonged length-of-stay in patients undergoing delayed CN.
Conclusion
Using a large, national, registry-based cohort, we provide the first report of survival outcomes in mRCC patients treated with CN combined with modern IO. Our findings support an oncologic role for CN in the ICI era and provide preliminary evidence regarding the timing and safety of CN relative to IO administration.
Keywords: cytoreductive nephrectomy, immunotherapy, survival, metastatic renal cell carcinoma
INTRODUCTION
Kidney cancer accounts for nearly 15,000 deaths annually in the United States (U.S.).1 At diagnosis, approximately 30–40% of patients already harbor metastatic disease,2 and 5-year survival rates for these patients have traditionally been dismal, ranging from 0–20%.3–5 Recently, immune checkpoint inhibitors (ICI) have revolutionized the contemporary management of immunogenic malignancies, including metastatic renal cell carcinoma (mRCC). The seminal CheckMate 025 trial heralded the modern immunotherapy (IO) era for mRCC resulting in the approval of the first ICI agent (nivolumab) to treat mRCC in 2015.6 Thereafter, several combination ICI regimens gained frontline approval based on results of the CheckMate 214 (nivolumab and ipilimumab),7 KEYNOTE-426 (pembrolizumab and axitinib),8 and JAVELIN Renal 101 (avelumab and axitinib)9 trials.
With this new class of therapy, the role for surgically removing the primary tumor remains unknown. The first trials to investigate the benefit of offering cytoreductive nephrectomy (CN) in mRCC were conducted in the cytokine era.10,11 A combined analysis of these trials, which compared interferon-alpha (IFN-α) alone versus IFN-α plus CN, revealed a 31% decrease in the risk of death and a median survival advantage of 5.8 months in support of CN.12 Survival benefits with CN in the targeted era have also been seen in multiple retrospective studies, including population-based analyses of the Surveillance, Epidemiology, and End Results (SEER) registry and National Cancer Database (NCDB),13–17 along with a meta-analysis.18
The recent randomized, phase III CARMENA trial, however, challenged this notion by demonstrating non-inferiority of sunitinib alone compared to sunitinib combined with CN.19 While interpretation of the results from CARMENA is nuanced, the trial highlights the evolving question of when—and for whom—CN is indicated. Furthermore, as ICIs increasingly gain popularity among medical oncologists over sunitinib as the standard-of-care for treating mRCC, the relevance of the CARMENA trial in the ICI era is questionable. Without any published data regarding the role of combining CN with ICI, current clinical practice is guided by extrapolation from these earlier studies.
Thus, there is an urgent clinical need to elucidate the role, candidacy, and timing of offering CN to patients treated with ICI. Herein, using the NCDB, we provide the first report of survival outcomes in mRCC patients treated with CN combined with modern IO approaches versus IO alone. Among patients who received CN, we also evaluate the timing of CN in relation to IO administration and the safety of CN after IO.
METHODS
Data Source
The NCDB is sponsored by the American College of Surgeons (ACS) and the American Cancer Society, and it collects data on malignancies from ACS-Commission on Cancer (CoC)-accredited facilities. It includes approximately 70% of all malignancies diagnosed in the U.S. from over 1,500 facilities.20–22 Abstractors are trained using standard methodology to collect data on patient demographics, tumor characteristics, treatments, and survival data.22 Institutional review board approval was waived because NCDB data are deidentified for both patient and facility.
Study Population
There were 96,329 cases of renal cancer reported to the NCDB between 2015 and 2016. As ICI approval for mRCC was first granted in 2015,6 cases preceding 2015 were excluded from the initial pre-screened cohort. We performed aconservative selection process for our final analysis cohort (Supplementary Figure S1). The International Classification of Disease for Oncology 3rd edition was used to identify patients diagnosed with predominant clear cell RCC histology (codes 8005, 8310, 8312, and 8316), and only patients metastatic at diagnosis were included. Receipt of IO was required for inclusion, and patients who received any non-IO systemic therapies were excluded to eliminate any potential confounding effects on survival outcomes. As a surrogate for surgical candidacy, patients with a Charlson-Deyo comorbidity score >2 were also excluded. CN was identified by surgery primary-site codes (40, 50, 70, and 80), which records primary surgical treatment at any CoC-affiliated hospital. After exclusions, our final cohort consisted of 391 patients diagnosed with clear cell mRCC between 2015–2016 who were treated with IO +/− CN and no other systemic therapies.
Variables
Variables of interest included patient demographics, performance of CN, presence of sarcomatoid features, primary tumor size, cT stage, cN stage, presence of bone, brain, liver, or lung metastases, number of known metastatic sites, and time to receipt of IO from diagnosis. Among patients who underwent CN, additional variables of interest included performance of lymph node dissection, Fuhrman grade, pT stage, pN stage, lymphovascular invasion (LVI), time to surgery from diagnosis, surgical margin status, inpatient length-of-stay (LOS) for the operative admission, 30-day readmission rates following surgery, and timing of CN in relation to IO administration (IO administered before CN (delayed CN) versus upfront CN before IO).
Clinical staging was identified by the American Joint Committee (AJCC) cancer staging manual, 7th edition. When reported, differences between the clinical (cT) stage at diagnosis and pathologic (pT) stage of the primary tumor were compared. Pathologic downstaging was defined as any improvement in pT substage relative to cT substage, and pathologic upstaging was defined as any worsening in pT substage relative to cT substage. Complete pathologic response of the primary tumor was defined as pT0 stage. Vitality status was reported only for patients diagnosed in 2015, as follow-up data for 2016 diagnoses remains immature.
Outcomes and Statistical Analyses
The primary endpoint was overall survival (OS), stratified by the performance of CN (CN+IO versus IO alone). Baseline clinicopathologic characteristics were compared between the two cohorts using chi-square and independent-sample Mann-Whitney U tests for categorical and continuous variables, respectively. Missing data were excluded from comparative analyses. OS was compared between the two cohorts using Kaplan-Meier methods, and differences were analyzed with the log-rank statistic. Clinicopathologic predictors for OS were assessed using multivariable Cox regression analyses. Multivariable analyses incorporated models involving a priori selection of clinically-relevantcovariates and contingent selection of covariates based on significance in univariable analysis.
Secondarily, we sought to evaluate whether the timing of IO administration in relation to CN impacts OS, pathologic downstaging, and perioperative outcomes. Patients who received CN were stratified according to the timing of first IO administration (before versus after CN).
Clinicopathologic characteristics, surgical margin status, perioperative outcomes, and OS were compared between groups. Predictors for OS among patients who received CN+IO were identified using multivariable Cox regression analyses.
All statistical analyses were conducted using SPSS version 25.0 (IBM, Armonk, NY). Two-sided statistical significance was defined as p<0.05.
RESULTS
Impact of CN in patients treated with IO
Our final cohort consisted of 391 patients (183 diagnosed in 2015, 208 diagnosed in 2016), including 221 (56.5%) who received CN+IO and 170 (43.5%) who received IO only (Table 1). Patients who received CN were younger; baseline demographics and Charlson-Deyo comorbidity score were otherwise similar between groups. Patients who underwent CN also had a larger median primary tumor size. The frequency of clinically positive nodes and hepatic metastasis was higher in the IO only group; however, the frequency of brain, bone, and pulmonary metastases was comparable between groups, with no significant difference in the number of known metastatic sites. Sarcomatoid features were present in 22 patients (5.6%) and similarly distributed between groups.
Table 1.
Baseline clinicopathologic characteristics of the entire IO cohort, stratified by receipt of CN.
| IO only | CN + IO | p-value* | |
|---|---|---|---|
| Number of patients | 170 | 221 | - |
|
Diagnosis year [# (% for each year)] −2015 −2016 |
69 (37.7%) 101 (48.6%) |
114 (62.3%) 107 (51.4%) |
0.032 |
| Median age (IQR), yrs. | 64 (57–72) | 57 (51–64) | <0.001 |
| Male sex (%) | 70.6 | 75.6 | 0.299 |
|
Race (%)
-White -Black -Hispanic -Asian/Other |
82.9 6.5 8.8 1.8 |
85.1 4.5 6.8 3.6 |
0.489 |
|
Charlson-Deyo comorbidity score (%)
−0 −1 −2 |
74.1 20.6 5.3 |
80.5 14.9 4.5 |
0.302 |
| Presence of sarcomatoid features (%) | 6.5 | 5.0 | 0.659 |
| Median primary tumor size (IQR), cm | 8.0 (5.8–11.0) | 9.7 (7.4–12.0) | <0.001 |
|
cT stage (%)
-cT0 -cT1 -cT2 -cT3 -cT4 -cTx |
1.8 22.4 22.4 22.4 9.4 21.8 |
0 15.4 35.7 35.7 5.0 8.1 |
0.001 |
|
cN stage (%) -cN0 -cN1 -cNx |
52.9 31.7 12.9 |
67.0 24.4 8.6 |
0.015 |
|
Presence of bone metastases (%)
-Yes -No -Unknown |
36.5 52.3 11.2 |
33.0 61.1 5.9 |
0.271 |
|
Presence of brain metastases (%)
-Yes -No -Unknown |
10.0 78.2 11.8 |
6.3 88.3 5.4 |
0.132 |
|
Presence of liver metastases (%) -Yes -No -Unknown |
20.0 67.6 12.4 |
7.7 86.9 5.4 |
<0.001 |
|
Presence of lung metastases (%) -Yes -No -Unknown |
58.8 29.4 11.8 |
66.5 27.6 5.9 |
0.421 |
|
Presence of bone, liver, or brain metastases (%) -Yes -No -Unknown |
52.9 36.5 10.6 |
42.1 52.5 5.4 |
0.008 |
|
Number of known metastatic sites (%)
−1 −2 −3 −4 -No bone, brain, liver, or lung involvement -No information |
48.8 27.1 5.9 1.2 6.5 10.6 |
59.3 20.8 3.6 0.5 10.4 5.4 |
|
| Median time to receipt of IO from diagnosis (IQR), days | 51 (32–82) | 91 (59–119) | <0.001 |
P-values are 2-sided and calculated by chi-square or Mann-Whitney U tests for categorical or continuous variables, respectively, after exclusion of missing values. P<0.05 indicates significance.
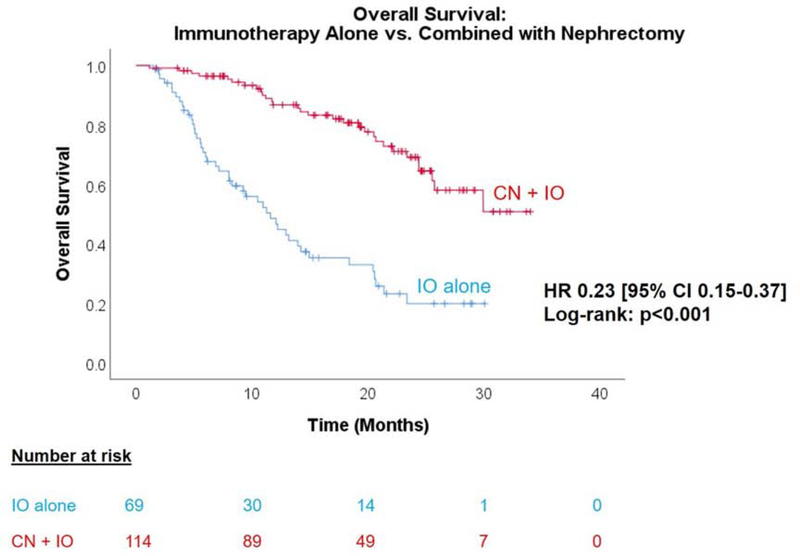
After a median follow-up of 14.7 months among the 183 patients with available outcomes data (2015 diagnoses), there were 75 overall deaths (41%). Patients who underwent CN+IO had significantly better OS than those who received IO alone (HR 0.23 [95% CI 0.15–0.37], Figure 1). Median OS was not reached in the CN+IO group, compared to 11.6 months in the IO-only group (p<0.001).
Figure 1.
Kaplan-Meier analysis of OS in mRCC patients treated with IO, stratified by receipt of CN. Median OS for CN+IO was not reached, versus 11.6 months (95% CI 8.5–14.7 months) for IO alone (HR 0.23 [95% CI 0.15–0.37], log-rank p<0.001).
To account for confounding variables known to affect survival in mRCC, we performed multivariable Cox regression analyses to identify predictors of OS using a model defined a priori and a separate model in which selected covariates were contingent on significance in univariable analysis (Table 2). On univariable analysis, we found that the presence of hepatic metastases predicted significantly worse OS (HR 2.68, p=0.002). On multivariable analyses, however, receipt of CN remained the strongest and only independent predictor for OS, using both the a priori and contingent models.
Table 2:
Univariable (UVA) and multivariable (MVA) Cox regression analysis to identify predictors for worse OS in the IO cohort with outcomes data available (2015 diagnoses only). Two MVA models were tested, including a “contingent” model developed based on covariates found to be significant on UVA and an “a priori” model based on prespecified covariates. Total mortality events: n=75.
| UVA | “Contingent” MVA | “A priori” MVA | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value* | HR (95% CI) | p-value* | HR (95% CI) | p-value* | |
| CN performed | 0.23 (0.15–0.37) | <0.001 | 0.24 (0.14–0.41) | <0.001 | 0.22 (0.11–0.42) | <0.001 |
| Age | 1.02 (1.00–1.04) | 0.097 | 1.00 (0.97–1.04) | 0.785 | ||
| Male sex | 0.99 (0.60–1.62) | 0.955 | ||||
|
Race
-White -Black -Hispanic -Asian/Other |
Ref. 1.05 (0.38–2.90) 1.15 (0.53–2.51) 0.69 (0.17–2.82) |
Ref. 0.925 0.731 0.605 |
||||
|
Charlson-Deyo comorbidity score
−0 −1 −2 |
Ref. 1.62 (0.94–2.79) 0.54 (0.13–2.22) |
Ref. 0.082 0.393 |
||||
| Sarcomatoid features | 0.71 (0.18–2.91) | 0.637 | ||||
| Primary tumor size | 1.00 (0.99–1.00) | 0.199 | ||||
| Locally advanced cT stage (cT3–4) | 0.92 (0.54–1.57) | 0.760 | 0.86 (0.43–1.71) | 0.661 | ||
| cN1 stage | 1.23 (0.73–2.06) | 0.432 | 1.17 (0.60–2.28) | 0.642 | ||
| Presence of bone metastases | 1.30 (0.77–2.21) | 0.322 | ||||
| Presence of brain metastases | 1.76 (0.83–3.73) | 0.142 | ||||
| Presence of liver metastases | 2.68 (1.45–4.93) | 0.002 | ||||
| Presence of lung metastases | 0.95 (0.53–1.70) | 0.855 | ||||
| Presence of bone, liver, or brain metastases | 2.01 (1.14–3.52) | 0.015 | 1.62 (0.91–2.87) | 0.098 | 1.20 (0.60–2.38) | 0.607 |
|
Number of known metastatic sites
−1 −2 −3 −4 -No bone, brain, liver, or lung involvement |
1.16 (0.35–3.84) 2.54 (0.76–8.47) 1.70 (0.38–7.60) 7.94 (0.81–77.88) Ref. |
0.813 0.129 0.489 0.075 Ref. |
||||
HR: hazard ratio; CI: confidence interval; UVA: univariable analysis; MVA: multivariable analysis
P<0.05 indicates significance
Impact of CN timing versus IO administration
Next, among patients undergoing CN, we sought to evaluate whether the treatment sequence (CN followed by IO versus IO followed by CN) affected outcomes. Pathologic characteristics and perioperative outcomes of the CN cohort are summarized in Supplementary Table S1. Of the 221 patients, 197 underwent upfront CN, while 24 received IO before CN (including 9 who had continuation of IO following CN). Comparing upfront versus delayed CN groups (Table 3), patients who received IO first tended to be older and more likely to have bone metastases, but pathologically, they tended to have lower Fuhrman grade, smaller tumor size, lower pT stage, and less likelihood of LVI. In contrast, patients who underwent upfront CN were more likely to have pulmonary metastases. Rates of brain, liver, and pathologically positive lymph node (pN1) metastases were similar between groups.
Table 3.
Baseline clinicopathologic characteristics of the patients who underwent CN, stratified by timing of IO administration.
| IO before CN | Upfront CN | p-value* | |
|---|---|---|---|
| Number of patients | 24 | 197 | - |
|
Diagnosis year [# (% for each year)] −2015 −2016 |
13 (11.4%) 11 (10.3%) |
101 (88.6%) 96 (89.7%) |
0.832 |
| Median age (IQR), yrs. | 65 (56–70) | 56 (51–63) | 0.002 |
| Male sex (%) | 70.8 | 76.1 | 0.616 |
|
Race (%)
-White -Black -Hispanic -Asian/Other |
79.2 12.5 8.3 0 |
85.8 3.6 6.6 4.1 |
0.174 |
|
Charlson-Deyo comorbidity score (%)
−0 −1 −2 |
75.0 20.8 4.2 |
81.2 14.2 4.6 |
0.691 |
| Presence of sarcomatoid features (%) | 4.2 | 5.1 | 1.000 |
|
Fuhrman grade (%)
−1 −2 −3 −4 -Unknown |
0 25.0 29.2 8.3 37.5 |
0.5 11.7 34.0 35.0 18.8 |
0.035 |
|
Lymphovascular invasion (%)
-Yes -No -Unknown |
8.3 54.2 37.5 |
34.0 37.6 28.4 |
0.013 |
| Median primary tumor size (IQR), cm | 6.8 (5.0–9.4) | 10.0 (7.6–12.5) | 0.001 |
|
cT stage (%)
-cT1 -cT2 -cT3 -cT4 -cTx |
37.5 16.7 37.5 4.2 4.2 |
12.7 38.1 35.5 5.1 8.6 |
0.012 |
|
pT stage (%)
-pT0 -pT1 -pT2 -pT3 -pT4 -pTx |
8.3 20.8 4.2 41.7 8.3 16.7 |
0 6.1 13.7 69.5 7.6 3.0 |
<0.001 |
|
Difference between initial cT stage and pT stage (%) -pT<cT (pathologic downstaging from diagnosis) -pT = cT (unchanged from diagnosis) -pT>cT (pathologic upstaging from diagnosis) -Unable to determine |
8.3 58.3 16.7 16.7 |
4.6 48.2 37.1 10.2 |
0.156 |
|
cN stage (%) -cN0 -cN1 -cNx |
75.0 20.8 4.2 |
66.0 24.9 9.1 |
0.803 |
|
pN stage (%)
-pN0 -pN1 -pNx |
50.0 8.3 41.7 |
47.7 19.8 32.5 |
0.351 |
|
Presence of bone metastases (%)
-Yes -No -Unknown |
70.8 29.2 0 |
28.4 65.0 6.6 |
<0.001 |
|
Presence of brain metastases (%)
-Yes -No -Unknown |
0 100 0 |
7.1 86.8 6.1 |
0.378 |
|
Presence of liver metastases (%) -Yes -No -Unknown |
8.3 91.7 0 |
7.6 86.3 6.1 |
1.000 |
|
Presence of lung metastases (%) -Yes -No -Unknown |
50.0 50.0 0 |
68.5 24.9 6.6 |
0.030 |
|
Presence of bone, liver, or brain metastases (%)
-Yes -No -Unknown |
70.8 29.2 0 |
38.6 55.3 6.1 |
0.008 |
|
Number of known metastatic sites (%)
−1 −2 −3 −4 -No bone, brain, liver, or lung involvement -No information |
54.2 25.0 8.3 0 12.5 0 |
59.9 20.3 3.0 0.5 10.2 6.1 |
0.727 |
| Median time to surgery from diagnosis (IQR), days | 123 (107–176) | 21 (7–38) | <0.001 |
| Median time to receipt of IO from diagnosis (IQR), days | 51 (40–60) | 92 (68–122) | <0.001 |
|
Surgical margins (%)
-Negative -Positive -Unknown |
91.7 0 8.3 |
82.2 14.7 3.0 |
0.050 |
| Median inpatient post-operative length-of-stay (IQR), days | 4.0 (2.5–4.5) | 3.0 (2.0–5.0) | 0.369 |
|
30-day post-operative readmission (%)
-Yes -No -Unknown |
0 100 0 |
4.6 91.4 4.1 |
0.602 |
P-values are 2-sided and calculated by chi-square or Mann-Whitney U tests for categorical or continuous variables, respectively, after exclusion of missing values. P<0.05 indicates significance.
Two patients with initial clinical stages of cT3a and cT2b achieved complete pathologic response in the primary tumor (pT0) after IO. Pathologic downstaging of the primary tumor was twice as frequent in patients who underwent CN after IO compared to those who underwent upfront CN, whereas pathologic upstaging was more than twice as frequent for the latter, though statistical significance was not achieved (Supplementary Figure S2). Likewise, the pN1 rate was twice as high in patients who did not receive IO before CN, though this was not significant.
The median time to surgery was delayed by 102 days in patients who received IO first, whereas median time to first IO administration was delayed by 41 days in patients who underwent upfront CN. With respect to safety of performing CN after prior IO, there were notably no positive surgical margins, 30-day readmissions, or prolonged LOS in patients undergoing delayed CN (Table 3). Median (IQR) LOS was 4.0 (2.5–4.5) days in patients undergoing CN after IO, compared to 3.0 (2.0–5.0) days in those undergoing upfront CN, p=0.369.
Among patients with outcomes data available, there were 30 deaths (26%) over a median follow-up of 18.9 months, including only 1 death in the delayed CN group (8%) at 21 months after diagnosis and 29 deaths in the upfront CN group (29%). On Kaplan-Meier analysis for OS, although a statistically significant difference was not achieved (Supplementary Figure S3), possibly attributable to low sample size, the median OS for the delayed CN group was not reached, compared to 30 months for the upfront CN group (HR 0.25 [95% CI 0.03–1.83]; log-rank p=0.139). On univariable Cox regression analysis, pN1 stage and the presence of brain metastases were the only significant predictors for worse OS (Table 4). Given the number of events, an a priori multivariable Cox regression model was not deployed to mitigate the risk of overfitting. After adjusting for timing of CN, pN1 stage, and the presence of bone, liver, or brain metastases, no predictors were significant on multivariable analysis.
Table 4.
Univariable (UVA) and multivariable (MVA) Cox regression analysis to identify predictors for worse OS in patients who underwent both IO and CN with outcomes data available (2015 diagnoses only). A “contingent” MVA model was developed based on covariates found to be significant on UVA. No a priori MVA was conducted due to limited mortality events (n=30).
| UVA | MVA | |||
|---|---|---|---|---|
| HR (95% CI) | p-value* | HR (95% CI) | p-value* | |
| Receipt of IO before CN | 0.25 (0.03–1.83) | 0.172 | 0.63 (0.07–5.49) | 0.674 |
| Age | 0.98 (0.94–1.02) | 0.359 | ||
| Male sex | 1.23 (0.54–2.77) | 0.621 | ||
|
Race
-White -Black -Hispanic -Asian/Other |
Ref. Insuff. 1.18 (0.36–3.92) 2.03 (0.47–8.68) |
Ref. Insuff. 0.785 0.340 |
||
|
Charlson-Deyo comorbidity score
−0 −1 −2 |
Ref. 1.26 (0.51–3.10) 0.73 (0.10–5.45) |
Ref. 0.618 0.763 |
||
| Sarcomatoid features | 1.83 (0.25–13.61) | 0.555 | ||
| Fuhrman grade 3–4 | 1.34 (0.45–3.94) | 0.599 | ||
| Lymphovascular invasion | 1.72 (0.75–3.95) | 0.200 | ||
| Primary tumor size | 1.00 (0.99–1.01) | 0.730 | ||
| Lymph node dissection performed | 1.24 (0.59–2.61) | 0.571 | ||
| Locally advanced cT stage (cT3–4) | 0.70 (0.29–1.68) | 0.429 | ||
| Locally advanced pT stage (pT3–4) | 0.80 (0.32–2.00) | 0.628 | ||
| pT ≤ cT (downstaged or stable pT stage) | 0.86 (0.38–1.94) | 0.704 | ||
| cN1 stage | 1.20 (0.52–2.75) | 0.666 | ||
| pN1 stage | 2.70 (1.02–7.17) | 0.046 | 2.80 (0.93–8.39) | 0.066 |
| Presence of bone metastases | 0.93 (0.41–2.13) | 0.867 | ||
| Presence of brain metastases | 3.31 (1.23–8.95) | 0.018 | ||
| Presence of liver metastases | 1.57 (0.46–5.31) | 0.471 | ||
| Presence of lung metastases | 1.38 (0.52–3.70) | 0.522 | ||
| Presence of bone, liver, or brain metastases | 1.51 (0.67–3.41) | 0.321 | 1.81 (0.59–5.61) | 0.303 |
|
Number of known metastatic sites
−1 −2 −3 -No bone, brain, liver, or lung involvement |
0.66 (0.15–3.00) 1.37 (0.29–6.48) 1.68 (0.28–10.16) Ref. |
0.590 0.690 0.572 Ref. |
||
| Positive surgical margin | 0.88 (0.26–2.94) | 0.835 | ||
| Inpatient length-of-stay | 1.11 (0.99–1.25) | 0.064 | ||
| 30-day readmission | 2.09 (0.63–6.98) | 0.229 | ||
HR: hazard ratio; CI: confidence interval; UVA: univariable analysis; MVA: multivariable analysis
P<0.05 indicates significance
DISCUSSION
Using a large, national, registry-based cohort that captures approximately 70% of all malignancies diagnosed in the U.S.,20–22 we provide the first report of survival outcomes in mRCC patients treated with CN combined with modern IO approaches. Patients who received CN+IO exhibited significantly better OS than those who received IO alone. Using multiple multivariable models, receipt of CN remained the strongest and only independent predictor for OS. Furthermore, performing CN after prior IO appeared safe, and these patients demonstrated pathologically favourable tumor stage and grade relative to patients undergoing upfront CN. Delayed initiation of IO by approximately 6 weeks in these patients did not worsen survival outcomes. Taken together, our analyses support an oncologic role for CN in the modern IO era and provide preliminary evidence regarding the timing of CN relative to IO administration.
The theoretical advantages of offering CN in mRCC include the reduction of de novo metastases, facilitation of spontaneous regression, or palliation of malignant symptoms or complications.23 These perceived benefits must be weighed against perioperative morbidity and mortality, delayed receipt of systemic therapies, or adverse impact on immunity, disease progression, or quality-of-life.23 Mechanistically, ICI uniquely block inhibitory signaling mediated through CTLA-4 or PD-1/PD-L1 pathways and, in turn, enhance T-cell activation, proliferation, and infiltration in tumors to elicit an immune-mediated anti-tumoral response.24 Despite the immunomodulation induced by these agents, there were no 30-day readmissions or positive margins in patients who underwent CN after prior IO, and post-operative LOS was similar to treatment-naïve patients, attesting to the surgical safety of this approach. In an earlier institutional series of 11 cases, we similarly found nephrectomy to be both feasible and safe after receipt of ICI based on favorable perioperative outcomes.25
The rationale for combining CN with IO stems from trials conducted in the cytokine era,10–12 which defined the first generation of IO for mRCC. In those trials (SWOG 8949, EORTC 30947), combining CN with IFN-α amounted to a 31% decrease in mortality risk and a median OS advantage of 5.8 months over treatment with IFN-α alone.12 While encouraging, those results afford considerable room for improvement, which may now be possible with the approval of more selective forms of IO. Furthermore, the benefits of combining surgical resection of the primary tumor with contemporary ICI agents have been shown for other immunogenic cancer types, including lung cancer26 and melanoma,27,28 and RCC would be a logical extension. In these studies, ICI administration prior to surgery was safe and associated with favorable pathologic response that correlated with molecular biomarkers. Although such biomarkers cannot be assessed using the NCDB, ongoing and future studies that prospectively evaluate CN after prior ICI provide a unique opportunity to study the treated nephrectomy tissue to identify putative molecular biomarkers.
Among the 20 patients who had pT stage recorded after CN post-IO in the present cohort, 2 (10%) exhibited complete pathologic response in the primary tumor, concordant with our institutional experience.25 Although limited by sample size and lack of pre-treatment pathologic information, the downward stage and grade migration, smaller tumor size at CN, and lower frequency of LVI in patients who underwent delayed versus upfront CN are encouraging. Published literature regarding CN after ICI currently remains limited to case reports.29–31 In these reports, tumors that responded well to ICI tended to be infiltrated with inflammatory cells and high immunohistochemical expression of PD-1, PD-L1, and CD8, while a non-inflamed phenotype was reported in non-responding areas. These case reports attest to not only the feasibility of CN after ICI exposure, but also the potential role of tissue biomarkers in predicting RCC response to ICI. An integration of clinical, genomic, transcriptomic, and/or immunohistochemical expression profiles will likely have an important role in identifying which patients would derive oncologic benefit from ICI combined with locoregional control.
An important aspect to highlight is the critical role of patient selection in offering CN.32 Traditional characteristics associated with better outcomes after CN in the targeted therapy era include good performance status, absence of brain, hepatic, or osseous metastases, absence of sarcomatoid features, and the ability to debulk the majority of the tumor burden.23,33,34 In the CARMENA trial, patients with poorer risk disease and large metastatic burden outside the primary tumor were notably included, which may have contributed to the results of the study.19 As performance status and other variables that comprise validated prognostic variables for mRCC3,35 are not included in the NCDB, we used Charlson-Deyo comorbidity score <2 as a surrogate for surgical candidacy. Notably, the presence of sarcomatoid features, osseous metastases, brain metastases, and known tumor burden were similar between the CN+IO and IO only groups, and on multivariable analysis, none of these variables was significant for poor OS. Hence, there may be a need to revisit criteria for CN candidacy in the ICI era. Future studies will also need to explore the validity of current prognostic models in risk stratifying mRCC patients treated with ICI.
Our findings should be interpreted within the limitations of an observational study design and the limitations of the data captured by the NCDB. Selection bias (evidenced by a skew towards older age and more hepatic and nodal metastases in the IO-only group) and clinical risk stratification (which could not be accommodated by NCDB) could have influenced the decision to offer CN. All attempts to account for observed covariates were made using multivariable analyses, yet there are likely unaccounted confounders. The NCDB does not provide information regarding the type of IO regimen, number of cycles, or duration between the last IO treatment and surgery. Thus, it is not known whether patients received ICI or cytokines, which would have also been classified as IO per the NCDB. Hence, we focused our analysis on “modern IO approaches” (2015–2016) to capture contemporary nationwide strategies, which have largely moved away from cytokine therapy. Unfortunately, follow-up data for patients diagnosed in 2016 is immature, limiting our outcomes analysis to 2015 diagnoses. Future iterations of NCDB are awaited for more mature follow-up data. The NCDB also does not provide information on clinical response/progression or treatment-related adverse events, limiting analytical endpoints. While we employed a rigorous approach to patient selection to minimize heterogeneity, this conservative approach yielded only 24 patients who received IO before CN, thus limiting our ability to draw conclusions on the optimal timing of CN.
Despite these limitations, our national registry-based analysis represents the first contemporary report on oncologic outcomes combining CN with IO in the ICI era for mRCC. Results from ongoing and future clinical trials will be needed to further elucidate the role and timing of CN in the setting of ICI.36 Indeed, with the recent approval of combination ICI and anti-angiogenic regimens, the evolving role of CN will remain a moving target.
CONCLUSION
We use a large, national, registry-based cohort to report on survival outcomes in mRCC patients treated with modern immunotherapy and CN. Using multivariable analyses, patients who received CN with IO had better OS than those treated with IO alone. Performing CN after prior IO appears to be safe with pathologically favourable tumor characteristics. Our results support the role for CN in the modern IO era and call for prospective validation.
Supplementary Material
HIGHLIGHTS.
Using the NCDB, patients undergoing CN with modern IO for mRCC exhibited better OS than those treated with IO alone.
CN after prior IO is safe with pathologically favorabletumor characteristics.
Defining the role for CN in the modern IO era warrants prospective validation.
Acknowledgments
FUNDING
This work was supported, in part, by the Ruth L. Kirschstein National Research Service Award T32 CA136515-09 (N.S.), the University of Texas Southwestern Medical Center Physician Scientist Training Program (N.S.), and Dedman Family Scholarship in Clinical Care (A.B.).
Financial Disclosures/Funding Source: This work was supported, in part, by the Ruth L. Kirschstein National Research Service Award T32 CA136515-09 (N.S.), the University of Texas Southwestern Medical Center Physician Scientist Training Program (N.S.), and Dedman Family Scholarship in Clinical Care (A.B.).
Footnotes
CONFLICTS OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NNA, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. [DOI] [PubMed] [Google Scholar]
- 4.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23(4):832–841. [DOI] [PubMed] [Google Scholar]
- 5.MotzerRJ Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116–1127. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. The New England journal of medicine. 2001;345(23):1655–1659. [DOI] [PubMed] [Google Scholar]
- 11.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet (London, England). 2001;358(9286):966–970. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. The Journal of urology. 2004;171(3):1071–1076. [DOI] [PubMed] [Google Scholar]
- 13.Patel MI, Beattie K, Bang A, Gurney H, Smith DP. Cytoreductive nephrectomy for metastatic renal cell carcinoma: inequities in access exist despite improved survival. Cancer Med. 2017;6(10):2188–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsao CK, Small AC, Kates M, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy in the United States: a SEER analysis. World J Urol. 2013;31(6):1535–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore ME, Szczylik C, Porta C, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer. 2015;113(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna N, Sun M, Meyer CP, et al. Survival Analyses of Patients With Metastatic Renal Cancer Treated With Targeted Therapy With or Without Cytoreductive Nephrectomy: A National Cancer Data Base Study. J Clin Oncol. 2016;34(27):3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti SL, Thomas IC, Hagedorn JC, et al. Utilization of cytoreductive nephrectomy and patient survival in the targeted therapy era. Int J Cancer. 2014;134(9):2245–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrelli F, Coinu A, Vavassori I, et al. Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma Treated With Targeted Therapies: A Systematic Review With a Meta-Analysis. Clin Genitourin Cancer. 2016;14(6):465–472. [DOI] [PubMed] [Google Scholar]
- 19.Mejean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. The New England journal of medicine. 2018;379(5):417–427. [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 22.Facility Oncology Registry Data Standards (FORDS): revised for 2016. https://www-facs-org.foyer.swmed.edu/quality-programs/cancer/ncdb/registrymanuals/cocmanuals/fordsmanual.
- 23.Pindoria N, Raison N, Blecher G, Catterwell R, Dasgupta P. Cytoreductive nephrectomy in the era of targeted therapies: a review. BJU international. 2017;120(3):320–328. [DOI] [PubMed] [Google Scholar]
- 24.Ascierto PA, Addeo R, Carteni G, et al. The role of immunotherapy in solid tumors: report from the Campania Society of Oncology Immunotherapy (SCITO) meeting, Naples 2014. J Transl Med. 2014;12:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singla N, Elias, R., Ghandour, R., et al. Safety and feasibility of nephrectomy after receipt of immune checkpoint inhibitors for renal cell carcinoma. J Clin Oncol. 2019;37(7_suppl):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England journal of medicine. 2018;378(21):1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woldu SL, Brugarolas J, Kapur P, Margulis V. What is the role of nephrectomy following complete response to checkpoint inhibitors? Urol Case Rep. 2018;18:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikarashi D, Kato Y, Katagiri H, et al. Case of complete response to neoadjuvant therapy using nivolumab in a patient with metastatic renal cell carcinoma. Int J Urol. 2018;25(6):630–632. [DOI] [PubMed] [Google Scholar]
- 31.Labbate C, Hatogai K, Werntz R, et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghandour RA, Singla N, Margulis V. The use of cytoreductive nephrectomy in patients with renal cell carcinoma. Expert Rev Anticancer Ther. 2019;19(5):405–411. [DOI] [PubMed] [Google Scholar]
- 33.Bhindi B, Abel EJ, Albiges L, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. European urology. 2018. [DOI] [PubMed] [Google Scholar]
- 34.Massari F, Di Nunno V, Gatto L, et al. Should CARMENA Really Change our Attitude Towards Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma? A Systematic Review and Meta-Analysis Evaluating Cytoreductive Nephrectomy in the Era of Targeted Therapy. Target Oncol. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. [DOI] [PubMed] [Google Scholar]
- 36.Kim HL, Mayerson E, Lara PN, et al. Considerations for the Next Clinical Trial Evaluating the Role of Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma. Eur Urol Focus. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.