Abstract
Background & Aims:
There is controversy over the utility of symptoms, examination, and tests for diagnosis of rectal evacuation disorders (REDs) or slow-transit constipation (STC). We aimed to ascertain the pooled prevalence, sensitivity, specificity, and likelihood ratios for clinical parameters to determine pretest and post-test probabilities of diagnoses of RED and STC without RED.
Methods:
We searched the MEDLINE and PUBMED databases since 1999 for studies that used binary data to calculate sensitivity, specificity, and likelihood ratios to determine the diagnostic utility of history, symptoms, and tests for RED and STC. RED and STC were defined based on confirmation by at least 1 objective anorectal test or colonic transit test. Controls had normal test results based on the specific protocol in each study.
Results:
We reviewed 100 articles; 63 studies of RED and 61 studies of STC met the inclusion criteria. Among 3364 patients with chronic constipation, objective tests demonstrated: RED alone, 27.2%; normal transit constipation alone, 37.2%; STC alone, 19.0%; and RED with STC, 16.6%. To diagnose RED, discriminant features were: urinary symptoms (specificity, 100%; likelihood ratio, above 10; 58 patients), less than 2 findings of dyssynergia in a digital rectal exam (sensitivity, 83.2%; negative likelihood ratio, 0.2; 462 patients) and rectoanal pressure gradient below –40 mm Hg with high anal pressure during straining (specificity, 100%; likelihood ratio, above 10; 101 patients). The features most strongly associated with STC alone were call to stool (specificity, 91.5%; likelihood ratio, 10.5; 75 patients) and absence of abdominal distension, fullness, or bloating (sensitivity, 92.9%; negative likelihood ratio, 0.1; 93 patients)
Conclusions:
In a systematic review, we found specific symptoms, lack of dyssynergia in a digital rectal exam, and findings on anorectal manometry to be highly informative and critical in evaluation of RED and STC.
Keywords: Identification, dyssynergia, NTC, BSFS
INTRODUCTION
The identification of rectal evacuation disorders (RED) and slow transit constipation (STC) among patients with chronic idiopathic constipation (CIC) or irritable bowel syndrome with constipation (IBS-C) constitutes an important step in the diagnosis and selection of treatment of constipation.1 RED is diagnosed in at least 25% of patients with chronic constipation in tertiary referral practice.2 In epidemiological studies, at least 25% of patients with symptoms of chronic constipation endorse symptoms suggestive of RED such as excessive straining or sense of incomplete evacuation.3 Guidelines suggest that anorectal manometry (ARM) should be the first functional study, ahead of transit measurements.1 However, there is still considerable controversy regarding the diagnostic criteria for RED. For example, even though four different phenotypes of dyssynergic defecation are recognized,4 ARM is not widely available in primary care or secondary gastroenterology practices outside referral centers. In addition, recommended criteria for normal balloon expulsion time (BET) range from 60 to 120 seconds, and others have questioned whether high resolution anorectal manometry (HR-ARM) is an expensive hobby or worth every penny.5 We recently showed that, in a tertiary referral practice in 449 consecutive patients evaluated by a single gastroenterologist, BET of 22 seconds had a sensitivity of 78% at a specificity 70%, whereas at 60 seconds there was 39% sensitivity at 93% specificity.6
Given these considerations, it is worth assessing what clinical indicators might be recommended to identify RED and STC without RED to optimize clinical evaluation of patients with CIC. We previously reported that, when the rectal area identified by gas and/or stool content (between the inferior margin of the sacroiliac joints and the superior margin of the pubis) exceeds 900mm2, there is a 75% likelihood of diagnosing RED.7 However, such an approach requires some radiation exposure and is not necessarily recommended unless the patient has undergone abdominal radiograph or computerized tomography typically performed for other indications.
Our objective was to review the literature on clinical features (history and examination) and first-line testing such as BET and ARM to estimate quantitative information on their diagnostic utility as compared to objective anorectal and transit tests. This review and analysis was designed to provide pooled sensitivity, specificity, and likelihood ratios (positive or negative) for each individual parameter, with the overall objective to enhance the pre- and post-test probabilities of RED, and STC without RED when clinicians assess patients with chronic constipation. A secondary objective was to assess the diagnostic utility of abuse history, laboratory results, and colonoscopy in chronic constipation.
METHODS
Collection and Selection of Articles
Search strategy/inclusion criteria
In April 2019, we used the MEDLINE and PUBMED search terms “chronic constipation,” “functional constipation,” “benign anorectal disorders,” “dyssynergic defecation,” “rectal evacuation disorder,” and “slow transit constipation” using the filters “human,” “review,” “systematic review,” “meta-analysis,” and “technical report” that were published since 2013 when the most recent American Gastroenterological Association Technical Review on Constipation was published.8 From these results, we reviewed additionally cited clinical reviews, primers, guidelines, and consensus statements on the diagnosis of CIC published since 1999 when the previous American Gastroenterological Association Technical Review on anorectal testing was published.9
The Supplement Materials include detailed information on the collection and selection of articles including exclusion criteria, criteria used to define gold standard for differentiation from control data, as well as calculations of the proportions of patients in each category (e.g., STC, RED), true or false positives or negatives, sensitivity, specificity, and likelihood ratios. Supplementary Tables B1 and B2 provide detailed description of how RED and STC were objectively defined as there was significant heterogeneity in the definitions across studies. Thus, Supplementary Table B2 includes articles which allowed discrimination of concurrent RED + STC (“total STC”) compared to STC without RED on at least one objective anorectal test (“STC alone”). Conversely, if patients were categorized as having RED + STC, they were included as having RED (“total RED”) in Supplementary Table B1.
RESULTS
Selection of Articles
Our literature search yielded a total of 100 summary articles (Supplement A) which were individually reviewed for cited articles pertinent to diagnostic utility in chronic constipation. The aforementioned inclusion/exclusion criteria yielded a total of 63 eligible articles for the diagnosis of RED (Supplement A1) and 61 articles for the diagnosis of total STC with or without RED (Supplement A2). In addition, 16 articles were included for STC alone (Supplement A3). This entire list of 100 references is also provided in the Supplemental Materials.
Prevalence of Constipation Phenotypes
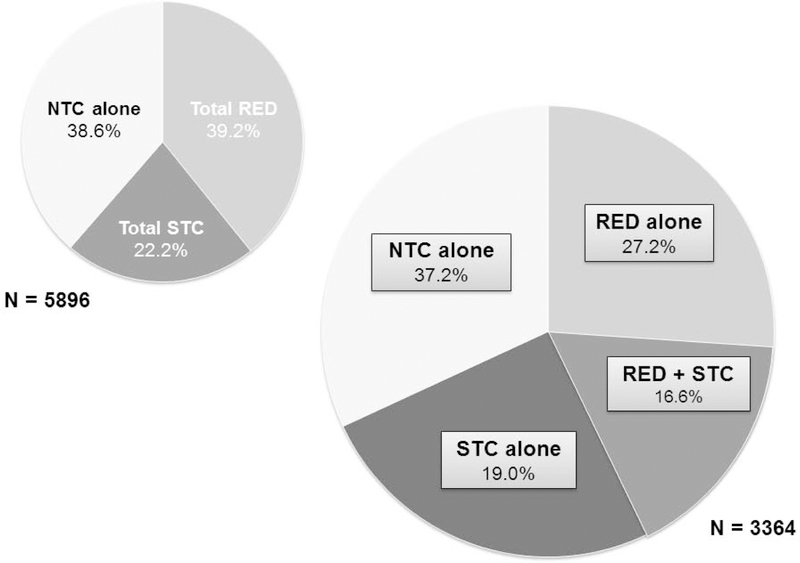
The global prevalence of RED, STC, and NTC, as well as the various subtypes are reported in Supplement Table C, stratified regionally into USA, European, and Asian study populations. Only 2 studies used criteria based solely on symptoms from community samples (total RED prevalence 10.4%, total 2031 participants).3,10 The pooled global prevalence of 5896 patients with functional constipation and objective testing, typically in academic centers, was 39.2% total RED (RED alone, RED + STC), 22.2% total STC (STC alone, RED + STC), and 38.6% NTC alone (Figure 1). Among the 3364 patients where subtypes could be further delineated, the majority of patients had RED (43.8% total, 27.2% RED alone, RED + STC 16.6%), followed by NTC alone (37.2%) and STC (35.6% total, 19.0% STC alone). The highest prevalence of total RED was in Asian studies (49.0%) and the highest prevalence of STC alone was in European studies (19.3%).
Figure 1:
Prevalence of chronic idiopathic constipation phenotypes based on entire literature cited (n=5897) and literature that differentiates RED or STC alone from RED with STC (n=3364).
Features in the History
Utility of symptoms in diagnosing RED with or without STC
Tables 1–3 and Supplement Table D report all the diagnostic parameters investigated. Those with clinical significance are highlighted in grey [SN and SP ≥85% (with IQR [being the range from the 25th to 75th percentile] <20%), +LR ≥2 (IQR <1.0), −LR <0.5 (IQR ≤0.5)], with the strongest clinical significance highlighted in black [SN and SP ≥95% (IQR <20%), +LR ≥5 (IQR <1.0), −LR ≤0.2 (IQR <0.5)] and summarized in Figure 2. The symptoms evaluated in the diagnosis of RED (with or without STC) and STC alone are listed in Table 1. The strongest positive clinical predictors of RED were urinary symptoms such as nocturia, hesitancy, strain to start stream, weak/prolonged urinary stream (all LR >10).
Table 1:
Diagnostic utility of symptoms in diagnosing RED and STC alone
| Symptoms | SN (%) | SP (%) | (+) LR | (−) LR | SN (%) | SP (%) | (+) LR | (−) LR | SN (%) | SP (%) | (+) LR | (−) LR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosing RED with or without STC | Diagnosing STC alone | Diagnosing RED alone | ||||||||||
| Straining1–15 | 88.1 (76.1–93.3) | 35.0 (21.2–42.1) | 1.1 (1.0–1.5) | 0.7 (0.3–1.2) | 67.9 (55.9–78.6) | 40.7 (30.0–49.6) | 1.1 (0.8–1.9) | 0.9 (0.6–1.3) | 76.0 (69.2–82.7) | 40.7 (40.3–41.0) | 1.3 (1.2–1.4) | 0.6 (0.4–0.8) |
| Hard or lumpy stools1–3, 5–11, 13–15 | 73.7 (55.1–79.2) | 33.1 (26.9–45.6) | 1.0 (0.8–1.1) | 0.8 (0.7–1.0) | 52.2 (46.4–60.6) | 43.1 (36.7–49.2) | 1.0 (0.9–1.0) | 1.1 (1.0–1.1) | 72.0 (71.2–72.9) | 42.4 (35.4–49.5) | 1.3 (1.2–1.5) | 0.8 (0.6–0.9) |
| Sensation of incomplete evacuation1, 2, 4–16 | 80.6 (20.0–90.7) | 38.2 (27.9–57.0) | 1.1 (1.0–1.7) | 0.7 (0.2–0.9) | 68.9 (51.3–78.3) | 48.3 (31.0–61.5) | 1.1 (0.8–1.6) | 1.0 (0.6–2.2) | 80.5 (77.3–83.7) | 48.3 (44.1–52.4) | 1.6 (1.4–1.8) | 0.4 (0.3–0.5) |
| Anorectal blockage/obstruction sensation1, 3, 6–11, 13–15 | 69.7 (46.4–76.9) | 61.3 (37.2–77.6) | 1.4 (1.2–1.6) | 0.8 (0.6–0.8) | 28.6 (18.1–44.5) | 73.3 (51.8–76.9) | 0.9 (0.6–1.0) | 1.1 (1.1–1.2) | 49.9 (47.1–52.6) | 76.9 (75.1–78.7) | 2.2 (2.0–2.5) | 0.7 (0.6–0.7) |
| Digital or manual maneuvers1, 2, 4–15, 17 | 46.1 (37.3–52.3) | 58.3 (51.8–75.6) | 1.1 (0.9–1.3) | 0.9 (0.8–1.1) | 11.5 (7.1–34.2) | 72.4 (58.3–81.3) | 0.6 (0.1–1.2) | 1.1 (0.9–1.1) | 46.4 (45.6–47.3) | 74.8 (66.6–83.1) | 3.2 (2.2–4.1) | 0.7 (0.6–0.7) |
| Infrequent BM ≤3/wk1–3, 5–11, 13, 15 | 45.6 (31.6–59.5) | 60.0 (56.0–70.2) | 1.1 (1.1–1.2) | 0.9 (0.8–1.0) | 61.8 (53.2–72.5) | 51.1 (37.6–62.0) | 1.4 (1.1–1.5) | 0.6 (0.5–1.0) | 51.9 (39.1–59.8) | 61.7 (51.0–62.0) | 1.1 (0.9–1.2) | 0.8 (0.8–1.0) |
| Laxative and/or enema dependent1–3, 17 | 64.4 (54.9–73.1) | 61.7 (53.2–70.0) | 1.2 (1.2–2.5) | 0.8 (0.5–0.8) | 42.9 (39.2–58.7) | 48.2 (41.6–59.2) | 1.0 (0.9–1.4) | 1.0 (0.9–1.2) | 53.7 | 76.7 | 1.4 | 0.8 |
| Suppository use17 | 13.5 | 93.8 | 2.2 | 0.9 | ||||||||
| Awareness of sphincter contraction during BM18 | 93.9 | 66.0 | 2.8 | 0.1 | ||||||||
| Anal/rectal pain1–3, 13 | 33.7 (19.8–51.7) | 65.3 (54.9–72.5) | 1.1 (0.7–1.5) | 0.9 (0.7–1.5) | 31.6 | 49.5 | 0.6 | 1.4 | 51.9 | 66.7 | 1.6 | 0.7 |
| Abdominal discomfort/pain3, 4, 15 | 46.7 (46.3–62.6) | 50.0 (31.7–53.8) | 0.9 (0.9–1.0) | 1.1 (1.0–1.3) | ||||||||
| Abdominal distension/fullness/bloating2, 4, 5, 19 | 87.1 (85.1–90.6) | 48.3 (34.1–62.4) | 2.3 (1.7–2.9) | 0.4 (0.3–0.5) | 92.9 (77.0–96.4) | 76.6 | 4.0 | 0.1 | ||||
| Duration on toilet ≥10mins1, 20 | 36.6 (29.1–44.1) | 70.9 (65.5–76.4) | 1.2 (1.2–1.3) | 0.9 (0.8–0.9) | 54.3 | 60.0 | 1.4 | 0.8 | 61.1 | 60.0 | 1.5 | 0.6 |
| Position other than sitting to defecate1, 7 | 40.7 (29.0–52.5) | 91.7 | 2.0 | 0.9 | 25.7 | 91.7 | 3.1 | 0.8 | 20.4 | 91.7 | 2.4 | 0.9 |
| Lack of call to stool2, 21 | 44.6 (32.5–56.8) | 51.8 (30.1–73.4) | 7.0 (3.6–10.4) | 4.8 (2.6–7.1) | 10.7 | 8.5 | 0.1 | 10.5 | ||||
| Feeling of call to stool2, 21 | 55.4 (43.2–67.5) | 48.2 (26.6–69.9) | 4.8 (2.6–7.1) | 7.0 (3.6–10.4) | 89.3 | 91.5 | 10.5 | 0.1 | ||||
| Lack of response to fiber (30g/d x 6 weeks)22 | 62.3 | 77.5 | 2.8 | 0.5 | 71.7 | 77.5 | 3.2 | 0.4 | ||||
| UGI symptoms* 2 | 11.9 (10.2–24.6) | 25.5 (22.3–35.1) | 0.2 (0.1–0.4) | 3.5 (2.4–4.1) | 55.6 (33.7–61.9) | 25.5 (22.3–35.1) | 0.5 (0.3–0.8) | 2.8 (1.9–3.6) | ||||
| Urinary symptoms2, 7, 23 | 46.4 (32.1–51.8) | 96.7 (96.7–100) | >10 | 0.5 (0.5–0.7) | ||||||||
| Frequency increased (>7 times/d)7 | 35.7 | 83.3 | 2.1 | 0.8 | ||||||||
| Urgency7 | 60.7 | 93.3 | 9.1 | 0.4 | ||||||||
| Nocturia7 | 21.4 | 100 | >10 | 0.8 | ||||||||
| Hesitancy7 | 46.4 | 100 | >10 | 0.5 | ||||||||
| Strain to start stream7 | 21.4 | 100 | >10 | 0.8 | ||||||||
| Strain to empty7 | 50.0 | 96.6 | 15 | 0.5 | ||||||||
| Weak or prolonged urinary stream7 | 57.1 | 100 | >10 | 0.4 | ||||||||
| Incomplete urinary emptying sensation7 | 53.5 | 96.7 | 16.1 | 0.5 | ||||||||
| Interrupted urinary stream7 | 42.9 | 96.7 | 12.9 | 0.6 | ||||||||
Data presented as median (Q1,Q3). If no IQR present, this is due to only one reference with the pertinent information.
Reference numbers in brackets are found in the Supplemental reference list.
Nausea, vomiting, dyspepsia, heartburn, or borborygmi
Grey = SN/SP ≥85% with IQR <20%; (+)LR ≥2 with IQR <1.0, (−) LR ≤0.5 with IQR <0.5;
Black = SN/SP ≥95% with IQR <20%, (+) LR ≥5 with IQR <1.0, (−) LR ≤0.2 with IQR <0.
Table 3:
Diagnostic utility of physiology and imaging testing in diagnosing RED
| Finding | SN (%) | SP (%) | (+) LR | (−) LR |
|---|---|---|---|---|
| BET overall3, 4, 8, 9, 13, 26, 31–45 | 66.5 (43.2–80.9) | 78.7 (68.8–89.4) | 2.7 (1.4–5.1)+ | 0.4 (0.3–0.8) |
| Inability to expel alone31–33, 35, 36, 38 | 53.8 (30.7–72.9) | 73.9 (69.1–88.5) | 2.4 (2.1–2.9) | 0.5 (0.4–0.8) |
| Weight required to expel >200g45 | 26.8 | 89.7 | 2.6 | 0.8 |
| Expulsion time >22s* 30 | 77.8 | 69.8 | 2.6 | 0.3 |
| Expulsion time >60s3, 13, 41–44 | 76.9 (74.6–87.5) | 71.4 (60.4–83.3) | 2.7 (2.5–4.2) | 0.3 (0.1–0.4) |
| Expulsion time >120s13, 34, 39, 43, 44 | 66.3 (57.1–78.3) | 86.8 (82.9–93.1) | 5.6 (4.3–7.0)+ | 0.3 (0.2–0.4) |
| Expulsion time >180s4, 9, 13, 43 | 49.3 (36.4–68.8) | 91.4 (81.8–100) | 3.5 (2.5–4.5)+ | 0.5 (0.3–0.7) |
| ARM, HR-ARM, HD-ARM any abnormality23, 27, 46–52 | 77.3 (66.7–92.9 | 76.2 (67.2–84.1) | 2.4 (2.2–4.5)+ | 0.3 (0.2–0.5) |
| ARM pr-pc and/or impaired anal relaxation27, 46, 48–50 | 84.2 (71.4–92.9) | 76.3 (71.2–78.6) | 2.4 (2.2–3.6) | 0.2 (0.1–0.3)# |
| HR-ARM, HD-ARM pr-pc and/or impaired anal relaxation23, 47, 51, 52 | 72.0 (66.7–79.7) | 78.2 (57.9–89.2) | 2.3 (1.7–3.8)+ | 0.4 (0.3–0.5) |
| Paradoxical puborectalis contraction52 | 12.4 | 77.7 | 0.6 | 1.1 |
| Impaired anal relaxation <20% baseline52 | 31.2 | 82.4 | 1.8 | 0.8 |
| Negative rectoanal pressure gradient (<–40mmHg) + high pressure band at anus23 | 32.4 | 100 | >10 | 0.7 |
| Inadequate rectal pressure <45 mmHg^* 52 | 43.0 | 81.0 | 2.3 | 0.7 |
| Type I-IV Dyssynergic Defecation23, 52 | 82.2 (79.7–84.6) | 52.9 (36.4–69.3) | 3.2 (2.2–4.3) | 0.5 (0.4–0.6) |
| Type I Dyssynergic Defecation^ 52 | 20.0 | 63.5 | 0.5 | 1.3 |
| Type II Dyssynergic Defecation^ 52 | 4.7 | 9.2 | 0.6 | 1.0 |
| Type III Dyssynergic Defecation^ 52 | 16.5 | 84.7 | 1.1 | 1.0 |
| Type IV Dyssynergic Defecation^ 52 | 45.9 | 80.0 | 2.3 | 67.6 |
| Rectal hyposensitivity$21, 53 | 52.6 (46.0–59.2) | 55.8 (42.2–69.4) | 1.6 (1.3–2.0) | 1.0 (0.8–1.1) |
| Rectosigmoid Predominance on Imaging30, 34, 54–57 | 54.4 (47.3–57.6) | 73.3 (59.2–85.1) | 1.5 (1.5–2.0)+ | 0.6 (0.6–0.8) |
| Radiopaque Markers@34, 54, 55, 57 | 56.9 (53.0–61.2) | 80.8 (68.2–91.7) | 2.0 (1.8–3.6)+ | 0.5 (0.5–0.6) |
| Scintigraphy (left sided vs. generalized delay)56 | 53.3 | 53.7 | 1.2 | 0.9 |
| CT abd/pelvis | ||||
| Rectal area on scout film >9cm2 30 | 39.5 | 73.8 | 1.5 | 0.8 |
| Rectal gas volume >20mL* 58 | 38.1 | 89.1 | 3.5 | 0.7 |
| Max rectal gas transaxial area >10cm2* 58 | 24.6 | 90.9 | 2.7 | 0.8 |
Data presented as medians (Q1, Q3)
Grey = SN/SP ≥85% with IQR <20%; (+)LR ≥2 with IQR <1.0, (−) LR ≤0.5 with IQR <0.5
Black = SN/SP ≥95% with IQR <20%, (+) LR ≥5 with IQR <1.0, (−) LR ≤0.2 with IQR <0.5
Reference numbers in brackets are found in the Supplemental reference list.
Pr-pc: puborectalis paradoxical contraction on simulated defecation
Reference 50 excluded from –LR calculation due to 100% sensitivity
SN/SP taken from documented values without raw values from reported regression analysis, thus unable to pool
Gold standard was symptomatic criteria (Rome III FC + CCCS >12) versus healthy asymptomatic controls, absolute values not available, thus unable to pool
Rectal hyposensitivity is defined as elevation of ≥1 of three rectal sensory threshold volumes beyond normal range during simple balloon distension. Thresholds for men: first sensation volume >160mL, desire to defecate volume >230mL, or max tolerated volume >315 mL in men. Thresholds for women: first sensation >120mL, desire to defecate >210mL, max tolerated volume >325mL.
Significant heterogeneity in methods used even within same modality
Figure 2: Summary of most predictive factors in chronic idiopathic constipation.
*urinary symptoms include nocturia, hesitancy, strain to start stream/empty bladder, and weak or prolonged stream (58 patients)
# Patients who are aware of anal sphincter contraction during BM were more likely to have RED (+) LR 2.8, whereas those who are unaware of sphincter contraction are less likely to have RED (−) LR 0.1 (83 patients) @ Dyssynergia overall: 2 of 4 criteria during simulated defecation: inability to contract abdominal muscles, inability to relax anal sphincter, paradoxical anal contraction, absent perineal descent
PR = puborectalis muscle
Strong negative associations were patient lack of awareness of their anal sphincter contracting during defecation (SN 93.9%, −LR 0.1); such patients were less likely to have objective RED. Specifically, this means that, if patients were unaware of anal sphincter contraction during defecation, they were less likely to have RED. Position other than sitting to defecate was associated with 91.7% specificity and positive LR 2.0.
Symptoms which did not meet any clinical significance thresholds included hard or lumpy stools, incomplete evacuation sensation, sensation of anorectal blockage/obstruction, digital or manual maneuvers, infrequent bowel movements <3/week, anal/rectal pain, abdominal discomfort/pain, duration on toilet >10 minutes, lack of presence of call to stool, or upper GI symptoms such as nausea and vomiting.
Utility of symptoms in diagnosing RED alone (without STC)
When stratifying those studies which distinguished patients with RED alone without STC (Table 1, Supplementary Table E), a position other than sitting to defecate had a positive association with RED (SP 91.7%, +LR 2.4). Negative associations with RED alone was lack of sensation of incomplete evacuation [SN 80.5% (IQR 6.4%); −LR 0.4 (IQR 0.2)]. This observation contrasts with RED with or without STC where lack of sensation of incomplete evacuation was less predictive [SN 80.6% (IQR 70.7%); −LR 0.7 (IQR 0.7)].
Utility of symptoms in diagnosing STC alone
These data are shown in Tables 1 and 4. The most clinically significant symptoms for predicting the presence of STC alone were feeling of the call to stool (SP 91.5%, +LR 10.5), while helpful symptoms for predicting the absence of STC alone were lack of abdominal distension/fullness/bloating [SN 92.9% (IQR 19.4%), −LR 0.1] and feeling the call to stool (SN 89.3%, +LR 10.5, −LR 0.1). Thus, feeling the call to stool is useful as a marker of needing to defecate in patients with STC; it is also useful when absent as a feature of patients who typically have significant constipation unassociated with sensation of needing to defecate.
Table 4:
Diagnostic utility of testing in predicting STC alone
| Finding | SN (%) | SP (%) | (+) LR | (−) LR |
|---|---|---|---|---|
| Imaging | ||||
| Scintigraphy: generalized vs. left sided delay* 56 | 53.7 | 53.3 | 1.2 | 0.9 |
| Rectal gas area <5cm2 on CT abd/pelvis scout film59 | 88.2 | 23.7 | 1.2 | 0.5 |
| High stool burden on AXR via Leech method60 | 79.3 | 55.2 | 1.8 | 0.4 |
| Physiology Testing | ||||
| BET inability to expel (Total STC vs. NTC)32 | 80.0 | 33.3 | 1.2 | 0.6 |
| Rectal hyposensitivity vs. normal sensation$21 | 80.0 | 36.4 | 1.3 | 0.5 |
| Normal sensation vs. rectal hyposensitivity$21 | 20.0 | 63.6 | 0.6 | 1.3 |
| Wireless motility capsule vs. ROM^ | insufficient evaluation to predict STC | |||
| Total STC vs. NTC61–63 | 85.7 (82.7–86.3) | 66.7 (54.8–78.7) | 2.6 (2.1–5.6) | 0.2 (0.2–0.3) |
Data presented as medians (Q1,Q3)
Reference numbers in brackets are found in the Supplemental reference list.
Generalized STC: GC48 <3.6, Left sided delay: GC48 >3.8 + normal defecography
Rectal hyposensitivity is defined as elevation of >1 of three rectal sensory threshold volumes beyond normal range during simple balloon distension. Thresholds for men: first sensation volume >160mL, desire to defecate volume >230mL, or max tolerated volume >315 mL in men. Thresholds for women: first sensation >120mL, desire to defecate >210mL, max tolerated volume >325mL.
Wireless motility capsule colon transit time >59h vs. abnormal ROM (various methods) as the gold standard
Grey = SN/SP ≥85% with IQR <20%; (+)LR ≥2 with IQR <1.0, (−) LR ≤0.5 with IQR <0.5
Black = SN/SP ≥95% with IQR <20%, (+) LR ≥5 with IQR <1.0, (−) LR ≤0.2 with IQR <0.5
Symptoms which did not meet our clinically significant thresholds were straining, hard or lumpy stools, incomplete evacuation sensation, sensation of anorectal blockage/obstruction, digital or manual maneuvers, infrequent bowel movements <3/week, laxative and/or enema dependence, anal/rectal pain, and upper gastrointestinal symptoms.
Utility of past medical history for diagnosis of RED or STC alone
Overall, the medical history was not as strongly predictive as symptoms for diagnosis of RED or STC alone (Supplement Table D). However, for diagnosing RED, history of abdominal surgery (SP 93.4% (IQR 8.2%) +LR 0.9 (IQR 0.3)), especially appendectomy (SP 93.4%, +LR 2.2) and cholecystectomy (SP 86.9%, +LR 0.6), ≥3 vaginal deliveries (SP 86.2%, +LR 0.3), and a history of caesarean section deliveries [SP 94.1% (IQR 2.4%); +LR 0.6 (IQR 0.0)] were useful. History of abdominal surgery was helpful for predicting STC alone [SP 94.4% (IQR 10.6%); +LR 0.9 (IQR 1.0)].
History of psychiatric disorders including depression or anxiety was not significantly associated with RED or STC alone. Other features in the history that did not meet our thresholds for clinical significance included constipation since childhood and/or >10 years, constipation since having any childbirth or being nulliparous, ≥1 vaginal deliveries, traumatic vaginal delivery (with perineal tear).
Features on Physical Examination
Utility of DRE in diagnosing RED
With a pooled sample of 1223 patients, an entirely normal digital rectal exam was associated with absence of RED [SN 75.1% (IQR 25.0%); −LR 0.3 (IQR 0.2)]. As shown in Table 2, when stratified into the individual components of the DRE, the findings most strongly associated with positive objective testing were paradoxical puborectalis contraction and/or impaired anal relaxation during simulated defecation [SP 58.9% (IQR 23.2%); +LR 2.0 (IQR 0.9)]. In addition, if patients did not have either of these findings, it was associated with negative objective testing [SN 81.8% (IQR 33.7%; −LR 0.3 (IQR 0.4)]. If patients did not have 2 of 4 findings of dyssynergia during simulated defecation (inability to contract abdominal muscles, inability to relax anal sphincter, paradoxical anal contraction, absent perineal descent), they were less likely to have RED [SN 83.2% (IQR 10.0), −LR 0.2 (IQR 0.1)]. There were several DRE findings that did not meet our thresholds for clinical significance including increased resting anal tone, inability to contract abdominal muscles during defecation, and puborectalis muscle tenderness to palpation.
Table 2:
Diagnostic utility of DRE in diagnosing RED
| Finding | SN (%) | SP (%) | (+) LR | (−) LR |
|---|---|---|---|---|
| DRE (any abnormality)24–30 | 75.0 (63.4–88.5) | 84.4 (55.9–87.6) | 4.8 (2.1–5.2) | 0.3 (0.2–0.4) |
| Paradoxical puborectalis contraction or impaired relaxation during defecation24, 25, 27–30 | 81.8 (55.4–89.2) | 58.9 (52.1–75.3) | 2.0 (1.7–2.6) | 0.3 (0.2–0.6) |
| Paradoxical puborectalis contraction24, 25, 27, 30 | 69.6 (55.0–87.2) | 56.1 (52.6–56.8) | 1.9 (1.7–2.9) | 0.4 (0.2–0.6) |
| Impaired anal relaxation29, 30 | 52.1 (30.8–73.4) | 68.1 (53.6–82.6) | 2.4 (2.0–2.8) | 0.5 (0.3–0.7) |
| Increased resting anal tone28, 30 | 49.1 (46.0–52.3) | 67.0 (60.8–73.1) | 1.8 (1.4–2.2) | 0.8 (0.7–0.9) |
| Increased anal squeeze28, 30 | 38.1 (29.2–47.1) | 93.7 (91.1–96.4) | 12.7 (8.8–16.6) | 0.7 (0.6–0.7) |
| Abnormal perineal descent (increased/decreased)26, 30 | 70.8 (63.7–78.0) | 62.6 (57.5–67.7) | 1.9 (1.9–2.0) | 0.4 (0.4–0.5) |
| Decreased/absent perineal descent26, 30 | 80.1 (77.5–82.6) | 68.5 (60.5–76.4) | 3.3 (2.5–4.1) | 0.3 (0.3–0.3) |
| Increased perineal descent26 | 18.2 | 89.7 | 1.8 | 0.9 |
| Inability to contract abdominal muscles during defecation29 | 71.0 | 52.3 | 1.5 | 0.6 |
| Puborectalis tenderness30 | 54.1 | 71.3 | 1.9 | 0.6 |
| No dyssynergia overall* 28, 29 | 83.2 (78.2–88.2) | 71.7 (65.2–78.1) | 3.5 (2.9–4.1) | 0.2 (0.2–0.3) |
Data presented as median (Q1,Q3). If no IQR present, this is due to only one reference with the pertinent information.
Reference numbers in brackets are found in the Supplemental reference list.
No dyssynergia overall is defined by absence of at least 2 of 4 findings during simulated defecation: 1) inability to contract abdominal muscle 2) inability to relax anal sphincter 3) paradoxical anal contraction 4) absence of perineal descent
Grey = SN/SP ≥85% with IQR <20%; (+)LR ≥2 with IQR <1.0, (−) LR <0.5 with IQR <0.5
Black = SN/SP ≥95% with IQR <20%, (+) LR ≥5 with IQR <1.0, (−) LR <0.2 with IQR <0.5
Features on Investigations
Utility of physiology and imaging testing in diagnosing RED
Table 3 shows that balloon expulsion testing overall and stratified by various testing thresholds was clinically significantly associated with objective testing confirming RED (most frequently defecography). If patients expelled the balloon in >60 seconds, they were likely to have RED [SN 76.9% (IQR 12.9%); −LR 0.3 (IQR 0.3)]. If they required >120 seconds to expel the balloon, they were more likely to have RED [SP 86.8% (IQR 10.2%); +LR 5.6 (IQR 2.7)]. When stratified for patients with RED alone, inability to expel a 50 mL balloon, despite addition of 200 g weight, had positive association with RED (SP 89.7%; +LR 2.6).
Since high-resolution and high-definition anorectal manometry (HR-ARM, HD-ARM) have only been developed in recent years, the majority of the literature evaluated conventional water perfusion ARM. Overall, if no abnormality was found on ARM, HR-, or HD-ARM, this finding was helpful for excluding RED [SN 77.3% (IQR 26.2%); −LR 0.3 (IQR 0.3)]. The most helpful individual parameters for diagnosis were presence of a negative rectoanal pressure gradient <–40mmHg with a high pressure band at the anus during defecation on HR-ARM (SP 100%, +LR >10) or paradoxical puborectalis muscle contraction and/or impaired anal relaxation on ARM [SN 84.2% (IQR 21.5%; −LR 0.2 (IQR 0.2)] or HR-ARM or HD-ARM [SN 72.0 (IQR 13.0%); −LR 0.4 (IQR 0.2)]. This means that the absence of the paradoxical puborectalis contraction or of impaired anal relaxation on manometry helps to exclude rectal evacuation disorder.
While the Rao classifications of dyssynergic defecation types I-IV4 were not predictive for the presence of RED, there was a large study of 170 patients that used symptomatic criteria to determine health versus disease in which dyssynergic defecation types I and II were more common in health than disease.11 If this study was removed, the dyssynergic defecation types I-IV were more predictive (SN 77.3%, SP 85.7%, +LR 5.4, −LR 0.3).
For imaging modalities (Table 3), the most predictive in diagnosing RED was computed tomography (CT) of the abdomen/pelvis with rectal gas volume >20mL (SP 89.1%, +LR 3.5) or a maximum rectal gas transaxial area >10cm2 (SP 90.9%, +LR 2.7). The predominant location of radiopaque markers (ROM) in the rectosigmoid (heterogeneous methodologies) and scintigraphy (left-sided versus generalized delays) were both not clinically significant for diagnosing RED. When stratifying for patients with RED alone, there was a negative association with RED for those who did not have an abnormal rectosigmoid transit time (SN 80.0%, −LR 0.4), implying that RED is associated with abnormal rectosigmoid transit time.
Utility of physiology and imaging testing in diagnosing STC alone
As shown in Table 4, if the rectal gas area on CT abdomen/pelvis was >5cm2, this was helpful for ruling out STC alone (SN 88.2%, −LR 0.5). Similarly, if there was not a high stool burden on abdominal x-ray as measured via the Leech method,12 STC alone was less likely (SN 79.3%, −LR 0.4). For physiology testing, if a patient did not have rectal hyposensitivity on simple balloon distension, they were less likely to have STC alone (SN 80.0%, −LR 0.5).
The wireless motility capsule (WMC) method for measuring colonic transit was compared to ROM testing for its accuracy in diagnosing total STC, as it was not clear from the articles if RED had been excluded in the patients tested. If the WMC did not show a colonic transit delay with colonic transit <59 hours, it was less likely the patient would have STC on ROM [SN 85.7% (IQR 3.6%); −LR 0.2 (IQR 0.1)].
Other Observations
Additional information on diagnostic utility of abuse history, labs, and colonoscopy in chronic constipation is included in the Supplement Materials.
DISCUSSION
Our comprehensive analysis provides valuable clinical tools for clinicians to generate evidence-based pre- and post-test probabilities in the diagnosis and, ultimately, the management of patients with CIC. Similar to previous studies, we found that the prevalence of RED was high in study populations regionally and collectively across the world (39.2%) among a pooled cohort of 5896 patients with objective testing. While studies previously have used statistical testing to search associations between clinical features such as history, symptoms, and first-line testing for objectively diagnosed RED or STC,13–15 our approach has focused on the translation of these data into the pragmatic clinical terminology of sensitivity, specificity, and likelihood ratios. This approach enhances the diagnostic acumen of providers who are managing patients with constipation. A summary of the most important features and associated positive and negative likelihood ratios is shown in Figure 2. Our analysis focused particularly on the identification of RED rather than STC for several reasons: first the overall prevalence of RED in patients with chronic constipation is close to 45% compared to <20% with STC alone; second, RED requires a completely different management with biofeedback-assisted retraining of the evacuation process, in contrast to NTC and STC for which treatment is similar and based on a trial of fiber, osmotic, secretory and stimulant laxatives. This is, at least in part, due to the fact that the pathobiology of STC is incompletely understood, other than variable levels of loss of interstitial cells of Cajal and sometimes contradictory reports of loss of intrinsic myenteric neurons within the colon, as reviewed elsewhere.16
A systematic review and meta-analysis of 261,040 subjects across 45 studies found a pooled prevalence of CIC based on symptom-based criteria of 14% (95% CI, 12–17%).17 Large community samples seeking to characterize those constipated patients with defecation disorders found a similar prevalence of 10.4% from a pooled total of 2031 patients.3,10 However, these studies were limited to symptom-based criteria rather than objective tests, which are often only available at academic centers. While disease patterns in academic centers are subject to referral bias that may not entirely reflect community practice, the consistently high percentage of RED across the United States (35.0%), Europe (43.3%), and Asia (49.0%) demonstrates the importance of considering this disorder in managing all patients presenting with CIC, regardless of the clinical setting. While motility experts recognize that RED is associated with STC,2 the sizeable number of patients with RED + STC (16.6%) further demonstrates the importance of excluding RED prior to testing for STC, and especially before referral for subtotal or total colectomy. Indeed, the experience of one tertiary referral practice1 showed that 12/324 patients diagnosed with RED had previously undergone colonic resection.
Symptom assessment has been central to diagnostic classifications of constipation and irritable bowel syndrome in the Rome criteria for functional bowel disorders.18 However, their role in determining the diagnostic phenotypes of RED and STC without RED have been controversial, especially in the absence of objective anorectal or transit testing. Our most clinically meaningful parameters for predicting RED were urinary symptoms, which are not included in the Rome IV guidelines for diagnostic clarification in patients presenting with constipation.18 While the Bristol Stool Fullness Scale is commonly used in clinical practice, our analysis did not find that a report of hard or lumpy stools was associated with RED with or without STC or STC alone. Conversely, a post hoc analysis of a previous study evaluating the correlation of the WMC with radiopaque marker testing19 found that BSFS <3 had adequate predictive value for delayed colonic transit (SN 82%, SP 83%), although it is unclear from the methodology if RED was excluded prior to transit testing.20 This disparity from our results could be due to the lack of formal BSFS assessment in older studies. While bowel frequency is often used as a correlate for constipation, a reported frequency of <3 bowel movements/week had poor correlation with transit testing in our study, similar to other findings demonstrating the poor reliability of patients’ reports of decreased stool frequency with subsequent 4-week bowel diaries.21
As stated earlier, the most predictive symptoms for RED were urinary symptoms, especially those included in the shortened American Urological Association Symptom Index Questionnaire (AUA-7).22 These could readily be employed in the pre-assessment of patients presenting with constipation. One recent meta-analysis of 9 studies and 19907 participants suggested lower urinary tract symptoms associated with IBS [OR 1.80 (1.34–2.42)].23 While the association of urinary symptoms and CIC has been described in children,24 there is less evidence supporting this relationship in adults with functional constipation, and our analysis identified a sample of 58 patients to assess the relationship with RED,25 suggesting that further study is required to better characterize this relationship.
Previous systematic reviews and meta-analyses have evaluated the utility of the DRE and the BET.26,27 DRE overall was found to have pooled sensitivity of 80% [64–90%] and specificity 84% [64–94%] similar to our findings; however, there was significant heterogeneity between studies (I2 = 91%).26 Due to the high variability of testing protocols and criteria across academic centers, significant heterogeneity also occurred in a recent meta-analysis investigating the optimal protocol for the BET (I2 = 98%).27 However, our analysis concurs with the conclusion that a balloon expulsion time cut off of <60 seconds is adequate for ruling out dyssynergic defecation,27 consistent with our finding for expulsion time >60 seconds (Table 3) [SN 76.9% (IQR 12.9%); −LR 0.3 (IQR 0.3)]. In addition, our study also found that a balloon expulsion time >120 seconds was helpful to rule in the disorder [SP 86.8% (IQR 10.2%); −LR 0.3 (IQR 0.2)]. Other older meta-analyses have evaluated other diagnostic modalities such as ARM, defecography, and anal ultrasound;28,29 however, there was also significant heterogeneity and the reported findings are less clinically translatable since they do not detail the prevalence or odds ratios. Given its novelty, HR-ARM was not evaluated in prior studies, in contrast to our analysis, and this is relevant since HR-ARM has become the predominant manometry method used in specialized centers.30
Implications for Clinicians
This study provides important implications for clinicians in practice, which are summarized in Figure 2 and detailed in the Supplemental Materials.
Limitations
Our study’s limitations include the current lack of gold standard criteria for RED and STC, precluding formal meta-analysis; this weakness is demonstrated by the significant heterogeneity found in previous meta-analyses.26–29 However, we propose that, until more formal consensus criteria are implemented and accepted as gold standard criteria, our study using objective rather than subjective symptomatic criteria for RED and STC provides clinicians with a comprehensive collection of clinical parameters to augment their clinical diagnostic acumen and enhance the value of their care through judicious performance and interpretation of advanced anorectal and transit testing.
Another limitation is the heterogeneous prevalence of both RED and STC without RED across studies used for pooled analysis (Supplement Table C) in addition to numerous case control studies preventing the accurate calculation of positive/negative predictive values. However, our calculation of likelihood ratios from pooled sensitivities/specificities still provides clinicians with similar tools for directing post-test probability in the setting of a positive or negative symptom, DRE finding, or test result. Finally, given the large scope of the analysis and the previously stated heterogeneity within the patients with CIC or functional constipation, these results cannot be extrapolated to patients presenting with constipation-predominant IBS, fecal incontinence, or pediatric populations.
While we have conducted as systematic a review of the literature as possible, a limitation of our paper is that we did not follow standard reporting guidelines.
In summary, our comprehensive analysis highlights the importance of clinical evaluation for directing diagnosis and treatment of patients with CIC, as well as the high prevalence of RED across studies. Urinary symptoms were predictive for RED, while lack of call to stool or abdominal distension, fullness, or bloating was predictive of not having STC. These findings, in conjunction with a careful DRE, can generate a robust post-test probability to interpret anorectal and transit testing with improved diagnostic accuracy.
Supplementary Material
Need to Know.
Background:
Chronic constipation is one of the most common gastrointestinal symptoms; it is important to identify clinical features of and diagnostic tests for detection of rectal evacuation disorders.
Findings:
Hard, lumpy stools; urinary symptoms; poor anal relaxation and increased anal squeeze on rectal examination; prolonged balloon expulsion time; and recto–anal pressure gradient with high anal pressure on manometry can be used to identify patients with RED.
Implications for patient care:
When first-line therapies do not resolve chronic constipation, gastroenterologists should use clinical discriminating factors to identify patients who might have RED and select appropriate diagnostic tests and treatment.
Acknowledgement:
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Grant support: Dr. Camilleri is supported by grant R01-DK115950 from National Institutes of Health. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Michael Camilleri receives research support from Allergan and Takeda.
Abbreviations:
- ARM
anorectal manometry
- BET
balloon expulsion test
- BM
bowel movement
- CIC
chronic idiopathic constipation
- CRC
colorectal cancer
- DEF
defecography
- DRE
digital rectal exam
- EMG
electromyography
- HR-, HD-ARM
high resolution-, high definition anorectal manometry
- HV
healthy volunteers
- IBS
irritable bowel syndrome
- +/−LR
positive/negative likelihood ratio
- NTC
normal transit constipation
- OR
odds ratio
- RED
rectal evacuation disorder
- ROM
radiopaque marker
- SN
sensitivity
- SP
specificity
- STC
slow transit constipation
- TP/FP
true/false positive
- TN/FN
true/false negative
- US
ultrasound
- WMC
wireless motility capsule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Justin Brandler has no conflicts of interest.
References
- 1.Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers 2017;3:17095. [DOI] [PubMed] [Google Scholar]
- 2.Nullens S, Nelsen T, Camilleri M, et al. Regional colon transit in patients with dyssynergic defaecation or slow transit in patients with constipation. Gut 2012;61:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talley NJ, Weaver AL, Zinsmeister AR, et al. Functional constipation and outlet delay: a population-based study. Gastroenterology 1993;105:781–790. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 2016;13:295–305. [DOI] [PubMed] [Google Scholar]
- 5.Basilisco G, Bharucha AE. High-resolution anorectal manometry: An expensive hobby or worth every penny? Neurogastroenterol Motil 2017. August;29(8). doi: 10.1111/nmo.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chedid V, Vijayvargiya P, Halawi H, et al. Audit of the diagnosis of rectal evacuation disorders in chronic constipation. Neurogastroenterol Motil 2019;31: e13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Khemani D, Acosta A, et al. Rectal gas volume: Defining cut-offs for screening for evacuation disorders in patients with constipation. Neurogastroenterol Motil 2017. July;29(7). doi: 10.1111/nmo.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.