Abstract
Purpose:
Trials of adoptive natural killer (NK)-cell immunotherapy for hematological malignancies have thus far shown only marginal effects, despite the potent in-vitro anti-tumor activity of these cells. Homing of infused cells to tumor microenvironments is critical for efficacy but has not been well characterized. We established a novel method to track and quantify the distribution of adoptively transferred NK-cells using rhesus macaques (RMs) as a clinically relevant preclinical model.
Experimental Design:
RM NK-cells were expanded ex-vivo for 14–21 days, labeled with 89Zr-oxine complex and assessed for phenotype, function and survival. Trafficking of 89Zr-labeled ex-vivo-expanded NK-cells infused into RMs was monitored and quantitated by serial positron emission tomography (PET)/computed tomography (CT) (n=3, 2.05 ± 0.72 MBq, 23.5 ± 2.0×106 NK-cells/kg) and compared to that of 89Zr-labeled non-expanded NK-cells, apoptotic NK-cells and hematopoietic stem and progenitor cells (HSPCs).
Results:
NK-cells retained sufficient levels of 89Zr for accurate in-vivo tracking for 7 days. 89Zr-labeling did not alter cellular phenotype, viability or function. PET/CT showed NK-cells initially localized in the lungs, followed by their migration to the liver, spleen and, at low levels, bone marrow (BM). One day following transfer, only 3.4% of infused NK-cells localized to the BM vs 22.1% of HSPCs. No clinical side effects were observed, and dosimetry analysis indicated low organ radio-exposures of 6.24 mSv/MBq (spleen) or lower.
Conclusions:
These data support translation of this technique to humans to track the distribution of adoptively infused cells and to develop novel techniques to improve immune cell homing to tumor microenvironments.
Keywords: Natural killer cells, Cell tracking, Positron emission tomography, Zirconium-89, Rhesus macaque
Introduction
Adoptive-transfer of allogeneic and autologous NK-cells is being investigated clinically for the treatment of malignancies. Despite their capacity to induce cellular cytotoxicity, NK-cells maintain self-tolerance regulated by the interaction of activating and inhibitory receptors with self-MHC class I molecules and their derivatives (1–4). The lack of a requirement for prior sensitization and the ability to induce tumor cytotoxicity in an antigen-independent manner without causing graft-versus-host disease (5) may offer advantages to adoptive NK-cell immunotherapy compared to approaches using T-cells. Although regression of malignancies in humans with advanced cancers has been observed, overall efficacy of NK-cell based immunotherapy remains marginal (6–8). One potential limitation of adoptive NK-cell therapy for leukemia and other hematological malignancies may be the inability of NK-cells to efficiently traffick to bone marrow (BM) where these malignancies primarily reside (9,10). Most preclinical adoptive NK-cell studies have used tissue harvesting to identify transferred cells. Such approaches preclude longitudinal analysis of cell fate and are problematic in humans.
Imaging adoptively transferred cells in-vivo could provide a useful method to assess the capacity of NK-cell homing to desired locations, such as tumors. Among the cell tracking imaging methods clinically available, magnetic resonance imaging (MRI) with ex-vivo superparamagnetic iron oxides (SPIOs) cell labeling permits high resolution without ionizing radiation, however, interpretation and quantitation are difficult and the SPIOs remain in the tissue after labeled-cell death (11). Indium-111 (111In)-oxine for single-photon emission computed tomography imaging has been used to label hematopoietic cells, but the high 111In doses required can impair cellular viability and function (12,13).
A 89Zr-oxine complex was recently developed as a cell labeling agent for tracking cells utilizing positron emission tomography (PET) (14). 89Zr has a half-life of 78.4 hours, ideal for tracking labeled cells over multiple days. With inherently higher sensitivity and spatial resolution of PET (15), studies in various murine models have established that only extremely low doses of 89Zr-oxine are necessary to track labeled cells for up to 7 days, with minimal to no deleterious radio-toxicity (14,16,17).
Here, we present a method to track adoptively transferred ex-vivo-expanded NK-cells using 89Zr-oxine cell labeling and PET imaging, validated in-vivo in a clinically relevant large animal model using rhesus macaques (RMs). Tissue 89Zr-distribution accurately quantitated NK-cell trafficking with low radio-exposure to organs, suggesting this method can be safely translated to humans.
Materials and methods
Animal care
All RM experiments were performed in accordance with a protocol approved by the institutional animal care and use committee. RMs were monitored daily in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved facility.
Collection and purification of NK-cells and expansion
See Supplementary Methods for material sources and details. Briefly, RM or human NK-cells were first enriched by either a) T-cell depletion of peripheral blood mononuclear cells (PBMCs) alone or b) additional NK-cell selection with NKp80 for RM or CD56 for human NK-cells. Enriched NK-cells were then expanded for 14–21 days as per an ongoing clinical trial of adoptive NK-cell therapy ( NCT00720785) (18,19), by co-culturing with an irradiated (100 Gy) human Epstein-Barr virus transformed lymphoblastoid cell line (SMI-EBV-LCL, authenticated 3/4/2009 by The Center for Cell and Gene Therapy using HLA typing) in X-VIVO 20 medium with 10% RM or human serum, supplemented with 500 IU/ml recombinant human interleukin (IL)-2. For experiments using non-expanded RM NK-cells, PBMCs obtained by apheresis and density-gradient centrifugation (Ficoll, GE Healthcare) were magnetically depleted of CD3+ (T-cells), CD20+ (B-cells), and CD14+ cells (monocytes), before positive-selection of NK-cells with anti-NKp80, a marker present on the vast majority of RM NK-cells, in contrast to CD56, which is downregulated in a population of mature NK-cells in RM (20).
Collection and purification of hematopoietic stem and progenitor cells
RM CD34+ hematopoietic stem and progenitor cells (HSPCs) were purified as described following mobilization with granulocyte-colony stimulating factor and plerixafor, apheresis and then immunomagnetic selection (21,22) (Supplementary Methods). Eight million CD34+ HSPCs with a purity of 92.3% were isolated and labeled with 89Zr-oxine prior to PET/CT studies.
Synthesis of 89Zr-oxine complex and cell labeling.
89Zr-oxine complex was synthesized from 89ZrCl4 produced at the institutional cyclotron facility (23) and oxine, as previously reported (14,16). RM or human NK-cells in phosphate-buffered saline (PBS) were incubated with 89Zr-oxine solution (63–100 kBq/106 cells) at a 30:1 volume ratio for 15 minutes, washed twice in the culture medium and transferred to a new tube in culture medium for in-vitro assays or in PBS for PET/CT studies. CD34+ HSPCs were labeled in a similar manner.
Impact of 89Zr-labeling on NK-cell surface phenotype, viability, function, and 89Zr-activity retention
NK-cells with and without 89Zr-oxine labeling were cultured in medium without IL-2 and, at indicated time points, examined for CD16, CD56, NKG2A, annexin V, Ki67 and CD107a expression by flow cytometry (FACSCalibur or LSRII, BD Biosciences) or assessed for cell number and 89Zr-activity retention (see Supplementary Methods for antibody and reagent information and details). Ki67 staining was performed using the cells fixed and permeabilized with −20°C 80% ethanol. To evaluate degranulation, a surrogate for cytotoxic function, at each time point, 5×106 NK-cells were co-cultured with 5×106 K562 target cells (human chronic myeloid leukemia in blast crisis) purchased from American Type Culture Collection (ATCC, authenticated on 3/8/2019 by ATCC using short-tandem repeat profiling) for 3 hours and NK-cell CD107a expression was analyzed. Viable cell quantitation was performed using 0.4% trypan blue dye (Thermo Fisher Scientific). The cell suspension was spun, and 89Zr-activity of the pellet was measured by a γ-counter (γ-WIZARD2, Perkin Elmer) and corrected for 89Zr-activity decay.
Assessing whether 89Zr transferred from 89Zr-oxine-labeled NK-cells to neighboring cells
89Zr-oxine-labeled human NK-cells from healthy donors were cultured alone or with different cell types (non-89Zr-labeled) for 24–36 hours. Thereafter, the two cell types were dissociated by magnetic separation and 89Zr-activity was measured for each population (Supplementary Methods).
Tracking cells by a clinical PET/CT imager
Supplementary Table S1 summarizes the 89Zr-oxine-labeled cell doses infused to RMs in this study. All RMs (weight 6.4 ± 0.9 kg) received a continuous intravenous infusion of deferoxamine (Desferal) beginning immediately before 89Zr-oxine-labeled cell transfer until day 7, using first a 15 mg/kg/hour loading dose for 4 hours then 3 mg/kg/hour for the remainder of the study. RMs underwent serial PET/CT imaging for up to 7 days using a clinical PET/CT scanner (Supplementary Methods). In addition to autologous, ex-vivo-expanded NK-cells, 3 RMs served as controls to track 3 alternative autologous cell populations; a) non-expanded NK-cells, b) apoptotic NK-cells, and c) HSPCs. VivoQuant software (inviCRO LLC) was used to fuse the maximum intensity projection (MIP) PET images with CT.
Quantitation of PET images
To quantitate 89Zr distribution, contours were drawn on the acquired PET/CT images delineating organs and whole-body using MIMvista (MIM software). Standardized uptake value (SUV) was calculated by the formula: SUV = [Bq/ml (decay corrected) in tissue] / [Bq of the cells injected/body weight (grams)]. Percentage of injected dose (% ID) was calculated by decay corrected activity in the tissue divided by the injected dose.
Dosimetry
Dosimetry estimates were derived using the organs showing high 89Zr-activity from which residence times were measured. The residence times were converted to human equivalent and used as input parameters into OLINDA 1.1 (Vanderbilt University). See Supplementary Methods.
Liver biopsy
Expanded autologous RM NK-cells were labeled with 2 μM CellTracker Orange CMRA (Thermo Fisher Scientific) according to the manufacturer’s instructions, then intravenously infused into the RM (5×107 cells/kg). One day later, a percutaneous liver biopsy was performed under anesthesia. The fresh biopsy samples were analyzed using an inverted Leica SP5 five-channel confocal multiphoton microscope (Leica Microsystems), employing 561-nm excitation and 570–640nm emission range (Supplementary Methods). Flow cytometry analysis (Fortessa, BD Biosciences) was also performed on liver tissue mashed into suspension and stained with aqua live/dead stain (Thermo Fisher Scientific).
Statistical analysis
NKG2A expression was analyzed by Wilcoxon test. CD56/CD16 and CD107a expression within each time point, and expression of annexin V and Ki67 as well as cellular viability over time, were analyzed by repeated measure ANOVA with Sidak’s test. Friedman test with Dunn’s correction was used to compare CD107a expression at multiple time points. 89Zr transfer from 89Zr-labeled NK-cells to CD19+ cells or PBMCs was analyzed by Wilcoxon test. Prism ver.8.2.0 (GraphPad Software) was used for all statistical analyses. The results are indicated as mean ± standard deviation (s.d.) and p-values less than 0.05 were considered significant.
Results
89Zr-oxine labeling does not alter the phenotype, viability nor function of expanded NK-cells
We first examined whether labeling RM and human NK-cells with 89Zr-oxine complex had any impact on NK-cell phenotype. RM and human NK-cells labeled at 19.5 ± 4.0 kBq/106 cells and 20.2 ± 4.0 kBq/106 cells, respectively, were used in all in-vitro experiments. Flow cytometry analysis indicated that expression of CD56 and CD16 (RM: n=9, human: n=7), and NKG2A (RM: n=8), was not altered immediately (3 hours) after 89Zr-oxine labeling (Fig. 1A and B) or over the subsequent 24 hours of culture (n=3, Supplementary Fig. S1). These conditions were chosen to mimic an in-vivo environment in patients not receiving exogenous IL-2.
Figure 1. 89Zr-oxine-labeled NK-cells have normal cellular function and do not transfer their label.
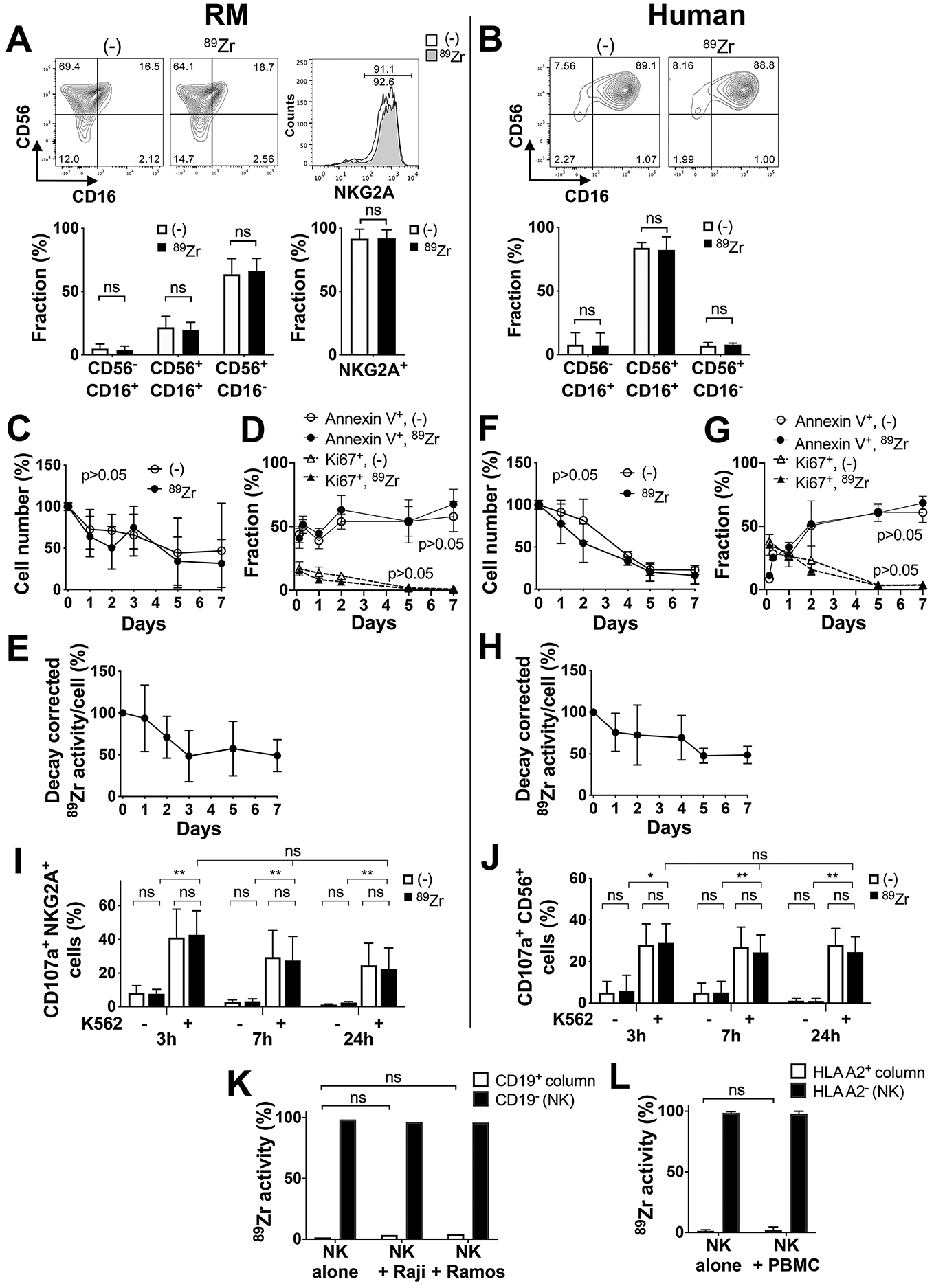
A. Flow cytometry analysis showed that the majority of ex-vivo-expanded RM NK-cells expressed NKG2A (n=8) and were CD56+CD16− (n=9), which was not altered by 89Zr-oxine labeling. B. The majority of ex-vivo-expanded human NK-cells were CD56+CD16+ (n=7), which was not altered by 89Zr-labeling. C. RM NK-cells without (open circle) or with 89Zr-oxine labeling (filled circle) showed similar viability over time when cultured in media without IL-2 (n=11). D. Flow cytometry analysis indicated that early after harvest from ex-vivo expansion cultures, a small fraction of RM NK-cells continued to proliferate (Ki67+, 89Zr-labeled: filled triangle, non-labled: open triangle) while annexin V+ apoptotic/necrotic cells increased over time (89Zr-labeled: filled circle, non-labled: open circle), neither of which were impacted by 89Zr-oxine labeling. E. Decay corrected 89Zr-activity per RM NK-cell declined at early time points and plateaued later. F. Similar to RM NK-cells, the number of viable human NK-cells gradually declined, with (filled circle) and without (open circle) 89Zr-labeling (n=9). G. Human NK-cells continued to proliferate (Ki67+) early after harvest from ex-vivo expansion cultures, regardless of 89Zr-labeling, while annexin V+ NK-cells increased over time. H. 89Zr-activity per human NK-cell declined over time (n=6). I and J. Degranulation of both RM (I, n=3) and human (J, n=3) NK-cells (cell surface CD107a) against K562 target cells, analyzed by flow cytometry, was similar regardless of 89Zr-labeling 3, 7, and 24 hours after labeling (*: p<0.05, **: p<0.01, ns: p>0.05). K. 89Zr-oxine-labeled human NK-cells (5×105 cells) were cultured alone or with CD19+ Raji or Ramos cells (5×105 cells) for 24–36 hours, then underwent CD19 positive selection. 89Zr did not transfer to Raji or Ramos cells (n=3). L. 89Zr-labeled HLA-A2 negative human NK-cells (8×105 cells) were cultured alone or with HLA-A2 positive PBMCs (8×105 cells) for 24–36 hours, then underwent HLA-A2 positive selection. 89Zr did not transfer to PBMCs (n=3).
We next assessed the viability of labeled cells and retention of 89Zr. 89Zr-oxine-labeled and non-labeled RM NK-cells showed similar viability and similar decline in live cell number (Fig. 1C, n=11) over time, as well as a similar increase in the percentage of the annexin V+ apoptotic/necrotic cells (Fig. 1D, n=3). Some cells proliferated at early time points, as indicated by Ki67 expression, which is likely due to ligering effect of IL-2 used for ex-vivo expansion (Fig. 1D, n=3). As a result, 89Zr-radioactivity per cell declined to approximately 50% of initial 89Zr-activity by day 3 (Fig. 1E, n=6). Similar findings were observed with human NK-cells (Fig. 1F: n=9, G: n=3, H: n=6). 89Zr labeling did not impair the cytotoxic function of RM (Fig. 1I, n=3) or human (Fig 1J, n=3) NK-cells; degranulation, assessed by measuring NK-cell surface CD107a expression up to 24 hours following co-culture with K562 tumor cells, was similar between 89Zr-labeled and non-labeled NK-cells.
89Zr is not readily transferred from the labeled NK-cells to neighboring cells
To exclude the possibility that 89Zr could transfer from labeled to non-labeled cells in-vivo, which would confound the interpretation of 89Zr signals on PET/CT, we assessed whether 89Zr could be transferred from labeled NK-cells to adjacent cells ex-vivo by passive diffusion. When 89Zr-oxine-labeled human NK-cells were co-cultured with Raji or Ramos cells for 24–36 hours, >96% of 89Zr-activity remained within the NK-cells after re-purification of the co-cultured cells (Fig. 1K, n=3). More importantly, the radioactivity detected in the CD19+ fraction (either Raji or Ramos cells) did not differ from background radioactivity measured when a sham CD19+ cell selection was performed on 89Zr-labeled NK-cells alone. Similar results were found when NK-cells from 3 HLA-A2 negative donors were labeled with 89Zr-oxine and co-cultured with HLA-A2 positive PBMCs. The 89Zr-activity in the immunomagnetically-selected HLA-A2 positive fraction was equivalent to the background radioactivity obtained from a sham HLA-A2 positive selection of 89Zr-labeled HLA-A2 negative NK-cells alone (Fig. 1L, n=3). These data suggest that 89Zr-oxine-labeled NK-cells retain the radiolabel without significant transfer to neighboring cells.
PET/CT imaging provides clear visualization of the trafficking of adoptively transferred 89Zr-oxine-labeled NK-cells in RMs.
Sequential PET/CT imaging of autologously-transferred 89Zr-oxine-labeled expanded NK-cells (n=3, 2.05 ± 0.72 MBq, 13.6 ± 5.3 kBq/106 cells, 23.5 ± 2.0×106 NK-cells/kg, Supplementary Table S1) showed initial localization to the lungs, followed by migration to the liver and spleen (Fig. 2A). The majority of NK-cells remained in these organs until day 7, when the experiment concluded. 89Zr-accumulation in the bladder indicates effective renal clearance of free 89Zr by deferoxamine. Low levels of radioactivity were observed within the BM, as shown in an axial PET/CT section of the 4th lumber vertebrae with 89Zr-activity localized in the center of the spinal body, the location of vertebral marrow (Fig. 2B). In contrast, measurable labeled NK-cells were not localized within lymph nodes. Quantitation of organ 89Zr-activity on acquired images showed the SUV of the liver and spleen reached an average of 14.4 and 25.3 respectively on day 1, in contrast to the BM which only reached an SUV of 0.64 (Fig. 2C and Supplementary Fig. S2A which shows the same data with magnification of the X-axis up to day 1). The percentage of 89Zr-activity measured in the liver corresponded to 31.3 ± 8.1% and 34.7 ± 11.8% of the infused 89Zr-activity on days 1 and 2 respectively, indicating that approximately one third of the transferred cells had trafficked to the liver (Fig. 2D and Supplementary Fig. S2B for magnification up to day 1). In contrast, only approximately 3% of the cells homed to the spleen and BM at these time points. As expected, whole-body radioactivity declined over time (Fig. 2E), likely due to renal clearance of 89Zr released from dead cells and chelated by deferoxamine, suggesting that one third and one half of the transferred ex-vivo-expanded NK-cells had died by days 2 and 7, respectively.
Figure 2. PET/CT imaging of autologous 89Zr-oxine-labeled NK-cells enables visualization and quantitation of NK-cell trafficking after adoptive-transfer.
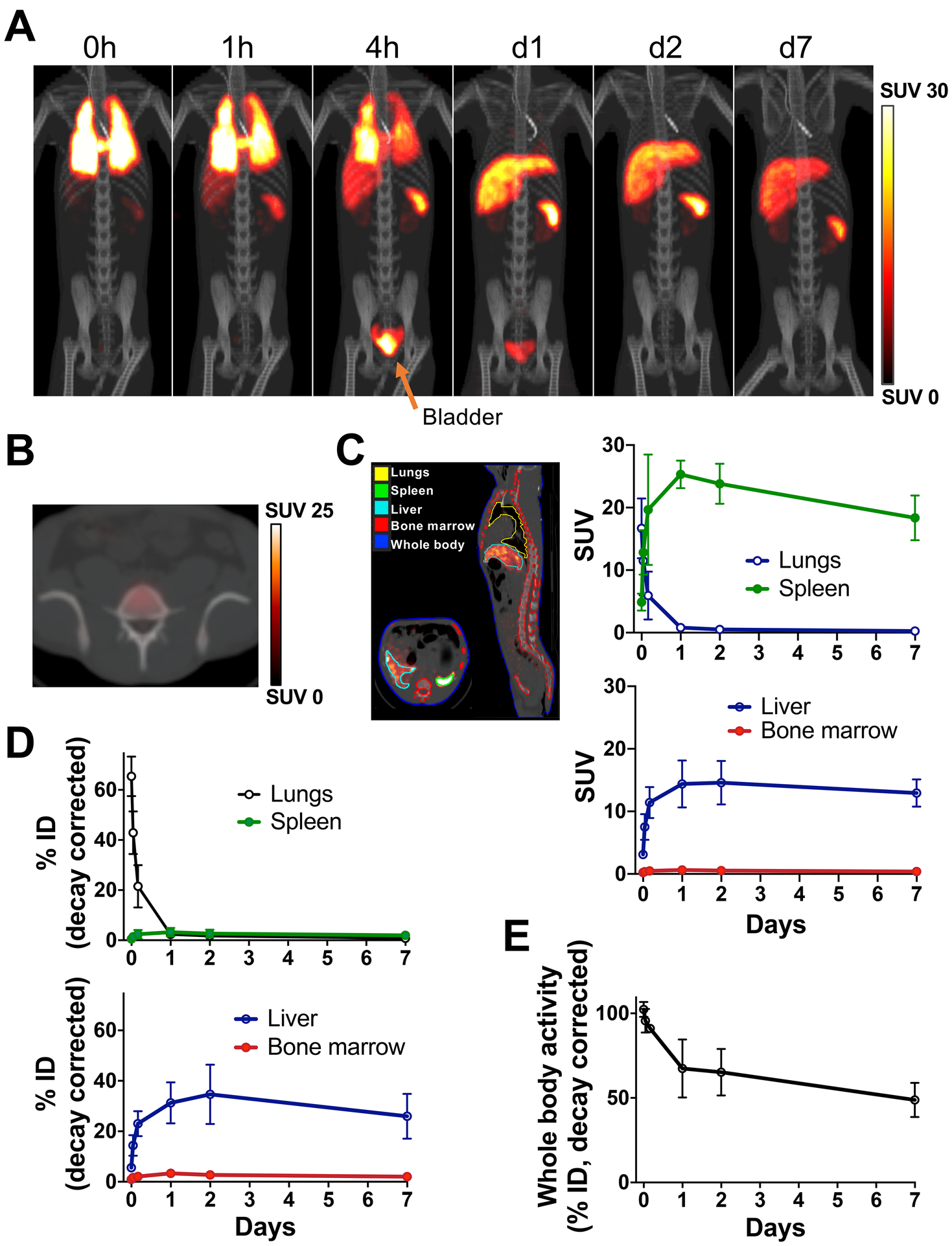
A. Autologous 89Zr-labeled expanded NK-cells transferred to RMs were tracked by PET/CT imaging, with continuous deferoxamine infusion, for up to 7 days (n=3, 2.05 ± 0.7 MBq, 13.7± 5.2 kBq/106 cells, 23.5 ± 2.0×106 cells/kg, representative set of images). B. Axial PET/CT image of mid-4th lumber spine on day 1 showed minimal homing of NK-cells to the BM area of the spinal body. C. Kinetics of SUV values (right) obtained from images by drawing the contours on the organs (left) suggested that 89Zr-labeled NK-cells migrated from the lungs to the liver and spleen. See Supplementary Fig. S2A for magnification of early time points. D. % ID curves indicated that most cells were in the liver while homing to the BM was limited. Also see Supplementary Fig. S2B. E. Whole-body activity declined over time, indicating death of transferred 89Zr-labeled cells and subsequent 89Zr excretion from the body.
Deferoxamine infusion results in rapid renal clearance of 89Zr released from apoptotic NK-cells
It is well documented that free 89Zr can be taken up by bone (24). Our previous study in mice using 89Zr-labeled cells demonstrated that deferoxamine infusion can prevent bone uptake of free 89Zr (16). To confirm that continuous infusion of deferoxamine efficiently bound and then excreted free 89Zr released from dead cells in RM and that the 89Zr-signals observed in Fig. 2A are from live cells, we performed a cell tracking study using ex-vivo-expanded NK-cells that had become apoptotic as a consequence of removing IL-2 from the medium used to culture cells ex-vivo (276 kBq, 15 kBq/106 cells, 3.5×106 cells/kg, Supplementary Table S1). In contrast to RMs receiving viable NK-cells, we observed significant radioactivity in the kidneys and bladder when the initial scan (20 minutes duration) was conducted (Fig. 3A) following the apoptotic NK-cell transfer, consistent with rapid renal clearance of 89Zr. Although some 89Zr-labeled apoptotic NK-cells were initially observed in the lungs and liver, by 4 hours, virtually all the 89Zr had moved to the bladder. On day 1, only 8.3 % of the total administered 89Zr remained in the body. These data show that deferoxamine chelation is effective in preventing transfer of free 89Zr from dying and/or dead cells into bone or other cellular populations with phagocytic capacity. Of note, following the infusion of apoptotic cells, a small amount of 89Zr accumulated in the gallbladder (1.5% ID at 4 hours), suggesting low-level clearance of 89Zr via the liver into the gallbladder.
Figure 3. Deferoxamine enhances renal clearance of 89Zr released from dead/dying cells.
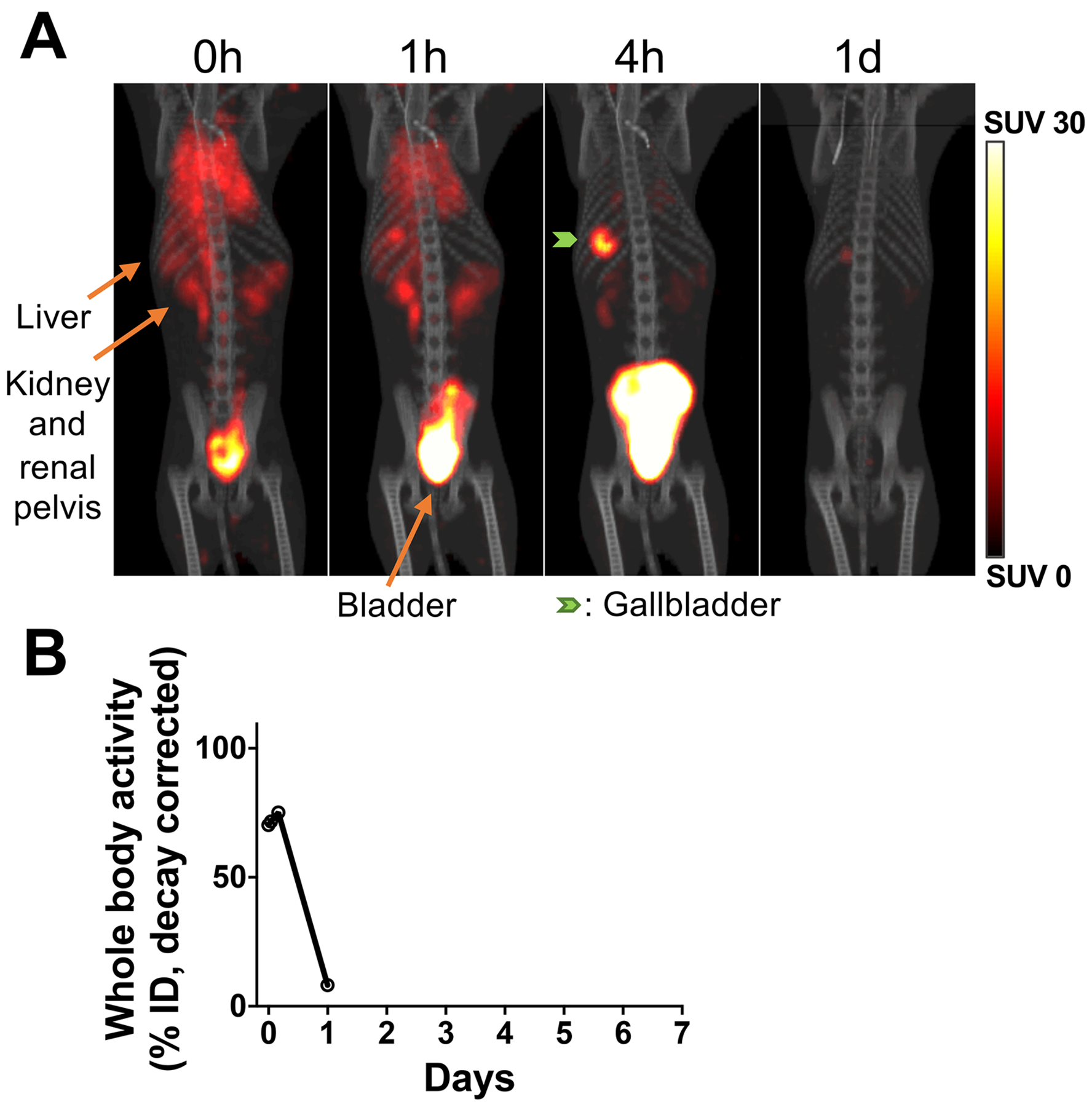
A. 89Zr-labeled apoptotic NK-cells were tracked in a RM (276 kBq, 3.5×106 cells/kg) by PET/CT under continuous deferoxamine infusion (n=1). 89Zr released from dying/dead cells was quickly chelated by deferoxamine and excreted in the urine. By 4 hours, most of the activity was observed in the bladder. B. Whole-body radioactivity decreased to 8% within 1 day.
Both freshly isolated and ex-vivo-expanded NK-cells showed limited BM homing compared to HSPCs
To assess whether ex-vivo-expansion may have altered the homing profile of NK-cells, the same 89Zr-oxine labeling and PET/CT approach was employed using non-expanded RM NK-cells (185 kBq, 6.9 kBq/106 cells, 4.0×106 cells/kg, Supplementary Table S1). The cell number (and therefore 89Zr-activity) infused was low because the number of NK-cells obtainable by purification from blood, without expansion, was limited. Compared to expanded NK-cells (Fig. 4A), non-expanded NK-cells distributed slightly less to liver and more to spleen, but their relative homing to the BM appeared similarly low (Fig. 4B). For comparison, we tracked 89Zr-oxine-labeled CD34+ HSPCs, a cell population known to efficiently localize and engraft in the BM (355 kBq, 44.4 kBq/106 cells, 1.2×106 cells/kg, Supplementary Table S1). In contrast to NK-cells, HSPCs showed robust trafficking to the BM, although infused cell number and 89Zr-activity were also low due to the limitation imposed by the number of these rare cells available for labeling (Fig. 4C, Supplementary S3A). The BM % ID on day 1 for expanded NK-cells, non-expanded NK-cells and HSPCs were 3.4%, 2.4% and 22.1%, respectively (Fig. 4D, Supplementary Fig. S3B). These results show labeled HSPCs can still home to BM and validate the sensitivity of this approach for tracking cell populations of interest to the BM. Importantly, they show that only a small fraction of adoptively transferred NK-cells trafficked to BM, suggesting the capability of infused NK-cells to home to normal BM is limited.
Figure 4. PET/CT imaging shows minimal homing of ex-vivo-expanded and non-expanded NK-cells to the BM compared to HSPCs.
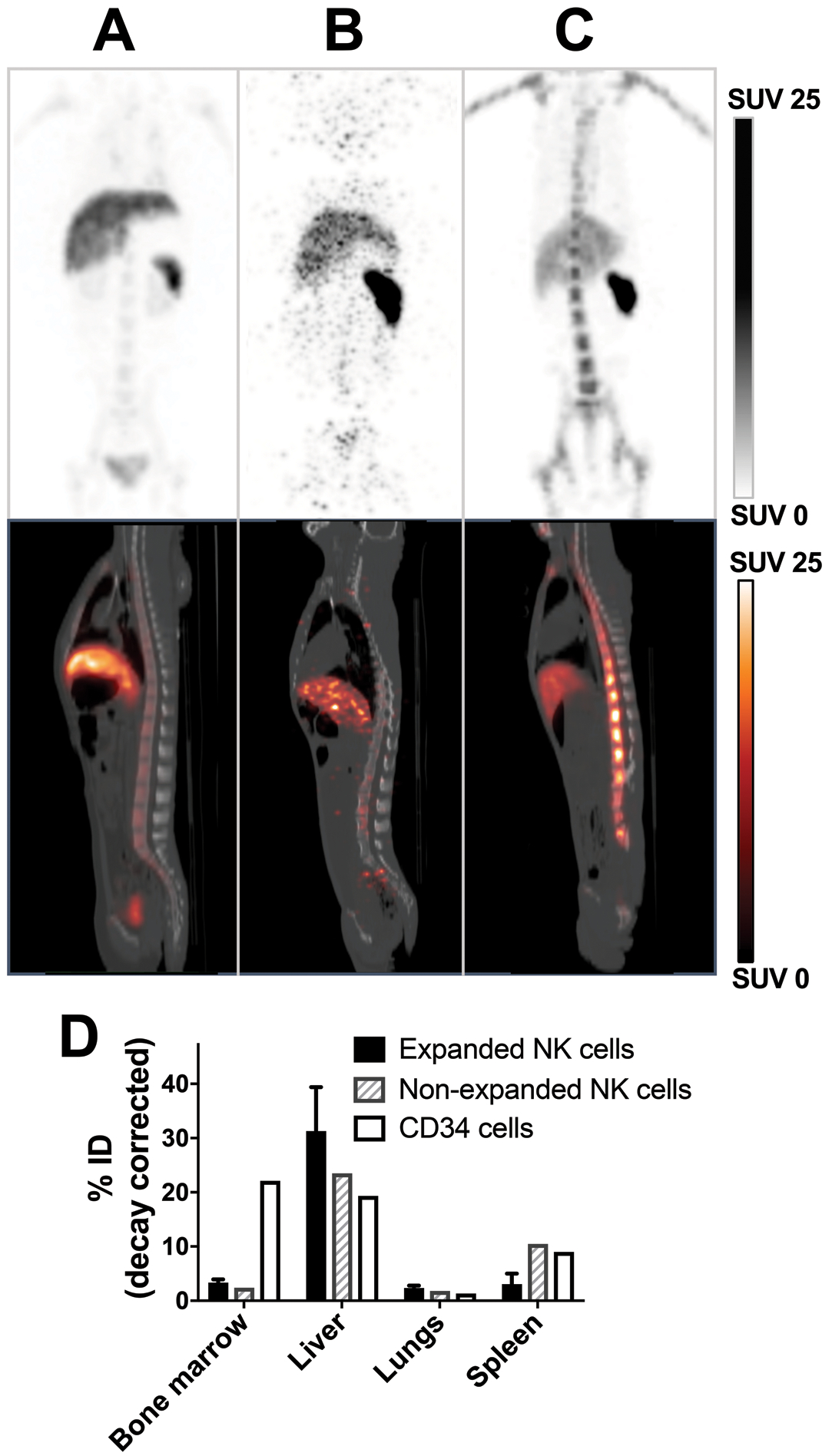
MIP PET (top) and sagittal PET/CT images acquired 1 day after transfer of 89Zr-oxine-labeled expanded NK-cells (A, 2.59 MBq, 15.2 kBq/106 cells, 21.9×106 cells/kg, representative images of 3 independent experiments), non-expanded NK-cells (B, 185 kBq, 6.9 kBq/106 cells, 4.0×106 cells/kg, n=1) and HSPCs (C, 355 kBq, 44.4 kBq/106 cells, 1.2×106 cells/kg, n=1) are presented. D. Quantitation of the day 1 images indicated that both NK-cell populations homed to the BM far less than HSPCs. See Supplementary Fig. S3 for axial PET/CT image of mid-4th lumber spine 1 day after transfer of 89Zr-oxine-labeled HSPCs and migration kinetics.
Liver biopsy shows presence of transferred NK-cells in the liver
To confirm that the radioactivity detected in the liver by PET/CT following the infusion of 89Zr-oxine-labeled NK-cells (Fig. 2A) reflected viable NK-cells that had homed to this organ, a liver biopsy was performed 24 hours following the infusion of CMRA-labeled NK-cells (Fig. 5A). Microscopic analysis of the collected samples showed CMRA-labeled NK-cells residing in the liver (Fig. 5B). Furthermore, flow cytometry analysis of the biopsy sample confirmed the presence of viable CMRA-positive NK-cells within the liver (CD56+ NK-cells represented 0.29 % of the liver lymphocyte population, Fig. 5C).
Figure 5. Biopsy confirms PET/CT imaging studies showing adoptively transferred ex-vivo-expanded NK-cells reside in the liver.
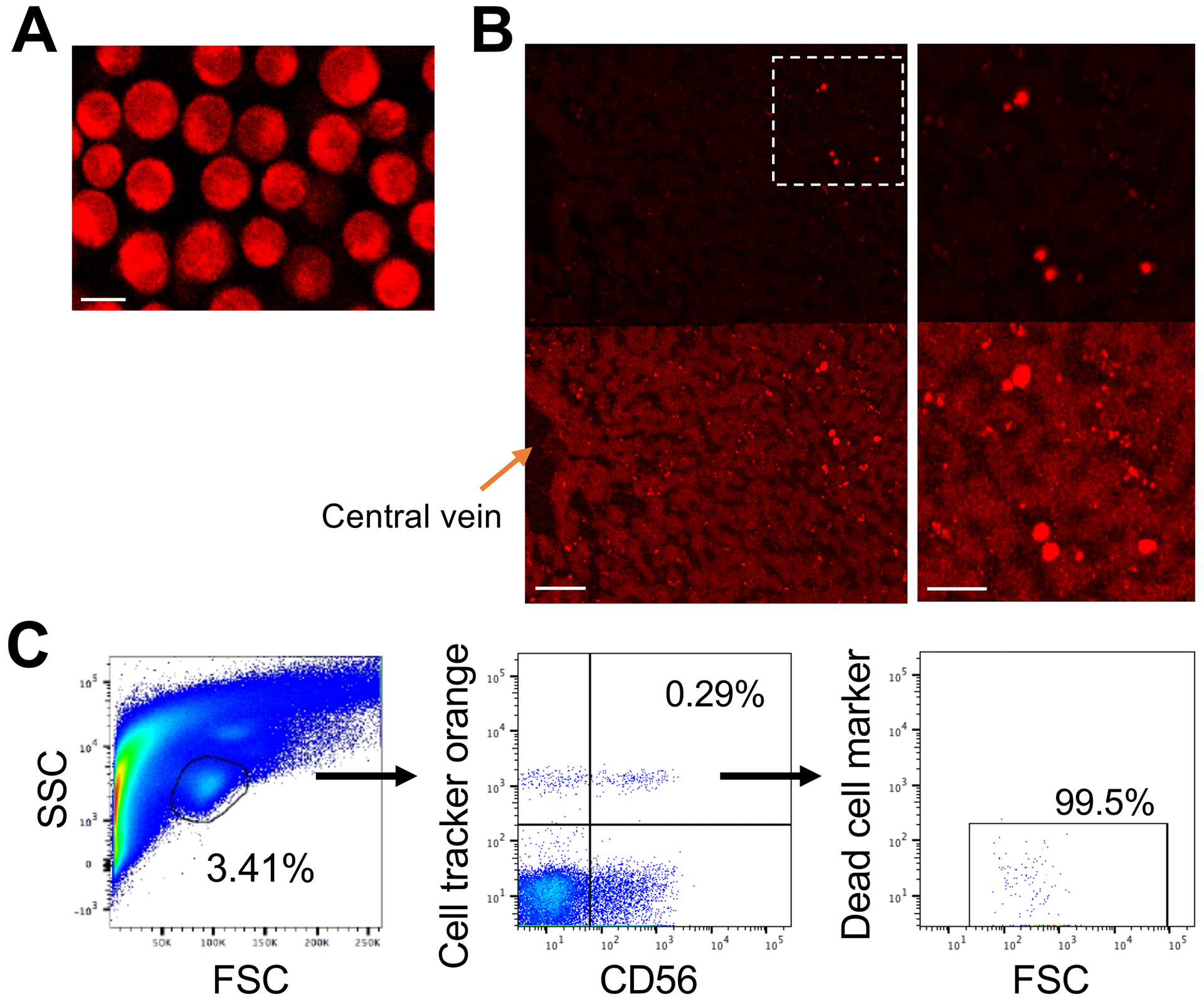
A. Expanded NK-cells were labeled with CMRA (bar indicates 10 μm). B. A biopsy obtained 24 hours following adoptive-transfer (5×107 cells/kg) showed presence of CMRA-labeled NK-cells in the liver (representative image). Brighter images are shown at the bottom to demonstrate liver structures. The right panels show magnified views of the outlined area in the left panel. The bars indicate 100 μm (left) and 50 μm (right). C. Flow cytometry analysis of the biopsy samples showed the presence of viable CMRA-labeled CD56+ NK-cells in the liver.
Safety profile of 89Zr-oxine-labeled NK-cell PET suggests this study approach is translatable to humans.
RMs did not have any changes in their vital signs, weight or clinical exam following the infusion of 89Zr-oxine-labeled cells. Dosimetry in humans, extrapolated from distribution of 89Zr-labeled NK-cells in RMs, indicated that the highest radio-exposure was observed in the spleen and liver (6.24 and 3.88 mSv/MBq, respectively, Table 1). When considering the U.S. Nuclear Regulatory Commission safety guideline limits of 500 mSv per organ or 50 mSv total body, per year, our data suggest that 89Zr-labeled NK-cells below 80.1 MBq (2.17 mCi)/70 kg doses [i.e. 1.14 MBq (30.9 μCi)/kg doses] per year could be infused safely into humans while allowing for tracking of the cells to locations of interest.
Table 1.
Human dosimetry estimates for 89Zr-oxine labeled NK cells
| Organs | mSv/MBq (mean ± s.d.) |
|---|---|
| Liver | 3.880 ± 1.228 |
| Lungs | 1.400 ± 0.323 |
| Red Marrow | 0.319 ± 0.045 |
| Spleen | 6.240 ± 3.606 |
| Total Body | 0.317 ± 0.048 |
| Effective Dose (mSv/MBq) | 0.675 ± 0.135 |
| 1. n=3 | |
| 2. Extrapolated to 70 kg adult humans. | |
Discussion
Although pilot trials have established that adoptive NK-cell transfer can induce anti-tumor effects, the overall efficacy to date of NK-cell-based immunotherapy has been limited (6–8). One reason may be that infused NK-cells are simply not migrating through the blood stream to relevant target organs where the malignancies reside, such as the BM or lymph nodes for hematological malignancies. In this study, we developed a method of tracking NK-cells in-vivo using 89Zr-oxine ex-vivo cell labeling and PET/CT imaging in RM. RM NK-cells have similar phenotypic and functional properties to human NK-cells and can be expanded ex-vivo using methods currently utilized in clinical trials (20). In contrast, mouse NK-cells have significant phenotypic and functional divergence from human NK-cells. For instance, most mouse NK-cells lack Fas and TNF-related apoptosis-inducing ligand used to kill tumor targets (25,26), and they do not possess killer-cell immunoglobulin-like receptors (27,28) which play a critical role in regulating NK-cell cytotoxicity. Further, most conditions used for expanding human NK-cells ex-vivo are ineffective in expanding murine NK-cells. Taken altogether, these factors favor the use of RMs over mice for studies exploring NK-cell trafficking.
The BM is the site of origin and proliferation of many hematological malignancies. NK-cells are known to express BM homing receptors such as CXCR4 (29) and CD62L (30). However, CXCR4 may be downregulated during ex-vivo NK-cell expansion (29,30) and E-selectin ligands expressed on expanded NK-cells are primarily non-glycosylated, making them inactive for binding E-selectin which is expressed on BM endothelial cells (31). These, as well as other factors, may in part account for the limited BM trafficking of ex-vivo-expanded NK-cells that we observed in our study. However, it is important to consider that we also observed that non-expanded NK-cells had poor homing to the BM. Migration of NK-cells to lymph nodes was also not observed, consistent with low expression and/or down-regulation of CCR7 reported during culture (32). In contrast, as would be expected, 89Zr-oxine-labeled HSPCs showed robust trafficking to the BM. Collectively, the low level trafficking observed with adoptively transferred NK-cells to the BM of RMs may in part explain the limited efficacy observed in clinical trials exploring adoptive NK-cell therapy for BM malignancies. It is possible that NK-cells would show superior homing or may only home to the bone marrow infiltrated by malignant cells. Unfortunately, we lack primate models for hematological malignancies to address this interesting question.
We observed high levels of liver homing of transferred NK-cells. Recent studies have revealed that high numbers of NK-cells, consisting of 30–40% lymphocytes, reside in the liver (33). Attraction and retention of NK-cells in the liver are considered to be mediated by chemokines such as CCL3, CCL5, and CXCL16, but only the CD56bright hepatic NK-cells have been characterized to express receptors for these ligands (34,35). In the context of our study’s findings, a characterization of liver homing receptors on NK-cells expanded using a variety of different approaches would be of interest. Modification of expansion conditions (9,10) or genetic modification of NK-cells to over-express BM homing receptors (29) or to under-express liver homing receptors may improve trafficking of these cells to the BM. 89Zr-oxine cell tracking could be a very useful tool to assess the impact of such maneuvers to alter NK-cell homing to the BM, lymph nodes, and other target tissues.
Previous studies suggest that, following 89Zr-oxine cell membrane permeabilization, 89Zr is retained in viable cells (14,16). Cell survival in culture showed substantial donor-to-donor variation, likely accounting for the large standard deviations observed in the calculated 89Zr-activity per cell. Further, following harvesting of ex-vivo-expanded NK-cells, some degree of proliferation continued to occur even after cells were labeled with 89Zr-oxine. We believe this persisting NK-cell proliferation likely caused the decline of 89Zr-activity/cell observed at earlier time points. In this context, 89Zr-oxine-labeled cells that are damaged or die following adoptive-transfer would be expected to release free 89Zr. Whole body 89Zr retention after labeled-NK-cell transfer was higher than expected from extrapolation of in-vitro retention over time. Better in-vivo survival of NK-cells could perhaps be explained by support from endogenous cytokines such as IL-15, or microenvironmental survival signals provided by tissues.
Although the majority of released 89Zr is excreted via the kidneys (14), it is possible that a fraction of free 89Zr could bind to hydroxyapatite in bone resulting in high 89Zr-signals in the bones (24), potentially confounding the accurate quantitation of cellular homing to the BM. To preclude this possibility in our experiments, RMs received a continuous infusion of deferoxamine, the most commonly used and clinically applicable chelator for 89Zr (36), to chelate and promote the renal excretion of any free 89Zr. In our previous murine studies, multiple intramuscular injections of deferoxamine reduced bone uptake of free 89Zr (14,16). As RM studies are limited by regulations and availability, it was not feasible to perform control 89Zr-oxine labeled NK-cell tracking in the absence of deferoxamine. Although bone uptake of 89Zr would spuriously inflate calculated marrow localization, the very low BM localization observed with 89Zr-labeled NK-cells in our study does not confound our conclusions.
The experiment infusing 89Zr-labeled apoptotic NK-cells, confirmed the efficiency of deferoxamine chelation and suggests that deferoxamine minimizes the direct transfer of 89Zr from dead/dying cells to phagocytes. Of note, in-vitro experiments did not detect 89Zr transfer from live 89Zr-oxine-labeled NK-cells to non-labeled cells when two cell populations were co-cultured, including PBMCs containing cells with phagocytic properties. These characteristics of 89Zr-retention within live cells and release and rapid clearance of 89Zr from the circulation after cell death are essential for accurate interpretation of imaging studies using radiolabeled agents for in-vivo cell tracking. Importantly, liver biopsy revealed that viable CMRA-labeled NK-cells were present in a RM following adoptive infusion of NK-cells, confirming the findings of the PET studies. Further, the deferoxamine doses we used were the same as those administered to humans treated with this agent for iron overload, establishing the clinical applicability of this strategy for patients receiving 89Zr-oxine-labeled NK-cells.
The PET studies provided a good dosimetry model to estimate potential radio-exposure in humans. The spleen and liver received the highest exposure among organs, however, the doses were still well within safety guidelines when no more than 157.7 MBq (4.26 mCi)/70 kg doses (i.e. 1.14 MBq (30.9 μCi)/kg doses) are administered to humans. This corresponds to 83.8×106 NK-cells/kg when the cells were labeled with a specific activity of 13.6 kBq/106 cells used in this study. We observed high background noise with the PET/CT images of non-expanded NK-cells when only 185 kBq (27.2 kBq/kg) was infused, due to the small number of NK-cells we were able to purify from PBMCs, suggesting this dose is at the detection limit of PET/CT. On the other hand, the dose used for HSPCs (355 kBq, 54.6 kBq/kg) generated high quality images with low background noise. Based on these results, we estimate that an approximately 55 kBq (1.5 μCi)/kg dose of 89Zr-oxine-labeled cells would be sufficient to provide good quality images by PET/CT to accurately track these cells in-vivo. These data indicate that in future clinical trials evaluating adoptive NK-cell infusions in humans, where cell numbers in the order of 5–10 × 108 cells/kg are typically infused, only a small fraction of the cells being infused would need to be labeled to provide robust qualitative and quantitative information on cellular trafficking.
In conclusion, 89Zr-oxine labeling of NK-cells followed by PET/CT imaging represents a promising new tool to track the in-vivo fate of adoptively transferred NK-cells. The RM model presented here provides a method to evaluate and optimize various strategies to improve NK-cell trafficking and homing to the BM, lymph nodes, and other locations where hematological malignancies and tumors reside. These preclinical in-vitro and in-vivo data suggest this technology could be safely extended to humans and could be applied to other cellular populations besides NK-cells.
Supplementary Material
TRANSLATIONAL RELEVANCE.
The success of adoptive immunotherapy for hematological malignancies may require infused cells to traffick to the bone marrow (BM) where malignant cells often reside. We developed a novel method to track and quantify organ distribution of adoptively transferred natural killer (NK)-cells using 89Zr-oxine ex-vivo cell labeling and positron emission tomography (PET). Low 89Zr-oxine doses required for imaging did not alter NK-cell viability or function. Following autologous infusion into rhesus macaques, labeled NK-cells were tracked for 7 days, passing through the lungs, then localizing primarily to the liver, spleen and, at low levels, the BM. No toxicity was observed, and dosimetry estimates extrapolated to humans suggested tracking could be achieved with very low radio-exposure to organs. These data suggest that 89Zr-oxine-labeled NK-cell PET can be safely translated to humans for tracking distribution of adoptively infused cells and for assessing cell modification techniques to improve immune cell homing to tumor microenvironments.
Acknowledgements
We thank Marvin L Thomas, Theresa M. Engels, Randall R. Clevenger, Timothy Hunt, Kenneth R. Jeffreys, Mark Metzger, Allen E. Krouse, Sandra D. Price, Robert E. Donahue, Robert F. Hoyt, Jr., Karen J. Keeran, Arthur Zetts and Shawn Kozlov for animal care, Lawrence P. Szajek for generating 89ZrCl4, and Gideon K. Kwarteng, Philip C. Eclarinal and Alicia Forest for acquiring PET/CT, and Daniela A. Malide for microscopic analysis of the biopsy samples. We also wish to acknowledge the NHLBI and NCI intramural research programs, and the Dean R. O’Neill and Edward Rancic Cancer Fellowship for their support of this research.
Financial support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the National Heart, Lung, and Blood Institute, Division of Intramural Research. Regarding S. Adler; This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of interest: The authors declare no competing financial interests. The authors N.S. and P.L.C. have filed U.S. Patent Applications for generation and application of the 89Zr-oxine complex. The author R.W.C. has filed a U.S. Patent Application for the method used in this study to expand NK-cells ex-vivo.
References
- 1.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev 2006;20(3):123–37 doi 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Caligiuri MA. Human natural killer cells. Blood 2008;112(3):461–9 doi 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011;331(6013):44–9 doi 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bern MD, Parikh BA, Yang L, Beckman DL, Poursine-Laurent J, Yokoyama WM. Inducible down-regulation of MHC class I results in natural killer cell tolerance. The Journal of experimental medicine 2019;216(1):99–116 doi 10.1084/jem.20181076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev 2006;214:202–18 doi 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 6.Bjorklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(8):1834–44 doi 10.1158/1078-0432.CCR-17-3196. [DOI] [PubMed] [Google Scholar]
- 7.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010;28(6):955–9 doi 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011;13(1):98–107 doi 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JS, Rooney CM, Curtsinger J, McElmurry R, McCullar V, Verneris MR, et al. Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: implications for clinical therapy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2014;20(8):1252–7 doi 10.1016/j.bbmt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grzywacz B, Moench L, McKenna D Jr., Tessier KM, Bachanova V, Cooley S, et al. Natural Killer Cell Homing and Persistence in the Bone Marrow After Adoptive Immunotherapy Correlates With Better Leukemia Control. Journal of immunotherapy (Hagerstown, Md : 1997) 2019;42(2):65–72 doi 10.1097/cji.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cianciaruso C, Pagani A, Martelli C, Bacigaluppi M, Squadrito ML, Lo Dico A, et al. Cellular magnetic resonance with iron oxide nanoparticles: long-term persistence of SPIO signal in the CNS after transplanted cell death. Nanomedicine (Lond) 2014;9(10):1457–74 doi 10.2217/nnm.14.84. [DOI] [PubMed] [Google Scholar]
- 12.Gholamrezanezhad A, Mirpour S, Ardekani JM, Bagheri M, Alimoghadam K, Yarmand S, et al. Cytotoxicity of 111In-oxine on mesenchymal stem cells: a time-dependent adverse effect. Nuclear medicine communications 2009;30(3):210–6 doi 10.1097/MNM.0b013e328318b328. [DOI] [PubMed] [Google Scholar]
- 13.Gildehaus FJ, Haasters F, Drosse I, Wagner E, Zach C, Mutschler W, et al. Impact of indium-111 oxine labelling on viability of human mesenchymal stem cells in vitro, and 3D cell-tracking using SPECT/CT in vivo. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging 2011;13(6):1204–14 doi 10.1007/s11307-010-0439-1. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Wu H, Asiedu KO, Szajek LP, Griffiths GL, Choyke PL. (89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies. Radiology 2015;275(2):490–500 doi 10.1148/radiol.15142849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nuclear medicine communications 2008;29(3):193–207 doi 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 16.Asiedu KO, Koyasu S, Szajek LP, Choyke PL, Sato N. Bone Marrow Cell Trafficking Analyzed by (89)Zr-oxine Positron Emission Tomography in a Murine Transplantation Model. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(11):2759–68 doi 10.1158/1078-0432.Ccr-16-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asiedu KO, Ferdousi M, Ton PT, Adler SS, Choyke PL, Sato N. Bone marrow cell homing to sites of acute tibial fracture: (89)Zr-oxine cell labeling with positron emission tomographic imaging in a mouse model. EJNMMI Res 2018;8(1):109 doi 10.1186/s13550-018-0463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg M, Lundqvist A, McCoy P Jr., Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009;11(3):341–55 doi 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Childs RW, Berg M. Bringing natural killer cells to the clinic: ex vivo manipulation. Hematology Am Soc Hematol Educ Program 2013;2013:234–46 doi 10.1182/asheducation-2013.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong HS, Rajakumar PA, Billingsley JM, Reeves RK, Johnson RP. No monkey business: why studying NK cells in non-human primates pays off. Front Immunol 2013;4:32 doi 10.3389/fimmu.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida N, Bonifacino A, Krouse AE, Metzger ME, Csako G, Lee-Stroka A, et al. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34+ cells mobilized by G-CSF and plerixafor. Exp Hematol 2011;39(7):795–805 doi 10.1016/j.exphem.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue RE, Kuramoto K, Dunbar CE. Large animal models for stem and progenitor cell analysis. Curr Protoc Immunol 2005;Chapter 22:Unit 22A 1 doi 10.1002/0471142735.im22a01s69. [DOI] [PubMed] [Google Scholar]
- 23.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nuclear medicine and biology 2009;36(7):729–39 doi 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou DS, Ku T, Smith-Jones PM. In vivo biodistribution and accumulation of 89Zr in mice. Nuclear medicine and biology 2011;38(5):675–81 doi 10.1016/j.nucmedbio.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. The Journal of experimental medicine 1998;188(12):2375–80 doi 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol 1999;163(4):1906–13. [PubMed] [Google Scholar]
- 27.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172(5):2731–8 doi 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 28.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol 2018;9:1869 doi 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy E, Reger R, Segerberg F, Lambert M, Leijonhufvud C, Baumer Y, et al. Enhanced Bone Marrow Homing of Natural Killer Cells Following mRNA Transfection With Gain-of-Function Variant CXCR4(R334X). Front Immunol 2019;10:1262 doi 10.3389/fimmu.2019.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terren I, Mikelez I, Odriozola I, Gredilla A, Gonzalez J, Orrantia A, et al. Implication of Interleukin-12/15/18 and Ruxolitinib in the Phenotype, Proliferation, and Polyfunctionality of Human Cytokine-Preactivated Natural Killer Cells. Front Immunol 2018;9:737 doi 10.3389/fimmu.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov 2015;14(7):487–98 doi 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 32.Somanchi SS, Senyukov VV, Denman CJ, Lee DA. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp 2011;48:e2540 doi 10.3791/2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43(2 Suppl 1):S54–62 doi 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 34.Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun 2016;66:40–50 doi 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol 2016;16(5):310–20 doi 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- 36.Price TW, Greenman J, Stasiuk GJ. Current advances in ligand design for inorganic positron emission tomography tracers (68)Ga, (64)Cu, (89)Zr and (44)Sc. Dalton Trans 2016;45(40):15702–24 doi 10.1039/c5dt04706d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


