Abstract
Major depressive disorder (MDD) is a disabling mental disorder worldwide. Several animal models have been used to study the neurobiology of this disorder, including the learned helplessness (LH) paradigm, in which susceptible animals show helpless behavior indicated by fails to escape a controllable footshock. This behavior has been associated with a downregulation of ventral tegmental area (VTA) dopamine (DA) system activity. The prelimbic portion of the prefrontal cortex (plPFC) plays an important role in the modulation of helpless behavior, but so far there is no evidence indicating that its developmental disruption alters susceptibility to helpless behavior. We investigated the impact of plPFC lesion performed at adolescence (postnatal day 31–33) or adulthood (postnatal day 70–72) on anxiety responses (elevated plus-maze), susceptibility to helpless behavior, and the VTA DA system activity in adult Sprague-Dawley rats. Whereas adult plPFC lesions induced neither anxiety responses nor increased susceptibility to helpless behavior (plPFC lesion: 33.3% of helplessness; controls: 30.8% of helplessness rats), adolescent plPFC lesions induced anxiety responses and increased the proportion of rats showing helpless at adulthood (plPFC lesion: 92.3% helplessness; controls: 42.1% helplessness rats). Moreover, only helpless rats in the groups showed a decreased VTA DA system population activity that was confined to the medial portion of the VTA. These findings suggest that the impairment of plPFC activity during adolescence occurs during a critical window for the development of helpless behavior in adult rats, indicating that predisposition or early life adverse events that impair plPFC activity may enhance susceptibility to depression in adulthood.
Keywords: depression, dopamine, prefrontal cortex, adolescence
1. Introduction
Major depressive disorder (MDD) is a disabling disorder with high prevalence worldwide (Kessler and Bromet, 2013). MDD is complex and involves a multitude of symptoms, such as depressed mood, loss of interest or pleasure (i.e., anhedonia), fatigue, and sleep problems for 2 weeks or more (American Psychiatric Association, 2013). An important external factor that can predict the development of depression is stress (Richter-Levin and Xu, 2018). Several animal models based on stressful situations are used to study depression, such as chronic mild stress (CMS), social defeat, and learned helplessness (LH) (Czéh et al., 2016; Muir et al., 2018). The LH paradigm, in particular, is based on the inability of the individual to escape from an aversive controllable situation due to a previous unpredictable/uncontrollable stress (Maier and Seligman, 2016; Seligman and Maier, 1967). It is proposed that LH induces behavioral changes that have some similarity with those observed in depressive patients, such as anhedonia (Sanchis-Segura et al., 2005).
Anhedonia and helpless behavior have been associated with a decreased activity of the dopamine (DA) reward system (Nestler and Carlezon, 2006; Wise, 2008). Rats undergoing LH, CMS and cold stress models for depression all show a hypodopaminergic state indicated by a decrease in the activity of ventral tegmental area (VTA) DA neurons (Valenti et al., 2012; Chang and Grace, 2014; Moreines et al., 2017; Rincón-Cortés and Grace, 2017; Douma and de Kloet, 2019; Kaufling, 2019). In addition, optogenetic inhibition of the activity of VTA DA neurons has been described to induce a depression-like phenotype (Tye et al., 2013). Therefore, the downregulation of the DA system seems to be critically related to a depressive-like phenotype, including helpless behavior (Belujon and Grace, 2017, 2014).
Of the many brain regions involved in the regulation of stress responses, the medial prefrontal cortex (mPFC) plays a prominent role (McEwen and Morrison, 2013). There are two subdivisions of the mPFC that have been linked with stress and affect regulation. The infralimbic PFC (ilPFC) exhibits an excitatory projection to the amygdala (Likhtik et al., 2006) and activation of this pathway leads to increased anxiety (Radley et al., 2006) and with repeated stress, depression-like behaviors (Chang and Grace, 2014). In contrast, the prelimbic PFC (plPFC) has projections to GABAergic neurons in the amygdala (Rosenkranz and Grace, 2002) and has been shown to attenuate amygdala responsivity to stressors (Figueiredo et al., 2003; Jones et al., 2011; Radley et al., 2006; Rosenkranz and Grace, 2002). Thus, a plPFC dysfunction could increase amygdala responsivity to stress, which in turn could facilitate the impact of stress. We found previously that adolescent plPFC lesions increased vulnerability to the development of schizophrenia-like changes induced by stress during adolescence in rats (Gomes and Grace, 2017). Concerning depression, studies investigating LH neurobiology demonstrate an important role of plPFC activity in detecting stress controllability via inputs to the dorsal raphe nucleus (DRN) (Amat et al., 2008, 2005; Maier and Seligman, 2016), indicating that the plPFC provides a top-down control over helpless behavior.
Adolescence represents a critical period in the maturation of the PFC (Caballero and Tseng, 2016) and adversities experienced during this period of neurodevelopment have a profound impact on the emergence of several psychiatric disorders, including depression (Andersen and Teicher, 2008; Brenhouse and Andersen, 2011; Spear, 2013; Keshavan et al., 2014; Marín, 2016). However, there is no evidence examining whether developmental disruption of the plPFC affects susceptibility to helpless behavior. Thus, we investigated the impact of plPFC lesion, performed at adolescence or adulthood, on the susceptibility to helpless behavior and its corresponding effects on VTA DA system activity in adult Sprague–Dawley rats.
2. Experimental procedures
2.1. Animals
Sixteen pregnant Sprague–Dawley rats (Envigo, Indianapolis, USA) were obtained on gestational day 14 and each pregnant rat was housed individually in ventilated plastic breeding tubs. Male offspring (81 rats) were weaned on postnatal day (PD) 21 and housed in groups of two or three with littermates. Male rats were used in this study to match prior work; future work extending to females will require study into the precise timing of the insult given differences in puberty onset and susceptibility to LH. Forty-two rats at PD 31–33 were subjected to the plPFC lesion surgery (24 sham and 18 lesioned animals) and used for behavior/electrophysiology experiments at adulthood (>PD65). Thirty-nine adult rats at PD 70–72 were subjected to adult plPFC lesion surgery (19 sham and 20 lesioned rats) and tested for behavior/electrophysiology after at least 10 days of recovery. All rats were housed under standard housing conditions: 12h/light-dark cycle (regular light cycle: lights on at 7 a.m.; reverse light cycle: lights on at 7 p.m.) and temperature-controlled room (22±1°C), with free access to food and water. Behavioral experiments were performed during the lights-off cycle. The procedures were carried out following the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
2.2. Experimental Design
We tested whether a lesion of the plPFC during either adolescence or adulthood would increase the susceptibility of adult Sprague–Dawley rats to LH. The plPFC lesion was induced by infusing ibotenic acid bilaterally into the plPFC in rats at PD31–33 (adolescence) or PD70–72 (adulthood). As adults, rats were tested in the elevated plus-maze (EPM) and then subjected to LH two days later. Four days after LH, activity patterns of VTA DA neurons were assessed. A separate group of animals that received saline (sham) or ibotenic acid injection during adolescence (PD31) and adulthood (PD70) were tested in the EPM and the VTA DA recordings at adulthood, without being exposed to LH. The injections were randomized by experimenters and blinded for the behavioral and electrophysiological experiments.
2.3. plPFC lesion
The rats were anesthetized with isoflurane and fixed in a stereotaxic frame. Ibotenic acid (5μg/0.5μl; Sigma) or saline (0.9% NaCl) was infused bilaterally (0.5μl) into the plPFC using a 10μl Hamilton syringe and infusion pump (11 plus Harvard apparatus; Rate: 200μl/min). The plPFC coordinates used in adolescent rats were: +2.9 mm antero-posterior, ±0.5 mm mediolateral from bregma, − 3.1 mm ventral from the skull surface, these coordinates were based on a previous study from our group (Gomes and Grace, 2017); and for adults: + 3.2 mm antero-posterior, ± 0.7 mm mediolateral from bregma, − 4.1 mm ventral from skull surface, according to Paxinos and Watson (2007) (Paxinos and Watson, 2007). A subcutaneous injection of the nonsteroidal anti-inflammatory carprofen (Rimadyl, Zoetis Inc) was administered for post-operative analgesia at the end of the surgery. Medicated food (MediGel®CPF) was also provided in the rat homecage. The animals were kept together with their respective littermates during recovery. Histological verification of the lesion was evaluated at adulthood (Figure1A–B), after the electrophysiology recordings of DA neurons in the VTA.
Figure 1.
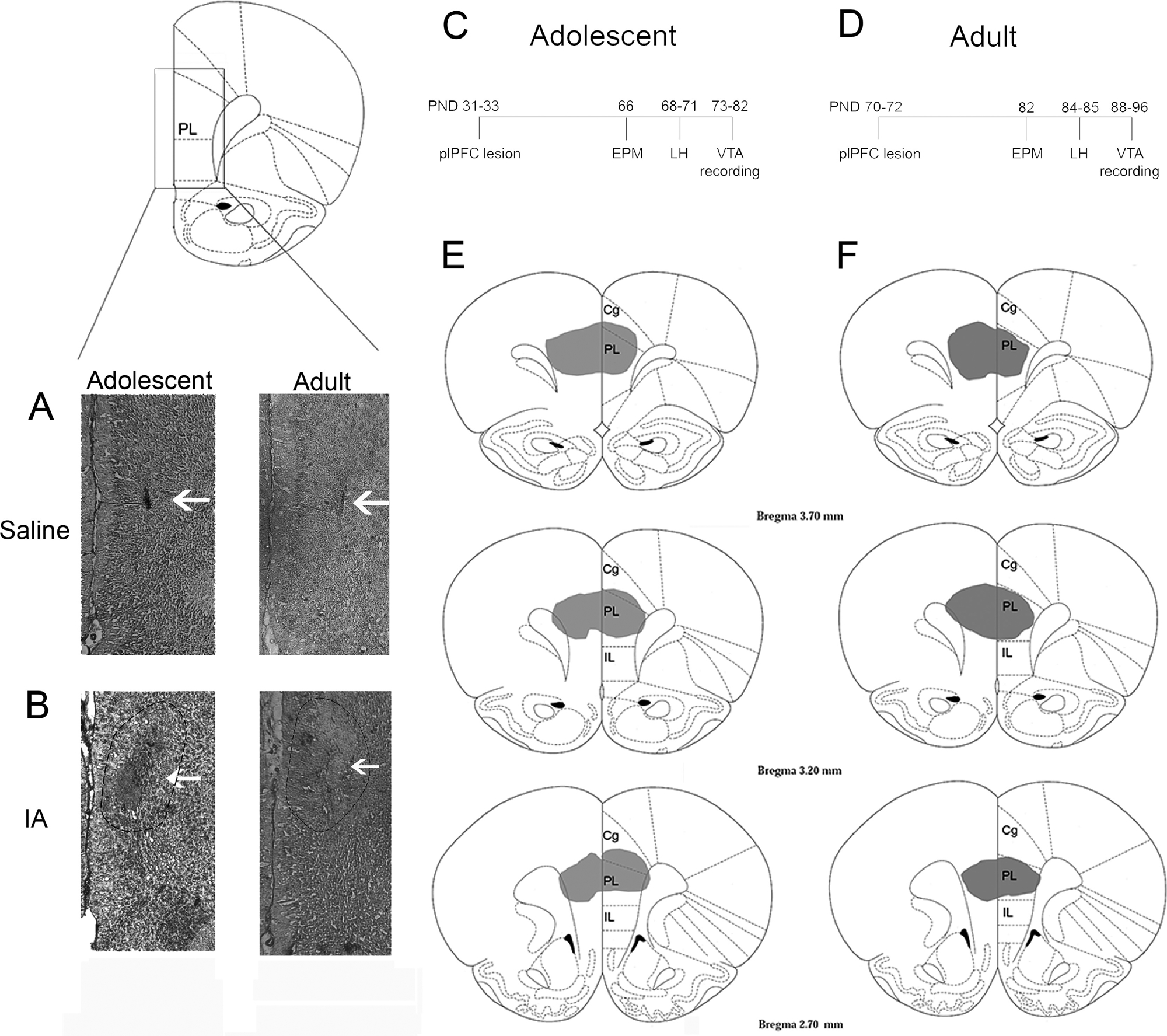
Schematic representation of lesion extent in the plPFC. Representative photomicrographs of saline (A) and ibotenic acid (IA; B) injections into plPFC of adolescent and adult rats. Time course of the adolescent (C) and adult (D) plPFC lesion experiments. Coronal section illustration of the extent of the ibotenic acid lesion in adolescent rats (E) and adult rats (F). Cg: cingulate; PL: prelimbic PFC; IL: infralimbic PFC.
2.4. Elevated plus-maze (EPM)
The EPM consisted of two opposite open (50 cm × 10 cm) and enclosed (40 cm high opaque wall) arms crossed at a right angle and located 50 cm above the floor. In the test, rats were placed in the center of the maze facing an enclosed arm. The test was recorded over 5 minutes and the percentage of time and entrances into the open arms were measured as an index of anxiety. The number of total entries was evaluated as an index of locomotor activity.
2.5. Learned Helplessness (LH)
The LH paradigm has been widely used as a model of stress inducing depression-like behaviors and was based on a previous study from our group (Belujon and Grace, 2014). The apparatus consisted of a two-chamber shuttle box with grid floor and stainless-steel rods plugged into a shock generator (Med Associates, USA). On day 1, the rats were subjected to inescapable stress session, consisting of 120 inescapable and uncontrollable footshocks (1 mA; 7–22 s interval duration; 35–85 s interval between the footshock) in one side of the chamber. In the next day, the animals were tested for helpless behavior in the active avoidance session. The rats were first acclimated to the shuttle box for 5 minutes (both sides), following by 25 trials of unexpected and escapable footshock. The trial started with a 5-second tone and a light followed by a 0.8 mA footshock. The termination of shock required two crossings in the shuttlebox (FR2) or a maximum of 15 seconds in case of no crossing response. The number of escape failures and the mean escape latency were evaluated during 25 FR2 trials. More than 10 escape failures and 8 seconds of the mean latency to escape were used as a criterion for helpless behavior, according to a previous study by our group (Belujon and Grace, 2014).
2.6. In vivo extracellular VTA DA neuron recordings
Rats were anesthetized with chloral hydrate (400mg/kg, i.p., Sigma), following by fixation in the stereotaxic frame. A thermostatically-controlled heating pad was used to maintain body temperature at 37 °C. Electrodes pulled from Omegadot 2.0 mm glass tubing were used for in vivo extracellular recordings of DA neurons in the VTA. The electrode tip was broken back under microscopic control to an impedance of 6–16 MΩ and filled with 2M NaCl containing 2% Sky Blue dye (Sigma). The electrodes were lowered into VTA (− 5.3 mm posterior from bregma, 0.6 mm lateral to the midline and 6.5–9.0 mm ventral from brain surface). Six to nine vertical tracks were sampled within the VTA in a fixed pattern (Figure 2D, Belujon and Grace, 2014). The parameters evaluated were: population activity (number of spontaneously active DA neurons per track), firing rate and percentage of spikes occurring in bursts. These parameters were also evaluated in relation to medial, central and lateral portions of VTA. The identification of DA neurons was based on well-established electrophysiology characteristics (Figure 2, Grace and Bunney, 1983; Ungless and Grace, 2012). In the VTA recordings, some animal data were excluded due to electrode misplacement. Five rats (3 from the saline group and 2 from the ibotenic acid group) were excluded in the adolescent lesion experiment due to incorrect electrode placements.
Figure 2.
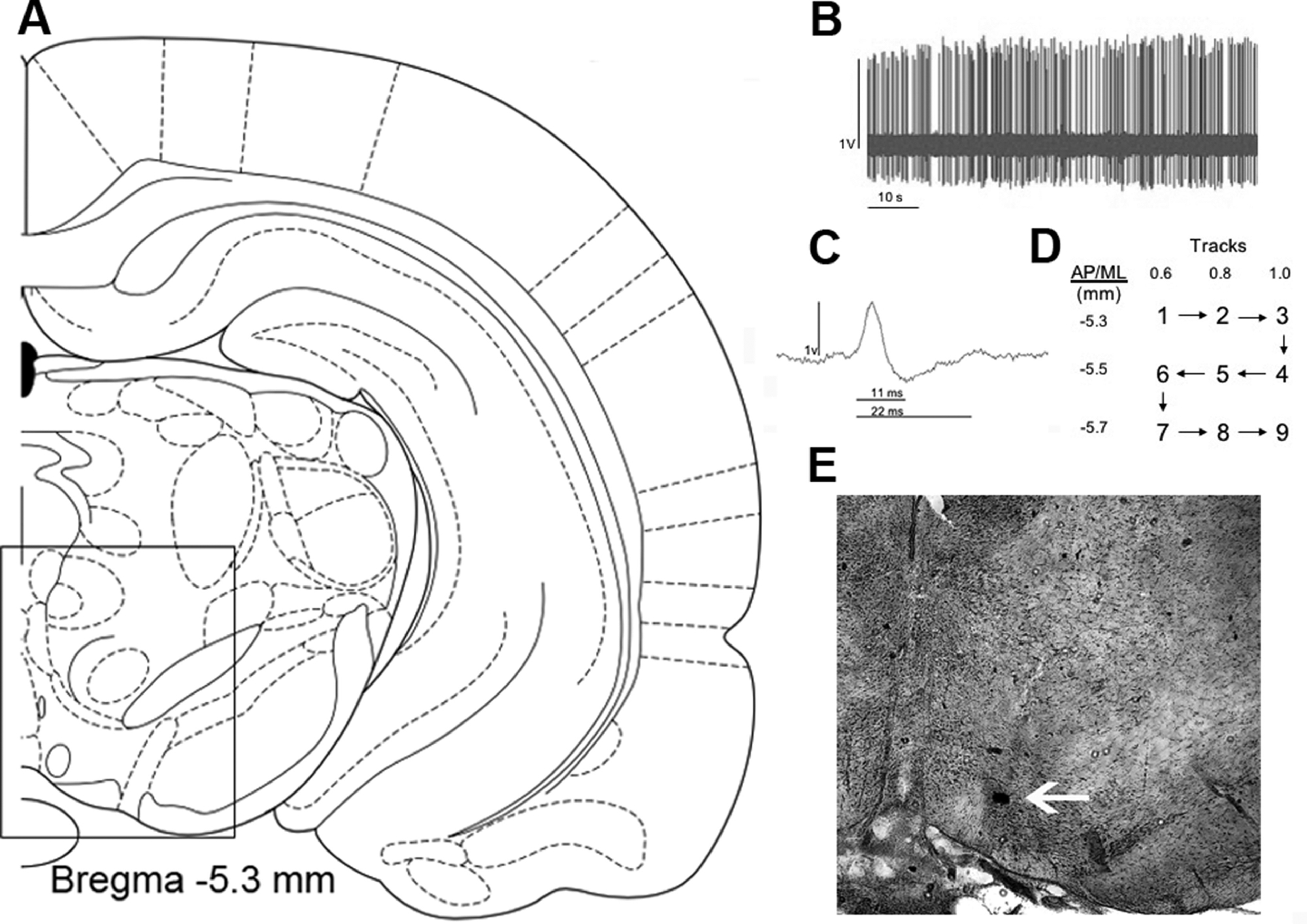
Electrophysiological properties of dopaminergic neuron recording in the VTA (A). Spontaneous activity tracing recorded over 1 min (B). A representative dopaminergic neuron waveform (C). The pattern of tracks performed during the recording in the VTA (D) and representative photomicrography of an electrode localization in the VTA (E). (A) was adapted from Paxinos and Watson, 2007.
2.7. Histology
The electrode location was marked with an electrophoretic ejection of Chicago Sky Blue dye (−20μA constant negative current, 20 min) at the end of VTA recordings (Figure 2E). Euthanasia was induced by the administration of a lethal dose of chloral hydrate (additional 400mg/kg).
The rats were decapitated and the brains removed. The brains were fixed in 8% paraformaldehyde for approximately 48h followed by 25% sucrose solution for cryoprotection. After saturation, the brains were frozen and sliced coronally (PFC, 40 μm; VTA, 60 μm) using a cryostat (Leica Frigocut 2800). PFC and VTA slices were mounted on gelatin-coated slides, followed by staining with a combination of neutral red and cresyl violet.
2.8. Statistical Analyses
Behavior and electrophysiology data were expressed as mean ± SEM and analyzed using Student’s t-test or 2-way ANOVA with lesion (ibotenic acid or saline) and condition [(1) not exposed to the LH and (2) exposed to the LH - helplessness (3) exposed to the LH - nonhelplessness] as main factors followed by the Tukey’s post hoc test. The percentage of rats showing helpless behavior was analyzed using the Chi-square test. p<0.05 was defined as significant.
3. Results
3.1. Adolescent plPFC lesion promotes anxiety and increases susceptibility to helpless behavior in adulthood.
The extents of the plPFC lesions performed during adolescence or adulthood are illustrated in Figure 1E–F.The plPFC lesion was equivalent in both nonhelpless and helpless rats in the adolescent and adult experiments. Similar to our previous findings with juvenile (PD24) plPFC lesions (Gomes and Grace, 2017), we found that the plPFC lesion at PND 31–33 (n=18) induced anxiety responses at adulthood, as indicated by a decrease in the percentage of time (t40=4.01, p<0.05, t-test, Figure 3A) and entries into the open arms in the EPM compared to controls (n=24; t40=3.27 p<0.05, t-test, Figure 3B). These changes were not driven by altered locomotor activity since no significant difference was observed for the number of entries into all arms of the EPM (p>0.05, t-test, Figure 3C). Concerning LH, adolescent plPFC lesions increased susceptibility to helpless behavior at adulthood (z=7.52, p<0.05, Chi-square test, Figure 3D). Whereas the percentage of adult rats with plPFC lesions during adolescence showing helpless behavior was 92.3% (12 of 13 total rats), only 42.1% of sham rats (8 of 19 total rats) developed helpless behavior. Adolescent plPFC lesions (n=13) increased the latency to escape (t30=2.92, p<0.05, t-test, Figure 3F) and the number of escape failures (t30=2.82, p<0.05, test-t, Figure 3E) compared to the saline-injected group (n=19). In addition, 2-way ANOVA indicated an effect of the condition for the latency to escape (F1,28=40.76, p<0.05) and number of escape failures (F1,28=78.63, p<0.05), but with no effect of the lesion when the animals were separated based on the behavior (p>0.05) or an interaction between condition and lesion (p>0.05). And, as expected, post-hoc analysis showed an increase in the latency to escape (p<0.05; Tukey’s test) and the number of escape failures (p<0.05; Tukey’s test) in helplessness rats compared to nonhelplessness rats in both groups (Figure 3E and 3F).
Figure 3.
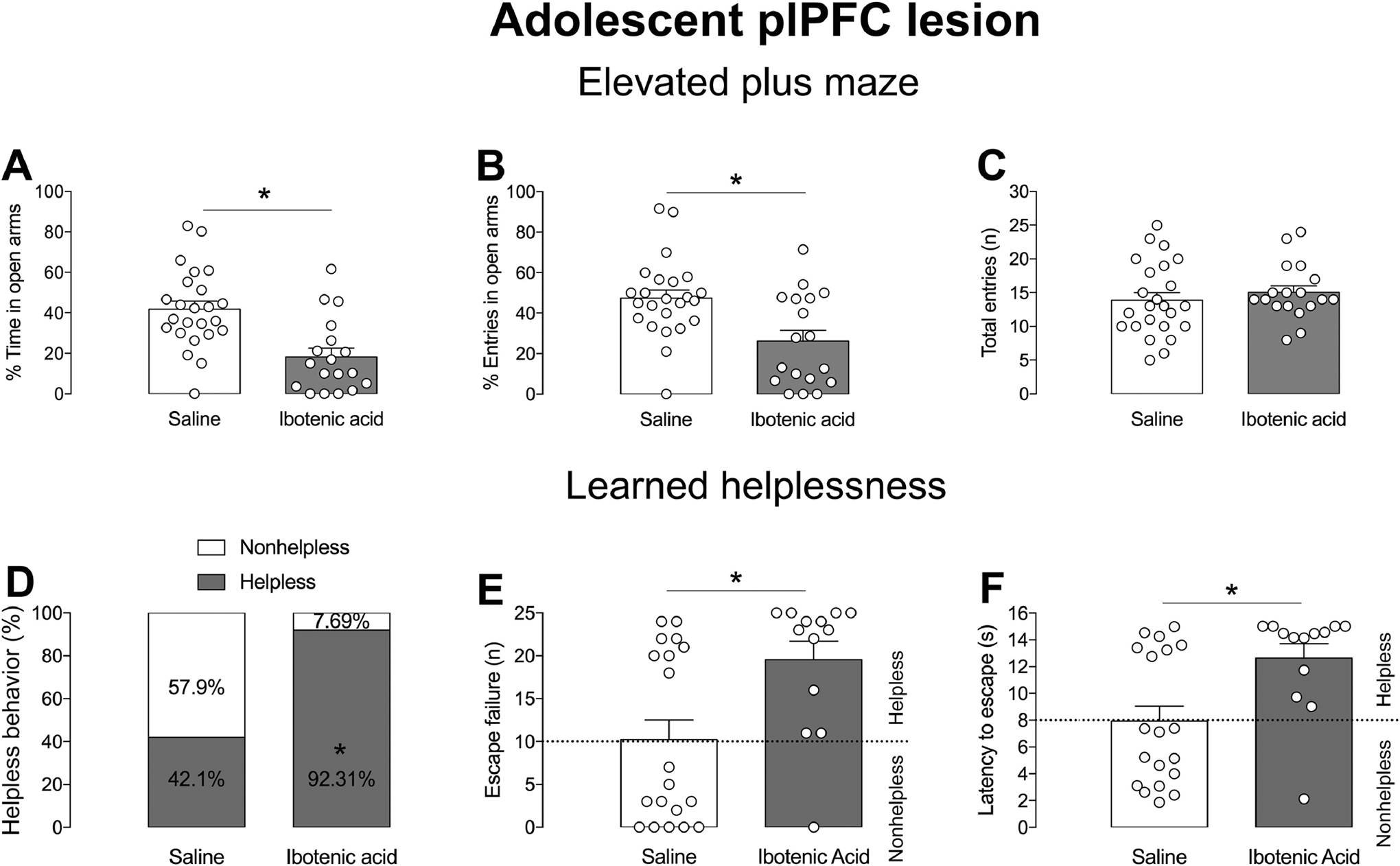
plPFC disruption during adolescence induces an anxiety-like state and increases the susceptibility to helpless behavior in adults. Adolescent plPFC lesion (A) decreased the percentage of time (A) and the number of entries (B) into open arms of the EPM at adulthood. The total number of entries was not affected by the adolescent plPFC lesion (C). * p<0.05, t-test. In the LH experiment, adolescent plPFC lesion increased the percentage of rats showing helpless behavior (D), the number of escape failures (E) and latency to escape (F) in the LH paradigm at adulthood (*p<0.05, t-test).
Regarding VTA DA system activity, 2-way ANOVA showed an effect of the condition for number of spontaneously active DA neurons in the VTA (F1,33=8.61, p<0.05), but with no effect of the lesion (p>0.05) or an interaction between condition and lesion (p>0.05). In addition, post-hoc analysis indicated a reduction in VTA DA neuron population activity in adult rats with adolescent plPFC lesion exposed to LH (n=11 rats; 44 cells; 0.65 ± 0.6 cells/track; p<0.05, Tukey’s test, Figure 4A) compared to sham animals exposed to LH (n=16 rats; 97 cells; 0.99 ± 0.1 cells/track). In contrast, no difference was observed between sham (n=5; 39 cells; 1.11 ± 0.04 cells/track) and adolescent plPFC lesioned animals that were not exposed to LH (n=5; 46 cells; 1.22 ± 0.11 cells/track; Figure 4A).
Figure 4.
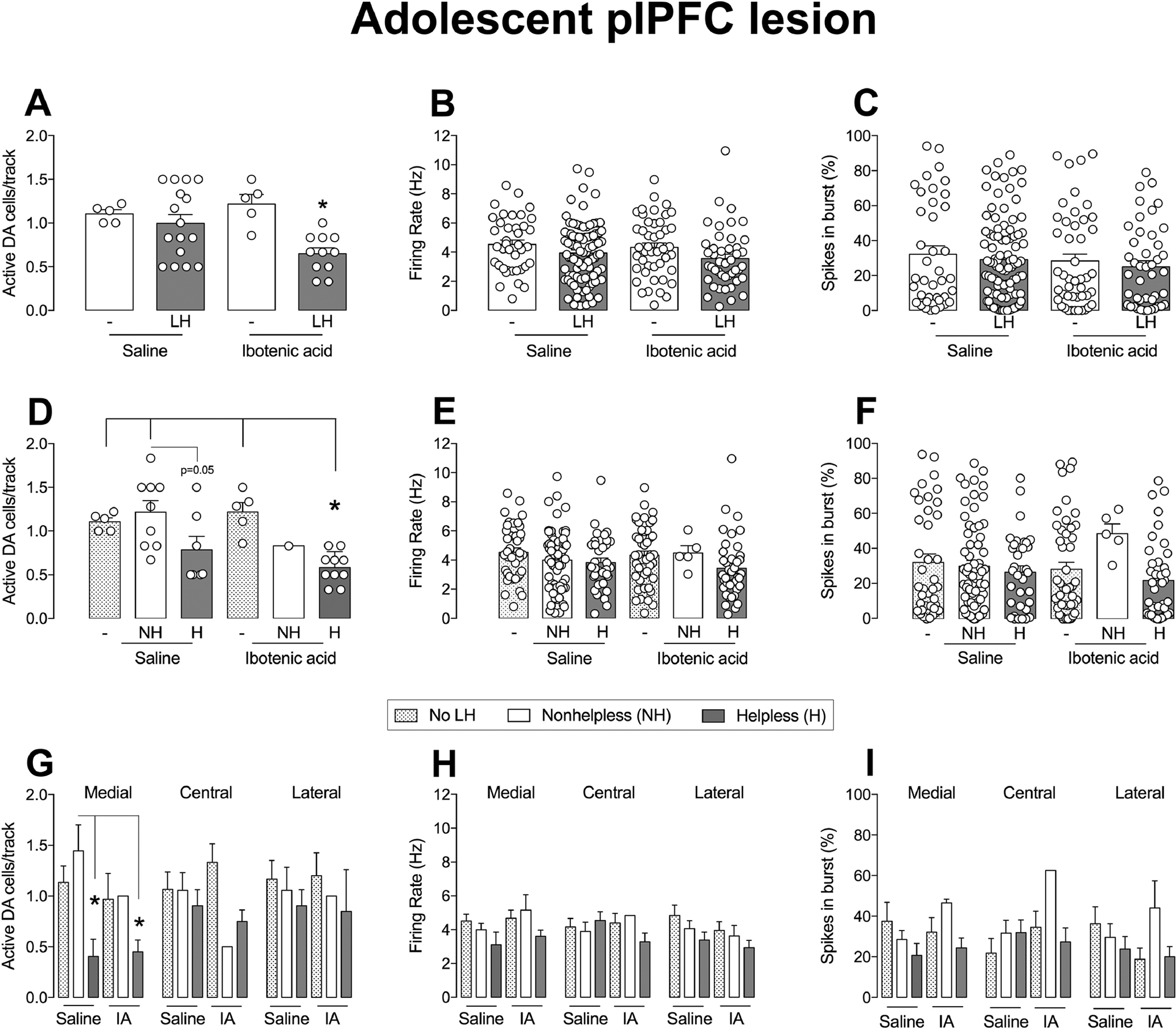
plPFC disruption during adolescence decreases the number of spontaneously active DA neurons in the VTA of helpless rats. The plPFC lesion in adolescent rats decreased the number of spontaneously active VTA DA cells/track compared to intact rats after exposure to learned helpless in adult rats (A; *p<0.05), with no change in firing rate (B) or the percentage of spikes in bursts (C). The number of VTA DA cells/track in helpless rats in the ibotenic acid group (D) is decreased in relation to no LH/nonhelpless rats of the saline groups and no LH of ibotenic acid group (*p<0.05). No statistical difference was found for firing rate (E) or in the percentage of spikes in bursts (F) of helpless/nonhelpless rats in saline and ibotenic acid groups. Helpless rats in saline and ibotenic acid group exhibit a reduction in the number of active DA cells specifically in the medial portion of VTA compared to nonhelpless and rats not subjected to LH (*p<0.05), but not in central and lateral portion (G). The medial/central/lateral VTA firing rate (H) and percentage of burst activity (I) were not affected by treatment or helpless behavior. NH: nonhelpless; H: helpless; LH: learned helplessness; IA: ibotenic acid. *p<0.05, ANOVA followed by Tukey’s post hoc analysis.
In addition, the firing rate and percentage of spikes in bursts of identified VTA DA neurons were not different among the groups (all factors, p>0.05, 2-way ANOVA, Figure 4B–C). When we analyzed the data on the VTA DA system activity to evaluate changes among animals not exposed to LH, nonhelplessness, and helplessness animals, as previously reported (Belujon and Grace, 2014), we found that helpless rats, in both groups (with lesion or not), showed a reduction of VTA DA neuron population activity (condition: F2,31=8.20, p<0.05; lesion: p>0.05; interaction: p>0.05; 2-way ANOVA). Animals with adolescent plPFC lesion showing helpless behavior had a decreased number of spontaneously active VTA DA neurons (n=10; 39 cells; 0.58 ± 0.06 cells/track) compared to naive rats (not subjected to LH) and nonhelplessness rats (p<0.05, Tukey’s test, Figure 4D). There was also a trend for a reduction in the number of spontaneously active VTA DA neurons in helplessness rats that received saline intra-plPFC during adolescence (n=7; 33 cells; 0.78 ± 0.15 cells/track; p=0.05, Tukey’s test) compared to nonhelplessness rats (n=9; 64 cells; 1.22 ± 0.13 cells/track). No significant difference was observed in the average firing rate and percentage of spikes in bursts between helpless and non-helpless animals of sham and lesioned groups (p>0.05 for condition, lesion, and interaction between condition and lesion, 2-way ANOVA, Figure 4E–F). The analysis of the distribution of active DA cells/track among the medial, central and lateral portions of the VTA demonstrated that the decreased VTA DA neuron population activity observed in either sham or lesioned helpless rats was confined to the medial portion (condition: F2,31=4.59, p<0.05; lesion, p>0.05; interaction, p>0.05; 2-way ANOVA, p<0.05 helplessness rats vs. nonhelplessness rats and animals that were not subjected to LH for sham and lesioned rats, Tukey’s test, Figure 4G) No effect was observed in the average firing rate and burst activity of the DA neurons recorded in the medial portion of the VTA (all factors, p>0.05, 2-way ANOVA, Figure 4H–I). Also, no change in the number of active DA neurons, firing rate, and burst activity was found in the central and lateral portions of the VTA (all factors, p>0.05, 2-way ANOVA, Figure 4G–I).
3.2. The adult plPFC lesion did not change susceptibility to LH.
The adult plPFC lesion (n=20) did not induce anxiety responses as indicated by no change in the percentage of time (p>0.05, t-test, Figure 5A) and entries into the open arms in the EPM compared to controls (n=19; p>0.05, t-test, Figure 5B). No change was observed in the number of total entries into all arms of the EPM as well (p>0.05, t-test, Figure 5C), indicating that the adult plPFC lesion did not change locomotor activity. In addition, the adult plPFC lesion did not change the percentage of rats susceptible to helpless behavior (p>0.05, Chi-square test, Figure 5D). The percentage of rats with adult plPFC lesion showing helpless behavior was 33.3% (5 of 15 rats), while the percentage of sham rats showing helpless behavior was 30.8% (4 of 13 rats). These findings indicate that the adult plPFC lesion did not increase susceptibility to helpless behavior. Moreover, adult plPFC lesion (n=15) did not alter the latency to escape (p>0.05, test-t, Figure 5F) and the number of escape failures (p>0.05, t-test, Figure 5E) compared to saline-injected rats (n=13). 2-way ANOVA indicated an effect of the condition for the latency to escape (F1,24=148.4, p<0.05) and number of escape failures (F1,24=149.5, p<0.05), but with no effect of the lesion (p>0.05) or an interaction between condition and lesion (p>0.05). And, as expected, post-hoc analysis showed an increase in the latency to escape (p<0.05; Tukey’s test) and the number of escape failures (p<0.05; Tukey’s test) in helplessness rats compared to nonhelplessness rats (data not shown).
Figure 5.
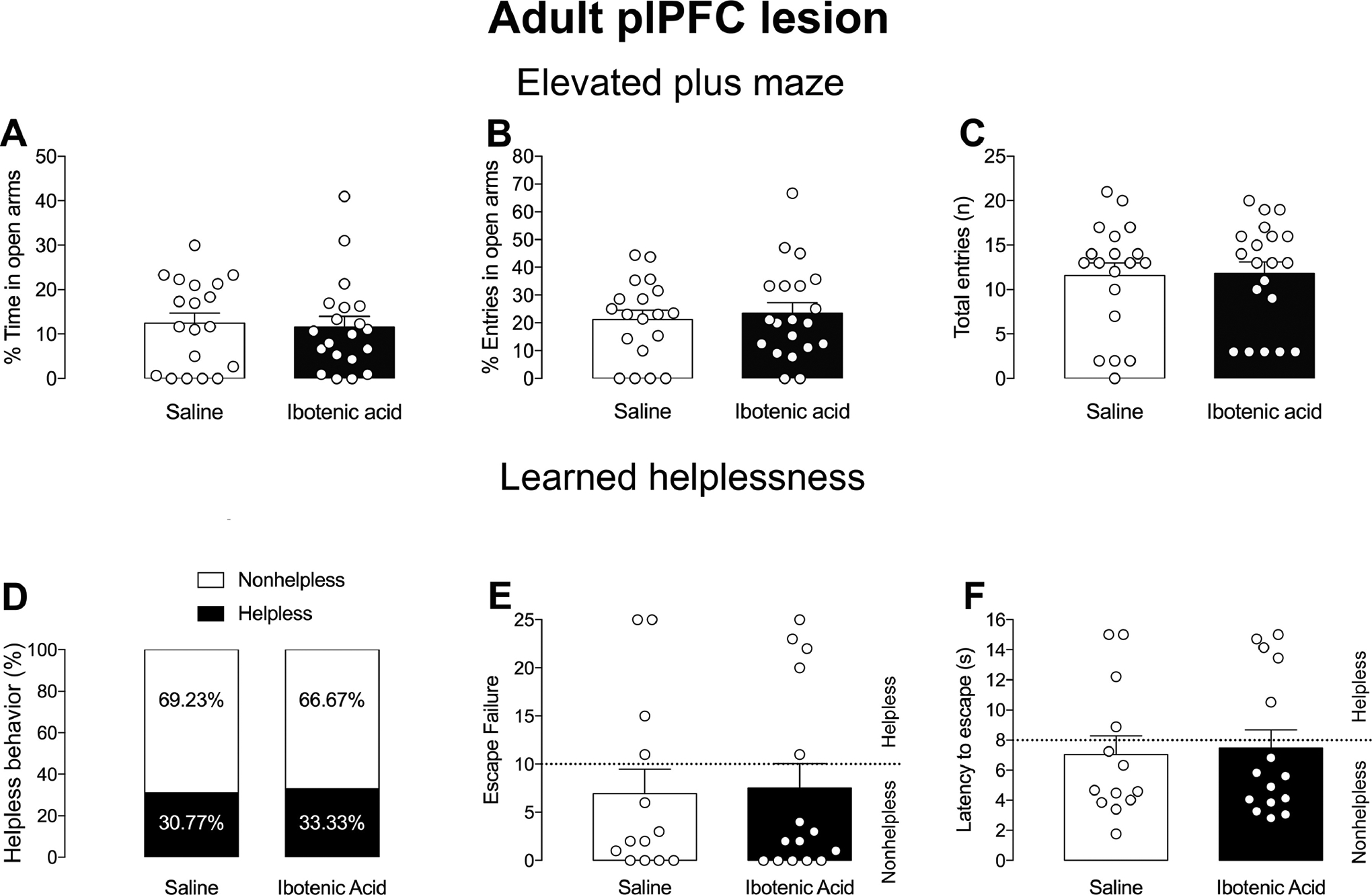
plPFC lesions in adult rats do not impact behavioral response in EPM/LH. Adult plPFC lesion did not change anxiety responses in the EPM, % Time (A), % Entrance in open arms (B) or Total entries (C). No changes in % of helpless behavior (D), number of escape failures (E) or latency to escape (F) in rats subjected to the LH paradigm.
For the recordings of VTA DA neurons, a 2-way ANOVA showed no effect of the condition (p>0.05), lesion (p>0.05) or an interaction between condition and lesion (p>0.05), indicating that the adult plPFC lesion did not affect the number of spontaneously active DA neurons in the VTA (n=15 rats; 90 cells; 0.81 ± 0.05 cells/track; Figure 6A) compared to sham animals exposed to LH (n=13 rats; 87 cells; 0.89 ± 0.09 cells/track) or to sham (n=6; 48 cells; 0.99 ± 0.08 cells/track) and adult plPFC lesioned animals that were not exposed to LH (n=5; 46 cells; 1.09 ± 0.14 cells/track; Figure 6A–C). The firing rate and percentage of spikes in bursts of identified VTA DA neurons were not different among all groups (all factors, p>0.05, 2-way ANOVA, Figure 6B–C). Helpless rats, in both lesioned and non-lesioned groups, showed a significant reduction of VTA DA neuron population activity (condition: F2,31=8.20, p<0.05; lesion: p>0.05; interaction: p>0.05; 2-way ANOVA; Figure 6D). plPFC lesioned rats showing helpless behavior (n=5; 28 cells; 0.44 ± 0.09 cells/track) had a decreased number of spontaneously active VTA DA neurons compared to naive rats (not subjected to LH; p<0.05, Tukey’s test) and nonhelpless lesioned rats (n=10, 62 cells, 0.91 ± 0.04 cells/track). Also, there was a reduction in the number of spontaneously active VTA DA neurons in helpless rats that received saline intra-plPFC (n=4; 15 cells; 0.54 ± 0.08 cells/track; p<0.05, Tukey’s test) compared to nonhelpless sham rats (n=9; 72 cells; 1.04 ± 0.09 cells/track). No significant difference was observed in the firing rate and percentage of spikes in bursts between helpless and non-helpless animals of sham and lesioned groups (all factors, p>0.05, 2-way ANOVA, Figure 6E–F). The analysis of the distribution of active DA cells/track in relation to region of the VTA indicated a significant reduction of DA neurons firing in the medial portion of the VTA of either sham or lesioned helpless rats (condition: F2,33=7.24; lesion, p>0.05; interaction, p<0.05, 2-way ANOVA, p<0.05 helplessness rats vs. nonhelpless rats and animals that were not subjected to LH for sham and lesioned rats Tukey’s test, Figure 6G). No effect was observed in the firing rate and burst activity of the DA neurons recorded in the medial portion of the VTA (all factors, p>0.05, 2-way ANOVA, Figure 6H–I). Also, no change in the number of active DA neurons, firing rate, and burst activity was found in the central and lateral portions of the VTA (all factors, p>0.05, 2-way ANOVA, Figure 6G–I). Thus, in summary, all helpless animals independent of treatment condition exhibited reduced VTA DA neuron population activity.
Figure 6.
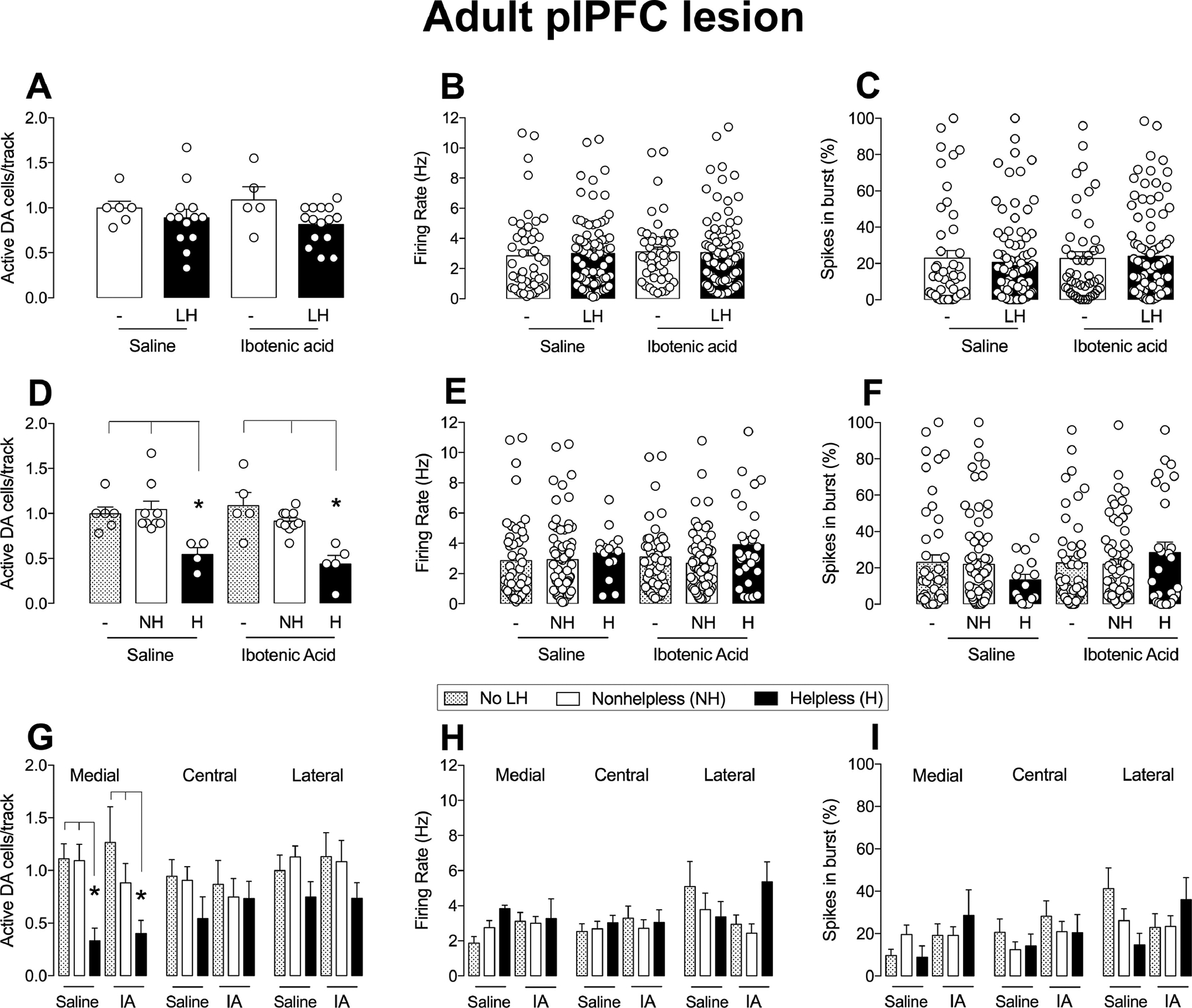
The adult plPFC lesion had no effect on VTA DA neuron activity. (A) The plPFC lesion in adult rats did not affect the number of spontaneously active VTA DA cells/track (A), firing rate (B) or the percentage of spikes in bursts (C) after exposure to learned helplessness. The number of active DA cells/track is reduced in helpless rats in both saline and ibotenic acid groups (D) in relation to nonhelpless or animals not subjected to LH (*p<0.05). No statistical difference was found for (E) firing rate or in (F) the percentage of spikes in bursts of helpless/nonhelpless rats in saline and ibotenic acid groups. Helpless rats in both treatment groups had a decrease in the number of active DA cells in the medial portion of VTA compared with nonhelpless rats and rats not subjected to LH, but not central and lateral portions (G). The medial/central/lateral VTA firing rate (H) and percentage of burst activity (I) were not affected by treatment or presence of helpless behavior. NH: nonhelpless; H: helpless; LH: learned helplessness; no LH: not subjected to learned helplessness. *p<0.05, ANOVA followed by Tukey’s post hoc analysis.
4. Discussion
The present study demonstrated that disruption of the plPFC in adolescent rats increases, at adulthood, anxiety and the susceptibility to helpless behavior in the LH paradigm, and the helpless behavior was accompanied by a decrease in the number of spontaneously active DA neurons in the medial VTA. In contrast, the adult plPFC lesion did not impact anxiety or susceptibility to helpless behavior. Together, these data suggest that adolescence represents a sensitive period for the development of susceptibility to depressive-like changes and PFC maturation since a PFC disruption during this period lead to an increased late vulnerability to helpless behaviors.
PFC dysfunction has been described in depression in humans (Drevets et al., 2008; Rogers et al., 2004). Similarly, the involvement of the PFC in depression-like states has also been demonstrated in animal models. In particular, the plPFC plays an important role in the modulation of behavioral changes induced by LH (Amat et al., 2008, 2005; Maier and Seligman, 2016), which has been associated with the involvement of the plPFC activity in the detection of stress controllability in the first-day session of this model. In the LH model, the stress session on day 1 can be controllable or uncontrollable, which affects the animal response in the escape session (day2). The animal is more susceptible to helpless behavior when they do not detect control during the stress session. Thus, the detection of stress controllability determines the animal’s action, suggesting that a previous intense and uncontrollable stress generally leads to incapacity and inability to act in future aversive events (Maier and Seligman, 2016). It is proposed that in LH, plPFC activity during the first session of stress detects controllability via direct projections to the DRN that ultimately inhibits this nucleus (Amat et al., 2005). plPFC inactivation during controllable stress (escapable shocks) leads to helpless behavior (Amat et al., 2005) and its activation during uncontrolled stress (inescapable shocks) does not promote this behavior (Amat et al., 2008), demonstrating the importance of the plPFC in top-down control over helpless outcome. We performed the experiment using only the uncontrollable stress paradigm and not controllable stress in day 1, because the plPFC inactivation in adult rats during controllable stress has already been reported to affect the escape session on the following day (Amat et al., 2005), and this could be a confounding factor for interpretation of susceptibility to helpless behavior in plPFC lesion rats. Overall, a plPFC dysfunction could impair the detection of stress controllability during the escape session and increase helpless behavior. Therefore, we hypothesized that a developmental plPFC dysfunction impairs the vulnerability to helpless behavior later in life. This was indeed demonstrated by our findings showing increased susceptibility to helpless behavior in animals with adolescent plPFC lesions. Thus, the increased susceptibility to helpless behavior in animals with adolescent lesion of the plPFC indicates that neurodevelopmental changes in the PFC may increase the susceptibility of an individual to develop depression later in life after exposure to stressful events. Interestingly, the adult plPFC lesion did not increase the susceptibility to helpless behavior or induce an anxiety-like response; these findings are similar to those showing no change in the escape session of the LH when the plPFC is inactivated prior to the exposure to uncontrollable stress (Amat et al., 2005).
The animals subjected to surgery during either adolescence or adulthood did not demonstrate a substantial difference in susceptibly to helpless behavior when compared to the saline groups (42% in adolescence and 30.77% in adulthood), suggesting that the timing of the surgery is not the factor leading to an increased expression of helpless behavior. It is also worth noting that while adolescent plPFC lesioned animals started testing 33 days post-lesion, adult plPFC lesioned animals started testing 10 days post-lesion. Although we did not observe any change in anxiety and helpless behavior 10 days post-lesion in adult-lesioned rats and it is unlikely that these animals would present behavioral changes 30 days post-lesion due to the functional reorganization that is described to take place after the excitotoxic lesion induced by ibotenic acid. In fact, it has been shown that this functional reorganization may promote adaptative mechanisms to regulate stress response and behavior (Vaidya et al., 2019). In the case of the adolescent plPFC lesion, despite some functional reorganization that can take place, we believe that the lesion at this period my impact plPFC maturation leading to a developmental dysfunction of this brain region which in turn drives the increased susceptibility to helpless behavior later in life.
The PFC represents one of the last brain areas to mature in rats, and it happens mainly in late adolescence (Caballero et al., 2016; Davey et al., 2008; Gogtay et al., 2004). In addition, MDD can develop during adolescence (Kessler et al., 2001) and the early onset is related to a greater illness burden throughout life (Zisook et al., 2007). The adolescence period also represents a time in which the connectivity of the PFC with other cortical and subcortical areas emerge, achieving an optimal excitatory-inhibitory balance (Caballero et al., 2016; Caballero and Tseng, 2016; Zimmermann et al., 2019). The plPFC lesion during this period would impair maturation and also remove its connectivity with other structures, diminishing its role in stress controllability and increasing the vulnerability to adverse events and their consequences during development. In this context, it was found that alterations in cortical neurodevelopment are associated with the duration of depression and the number of depressive episodes in humans (Schmitgen et al., 2019). Other evidence points to PFC dysfunction during adolescence in the emergence of depression. The onset of early depression and PFC maturation share the same period during adolescence (Davey et al., 2008). We found that only 1 of 13 rats with adolescent PFC lesion did not become helpless after the exposure to LH at adulthood. Although this of course precludes appropriate statistical analysis, this reinforces the idea that lesion of the PFC during adolescence leads to a greater vulnerability to stress-induced depression phenotype at adulthood. In addition, similar to what we observed previously (Gomes and Grace, 2017), our results demonstrated that adolescent plPFC lesion also induced anxiety-like responses in the EPM at adulthood. Anxiety is a common comorbid condition associated with depression (Gaspersz et al., 2018).
The plPFC is described as a key brain area in the modulation of the stress response, mostly by attenuating the deleterious effects of stress (Arnsten, 2015; Datta and Arnsten, 2019; McEwen and Morrison, 2013). This has been associated with the inhibitory influence that the plPFC has on amygdala activity, particularly in the basolateral amygdala (BLA) in rats (Hariri et al., 2003; Rosenkranz and Grace, 2002, 2001). In humans, the PFC attenuates emotional responses mediated by the amygdala (Hariri et al., 2003), and PFC dysfunction is related to hyperactivity of the amygdala in depressed patients (Murray et al., 2011). In addition, individuals with cognitive vulnerability to depression exhibit PFC hypoactivation and amygdala hyperactivation in response to emotional stimuli (Zhong et al., 2011). These data suggest that the PFC-BLA pathway is involved in neurobiological changes in depressed patients and that a PFC dysfunction may alter amygdala responsivity to stress which in turn can lead to maladaptive behaviors. Moreover, adolescence is a critical period for the development of the amygdala and PFC and also their connectivity in humans, which increases with age (Gee et al., 2013; Perlman and Pelphrey, 2011; Tottenham and Gabard-Durnam, 2017), and dysfunction in these areas can have a great impact on the development of affective disorders later in life. Young adults with early-onset depression demonstrate amygdala hyperactivity to threat-related faces and this activity is associated with symptom severity (Mingtian et al., 2012). Increased activity of the amygdala also has been described in adolescents with depressive symptoms (Beesdo et al., 2009; Yang et al., 2010; Forbes et al., 2011; Hall et al., 2014), demonstrating that amygdala hyperactivity during adolescence might represent a vulnerability state to the development of depression. Therefore, while certainly not conclusive, these data strongly implicate the amygdala-plPFC in this effect.
Regarding mPFC-BLA connectivity maturation in rodents, a large increase in fiber proliferation between PD10–30 has been reported (Arruda-Carvalho et al., 2017), reaching a maximal pruning and fiber density at PD25 that remains stable until PD45 (Cressman et al., 2010). After PD45 the anatomical connectivity decreases until adulthood (Arruda-Carvalho et al., 2017; Cressman et al., 2010). Functionally, the mPFC-BLA connectivity seems to achieve an adult-like state around PD40 (Arruda-Carvalho et al., 2017; Selleck et al., 2018). Therefore, these data suggest that the maturational window of PFC-BLA connectivity starts around PD25, supporting that the time-point used in our study to perform the plPFC lesion (PD31) may represent an important neurodevelopmental period for plPFC-BLA connectivity. Thus, the increased susceptibility to helpless behavior in animals with adolescent plPFC lesion may be a consequence of a plPFC inability to control amygdala responsivity to stress during this period. Consequently, changes in intrinsic PFC activity or connectivity can be essential mechanisms to promote the increased susceptibility to a depression-like state.
Stress during adolescence has a negative impact on PFC, hippocampus, and BLA anatomy, and could influence the emergence of psychiatric disorders by affecting the neurodevelopmental state of the PFC (Andersen and Teicher, 2008; Keshavan et al., 2014; Romeo, 2017). The changes induced in the PFC by social stress in adolescence are reported to be long-lasting in rats (Eiland et al., 2012; Leussis et al., 2008). However, the exact consequence of other stress types during adolescence on adult pathology is still not clear. We previously found that plPFC lesions in juvenile rats (PND 24) together with mild stress during adolescence (PD31–40) led to schizophrenia-like changes, including an increased population activity of DA neurons projecting to the associative striatum (Gomes and Grace, 2017). In these experiments, we show that adolescent plPFC disruption increases susceptibility to a depression-like state after exposure to stressful events in adulthood but without adolescent stress exposure. Altogether, these findings indicate that plPFC dysfunction during the juvenile/adolescence period can increase the impact of stress on susceptibility for the development of schizophrenia or depression, with the timing of the insult determining the pathology.
Regarding the changes in the VTA DA system, it was observed that acute inactivation of the plPFC led to decreased VTA DA population activity (Patton et al., 2013), but the juvenile plPFC lesion did not change the VTA DA system in adult animals (Gomes and Grace, 2017), similar to our plPFC lesion data in adolescent rats. It is possible that the lesion of the plPFC could promote an adaption in the pathways involved in the control of dopaminergic activity in the VTA during basal conditions. However, adolescent plPFC lesioned or sham animals showing helpless behavior presented a decreased VTA DA neuron population activity. Therefore, the plPFC lesion during adolescence demonstrated an extensive impact on the DA system; an effect that is probably due to the increased susceptibility to helpless behavior that was observed in this group. This is consistent with previous results from our group showing that the reduction in dopaminergic activity in the VTA consistently occurs with helpless behavior in the LH model (Belujon and Grace, 2014), with rats subjected to CMS (Chang and Grace, 2014; Moreines et al., 2017; Rincón-Cortés and Grace, 2017; Neves and Grace, 2019) and also with a combination of repeated footshock/restraint stress during adulthood (Gomes et al., 2019). Thus, we have shown that changes related to depression in rats are linked to the hypodopaminergic state in the VTA, similar to the downregulation of the DA system that is described in depression patients (Lambert et al., 2000; Nestler and Carlezon, 2006; Reddy et al., 1992). Moreover, we also found that the downregulation of the DA system is restricted to the medial portion of VTA in helplessness rats in both treatment groups and independent of the timing of the surgery. This is consistent with previous data from our group demonstrating that in animal models to study depression there is a reduction in active DA neurons in more medial regions of the VTA (Belujon and Grace, 2014; Moreines et al., 2017). The medial VTA is known to project to the nucleus accumbens shell, an area involved with reward-related events (Grace, 2016; Ikemoto, 2007); thus the reduction of the spontaneous active DA cells in the medial portion of the VTA in helpless rat may play an essential role in the reward-related behavioral consequences in the LH model. Supporting this idea, the reduction in the DA reward system is also consistent with the presence of anhedonia previously described in animals subjected to CMS (Tye et al., 2013) and LH (Sanchis-Segura et al., 2005), and support the translational neurobiological changes in animal models for the study of depression (Wise, 2008). We did not test the reward sensitivity in our study after LH. Nonetheless, the downregulation of dopaminergic activity in the medial portion of VTA can indirectly support the idea of a decreased reward response, as demonstrated by previous studies mentioned above.
In conclusion, our results support the role of plPFC dysfunction in susceptibility to the development of anxiety- and depressive-like behaviors during adulthood. These data reinforce the idea that adolescence represents a sensitive period of vulnerability for the development of psychiatric disorders and also is a critical period of maturation of normal PFC function. A plPFC dysfunction that may arise due to a genetic predisposition or external influence, like stress, during the adolescent period might have an important impact on the susceptibility to mood disorders in adults.
Highlights.
Adolescent plPFC disfunction increases vulnerability to helpless behavior at adulthood.
Adult plPFC disruption did not change vulnerability to helpless behavior.
Helpless behavior is associated with blunted dopaminergic activity in the medial ventral tegmental area.
Acknowledgments
The authors wish to thank Niki MacMurdo and Christy Smolak for technical assistance and Millie Rincón-Cortés for the valuable inputs to the manuscript.
Role of Funding Source
This study was funded by US National Institutes of Health (NIH; MH101180 to AAG). The NIH had no further role in the design, analysis and interpretation of the experiments; in writing and decision to submit the paper for publication. FVG received a São Paulo Research Foundation Young Investigator grant (FAPESP - 2018/ 17597-3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest
AAG has received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron, Takeda, and Minerva. DLU and FVG declare no conflict of interest.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF, 2005. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci 8, 365–371. 10.1038/nn1399 [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF, 2008. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience 154, 1178–1186. 10.1016/j.neuroscience.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders, Fifth Ed. ed. American Psychiatric Association Publishing. [Google Scholar]
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 31, 183–191. 10.1016/j.tins.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, 2015. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci 18, 1376–1385. 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu W-C, Cummings KA, Clem RL, 2017. Optogenetic Examination of Prefrontal-Amygdala Synaptic Development. J. Neurosci. Off. J. Soc. Neurosci 37, 2976–2985. 10.1523/JNEUROSCI.3097-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau JYF, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen H-U, Leibenluft E, Ernst M, Pine DS, 2009. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch. Gen. Psychiatry 66, 275–285. 10.1001/archgenpsychiatry.2008.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA, 2017. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol 20, 1036–1046. 10.1093/ijnp/pyx056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA, 2014. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol. Psychiatry 76, 927–936. 10.1016/j.biopsych.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL, 2011. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev 35, 1687–1703. 10.1016/j.neubiorev.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Granberg R, Tseng KY, 2016. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev 70, 4–12. 10.1016/j.neubiorev.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng KY, 2016. GABAergic Function as a Limiting Factor for Prefrontal Maturation during Adolescence. Trends Neurosci 39, 441–448. 10.1016/j.tins.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Grace AA, 2014. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol. Psychiatry 76, 223–230. 10.1016/j.biopsych.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H, 2010. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J. Comp. Neurol 518, 2693–2709. 10.1002/cne.22359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Fuchs E, Wiborg O, Simon M, 2016. Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 293–310. 10.1016/j.pnpbp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Datta D, Arnsten AFT, 2019. Loss of Prefrontal Cortical Higher Cognition with Uncontrollable Stress: Molecular Mechanisms, Changes with Age, and Relevance to Treatment. Brain Sci 9 10.3390/brainsci9050113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB, 2008. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci. Biobehav. Rev 32, 1–19. 10.1016/j.neubiorev.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Douma EH, de Kloet ER, 2019. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev 108, 48–77. 10.1016/j.neubiorev.2019.10.015 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML, 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct 213, 93–118. 10.1007/s00429-008-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS, 2012. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37, 39–47. 10.1016/j.psyneuen.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP, 2003. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur. J. Neurosci 18, 2357–2364. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE, 2011. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev. Neuropsychol 36, 429–452. 10.1080/87565641.2010.550178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspersz R, Nawijn L, Lamers F, Penninx BWJH, 2018. Patients with anxious depression: overview of prevalence, pathophysiology and impact on course and treatment outcome. Curr. Opin. Psychiatry 31, 17–25. 10.1097/YCO.0000000000000376 [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, 2013. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. Off. J. Soc. Neurosci 33, 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A 101, 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Grace AA, 2017. Prefrontal Cortex Dysfunction Increases Susceptibility to Schizophrenia-Like Changes Induced by Adolescent Stress Exposure. Schizophr. Bull 43, 592–600. 10.1093/schbul/sbw156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Zhu X, Grace AA, 2019. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol. Psychiatry 10.1038/s41380-019-0514-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, 2016. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci 17, 524–532. 10.1038/nrn.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1983. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience 10, 301–315. 10.1016/0306-4522(83)90135-5 [DOI] [PubMed] [Google Scholar]
- Hall LMJ, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, Mueller BA, Lim KO, Cullen KR, 2014. An fMRI study of emotional face processing in adolescent major depression. J. Affect. Disord 168, 44–50. 10.1016/j.jad.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR, 2003. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry 53, 494–501. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, 2007. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev 56, 27–78. 10.1016/j.brainresrev.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Myers B, Herman JP, 2011. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol. Behav 104, 266–271. 10.1016/j.physbeh.2011.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, 2019. Alterations and adaptation of ventral tegmental area dopaminergic neurons in animal models of depression. Cell Tissue Res 377, 59–71. 10.1007/s00441-019-03007-9 [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JYF, Lewis DA, Paus T, 2014. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry 1, 549–558. 10.1016/S2215-0366(14)00081-9 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K, 2001. Mood disorders in children and adolescents: an epidemiologic perspective. Biol. Psychiatry 49, 1002–1014. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ, 2013. The epidemiology of depression across cultures. Annu. Rev. Public Health 34, 119–138. 10.1146/annurev-publhealth-031912-114409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Agren H, Friberg P, 2000. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch. Gen. Psychiatry 57, 787–793. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Lawson K, Stone K, Andersen SL, 2008. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synap. N. Y. N 62, 185–192. 10.1002/syn.20483 [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Popescu AT, Paré D, 2006. Identification of basolateral amygdala projection cells and interneurons using extracellular recordings. J. Neurophysiol 96, 3257–3265. 10.1152/jn.00577.2006 [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP, 2016. Learned helplessness at fifty: Insights from neuroscience. Psychol. Rev 123, 349–367. 10.1037/rev0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, 2016. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat. Med 22, 1229–1238. 10.1038/nm.4225 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH, 2013. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29. 10.1016/j.neuron.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingtian Z, Shuqiao Y, Xiongzhao Z, Jinyao Y, Xueling Z, Xiang W, Yingzi L, Jian L, Wei W, 2012. Elevated amygdala activity to negative faces in young adults with early onset major depressive disorder. Psychiatry Res 201, 107–112. 10.1016/j.pscychresns.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Grace AA, 2017. Involvement of Infralimbic Prefrontal Cortex but not Lateral Habenula in Dopamine Attenuation After Chronic Mild Stress. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 42, 904–913. 10.1038/npp.2016.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J, Lopez J, Bagot RC, 2018. Wiring the depressed brain: optogenetic and chemogenetic circuit interrogation in animal models of depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 10.1038/s41386-018-0291-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP, Drevets WC, 2011. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol. Psychiatry 69, e43–54. 10.1016/j.biopsych.2010.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA, 2006. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159. 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Neves GA, Grace AA, 2019. α7 nicotinic receptor full agonist reverse basolateral amygdala hyperactivity and attenuation of dopaminergic neuron activity in rats exposed to chronic mild stress. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol 29, 1343–1353. 10.1016/j.euroneuro.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, Grace AA, 2013. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J. Neurosci. Off. J. Soc. Neurosci 33, 16865–16873. 10.1523/JNEUROSCI.2449-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The Rat Brain in Stereotaxic Coordinates, 6th ed. Academic Press, San Diego. [Google Scholar]
- Perlman SB, Pelphrey KA, 2011. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol 108, 607–620. 10.1016/j.jecp.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE, 2006. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci. Off. J. Soc. Neurosci 26, 12967–12976. 10.1523/JNEUROSCI.4297-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS, 1992. CSF amine metabolites in depression. Biol. Psychiatry 31, 112–118. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Xu L, 2018. How could stress lead to major depressive disorder? IBRO Rep 4, 38–43. 10.1016/j.ibror.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, Grace AA, 2017. Sex-Dependent Effects of Stress on Immobility Behavior and VTA Dopamine Neuron Activity: Modulation by Ketamine. Int. J. Neuropsychopharmacol 20, 823–832. 10.1093/ijnp/pyx048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N, 2004. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci. Res 50, 1–11. 10.1016/j.neures.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Romeo RD, 2017. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res 1654, 185–191. 10.1016/j.brainres.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA, 2002. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J. Neurosci. Off. J. Soc. Neurosci 22, 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA, 2001. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J. Neurosci. Off. J. Soc. Neurosci 21, 4090–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R, Henn FA, Vollmayr B, 2005. Reduced sensitivity to sucrose in rats bred for helplessness: a study using the matching law. Behav. Pharmacol 16, 267–270. [DOI] [PubMed] [Google Scholar]
- Schmitgen MM, Depping MS, Bach C, Wolf ND, Kubera KM, Vasic N, Hirjak D, Sambataro F, Wolf RC, 2019. Aberrant cortical neurodevelopment in major depressive disorder. J. Affect. Disord 243, 340–347. 10.1016/j.jad.2018.09.021 [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF, 1967. Failure to escape traumatic shock. J. Exp. Psychol 74, 1–9. [DOI] [PubMed] [Google Scholar]
- Selleck RA, Zhang W, Samberg HD, Padival M, Rosenkranz JA, 2018. Limited prefrontal cortical regulation over the basolateral amygdala in adolescent rats. Sci. Rep 8, 17171 10.1038/s41598-018-35649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2013. Adolescent neurodevelopment. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med 52, S7–13. 10.1016/j.jadohealth.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Gabard-Durnam LJ, 2017. The developing amygdala: a student of the world and a teacher of the cortex. Curr. Opin. Psychol 17, 55–60. 10.1016/j.copsyc.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, Kim S-Y, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K, 2013. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541. 10.1038/nature11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA, 2012. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35, 422–430. 10.1016/j.tins.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AR, Pujara MS, Petrides M, Murray EA, Fellows LK, 2019. Lesion Studies in Contemporary Neuroscience. Trends Cogn. Sci 23, 653–671. 10.1016/j.tics.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Gill KM, Grace AA, 2012. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur. J. Neurosci 35, 1312–1321. 10.1111/j.1460-9568.2012.08038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 2008. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox. Res 14, 169–183. 10.1007/BF03033808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP, 2010. Adolescents with major depression demonstrate increased amygdala activation. J. Am. Acad. Child Adolesc. Psychiatry 49, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S, 2011. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol. Psychol 88, 233–242. 10.1016/j.biopsycho.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Zimmermann KS, Richardson R, Baker KD, 2019. Maturational Changes in Prefrontal and Amygdala Circuits in Adolescence: Implications for Understanding Fear Inhibition during a Vulnerable Period of Development. Brain Sci 9 10.3390/brainsci9030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, Gilmer WS, Dresselhaus TR, Thase ME, Nierenberg AA, Trivedi MH, Rush AJ, 2007. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 164, 1539–1546. 10.1176/appi.ajp.2007.06101757 [DOI] [PubMed] [Google Scholar]


