Abstract
Chronic overlapping pain conditions (COPCs) are conditions that share several clinical characteristics and symptomatology, are usually considered idiopathic in nature and are frequently comorbid. Currently, there is no established inclusion criteria to determine which conditions should be included under this umbrella term despite different systems proposed. Persistent dentoalveolar pain disorder (PDAP), also referred to as atypical odontalgia and thought to be a component of persistent idiopathic facial pain, is a chronic pain condition that manifests as a persistent tooth pain or pain over a dentoalveolar site formerly occupied by a tooth in the absence of detectable pathology during clinical or radiological exam. PDAP is considered idiopathic in nature and its pathophysiological mechanisms are not fully understood. Our objective was to investigate if PDAP fits the conceptual paradigm of COPC given its characteristics and commonalities with other COPC, based on published literature identified through a scoping review. We found that PDAP fits 16 out of 18 common characteristics among COPCs, and based on this finding, we discuss the implications of PDAP being considered a COPC.
Keywords: atypical odontalgia, phantom tooth pain, functional somatic syndromes, central sensitization, Persistent Dentoalveolar Pain Disorder
1 |. INTRODUCTION
Chronic pain is considered a major health issue affecting around 20% of people worldwide and being accounted for 15% to 20% of all medical visits (Treede et al., 2015). Chronic pain significantly impacts patients’ well-being and quality of life, affecting their mood, coping resources, expectations, sleep quality and physical function. Moreover, it is highly associated with disability and suicidal risk (Duenas, Ojeda, Salazar, Mico, & Failde, 2016; Racine, 2018; Turk, Fillingim, Ohrbach, & Patel, 2016) and its cost has been estimated to be approximately 635 billions of dollars in the United States of America (Gaskin & Richard, 2012).
Chronic overlapping pain conditions (COPCs) are conditions that share several clinical characteristics and symptomatology, they are usually considered idiopathic in nature and are frequently comorbid between them. Several features of COPCs make them significant contributors to the aforementioned burden related to chronic pain including their prevalence, difficulty in arriving at a proper diagnosis, and the challenge associated with their management (Maixner, Fillingim, Williams, Smith, & Slade, 2016). Being a relatively new term used in the literature, COPC have also been referred to as functional somatic syndromes (FSS), often associated with a more “psychological in nature” connotation (Barsky & Borus, 1999; Wessely, Nimnuan, & Sharpe, 1999), or as central sensitivity syndromes (CSS), based on putative pathophysiological mechanisms related to central sensitization (Yunus, 2007b). COPC usually affect more females and their diagnosis is mainly arrived at by exclusion, similarly to FSS and CSS. Conditions commonly described as COPCs (or FSS or CSS) in the literature include, but are not limited to, temporomandibular disorders (TMD), fibromyalgia (FM), irritable bowel syndrome (IBS), vulvodynia, myalgic encephalomyelitis/chronic fatigue syndrome, bladder pain syndrome/interstitial cystitis (BPS/IC), endometriosis, chronic tension-type headache, migraine headache, and chronic lower back pain (Maixner et al., 2016; Wessely et al., 1999; Yunus, 2007a, 2015). For simplicity and clarity, we will refer to any of these conditions from here on as COPCs, regardless if they were previously described as FSS or CSS by earlier literature. COPCs are prevalent in the population at large (Maixner et al., 2016). For instance, it was estimated that TMD can affect between 5% and 12% of the general population (National Institute of Dental and Craniofacial Research) while IBS could affect up to 44 million individuals (T. V. Macfarlane, Blinkhorn, Davies, & Worthington, 2003).
COPCs are frequently associated with the presence of pain amplification (Maixner et al., 2016; Yunus, 2007b), a term used to describe increased sensitivity to pain due to abnormal neural networks function within the central nervous system (Woolf, 2014). Moreover, COPCs are associated to several symptoms and psychosocial issues including fatigue, sleep disturbances, cognitive impairment, physical dysfunction, anxiety, anger and depression (Diatchenko, Nackley, Slade, Fillingim, & Maixner, 2006; Maixner et al., 2016). Due to their characteristics, the difficulties to arrive at a proper diagnosis and develop an adequate treatment plan, these conditions have considerable impact on individual well-being and quality of life (Maixner et al., 2016).
It has been postulated that each condition considered as a COPC likely has not only shared but also unique pathophysiological mechanisms (Maixner et al., 2016). Symptom amplification, victimization, psychological trauma, distress and psychiatric disorders were firstly proposed as possible mechanisms for the presence of these medically unexplained conditions (Aaron & Buchwald, 2001; Barsky & Borus, 1999). Currently, a biopsychosocial model seems to be the most emergent and accepted theory (Bourke, Langford, & White, 2015). In this model, individuals with a genetic predisposition may start experiencing symptoms after an environmental stressor triggers neuronal changes which facilitate pain or sensory abnormalities in a complex process involving central sensitization (CS) (Ablin & Clauw, 2009; Arnold et al., 2004; Buskila, Sarzi-Puttini, & Ablin, 2007; Gracely & Schweinhardt, 2015; Kato, Sullivan, Evengard, & Pedersen, 2006; Meeus & Nijs, 2007; Woolf, Thompson, & King, 1988; Yunus, 2007a). Multiple psychosocial factors including somatic symptom burden, negative affect/mood, environmental stress, and the presence of comorbid COPCs are thought to add to the risk of onset and persistence of COPCs (Edwards, Dworkin, Sullivan, Turk, & Wasan, 2016; Warren, Langenberg, & Clauw, 2013).
What constitutes a COPC is currently vaguely defined, with no established inclusion/exclusion criteria to determine which conditions should be included under this umbrella term. A clear delimitation of which clinical entities should be considered a COPC is needed in order to develop a better grouping or classification system, with the aims of improving diagnosis and treatment strategies as well as to further research of these pain conditions. Previous attempts to better delimitate and define COPCs were made, mainly based in common symptoms and characteristics. For example, when proposing BPS/IC as a condition fitting the COPC concept (originally reported by the author as FSS), Warren performed a comprehensive review of three conditions (chronic fatigue syndrome, fibromyalgia, irritable bowel syndrome) and gathered 18 characteristics that are considered common to them (Warren, 2014). He then proceeded to investigate if those 18 characteristics were present in patients with BPS/IC. finding that 16 out of those 18 characteristics were present in BPS/IC patients and concluding that BPS/IC should be considered as one of the FSS - in other words a COPC (Warren, 2014).
Another study identified eight profiles characterized by specific combinations of symptoms, highlighting the importance of specific combinations that could determine different subtypes of COPCs (Eliasen et al., 2018). Using a similar strategy as Warren (Warren, 2014), Levitt et al. proposed the inclusion of dry eye as a COPC based on its multiple comorbidities and central pain processing abnormalities reflective of CS manifestation (Levitt et al., 2017). Their rationale was mainly based in the co-existence of dry eye with other COPCs, shared pathologic neuroplasticity features (spontaneous pain, allodynia, hyperalgesia, and persistent pain after the resolution of the initial injury), and the presence of somatosensory dysfunction (peripheral and central). They also suggested shared genetic factors that could explain the underlying mechanisms associated with the observed comorbidities among COPCs (Levitt et al., 2017).
To determine if burning mouth syndrome (BMS) was associated with COPCs, Moisset and collaborators assessed systematically the co-occurrence of pain symptoms and trigeminal/extra-trigeminal somatosensory sensitivity in patients with this condition (Moisset, Calbacho, Torres, Gremeau-Richard, & Dallel, 2016). They found an association between BMS and different COPCs such as headaches, TMD and fibromyalgia among others. However, the prevalence of these symptoms was not different from age-matched members of general population. In addition, quantitative sensory testing (QST) studies revealed inconsistent evidence of abnormal cutaneous trigeminal and extra-trigeminal somatosensory sensitivity in patients with BMS (Moisset et al., 2016). Therefore, it appears that there is scant evidence supporting BMS as a COPC (Moisset et al., 2016).
Persistent dentoalveolar pain disorder (PDAP) is another chronic pain condition presenting within the orofacial region. Also referred to as atypical odontalgia, phantom tooth pain or painful traumatic trigeminal neuropathy, as well as thought to be a component of persistent idiopathic facial pain. PDAP presents as a persistent pain in an intraoral area currently or previously occupied by a tooth with no evident clinical or radiographical signs of pathology (Brooke, 1980; Durham & Nixdorf, 2014; Marbach, 1978; D. Nixdorf & Moana-Filho, 2011; D. R. Nixdorf et al., 2012; Rees & Harris, 1979). PDAP is considered idiopathic in nature, with its pathophysiological mechanisms not yet fully understood (Malacarne, Spierings, Lu, & Maloney, 2018; D. R. Nixdorf et al., 2012). Authors have previously proposed that PDAP has a vascular or psychogenic origin due to its relationship to depression (Graff-Radford & Solberg, 1992; Rees & Harris, 1979), while posterior literature suggests that PDAP presents an important neuropathic component due to its association to trauma and dental procedures in some cases (Baad-Hansen, List, Jensen, & Svensson, 2006; Baad-Hansen, List, Kaube, Jensen, & Svensson, 2006; Baad-Hansen et al., 2013; List et al., 2007a). Additionally, PDAP has been considered a more anatomically restricted version of persistent idiopathic facial pain or atypical facial pain (Benoliel & Gaul, 2017; Reik, 1984).
PDAP is frequently comorbid with TMD (Baad-Hansen, Leijon, Svensson, & List, 2008) and chronic tension type headaches (TTH) (Baad-Hansen et al., 2008). Moreover, depression, somatization, other psychiatric conditions and sleep disturbances have been reported in individuals with PDAP (Baad-Hansen et al., 2008; Ciaramella, Paroli, Lonia, Bosco, & Poli, 2013; Miura et al., 2018), who also present more jaw related disability, worse psychosocial functioning, and lower quality of life when compared to controls (Durham, Exley, John, & Nixdorf, 2013; Durham & Nixdorf, 2014; List et al., 2007a; Shueb, Nixdorf, John, Alonso, & Durham, 2015).
Given the clinical characteristics of PDAP, our objective was to investigate if PDAP fits the conceptual paradigm of COPCs based on the available literature. For that purpose, we decided to use the method proposed by Warren (Warren, 2014) given that, since none of the previously reported eligibility criteria are validated to date, it is our opinion that Warren’s method is the most systematic approach, being feasible to implement and easy to reproduce. Therefore, we conducted a literature search in PubMed on June 1st 2019 using the terms “persistent dentoalveolar pain”, “atypical odontalgia”, “phantom tooth pain”, “painful posttraumatic trigeminal neuropathy”, “non-odontogenic tooth pain”, “continuous neuropathic orofacial pain”, “idiopathic toothache” and “persistent idiopathic facial pain”. In addition, we hand-searched citations included in published literature reviews about PDAP and similar conditions for any additional reports not included in the PubMed search results. Our goal was to perform a scoping literature review (Pham et al., 2014), as opposed to a systematic one.
2 |. CAN BE PDAP CONSIDERED A COPC?
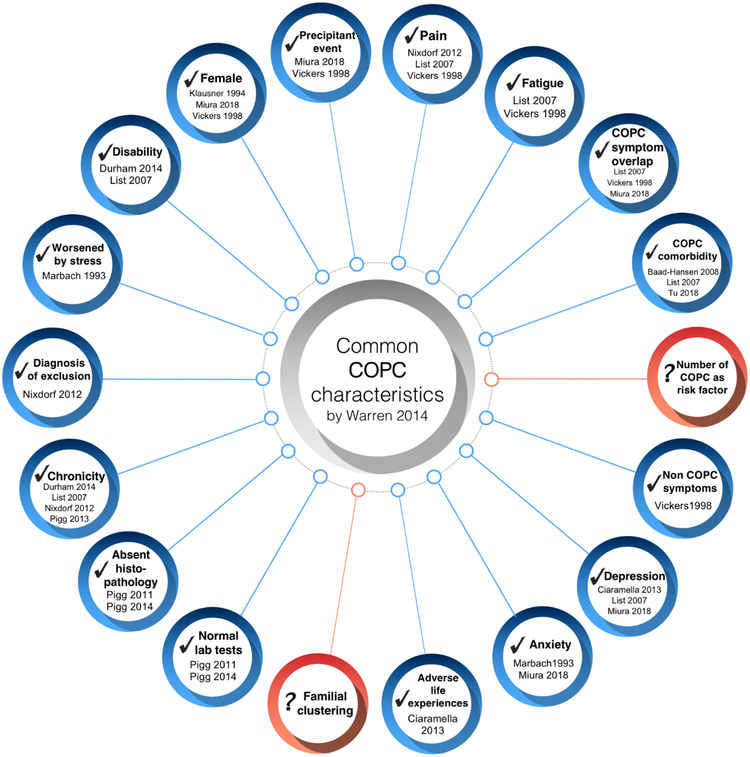
We identified 380 individual citations from our PubMed and hand searches. From those, we excluded reviews and non-English publications as we aimed to identify publications reporting clinical trials, observational studies, case reports and/or case series that included PDAP patient samples, in order to determine if the 18 characteristics proposed by Warren were present in individuals with PDAP. From the information gathered from all publications fitting our criteria, we found that 16 out of the 18 characteristics described by Warren were present in PDAP patients (Fig. 1). The identified characteristics are described in more detail below:
Figure 1.
Persistent dentoalveolar pain disorder characteristics in relation to those commonly reported with chronic overlapping pain conditions (adapted from Warren 2014). Blue circles indicate met characteristics, while red circles represent unmet characteristics.
2.1 |. Female predominance
Several studies reported that PDAP affects more women, with females being affected in 65% to 86% of the cases (Klausner, 1994; Miura et al., 2018; Vickers, Cousins, Walker, & Chisholm, 1998).
2.2 |. Occurrence after a precipitating event
PDAP is frequently associated with some kind of precipitating event, the most common being dental procedures (55% of cases) (Miura et al., 2018) but also including infection or dental trauma (Vickers et al., 1998). Other dental procedures such as root canal treatments (RCT), tooth/teeth extractions, dental prosthesis adjustments, dental fillings and implants were also associated with PDAP development (Miura et al., 2018; Vickers et al., 1998). However, one study reported that about 64% of patients presenting with intraoral pain that fit the PDAP definition described no specific cause or triggering event for it (Ram, Teruel, Kumar, & Clark, 2009).
2.3 |. Presence of pain
By definition, PDAP is associated with pain and/or dysesthesia present in an area currently or previously occupied by a tooth or teeth (D. R. Nixdorf et al., 2012). Symptom descriptors are variable but usually include “dull”, “achy”, “throbbing” or “sharp” (Vickers et al., 1998). The mean pain intensity fluctuates around an approximated mean of 5.6 (±1.9 SD) on 0 to 10 numerical rating scale, lasting an average of 7.7 years (±7.1 SD) (List et al., 2007a).
2.4 |. Presence of fatigue
There are reports of PDAP patients feeling tired daily (Tu et al., 2018), and they frequently describe the pain as tiredness (Vickers et al., 1998). Moreover, they also report less vitality when compared to controls (List et al., 2007a).
2.5 |. Overlap with other COPC symptoms
As stated in Warren 2014 (Warren, 2014), this characteristic is less defined and unspecific, therefore less consistent when compared to the others. Nonetheless, PDAP patients may present symptoms such as unspecific headaches, widespread bodily pain, and sleep disturbances than healthy controls (List et al., 2007a; Miura et al., 2018).
2.6 |. Comorbidity with other COPCs
PDAP has been associated with presence of COPCs affecting the head and face area. It has been reported that TMD may be present in 50% of PDAP cases (List et al., 2007a) and TTH in 46% of the cases (Baad-Hansen et al., 2008).
2.7 |. Presence of other non-COPC symptoms
Patients with PDAP have also reported numbness and tingling sensation in the face, dizziness, taste alterations, and difficulty in nose breathing among others (Vickers et al., 1998).
2.8 |. Depression
It has been reported that depressive disorders are frequently associated with PDAP (Miura et al., 2018), and that PDAP patients score higher in depression inventories when compared to controls (Ciaramella et al., 2013; List et al., 2007b), with reports of 48% of PDAP patients exhibiting severe depression (List et al., 2007a).
2.9 |. Anxiety
Increased anxiety has been linked to PDAP since early studies (Marbach, 1993). It was reported that comorbid anxiety disorders (i.e. generalized anxiety disorder, panic disorder) are present in about 10% of PDAP patients (Miura et al., 2018).
2.10 |. Adverse life experiences
PDAP patients reported a high number of life distressing events in the period immediately before or in coincidence with tooth extraction when compared to other pain disorders such as TMD or trigeminal neuralgia (Ciaramella et al., 2013).
2.11 |. Normal tests and absent histopathology
Normal tooth pulp testing outcomes and radiological findings are commonly reported following clinical evaluation of PDAP patients when using imaging procedures such as panoramic and periapical radiographs, cone beam computed tomography (Pigg, List, Petersson, Lindh, & Petersson, 2011), and magnetic resonance imaging (Pigg, List, Abul-Kasim, Maly, & Petersson, 2014).
2.12 |. Chronicity
PDAP is defined as a persistent condition (pain present at least eight hours per day, ≥15 days or more per month for ≥3 months) (D. R. Nixdorf et al., 2012). As previously mentioned, it has been estimated that PDAP can be present in these patients up to 7.7 years (±7.1 SD) (List et al., 2007a).
2.13 |. Diagnosis by exclusion
By definition, the diagnosis of PDAP is arrived at by exclusion. Therefore, evaluation of other diseases or disorders that can cause similar pain (i.e. odontogenic pain) is mandatory before arriving at this diagnosis (D. R. Nixdorf et al., 2012).
2.14 |. Worsened by stress
Studies indicate that 66% of PDAP patients present emotional stress that accompanies the pain, predominantly due to health, family, financial, or pain-related factors. Moreover, other reports also point towards the aggravation of PDAP pain-related intensity due to stress episodes (Marbach, 1993).
2.15 |. Disability
PDAP patients reported experiencing increased disability related to jaw function, emotions and communication when compared with pain-free controls (List et al., 2007a) and a high negative impact across a wide variety of everyday activities including employment, personal relationships and social activities (Durham & Nixdorf, 2014).
2.15 |. Unmet COPC characteristics
Two characteristics mentioned by Warren, namely “numbers of COPC as risk factor for the development of PDAP” and “familial clustering”, have not been reported yet in the PDAP literature to our knowledge. Genetic studies or self-reports data on familial predominance are lacking, and although longitudinal studies are available for chronic pain development following RCT, assessment of comorbidity with other COPC was not adequately measured and therefore this characteristic cannot currently be determined (D. R. Nixdorf et al., 2016).
Moreover, the presence of CS and somatosensory dysfunction seem to be key for a condition to be considered as COPC (Yunus, 2007b), and research is currently under way to better define and operationalize the CS concept and refine measurement instruments (den Boer et al., 2019). In fact, the development of a classification system targeting multiple dimensions of COPC symptomatology that would allow improved identification of patients presenting with COPC has also been recently proposed (Maixner et al., 2016). Studies have demonstrated that PDAP patients present with altered QST parameters in mechanical and thermal detection and pain thresholds, presenting both sensory gain and sensory loss (Baad-Hansen et al., 2013; Porporatti, Costa, Stuginski-Barbosa, Bonjardim, Conti, et al., 2015) suggesting the presence of CS, which does not exclude the possibility of an overlapping neuropathic pain component. PDAP patients also exhibited abnormal pain facilitation as measured with temporal summation of pain (Baad-Hansen et al., 2013; Porporatti, Costa, Stuginski-Barbosa, Bonjardim, & Conti, 2015; Zagury et al., 2011), altered blink reflexes (List, Leijon, Helkimo, Oster, & Svensson, 2006) and impaired pain inhibition assessed with condition pain modulation (CPM) testing (Nasri-Heir et al., 2015). Although one study found that application of topical anesthetic to the painful area in PDAP patients produced reduction in clinical pain intensity and increase in mechanical and pain detection thresholds suggesting predominance of a peripheral component (Porporatti, Costa, Stuginski-Barbosa, Bonjardim, & Conti, 2015), another study found only partial reduction in spontaneous PDAP pain after local anesthetic infiltrations (Baad-Hansen, List, Kaube, et al., 2006), suggesting a central component for spontaneous PDAP pain in at least part of these patients. The latter finding is partially supported by the previously mentioned study that found impaired pain inhibition in PDAP patients compared to controls (Nasri-Heir et al., 2015).
However, a different study that tested CPM in PDAP cases and controls did not find such endogenous pain inhibition impairment (Baad-Hansen, List, Kaube, et al., 2006). Other study supporting central amplification of pain in PDAP patients showed greater activation in brain regions related to the sensory-discriminative and cognitive components of pain perception in patients relative to pain-free controls following matched dentoalveolar pressure intensity stimulation (Moana-Filho, Bereiter, & Nixdorf, 2015). Put together, these findings suggest dysfunction of endogenous pain modulation mechanisms and brain-related pain amplification following dentoalveolar mechanical painful stimulus during functional magnetic resonance imaging (Moana-Filho et al., 2015; Nasri-Heir et al., 2015) similarly to other proposed COPCs such as TMD (Moana-Filho, Herrero Babiloni, & Nisley, 2019; Moana-Filho, Herrero Babiloni, & Theis-Mahon, 2018) and fibromyalgia (O’Brien, Deitos, Trinanes Pego, Fregni, & Carrillo-de-la-Pena, 2018; Sawaddiruk, Paiboonworachat, Chattipakorn, & Chattipakorn, 2017).
3 |. IMPLICATIONS OF PDAP BEING A COPC
The pathophysiological mechanisms underlying PDAP are still not fully understood. The present report may offer another perspective regarding PDAP as a pain disorder, which can be useful to further understand, diagnose, and treat this condition. PDAP is frequently considered a neuropathic condition, defined as pain caused by a lesion or disease of the somatosensory nervous system (Treede et al., 2008). In this context, the presence of an injury caused by an event (i.e., trauma or dental procedure) and loss of function (i.e., sensory loss as assessed with QST) are considered two key features for its categorization as a neuropathic pain condition (Finnerup et al., 2016; Scholz et al., 2019). Nonetheless, it must be kept in mind the following: a) some reports indicate that more than half of PDAP cases are not associated with traumatic events or dental procedures (Ram et al., 2009); and b) although results are mixed, it appears that PDAP patients present more consistently with sensory gain than sensory loss when assessed with QST (Baad-Hansen et al., 2013; Porporatti et al., 2017; Porporatti, Costa, Stuginski-Barbosa, Bonjardim, Conti, et al., 2015). Hence, the categorization of PDAP as one of COPCs with or without a neuropathic component may be considered in some cases. This is of clinical significance, because the treatment and resultant prognosis of neuropathic pain diagnoses are markedly different than fibromyalgia as an example of COPC, as evidenced by the differential response to antiepileptic medications (Wiffen et al., 2013) as well as different approaches to treatment (Deng, Luo, Hu, Fang, & Liu, 2016; G. J. Macfarlane et al., 2017). This is the point of classifying pain disorders within a diagnostic taxonomy insofar as there needs to be inherent meaning in making a distinction between entities (Bayne, Hohwy, & Owen, 2017; Ceusters, Michelotti, Raphael, Durham, & Ohrbach, 2015; Fillingim et al., 2014).
Psychological trauma and posttraumatic stress disorder (PTSD) have also been associated with COPCs. For instance, individuals who reported exposure to trauma were 2.7 times [95% confidence interval = 2.3, 3.1] more likely to have a COPC, with the magnitude of the association with PTSD significantly larger than with sexual or physical abuse (Afari et al., 2014). The only report on this issue in the PDAP literature suggests that this may not be the case in PDAP patients, as only one patient out of 383 presented with trauma and stress related disorder (Miura et al., 2018). However, given the importance of this characteristic in other COPCs, such relationship should be further clarified by future investigations.
A major implication of PDAP being a COPC is that it could be a risk factor for the development of other COPCs and vice versa (Warren et al., 2013). Therefore, the presence of PDAP could be associated with other COPCs especially those presenting within the trigeminal innervation territory such as TMD, migraine, or tension type headaches. While speculative, these considerations implicate a call for attention towards PDAP among health practitioners beyond dentists, such as other medical specialists likely to encounter these patients: neurologists, rheumatologists, or pain specialists. Therefore, an increase in public awareness about PDAP is warranted. Ideally, multidisciplinary medical team management should be offered to COPC patients, and in this context improved communication between dentists, physicians and other health care providers becomes essential.
Some limitations must be taken into consideration when interpreting our findings. As previously mentioned, there is not yet a validated method for defining and/or identifying a given pain condition as COPC. Thus, conclusions from investigations aimed at evaluating such possibility – as such as the present - should be regarded as tentative, at least until an accepted COPC definition and inclusion/exclusion criteria for the pain conditions are available. Furthermore, PDAP literature is modest on peer-reviewed reports describing this patient population’s characteristics when compared to other COPCs, which partially explain the recommended caution when interpreting the present findings.
4 |. CONCLUSIONS
Based on the presence of several common characteristics typically attributed to COPCs, we posit that PDAP could be considered a COPC. This hypothesis, if supported by further studies aimed at addressing the gaps in knowledge as reported here, could help clarify pathophysiological mechanisms of PDAP and improve its diagnosis and management. In addition, improving our knowledge about PDAP as one COPC could have positive repercussions to better understand the development and management of comorbid COPCs. Finally, increased awareness about PDAP among dentists, physicians and other health care providers is warranted as a multidisciplinary and holistic approach seems to be the most appropriate option to manage COPCs.
Acknowledgements:
We would like to acknowledge Gabrielle Beetz (University of Montreal) for her collaboration in the design and development of the figure. Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number K99DE027414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: none
REFERENCES
- Aaron LA, & Buchwald D (2001). A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med, 134(9 Pt 2), 868–881. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11346323 [DOI] [PubMed] [Google Scholar]
- Ablin K, & Clauw DJ (2009). From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am, 35(2), 233–251. doi: 10.1016/j.rdc.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, & Cuneo JG (2014). Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med, 76(1), 2–11. doi: 10.1097/PSY.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LM, Hudson JI, Hess EV, Ware AE, Fritz DA, Auchenbach MB, … Keck PE Jr. (2004). Family study of fibromyalgia. Arthritis Rheum, 50(3), 944–952. doi: 10.1002/art.20042 [DOI] [PubMed] [Google Scholar]
- Baad-Hansen L, Leijon G, Svensson P, & List T (2008). Comparison of clinical findings and psychosocial factors in patients with atypical odontalgia and temporomandibular disorders. J Orofac Pain, 22(1), 7–14. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18351030 [PubMed] [Google Scholar]
- Baad-Hansen L, List T, Jensen TS, & Svensson P (2006). Increased pain sensitivity to intraoral capsaicin in patients with atypical odontalgia. J Orofac Pain, 20(2), 107–114. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16708828 [PubMed] [Google Scholar]
- Baad-Hansen L, List T, Kaube H, Jensen TS, & Svensson P (2006). Blink reflexes in patients with atypical odontalgia and matched healthy controls. Exp Brain Res, 172(4), 498–506. doi: 10.1007/s00221-006-0358-1 [DOI] [PubMed] [Google Scholar]
- Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, & Svensson P (2013). Intraoral somatosensory abnormalities in patients with atypical odontalgia--a controlled multicenter quantitative sensory testing study. Pain, 154(8), 1287–1294. doi: 10.1016/j.pain.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky AJ, & Borus JF (1999). Functional somatic syndromes. Ann Intern Med, 130(11), 910–921. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10375340 [DOI] [PubMed] [Google Scholar]
- Bayne T, Hohwy J, & Owen AM (2017). Reforming the taxonomy in disorders of consciousness. Annals of Neurology, 82(6), 866–872. doi: 10.1002/ana.25088 [DOI] [PubMed] [Google Scholar]
- Benoliel R, & Gaul C (2017). Persistent idiopathic facial pain. Cephalalgia, 37(7), 680–691. doi: 10.1177/0333102417706349 [DOI] [PubMed] [Google Scholar]
- Bourke JH, Langford RM, & White PD (2015). The common link between functional somatic syndromes may be central sensitisation. J Psychosom Res, 78(3), 228–236. doi: 10.1016/j.jpsychores.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Brooke RI (1980). Atypical odontalgia. A report of twenty-two cases. Oral surgery, oral medicine, and oral pathology, 49(3), 196–199. [DOI] [PubMed] [Google Scholar]
- Buskila D, Sarzi-Puttini P, & Ablin JN (2007). The genetics of fibromyalgia syndrome. Pharmacogenomics, 8(1), 67–74. doi: 10.2217/14622416.8.1.67 [DOI] [PubMed] [Google Scholar]
- Ceusters W, Michelotti A, Raphael KG, Durham J, & Ohrbach R (2015). Perspectives on next steps in classification of oro-facial pain - part 1: role of ontology. J Oral Rehabil, 42(12), 926–941. doi: 10.1111/joor.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramella A, Paroli M, Lonia L, Bosco M, & Poli P (2013). Biopsychosocial aspects of atypical odontalgia. ISRN Neurosci, 2013, 413515. doi: 10.1155/2013/413515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer C, Dries L, Terluin B, van der Wouden JC, Blankenstein AH, van Wilgen CP, … van der Horst HE (2019). Central sensitization in chronic pain and medically unexplained symptom research: A systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res, 117, 32–40. doi: 10.1016/j.jpsychores.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Deng Y, Luo L, Hu Y, Fang K, & Liu J (2016). Clinical practice guidelines for the management of neuropathic pain: a systematic review. BMC Anesthesiol, 16, 12. doi: 10.1186/s12871-015-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Nackley AG, Slade GD, Fillingim RB, & Maixner W (2006). Idiopathic pain disorders--pathways of vulnerability. Pain, 123(3), 226–230. doi: 10.1016/j.pain.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Duenas M, Ojeda B, Salazar A, Mico JA, & Failde I (2016). A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res, 9, 457–467. doi: 10.2147/JPR.S105892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J, Exley C, John MT, & Nixdorf DR (2013). Persistent dentoalveolar pain: the patient’s experience. J Orofac Pain, 27(1), 6–13. doi: 10.11607/jop.1022 [doi] [DOI] [PubMed] [Google Scholar]
- Durham J, & Nixdorf DR (2014). Healthcare pathway and biopsychosocial impact of persistent dentoalveolar pain disorder: a qualitative study. International endodontic journal, 47(12), 1151–1159. doi: 10.1111/iej.12263 [doi] [DOI] [PubMed] [Google Scholar]
- Edwards RR, Dworkin RH, Sullivan MD, Turk DC, & Wasan AD (2016). The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain, 17(9 Suppl), T70–92. doi: 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasen M, Schroder A, Fink P, Kreiner S, Dantoft TM, Poulsen CH, … Jorgensen T (2018). A step towards a new delimitation of functional somatic syndromes: A latent class analysis of symptoms in a population-based cohort study. J Psychosom Res, 108, 102–117. doi: 10.1016/j.jpsychores.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, … Wesselmann U (2014). The ACTTION-American Pain Society Pain Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain, 15(3), 241–249. doi: 10.1016/j.jpain.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, … Jensen TS (2016). Neuropathic pain: an updated grading system for research and clinical practice. Pain, 157(8), 1599–1606. doi: 10.1097/j.pain.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, & Richard P (2012). The economic costs of pain in the United States. J Pain, 13(8), 715–724. doi: 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Gracely RH, & Schweinhardt P (2015). Programmed symptoms: disparate effects united by purpose. Curr Rheumatol Rev, 11(2), 116–130. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26088212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford SB, & Solberg WK (1992). Atypical odontalgia. J Craniomandib Disord, 6(4), 260–265. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1298761 [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, & Pedersen NL (2006). Importance of genetic influences on chronic widespread pain. Arthritis Rheum, 54(5), 1682–1686. doi: 10.1002/art.21798 [DOI] [PubMed] [Google Scholar]
- Klausner JJ (1994). Epidemiology of chronic facial pain: diagnostic usefulness in patient care. J Am Dent Assoc, 125(12), 1604–1611. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7798480 [DOI] [PubMed] [Google Scholar]
- Levitt AE, Galor A, Chowdhury AR, Felix ER, Sarantopoulos CD, Zhuang GY, … Levitt RC (2017). Evidence that Dry Eye Represents a Chronic Overlapping Pain Condition. Mol Pain, 13, 1744806917729306. doi: 10.1177/1744806917729306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List T, Leijon G, Helkimo M, Oster A, Dworkin SF, & Svensson P (2007a). Clinical findings and psychosocial factors in patients with atypical odontalgia: a case-control study. J Orofac Pain, 21(2), 89–98. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17547120 [PubMed] [Google Scholar]
- List T, Leijon G, Helkimo M, Oster A, Dworkin SF, & Svensson P (2007b). Clinical findings and psychosocial factors in patients with atypical odontalgia: A case-control study. J Orofac Pain, 21(2), 89–98. Retrieved from <Go to ISI>://WOS:000246263400002 [PubMed] [Google Scholar]
- List T, Leijon G, Helkimo M, Oster A, & Svensson P (2006). Effect of local anesthesia on atypical odontalgia - A randomized controlled trial. Pain, 122(3), 306–314. doi: 10.1016/j.pain.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, … Jones GT (2017). EULAR revised recommendations for the management of fibromyalgia. Annals of the Rheumatic Diseases, 76(2), 318–328. doi: 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- Macfarlane TV, Blinkhorn AS, Davies RM, & Worthington HV (2003). Association between local mechanical factors and orofacial pain: survey in the community. J Dent, 31(8), 535–542. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14554070 [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim RB, Williams DA, Smith SB, & Slade GD (2016). Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain, 17(9 Suppl), T93–T107. doi: 10.1016/j.jpain.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacarne A, Spierings ELH, Lu C, & Maloney GE (2018). Persistent Dentoalveolar Pain Disorder: A Comprehensive Review. J Endod, 44(2), 206–211. doi: 10.1016/j.joen.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Marbach JJ (1978). Phantom tooth pain. J Endod, 4(12), 362–372. doi: 10.1016/S0099-2399(78)80211-8 [DOI] [PubMed] [Google Scholar]
- Marbach JJ (1993). Is phantom tooth pain a deafferentation (neuropathic) syndrome? Part II: Psychosocial considerations. Oral Surg Oral Med Oral Pathol, 75(2), 225–232. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8426723 [DOI] [PubMed] [Google Scholar]
- Meeus M, & Nijs J (2007). Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol, 26(4), 465–473. doi: 10.1007/s10067-006-0433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Tu TTH, Shinohara Y, Mikuzuki L, Kawasaki K, Sugawara S, … Toyofuku A (2018). Psychiatric comorbidities in patients with Atypical Odontalgia. J Psychosom Res, 104, 35–40. doi: 10.1016/j.jpsychores.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Moana-Filho EJ, Bereiter DA, & Nixdorf DR (2015). Amplified Brain Processing of Dentoalveolar Pressure Stimulus in Persistent Dentoalveolar Pain Disorder Patients. J Oral Facial Pain Headache, 29(4), 349–362. doi: 10.11607/ofph.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moana-Filho EJ, Herrero Babiloni A, & Nisley A (2019). Endogenous pain modulation assessed with offset analgesia is not impaired in chronic temporomandibular disorder pain patients. J Oral Rehabil. doi: 10.1111/joor.12832 [DOI] [PubMed] [Google Scholar]
- Moana-Filho EJ, Herrero Babiloni A, & Theis-Mahon NR (2018). Endogenous pain modulation in chronic orofacial pain: a systematic review and meta-analysis. Pain, 159(8), 1441–1455. doi: 10.1097/j.pain.0000000000001263 [DOI] [PubMed] [Google Scholar]
- Moisset X, Calbacho V, Torres P, Gremeau-Richard C, & Dallel R (2016). Co-occurrence of Pain Symptoms and Somatosensory Sensitivity in Burning Mouth Syndrome: A Systematic Review. PLoS ONE, 11(9), e0163449. doi: 10.1371/journal.pone.0163449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri-Heir C, Khan J, Benoliel R, Feng C, Yarnitsky D, Kuo F, … Eliav E (2015). Altered pain modulation in patients with persistent postendodontic pain. Pain, 156(10), 2032–2041. doi: 10.1097/j.pain.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research. (July 2018). Facial Pain. Retrieved from https://www.nidcr.nih.gov/research/data-statistics/facial-pain
- Nixdorf D, & Moana-Filho E (2011). Persistent dento-alveolar pain disorder (PDAP): Working towards a better understanding. Rev Pain, 5(4), 18–27. doi: 10.1177/204946371100500404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf DR, Drangsholt MT, Ettlin DA, Gaul C, De Leeuw R, Svensson P, … International RDCTMDC (2012). Classifying orofacial pains: a new proposal of taxonomy based on ontology. Journal of oral rehabilitation, 39(3), 161–169. doi: 10.1111/j.1365-2842.2011.02247.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf DR, Law AS, Lindquist K, Reams GJ, Cole E, Kanter K, … National Dental PCG (2016). Frequency, impact, and predictors of persistent pain after root canal treatment: a national dental PBRN study. Pain, 157(1), 159–165. doi: 10.1097/j.pain.0000000000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien AT, Deitos A, Trinanes Pego Y, Fregni F, & Carrillo-de-la-Pena MT (2018). Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J Pain, 19(8), 819–836. doi: 10.1016/j.jpain.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Pham MT, Rajic A, Greig JD, Sargeant JM, Papadopoulos A, & McEwen SA (2014). A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods, 5(4), 371–385. doi: 10.1002/jrsm.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigg M, List T, Abul-Kasim K, Maly P, & Petersson A (2014). A comparative analysis of magnetic resonance imaging and radiographic examinations of patients with atypical odontalgia. J Oral Facial Pain Headache, 28(3), 233–242. doi: 10.11607/ofph.1230 [DOI] [PubMed] [Google Scholar]
- Pigg M, List T, Petersson K, Lindh C, & Petersson A (2011). Diagnostic yield of conventional radiographic and cone-beam computed tomographic images in patients with atypical odontalgia. Int Endod J, 44(12), 1092–1101. doi: 10.1111/j.1365-2591.2011.01923.x [DOI] [PubMed] [Google Scholar]
- Porporatti AL, Bonjardim LR, Stuginski-Barbosa J, Bonfante EA, Costa YM, & Rodrigues Conti PC (2017). Pain from Dental Implant Placement, Inflammatory Pulpitis Pain, and Neuropathic Pain Present Different Somatosensory Profiles. J Oral Facial Pain Headache, 31(1), 19–29. doi: 10.11607/ofph.1680 [DOI] [PubMed] [Google Scholar]
- Porporatti AL, Costa YM, Stuginski-Barbosa J, Bonjardim LR, & Conti PC (2015). Effect of topical anaesthesia in patients with persistent dentoalveolar pain disorders: A quantitative sensory testing evaluation. Arch Oral Biol, 60(7), 973–981. doi: 10.1016/j.archoralbio.2015.02.027 [DOI] [PubMed] [Google Scholar]
- Porporatti AL, Costa YM, Stuginski-Barbosa J, Bonjardim LR, Conti PC, & Svensson P (2015). Quantitative methods for somatosensory evaluation in atypical odontalgia. Braz Oral Res, 29. doi: 10.1590/1807-3107BOR-2015.vol29.0020 [DOI] [PubMed] [Google Scholar]
- Racine M (2018). Chronic pain and suicide risk: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry, 87(Pt B), 269–280. doi: 10.1016/j.pnpbp.2017.08.020 [DOI] [PubMed] [Google Scholar]
- Ram S, Teruel A, Kumar SK, & Clark G (2009). Clinical characteristics and diagnosis of atypical odontalgia: implications for dentists. J Am Dent Assoc, 140(2), 223–228. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19188419 [DOI] [PubMed] [Google Scholar]
- Rees RT, & Harris M (1979). Atypical odontalgia. Br J Oral Surg, 16(3), 212–218. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/285723 [DOI] [PubMed] [Google Scholar]
- Reik L Jr. (1984). Atypical odontalgia: a localized form of atypical facial pain. Headache, 24(2), 222–224. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6571407 [DOI] [PubMed] [Google Scholar]
- Sawaddiruk P, Paiboonworachat S, Chattipakorn N, & Chattipakorn SC (2017). Alterations of brain activity in fibromyalgia patients. J Clin Neurosci, 38, 13–22. doi: 10.1016/j.jocn.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, … Classification Committee of the Neuropathic Pain Special Interest, G. (2019). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain, 160(1), 53–59. doi: 10.1097/j.pain.0000000000001365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shueb SS, Nixdorf DR, John MT, Alonso BF, & Durham J (2015). What is the impact of acute and chronic orofacial pain on quality of life? J Dent, 43(10), 1203–1210. doi: 10.1016/j.jdent.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, … Serra J (2008). Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology, 70(18), 1630–1635. doi:01.wnl.0000282763.29778.59 [pii] 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, … Wang SJ (2015). A classification of chronic pain for ICD-11. Pain, 156(6), 1003–1007. doi: 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu TTH, Miura A, Shinohara Y, Mikuzuki L, Kawasaki K, Sugawara S, … Toyofuku A (2018). Evaluating Burning Mouth Syndrome as a Comorbidity of Atypical Odontalgia: The Impact on Pain Experiences. Pain Pract, 18(5), 580–586. doi: 10.1111/papr.12647 [DOI] [PubMed] [Google Scholar]
- Turk DC, Fillingim RB, Ohrbach R, & Patel KV (2016). Assessment of Psychosocial and Functional Impact of Chronic Pain. J Pain, 17(9 Suppl), T21–49. doi: 10.1016/j.jpain.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Vickers ER, Cousins MJ, Walker S, & Chisholm K (1998). Analysis of 50 patients with atypical odontalgia. A preliminary report on pharmacological procedures for diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 85(1), 24–32. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9474610 [DOI] [PubMed] [Google Scholar]
- Warren JW (2014). Bladder pain syndrome/interstitial cystitis as a functional somatic syndrome. J Psychosom Res, 77(6), 510–515. doi: 10.1016/j.jpsychores.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Warren JW, Langenberg P, & Clauw DJ (2013). The number of existing functional somatic syndromes (FSSs) is an important risk factor for new, different FSSs. J Psychosom Res, 74(1), 12–17. doi: 10.1016/j.jpsychores.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Wessely S, Nimnuan C, & Sharpe M (1999). Functional somatic syndromes: one or many? Lancet, 354(9182), 936–939. doi: 10.1016/S0140-6736(98)08320-2 [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice AS, … Kalso EA (2013). Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev(11), CD010567. doi: 10.1002/14651858.CD010567.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2014). What to call the amplification of nociceptive signals in the central nervous system that contribute to widespread pain? Pain, 155(10), 1911–1912. doi: 10.1016/j.pain.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW, & King AE (1988). Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J Physiol (Paris), 83(3), 255–266. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3272296 [PubMed] [Google Scholar]
- Yunus MB (2007a). Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum, 36(6), 339–356. doi: 10.1016/j.semarthrit.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Yunus MB (2007b). Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol, 21(3), 481–497. doi: 10.1016/j.berh.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Yunus MB (2015). Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev, 11(2), 70–85. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26138918 [DOI] [PubMed] [Google Scholar]
- Zagury JG, Eliav E, Heir GM, Nasri-Heir C, Ananthan S, Pertes R, … Benoliel R (2011). Prolonged gingival cold allodynia: a novel finding in patients with atypical odontalgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 111(3), 312–319. doi: 10.1016/j.tripleo.2010.10.008 [DOI] [PubMed] [Google Scholar]