Abstract
The substantial social and economic burden attributable to smoking is well‐known, with heavy smokers at higher risk of chronic disease and premature mortality than light smokers and nonsmokers. In aging societies with high rates of male smoking such as in East Asia, smoking is a leading preventable risk factor for extending lives (including work‐lives) and healthy aging. However, little is known about whether smoking interventions targeted at heavy smokers relative to light smokers lead to disproportionately larger improvements in life expectancy and prevalence of chronic diseases and how the effects vary across populations. Using a microsimulation model, we examined the health effects of smoking reduction by simulating an elimination of smoking among subgroups of smokers in South Korea, Singapore, and the United States. We found that life expectancy would increase by 0.2 to 1.5 years among light smokers and 2.5 to 3.7 years among heavy smokers. Whereas both interventions led to an increased life expectancy and decreased the prevalence of chronic diseases in all three countries, the life‐extension benefits were greatest for those who would otherwise have been heavy smokers. Our findings illustrate how smoking interventions may have significant economic and social benefits, especially for life extension, that vary across countries.
Keywords: healthy aging, heavy smokers, microsimulation, Singapore, smoking interventions, South Korea, tobacco control, United States
1. INTRODUCTION
Market failures provide an economic rationale for smoking interventions to address two information failures. 1 First, there is an information failure about the health risk of smoking; smokers either do not appreciate the health damages correctly (Khwaja, Silverman, Sloan, & Wang, 2009) or their time preferences are inconsistent (Sloan & Wang, 2008). Second, there is an information failure about the addictive potential of smoking; many smokers underestimate the risk of becoming addicted and, once addicted, face high costs in trying to quit. Due to these information failures, the individual's choice of smoking can be suboptimal from a social point of view even if the private choice of smoking is rational. This justifies government intervention in smoking through strategies such as the provision of information, regulation, and taxation. Specifically, smoking interventions have the potential to increase social welfare by decreasing smoking‐related diseases and increasing life expectancy. Research and policy experience confirm that smoking cessation programs can reduce cancer risk (National Center for Chronic Disease Prevention and Health Promotion [United States] Office on Smoking and Health, 2014), lower direct and indirect costs to payers, employers, and employees (Rasmussen, Prescott, Sorensen, & Sogaard, 2005), and increase work productivity (Halpern, 2001), potentially also extending the productive work life of older adults.
A strong dose‐response relationship between cigarette consumption and specific diseases is well‐documented (Burns, 2003). 2 However, little is known about whether smoking interventions targeted at heavy smokers relative to light smokers lead to disproportionately larger improvements in life expectancy and prevalence of chronic diseases and how the effects vary across populations. Various smoking intervention strategies have been employed, such as group behavioral therapy, bupropion, intensive physician advice, nicotine replacement therapy, individual counselling, telephone counselling, nursing interventions, and tailored self‐help (Lemmens, Oenema, Knut, & Brug, 2008). Many studies of these strategies do not account for the heterogeneity of baseline smoking intensity. Indeed, heavy smokers are different from light smokers in several ways. For example, heavy smokers were more likely to report greater difficulty quitting, troubled by withdrawal symptoms, and experienced stronger urges and cravings than light smokers (Killen, Fortmann, Telch, & Newman, 1988). This suggests that the health impact of smoking cessation programs will be more pronounced among heavy smokers, although the cost may also be greater for any intervention successful in inducing heavy smokers to quit.
Furthermore, it is expected that the impacts of smoking programs vary by country. Differences in smoking norms (European tobacco control status report, 2014), tobacco policies, anti‐smoking interventions (Klumbiene, Sakyte, Petkeviciene, Prattala, & Kunst, 2015), and their interaction with socio‐demographic factors (Hiscock, Bauld, Amos, Fidler, & Munafo, 2012) give rise to distinctive patterns of smoking intensity across cohorts and different impacts of smoking reduction policies. In particular, the Asia‐Pacific region is of interest because Asian countries are aging rapidly and projected to have continually increasing proportions of older adults in the coming decades (Lemmens et al., 2008). Moreover, high‐income East Asia has a higher prevalence of male smoking and lower prevalence of obesity than the United States, suggesting differential impacts of tobacco control. Given the speed of aging in these countries and fiscal concerns about the sustainability of health insurance systems if the burden of chronic disease escalates significantly, it is crucial to understand the future health burdens associated with such a high proportion of elderly (Chen et al., 2019) and the potential health gains from reducing the leading preventable risk factor.
In this paper, we examined the potential improvement in health if we reduce smoking among light and heavy smokers to zero, for three distinct populations of older adults—South Korea, Singapore, and the United States. To accomplish this task, we developed a dynamic microsimulation model to estimate the health gains of reducing smoking among heavy smokers and light smokers. Microsimulation models are powerful tools for assessing the value of health promotion and simulating counterfactual scenarios. By modelling individual health trajectories while taking account of competing risks, 3 such models can provide valuable information about the impact of interventions and how they may interact with the changing demographics and socioeconomic profile of a population to determine future health. Over recent decades, South Korea and Singapore, and to a lesser degree the United States, have experienced rapid increases in living standards and in life expectancy, alongside fertility reductions so that the future middle‐aged and elderly population will differ significantly from that of the past. We developed and applied microsimulation models tailored to the demographic and epidemiological context in the three economies and compared the gains in survival and reduction in chronic disease prevalence from a given reduction in smoking and how these impacts vary depending on initial smoking intensity. We hypothesize that smoking reduction substantially increases life expectancy and reduces the burden of chronic diseases such as cancer, lung disease, and heart disease. This experiment allows us to measure the effect of smoking interventions targeting heavy smokers compared with light smokers. By modifying smoking intensity, we can quantify the associated changes in mortality and the prevalence of major diseases in each country.
To illustrate that the health effects of smoking reduction depend on the heterogeneity of individuals who smoke, we simulated two policy scenarios: eliminating smoking among light (30th percentile) and heavy (70th percentile) smokers in each country. We find that reducing smoking prevalence to zero leads to disproportionately greater increases in life expectancy for the heavy smokers than light smokers across these countries, while reducing the prevalence of key chronic diseases, depending on offsetting effects (e.g., lower or delayed incidence but fewer fatal events, thus increasing the number living with diseases).
The paper proceeds as follows. Section 2 describes our data, sample, and outcome measures. Section 3 describes our methodology. Section 4 provides the results, whereas Section 5 discusses our findings and concludes.
2. DATA AND MEASUREMENT
2.1. Data
To develop the microsimulation models, we analyzed individual‐level panel data from each country: (a) the Korean Longitudinal Study of Aging (KLoSA) for South Korea, (b) the Health and Retirement Study (HRS) for the United States, and (c) the Singapore Chinese Health Study (SCHS) for Singapore. We provide a short description of each; for more detailed information about the estimation and validation of each model, please see the Supporting information.
The Korean Future Elderly Model (FEM) simulates the population of Korea age 51 and older based on a range of relevant demographic and health data drawn primarily from the harmonized KLoSA. KLoSA is a nationally representative longitudinal survey of the South Korean population aged 45 years or older. The survey has been conducted every 2 years starting from 2006. The harmonized KLoSA data includes a set of harmonized variables suitable for cross‐country analysis based on the first four waves of KLoSA, with 10,254, 8,688, 7,920, and 7,486 respondents, respectively. We used this harmonized KLoSA data for all model variables except the prevalence of lung disease because lung disease appears to be substantially underreported in KLoSA compared with epidemiological estimates. Indeed, according to credible sources, the prevalence of chronic obstructive pulmonary disease (COPD) in South Korea was 13–14% between 2010 and 2015, but only 2.8% were diagnosed as having COPD (Hwang, Park, & Yoo, 2017). To adjust for this known discrepancy, we used the Korea National Health and Nutrition Examination Survey health examination data from 2010 to 2015 to estimate lung disease prevalence and incidence instead of KLOSA's self‐reports (Kweon et al., 2014). We categorized an individual as having COPD lung disease if his forced expiratory volume in 1 s divided by forced vital capacity was less than 0.7 (Hwang et al., 2017).
The Singapore FEM simulates the population of older adults of Chinese ethnicity in Singapore. The population estimates are based on the demographic and health status variables included in the SCHS, a population‐based prospective cohort study of ethnic Chinese in Singapore. The SCHS is conducted once every 6 years. The baseline study (n = 63,257) was collected between 1993 and 1999; the first follow‐up (n = 52,325) was collected between 1999 and 2004, and the second follow‐up (n = 39,528) was collected between 2006 and 2010. The Singapore Multi‐Ethnic Cohort Study was used to predict asthma and lung disease prevalence.
The U.S. FEM simulates representative cohorts of people aged 51 or older based on the HRS, a biennial survey of Americans age 51 or older that began in 1992. For each individual, the FEM predicts health conditions and functional status for the next 2 years, given initial demographic characteristics and health states. Both functional status and the likelihood of developing a health condition depend on age, sex, education, race, ethnicity, body mass index, smoking status, and health at the time of entry into the study.
2.2. Outcome measures
Our outcomes were life expectancy and the prevalence of key chronic diseases. For the latter, we focused on three major chronic diseases shown to be causally related to smoking: cancer, lung disease, and heart disease.
2.3. Explanatory variables
The key explanatory variable captures cigarette smoking history. Our smoking intensity variable (pack‐years) is the cumulative number of packs of cigarettes a person had smoked up to age 50, expressed as the equivalent number of packs per day for a single year, or as the equivalent number of years smoking a single pack per day. For example, this smoking intensity variable would be 10 pack‐years if a person smoked one pack a day for 10 years, or two packs a day for 5 years, or half a pack per day for 20 years. This number provides a proxy for smoking intensity of each person over his or her first 50 years. We then categorized the intensity of smoking based on an individual's percentile ranking in the country‐specific distribution of cumulative pack‐years before age 50. Other covariates include age, sex, education, and health status risk factors (body mass index) and health status in the previous survey wave (see Table S1a–c).
2.4. Descriptive statistics
We provide descriptive statistics in Table 1, which summarize the characteristics of the population used to estimate the health transition model for each country. The population used to estimate the health transition model only included males in South Korea, whereas in the United States and Singapore, both genders were included. The percentage of current smokers was higher among men in South Korea (36%) than among men and women in the United States and Singapore (14%, and 15%, respectively). However, focusing only on men, the prevalence of current smokers was 16% in the United States and 26% in Singapore. As shown in Table 1, the heaviest smokers in all three countries reported more than 100 cumulative pack‐years, meaning three packs per day for more than 30 years. The distribution of smoking intensity is wider in the United States, with the fewest pack‐years for average light smokers (30th percentile) and the largest number of pack‐years for the average heavy smoker (70th percentile).
Table 1.
Summary of the population used to estimate health transitions in Korea, Singapore, and the United States
| Variable, mean (SD) | Koreab | United Statesc | Singapored |
|---|---|---|---|
| Male | 1.0 (0.00) | 0.45 (0.50) | 0.45 (0.50) |
| Age | 65.0 (10.7) | 67.3 (9.90) | 69.8 (6.01) |
| Current smoker | 0.36 (0.48) | 0.14 (0.34) | 0.15 (0.35) |
| Ever smoked | 0.65 (0.48) | 0.57 (0.50) | 0.37 (0.48) |
| Smoke packs to age 50, mean (min–max) | 12.8 (0–110) | 16.3 (0–352) | 10.2 (0–107) |
| Body mass index | 23.1 (2.6) | 28.0 (5.8) | 23.4 (3.2) |
| Heart Disease | 0.07 (0.25) | 0.22 (0.41) | 0.10 (0.39) |
| Hypertension | 0.33 (0.47) | 0.53 (0.50) | 0.53 (0.50) |
| Stroke | 0.06 (0.24) | 0.08 (0.27) | 0.05 (0.23) |
| Cancer | 0.04 (0.19) | 0.09 (0.29) | 0.05 (0.21) |
| Diabetes | 0.16 (0.36) | 0.14 (0.35) | 0.20 (0.40) |
| Lung diseasea | 0.22 (0.41) | 0.19 (0.39) | 0.12 (0.33) |
| Death | 0.05 (0.20) | 0.04 (0.20) | 0.19 (0.39) |
Note. “Smoke packs to age 50” is the cumulative number of packs of cigarettes a person self‐reports as having smoked up to age 50, expressed as the equivalent number of packs per day for a single year.
In Korea, lung disease for males was modelled from the Korea National Health and Nutrition Examination Survey. If forced expiratory volume in 1 s/forced vital capacit y< 0.7, then we considered the individual to have chronic obstructive pulmonary disease. In the United States, we used the Health and Retirement Study to model lung disease based on the question: “Has a doctor ever told you that you have chronic lung disease such as chronic bronchitis or emphysema?" In Singapore, we used the Multi‐Ethnic Cohort based on the question: “Have you ever had asthma or other lung disease?"
The Korea transition population was based on 2‐year panel data for males (n = 11,039) using the Korean Longitudinal Study of Aging, from year 2008 to 2012.
The U.S. transition population was based on 2‐year panel data for males and females (n=123,234) using the Health and Retirement Study, from year 2000 to 2012.
The Singapore transition population was based on 6‐year panel data for males and females (n = 64,995) using the Singapore Chinese Health Study, from year 1999 to 2010.
Table 2 provides a summary of the cohort used to simulation. Our simulations focused on male smokers. The mean cumulative pack‐years among male light smokers at age 50 was 9.9, 20.6, and 5.8 in South Korea, the United States, and Singapore, respectively. Given the relatively skewed distribution of smoking, heavy male smokers had substantially higher mean cumulative pack‐years: 39.9, 35.3, and 43.1 in South Korea, the United States, and Singapore, respectively. This means that among the heaviest smokers in Singapore at age 50, the average heavy smoker accumulated exposure to the equivalent of two packs per day for more than 20 years. These smoking intensity variables illustrate that for a given reduction in the prevalence of smoking, the health gains are likely to be disproportionately greater for those interventions successfully impacting heavy smokers. Our microsimulation quantifies this effect for each population.
Table 2.
Summary of simulating cohort in Korea, Singapore, and the United States
| Korea | United States | Singapore | |
|---|---|---|---|
| Percentage of current smokers in initial cohort | 27.9 | 29.5 | 9.78 |
| Mean cumulative pack‐years of light smokersa (30th percentile smokers) | 9.90 (4.01) | 20.6 (5.07) | 5.83 (4.03) |
| Mean cumulative pack‐years of heavy smokersb (70th percentile smokers) | 39.9 (16.2) | 35.3 (5.16) | 43.1 (15.9) |
Light smokers were defined as having less than the 30th percentile of cumulative pack‐years at age 50 for each country. Mean cumulative pack‐years for male light smokers in Korea, the United States, and Singapore were 9.9, 20.6, and 5.8 pack‐years, respectively. Pack‐years are defined as the cumulative number of packs of cigarettes a person self‐reports as having smoked up to age 50, expressed as the equivalent number of packs per day for a single year.
Heavy smokers were defined as having greater than the 70th percentile of cumulative pack‐years at age 50 for each country. Mean cumulative pack‐years for male heavy smokers in Korea, the United States, and Singapore were 39.9, 35.3, and 43.1 pack‐years, respectively.
3. METHODOLOGY
3.1. Microsimulation
We used a microsimulation model to take into account individual‐level heterogeneity and its cohort‐level impacts. A microsimulation model follows individual‐level health trajectories; other models typically average health trajectories across a specific cohort. We conducted microsimulations using the FEM, which was initially developed by Goldman, Shekelle, Bhattacharya, Hurd, and Joyce (2004) in the United States. As described above, adaptations of the FEM have been developed for Korea (under review) and Singapore using country‐specific data sets (Chen et al., 2019).
We first estimated a health transition model, which calculates transition probabilities across various health states based on the individual's current characteristics. The transition model calculates the probabilities of transiting across various health states. We modelled the incidence of six major chronic diseases (cancer, diabetes, heart disease, hypertension, lung disease, and stroke) and all‐cause mortality. These health transitions depend on several predictors such as age, sex, education, risk factors (body mass index and cumulative pack‐years smoked before the age of 50), and health status in the previous wave (Tables S1a–c).
Second, we used these health transition estimation results to predict the health outcomes of a cohort as that cohort ages. Beginning with a cohort of middle‐aged individuals for each country, we simulated the health trajectory for each individual through to death. In each period, those who survive to the end of that period move to the following period, proceeding through the health transition model as before. This process is repeated until we reach the extinction of the cohort in the simulation. No new individuals are introduced at any point in the simulation.
We used probit regression to estimate the probability of transition to each health condition. All diseases are assumed to be absorbing states (i.e., recovery is not possible), consistent with the question asked in the underlying population surveys: “Have you ever been told by a doctor that you have X?” In South Korea, variables were measured with a 2‐year lag among the males in the KLoSA, whereas for the United States, the 2‐year health transition model incorporated both males and females in the HRS. In Singapore, the 6‐year transition from the SCHS also included both males and females. Korea's transition population focused on men because of the very low rate of reported smoking, as well as the known underreporting, among women in South Korea (Park, Kim, Nam, & Hong, 2014). To focus on the most affected population with the most reliable data and the population targeted in national policy, the South Korea simulation only included men. For the United States and Singapore, the prevalence of smoking among women was nontrivial, although lower than among men; therefore, for the United States and Singapore, we modelled health transitions based on both genders. However, for ease of comparison between the three countries, we report results for the impact of smoking reduction only on males.
In all three countries, transition probabilities for each health condition were estimated only using individuals who did not currently suffer from that health condition but were observed to have that specific condition in the subsequent wave. For the Korean and the U.S. simulations, we used an initial population aged 51 and 52 taken from the KLoSA and HRS, respectively, for the year 2012. For Singapore, we start with a cohort in 2010 between 55 and 60 years old.
Third, we implemented the health transition model using Monte Carlo decision‐making as follows. We drew a random variable from a uniform distribution and compared it with the relevant transition probability. If an individual's transition probability is larger than the random variable, we assigned the predicted health status change to that individual. We applied this decision‐making to all individuals at each period of the simulation. We present the simulation outcomes graphically with 95% Monte Carlo confidence intervals. To calculate the Monte Carlo confidence intervals, we repeated the sampling process described above 1,000 times and used the 2.5 and 97.5 percentiles as the credible interval, 4 similar to other studies (Chen et al., 2019; O'Brien et al., 2009; Pericchi & Walley, 1991).
3.2. Smoking intervention simulation
We simulated two policy scenarios to illustrate that the health effects of smoking reduction depend on the heterogeneity of treated individuals: eliminating smoking among light (30th percentile) and heavy (70th percentile) smokers. Both simulations first reduce the smoking intensity variable (capturing the cumulative number of packs of cigarettes a person had smoked up to age 50) and then measure the later‐life health of those individuals, including the effect on the life expectancy and chronic disease.
Our first simulation envisions a policy that has its maximum effect on light smokers only. We implement this scenario in our simulation by running a counterfactual version of our simulation model, in which the probability of becoming a light smoker is set to zero. We run an analogous simulation for the prevention of heavy smoking.
In order to isolate the effect of the smoking interventions, we show the difference in outcomes for those who were affected by the intervention, a treatment‐on‐the‐treated analysis. To do so, we first simulate the health trajectory for each individual based on their actual smoking intensity up to age 50 (termed “pre‐treatment”). Then we simulate the intervention scenario for each individual, assuming they had never smoked (i.e., pack‐years was set to zero, termed “post‐treatment”). We report the difference in health outcomes for the same individuals, pre‐treatment compared with post‐treatment. The measured gains in life expectancy are all conditional upon an individual surviving to age 50.
4. RESULTS
4.1. Simulation results: Survival benefits of smoking reduction
The simulations confirm that a smoking reduction can achieve significant improvements in lifetime health as measured by survival and that the effects are heterogeneous. The difference in cumulative exposure to the harmful effects of smoking among light and heavy smokers suggests that for a given reduction in smoking prevalence, the health and survival benefits will be substantially greater for interventions that successfully target heavy smokers. Moreover, both the initial smoking intensity (even within the “light” and “heavy” categories) and other population‐ or country‐specific factors shape the life‐years gained from a given reduction in smoking.
Our simulations showed that eliminating smoking among light smokers (those in the bottom 30th percentile of smoking intensity in each country) increased remaining life expectancy by small but nontrivial amounts (Table 3): Life expectancy at age 52 increased by 0.21 year (35.8 years before intervention and 36.0 years after intervention) in South Korea and by 1.50 years in the United States (29.5 years before intervention and 31.0 years after intervention). Similarly, in Singapore, post‐intervention life expectancy at age 58 increased by 0.55 year (29.0 years before the intervention and 29.6 years after intervention).
Table 3.
Remaining life expectancy of older male adults, pre‐ and post‐treatment, in Korea, Singapore, and the United States
| Smoking cessation in light smokersc | Smoking cessation in heavy smokersd | Difference: Life‐year gain from not smoking | ||||
|---|---|---|---|---|---|---|
| Pre‐treated | Post‐treated | Pre‐treated | Post‐treated | Light smokers | Heavy smokers | |
| Koreaa | 35.81 | 36.02 | 32.65 | 35.01 | 0.21 | 2.36 |
| USa | 29.52 | 31.02 | 26.34 | 28.86 | 1.50 | 2.52 |
| Singapore2 | 29.01 | 29.56 | 24.22 | 27.93 | 0.55 | 3.71 |
Life expectancy at age 51.5 for Korea and the United States.
Life expectancy at age 57.5 for Singapore.
Light smokers were defined as having less than the 30th percentile of cumulative pack‐years at age 50 for each country. Mean cumulative pack‐years for male light smokers in Korea, the United States, and Singapore were 9.9, 20.6, and 5.8 pack‐years, respectively. Pack‐years are defined as the cumulative number of packs of cigarettes a person self‐reports as having smoked up to age 50, expressed as the equivalent number of packs per day for a single year.
Heavy smokers were defined as having greater than the 70th percentile of cumulative pack‐years at age 50 for each country. Mean cumulative pack‐years for male heavy smokers in Korea, the United States, and Singapore were 39.9, 35.3, and 43.1 pack‐years, respectively.
By contrast, eliminating smoking among heavy smokers yielded disproportionately larger gains in survival for the same percentage reduction in smoking prevalence. An intervention that converted heavy smokers to nonsmokers increases life expectancy at age 52 by 2.36 years (32.6 years before the intervention and 35.0 years after intervention) in South Korea and by 2.52 years in the United States (26.3 years before the intervention and 28.9 years after intervention). Similarly, in Singapore, post‐intervention life expectancy at age 58 increased by 3.71 years (24.2 years before the intervention and 27.9 years after intervention).
These simulations illustrate the disproportional life expectancy gains from different scenarios of 30% smoking reduction, at the two extreme ends of the distributions in each country: (a) smoking cessation among the lightest 30% of smokers, compared with (b) smoking cessation among the heaviest 30% of smokers. In South Korea, for example, the former yields 0.21 additional life‐years and the latter yields 2.36 additional life‐years—an 11‐fold greater increase in survival, for the same 30% reduction in smoking prevalence. This 11‐fold greater increase in life‐years gained is much larger than the fourfold difference in average pack‐years between light and heavy smokers (9.9 vs. 39.9 years).
Figure 1 shows the percentage point increase in survival between the pre‐ and post‐treated light smokers and heavy smokers in each country. As expected, all smokers experience some increase in survival from eliminating lifetime smoking, with the greatest gains among heavy smokers. 5 Our simulations project that if light smokers quit smoking, the mean increase in survival probability at age 65 in South Korea would be 0.28 percentage points (95.1% pre‐intervention vs. 95.3% post‐intervention), 2.17 percentage points in the United States (84.6% pre‐intervention vs. 86.7% post‐intervention), and 1.00 percentage point in Singapore (82.6% pre‐intervention vs. 83.6% post‐intervention; see Figure S1). By contrast, if heavy smokers quit smoking, the mean increase in survival probability at age 65 years in South Korea was 1.51 percentage points (93.5% pre‐intervention vs. 95.0% post‐intervention), 4.69 percentage points in the United States (78.4% pre‐intervention vs. 83.0% post‐intervention), and 9.00 percentage points in Singapore (72.4% pre‐intervention vs. 81.4% post‐intervention). These simulations suggest that smoking reduction could yield relatively large health benefits accruing before the traditional retirement years, especially from interventions reducing smoking among heavy smokers.
Figure 1.
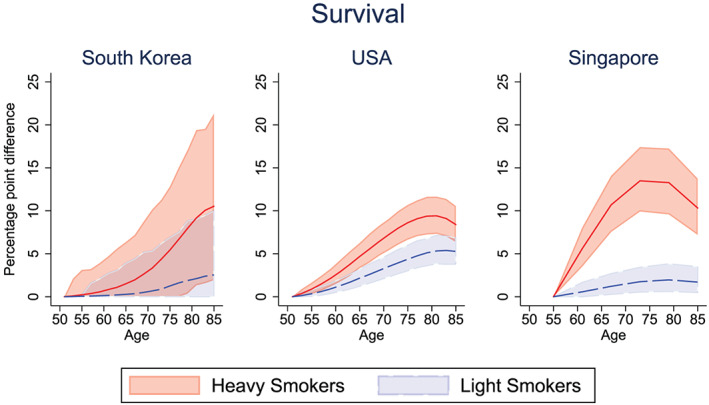
Percentage point increase in survival if never smoked, for light and heavy male smokers. Solid lines (red = “heavy smokers,” blue = “light smokers) represent the mean difference in survival between pre‐ and post‐intervention. Confidence bounds represent the 95% credible interval from Monte Carlo uncertainty of 1,000 bootstrap samples [Colour figure can be viewed at wileyonlinelibrary.com]
4.2. Simulation results: Chronic disease benefits of smoking reduction
Part of the health gains from smoking reduction flows through the reduction in the incidence of chronic disease. Of course, improved survival also means that individuals will survive to suffer from chronic disease (i.e., those who might have suffered a fatal heart attack will instead live with heart disease). We examine the net impact of smoking reduction on a given cohort of smokers in each country. Figures 2, 3, and 4 show the percentage point reduction in the prevalence of selected chronic diseases, comparing the pre‐ and post‐treated outcomes for light and heavy smokers. The corresponding Figures S2, S3, S4 show the prevalence (rather than the reduction in prevalence) of cancer, lung disease, and heart disease in the pre‐ and post‐treatment groups for the light and heavy smokers in South Korea, the United States, and Singapore, respectively.
Figure 2.
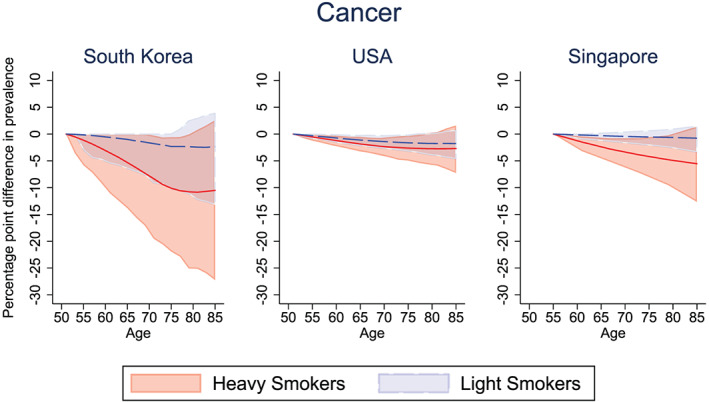
Percentage point difference in cancer prevalence between the pre‐treated and post‐treated group, among light and heavy male smokers. Solid lines (red = “heavy smokers,” blue = “light smokers”) represent the mean difference in cancer prevalence between pre‐ and post‐intervention. Confidence bounds represent the 95% credible interval from Monte Carlo uncertainty of 1,000 bootstrap samples [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
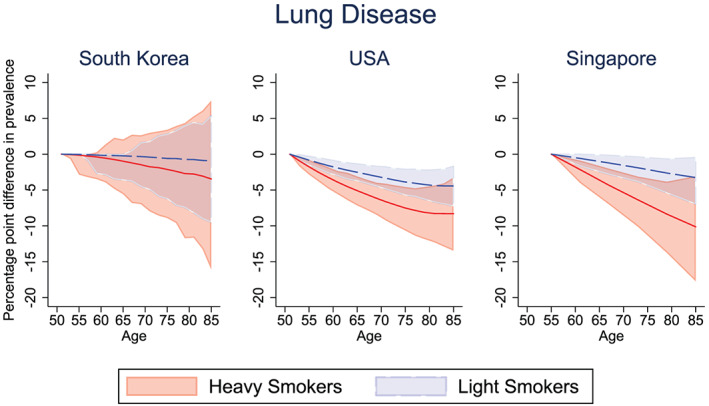
Percentage point difference in the prevalence of lung disease between the pre‐treated and post‐treated group, among light and heavy male smokers. Solid lines (red = “heavy smokers,” blue = “light smokers”) represent the mean difference in lung disease prevalence between pre‐ and post‐intervention. Confidence bounds represent the 95% credible interval from Monte Carlo uncertainty of 1,000 bootstrap samples [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
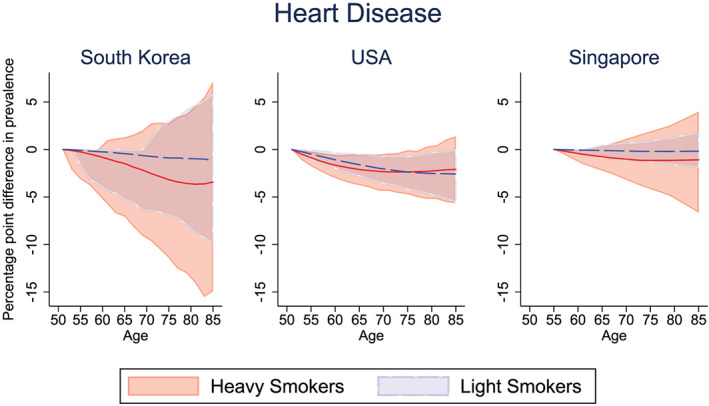
Percentage point difference in heart disease prevalence between the pre‐treated and post‐treated group, among light and heavy male smokers. Solid lines (red = “heavy smokers,” blue = “light smokers”) represent the mean difference in heart disease prevalence between pre‐ and post‐intervention. Confidence bounds represent the 95% credible interval from Monte Carlo uncertainty of 1,000 bootstrap samples [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2 shows the impact of smoking reduction on the burden of cancer among the cohort of smokers. The reduction in cancer prevalence is unsurprisingly larger for heavy smokers (70th percentile) than for light smokers (30th percentile) in each country, although the absolute magnitude of the reductions depends on the national context. If light smokers quit smoking, the mean decrease in cancer prevalence at age 65 was 1.01 percentage points in South Korea (6.70% pre‐intervention vs. 5.69% post‐intervention; Figure S2), 1.11 percentage points in the United States (16.5% pre‐intervention vs. 15.3% post‐intervention), and 0.30 percentage points in Singapore (11.9% pre‐intervention vs. 11.6% post‐intervention). Among heavy smokers, the corresponding mean decrease in cancer prevalence at age 65 was 5.31 percentage points in South Korea (9.88% pre‐intervention vs. 4.57% post‐intervention), 1.85 percentage points in the United States (26.1% pre‐intervention vs. 24.3% post‐intervention), and 2.38 percentage points in Singapore (13.2% pre‐intervention vs. 10.9% post‐intervention).
Figure 3 shows the impact of smoking reduction intervention on the burden of lung disease among the cohort of smokers. In this case, eliminating lifetime exposure to smoking among light smokers leads to a mean decrease in lung disease prevalence at age 65 of 0.22 percentage points in South Korea (25.6% pre‐intervention vs. 25.3% post‐intervention; Figure S3), 2.52 percentage points in the United States (8.91% pre‐intervention vs. 6.39% post‐intervention), and 1.02 percentage points in Singapore (5.12% pre‐intervention vs. 4.11% post‐intervention). Eliminating smoking among otherwise heavy smokers is shown to result in a mean decrease in lung disease prevalence at age 65 of 0.98 percentage points in South Korea (28.3% pre‐intervention vs. 27.3% post‐intervention), 5.09 percentage points in the United States (23.6% pre‐intervention vs.18.5% post‐intervention), and 3.61 percentage points in Singapore (14.2% pre‐intervention vs. 10.6% post‐intervention).
Finally, Figure 4 shows the impact of our simulated smoking interventions on the burden of heart disease among the cohort of smokers. Smokers are at greater risk for diseases that affect the heart and blood vessels (cardiovascular disease). Converting light smokers into nonsmokers yields a mean decrease in heart disease prevalence at age 65 of 0.44 percentage points in South Korea (12.0% pre‐intervention vs. 11.6% post‐intervention; Figure S4), 1.61 percentage points in the United States (23.8% pre‐intervention vs. 22.2% post‐intervention), and 0.11 percentage points in Singapore (14.6% pre‐intervention vs. 14.4% post‐intervention). Converting heavy smokers into nonsmokers yields a mean decrease in heart disease prevalence at age 65 of 1.48 percentage points in South Korea (10.3% pre‐intervention vs. 8.81% post‐intervention), 2.10 percentage points in the United States (53.2% pre‐intervention vs. 51.1% post‐intervention), and 0.76 percentage points in Singapore (14.8% pre‐intervention vs. 14.0% post‐intervention). Although we predict a decline in heart disease prevalence in all countries, we can see that the credible interval overlap contains the null in simulations for both light and heavy smokers.
5. DISCUSSION
5.1. Comparisons across the three countries
Microsimulation models allow analysts to study the interaction of differing population‐specific conditions in shaping individual‐level health trajectories and the cohort‐ and population‐level effects of policy intervention scenarios. Such models provide an apparatus to help understand why effects differ across countries, including the extent to which they arise from differences in the initial populations, or in the incidence rates from smoking to disease, or in conditional mortality rates. 6 We find significant differences in smoking reduction effects across the three countries from each of these sources.
First, the effect of the smoking intervention differs across the three countries in part because of differences in the smoking prevalence and intensity of the initial populations. The baseline smoking prevalence of current smokers in the simulation cohort was 27.9% in Korea, 29.5% in the United States, and 9.78% in Singapore. The mean cumulative pack‐years of the light versus heavy smokers was 9.9 versus 39.9 pack‐years in Korea; 20.6 versus 35.3 pack‐years in the United States, and 5.8 versus 43.1 pack‐years in Singapore (Table 2). For light smokers, the life‐year gain from not smoking was 1.5 years in the United States, 0.21 years in Korea, and 0.55 years in Singapore (Table 3). One of the reasons for these large gains should be explained by the large smoking intensity of light smokers in United States. Similarly, the higher baseline intensity also helps to explain why heavy smokers in Singapore are projected to experience the largest life‐year gains from quitting.
Second, the incidence rates from smoking to diseases differ across populations. For example, smoking can cause lung disease in any population by damaging airways and the small air sacs (alveoli) found in one's lungs. Lung diseases caused by smoking include COPD, which includes emphysema and chronic bronchitis. However, the incidence of such diseases may differ based on other risk factors in each country, the differing socioeconomic gradients in smoking, and the interaction with local tobacco‐control policies. Heterogeneity also arises from differences in the quality or potency of the cigarettes consumed, not just the overall number of pack‐years (Gibson & Kim, 2019).
The importance of differentiating initial conditions and transition paths in each country is well illustrated by the health transition models for each population, as they constitute the heart of our microsimulation. The health transition models determine the probabilities of health states in the next period, conditional on all the country‐specific factors shaping individual health trajectories. The marginal effects of the health transition models for South Korea, the United States, and Singapore are shown in Table S1a–c. In South Korea, other things being equal, an additional pack‐year of smoking before the age of 50 increases the probability of developing cancer by 0.026% (p < .001), heart disease by 0.007% (p > .05), and lung disease by 0.004% (p > .05), from a 2‐year transition (Table S1a). In the United States, an additional pack‐year of smoking before the age of 50 increases the probability of developing cancer by 0.01% (p < .001), heart disease by 0.02% (p < .001), and lung disease by 0.03% (p < .001), from a 2‐year transition (Table S1b). In Singapore, an additional pack‐year before the age of 50 increases the incidence of cancer by 0.011% (p < .01), heart disease by 0.015% (p < .01), and lung disease by 0.21% (p < .001), from a 6‐year transition (Table S1c).
Third, the mortality rates conditional on smoking and disease may differ across populations because of other known or unknown risk factors. Our health transition models estimated that holding other factors constant, smoking reduction before age 50 decreases the probability of mortality by the largest amount for smokers in Singapore, followed by the United States and Korea (see Tables S1a to S1c).
Combining these three dimensions and comparing across the three populations, we see that the life‐year gain from heavy smokers quitting well exceeds that of light smokers quitting in each country, but the magnitudes differ substantially: 11.2 times for South Korea, 6.8 times for Singapore, and 1.7 times for the United States. In fact, Americans suffer from many risk factors (such as obesity) to a greater extent than the Asian counterparts we study, such that life expectancy for U.S. light smokers who quit (51.5 + 31.02 = 82.52 years) is lower than the life expectancy of South Korean heavy smokers (51.5 + 32.65 = 84.15 years; see Table 3). Similarly, our simulations project that if light smokers quit smoking, the mean increase in survival probability at age 65 was largest in the United States, but the post‐intervention survival probability (86.7%) was still lower than the pre‐intervention survival among their light‐smoking counterparts in South Korea (95.1%).
5.2. Limitations of the study
Our study has several limitations. First, modelling this complex environment necessarily reflects only the best available information, requiring caution in interpreting our projections. In 2015, Singapore had an ethnic composition of around 74% Chinese, 14% Malays, 9% Indians, and 3% of other races. The SCHS contains data only for the Chinese; thus, health transitions for ethnic minorities such as the Malays and Indians are not included. As a result, the Singapore projections may be an underestimate as these minority groups have been shown to bear a greater chronic disease burden (Lee et al., 2009; Ministry of Trade and Industry and Department of Statistics (n.d.); Sharma, Tsivgoulis, Teoh, Ong, & Chan, 2012; Venketasubramanian et al., 2005; Williams & Mohammed, 2009). We were also unable to model shorter disease dynamics in Singapore as our transitional probabilities were estimated based on the survey with a 6‐year median follow‐up. However, as we are comparing the impact of smoking cessation in low‐ and high‐intensity smokers for each country, the difference in years between survey waves may not necessarily bias our results. Comparisons are also imperfect because Singapore's cohort is slightly older, starting at age 55 to 60 years, whereas the initial cohorts for both South Korea and the United States are age 51 to 52 years. Nevertheless, as most diseases tend to occur later in life, we do not expect our projections to be significantly biased by that difference. Finally, the microsimulation models necessarily incorporate a series of assumptions about the future. For example, they assume that except for the intervention, all other current policies and health system features continue into the future, and that age‐ and sex‐specific health transitions will be the same as those modelled with the baseline populations of each country in 2010 and 2012. In addition, we were unable to account for possible improvements in future health states due to screening or technological innovations in medical care. Our microsimulation models do not incorporate certain potential macroeconomic effects, such as the impact of avoided treatment costs on physical capital accumulation or impact of reduced morbidity on cohort productivity (Bloom et al., 2018). Further work on smoking projections should account for the latent dynamics, which underlie the burden of smoking‐related diseases. Regular future updates to the models and subsequent policy reviews are an important part of ensuring the continued value and relevance of the model for simulating smoking interventions and other policies in each country.
6. CONCLUSION
We simulated reducing smoking to zero among heavy smokers and light smokers in the three countries, comparing and contrasting the impact on survival and prevalence of chronic disease among the cohorts of those treated by the simulated intervention. We show that there are differentially positive health effects from smoking reduction, even after accounting for the heterogeneity of treated individuals, under two policy simulation scenarios: eliminating smoking among light (30th percentile) and heavy (70th percentile) smokers. We find that life expectancy increases by 0.2 to 1.5 years among light smokers and 2.5 to 3.7 years among heavy smokers, with a variable reduction in the prevalence of key chronic diseases in South Korea, the United States, and Singapore. These heterogeneous gains can be weighed against the differential marginal cost of interventions that persuade a heavy or light smoker to stop (or never to start). More research would be valuable to understand cost‐effective strategies for reducing smoking and other risk factors causing premature mortality and chronic disease morbidity in given historical and cultural contexts, to reap the health and productivity gains from healthy aging.
We thank Dr Hwee Lin Wee (National University of Singapore (NUS)) for data access to the Multi‐Ethnic Cohort study in Singapore and Dr Alex R Cook (NUS) for access to the Demographic Epidemiological Model of Singapore (DEMOS) results. The authors gratefully acknowledge funding from the National Medical Research Council (HSRG‐0077/2017 and HSRGMH18may‐0002) and the National Institute On Aging of the National Institutes of Health (P30AG024968). The Singapore Chinese Health Study was supported by the US NIH (R01CA144034 and UM1CA182876). Dr Koh WP was supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013). Dr Bhattacharya is grateful for support from the National Institutes of Health, and in particular from the National Institute on Aging (P30AG17253 and P30AG024968) for the conduct of the study. Dr Eggleston is grateful for a Shorenstein Asia‐Pacific Research Center faculty research award for innovations in healthy aging. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICTS OF INTEREST
The authors do not have conflicts of interest to report.
DISCLAIMER
There are no disclaimers to report. The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of funders or institutions of belonging.
Supporting information
Data S1:
Supplementary Information
Data S2:
Supplementary Information
Data S3:
Supplementary Information
Data S4:
Supplementary Information
Data S5:
Supplementary Information
ACKNOWLEDGEMENTS
Kim D, Chen C, Tysinger B, et al. Smoking, life expectancy, and chronic disease in South Korea, Singapore, and the United States: A microsimulation model. Health Economics. 2019;1–13. 10.1002/hec.3978
Footnotes
Myriad arguments have been invoked to support tobacco control policies, ranging from externalities of second‐hand smoke and redistributive concerns regarding burdens of medical spending, to self‐control problems (“internalities”) and libertarian paternalism. Our focus is on efficiency rather than redistributive effects and on the social benefits from life extension.
For example, for cardiovascular mortality, light smoking (4–7 cigarettes per day) has about 70% of the effect of heavy smoking(≥23 cigarettes per day; Arden et al., 2009).
A reduction in mortality from one cause of death inevitably and mechanically increases mortality from other causes of death because every individual eventually dies from a given cause. We account for this “competing risk” phenomenon in our microsimulation model by including the interrelationship between chronic diseases and mortality.
The 100(1−α)% credible interval is ( where (θ i ,i = 1,2,…,n) is the Markov chain Monte Carlo sample. For the 95% credible interval, we extract the 25th observation (2.5%) and the 975th observation (97.5%) of our 1,000 Markov chain Monte Carlo samples. (Chen and Shao, Monte Carlo Estimation of Bayesian Credible and HPD Intervals. Journal of Computational and Graphical Statistics, 1999. 8(1): p. 69‐92.)
Figure S1 shows the survival probability for the pre‐ and post‐treatment groups for the light and heavy smokers in South Korea, the United States, and Singapore.
We are indebted to a reviewer for framing the comparison in this way.
REFERENCES
- Arden, P. C. , Burnett, R. T. , Daniel, K. , Michael, J. , Yuanli, S. , Calle, E. E. , & Thun, M. J. (2009). Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke. Circulation, 120(11), 941–948. 10.1161/CIRCULATIONAHA.109.857888 [DOI] [PubMed] [Google Scholar]
- Bloom, D. E. , Chen, S. , Kuhn, M. , McGovern, M. E. , Oxley, L. , & Prettner, K. (2018). The economic burden of chronic diseases: Estimates and projections for china, japan, and south korea. The Journal of the Economics of Ageing, n/a, 100163. 10.1016/j.jeoa.2018.09.002 [DOI] [Google Scholar]
- Burns, D. M. (2003). Tobacco‐related diseases. Seminars in Oncology Nursing, 19(4), 244–249. 10.1053/j.soncn.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Cervero Liceras, F. , Flasche, S. , Sidharta, S. , Yoong, J. , Sundaram, N. , & Jit, M. (2019). Effect and cost‐effectiveness of pneumococcal conjugate vaccination: A global modelling analysis. The Lancet Global Health, 7(1), e5–e67. 10.1016/S2214-109X(18)30422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Lim, J. T. , Chia, N. C. , Wang, L. , Tysinger, B. , Zissimopoulos, J. , … Yoong, J. (2019). The long‐term impact of functional disability on hospitalization spending in singapore. The Journal of the Economics of Ageing, 14, 100193. 10.1016/j.jeoa.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European tobacco control status report (2014). WHO Regional Office for Europe. Retrieved from http://www.who.int/iris/handle/10665/128698
- Gibson, J. , & Kim, B. (2019). The price elasticity of quantity, and of quality, for tobacco products. Health Economics, 28(4), 587–593. 10.1002/hec.3857 [DOI] [PubMed] [Google Scholar]
- Goldman, D. P. , Shekelle, P. G. , Bhattacharya, J. , Hurd, M. , & Joyce, G. F. (2004). Health status and medical treatment of the future elderly. Retrieved from http://www.dtic.mil/docs/citations/ADA427404
- Halpern, M. T. (2001). Impact of smoking status on workplace absenteeism and productivity. Tobacco Control, 10(3), 233–238. 10.1136/tc.10.3.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock, R. , Bauld, L. , Amos, A. , Fidler, J. A. , & Munafo, M. (2012). Socioeconomic status and smoking: A review. Annals of the New York Academy of Sciences, 1248, 107–123. 10.1111/j.1749-6632.2011.06202.x [DOI] [PubMed] [Google Scholar]
- Hwang, Y. I. , Park, Y. B. , & Yoo, K. H. (2017). Recent trends in the prevalence of chronic obstructive pulmonary disease in korea. Tuberculosis Respiratory Disease, 80(3), 226–229. 10.4046/trd.2017.80.3.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja, A. , Silverman, D. , Sloan, F. , & Wang, Y. (2009). Are mature smokers misinformed? Journal of Health Economics, 28(2), 385–397. 10.1016/j.jhealeco.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Killen, J. D. , Fortmann, S. P. , Telch, M. J. , & Newman, B. (1988). Are heavy smokers different from light smokers?: A comparison after 48 hours without cigarettes. JAMA, 260(11), 1581–1585. 10.1001/jama.1988.03410110089033 [DOI] [PubMed] [Google Scholar]
- Klumbiene, J. , Sakyte, E. , Petkeviciene, J. , Prattala, R. , & Kunst, A. E. (2015). The effect of tobacco control policy on smoking cessation in relation to gender, age and education in lithuania, 1994‐2010. BMC Public Health, 15(1), 181. 10.1186/s12889-015-1525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon, S. , Kim, Y. , Jang, M. , Kim, Y. , Kim, K. , Choi, S. , … Oh, K. (2014). Data resource profile: The korea national health and nutrition examination survey (KNHANES). International Journal of Epidemiology, 43(1), 69–77. 10.1093/ije/dyt228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R. , Chan, S. , Chan, Y. , Wong, J. , Lau, D. , & Ng, K. (2009). Impact of race on morbidity and mortality in patients with congestive heart failure: A study of the multiracial population in singapore. International Journal of Cardiology, 134(3), 422–425. 10.1016/j.ijcard.2007.12.107 [DOI] [PubMed] [Google Scholar]
- Lemmens, V. , Oenema, A. , Knut, I. K. , & Brug, J. (2008). Effectiveness of smoking cessation interventions among adults: A systematic review of reviews. European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP), 17(6), 535–544. 10.1097/CEJ.0b013e3282f75e48 [DOI] [PubMed] [Google Scholar]
- Ministry of Trade and Industry, & Department of Statistics . (n.d.) Singapore residents by age group, ethnic group and gender. Retrieved from https://data.gov.sg/dataset/resident‐population‐by‐ethnicity‐gender‐and‐age‐group
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . (2014). No title. doi: NBK179276 [bookaccession]
- O'Brien, K. L. , Wolfson, L. J. , Watt, J. P. , Henkle, E. , Deloria‐Knoll, M. , McCall, N. , … Hib and Pneumococcal Global Burden of Disease Study Team (2009). Burden of disease caused by streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet (London, England), 374(9693), 893–902. 10.1016/S0140-6736(09)61204-6 [DOI] [PubMed] [Google Scholar]
- Park, M. B. , Kim, C. , Nam, E. W. , & Hong, K. S. (2014). Does South Korea have hidden female smokers: Discrepancies in smoking rates between self‐reports and urinary cotinine level. BMC Women's Health, 14(156), 15–156. 10.1186/s12905-014-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericchi, L. R. , & Walley, P. (1991). Robust Bayesian credible intervals and prior ignorance. International Statistical Review/Revue Internationale De Statistique, 59(1), 1–23. 10.2307/1403571 [DOI] [Google Scholar]
- Rasmussen, S. R. , Prescott, E. , Sorensen, T. I. , & Sogaard, J. (2005). The total lifetime health cost savings of smoking cessation to society. European Journal of Public Health, 15(6), 601–606. doi:cki024 [pii] [DOI] [PubMed] [Google Scholar]
- Sharma, V. K. , Tsivgoulis, G. , Teoh, H. L. , Ong, B. K. C. , & Chan, B. P. L. (2012). Stroke risk factors and outcomes among various asian ethnic groups in singapore. Journal of Stroke and Cerebrovascular Diseases, 21, 299–304. 10.1016/j.jstrokecerebrovasdis.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Sloan, F. A. , & Wang, Y. (2008). Economic theory and evidence on smoking behavior of adults. Addiction, 103(11), 1777–1785. 10.1111/j.1360-0443.2008.02329.x [DOI] [PubMed] [Google Scholar]
- Venketasubramanian, N. , Tan, L. C. , Sahadevan, S. , Chin, J. J. , Krishnamoorthy, E. S. , Hong, C. Y. , & Saw, S. M. (2005). Prevalence of stroke among Chinese, Malay, and Indian singaporeans: A community‐based tri‐racial cross‐sectional survey. Stroke, 36(3), 551–556. doi:01.STR.0000155687.18818.13 [pii]. 10.1161/01.STR.0000155687.18818.13 [DOI] [PubMed] [Google Scholar]
- Williams, D. R. , & Mohammed, S. A. (2009). Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine, 32(1), 20–47. 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Supplementary Information
Data S2:
Supplementary Information
Data S3:
Supplementary Information
Data S4:
Supplementary Information
Data S5:
Supplementary Information


