Abstract
Background & Aims:
Female sex hormones affect several non-reproductive organs but little is known about their effects on the liver during a normal menstrual cycle. We aimed to investigate the association between sex hormones and liver enzymes in healthy menstruating women.
Methods:
We performed a post-hoc analysis of data from the BioCycle study, a longitudinal cohort study designed to determine the association of sex hormones with markers of oxidative stress during the menstrual cycle. We analyzed data collected from 259 menstruating women, over 1–2 menstrual cycles, who had as many as 16 separate office visits, timed by fertility monitors. Levels of liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase, and alkaline phosphatase (ALKP), bilirubin, and lipids were measured by laboratory assays.
Results:
We found a natural cyclic pattern for liver enzymes, with transaminases and ALKP peaking in the mid-follicular phase and reaching a trough in the late luteal phase; the peak to trough differences were 4.0±4.9 U/L for ALT and 8.8±4.0 U/L for ALKP. Levels of ALT were significantly and negatively associated with levels of progesterone on the preceding visit (P=5×10–4), whereas level of ALKP was negatively associated with level of estrogen (P=.007) and progesterone (P=1×10–11). Food and alcohol intake did not modify the association. The amplitude of ALT fluctuation was greater in African Americans and decreased with age. Fluctuations in levels of ALT were smaller in women with a body mass indices >30 kg/m2 (P=.03). During menstrual fluctuation, 49% of participants had ALT values both above and below the normal cut-off value (19 U/L).
Conclusions:
Levels of liver enzymes fluctuate during the normal menstrual cycle, possibly mediated by progesterone, and the fluctuation varies with age and body mass index. These findings indicate the importance of accounting for phase of menstrual cycle when interpreting liver enzyme measurements in menstruating women.
Keywords: AST, Rhythm, BMI, estrogen receptor
Introduction
The female menstrual cycle comprises of a coordinated rhythmic fluctuation of hormonal activity. Beyond the cyclic effect on reproductive organs, during the menstrual cycle there are also changes to non-reproductive systems, for example body temperature,1 bone2 and the immune system,3 with the main effectors appearing to be the steroid sex hormones estrogen and progesterone. Recently, data from the BioCycle study demonstrated rhythmic fluctuations in multiple nutritional and metabolic parameters.4–8
Sex hormones have been known to affect the liver, and particularly hepatic lipid metabolism. The liver expresses estrogen receptor (ER) α, which, in response to estrogen (and possibly progesterone), affects hepatic gene expression 9 and controls hepatic lipid metabolism.10 Ovarian dysfunction states such as Turner’s syndrome 11 or polycystic ovary syndrome 12 are associated with excess hepatic fat deposition, surgical oophorectomy increases the risk of non-alcoholic fatty liver disease (NAFLD) 13 and menopause increases the severity of fibrosis in women with preexisting non-alcoholic steatohepatitis (NASH).14 Furthermore, treatment with the selective estrogen receptor modulator (SERM) tamoxifen is commonly associated with an increase in liver enzymes and development of steatohepatitis.15
Despite the breadth of information on the effects of steroid sex hormones on the liver in various disease states, little is known regarding their interaction with the liver during the menstrual cycle in healthy premenopausal women. We hypothesized that serum liver enzyme activity will fluctuate during the menstrual cycle due to the effect of sex hormones on liver fat metabolism.
Methods
BioCycle study
This is a post-hoc analysis of data from the BioCycle study, a prospective longitudinal cohort study designed to determine the association of sex hormones with markers of oxidative stress during the menstrual cycle.7 Briefly, 259 healthy premenopausal women, aged 18–44, with a regular menstrual cycle were recruited. Exclusion criteria included the use of hormonal contraceptives, pregnancy or breast-feeding, BMI>35, chronic use of medications, alcohol or substance dependency and liver disease requiring treatment.7
Participants were provided with a urine-based fertility monitor and timed their visits according to daily monitor prompts.7 Visits were timed to the menstrual phase (approximate day 2 of a 28-day cycle), mid-follicular (day 7), late follicular (day 12), luteinizing hormone (LH) / follicle stimulating hormone (FSH) surge (day 13), ovulation (day 14), early luteal (day 18), mid-luteal (day 22) and late luteal (day 27) for two consecutive cycles. During each clinic visit, fasting blood was collected, as well as information from questionnaires and diaries. The University at Buffalo Health Sciences Institutional Review Board (IRB) served as the IRB designated by the National Institutes of Health under a reliance agreement and approved the study, and all participants provided written informed consent.
Measurements
Liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALKP), bilirubin, and lipids were measured using standard laboratory techniques.7 Insulin, progesterone, LH and FSH levels were measured using a chemiluminescent enzyme immunoassay and estrogen levels were measured using a radio immunoassay.
Baseline physical activity was assessed using the international physical activity questionnaire (IPAQ) and nutrient intake using the food frequency questionnaire (FFQ). On cycle visits, physical activity, medication use and alcohol intake were obtained from participant diaries and nutrient intake from a 24-hour food recall questionnaire. Weight was measured at each visit.
Statistical Analyses
To allow comparison between participants with different cycle lengths, all cycles were normalized to a 28-day length and a standardized day was assigned to each visit according to the days listed above (e.g. the LH surge visit was defined as standardized day 13 of a 28-day cycle, even if it occurred on day 15 of a 32-day cycle).16
Liver enzyme and hormone levels were log-transformed for normality before statistical analyses. Significance testing for differences of baseline parameters across BMI categories was performed using chi-square or ANOVA, as appropriate.
The periodicity of liver enzyme levels and their association with fixed variables (collected at baseline) was tested using nonlinear mixed regression models with harmonic terms that model menstrual cycle patterns.17 The three parameters assessed by the models included: 1) mean level around which liver enzymes fluctuate across the cycle, 2) fluctuation amplitude, and 3) phase shift.17 (Supplementary Figure 1). Review of prior literature and statistical testing for confounding was used for identification of covariates.
Linear mixed-effects models were used to determine the association between time-dependent changes in liver enzymes and sex hormones. We tested three different models of possible association between liver enzymes and sex hormones. The first (“same-day”) model compared liver enzyme levels with estrogen and progesterone levels drawn on the same day. In addition, we determined the association between sex hormones on a given visit and liver enzymes measured in the following visit to account for a possible lag-time in the effect of hormones on liver enzymes (“lag model”). A third model (“slope model”) assessed the association between the slope of change of sex hormones between two visits and liver enzyme levels at the latter visit.
As a measure of individual “fluctuability”, we fit each participant’s ALT or ALKP values to a sine wave model using least-squares nonlinear regression. The model R2 for each individual was used as an estimate of how well her liver enzymes fit a cyclic pattern.
SPSS version 20.0 and SAS version 9.2 (SAS Institute, Cary, NC) were used for statistical analyses.
RESULTS
Participant Characteristics
259 women participated in the BioCycle study. Four participants were excluded from the analysis – one because of persistently elevated liver enzymes consistent with chronic hepatitis and three for transient elevations >100 U/L, consistent with an episode of acute hepatitis. The characteristics of the remaining 255 participants are detailed in Table 1. As expected, women with a higher BMI had significantly higher ALT, ALKP, and triglycerides, as well as a trend for higher cholesterol and lower HDL.
Table 1 –
Participant characteristics overall and stratified by BMI
| Total | BMI ≤ 25 | BMI 25–30 | BMI ≥ 30 | P-value | |
|---|---|---|---|---|---|
| n | 255 | 163 | 66 | 26 | |
| Age (years) | 27.4±8.2 | 26.4 ±7.9 | 29.5±8.6 | 28.4±8.3 | 0.02 |
| Race | 0.16 | ||||
| White | 152 (60%) | 94 (58%) | 41 (54%) | 17 (65%) | |
| African-American | 49 (19%) | 27 (17%) | 16 (21%) | 6 (23%) | |
| Asian | 54 (21%) | 42 (26%) | 19 (25%) | 3 (12%) | |
| Acetaminophen Use | 60 (24%) | 36 (22%) | 18 (24%) | 6 (23%) | 0.70 |
| Other pain medication | 105 (41%) | 60 (37%) | 32 (42%) | 13 (50%) | 0.17 |
| Energy intake* (Kcal/d) | 1610±383 | 1607±360 | 1610±405 | 1636±459 | 0.93 |
| Alcohol consumption* (drinks/d) | 0.38±0.7 | 0.35±0.6 | 0.38±0.7 | 0.55±0.8 | 0.38 |
| ALT† (u/L) | 16.6±7.0 | 15.8±6.0 | 17.8±8.4 | 19.0±8.2 | 0.007 |
| ALKP† (u/L) | 51.5±14.3 | 49.6±13.2 | 51.5±15.2 | 63.7±12.6 | <0.001 |
| HDL† (mg/dL) | 50.0±11.5 | 51.3±11.3 | 48.3±11.1 | 46.4±12.5 | 0.065 |
| Total Cholesterol † (mg/dL) | 163.4±29.0 | 160.2±25.1 | 167.1±33.6 | 174.2±35.1 | 0.06 |
| Triglycerides† (mg/dL) | 59.3±28.0 | 54.8±25.3 | 66.3±29.0 | 70.7±35.4 | 0.002 |
| Bilirubin† (mg/dL) | 0.8±0.3 | 0.8±0.3 | 0.8±0.3 | 0.7±0.3 | 0.65 |
Averaged across all visits
Values from first visit
Liver Enzyme Changes Across the Menstrual Cycle
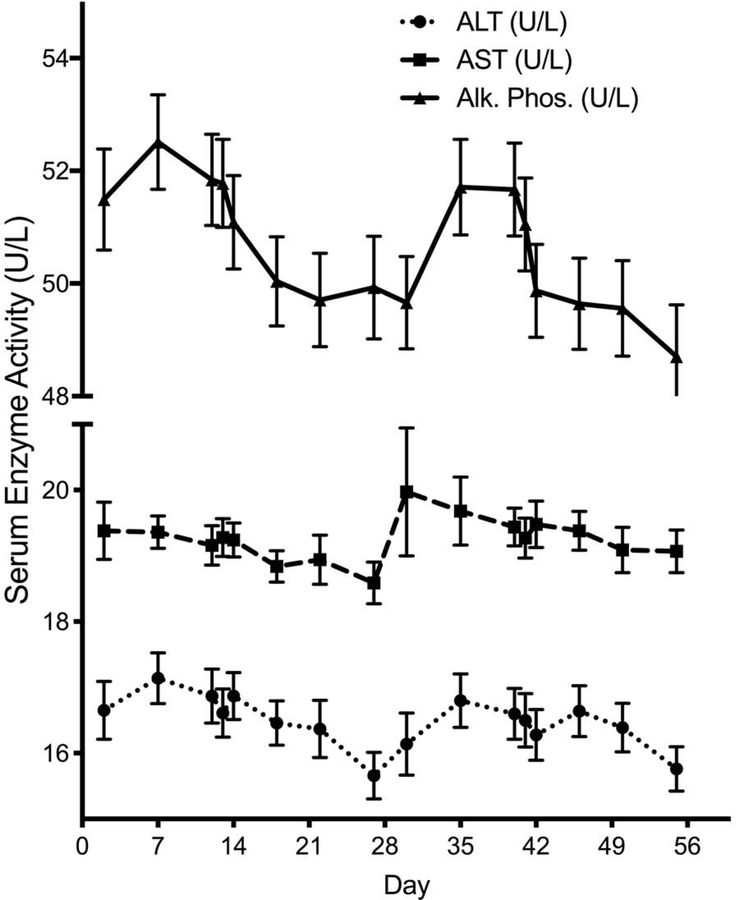
ALT and ALKP exhibited a cyclic pattern of fluctuation throughout the menstrual cycle, peaking at the mid-follicular phase and reaching a trough at the late luteal phase (Figure 1) with an average (per-subject) peak-to-trough difference of 4.0±4.9 U/L for ALT and 8.8±4.0 U/L for ALKP. Alkaline phosphatase activity started declining earlier than ALT in the menstrual cycle, with a plateau during most of the luteal phase. A minimal notch-like decrease in ALT levels of approximately 0.25 U/L was seen mid-ovulation (p=0.007 for the difference between day 12 and 13). AST activity demonstrated mild changes that were not as pronounced and hence, further analysis was limited to ALKP and ALT.
Figure 1 -. Liver enzyme activities across the menstrual cycle.
Lines denotes mean and error bars, SEM. Alk. Phos, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Harmonic regression confirmed a cyclic pattern, demonstrating an amplitude of fluctuation that was markedly greater than zero for both ALT (p<10−100) and ALKP (p<10−100).
Association of Liver Enzyme Fluctuation with Sex Hormones
Given the cyclic fluctuations in liver enzymes and sex hormone levels (supplementary figure 2), we tested whether the two are associated, using linear mixed effects models. We tested both a “same-day” model as well as the “lag model” accounting for a possible lag between the change in sex hormone level and that of the enzymes. A third “slope model” was examined but was non-contributory (data not shown).
ALT showed a negative association with same-day progesterone but not with same-day estrogen (Table 2). Using the lag model, the association with progesterone was stronger in magnitude and highly significant and a negative association was also seen with estrogen. On a multivariate analysis including both estrogen and progesterone, estrogen was not significantly associated with ALT (Table 2). Thus, ALT seems to be predominantly associated with progesterone, where the rise in progesterone during the luteal phase is driving a decline in ALT levels towards the end of that phase, and in contrast, the decrease in progesterone levels with menses allows ALT to return to baseline.
Table 2 -.
Association of ALT and ALKP with sex hormones
| Multivariate adjusted3 | |||||||
|---|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | ||
| ALT | Same-time Model | ||||||
| Estrogen | 0.002 | 0.59 | 0.005 | 0.21 | 0.01 | 0.14 | |
| Progesterone | −0.004 | 0.023 | −0.006 | 0.011 | −0.009 | 0.013 | |
| Lag Model | |||||||
| Estrogen | −0.014 | 0.0003 | −0.007 | 0.069 | −0.009 | 0.16 | |
| Progesterone | −0.015 | 6X10−12 | −0.014 | 8X10−10 | −0.014 | 0.0005 | |
| ALKP | Same-time Model | ||||||
| Estrogen | 0.0008 | 0.72 | 0.008 | 0.0004 | 0.008 | 0.03 | |
| Progesterone | -0.012 | 1X10−25 | −0.013 | 2X10−28 | −0.014 | 8X10−13 | |
| Lag Model | |||||||
| Estrogen | −0.018 | 8X10−16 | −0.011 | 2X10−6 | −0.009 | 0.007 | |
| Progesterone | −0.017 | 1X10−40 | −0.015 | 2X10−31 | −0.016 | 1X10−11 | |
Univariate models including either estrogen or progesterone
Multivariate model including both estrogen and progesterone
Multivariate model including both estrogen and progesterone, adjusted for daily alcohol consumption and daily caloric intake
ALKP was associated with progesterone only on the same day model, but on the lag model was negatively associated with both estrogen and progesterone, in both the univariate and the multivariate models (Table 2).
Since alcohol and food intake could potentially affect liver enzymes acutely, we performed a multivariate analysis, controlling for both. Neither daily alcohol nor daily caloric intake were significantly associated with daily liver enzyme levels, and their inclusion in the model had a negligible effect on the magnitude and significance of the association with sex hormones (Table 2). We performed a similar analysis limited to subjects with normal BMI (≤25 kg/m2, n=163) which yielded results that are similar overall to the analysis with the entire cohort (Supplemetary Table 1).
Baseline parameters associated with liver enzyme fluctuation
We used a harmonic regression analysis to determine which baseline parameters are affecting the cyclic fluctuation of ALT and ALKP. Associations were tested with the mean enzyme level around which the fluctuations occur (Supplementary Figure 1), the amplitude of the fluctuation and its phase.
The mean level of ALT was significantly increased with increasing age, BMI and with the use of non-acetaminophen painkillers (Table 3). Asian women had lower ALT mean level and a trend towards a lower mean was seen in African-American women compared to white women. The fluctuation amplitude of ALT was modified by age, with amplitude declining by approximately 13% for each 1-year increase. African-American women had nearly double the ALT fluctuation amplitude compared to whites (Table 3). The mean level of ALKP was strongly associated with BMI and race but no parameter modified ALKP cyclic fluctuations. Importantly, average alcohol consumption and the use of acetaminophen or other painkillers during the menstrual cycle were not associated with the changes in liver enzymes (Table 3).
Table 3 -.
Baseline parameters affecting liver enzyme fluctuation
| ALT | Mean Level | Fluctuation Amplitude | Phase Shift | |||
|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Age | 1.0047 | 0.01 | 0.8743 | 0.025 | 0.0973 | 0.019 |
| African American race vs. White | 0.9283 | 0.059 | 1.9160 | 0.021 | 1.1156 | 0.5411 |
| Asian race vs. White | 0.8647 | 0.0002 | 0.6134 | 0.40 | 1.2672 | 0.5346 |
| BMI | 1.0111 | 0.005 | 0.9392 | 0.12 | −0.0807 | 0.0036 |
| Average caloric consumption | 1.0000 | 0.14 | 1.0002 | 0.08 | −0.0003 | 0.11 |
| Average alcohol consumption | 1.0039 | 0.13 | 0.5650 | 0.37 | 0.5075 | 0.31 |
| Acetaminophen | 1.0354 | 0.34 | 1.2505 | 0.46 | 0.1636 | 0.42 |
| Pain killers | 1.0650 | 0.043 | 0.7437 | 0.35 | −0.2495 | 0.20 |
| ALKP | Mean Level | Fluctuation Amplitude | Phase Shift | |||
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Age | 0.9966 | 0.062 | 0.9935 | 0.47 | −0.0110 | 0.051 |
| African American race vs. White | 1.0987 | 0.0146 | 0.7476 | 0.2049 | 1.1311 | 0.3850 |
| Asian race vs. White | 0.9228 | 0.0315 | 0.9554 | 0.7957 | 1.0801 | 0.4868 |
| BMI | 1.0188 | 6X10−7 | 0.9690 | 0.11 | −0.0253 | 0.046 |
| Average caloric consumption | 1.0000 | 0.51 | 1.0001 | 0.58 | 0.0000 | 0.97 |
| Average alcohol consumption | 0.9986 | 0.36 | 1.0681 | 0.11 | 0.0030 | 0.92 |
| Acetaminophen | 0.9610 | 0.26 | 1.1953 | 0.25 | 0.1044 | 0.28 |
| Pain killers | 1.0028 | 0.93 | 0.8321 | 0.22 | −0.1590 | 0.09 |
To rule out a dilutional effect (secondary to volume retention) as a cause for the decline in liver enzymes at the end of the luteal phase, we analyzed the association between the percent change in enzymes relative to baseline and the percent change in body weight during the study period, which predominantly reflects volume changes. No association was seen between weight change and liver enzyme change.
Effect of BMI on Individual Fluctuations of ALT
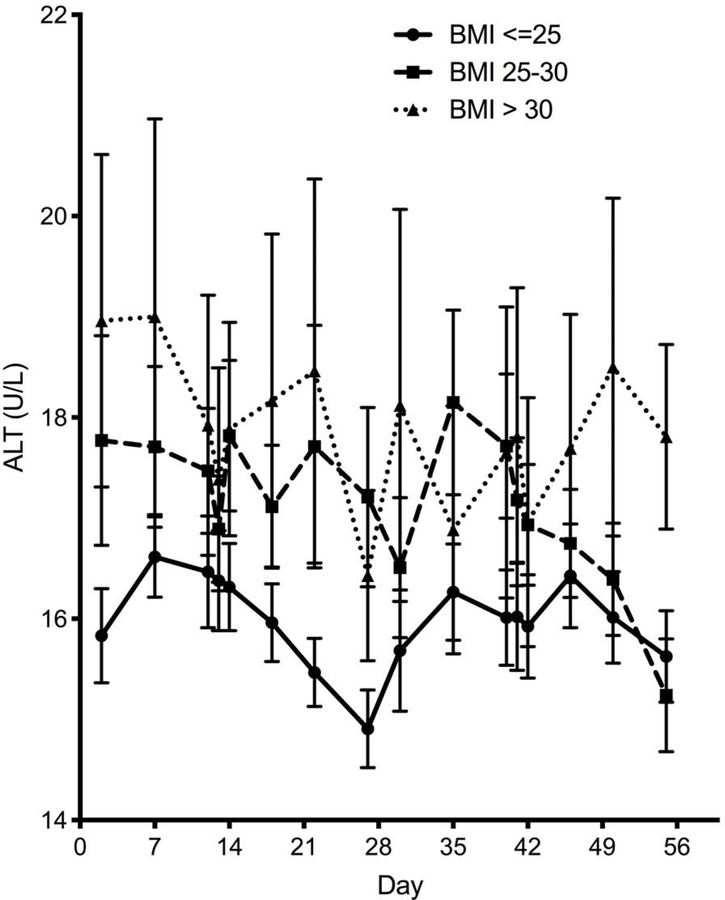
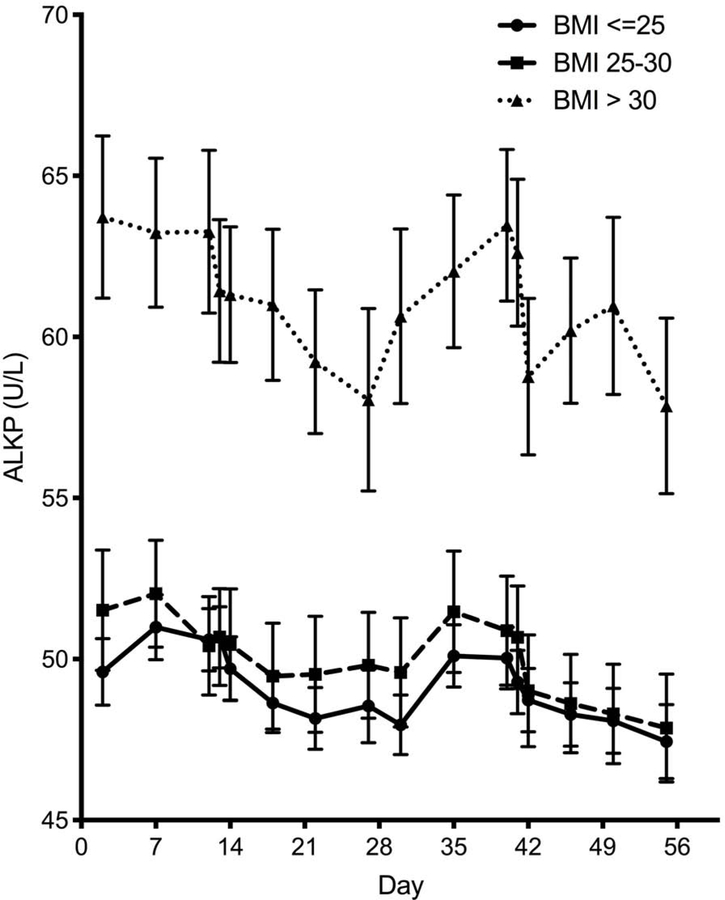
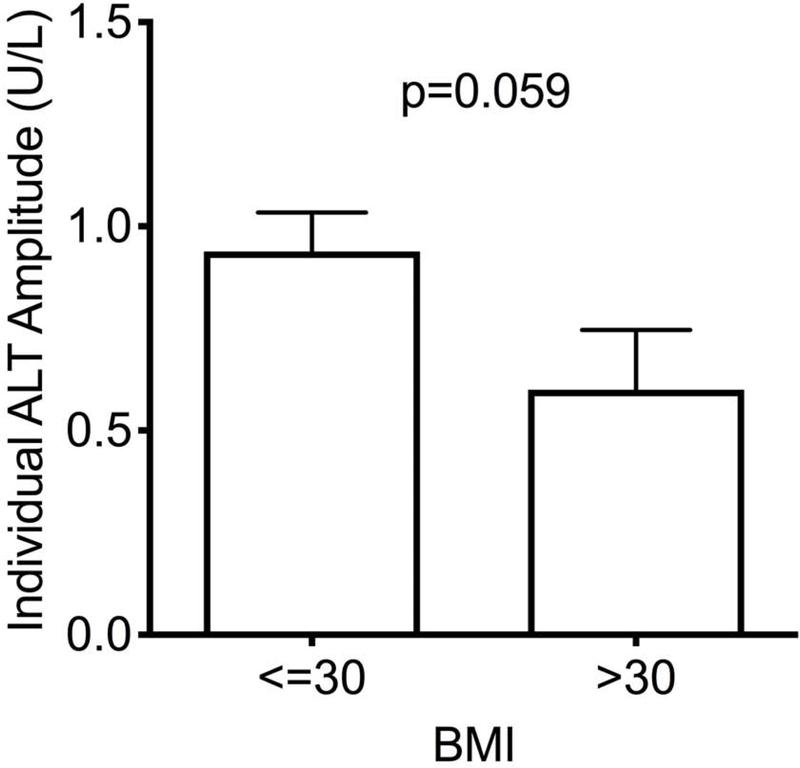
As previously noted, a higher BMI was associated with a higher mean level of ALT and ALKP (Figure 2). Interestingly, the cyclic fluctuation pattern of ALT appears to be lost with a higher BMI category (Figure 2a), whereas BMI does not exert a similar attenuating effect on the flucating pattern of ALKP (Figure 2b).
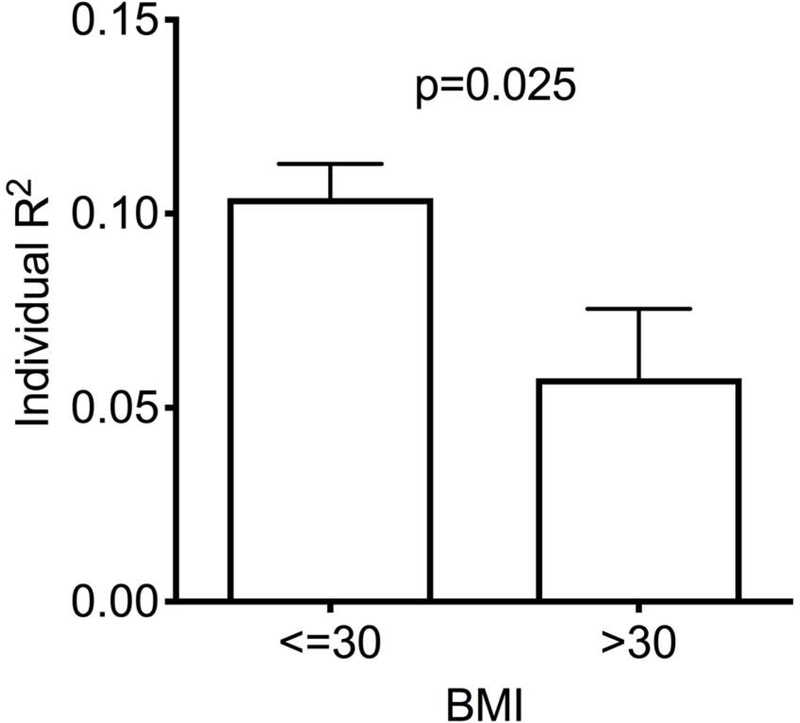
Figure 2 – Impact of BMI on liver enzyme fluctuations.
Temporal variation of ALT (A) and alkaline phosphatase (B) throughout the menstrual cycle by BMI category. Lines denotes mean and error bars, SEM. (C) The ability to fit each subject’s ALT to a sine wave (by individual R2) according to BMI category. Mean±SEM. (D) Individual amplitudes of ALT by BMI category. Mean±SEM. ALKP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index.
We assessed the “fluctuability” in each individual participant by how well her liver enzymes fit a sinusoidal cyclic pattern. ALT levels were less likely to follow a sine pattern in participants with a BMI>30, resulting in significantly lower individual fluctuability R2 values (Figure 2c, p=0.025), and a trend towards lower amplitude of fluctuation (Figure 2d, p=0.059). No differences in ALKP fluctuability were seen between BMI groups.
The loss of ALT fluctuation with higher BMI could be due to obesity itself, or due to underlying non-alcoholic fatty liver disease (NAFLD), which is commonly associated with obesity. As liver imaging studies to diagnose NAFLD were not performed in the test we utilized the mean ALT as a surrogate marker. The cyclic pattern of ALT through the menstrual cycle persisted for subjects across different categories of mean ALT, suggesting that underlying liver disease does not explain the loss of rhythmicity with high BMI (Supplementary Figure 3).
Proportion of Abnormal ALT
Although local laboratory reference ranges vary widely, the normal ALT for women is considered to be ≤19 U/L.18 Of the 210 women who had a normal ALT at baseline, 90 (43%) had at least one measurement that was abnormal and 72 (37%) had more than 25% of abnormal ALT values. Similarly, in the 43 women with baseline levels >19 U/L, 33 (77%) had at least one normal ALT value. As expected, women with >25% of abnormal ALT values had a higher average BMI (25.1±4.3 vs. 23.8±3.7 kg/m2, p=0.027). Overall, 124 women (49%) had ALT values that fluctuated both above and below the normal cutoff during the study period.
Discussion
Reproductive hormones have been shown to influence organs beyond the reproductive system.8, 19–22 Using data from BioCycle, the largest prospective study investigating multiple biochemical measurements in healthy menstruating women,7 we identified a rhythmic fluctuation of liver enzyme levels during the menstrual cycle that is tightly associated with the fluctuations in sex hormone levels.
We found that ALT and AST peak at the mid-follicular phase and reach a nadir just prior to menstruation; ALKP follows a similar pattern but is at nadir through most of the luteal phase. Alkaline phosphatase is predominantly expressed in the liver and bone, but it can also be derived from other tissues.23, 24 Thus, the ALKP fluctuations observed in our study may not reflect a liver origin and could represent subtle changes in sex hormone-sensitive tissues such as endometrium or cervical epithelium. In contrast, the changes in ALT level likely represent a hepatic source. ALT fluctuations appear to be driven predominantly by the rise in progesterone at the luteal phase, which induces a nadir in ALT levels just prior to menstruation. As progesterone levels rapidly fall at the end of the cycle, the inhibitory effect on ALT disappears and ALT levels recover and return to baseline. In contrast, the changes in estrogen levels do not affect ALT independently of progesterone.
There are several potential mechanisms to explain the association between sex hormones and ALT levels during the menstrual cycle. First, a purely dilutional effect secondary to water retention could be considered. However, this was ruled out by the absence of association between ALT and changes in weight (that mostly reflect volume changes) throughout the study period. A change in ALT driven by the use of acetaminophen, other pain killers, alcohol or caloric consumption was also ruled out. Finally, a direct effect of sex hormones on hepatocytes could be postulated. Konstandi et al9 identified cyclic changes in hepatic gene expression in a female mouse model during the estrous cycle. Of note, fluctuations were noted in the levels of several transcription factors including Srebp-1c, a major regulator of hepatic lipogenesis. Furthermore, the gene expression changes in response to sex hormone fluctuations appeared to be driven in part through modulation of hepatic insulin signaling. Thus, it is plausible that the fluctuations in ALT reflect an intrinsic effect of sex hormones on the liver, possibly via subtle changes in hepatic fat content.
The association between progesterone and the attenuation in ALT fluctuation is somewhat surprising as hepatocytes do not express the progesterone receptor,25, 26 although they do express the estrogen receptor. However, there is clear evidence for an effect of progesterone on hepatocyte function. Progesterone/progestin-based hormone replacement therapy in high doses can induce elevated transaminases.27 In patients with halothane-induced liver injury, higher serum progesterone levels correlate with more severe liver injury, whereas serum estrogen levels appears protective against such insult.28 Furthermore, in the murine model mentioned above,9 progesterone supplementation to ovariectomized animals led to expected changes in hepatic gene expression. The attenuated amplitude of ALT fluctuation seen in obese women with BMI>30 also supports our hypothesis that progesterone modulates ALT level as this same obese cohort was previously shown to have lower levels of progesterone compared to normal weight women (BMI<25).21 Similarly, the correlation between older age and variation in ALT amplitude may be explained by age-related changes in progesterone levels.29 The exact mechanism by which progesterone affects ALT fluctuation is unclear, and especially whether it is through a direct effect on the liver or indirectly through modulation of other pathways.
The normal physiological cycling of ALT and its association with sex hormones is present in normal weight women (Supplementary Table 1). However, with increased obesity, we found loss of this cyclic pattern, which could be postulated to be due to obesity itself modulating the sex hormones (as shown in this cohort21) or could be due to modulation of the hepatic response to the sex hormones by underlying NAFLD. The BioCycle trial did not collect imaging data to allow us to identify subejects with pre-existing NAFLD nor did it collect all of the values needed to calculate surrogate indices such as the fatty liver index (FLI)30. Instead, we used mean ALT itself as a surrogate marker of the presence of liver disease and found that ALT fluctuations over the menstrual cycle persist irrespective of basal ALT levels, hinting that obesity, rather than NAFLD, is a modulating factor of the fluctuability of ALT.
Cyclic changes in transaminases and ALKP, albeit small in magnitude, could have significant implications and we found that nearly half of the study participants had both normal and abnormal ALT levels within two menstrual cycles. Abnormal liver enzyme tests typically lead to workup and referral to specialists. Furthermore, liver enzymes are often tested routinely to monitor safety of medications in practice or in clinical trials. Finally, ALT and AST are components of several indices of liver disease severity that can be used to triage patients with NAFLD for hepatology referral, such as FIB-431 or the low ALT clinically significant NAFLD (LACSNA) score.32 Thus, recognition of the normal cycle-associated pattern in women is essential; when liver enzymes are mildly elevated in the middle of a menstural cycle, repeating the test at the end of the cycle or early menstruation could help avoid unnecessary referral and workup, especially in women with normal BMI.
A major strength of our study is that it is the largest cohort of its kind, with high frequency of prospective follow-up compared to other studies of menstrual cycle-related biochemical fluctuation. Zlontik et al performed a similar study of liver enzyme fluctuations, comparing 45 healthy women to 31 men at 4 time points over a one month period,33 but their study had a smaller sample size, no measurement at nadir (day 27), no data on weight, and remarkably low variation in standard deviations between subjects. Another strength is the use of fertility monitors to time visits, allowing for synchronization and correct assessment of the association between liver enzymes and phases of the menstrual cycle. Although our study clearly demonstrates the natural fluctuation of liver enzymes during the menstrual cycle, it is not able to conclusively determine the mechanism leading to that fluctuation and whether it truly reflects subtle changes in liver fat content. The other limitation is the aforementioned inability to rule out preexisting NAFLD.
In conclusion, our findings show that in healthy women there exists a menstrual fluctuation in transaminases which associates with sex hormone levels. Our data also reflects the importance of retesting transaminase levels in reproductive-age women if mildly abnormal, to account for the menstrual-associated variation. To understand the precise mechanism of progesterone on the liver physiology, future investigations involving imaging or mechanistic studies in animal models are warranted.
Supplementary Material
Need to Know.
Background:
Sex hormones have effects on extra-gonadal organs, but little is known about their effects on the liver.
Findings:
Levels of transaminases and alkaline phosphatase fluctuate rhythmically during the menstrual period; levels of alanine aminotransferase associate with levels of progesterone measured few days earlier. The fluctuation in level of alanine aminotransferase level is decreased in obese women.
Implications for patient care:
Abnormal increases in levels of transaminases and alkaline phosphate in otherwise healthy menstruating women should prompt repeat testing at the end of a menstrual cycle.
Acknowledgments
This work and EFS, SLM were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Number: HHSN275200403394C, HHSN275201100002I, Task 1 HHSN27500001). CWL, SJ, BN and YR were supported by the Intramural Research Program of NIDDK.
Abbreviations:
- LH
luteal hormone
- FSH
follicle stimulating hormone
- ALKP
alkaline phosphatase
- ALT
alanine aminotransferase
- BMI
body-mass index
- ER
estrogen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no conflict of interest.
References:
- 1.Barton DS. A Study of Temperature and Electric Potentials in the Menstrual Cycle. Yale J Biol Med. 1940;12(5):503–23. Epub 1940/05/01. [PMC free article] [PubMed] [Google Scholar]
- 2.Mozzanega B, Gizzo S, Bernardi D, et al. Cyclic variations of bone resorption mediators and markers in the different phases of the menstrual cycle. J Bone Miner Metab. 2013;31(4):461–7. Epub 2013/03/13. [DOI] [PubMed] [Google Scholar]
- 3.Kempe P, Eklund D, Hallin A, et al. Immune profile in relation to sex steroid cyclicity in healthy women and women with multiple sclerosis. J Reprod Immunol. 2018;126:53–9. Epub 2018/03/05. [DOI] [PubMed] [Google Scholar]
- 4.Gorczyca AM, Sjaarda LA, Mitchell EM, et al. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur J Nutr. 2016;55(3):1181–8. Epub 2015/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mumford SL, Browne RW, Schliep KC, et al. Serum Antioxidants Are Associated with Serum Reproductive Hormones and Ovulation among Healthy Women. J Nutr. 2016;146(1):98–106. Epub 2015/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mumford SL, Schisterman EF, Siega-Riz AM, et al. A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. J Clin Endocrinol Metab. 2010;95(9):E80–5. Epub 2010/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171–84. Epub 2009/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung EH, Zhang C, Mumford SL, et al. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95(12):5435–42. Epub 2010/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstandi M, Cheng J, Gonzalez FJ. Sex steroid hormones regulate constitutive expression of Cyp2e1 in female mouse liver. Am J Physiol Endocrinol Metab. 2013;304(10):E1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Torre S, Mitro N, Fontana R, et al. An Essential Role for Liver ERalpha in Coupling Hepatic Metabolism to the Reproductive Cycle. Cell Rep. 2016;15(2):360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostberg JE, Thomas EL, Hamilton G, et al. Excess visceral and hepatic adipose tissue in Turner syndrome determined by magnetic resonance imaging: estrogen deficiency associated with hepatic adipose content. J Clin Endocrinol Metab. 2005;90(5):2631–5. [DOI] [PubMed] [Google Scholar]
- 12.Kelley CE, Brown AJ, Diehl AM, et al. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J Gastroenterol. 2014;20(39):14172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Gualtieri MR, Cahoon SS, et al. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause. 2016;23(2):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(4):1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinowich L, Shibolet O. Drug Induced Steatohepatitis: An Uncommon Culprit of a Common Disease. Biomed Res Int. 2015;2015:168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mumford SL, Schisterman EF, Gaskins AJ, et al. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol. 2011;25(5):448–59. Epub 2011/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumford SL, Steiner AZ, Pollack AZ, et al. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. J Clin Endocrinol Metab. 2012;97(10):E1871–9. Epub 2012/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. Epub 2002/07/03. [DOI] [PubMed] [Google Scholar]
- 19.Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423–31. Epub 2012/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mumford SL, Dasharathy SS, Pollack AZ, et al. Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum Reprod. 2013;28(7):1853–62. Epub 2013/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung EH, Zhang C, Albert PS, et al. Adiposity and sex hormones across the menstrual cycle: the BioCycle Study. Int J Obes (Lond). 2013;37(2):237–43. Epub 2012/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human Reproduction Update. 2005;11(4):411–23. [DOI] [PubMed] [Google Scholar]
- 23.Siddique A, Kowdley KV. Approach to a patient with elevated serum alkaline phosphatase. Clin Liver Dis. 2012;16(2):199–229. Epub 2012/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price CP. Multiple forms of human serum alkaline phosphatase: detection and quantitation. Ann Clin Biochem. 1993;30 ( Pt 4):355–72. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 25.Petersen SL, Intlekofer KA, Moura-Conlon PJ, et al. Novel progesterone receptors: neural localization and possible functions. Front Neurosci. 2013;7:164 Epub 2013/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarpin KM, Graham JD, Mote PA, et al. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal. 2009;7:e009 Epub 2010/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LiverTox.nih.gov. [updated April 6, 2015; cited 2019 January 1]; Available from: https://livertox.nlm.nih.gov/Progestins.htm.
- 28.Toyoda Y, Endo S, Tsuneyama K, et al. Mechanism of exacerbative effect of progesterone on drug-induced liver injury. Toxicol Sci. 2012;126(1):16–27. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 29.Lipson SF, Ellison PT. Normative study of age variation in salivary progesterone profiles. J Biosoc Sci. 1992;24(2):233–44. Epub 1992/04/01. [DOI] [PubMed] [Google Scholar]
- 30.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33 Epub 2006/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davyduke T, Tandon P, Al-Karaghouli M, et al. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatology Communications. 2019;3(10):1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawrieh S, Wilson LA, Cummings OW, et al. Histologic Findings of Advanced Fibrosis and Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Who Have Normal Aminotransferase Levels. Am J Gastroenterol. 2019;114(10):1626–35. Epub 2019/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zlotnik A, Gruenbaum BF, Mohar B, et al. The effects of estrogen and progesterone on blood glutamate levels: evidence from changes of blood glutamate levels during the menstrual cycle in women. Biol Reprod. 2011;84(3):581–6. Epub 2010/10/29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.