Abstract
It is known that a highly dynamic communication among subcellular organelles (e.g., cytosol, endoplasmic reticulum (ER), mitochondria, and nucleus) dictate cellular behaviors. But little information exists on how the inter-organelle crosstalk impacts cancer cells due to the lack of approaches that manipulate inter-organelle communication in cancer cells. We unexpectedly found that a negatively charged, enzyme cleavable peptide enables the trafficking of histone protein (H2B), a nuclear protein, to the mitochondria in cancer cells. The peptide, denoted as MitoFlag, interacts with the nuclear location sequence (NLS) of H2B to block it entering nucleus. A protease on the mitochondria cleaves the Flag from the complex of MitoFlag and H2B to form assemblies that retain H2B on the mitochondria and facilitate the H2B entering mitochondria. Molecular validation of MitoFlag shows that adding NLS, replacing aspartic acid residues by glutamic acid residues, or changing L-aspartic acid to D-aspartic residue abolishes the trafficking of H2B into mitochondria of HeLa cells. As the first example of enzyme-instructed self-assembly (EISA) of a synthetic peptide for trafficking endogenous proteins, this work provides insights for understanding and manipulating inter-organelle communication in cells.
Keywords: Enzyme, self-assembly, peptide, mitochondria, Histone H2B, inter-organelle communication
Graphical Abstract
Carrying negative charge, MitoFlag(L-D) complexes with the nuclear protein, H2B, and traffics H2B to mitochondria via enzyme-instructed self-assembly (EISA). This result provides a supramolecular approach and molecular insights for understanding inter-organelle communication.

This communication reports the use of enzyme-instructed assemblies of peptides to enable the trafficking of a histone protein (H2B) to the mitochondria in cancer cells. Recent advances in cell biology reveal that inter-organelle communication play important roles in cellular signaling, especially during stress response.[1] Since cancer cells adapt well to stresses, inter-organelle communication in cancer cells may differ from that in normal cells. Although numbers of studies have associated inter-organelle communication with cancer,[2] there are few approaches to reveal or manipulate proteins to mitochondria in cancer cells. During our study of enzyme-instructed self-assembly (EISA),[3] we found that enzymatic cleavage of a negatively charged branched peptide (MitoFlag) is able to deliver cargos (either small molecules or exogenous proteins) selectively into to the mitochondria of cancer cells.[4] This intriguing discovery stimulates us to explore the use of EISA for manipulating a nuclear protein, H2B, to the mitochondria of cancer cells.
As a core histone protein, H2B normally forms nucleosomes with nuclear DNA after its synthesis in cytoplasm[5] and is absent from mitochondria. It is, however, possible for H2B to be in the mitochondria of dysregulated cells, such as cancer cells. In fact, proteomic analysis of a liver cancer cell has indicated that histones H2A and H2B are present in the mitochondrial membrane as integral membrane proteins and not bound directly to mitochondrial DNA (mtDNA) inside the mitochondria.[6] But it remains unclear how the histone proteins locate at mitochondria. A recent survey of the Protein Data Bank (PDB) shows that negatively charged peptide sequences are a common feature of histone-interacting proteins.[7] Since H2B is a core histone protein that interacts with negatively charged DNA to form chromatin in nucleosomes,[8] we hypothesize that the negatively charged peptide likely interacts with H2B, thus should be able to manipulate the trafficking of H2B (Scheme 1).
Scheme 1.

Trafficking histone H2B to mitochondria.
Our study shows that MitoFlag, containing an aspartic acid (D) repeat, traffics H2B to the mitochondria in cancer cells (HeLa and HepG2) (Scheme 1), but not in normal cells (HEK293). The molecular validation of MitoFlag shows that mixing with nuclear location sequence (NLS), replacing aspartic acid residues by glutamic acid residues, or changing L-aspartic acid to D-aspartic residue abolishes the trafficking of H2B to the mitochondria in HeLa cells. Revealing that enzymatic reaction of a negatively charged peptide traffics nuclear proteins to the mitochondria in cancer cells, this work indicates that EISA of small peptides as a useful approach for manipulating inter-organelle communication and providing insights for understanding the cross talks among organelles in cancer cells.
The structure of MitoFlag, a previously synthesized branch peptide,[4] is shown in Scheme 1. We denote it as MitoFlag(L-D), in which “L-D” indicates L-aspartic acid in the Flag tag[9] branch. Incubating HeLa cells with the nitrobenzofurazan (NBD) labeled derivative of MitoFlag(L-D), which is denoted as NBD-MitoFlag(L-D) (Scheme S1), reveals intensive fluorescence in the mitochondria of HeLa cells (Figure S1). This result confirms the mitochondrial localization of MitoFlag(L-D). Using the baculovirus encoding RFP-labeled histone H2B (H2B-RFP)[10] to transfect HeLa cells, we generated HeLa cells expressing H2B-RFP (labelled as HeLa_H2B-RFP). While the HeLa_H2B-RFP cells themselves exhibit red fluorescence exclusively in the nucleus (Figure S2), incubating MitoFlag(L-D) with the HeLa_H2B-RFP cells results in the fluorescence of H2B-RFP at mitochondria (Figure 1A). Immunofluorescence staining and western blot analysis using anti-H2B antibody confirm the presence of H2B-RFP in the mitochondria of the HeLa_H2B-RFP cells incubated with MitoFlag(L-D) (Figure S3). Immunostaining also indicates H2B in the mitochondria of the HeLa cells incubated with MitoFlag(L-D) (Figure S4). The results suggest that MitoFlag(L-D) traffics H2B-RFP and endogenous H2B to mitochondria. Moreover, the H2B-RFP level on mitochondrial outer membrane (MOM) is dominant, indicating MitoFlag(L-D) mostly traffics H2B-RFP to MOM. The observed H2B-RFP in mitochondrial matrix (MM) probably due to the protein import through TOM complex.[11] Treating HeLa_H2B-RFP cells by solvent control (PBS) or cisplatin (100 μM) hardly changes the distribution of H2B-RFP (Figures 1B and S5). These results exclude the possibility that the mitochondrial H2B-RFP originates from unregulated protein mislocalization in the HeLa cells with or without cell stress.[12] Moreover, MitoFlag(L-D) doped with less than one equivalent nuclear localization sequence of H2B[13] (NLSH2B, molar ratio 1: 0.25) fails to traffic H2B-RFP to the mitochondria of HeLa_H2B-RFP cells (Figure 1C), indicating that NLSH2B competes with H2B-RFP to interact with MitoFlag(L-D). The results above indicate that MitoFlag(L-D) interacts with H2B-RFP, likely through the NLS domain, to traffic H2B to mitochondria (Scheme 1).
Figure 1.
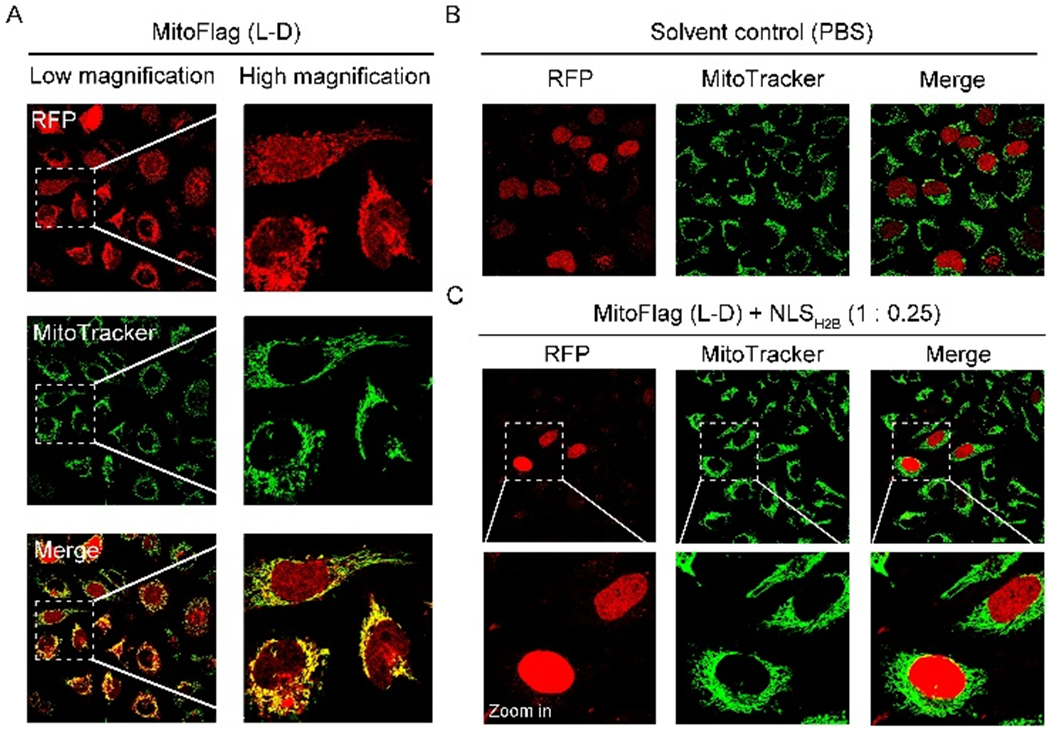
Fluorescent images of HeLa_H2B-RFP cells incubated with (A) MitoFlag(L-D) (200 μM, 24 h) (B) solvent control (PBS, 24 h), and (C) MitoFlag (L-D) (200 μM) mixed with the NLSH2B (1: 0.25, 24 h).
To understand the molecular features that enable the localization of H2B in mitochondria, we use L-glutamic acid or D-aspartic acid to replace the L-aspartic acid in the Flag tag and produce two derivatives of MitoFlag(L-D), MitoFlag(L-E) or MitoFlag(D-D), respectively (Figure 2A). Although the derivatives are negatively charged, H2B-RFP hardly localizes at the mitochondria of the HeLa_H2B-RFP cells incubated with MitoFlag(L-E) or MitoFlag(D-D) (Figure 2B and 2C). TEM images of MitoFlag(D-D) (200 μM), NLSH2B (50 μM), and the mixture of MitoFlag(D-D) (200 μM) and NLSH2B (50 μM) reveal small nanoparticles (Figure S6). However, MitoFlag(L-D) or MitoFlag(L-E) forms small nanoparticles or micelles at 200 μM (Figure 2D), which become large aggregates upon doping NLSH2B (50 μM, Figure 2D). The observations in TEM (Figure 2D) agree with the dissociation constants (Kd) of the binding between NLSH2B and MitoFlag(L-D), MitoFlag(L-E) and MitoFlag(D-D), which are 60.75, 107.94 and 184.81 µM, respectively. The Kd values are in the same magnitude of the electrostatic interaction between biomolecules.[14] Moreover, the critical micelle concentration (CMC) of MitoFlag(L-D) is 200 μM[4], and the CMCs of MitoFlag(L-E) and MitoFlag(D-D) are 193 and 148 μM, respectively (Figure S7). These results suggest that (i) all the MitoFlag derivatives are able to form multimers in solution (200 μM) and (ii) H2B can associate with MitoFlag(L-D) and MitoFlag(L-E) via NLSH2B, but hardly interacts with MitoFlag(D-D). While the hydrolysis rates of MitoFlag(L-D) and MitoFlag(L-E) by ENTK are comparable (about 80% hydrolyzed in 48 h, Figure S8) in cell free condition, adding NLSH2B hardly impedes the hydrolysis of MitoFlag(L-D) (Figure S9), as evidenced by a large amount of parallel aligned nanofibers (7±1 nm, made of Napffk(G)y) (Figure 2D) formed after adding ENTK. On the contrary, NLSH2B substantially inhibits the enzymatic hydrolysis of MitoFlag(L-E) catalyzed by ENTK (Figure S9), as supported by the formation of only sporadic nanofibers (9±1 nm) in the case of MitoFlag(L-E).
Figure 2.

(A) Molecular structures of MitoFlag(L-D), MitoFlag(L-E), and MitoFlag(D-D). (B, C) Fluorescent images of HeLa_H2B-RFP cells incubated with (B) MitoFlag(L-E) and (C) MitoFlag(D-D) (200 μM, 24 h). (D) TEM images of 200 μM MitoFlag(L-D), MitoFlag(L-E), the mixture of MitoFlag(L-D) or MitoFlag(L-E) with NLSH2B (50 μM), and the mixtures upon the addition of ENTK (5 U/mL, 24 h). (E) TEM images of mitochondria isolated from HeLa cells incubated with 200 μM MitoFlag(L-D) for 24 h. Scale bar = 100 nm.
The results above imply that MitoFlag(L-D), after being complexed with H2B, is still susceptible to mitochondrial proteases (e.g., ENTK), which agrees with the TEM images that MitoFlag(L-D) transforms into nanofibers (in 24 h) after the complexes approaching to the mitochondria of HeLa cells, as shown in Figure 2E. Since the particle-to-fiber transition originates from the proteolysis of MitoFlag(L-D)[4], the presence of nanofibers on the mitochondria of HeLa cells treated by MitoFlag(L-D) (200 μM, 24 h, Figure 2E) confirms the enzymatic cleavage of MitoFlag(L-D). Although the anti-ENTK staining shows some ENTK in place other than mitochondria, the formation of nanofibers on the mitochondria (Figure 2E) indicates that MitoFlag(L-D) mainly undergoes proteolysis on mitochondria. This aggregate-to-nanofiber transformation increases local viscosity and retains H2B on mitochondria. Unlike MitoFlag(L-D), MitoFlag(L-E) inefficiently undergoes such transformation (Figure. S10), and MitoFlag(D-D) can neither associate with H2B nor transform to nanofibers through enzymatic reaction (Figure S6 and S9). These MitoFlag derivatives hardly inhibit mammalian cells (e.g. HeLa, HEK293, and HepG2) at 200 μM (Figure S11), regardless of their self-assembling at mitochondria. These results indicate that the association of MitoFLag(L-D) with H2B via NLS and the enzyme-instructed transformation into nanofibers are critical for MitoFLag(L-D) to traffic H2B to the mitochondria of HeLa cells (Scheme 1).
To determine the efficiency of the mitochondrial trafficking of H2B via MitoFlag, we analyzed the fraction of H2B-RFP that remained in the nucleus and those trafficked to the mitochondria in the MitoFlag(L-D) treated HeLa_H2B-RFP cells using confocal fluorescence microscope. Results show that (Figure 3A, 3B and S12) around 25% of H2B-RFP protein present in nuclear (Nu) and 75% of the protein locate in mitochondria (Mito). In addition, 20% of HeLa_H2B-RFP cell have H2B-RFP only in nuclear, 10% of the cell exhibit H2B-RFP in both nuclear and mitochondria (Nu + Mito), and 70% of HeLa_H2B-RFP cell show the protein only in mitochondria.
Figure 3.

(A) Percentages of H2B-RFP protein in the nuclear (Nu) and mitochondria (Mito) of HeLa_H2B-RFP cells after the treatment of MitoFlag(L-D). (B) Percentage of HeLa_H2B-RFP cells exhibiting H2B-RFP in Nu, Mito and in both Nu and Mito after the treatment of MitoFlag(L-D). (C) Confocal fluorescent images of HeLa_PER-GFP cells before and (D) after the incubation with MitoFlag(L-D). (E) Confocal fluorescent images of HeLa_LAMP1-RFP cells before and (F) after the incubation with MitoFlag(L-D).
Though it appears that the electrostatic interaction is non-specific, the contribution from the sequence and conformation of the MitoFlag may increase the specificity for protein binding.To determine if MitoFlag selectively associates with the nuclear-localizing protein (e.g., H2B), we generate HeLa cell overexpressing peroxisome-targeting GFP[15] (HeLa_PER-GFP, Figure 3C) and RFP-fused LAMP1[16] (HeLa_LAMP1-RFP, Figure 3E), which are peroxisome and lysosome targeting proteins, respectively. Incubating HeLa_PER-GFP and HeLa_LAMP1-RFP with MitoFlag(L-D) hardly results in the mitochondrial localization of the overexpressed proteins (Figure 3D and 3F). These results indicate the specificity for MitoFlag to interact with H2B.
In order to examine whether the association of MitoFlag(L-D) with H2B protein complicates the application of the molecule as an on-target mitochondrial delivery agent, we determine the cell viability of HeLa and HeLa_H2B-RFP cells incubated with the mixture of Dox and MitoFlag(L-D). With the overexpression of H2B in HeLa_H2B-RFP cell, the intracellular interactions between MitoFlag(L-D) and H2B becomes more frequent compared to that in HeLa cell. If the protein-MitoFlag interaction significantly influences the mitochondria-targeting delivery of MitoFlag, the cell viability in HeLa and HeLa_RFP-H2B cells incubated with Dox and MitoFlag should be substantially different. Our results show that (i) MitoFlag(L-D) increased the cytotoxicity of Dox against HeLa and HeLa_RFP-H2B cells; (ii) the two different cells exhibited almost identical cell viability after the combination treatment (Figure S13). These results suggest that the effects of H2B or other proteins on the mitochondrial delivery ability of the MitoFlag(L-D) are negligible.
To validate the generality and selectivity of the MitoFlag(L-D)-induced mitochondrial localization of H2B, we produced HepG2[17] (liver cancer) and HEK293[18] (noncancerous cell line) cells that express H2B-RFP proteins, denoted as HepG2_H2B-RFP and HEK293_H2B-RFP, respectively. HEK293_H2B-RFP cells incubated with MitoFlag(L-D) or solvent control (PBS) exhibit the red fluorescence from H2B-RFP exclusively in the nucleus (Figure 3A), indicating that MitoFlag(L-D) is unable to traffic H2B to mitochondria in HEK293 cells. The red fluorescence of H2B-RFP presents in the mitochondria of HepG2_H2B-RFP cells incubated with MitoFlag(L-D) rather than with the solvent control (Figure 3B), suggesting that MitoFlag(L-D) traffics H2B to the mitochondria of HepG2 cells. These results imply that MitoFlag(L-D) traffics H2B to mitochondria in a cancer cell specific manner. To elucidate this selectivity, we visualized the ENTK in HeLa, HepG2 and HEK293 cells through immunofluorescence staining because the EISA of MitoFlag(L-D) on mitochondria is critical for the mitochondrial retention of H2B. The staining confirms the presence of ENTK in the mitochondria of HeLa and HepG2, but hardly in those of HEK293 cells (Figure S15). This result indicates that the cell-specific mitochondrial localization of H2B is resulted from the EISA of MitoFlag(L-D).
In conclusion, we show that enzymatic self-assembly of a Flag-tagged branch peptide to manipulate H2B, a nuclear protein, to the mitochondria in cancer cells. Such ability of manipulation diminishes when the concentration of the branch peptide is low (50 μM, Figure S2), indicating that the intracellular assembly of the branched peptide is also critical for trafficking H2B to mitochondria. This work illustrates that charge interactions (between Flag and NLS), stereochemistry (requiring MitoFlag(L-D)), enzymatic reaction (proteolysis catalyzed by ENTK), and dynamically forming assemblies (nanoparticle-to-nanofibers transition) collectively modulate the inter-organelle communication of H2B among cytosol, mitochondria, and nucleus of cancer cells. The principle revealed in this work support our hypothesis and likely is applicable to design other molecules that undergo enzymatic transformation[19] and forming higher-order assemblies[20] for manipulating the inter-organelle communication in cancer cells, which is a direction of our on-going research.
Supplementary Material
Figure 4.

Fluorescent images of (A) HEK293_H2B-RFP and (B) HepG2_H2B-RFP cells incubated with MitoFlag(L-D) (200 μM) and solvent control (PBS) for 24 h.
Acknowledgements
This work was partially supported by NIH (R01CA142746) and NSF (MRSEC-1420382).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Quiros PM, Mottis A, Auwerx J, Nat. Rev. Mol. Cell Biol 2016, 17, 213. [DOI] [PubMed] [Google Scholar]
- [2].a) Filadi R, Theurey P, Pizzo P, Cell Calcium 2017, 62, 1–15; [DOI] [PubMed] [Google Scholar]; b) Park H-K, Lee J-E, Lim J, Kang BH, Mol. Cancer 2014, 13, 148; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ahumada-Castro U, Silva-Pavez E, Lovy A, Pardo E, Molgo J, Cardenas C, Autophagy 2019, 15, 358–361; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Alpy F, Tomasetto C, Biochimie 2014, 96, 85–95; [DOI] [PubMed] [Google Scholar]; e) Yu SB, Pekkurnaz G, Journal of Molecular Biology 2018, 430, 3922–3941; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Doghman-Bouguerra M, Lalli E, Molecular and Cellular Endocrinology 2017, 441, 176–184. [DOI] [PubMed] [Google Scholar]
- [3].a) Feng Z, Han X, Wang H, Tang T, Xu B, Chem 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gao J, Zhan J, Yang Z, Adv. Mater. 2019, 1805798; [DOI] [PubMed] [Google Scholar]; c) Wu C, Zhang R, Du W, Cheng L, Liang G, Nano letters 2018, 18, 7749–7754; [DOI] [PubMed] [Google Scholar]; d) Wang Y, Hu X, Weng J, Li J, Fan Q, Zhang Y, Ye D, Angew. Chem. Int. Ed. 2019, 131, 4940–4944; [Google Scholar]; e) Zhou J, Xu B, Bioconjugate Chem. 2015, 26, 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) He H, Wang H, Zhou N, Yang D, Xu B, Chem. Commun. 2018, 54, 86–89; [DOI] [PubMed] [Google Scholar]; b) He H, Wang J, Wang H, Zhou N, Yang D, Green DR, Xu B, J. Am. Chem. Soc. 2018, 140, 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Bloch DP, Brack SD, J. Cell Biol. 1964, 22, 327–340; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chandrasekharan MB, Huang F, Sun Z-W, Proc. Nat. Acad. Sci. U.S.A 2009, 106, 16686–16691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choi Y-S, Jeong JH, Min H-K, Jung H-J, Hwang D, Lee S-W, Pak YK, Molecular Biosystems 2011, 7, 1523–1536. [DOI] [PubMed] [Google Scholar]
- [7].Chou CC, Wang AH, Mol Biosyst 2015, 11, 2144–2151. [DOI] [PubMed] [Google Scholar]
- [8].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, Molecular Biology of the Cell, 5th ed., Garland Science, New York, 2008. [Google Scholar]
- [9].Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, Urdal DL, Conlon PJ, Nat. Biotechnol. 1988, 6, 1204. [Google Scholar]
- [10].a) Ames RS, Kost TA, Condreay JP, Expert Opin. Drug Discovery 2007, 2, 1669–1681; [DOI] [PubMed] [Google Scholar]; b) Boyce FM, Bucher N, Proc. Nat. Acad. Sci. U.S.A 1996, 93, 2348–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahting U, Thun C, Hegerl R, Typke D, Nargang FE, Neupert W, Nussberger S, Cell Biol J. 1999, 147, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang X, Li S, Biochim. Biophys. Acta, Rev. Cancer 2014, 1846, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sheval EV, Musinova YR, in Proteins of the Nucleolus, Springer, 2013, pp. 175–196. [Google Scholar]
- [14].a) Sigal CT, Zhou W, Buser CA, McLaughlin S, Resh MD, Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 12253–12257; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yang J, Tsutsumi H, Furuta T, Sakurai M, Mihara H, Org. Biomol. Chem. 2004, 12, 4673–4681; [DOI] [PubMed] [Google Scholar]; c) Chen Y, Ludescher RD, Montville TJ, Appl. Environ. Microbiol 1997, 63, 4770–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wiemer EA, Wenzel T, Deerinck TJ, Ellisman MH, Subramani S, J. Cell Bio. 1997, 136, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Falcón-Pérez JM, Nazarian R, Sabatti C, Dell’Angelica EC, J. Cell Sci. 2005, 118, 5243–5255. [DOI] [PubMed] [Google Scholar]
- [17].Knowles BB, Aden DP, Google Patents, 1983.
- [18].Graham FL, Smiley J, Russell W, Nairn R, J. Gen. Virol 1977, 36, 59–72. [DOI] [PubMed] [Google Scholar]
- [19].a) Zhan J, Cai Y, He S, Wang L, Yang Z, Angew. Chem. Int. Ed. 2018, 57, 1813–1816; [DOI] [PubMed] [Google Scholar]; b) Kalafatovic D, Nobis M, Son J, Anderson KI, Ulijn RV, Biomaterials 2016, 98, 192–202; [DOI] [PubMed] [Google Scholar]; c) Hai Z, Li J, Wu J, Xu J, Liang G, J. Am. Chem. Soc. 2017, 139, 1041–1044; [DOI] [PubMed] [Google Scholar]; d) Ye D, Shuhendler AJ, Cui L, Tong L, Tee SS, Tikhomirov G, Felsher DW, Rao J, Nat. Chem. 2014, 6, 519; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Shi J, Fichman G, Schneider JP, Angew. Chem. Int. Ed. 2018, 130, 11358–11362; [Google Scholar]; f) Shi J, Schneider JP, Angew. Chem. Int. Ed. 2019; [Google Scholar]; g) Conte MP, Lau KA, Ulijn R, ACS Appl. Mater. Interfaces 2017, 9, 3266–3271; [DOI] [PubMed] [Google Scholar]; h) Tanaka A, Fukuoka Y, Morimoto Y, Honjo T, Koda D, Goto M, Maruyama T, J. Am. Chem. Soc. 2015, 137, 770–775; [DOI] [PubMed] [Google Scholar]; i) Pires RA, Abul-Haija YM, Costa DS, Novoa-Carballal R, Reis RL, Ulijn RV, Pashkuleva I, J. Am. Chem. Soc. 2015, 137, 576–579. [DOI] [PubMed] [Google Scholar]
- [20].Yin Q, Fu T-M, Li J, Wu H, Annu. Rev. Immunol. 2015, 33, 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


