Abstract
Purpose
Unlike most cancers, no clear epidemiological correlation between diabetes (Db) and malignant glioma progression exists. Because hyperglycemia activates proinflammatory pathways through the receptor for advanced glycation endproducts (RAGE), we hypothesized that Db can also promote malignant glioma progression.
Experimental Design
We compared the growth of two phenotypically diverse syngeneic glioma models in control and diabetic mice. Tumor growth and antitumor immune responses were evaluated in orthotopic and heterotopic models and correlated to RAGE and RAGE ligand expression.
Results
Irrespective of tumor implantation site, growth of a “classical” glioma model, GL261, increased in hyperglycemic mice and was mediated by upregulation of RAGE and its ligand, HMGB1. However, growth of a “mesenchymal” glioma subtype, K-Luc, depended on tumor implantation site. Whereas heterotopic K-Luc tumors progressed rapidly in Db mice, intracranial K-Luc tumors grew slower. We further showed that hyperglycemia inhibited the innate anti-tumor inflammatory responses in both models. Although this contributed to the accelerated growth of heterotopic tumors, suppression of tumor inflammatory responses dampened the growth of orthotopic K-Luc gliomas.
Conclusions
Hyperglycemia may enhance glioma growth through promotion of RAGE expression and suppression of antitumor immune responses. However, abrogation of the pro-inflammatory milieu in tumors may also dampen the growth of inflammatory glioma subtypes in the brains of diabetic mice. This dichotomy in glioma growth response to hyperglycemia may partly explain why conflicting epidemiological studies show both an increased risk and a protective effect of Db in patients with malignant gliomas.
Keywords: Brain tumor, Macrophage, Mice, RAGE
Introduction
Malignant gliomas are heterogeneous primary brain neoplasms with poor prognosis despite aggressive multimodal therapies. Patients with glioblastoma (GBM), most aggressive type of malignant glioma, are often treated with corticosteroids to reduce cerebral edema, but chronic use of steroids induces glucose intolerance and promotes the development of diabetes (Db). Individuals with Db have a significantly higher likelihood of developing malignant tumors (1–5), and also have a higher risk of cancer-related mortality compared to non-Db patients (6). In contrast to most cancers, however, epidemiologic studies have not demonstrated a clear correlation between Db and glioma risk or progression. Whereas, a few studies have suggested an increased risk of gliomas in subjects with pre-existing Db, others have reported an inverse association between blood glucose levels and glioma risk (7–10). Thus, the influence of Db on the development and progression of malignant gliomas is currently indeterminate.
The etiology of higher cancer-related mortality in patients with Db is unclear, but various mechanisms have been proposed. Hyperglycemia may contribute to tumor growth by increasing the availability of nutrients and generation of reactive oxygen species (ROS) that increases mutagenesis (11, 12). Another potential mechanism by which hyperglycemia promotes tumor growth is through generation of advanced glycation endproducts (AGEs) that perpetuate a chronic pro-tumorigenic inflammatory state (13). AGEs are generated by nonenzymatic glycation of proteins lipids or nucleic acids that accumulate in the tissues and cells of diabetic hosts (14). By binding to the receptor for advanced glycation endproducts (RAGE) and other receptors, AGEs may exert potent immune regulatory functions in disease pathogenesis. Engagement of RAGE by AGE or other ligands, activates multiple signaling pathways that leads to the activation of ERK1/2, AKT, p38 MAPK, JNK and NF-κB (15). Furthermore, upregulation of RAGE and its ligands have been linked to the development and progression of several cancers by facilitating and maintaining a chronic inflammatory state (16–19). Consistent with these observations, we also showed that ablation of RAGE in the tumor microenvironment (TME), abrogated tumor inflammation and angiogenesis in murine gliomas (20). As RAGE is upregulated in Db, we hypothesized that RAGE activation may contribute to tumor inflammation and glioma growth in hyperglycemic mice.
Because malignant gliomas are heterogeneous tumors, we examined two different syngeneic glioma models in streptozotocin (STZ)-induced Db mice. The immunogenic GL261 glioma that typically has a “nodular” phenotype, upregulated RAGE and propagated faster in hyperglycemia mice. But interestingly, the more invasive intracranial K-Luc glioma model that elicits a strong inflammatory reaction, was less tumorigenic in the brains of Db mice. Our findings suggest that dysregulation of tumor inflammatory responses to hyperglycemic may differentially impact glioma growth, and explain the conflicting epidemiological reports associating Db to gliomagenesis.
Materials and Methods
Reagents and cell lines
Murine GL261 glioma cells, RAW murine monocytes, and human THP-1 monocytes were purchased from American Type Culture Collection (ATCC, VA, USA). Luciferase-expressing KR158B cells (K-Luc), an invasive glioma cell line derived from spontaneous gliomas in Trp53/Nf1 double-mutant mice in Dr. Tyler Jacks laboratory, was a generous gift from Dr. John Sampson in 2011(21). GL261, K-Luc and RAW cells were cultured in DMEM medium, while THP-1 cells were cultured in RPMI 1640. Both media were supplemented with 10% FBS (BioWhittaker, Walkersville, MD), 100U/mL penicillin-G, 100 μg/mL streptomycin, and 0.01M HEPES buffer (Life Technologies, Gaithersburg, MD) in a humidified 5% CO2 atmosphere. Sterility, stability, and authenticity of each cell line was confirmed biannually by IDEXX Bioanalytics (ME, USA).
Induction of hyperglycemia in mice
To induce Db, male mice were injected intraperitoneally with streptozotocin (STZ, 50mg/kg/day for 5 days) as described previously (22). To avoid Db-induced complications and mortality, mice with severe hyperglycemia with glucose levels > 28 mM (~500 mg/dL) received insulin (1 U SQ/every 2–3 days) to maintain glucose levels of 17–22 mM (~300–400 mg/dL) prior to tumor implantation.
Tumor implantation and size measurements
Mice weighing 15–25 g (C57BL/6J background) were purchased from Jackson Laboratory (Bar Harbor, ME) and housed and handled in accordance to the guidelines and approval of City of Hope Institutional Animal Care and Use Committee under pathogen-free conditions. Cx3cr1gfpknock-in mice express gfp under control of the endogenous Cx3cr1 locus. Stereotactic intracranial (IC) tumor implantation was performed as described (23). Briefly, mice were anesthetized by ketamine (132 mg/kg, i.p.) and xylazine (8.8 mg/kg, i.p.) and implanted with 105 GL261 or K-Luc cells using a stereotactic head frame at a depth of 3 mm through a bur hole placed 2mm lateral and 0.5 mm anterior to the bregma. Bone wax was used to seal the small bur hole after IC tumor injection. Subcutaneous (SC) tumors were generated by injecting 106 GL261 or K-Luc cells suspended in PBS into the flanks.
For survival experiments, mice were sacrificed when they either developed signs of raised intracranial pressure or ulcerations in the SC (flank or scalp) tumors.
To assess IC tumor size, mice bearing 14-day old tumors were sacrificed, their brains harvested and sectioned at 10 μm thickness through the entire tumor. Every 5th brain slice was stained and used for tumor size measurements. For each tumor, 14 sections through the largest tumor area were used for size calculations. SC tumor size was serially measured with calipers.
Tumor macrophage depletion
Tumor-infiltrating macrophages were depleted using two separate techniques. Two days prior to tumor implantation, mice received daily PLX3397 (Plexxikon, 50 mg/kg/day, oral gavage) until they were sacrificed 14 days later for tumor size measurements (24). In separate experiments, Clodronate-containing liposomes (FormuMax, 20 mg, i.p.) were administered a day before and 1 week after tumor implantation before mice were sacrificed at 14 days (25).
Patient tissue collection
The study protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was reviewed and approved by the Institutional Review Board (IRB#17370) at City of Hope, Duarte, CA. Fresh tumor, peritumoral tissue and blood samples from 8 consecutive patients with newly-diagnosed GBM were collected at the time of surgery after obtaining written informed consent. Tissue samples were homogenized on ice in RIPA lysis buffer supplemented with protease inhibitor, separated into multiple aliquots and stored in liquid nitrogen. Blood samples were centrifuged at 1500 rpm for 5 mins, and the supernatants stored in liquid nitrogen. Tumor tissue and blood AGE protein adduct levels were measured by ELISA (STA-817, Cell Biolabs, Inc, USA). Serum glucose levels represent the average of three separate measurements collected during the perioperative course (pre-operative, intra-operative and one hour after surgery).
Real time RT-PCR, Western blot, and ELISA
Real-time quantitative PCR (qPCR) was performed with corresponding primers (Supplementary Table S1) using a TaqMan 5700 Sequence Detection System (Applied Biosystems, Foster City, CA) as described (26).
Western blots were performed as described (26) using primary antibodies specific for S100B (#ab41548, Abcam, Middlesex, NJ), full length RAGE (FL-RAGE, #ab3611, Abcam), HMGB1, and GAPDH (#2118s, Cell Signaling, USA).
ELISA for mouse IGF-1 (#MG100, R&D Systems, USA) and mouse AGE (#MBS2515909 MyBioSource, USA) were performed according to manufacturer instructions.
Flow cytometry analysis
Freshly harvested tumor and peritumoral tissue were minced and digested with trypsin (# 9341 Irvine Scientific, USA) for 20 minutes at 37°C. Tissue homogenate was then filtered through a 40 μm filter and prepped using Fixation/Permeabilization solution according to the manufacturer’s instructions (BD Pharmingen,San Diego, CA). Multiple-color FACS analyses was performed at the City of Hope Analytical Cytometry Core using a 3-laser CyAn immunocytometry system (Dako Cytomation, Fort Collins, CO), and data was analyzed using FlowJo software (TreeStar, San Carlos, CA) as described (27). PerCP-conjugated antibodies to mouse CD45 (Cat: 557235) was purchased from BD Pharmingen. Allophycocyanin-conjugated anti-mouse CD11b (Cat: 17–0112-82) was purchased from Abcam (Middlesex, NJ). FITC-conjugated anti-mouse MHCII (Cat: 11–5321-82) was purchased from Invitrogen (Carlsbad, CA). Secondary goat anti-rabbit-FITC (Cat: sc-2012) and secondary goat anti-mouse-FITC (Cat: sc-2010) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Tumor-associated macrophages (CD11bhigh CD45high) and microglia (CD11bhigh CD45low) were distinguished based on previously described FACS methodology (28).
Immunofluorescence staining
Frozen brain sections were prepared from naïve and tumor-bearing mice. Immediately after harvest, brains were fixed in paraformaldehyde for four hours before storage in 30% sucrose solution. Brains were embedded in O.C.T. (Tissue-Tek), and 10 μm sections were cut using a cryostat (Leica Microsystem Inc., Bannockburn, IL). Prior to immunofluorescence staining, slides were baked at 37°C overnight. After a one-hour block at room temperature, slides were incubated with Ki-67 (1:200, rabbit anti-mouse, Cell Signaling, Danvers, MA), RAGE (1:200, rabbit anti-mouse, Abcam), CD31 (1:20, rat anti-mouse, Abcam), HMGB1 (1:200, rabbit anti-mouse, Abcam), or AGE (1:200, rabbit anti-mouse, Abcam) primary antibodies for 1 hour. Slides were washed with PBS three times for 5 minutes and incubated with secondary antibody (Goat anti-rabbit Alexa Fluor 647or Goat anti-mouse Alexa Fluor 647, 1:200 dilution Life Technologies) for another hour. Tissue sections were mounted in Vectashield mounting medium containing 4060-diamidino-2-phenylindole (DAPI) (Vector, Burlingame, CA), imaged with AX-70 fluorescent microscopy (Leica Microsystems Inc.), and prepared by LSM Image Browser software (Zeiss, Dublin, CA).
3D Migration assay
GL261 or K-Luc cells were labeled with PKH26 according to manufacturer’s protocol (Sigma). Tumor cells (1×106) were resuspended in culture medium containing 3% Matrigel (Corning Inc.). A total of 5 μl of the mixture was placed at the center of the well in a form of a drop. After 3 hours, when cells formed a 3D colony in the Matrigel, each well was covered with a layer of culture medium containing 1% Matrigel and incubated overnight. On day one, cultures were replaced with medium supplemented with IL-6 or IL-1β (0, 25, or 50 ng/ml). Tumor spheres were imaged daily with an Axio Observer (Zeiss).
Primary monocyte cultures
Primary bone marrow-derived monocytes (BMM) were harvested from bone marrow of normal and Db mice as described before (20). After washing the bone marrow with cold PBS, cells were isolated and collected with Cell Strainer (BD Biosciences, San Jose, CA). After centrifugation (1500rpm, 8min), the isolated primary monocytes were cultured in L929-conditioned DMEM medium. Red blood cells and other non-adherent cells were removed by changing the culture medium at 24h.
AGE experiments
Glycated mouse and human albumin (Sigma, 0–100 μg/mL) were incubated with RAW and THP-1 cells, respectively, 24 hours prior to qPCR analysis.
Nanostring analysis
RNA from tumor and peritumoral brain tissue was extracted using miRNeasy FFPE kit (Qiagen cat# 217504). RNA concentration was assessed with the Nanodrop spectrophotometer ND-1000 and Qubit 3.0 Fluorometer (Thermo Scientific, CA). RNA fragmentation and quality control was determined by 2100 Bioanalyzer (Agilent, CA). RNA expression was analyzed by NanoString nCounter platform (NanoString Technologies, WA) using PanCancer Mouse Immune Profiling panel, which consists of 770 genes covering 24 different immune cell types and populations, and 30 common cancer antigens and genes that represent all categories of immune response. RNA was first hybridized with Codeset from gene panel at 65°C for 16 hours. Post-hybridization probe-target mixture was then purified and quantified with nCounter Digital Analyzer, and all data analysis was performed on nSolver (NanoString Technologies). Pathway Scores were calculated by nSolver Advance Analysis module to summarize the data from a pathway’s genes into a single score, using the first principal component (PC) of the expression data (29). In brief, PC analysis scores each sample using a linear combination of its gene expression values, weighting specific genes to capture the greatest possible variability in the data. The data is presented in a log2 scale. Increased score indicates increased overall expression.
Statistical analysis
Statistical comparison in all different experimental conditions was performed with GraphPad Prism software using two-way analysis of variance (ANOVA) or Student’s t-test. Survival was plotted using a Kaplan-Meir survival curve, and statistical significance was determined by the Log-rank (Mantel-Cox) test. A P value of less than 0.05 was considered significant.
Results
Hyperglycemia differentially impacts glioma growth
To evaluate the effect of hyperglycemia on tumor growth in vivo, male mice were injected with STZ to induce Db prior to tumor implantation. Within 2–3 weeks of Db induction, mice were then inoculated with intracranial (IC) glioma cells and monitored for signs of raised intracranial pressure over time. Because malignant gliomas are heterogenous tumors, two phenotypically distinct glioma models were studies. GL261 gliomas have a mutation in K-ras and have a nodular phenotype when implanted in the brains of syngeneic C57BL/6J strain (Supplementary Fig. 1). K-Luc gliomas, which were derived from Trp53/Nf1 double-mutant mice, are more invasive, and like mesenchymal GBM subtype, have a pronounced inflammatory myeloid cell infiltration (Supplementary Fig. 1).
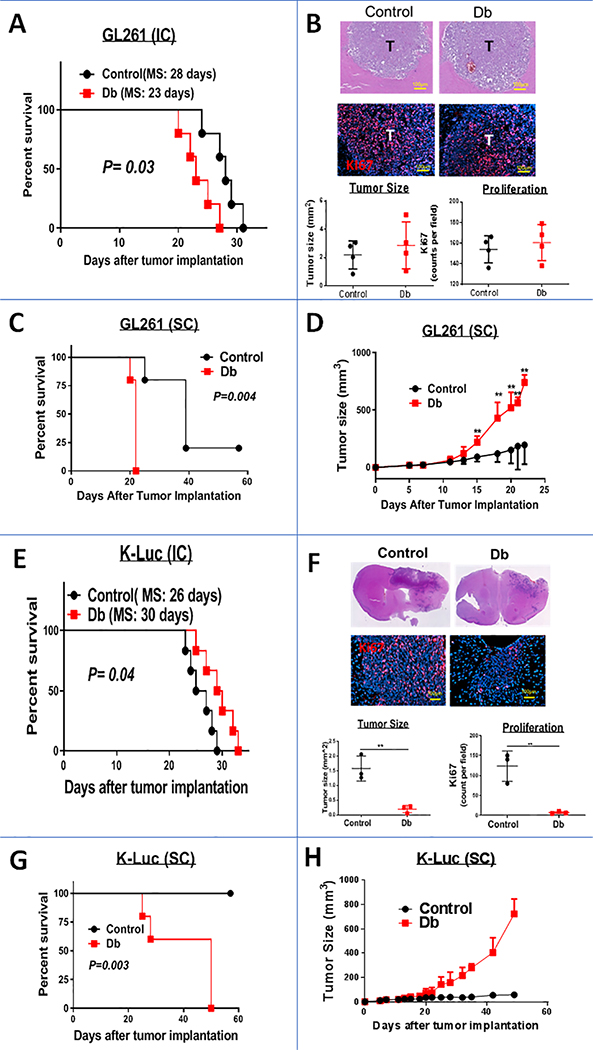
Within three weeks of IC implantation of GL261 gliomas, Db mice became symptomatic and died earlier than age and sex-matched control mice (Fig. 1A). However, the size and proliferation rate of tumors that were harvested two weeks after tumor implantation (before mice became symptomatic) were similar between the control and Db mice (Fig. 1B). To allow for monitoring of tumor growth over a longer period of time, mice were implanted with SC tumors. Compared to IC tumors, flank GL261 gliomas grew markedly faster in Db mice than the control group (Fig. 1C and D).
Figure 1:
Impact of hyperglycemia on glioma growth. A, Kaplan-Meier plot of control and diabetic (Db) mice implanted with IC GL261 gliomas (n=7 mice/group, MS: median survival). B, Tumor (T) histology (top panel) and proliferation (Ki67, bottom panel) of IC GL261 gliomas in control and Db mice two weeks after tumor implantation demonstrating similar tumor size and proliferation rates. Sections through the largest tumor area were used for size and Ki67 calculations (n=4 mice/group). Kaplan-Meier (C) and tumor size (D) plots of control and Db mice implanted with flank subcutaneous (SC) GL261 gliomas (n=7 mice/group) demonstrating more rapid tumor growth in Db mice. Data are shown as mean ± SD (**: P<0.01, compared to control group). E, Kaplan-Meier plot of control and Db mice implanted with IC K-Luc gliomas (n=6 mice/group, MS: median survival). F, Tumor histology and size (top panel), and proliferation (Ki67, bottom panel) of IC K-Luc gliomas two weeks after implantation demonstrating slower growth in Db mice. Four sections through the largest tumor area were used for size and Ki67 calculations (n=3 mice/group ± SD). Kaplan-Meier (G) and tumor size (H) plots of control and Db mice implanted with flank SC K-Luc gliomas (n=5 mice/group) demonstrating more rapid tumor growth in Db mice.
In contrast to the GL261 gliomas, survival of mice bearing IC K-Luc gliomas was longer in Db mice (Fig. 1E). In fact, most of K-Luc-bearing mice had to be sacrificed due to overgrowth of the extracranial extension of the tumor and not symptoms of raised intracranial pressure. Brain histology also demonstrated IC K-Luc tumors to be smaller and had a lower proliferation rate in Db mice than in control animals (Fig. 1F). But similar to the GL261 model, heterotopic K-Luc tumors that were implanted into the flanks, grew faster in Db mice than age-matched control animals (Fig. 1G and H). Because hyperglycemia did not affect GL261 or K-Luc growth in vitro (Supplementary Fig. 2), we evaluated the role of other extrinsic factors and TME on potentiating glioma growth in Db mice.
Insulin-like growth factor-1 is not altered in Db mice
Binding of insulin-like growth factor (IGF)-1 to its receptor activates the MAPK and the PI3K/AKT pathways to drive tumor growth and invasion (30). In type 2 Db, IGF-1 can be upregulated as a result of insulin resistance (31), whereas in type 1 Db, its production by the liver is reduced (32). Therefore, we studied IGF-1 concentration and its influence on the proliferation of GL261 and K-Luc gliomas. In contrast to the GL261 cells that have a mutation in K-ras and did not respond to upstream IGF-1 activation, K-Luc proliferation increased with high concentrations of IGF-1 (Fig. 2A). Nevertheless, serum IGF-1 levels were not significantly different between control and Db mice (Fig. 2B), excluding its role in promoting the growth of GL261 or K-Luc tumors in these experiments. Given that RAGE can be upregulated in Db, and because RAGE activation promotes tumor growth, we next measured the expression of RAGE and its ligands in each model.
Figure 2:
Impact of IGF-1 on glioma growth. A, IGF-1 did not affect GL261 proliferation (left panel) but did stimulate K-Luc growth in vitro (right panel). Data are shown as mean ± SD (*: P<0.05). B, IGF-1 levels were not significantly different in STZ-induced Db mice. Serum from control and IC tumor-bearing mice were collected two weeks after implantation and analyzed by ELISA.
Hyperglycemia induces RAGE expression
Within 2 weeks of STZ treatment (prior to tumor implantation), RAGE expression, increased in the livers, but not in the lungs of naïve Db mice (Fig. 3A and B). In the brain, however, RAGE was unchanged by immunostaining (Fig. 3A), but was markedly lower by Western blot analysis in Db mice (Fig. 3B); the discrepancy in RAGE measurement by these two techniques is unclear but may be due to differences in the detection of RAGE splice variants by each antibody (20). Nevertheless, these findings confirmed hepatic RAGE activation in Db mice prior to tumor implantation. We next measured RAGE expression in the tumor-bearing mice.
Figure 3:
Effect of hyperglycemia on RAGE expression. A, Tissue RAGE immunostains of naïve non-tumor bearing control and Db mice confirming upregulation of RAGE in the liver within 2 weeks of Db induction. B, Representative full-length (FL)-RAGE Western blots (top panel) and quantification (bottom panel) of various organs in naïve control and Db mice confirming RAGE upregulation in the liver. Interestingly, total FL-RAGE was decreased in Db brains. C, Representative FL-RAGE Western blot and quantification (top) and Ager expression (bottom) in control and Db mice bearing intracranial (IC) GL261 gliomas. D, Representative RAGE immunostains of IC and SC GL261 gliomas demonstrating upregulation of RAGE in Db mice. E, Representative FL-RAGE Western blots with quantification (top), and Ager expression (bottom) in control and Db mice bearing IC K-Luc gliomas. F, Representative RAGE immunostains of IC K-Luc gliomas illustrating similar tumor staining pattern in control and Db mice. Data are shown as mean ± SD (*: P<0.05, **: P<0.01).
Although RAGE expression (as measured by Western and qPCR) in the tumor-bearing brains (that contained both the GL261 tumors and the peritumoral brain tissue) was lower in Db mice (Fig. 3D), its expression in the tumor itself was higher in Db mice when assessed by immunostaining (Fig. 3E). Similarly, RAGE upregulation was noted in SC tumors in Db animals (Fig. 3E), confirming a link between RAGE activation and hyperglycemia. Because RAGE is expressed by endothelial cells, and its ablation can abrogate angiogenesis in GL261 gliomas (20), we also assessed tumor vascular density but did not see significant differences between control and Db mice (Supplementary Fig. 3).
In the K-Luc model, total RAGE expression was also lower in the tumors and peritumoral tissue in Db mice (Fig. 3F). But unlike GL261, RAGE expression did not significantly change in K-Luc tumors in response to hyperglycemia (Fig. 3G). Therefore, Db appeared to stimulate RAGE in GL261 gliomas, but not in the K-Luc tumors. To ascertain potential factors that activated the RAGE pathway in the GL261 gliomas, we next measured levels of various ligands that have been shown to stimulate RAGE.
HMGB1 is upregulated in Db mice
To investigate mechanisms of RAGE upregulation in the GL261 tumors, we first measured expression levels of AGE, which is a RAGE ligand that is commonly upregulated in Db. Surprisingly, neither serum (Fig. 4A) nor tissue-associated AGE levels (Fig. 4B) were significantly elevated in hyperglycemic mice. Similarly, expression of S100B, another RAGE ligand expressed by gliomas, was unchanged in GL261 tumors in Db mice (Fig. 4C). In contrast, tumor HMGB1 expression was markedly elevated in response to hyperglycemia (Fig. 4C and D). The increase in HMGB1 occurred locally as serum HMGB1 was not markedly different between the two groups in GL261-bearing mice (Fig. 4E). Although hyperglycemia did not promote GL261 proliferation in vitro (Supplementary Figure 2), it did upregulate HMGB1 expression by GL261 cells (Figure 4F). And because exogenous physiological concentrations of HMGB1 modestly increased GL261 proliferation in vitro (Fig. 4F), we conclude that release of HMGB1 into TME may have promoted RAGE expression and tumor growth in the Db IC GL261 model.
Figure 4:
Impact of Db on expression of RAGE ligands in IC gliomas. Serum and brain tissue from control and Db naïve and GL261-bearing mice (two weeks after tumor implantation) were collected and analyzed for RAGE ligands by ELISA and immunostaining. Db did not alter serum AGE (A) or brain AGE levels (B). C, Western blots of GL261 gliomas showed elevated HMGB1 (but not S100B) in Db mice. D, Immunostains of IC GL261 gliomas confirming upregulation of HMGB1 in Db mice. E, Serum HMGB1 was lower in naïve Db mice but was not altered in GL261-bearing animals. F, Physiological concentrations of HMGB1 induced GL261 growth in vitro. G, Representative HMGB1 Western blots with quantification (top), and Hmgb1 expression (bottom) in control and Db mice bearing IC K-Luc gliomas. Data are shown as mean ± SD (*: P<0.05, **: P<0.01). H, Representative HMGB1 immunostains of IC K-Luc gliomas illustrating lower tumor staining pattern in Db mice.
In contrast to the GL261 gliomas, the K-Luc model, (which grew slower in Db mice) had lower expression of HMGB1 under hyperglycemic conditions (Fig. 4 G and H), and did not propagate faster in response to exogenous HMGB1 (Supplementary Fig. 4). Collectively, these findings suggest that upregulation of HMGB1 under hyperglycemic conditions activated RAGE and promoted GL261 growth in Db mice, but not K-Luc tumors. Because secreted HMGB1 also promotes inflammation by activating RAGE and Toll-like receptor 4 (TLR4), we next compared tumor immune responses in control and Db mice.
Hyperglycemia suppresses tumor inflammation
To interrogate tumor inflammatory responses, IC GL261 gliomas from control and Db mice were dissected and analyzed by Nanostring. Interestingly, Pathway Scores for many processes involved in innate and adaptive immunity were significantly lower in Db mice (Fig. 5A). Analysis by qPCR supported the Nanostring data and confirmed a significant decrease in the expression of IL-1β and TNFα pro-inflammatory cytokines in Db mice (Fig. 5B). These findings were unexpected because we anticipated higher HMGB1 expression levels in Db gliomas to promote tumor inflammation. To further investigate the effect of hyperglycemia on tumor innate immune responses, myeloid-derived cells from Db and control brains were sorted and analyzed by FACS. Interestingly, the brains of naïve Db mice contained fewer number of resident microglia (Supplementary Fig. 5). In GL261-bearing mice, although the number and distribution of inflammatory cells were similar between the groups (Fig. 5C and D), expression of MHCII by CD11b+ myeloid-derived cells (microglia and macrophages) was lower in Db mice (Fig. 5E). These observations suggest that in addition to RAGE activation, suppression of anti-tumor immune responses may have also contributed to the accelerated growth of IC and SC GL261 tumors in hyperglycemic mice.
Figure 5:
Effect of Db on tumor inflammatory responses. A, To assess the overall inflammatory changes in tumors, intracranial (IC) GL261 gliomas from control and Db mice were dissected two weeks after implantation and analyzed by Nanostring. Pathway Scores for several inflammatory processes were suppressed in Db mice. B, RNA analysis also confirmed suppression of pro-inflammatory cytokines IL-1β and TNFα in Db mice. C, FACS analysis did not reveal a significant difference in the proportion of tumor-associated microglia (CD45low CD11b+), macrophages (CD45highCD11b+), or other leukocytes (CD45+CD11b−). D, Representative sections of GL261-bearing brains demonstrating similar distribution of microglia and macrophages (GFP+ cells) into tumors (T). Brains of normal and Db Cx3cr1gfp mice were harvested and sectioned two weeks after tumor implantation. E, Representative dot plots (left panel) and FACS quantification (right graph) demonstrating a decrease in the expression of MHCII by tumor-associated microglia and macrophages in Db mice.
Tumor AGE suppresses macrophage activation
In the GL261 glioma model, local tumor immune responses were suppressed in Db mice despite upregulation of HMGB1, a pro-inflammatory RAGE ligand. To interrogate why HMGB1 did not activate the innate immunity in Db mice, primary bone marrow-derived monocytes (BMM) were exposed to RAGE ligands at physiological concentrations. As expected, HMGB1 activated RAGE (by increasing Ager expression) (Fig. 6A), but it did not significantly stimulate IL-1β expression at physiological doses of < 150 ng/mL (Fig. 6B). Similarly, AGE (another RAGE ligand) stimulated IL-1β expression only at high concentrations (>30 μg/ml) (Fig. 6C). However, when BMM were pre-incubated with AGE, HMGB1-mediated IL-1β induction was suppressed (Fig. 6C). This suggests that AGE may have curtailed the pro-inflammatory activity of HMGB1 in the TME despite its upregulation in Db GL261 tumors.
Figure 6:
AGE suppresses macrophage activation. Primary bone marrow-derived mouse monocytes (BMM) were exposed to LPS/IFNγ (100/10 ng/ml; positive control), HMGB1, and AGE 24 hours prior to RT-PCR analysis. Although physiological concentrations of HMGB1 induced Ager expression (A), they did not stimulate Il-1β expression by BMM (B). C, High concentrations of AGE (30 μg/mL) and HMGB1 (500ng/mL) stimulated Il-1β expression, but pre-incubation of BMM with AGE suppressed their activation by HMGB1. D, Serum AGE concentration in 8 patients with newly-diagnosed GBM did not correlate with serum glucose concentrations. Serum glucose concentrations represent the average of three separate peri-operative measurements for each patient. E, Comparison of serum and tumor AGE levels in the same patient cohort revealing higher tumor AGE in three of the eight subjects (open circle represents superimposing data from two different patients). F, AGE stimulated the expression of IL1Β and TNFA by human THP-1 macrophages at physiological concentrations but suppressed their activation when these cells were preincubated with AGE 4 hours prior to exposure to LPS/IFNγ.
To investigate the relevance of these findings to humans, AGE levels were prospectively measured in 8 consecutive patients undergoing surgery for newly-diagnosed GBM. Serum AGE concentrations (~400μg/mL; Fig. 6D) were significantly higher in humans than those measured in tumor-bearing mice (~350ng/mL; Fig. 4A). But similar to mice, serum AGE levels did not rise with higher serum glucose concentrations in this small cohort (Fig. 6D). When normalized to total protein, GBM AGE levels were similar to serum levels except in three patients where they were markedly higher in tumor tissue (Fig. 6E). Furthermore, similar to the mice experiments, AGE alone stimulated THP-1 human macrophages at physiological levels but suppressed their activation in vitro (Fig. 6F). These findings suggest that AGE release into TME contributes to the suppression of the innate immune responses in gliomas.
In summary, these findings suggest that hyperglycemia may have contributed to GL261 tumor growth by both activating HMGB1-RAGE pathway and by suppressing anti-tumor immune responses. Yet, it was unclear why IC K-Luc gliomas grew significantly slower in the Db mice. Because K-Luc gliomas have a pronounced inflammatory myeloid cell infiltration, a characteristic of “mesenchymal” GBM subtype, and because Db suppressed macrophage activation, we next evaluated the impact of inflammation on K-Luc growth characteristics.
Inflammation promotes K-Luc glioma growth and invasion
As in GL261 gliomas, inflammatory Pathway Scores for processes involved in innate and adaptive immunity were significantly lower in Db K-Luc tumors (Fig. 7A). Furthermore, qPCR confirmed suppression of pro-inflammatory IL-1β and IL-6 cytokines in Db K-Luc gliomas relative to tumor-bearing control mice (Fig. 7B). Interestingly, expression of the immunosuppressive cytokine IL-10, was also lower in Db mice. Because IL-10 is expressed by myeloid-derived cells in gliomas (33), we next evaluated the proportion of infiltrating myeloid cells in these tumors. As predicted, intratumoral infiltration of myeloid-derived macrophages (i.e. CD11b+ CD45+) was significantly lower in Db mice (Fig. 7C and D). These findings confirmed that inflammatory responses to K-Luc gliomas were dampened in Db mice, similar to the GL261 model, but did not explain why IC K-Luc gliomas grew slower in Db mice. Accordingly, we tested whether tumor inflammation could directly influence the growth of K-Luc gliomas.
Figure 7:
Diabetes (Db) suppresses inflammatory responses in intracranial (IC) K-Luc gliomas. A, Nanostring Pathway Scores for inflammatory processes were suppressed in Db mice. B, Tumor RT-PCR analysis also confirmed suppression of cytokine expression in IC Db tumors. To correct for tumor size in A and B, control tumors were harvested two weeks, while Db tumors were collected three weeks after IC implantation. C, Representative immunostains of two-week old IC K-Luc gliomas showing a decrease in CD11b+ infiltration into tumors in Db mice. D, Representative dot plots (left) and FACS quantification (right) demonstrating a decrease in the proportion of tumor-infiltrating macrophages in Db mice. E, Inhibition of macrophage infiltration with PLX3397 (a CSF1R antagonist) inhibited the growth of IC K-Luc, but not GL261 gliomas. F, K-Luc cells were incubated with different concentrations of IL-6 (top) or IL-1β (bottom) and counted over 72 hrs. Neither cytokine stimulated cell growth. G, Microscopy images of 3D migration assay show enhanced invasiveness of K-Luc cells in the presence of IL-6 or IL-1β. Graphs (lower panel) show relative increase in sphere area. Data are shown as mean ± SD (*: P<0.05, **: P<0.01).
To confirm the pro-tumorigenic properties of inflammation on glioma growth, mice were treated with either PLX3397 (a CSF1R antagonist) or Clodronate-containing liposomes to inhibit the intratumoral trafficking of myeloid-derived cells (24, 25). As expected, the growth of K-Luc, but not GL261, gliomas were significantly inhibited with either PLX3397 (Fig. 7E) or Clodronate liposomes (Supplementary Fig. 6). In vitro studies further supported these observations: Although IL-1β and IL-6 (two cytokines that were lower in Db mice) did not affect K-Luc proliferation in vitro (Fig. 7F), they did promote K-Luc invasion in a 3D migration assay (Fig. 7G). These cytokines, however, had no impact on GL261 growth or invasion under similar conditions (Supplementary Fig. 7). Together, these results suggest that local suppression of tumor inflammation can have diverse influences on glioma growth. By suppressing antitumor responses, Db can facilitate immune evasion and promote tumor growth. Yet, inhibition of infiltrating myeloid-derived cells can also dampen the growth of inflammatory glioma subtypes that rely on local cytokines/chemokines for propagation and invasion.
Discussion
Hyperglycemia, a defining feature of diabetes, can directly contribute to tumor recurrence, metastasis, and cancer complications. Elevated circulating glucose levels provide abundant substrate for oxidative glycolysis, which regulates the cell growth, motility, and apoptosis of cancer cells (34). In the glioma models studied here, hyperglycemia did not directly stimulate cell proliferation in vitro, but it did differentially impact tumor growth in vivo by activating the RAGE pathway and by suppressing antitumor immune responses.
Under hyperglycemic conditions, ROS production can increase the release of RAGE ligands that further activate pro-inflammatory pathways through engagement of RAGE and Toll-like receptors (35). By maintaining a chronic inflammatory state, RAGE signaling has been linked to the development and progression of several neoplasms (16–19), and its blockade has been utilized to inhibit tumor growth, invasion, and angiogenesis in a variety of cancer models (36–40). Among the RAGE ligands measured here, only HMGB1 was up-regulated in both the IC and SC GL261 tumors from Db mice. An increase in RAGE expression, and stimulation of GL261 growth by exogenous HMGB1, suggested that HMGB1-RAGE interaction contributed to the accelerated growth of GL261 gliomas in Db mice. Our findings are consistent with previous reports that highlighted the role of HMGB1-RAGE interaction in glioma progression and invasion (41, 42).
Considering the key role of RAGE signaling in inflammation (43), we expected its upregulation in Db GL261 tumors to also trigger pro-inflammatory pathways. However, the overall tumor inflammation was significantly diminished in GL261 gliomas in Db mice despite upregulation of HMGB1. One explanation for this unexpected finding is the low concentration of tumor HMGB1 in this model. Our data suggest that intratumoral levels of HMGB1 (i.e. <100ng/ml) were lower than doses required to activate macrophages (i.e. >500 ng/ml). Another explanation for lack of HMGB1 pro-inflammatory activity in the GL261 tumors is the inhibitory effects of AGE. Although high levels of AGE activated RAGE and stimulated the expression of pro-inflammatory cytokines, lower levels dampened the activation of both murine and human macrophages by HMGB1 and LPS, respectively. The exact mechanism of this AGE-mediated inhibition is unclear but may be through competitive blockade of HMGB1 and LPS binding to RAGE. AGEs could also suppress the function of anti-tumor myeloid, T, and NK cells directly (44, 45). Nonetheless, our data suggest that intratumoral AGEs could potentially play a pivotal role in dysregulating anti-tumor immune responses in gliomas.
We also showed immune suppression in Db mice to be an important feature in both glioma models. Hyperglycemia suppresses different facets of immune function that include leukocyte trafficking, impaired killing mechanisms, and lymphocyte proliferation. Diabetic patients exhibit lower resistance to common infections when compared to healthy individuals partly due to reduced production of pro-inflammatory cytokines and generalized hypofunction of both innate and adaptive immune responses (46, 47). In the GL261 model, tumor microglia and macrophages expressed lower levels of MHC II, which reflected a deficiency in their antigen presenting function. These results are consistent with others that showed bone marrow dendritic cells from Db mice express lower levels of costimulatory molecules (CD80, CD86, 4–1BB, and CD40) as compared to non-Db animals (48). Our Nanostring analysis also confirmed suppression of the adaptive immune pathway in Db mice. This is consistent with others who reported STZ-induced Db animals display lower rates of T and B cell proliferation than non-Db groups (49, 50). Therefore, the observed accelerated growth of heterotopic SC GL261 and K-Luc tumors in Db mice is most likely due to suppression of systemic anti-tumor innate and adaptive immune responses.
Another interesting (but unexpected) finding in this study was the marked repression of IC K-Luc growth, whereas the same tumors propagated rapidly in the flanks of Db mice. Compared to the GL261 gliomas, K-Luc tumors have higher infiltration of myeloid cell (i.e. resident microglia and circulating monocytes). Since our initial characterization of tumor-associated myeloid cells in glioma models (28), there has been significant progress in understanding their heterogeneity and their contribution to glioma growth, angiogenesis, and invasion (51). In IC K-Luc tumors, both macrophage infiltration and expression of pro-inflammatory cytokines was markedly lower in Db mice. This immunosuppression may have been due to either a decrease in migration of circulating monocytes into K-Luc gliomas (52) or changes in TAM polarization under hyperglycemic conditions (53). Nonetheless, suppression of this pro-inflammatory milieu appeared to inhibit K-Luc tumorigenesis in Db mice.
Although our goal was to evaluate the effect of hyperglycemia induced by steroid usage, the STZ model used here emulates a type 1, and not type 2 Db, disease process. Because type 2 Db has lower severity and duration of hyperglycemia compared to type 1, the levels of RAGE and AGE may vary between the two diseases. Another limitation of the current study was the small human sample size. Future studies will measure expression of AGE and RAGE ligands in a larger patient cohort and will test the role of the RAGE axis on tumor inflammatory responses. Nevertheless, our findings affirm the impact of hyperglycemia on dysregulation of tumor inflammatory responses and highlight the role of intratumoral AGEs as potential immunosuppressive factors. Better characterization of local inflammatory conditions and their differential impact on the growth of tumor subtypes will be also invaluable in optimizing immunotherapy approaches to malignant gliomas.
Supplementary Material
Translational Relevance.
Epidemiological studies have not demonstrated a clear correlation between diabetes (Db) and malignant glioma progression. Given that hyperglycemia can facilitate tumor progression through several mechanisms, we hypothesized that the growth of gliomas will be more rapid in Db animals. Here, we show that the growth of gliomas can either accelerate or diminish in hyperglycemic mice and that this growth is highly dependent on glioma subtype, antitumor immune responses, and tumor microenvironment. These findings provide an explanation for the conflicting epidemiological reports that have shown both an increased risk and a protective effect of Db in patients with malignant gliomas.
Acknowledgements and grant support
The authors thank Chris Gandhi for editorial support. This work was supported by R01CA155769, R01 CA236500, Liam McGee Brain Tumor Fund and the Joan Traver Walsh Family Foundation. Research reported in this publication included work performed in the Analytical Cytometry and Molecular Pathology Cores supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Conflict of Interests: None
References
- 1.Mitri J, Castillo J, Pittas AG. Diabetes and risk of Non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31:2391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–74. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:369–80. [DOI] [PubMed] [Google Scholar]
- 6.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, Zheng Z, Huang P. Diabetes mellitus and the risk of glioma: a meta-analysis. Oncotarget. 2016;7:4483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seliger C, Ricci C, Meier CR, Bodmer M, Jick SS, Bogdahn U, et al. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro-Oncology. 2016;18:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartzbaum J, Edlinger M, Zigmont V, Stattin P, Rempala GA, Nagel G, et al. Associations between prediagnostic blood glucose levels, diabetes, and glioma. Scientific reports. 2017;7:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitahara CM, Linet MS, Brenner AV, Wang SS, Melin BS, Wang Z, et al. Personal history of diabetes, genetic susceptibility to diabetes, and risk of brain glioma: a pooled analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2014;23:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhou J, Wang T, Cai L. High level glucose increases mutagenesis in human lymphoblastoid cells. International journal of biological sciences. 2007;3:375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R, et al. Hyperglycemia, a neglected factor during cancer progression. BioMed research international. 2014;2014:461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad S, Khan H, Siddiqui Z, Khan MY, Rehman S, Shahab U, et al. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin Cancer Biol. 2018;49:44–55. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M Advanced protein glycosylatlon in diabetes and aging. Annual Review of Medicine. 1995;46:223–34. [DOI] [PubMed] [Google Scholar]
- 15.Sorci G, Riuzzi F, Giambanco I, Donato R. RAGE in tissue homeostasis, repair and regeneration. Biochim Biophys Acta. 2013;1833:101–9. [DOI] [PubMed] [Google Scholar]
- 16.Takada M, Koizumi T, Toyama H, Suzuki Y, Kuroda Y. Differential expression of RAGE in human pancreatic carcinoma cells. Hepatogastroenterology. 2001;48:1577–8. [PubMed] [Google Scholar]
- 17.Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–70. [DOI] [PubMed] [Google Scholar]
- 18.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. The Prostate. 2005;64:92–100. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes MK, Nigavekar SS, Arumugam T, Logsdon CD, Schmidt AM, Park JC, et al. RAGE activation by S100P in colon cancer stimulates growth, migration, and cell signaling pathways. Dis Colon Rectum. 2007;50:1230–40. [DOI] [PubMed] [Google Scholar]
- 20.Chen XB, Zhang LY, Zhang IY, Liang JL, Wang HQ, Ouyang M, et al. RAGE Expression in Tumor-Associated Macrophages Promotes Angiogenesis in Glioma. Cancer Research. 2014;74:7285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13:705–14. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y-S, Eun HS, Kim SY, Jeong J-M, Seo W, Byun J-S, et al. Hepatic immunophenotyping for streptozotocin-induced hyperglycemia in mice. Scientific reports. 2016;6:30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J, et al. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP, et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017;19:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poli A, Wang J, Domingues O, Planaguma J, Yan T, Rygh CB, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4:1527–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Zhang L, Zhang IY, Chen X, Da Fonseca A, Wu S, et al. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin Cancer Res. 2013;19:3764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao H, Zhang IY, Zhang L, Song Y, Liu S, Ren H, et al. S100B suppression alters polarization of infiltrating myeloid-derived cells in gliomas and inhibits tumor growth. Cancer Lett. 2018;439:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–61; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 29.Tomfohr J, Lu J, Kepler TB. Pathway level analysis of gene expression using singular value decomposition. BMC Bioinformatics. 2005;6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nature Reviews Cancer. 2004;4:505–18. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich N, Thuesen B, Jørgensen T, Juul A, Spielhagen C, Wallaschofksi H, et al. The Association Between IGF-I and Insulin Resistance. A general population study in Danish adults. 2012;35:768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teppala S, Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care. 2010;33:2257–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner S, Czub S, Greif M, Vince GH, Suss N, Kerkau S, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82:12–6. [DOI] [PubMed] [Google Scholar]
- 34.Joshi S, Liu M, Turner N. Diabetes and Its Link with Cancer: Providing the Fuel and Spark to Launch an Aggressive Growth Regime. BioMed research international. 2015;2015:390863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu XC, Abuduhadeer X, Zhang WB, Li T, Gao H, Wang YH. Knockdown of RAGE inhibits growth and invasion of gastric cancer cells. European journal of histochemistry : EJH. 2013;57:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaser AM, Huang Y, Zhou RR, Hu GS, Xiao MF, Huang ZB, et al. The Role of Receptor for Advanced Glycation End Products (RAGE) in the Proliferation of Hepatocellular Carcinoma. Int J Mol Sci. 2012;13:5982–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elangovan I, Thirugnanam S, Chen A, Zheng G, Bosland MC, Kajdacsy-Balla A, et al. Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochem Biophys Res Commun. 2012;417:1133–8. [DOI] [PubMed] [Google Scholar]
- 39.DiNorcia J, Lee MK, Moroziewicz DN, Winner M, Suman P, Bao F, et al. RAGE gene deletion inhibits the development and progression of ductal neoplasia and prolongs survival in a murine model of pancreatic cancer. J Gastrointest Surg. 2012;16:104–12; discussion 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. [DOI] [PubMed] [Google Scholar]
- 41.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. [DOI] [PubMed] [Google Scholar]
- 42.Angelopoulou E, Piperi C, Adamopoulos C, Papavassiliou AG. Pivotal role of high-mobility group box 1 (HMGB1) signaling pathways in glioma development and progression. J Mol Med (Berl). 2016;94:867–74. [DOI] [PubMed] [Google Scholar]
- 43.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in Inflammation and Cancer. Annual Review of Immunology. 2010;28:367–88. [DOI] [PubMed] [Google Scholar]
- 44.Price CL, Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2010;14:1806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstock P, Bezold V, Bork K, Scheffler J, Horstkorte R. Glycation interferes with natural killer cell function. Mech Ageing Dev. 2019;178:64–71. [DOI] [PubMed] [Google Scholar]
- 46.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, et al. Multiple immuno-regulatory defects in type-1 diabetes. The Journal of Clinical Investigation. 2002;109:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodacki M, Milech A, de Oliveira J, #233, Eg, #237, et al. NK Cells and Type 1 Diabetes. Clinical and Developmental Immunology. 2006;13:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi K, Satoh J, Oka Y. Lowered Expressions of the NF-κB Family Members in Dendritic Cells from NOD Mice are Associated with a Reduced Expression of GATA-2. Annals of the New York Academy of Sciences. 2008;1150:59–60. [DOI] [PubMed] [Google Scholar]
- 49.Rubinstein R, Genaro AM, Motta A, Cremaschi G, Wald MR. Impaired immune responses in streptozotocin-induced type I diabetes in mice. Involvement of high glucose. Clin Exp Immunol. 2008;154:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakowicz-Burkiewicz M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T. Diabetes-induced decrease of adenosine kinase expression impairs the proliferation potential of diabetic rat T lymphocytes. Immunology. 2006;118:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouma G, Coppens JMC, Lam-Tse W-K, Luini W, Sintnicolaas K, Levering WH, et al. An increased MRP8/14 expression and adhesion, but a decreased migration towards proinflammatory chemokines of type 1 diabetes monocytes. Clinical & Experimental Immunology. 2005;141:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan MA, Schultz S, Othman A, Fleming T, Lebron-Galan R, Rades D, et al. Hyperglycemia in Stroke Impairs Polarization of Monocytes/Macrophages to a Protective Noninflammatory Cell Type. J Neurosci. 2016;36:9313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.