Abstract
Inflexibility of the autonomic nervous system is relevant to depression vulnerability, but the downstream behavioral consequences of autonomic inflexibility are not well understood. Rumination, a perseverative thinking style that characterizes depression, is one candidate phenotype relevant to autonomic inflexibility. Undergraduates (N=134) completed a sadness induction while respiratory sinus arrhythmia was measured, and completed four waves of follow-up over twelve weeks during which rumination and stressful events were measured. Individuals with less parasympathetic flexibility had higher levels of trait rumination, and were more likely to ruminate in daily life, regardless of stress exposure, whereas individuals with more parasympathetic flexibility ruminated more only in the context of stress. These findings provide the first evidence that autonomic inflexibility may confer vulnerability to context-insensitive rumination. This work suggests a potential behavioral mechanism by which autonomic inflexibility leads to problems with self-regulation and depression, suggesting multiple avenues for intervention to target these markers of vulnerability.
Keywords: Stress, emotion regulation, respiratory sinus arrhythmia, rumination, Autonomic flexibility
Efforts to improve the prevention of depression, as well as the efficacy and durability of depression treatments, have increasingly focused on better understanding biobehavioral markers of depression as potential targets for novel treatments (Strawbridge et al., 2017). For instance, depression often is characterized by a loss of biological and behavioral flexibility that manifests as difficulty with adapting physiological and affective responses to meet situational demands (Kashdan & Rottenberg, 2010; Stange, Alloy, et al., 2017), and difficulty with disengaging from perseverative thinking processes such as rumination (Joormann & Vanderlind, 2014). Studying these aspects of inflexibility dimensionally, even in nonclinical samples, may have utility given evidence that biobehavioral markers of depression (as well as depression itself) typically are better categorized as dimensional rather than categorical in nature (Haslam et al., 2012; Liu et al., 2019).
One physiological index of flexibility relevant to affect regulation and symptoms of depression is parasympathetic nervous system activity, which can facilitate the modulation of behavioral and affective responses to meet contextual demands (Thayer & Lane, 2009). Parasympathetic flexibility represents the extent to which individuals show contextually appropriate changes in parasympathetic activation across different environmental or affective cues. Parasympathetic flexibility can be measured with respiratory sinus arrhythmia (RSA), a measure of variability in heart rate that occurs over the respiration cycle. During rest, the medial prefrontal cortex inhibits amygdala activation, indirectly enhancing parasympathetic activation via the vagus nerve, and resulting in higher levels of resting RSA (Thayer et al., 2012; Thayer & Lane, 2009). However, during an affective or environmental challenge (e.g., sadness), the parasympathetic nervous system withdraws its inhibitory input, resulting in reductions in RSA. This is thought to allow the body to conserve resources at rest and mobilize resources needed to flexibly and effectively respond to the challenge (Beauchaine & Thayer, 2015).
Indeed, research indicates that lower levels of resting RSA are associated with psychopathology such as depression (Rottenberg, 2007). Recent work also indicates that RSA reactivity (i.e., decreases in RSA) in response to sadness is an index of parasympathetic flexibility that is particularly relevant to the depressive state (Bylsma et al., 2014; Rottenberg et al., 2007; Stange, Alloy, et al., 2017; Yaroslavsky, Rottenberg, et al., 2013). Furthermore, individuals with less RSA reactivity to sadness may be vulnerable to experiencing future symptoms of depression and a poorer course of depression (Panaite et al., 2016; Yaroslavsky et al., 2014), particularly when encountering negative life events (Stange, Hamilton, Olino, et al., 2017) or in conjunction with other aspects of vulnerability (Hamilton et al., 2019; Stange, Hamilton, Fresco, et al., 2017b).
Despite evidence supporting the relevance of autonomic inflexibility to depression vulnerability, the downstream behavioral consequences of autonomic inflexibility are not well understood — in other words, why might such inflexibility confer vulnerability to depression? One potential explanation is that RSA may represent a biological index of the capacity for affect regulation (Thayer et al., 2012). Indeed, inflexible autonomic responses to sadness may interfere with the effective downregulation of negative affect (Stange, Hamilton, Fresco, et al., 2017a; Yaroslavsky, Bylsma, et al., 2013; Yaroslavsky et al., 2016). Prior cross-sectional studies of nonclinical samples also suggests that lower levels of RSA at rest are dimensionally associated with inflexible cognitive processes such as rumination (Ottaviani et al., 2016). Indeed, there are theoretical and empirical reasons to suspect acute bidirectional relationships between rumination and RSA. For instance, rumination represents a perseverative cognitive-affective response to loss or stress, which may lead acutely to decreases in RSA (Ottaviani et al., 2016), similar to the expected parasympathetic withdrawal that occurs in response to the stressor itself (Hamilton & Alloy, 2016). The reductions in RSA that occur as part of the stress-ruminative response reduce the parasympathetic resources available, thereby making it more difficult to modulate attention away from the stressor and to disengage from rumination. This may subsequently lead to more rumination and continued RSA withdrawal, resulting in a vicious cycle of cognitive and autonomic inflexibility.
In addition to acute bidirectional relationships between RSA and rumination, chronic relationships between blunted RSA responses and rumination also may exist over time. When RSA decreases are prolonged or experienced repeatedly over time (as occurs with rumination), a series of physiological defense systems are activated that lead to wear and tear on the systems, sometimes referred to as allostatic load (Juster et al., 2010). Over time, this load may lead to prolonged RSA withdrawal, thus reducing physiological sensitivity to context and leading to autonomic inflexibility. Central to the present investigation, it is possible that this autonomic inflexibility (which represents a lack of parasympathetic resources that facilitate regulation) could further enhance the likelihood of engaging in rumination when experiencing stress. However, no prospective studies to our knowledge have examined whether autonomic inflexibility might predispose individuals to subsequent rumination, perhaps helping to explain why autonomic inflexibility may lead to poor mental and physical health-related outcomes (Thayer et al., 2012). This would provide external validity to the hypothesis that autonomic inflexibility is a risk factor for rumination, which might have implications for intervention and prevention. For example, if parasympathetic inflexibility temporally precedes rumination, this suggests that augmenting parasympathetic flexibility (e.g., with RSA biofeedback) could reduce the tendency to ruminate.
In addition to understanding why parasympathetic inflexibility leads to poor outcomes, little is known about when (or in which contexts) rumination is most likely to occur for individuals with autonomic inflexibility. This would be useful to know for designing real-time interventions to target rumination in the contexts when rumination is likely to occur. Prior work suggests that rumination in daily life is more likely to occur in the context of stressors (Kircanski et al., 2018; Moberly & Watkins, 2008), but individuals may vary in the tendency to ruminate following stress (Kircanski et al., 2018). For example, individuals with autonomic inflexibility might be particularly likely to ruminate when they encounter more stress than usual (i.e., more “stress-reactive” rumination) (Robinson & Alloy, 2003).
In the present study, we examined relationships between autonomic inflexibility and rumination across several time scales, using between-subject and within-subject levels of assessment. We hypothesized that relative to individuals with more parasympathetic flexibility, individuals with less flexible autonomic responses to sadness (i.e., less RSA withdrawal) would have higher levels of trait rumination and prospective levels of rumination experienced in daily life (even after accounting for levels of trait rumination). We further hypothesized that parasympathetic inflexibility would amplify the relationship between exposure to stressful events and rumination in everyday life. Prior work suggests that rumination has two subcomponents: brooding, involving passive perseverative thought about one’s negative affect, and pondering, involving more active reflection and problem solving about one’s situation (Treynor et al., 2003). As brooding is the component of rumination that most commonly has been associated with adverse outcomes, we hypothesized that brooding, but not pondering, would be specifically associated with parasympathetic inflexibility.
Method
Participants and Procedure
This project represented a secondary analysis of data collected from 179 (58% female) undergraduate students at a large urban university. Ages ranged from 18 years to 50 years, with a mean age of 21.94 years (SD = 5.70). The sample was 64% Caucasian, 20% African American, 13% Asian, 1% Native American, 7% other race, and 9% Hispanic/Latino. Participants were recruited from undergraduate psychology classes and from flyers posted on campus. Potential participants were required to have fluency in English and either normal or corrected-to-normal vision. Students either received psychology course credit or were compensated in cash for participation.
After providing written informed consent, participants completed a baseline laboratory assessment that involved completing self-report questionnaires and an emotion induction task while heart rate and respiration were monitored. Four follow-up assessments were completed via online survey at three-week intervals (Times 2–5), and included measures of rumination and exposure to negative life events in the prior three weeks. At Time 5, participants were interviewed to verify that life events reported met a priori criteria, and occurred within the correct three-week interval.
To obtain a reliable estimate of each person’s own usual level of stress and the extent to which each observation deviates from this mean (i.e., an idiographic approach), the use of multiple observations centered on each participant’s mean is recommended (Abela & Hankin, 2008). Therefore, to qualify for inclusion in the prospective analyses, participants were required to have completed at least two of the four follow-up assessments (n = 134). Participants included in prospective analyses did not differ from participants excluded from analyses (n = 44) on any study variables or demographic characteristics (ps > .08). Number of follow-up assessments completed also was not associated with any study variables or demographic characteristics (ps > .06).
Measures
Parasympathetic Flexibility.
Electrocardiogram (ECG) and respiration were measured at the Time 1 assessment with a three-lead electrocardiogram and a Biopac BioHarness, and recorded with AcqKnowledge 4.3 software sampled at 1000Hz. Disposable Ag/AgCl electrodes were placed in a modified Lead-II configuration on the chest. ECG signals were amplified with a Biopac MP150 and ECG100 amplifier (Goleta, CA). Respiration was monitored with an RSP100C amplifier with a TSD100C respiratory transducer, which was placed around the chest, passing under the armpits and over the breastbone.
Participants watched a series of video clips, which were presented on a desktop computer approximately 24 inches in front of them. Film selection was based on criteria recommended by (Coan & Allen, 2007). To establish physiological parameters during a neutral baseline, participants first viewed a two-minute nature film clip (a documentary about Denali National Park). This was followed by a film clip depicting a boy distraught at the death of his father (the movie The Champ), which has been demonstrated to elicit sadness (Rottenberg et al., 2007; Stange, Hamilton, Fresco, et al., 2017a).
Expiration and inspiration events in the original respiration channel were located using a software algorithm in AcqKnowledge, based on the positive and negative peaks in a duplicated respiration channel that was high pass filtered at 0.05 Hz. Trained researchers visually inspected the labeling of the original respiration channel for artifacts and mis-labelings and manually corrected if necessary, following well-established procedures (Grossman et al., 1990; Rottenberg et al., 2007). ECG data were visually inspected for artifacts with the aid of a channel that computed momentary inter-beat interval; artifacts were manually adjusted as necessary (< 1% of heartbeats required adjustment). RSA was calculated using the well-validated peak-valley method (Grossman et al., 1990) using AcqKnowledge software. The inspiratory period was used as a window to identify the minimum heart period for each breath, whereas the expiratory period was used as a window to identify the maximum heart period for each breath. Windows of inspiration and expiration were extended forward by 750 milliseconds to account for the phase shifts that occur between respiration and heart period that are dependent on respiration rate (Eckberg, 1983; Grossman et al., 1990). Only minima that were immediately preceded by a longer interval and maxima that were immediately preceded by a shorter interval were considered valid (Grossman et al., 1990). RSA values for each respiration cycle then were averaged across each epoch of interest (i.e., neutral film, sad film). RSA was computed in milliseconds such that higher values reflected greater vagal tone (or parasympathetic activity). RSA reactivity was calculated as a residual difference score between rest and sad film conditions by including RSA terms from the neutral and sad films in statistical models (see Statistical Analyses section, below). Given that respiration rate (RR) typically is correlated with RSA (Bertsch et al., 2012; Kuehl et al., 2015; Overbeek et al., 2012; Ritz & Dahme, 2006), RSA scores during the neutral and sad films were regressed onto average RR during the respective epoch, yielding residual RSA scores that represent RSA after accounting for RR.
Life Events Scale (LES) and Interview (LEI).
The LES (Alloy & Clements, 1992) records the incidence of 134 major and minor life events in a variety of domains relevant to college students (e.g., school, finances, family, social and romantic relationships). Participants indicated the presence or absence of each event over the past three weeks. The LEI is administered by an interviewer, who evaluates the validity of these reports according to a priori criteria for event definition, thereby reducing subject report biases. Any events that do not meet the event definition criteria or that did not occur within the relevant interval(s) are excluded. For example, for the event “did poorly on or failed an exam or major project in an important class (i.e., grade less than C),” the LEI probes require that the exam or project be worth at least 20% of the final grade to be classified as major. Interviewers also must verify the timing of events by anchoring events to time of week, year, and time during the semester or school break, as well as in relation to the timing of other major events. Interviews were completed by phone or in person by a trained graduate student, who was blind to individuals’ RSA scores. The LES and LEI have shown excellent reliability and predictive validity (Alloy & Clements, 1992; Stange, Hamilton, Olino, et al., 2017). In the present study, internal consistency of the LES was good (αs = .88, .91, .89, and .84 at Times 2–5).
Ruminative Response Scale (Treynor et al., 2003).
The RRS (Trait version) is a 10-item, self-report measure that assesses the frequency of rumination in response to dysphoric mood. The scale contains five brooding items (e.g., “think about a recent situation, wishing it had gone better”) and five pondering items (e.g., “analyze recent events to try to understand why you are depressed”), which are scored on a Likert scale ranging from 1 (almost never) to 4 (almost always). The RRS has excellent internal consistency and validity (Armey et al., 2009; Treynor et al., 2003). In the present study, α = .74 for brooding, and α = .76 for pondering (measured at time 1). For the real-world assessments of rumination at times 2–5, the RRS contained the same items with instructions modified to indicate ruminative responses over the previous three weeks (at times 2–5, αs = .74–.84 for brooding, and αs = .77–.87 for pondering).
Beck Depression Inventory (BDI-II) (Beck, 1996).
The BDI-II assessed the severity of cognitive, affective, and somatic symptoms of depression during the previous three weeks. It has demonstrated good internal consistency and validity in undergraduate samples (Dozois et al., 1998; Storch et al., 2004). In the present study, αs = .87–.90 at times 1–5.
Positive and Negative Affect Scale (PANAS) — Brief Version (Mackinnon et al., 1999).
The PANAS — Brief version is a 10-item version of the original self-report measure (Watson, Clark, & Tellegen, 1988) that assesses current emotions and affective experiences. The PANAS is a commonly-used measure of affect in experimental studies, and it has excellent validity and reliability (Watson et al., 1988). For each word, participants were asked to “indicate to what extent you feel this way right now, that is, at the present moment,” on a scale ranging from 1 (very slightly or not at all) to 4 (extremely). The PANAS was administered after the neutral and sad films to evaluate shifts in affect. The present study focused on the negative affect (NA) subscale given its importance in loss events (e.g., the content of the sad film). It was used as both a manipulation check and to examine affective reactivity to sadness as a covariate in analyses of RSA reactivity. A difference score was computed by subtracting the post-neutral film NA score from the post-sad-film NA score to assess changes in negative affect during the sad film, with higher scores representing greater increases in NA. In the present study, NA had excellent internal consistency after the neutral (α = .90) and sad (α = .92) films.
Statistical Analyses
To test the hypothesis that autonomic inflexibility would be associated with trait brooding, linear regressions were conducted. Trait brooding or pondering served as the outcome variable; RSA during the sad film served as the focal predictor; and covariates included RSA responses during the neutral film, and changes in negative affect from the neutral to the sad films. Thus, the effect of RSA during the sad film represented the relationship between RSA reactivity to sadness (i.e., residual change in RSA during the sad film relative to the neutral film) and rumination, after accounting for the covariates.
Given the nested structure of the data (multiple observations of negative life events and real-world rumination within each person), multilevel modeling (MLM) (Raudenbush & Bryk, 2002) was used for analyses involving these measures. MLM is advantageous in terms of maximizing data usage because it can flexibly handle cases with missing data, so participants with missing data (e.g., participants who miss a follow-up visit) are not eliminated from the data analyses (however, as noted earlier, participants were excluded if they completed fewer than two follow-up assessments). Analyses were conducted with the Mplus 6.12 statistical software package (Muthén & Muthén, 2010), which allowed for use of full information maximum likelihood (FIML) estimation of data, using maximum likelihood estimation with robust standard errors.
To test the hypothesis that autonomic inflexibility would be associated with higher levels of real-world rumination, MLMs were conducted with the random intercept of real-world brooding or pondering serving as the outcome variable. At level 2, RSA during the sad film served as the focal predictor representing autonomic reactivity to sadness; level 2 covariates included RSA responses during the neutral film, baseline BDI (to account for individual differences in depression symptom severity), trait brooding or pondering (to evaluate effects of autonomic responses on real-world rumination, above and beyond habitual levels of rumination, assessed at wave 1), change in negative affect from after the neutral film to after the sad film (to account for individual differences in affective reactivity), and average levels of negative life events over waves 2–5.
To examine whether autonomic inflexibility would be associated with higher levels of real-world rumination particularly when individuals were exposed to more life stressors than normal for themselves, life events were added to the models as a level 1 predictor, along with a random slope reflecting the relationship between life events and rumination. These slopes estimated, for each person, the relationship between negative life events and rumination at each wave of follow-up. Idiographic (person-centered) approaches such as this may more accurately test theoretical vulnerability-stress models than is possible with nomothetic (sample mean-centered) approaches (Abela & Hankin, 2008; Stange et al., 2019). Slopes were predicted (moderated) by RSA during the sad and neutral films and change in negative affect from neutral to sad films. Significant interactions were probed by testing the simple slopes of life events on rumination at ± 1 standard deviation from the mean of the moderator of interest (RSA responses to the sad film). All measures of autonomic activity were standardized. At level 1, BDI at each wave was included as a covariate, along with rumination at the previous wave. Nonsignificant random effects of level 1 covariates were removed from models before interpreting results.
Results
Mean values of RSA were 102.68 ms (SD = 58.69) during the neutral film, and 92.28 ms (SD = 48.24) during the sad film. Manipulation checks indicated that in response to the sad film, RSA decreased (t(133) = 4.52, p < .001, d = .19), and negative affect increased as reported previously (Stange, Hamilton, Fresco, et al., 2017a). The mean number of life events across participants and waves was 8.05 (SD = 6.43); the mean BDI score across participants and waves was 7.00 (SD = 6.52); at baseline, 24% of the sample had BDI scores suggesting at least mild depression (BDI > 13), and 11% had at least moderate depressive symptoms (BDI > 20). Measures of habitual brooding and pondering were moderately correlated (r = .48, p < .001). Within MLMs, the intra-class correlations for intercept-only models predicting real-world brooding and pondering were .53 and .51, respectively, indicating that 51–53% of the variance in rumination occurred at the between-subject level (Level 2), whereas 47–49% of the variance occurred at the within-subject level (Level 1). Intercepts of real-world brooding and pondering were moderately correlated (r = .46, p < .001).
Are Inflexible Parasympathetic Responses to Sadness Associated with Habitual Rumination?
As hypothesized, after accounting for the influence of covariates, individuals with less flexible RSA responses to sadness (i.e., those who showed higher RSA or smaller decreases in RSA during sadness) had higher levels of trait brooding (B = 0.30, SE = 0.15, p = .05, model R2 = .07). In contrast, less flexible RSA responses to sadness were not significantly associated with trait pondering (B = 0.26, SE = 0.15, p = .09, model R2 = .03).1
Are Inflexible Parasympathetic Responses to Sadness Associated with Real-World Rumination?
A similar pattern of results emerged when examining real-world rumination. Consistent with hypotheses, individuals with less flexible RSA responses to sadness had significantly higher levels of real-world brooding across follow-up (Table 1; Figure 1), even after accounting for trait levels of brooding and baseline symptoms of depression. In contrast, RSA during the sad film was not significantly associated with real-world pondering (Table 1).
Table 1.
Multilevel Models with Parasympathetic Flexibility (Respiratory Sinus Arrhythmia Decreases in Response to Sad Film Relative to Neutral Film) and Person-Centered Stressful Life Event Exposure at Each Wave Predicting Real-World Rumination
| Model (Predictor) | B/γ | SE | p | ΔR2 |
|---|---|---|---|---|
| Predicting Real-World Brooding | .75 | |||
| Within-Subject Level (Level 1) | ||||
| RRS Brooding (t-1) | 0.18 | 0.07 | <.01 | .05 |
| BDI (t) | 0.18 | 0.04 | <.001 | .13 |
| Life Events Scale (LES) Slope | 0.05 | 0.04 | .24 | .01 |
| Associations with Levels of Brooding 1 (Level 2) | ||||
| RSA (Sad Film) | 0.65 | 0.32 | .04 | .03 |
| RSA (Neutral Film) | −0.27 | 0.31 | .39 | .01 |
| RRS Brooding | 2.02 | 0.17 | <.001 | .52 |
| T1 BDI | 0.06 | 0.02 | .02 | .06 |
| LES Mean | 0.05 | 0.02 | .04 | .05 |
| ΔNA | 0.18 | 0.20 | .35 | .01 |
| Associations between RSA and Brooding Responses to Stress (Level 2) | ||||
| RSA (Sad Film) × LES | −0.17 | 0.08 | .04 | .03 |
| RSA (Neutral Film) × LES | 0.18 | 0.08 | .03 | .04 |
| ΔNA × LES | −0.06 | 0.03 | .11 | .03 |
| Random Effects (Level 2) | ||||
| RRS Brooding (Random Intercept) | 6.49 | 0.74 | <.001 | .37 |
| LES (Random Slope) | <0.01 | 0.01 | .25 | <.01 |
| Predicting Real-World Pondering | .51 | |||
| Within-Subject Level (Level 1) | ||||
| RRS Pondering (t-1) | 0.09 | 0.06 | .13 | .02 |
| BDI (t) | 0.13 | 0.04 | <.001 | .07 |
| Life Events Scale (LES) Slope | 0.16 | 0.05 | <.01 | .07 |
| Associations with Levels of Pondering (Level 2) | ||||
| RSA (Sad Film) | −0.34 | 0.40 | .40 | .01 |
| RSA (Neutral Film) | 0.43 | 0.41 | .29 | .01 |
| RRS Pondering | 2.31 | 0.20 | <.001 | .50 |
| T1 BDI | 0.03 | 0.03 | .36 | .01 |
| LES Mean | 0.09 | 0.04 | .02 | .04 |
| ΔNA | 0.19 | 0.19 | .31 | .01 |
| Associations between RSA and Pondering Responses to Stress (Level 2) | ||||
| RSA (Sad Film) × LES | −0.07 | 0.07 | .36 | .01 |
| RSA (Neutral Film) × LES | 0.01 | 0.07 | .87 | <.01 |
| ΔNA × LES | −0.14 | 0.05 | <.01 | .06 |
| Random Effects (Level 2) | ||||
| RRS Pondering (Random Intercept) | 7.68 | 0.74 | <.001 | .45 |
| LES (Random Slope) | <0.01 | 0.01 | .19 | <.01 |
Note. RSA = Respiratory Sinus Arrhythmia; R2 for full model represents pseudo R2, the proportion of variance explained by the model relative to an unrestricted (intercept-only) model containing no predictors (Kreft & Leeuw, 1998; Singer, 1998). ΔR2 = partial R2 of given predictor (proportion of variance in rumination predicted) after accounting for covariates. Significant hypothesized predictors of interest are bolded.
Figure 1.
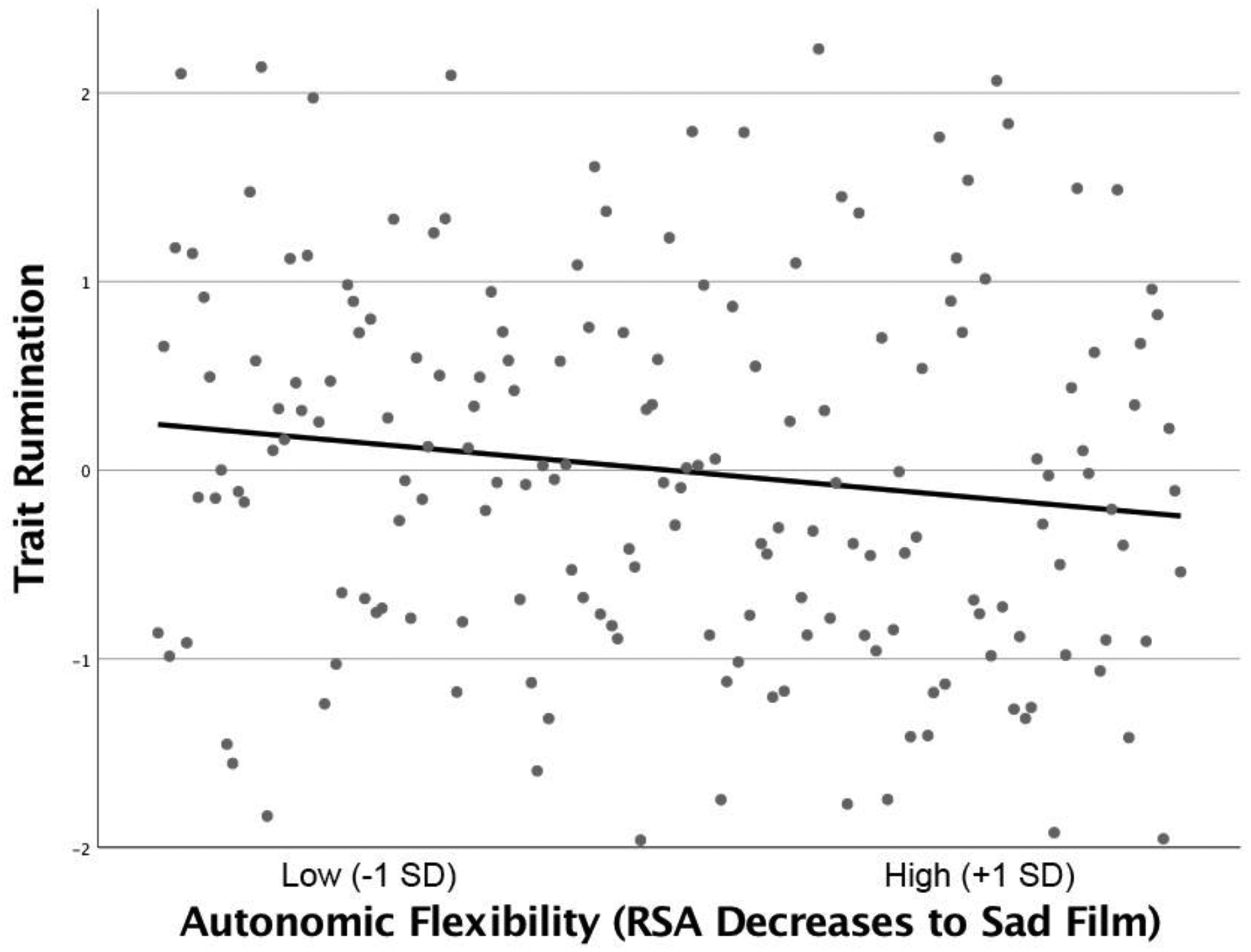
Association between parasympathetic flexibility (respiratory sinus arrhythmia decreases in response to sad film relative to neutral film) and real-world brooding (mean across up to four follow-up assessments).
Do Inflexible Parasympathetic Responses to Sadness Potentiate Rumination Following Stressors?
There was a significant interaction between RSA responses to sadness and stressful event exposure predicting real-world brooding (Figure 2), but not pondering (Table 1).2 However, inconsistent with hypotheses, individuals who demonstrated more inflexible RSA responses to sadness were not more likely to report brooding when they experienced more stressors than usual for themselves (B = −0.12, SE = 0.08, p = .15). In contrast, individuals who did demonstrate more flexible RSA responses to sadness were more likely to report brooding when they experienced more stressors than usual for themselves (B = 0.23, SE = 0.10, p = .03). In other words, whereas individuals with more flexible RSA responses ruminated more only when they were exposed to more stressors than usual, less flexible individuals displayed a high level of rumination regardless of how many stressors they experienced.
Figure 2.
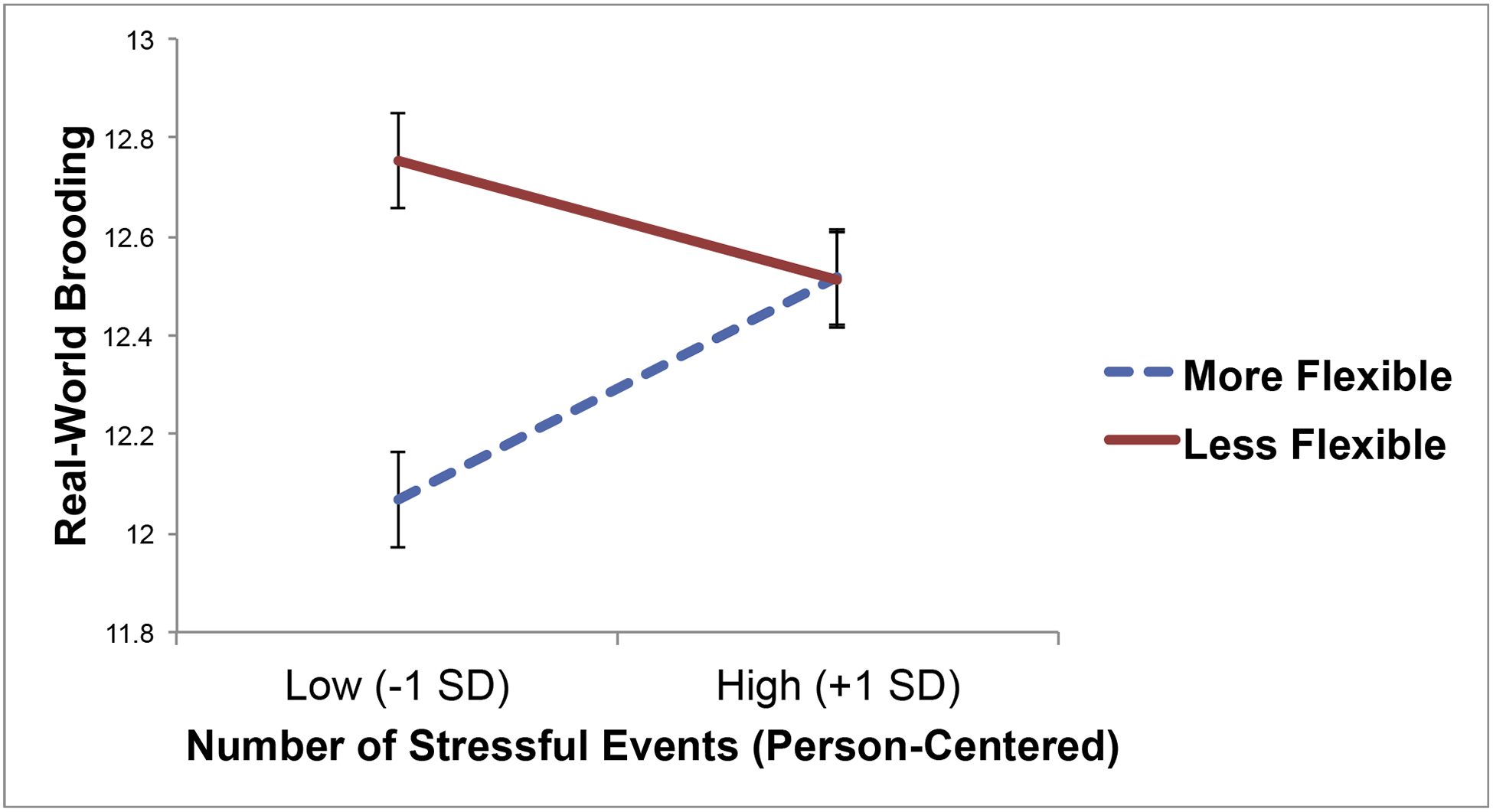
Cross-level interaction between parasympathetic flexibility (respiratory sinus arrhythmia decreases in response to sad film relative to neutral film) and person-centered stressful life event exposure at each wave predicting real-world brooding.
Although not the focus of our study, there also was a significant interaction between RSA responses to the neutral film and stressful event exposure predicting real-world brooding, but not pondering (Table 1). The form of this interaction was such that for individuals who demonstrated higher residual RSA responses to the neutral film (after statistically accounting for responses to the subsequent sad film), there was a significant positive relationship between stress exposure and brooding (B = 0.23, SE = 0.09, p = .01). In contrast, for individuals who demonstrated lower residual RSA responses to the neutral film, the relationship between stress exposure and brooding was not significant (B = −0.13, SE = 0.09, p = .18). However, when RSA during the sad film was not included in the model, RSA during the neutral film did not interact significantly with life events to predict brooding (B = .02, SE = .04, p = .57).
Discussion
We conducted the first prospective examination of autonomic inflexibility (indexed by a lack of RSA withdrawal to sadness) as a vulnerability factor for both habitual and real-world rumination. Results indicated that relative to individuals with greater autonomic flexibility, individuals with less autonomic flexibility had higher levels of trait rumination and were more likely to ruminate in daily life across twelve weeks of follow-up, even after accounting for the influence of trait rumination and symptoms of depression. We also examined the hypothesis that individuals with autonomic inflexibility would be particularly likely to ruminate when they were exposed to life stressors. Contrary to this hypothesis, individuals with autonomic inflexibility experienced high levels of rumination regardless of the context of stress, whereas individuals with more parasympathetic flexibility exhibited high rumination only in the context of higher stress. Together, these results provide initial evidence that inflexible autonomic responses to sadness may predispose individuals to rumination. This suggests a plausible behavioral mechanism by which autonomic inflexibility may lead to problems with self-regulation and vulnerability to depression.
Our findings build on prior experimental work suggesting that RSA decreases in response to rumination, and cross-sectional studies suggesting associations between lower resting RSA and trait rumination (Ottaviani et al., 2016). In contrast, our study provides the first evidence that inflexible autonomic responses to sadness (i.e., failing to show contextually appropriate decreases in RSA during a sad film) are associated with habitual rumination, and even confer vulnerability to future ruminative responses in daily life. Interestingly, at both the habitual and real-world levels of analysis, autonomic inflexibility predicted higher levels of brooding, but not pondering. This finding corroborates an existing literature that suggests that brooding may be the more maladaptive component of rumination that is more closely tied to depression (Alloy et al., 2017; Treynor et al., 2003). By predicting not only habitual (or trait) levels of brooding, but also state levels of brooding in daily life, these results provide external validity to autonomic inflexibility as a vulnerability factor for rumination. By identifying rumination as a downstream behavioral marker of autonomic inflexibility, our findings may help to explain why autonomic inflexibility may confer vulnerability to depression.
We also examined contexts when rumination occurred, hypothesizing that rumination in daily life would be more likely to occur when individuals were exposed to more stressful events than usual, and that autonomic inflexibility might potentiate these effects. In contrast with this hypothesis, although individuals with autonomic inflexibility had higher levels of brooding overall (i.e., a main effect), there was not a significant relationship between stressful event exposure and rumination among individuals with greater autonomic inflexibility. The pattern of the interaction was similar to a ceiling effect of rumination, in which individuals with autonomic inflexibility reported high levels of rumination regardless of their exposure to stressful events. In contrast, individuals with more parasympathetic flexibility demonstrated increases in rumination only when exposed to more stressors than usual. Although this pattern of results was inconsistent with the hypothesized interaction between autonomic inflexibility and stressful events, the results may speak to a theme highlighted by the broader literature on flexibility (Stange, Alloy, et al., 2017). Whereas individuals with less parasympathetic flexibility exhibit high levels of rumination regardless of stressor context, those with more parasympathetic flexibility demonstrate rumination only in the context of stress, perhaps representing a context-specific, or even contextually appropriate, pattern of rumination. Although it would be premature to assert that there are contexts in which any amount of rumination may be adaptive, in our study individuals with more parasympathetic flexibility showed perhaps a more flexible pattern of rumination — that is, ruminating more when stressors occurred (as would be expected in the general population), but more importantly, ruminating less when fewer stressors occurred. Thus, perhaps it is the fact that individuals with autonomic inflexibility appeared unable to disengage from rumination, even when there was less stress than usual, that particularly distinguishes inflexibility, and hence, vulnerability to problems such as depression (Stange, MacNamara, et al., 2017). In this sense, by establishing links between autonomic inflexibility and ruminative inflexibility (despite assessment across multiple contexts, methods, and levels of analysis), these results seem to corroborate theories that have proposed links between multiple components of flexibility that broadly represent context insensitivity (Stange, Alloy, et al., 2017).
Counterintuitively, we found that RSA levels during the neutral film were associated with a more positive relationship between stress exposure and brooding, but only when statistically controlling for RSA during the sad film. It is not entirely clear why the variance unique to RSA during the neutral film (which essentially served as a baseline or resting measure of RSA) would be associated with maladaptive outcomes such as brooding. However, RSA at rest and stimulus-driven reactivity in RSA may be supported by different processes. As RSA is thought to reflect a measure of regulatory effort (Butler et al., 2006), it is possible that for some individuals higher RSA during the neutral film reflected greater habitual level of effort to regulate. As rumination sometimes is conceptualized as an attempt to regulate one’s affect, it might make sense for brooding to be associated with a biomarker of regulatory effort such as RSA. Alternatively, a statistical explanation for this finding is possible. Given that RSA responses to the neutral and sad contexts were correlated, and that the relationship between RSA during the neutral film and brooding in response to life events only was seen when RSA responses to sadness was included as a covariate, it is possible that RSA responses to sadness served as a statistical suppressor variable for RSA responses to the neutral film in this model. This would artificially inflate the relationship between RSA during the neutral film and brooding in response to life events. This explanation would fit within the broader literature on RSA and rumination, in which meta-analytic results have suggested that resting RSA is associated with lower levels of rumination and depression (Ottaviani et al., 2016). In contrast, the relationship between RSA responses to sadness and rumination (when controlling for RSA responses to the neutral film) is best conceptualized as a residual change score, because the change from neutral to sad contexts is conceptually meaningful as a temporal change in RSA. The same cannot be said when interpreting RSA responses to the neutral film while controlling for RSA responses to the sad film, since the neutral film occurred before the sad film. However, future research should continue to examine whether the variance in RSA that is unique to neutral or resting contexts has utility in understanding perseverative thinking processes, and to explore what mechanisms and processes might explain this relationship.
Results of the present study have several potential implications for intervention. Knowing that autonomic inflexibility may confer vulnerability to rumination suggests that interventions could target either autonomic inflexibility or rumination, or other factors that may be relevant to both of these markers of vulnerability. For example, inhibitory control is implicated in both the suppression of sympathetic activation (Thayer et al., 2012) and the ability to disengage from rumination (Joormann & Vanderlind, 2014). Neuromodulatory approaches such as transcranial current stimulation and transcranial magnetic stimulation represent “top-down” (brain-to parasympathetic) methods for experimentally enhancing inhibitory control, with preliminary evidence for downstream effects on resting RSA (Makovac et al., 2017) and rumination (Vanderhasselt et al., 2017). Alternatively, “bottom-up” (parasympathetic-to-brain) approaches such as biofeedback may help individuals to improve parasympathetic activity at rest and in response to relevant stimuli (Lehrer, 2017). Wearable technologies are increasingly enabling real-time assessment of parasympathetic activity (Valenza et al., 2015), enabling biofeedback interventions to be implemented outside of the lab in the contexts where they may be needed most (Plans et al., 2019).
Additionally, mindfulness-based techniques may improve resting RSA (Krygier et al., 2013) and reduce the tendency to engage with ruminative thoughts (Cohen et al., 2017; Hawley et al., 2014). Several third-wave behavioral therapies, including mindfulness-based cognitive therapy (MBCT) (Segal et al., 2018), contain mindfulness-based components and either explicitly or indirectly target rumination. There also is evidence that MBCT reduces excessive functional connectivity within the brain’s default mode network (Taylor et al., 2013), which is involved in self-referential processing such as rumination and is elevated in depression. These types of interventions thus offer promise for targeting the antecedents of rumination and reducing downstream effects such as depression. Given the presence of substantial heterogeneity within depression, by measuring parasympathetic flexibility and rumination, future interventions might be tailored toward specific subgroups of individuals who stand to benefit the most from treatments that aim to modulate these targets.
This study had several strengths, including measuring habitual and real-world rumination, measuring parasympathetic flexibility in response to sadness, demonstrating associations across multiple methods of assessment, and the use of a prospective multi-wave study rather than a purely cross-sectional design. Despite these strengths, several limitations must be noted that can provide direction for future research. The study used a nonclinical sample, so generalizability outside of the context of university students and within clinical samples must be demonstrated in the future. Next, effect sizes were relatively small, suggesting the need to consider additional variables and contextual factors for identifying risk factors for rumination. Although we used a prospective design, we did not examine factors that may have predated low autonomic flexibility at wave 1, such as earlier life history of rumination or earlier life stressors. In addition, although we measured habitual rumination and ruminative responses across multiple waves of follow-up, we did not include a measure of rumination in response to the sad mood induction in the lab or rumination specifically in response to naturally occurring life stressors. Due to feasibility limitations, we were not able to examine potential cross-lagged, transactional relationships between autonomic inflexibility and rumination. Our current work is examining these constructs throughout the day in clinical samples, which will allow for a more fine-grained analysis of the temporal associations between autonomic inflexibility and rumination in daily life. Finally, although analyses controlled for respiration rate, the use of paced breathing might have allowed us to minimize the effects of respiration rate on RSA, a technique that could be considered in future research (Bertsch et al., 2012; Kuehl et al., 2015; Ritz & Dahme, 2006). Our decision not to use paced breathing was due to concern that this might reduce the effectiveness of the negative affect induction procedure by interfering with participants’ ability to fully attend to the film stimuli.
In conclusion, this study provides some of the first evidence that autonomic inflexibility may confer vulnerability to rumination. Furthermore, for individuals with autonomic inflexibility, this subsequent rumination may occur in a context-insensitive manner (regardless of the presence of stressful life events). This work suggests a potential behavioral mechanism by which autonomic inflexibility may lead to problems with self-regulation and vulnerability to depression, suggesting multiple avenues for intervention to target these markers of vulnerability.
Highlights.
The downstream behavioral consequences of autonomic inflexibility are not well understood.
Rumination is one candidate phenotype relevant to autonomic inflexibility.
Individuals with less parasympathetic flexibility had higher levels of trait rumination.
Less flexible individuals also were more likely to ruminate in daily life, regardless of stress exposure.
Suggests a potential behavioral mechanism by which autonomic inflexibility leads to problems with self-regulation and depression.
Acknowledgments
This work was supported by grants to Jonathan P. Stange from the National Institute of Mental Health (F31MH099761), the Association for Psychological Science, the American Psychological Foundation, and the American Psychological Association. Jonathan P. Stange was supported by grant 1K23MH112769-01A1 from NIMH. Jessica L. Hamilton was supported by T32HL082610 from National Heart Lung Blood Institute (NHBLI). Lauren B. Alloy was supported by NIMH Grant MH101168. David M. Fresco was supported by NHLBI Grant R01HL119977, NINR Grant P30NR015326, NCCIH Grant R61AT009867, and NICHD Grant R21HD095099.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RSA during the neutral film was not significantly associated with habitual brooding (r = .11, p = .21) or pondering (r = −.04, p = .64).
The interaction between changes in negative affect in response to the sad film and negative life events was a significant predictor of real-world pondering (Table 1). However, when the RSA terms were removed from the model, changes in negative affect in response to the sad film did not interact significantly with life events to predict brooding (B = −0.13, SE = .27, p = .65).
References
- Abela JRZ, & Hankin BL (2008). Cognitive vulnerability to depression in children and adolescence: A developmental psychopathology perspective In Abela JRZ & Hankin BL (Eds.), Handbook of Depression in Children and Adolescents (pp. 35–78). Guilford Press. [Google Scholar]
- Alloy LB, & Clements CM (1992). Illusion of control: Invulnerability to negative affect and depressive symptoms after laboratory and natural stressors. Journal of Abnormal Psychology, 101(2), 234–245. 10.1037/0021-843X.101.2.234 [DOI] [PubMed] [Google Scholar]
- Alloy LB, Salk R, Stange JP, & Abramson LY (2017). Cognitive vulnerability and unipolar depression In DeRubeis RJ & Strunk DR (Eds.), The Oxford handbook of mood disorders (pp. 142–153). [Google Scholar]
- Armey MF, Fresco DM, Moore MT, Mennin DS, Turk CL, Heimberg RG, Kecmanovic J, & Alloy LB (2009). Brooding and Pondering: Isolating the Active Ingredients of Depressive Rumination With Exploratory Factor Analysis and Structural Equation Modeling. Assessment, 16(4), 315–327. 10.1177/1073191109340388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Beck AT (1996). Beck Depression Inventory (2nd ed.). The Psychological Corporation. [Google Scholar]
- Bertsch K, Hagemann D, Naumann E, Schächinger H, & Schulz A (2012). Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology, 49(5), 672–682. 10.1111/j.1469-8986.2011.01341.x [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, & Gross JJ (2006). Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology, 43(6), 612–622. 10.1111/j.1469-8986.2006.00467.x [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, & Rottenberg J (2014). Respiratory Sinus Arrhythmia Reactivity in Current and Remitted Major Depressive Disorder: Psychosomatic Medicine, 76(1), 66–73. 10.1097/PSY.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2007). Handbook of Emotion Elicitation and Assessment. Oxford University Press. [Google Scholar]
- Cohen JN, Jensen D, Stange JP, Neuburger M, & Heimberg RG (2017). The Immediate and Long-Term Effects of an Intensive Meditation Retreat. Mindfulness, 8(4), 1064–1077. 10.1007/s12671-017-0682-5 [DOI] [Google Scholar]
- Dozois DJA, Dobson KS, & Ahnberg JL (1998). A psychometric evaluation of the Beck Depression Inventory—II. Psychological Assessment, 10(2), 83–89. 10.1037/1040-3590.10.2.83 [DOI] [Google Scholar]
- Eckberg DL (1983). Human sinus arrhythmia as an index of vagal cardiac outflow. Journal of Applied Physiology, 54(4), 961–966. [DOI] [PubMed] [Google Scholar]
- Grossman P, Beek J, & Wientjes C (1990). A Comparison of Three Quantification Methods for Estimation of Respiratory Sinus Arrhythmia. Psychophysiology, 27(6), 702–714. 10.1111/j.1469-8986.1990.tb03198.x [DOI] [PubMed] [Google Scholar]
- Hamilton JL, & Alloy LB (2016). Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clinical Psychology Review, 50, 67–79. 10.1016/j.cpr.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Stange JP, Burke TA, Franzen PL, & Alloy LB (2019). Sleep disturbance and physiological regulation among young adults with prior depression. Journal of Psychiatric Research, 115, 75–81. 10.1016/j.jpsychires.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam N, Holland E, & Kuppens P (2012). Categories versus dimensions in personality and psychopathology: A quantitative review of taxometric research. Psychological Medicine, 42(5), 903–920. 10.1017/S0033291711001966 [DOI] [PubMed] [Google Scholar]
- Hawley LL, Schwartz D, Bieling PJ, Irving J, Corcoran K, Farb NAS, Anderson AK, & Segal ZV (2014). Mindfulness Practice, Rumination and Clinical Outcome in Mindfulness-Based Treatment. Cognitive Therapy and Research, 38(1), 1–9. 10.1007/s10608-013-9586-4 [DOI] [Google Scholar]
- Joormann J, & Vanderlind WM (2014). Emotion Regulation in Depression: The Role of Biased Cognition and Reduced Cognitive Control. Clinical Psychological Science, 2(4), 402–421. 10.1177/2167702614536163 [DOI] [Google Scholar]
- Juster R-P, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kashdan TB, & Rottenberg J (2010). Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review, 30(7), 865–878. 10.1016/j.cpr.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Thompson RJ, Sorenson J, Sherdell L, & Gotlib IH (2018). The everyday dynamics of rumination and worry: Precipitant events and affective consequences. Cognition and Emotion, 32(7), 1424–1436. 10.1080/02699931.2017.1278679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft IGG, & Leeuw J. de. (1998). Introducing Multilevel Modeling. SAGE. [Google Scholar]
- Krygier JR, Heathers JAJ, Shahrestani S, Abbott M, Gross JJ, & Kemp AH (2013). Mindfulness meditation, well-being, and heart rate variability: A preliminary investigation into the impact of intensive Vipassana meditation. International Journal of Psychophysiology, 89(3), 305–313. 10.1016/j.ijpsycho.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Kuehl LK, Deuter CE, Richter S, Schulz A, Rüddel H, & Schächinger H (2015). Two separable mechanisms are responsible for mental stress effects on high frequency heart rate variability: An intra-individual approach in a healthy and a diabetic sample. International Journal of Psychophysiology, 95(3), 299–303. 10.1016/j.ijpsycho.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Lehrer PM (2017). Heart rate variability biofeedback and other psychophysiological procedures as important elements in psychotherapy. International Journal of Psychophysiology. 10.1016/j.ijpsycho.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Liu R, McArthur BA, Burke TA, Hamilton JL, Mac Giollabhui N, Stange JP, Hamlat EJ, Abramson LY, & Alloy LB (2019). A Latent Structure Analysis of Cognitive Vulnerability to Depression in Adolescence. Behavior Therapy, 50(4), 755–764. 10.1016/j.beth.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovac E, Thayer JF, & Ottaviani C (2017). A meta-analysis of non-invasive brain stimulation and autonomic functioning: Implications for brain-heart pathways to cardiovascular disease. Neuroscience & Biobehavioral Reviews, 74, 330–341. 10.1016/j.neubiorev.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Moberly NJ, & Watkins ER (2008). Ruminative self-focus, negative life events, and negative affect. Behaviour Research and Therapy, 46, 1034–1039. 10.1016/j.brat.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén Bengt. (2010). Mplus statistical analysis with latent variables: User’s guide. http://www.statmodel.com
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, & Brosschot JF (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142(3), 231–259. 10.1037/bul0000036 [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, van Boxtel A, & Westerink JHDM (2012). Respiratory sinus arrhythmia responses to induced emotional states: Effects of RSA indices, emotion induction method, age, and sex. Biological Psychology, 91(1), 128–141. 10.1016/j.biopsycho.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Panaite V, Hindash AC, Bylsma LM, Small BJ, Salomon K, & Rottenberg J (2016). Respiratory sinus arrhythmia reactivity to a sad film predicts depression symptom improvement and symptomatic trajectory. International Journal of Psychophysiology, 99, 108–113. 10.1016/j.ijpsycho.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plans D, Morelli D, Sütterlin S, Ollis L, Derbyshire G, & Cropley M (2019). Use of a Biofeedback Breathing App to Augment Poststress Physiological Recovery: Randomized Pilot Study. JMIR Formative Research, 3(1), e12227 10.2196/12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2002). Hierarchical Linear Models: Applications and Data Analysis Methods. SAGE. [Google Scholar]
- Ritz T, & Dahme B (2006). Implementation and Interpretation of Respiratory Sinus Arrhythmia Measures in Psychosomatic Medicine: Practice Against Better Evidence?: Psychosomatic Medicine, 68(4), 617–627. 10.1097/01.psy.0000228010.96408.ed [DOI] [PubMed] [Google Scholar]
- Robinson MS, & Alloy LB (2003). Negative Cognitive Styles and Stress-Reactive Rumination Interact to Predict Depression: A Prospective Study. Cognitive Therapy and Research, 18. [Google Scholar]
- Rottenberg J (2007). Cardiac vagal control in depression: A critical analysis. Biological Psychology, 74(2), 200–211. 10.1016/j.biopsycho.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, & Salomon K (2007). RSA fluctuation in major depressive disorder. Psychophysiology, 44(3), 450–458. 10.1111/j.1469-8986.2007.00509.x [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, & Teasdale J (2018). Mindfulness-Based Cognitive Therapy for Depression, Second Edition Guilford Publications. [Google Scholar]
- Singer JD (1998). Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. Journal of Educational and Behavioral Statistics, 23(4), 323–355. 10.3102/10769986023004323 [DOI] [Google Scholar]
- Stange JP, Alloy LB, & Fresco DM (2017). Inflexibility as a Vulnerability to Depression: A Systematic Qualitative Review. Clinical Psychology: Science and Practice, 24(3), 245–276. 10.1111/cpsp.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Fresco DM, & Alloy LB (2017a). Flexible parasympathetic responses to sadness facilitate spontaneous affect regulation. Psychophysiology, 54(7), 1054–1069. 10.1111/psyp.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Fresco DM, & Alloy LB (2017b). Perseverate or decenter? Differential effects of metacognition on the relationship between parasympathetic inflexibility and symptoms of depression in a multi-wave study. Behaviour Research and Therapy, 97, 123–133. 10.1016/j.brat.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Olino TM, Fresco DM, & Alloy LB (2017). Autonomic reactivity and vulnerability to depression: A multi-wave study. Emotion, 17(4), 602–615. 10.1037/emo0000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Kleiman EM, Mermelstein RJ, & Trull TJ (2019). Using ambulatory assessment to measure dynamic risk processes in affective disorders. Journal of Affective Disorders, 259, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, MacNamara A, Kennedy AE, Hajcak G, Phan KL, & Klumpp H (2017). Brain-behavioral adaptability predicts response to cognitive behavioral therapy for emotional disorders: A person-centered event-related potential study. Neuropsychologia. 10.1016/j.neuropsychologia.2017.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, & Roth DA (2004). Factor structure, concurrent validity, and internal consistency of the beck depression inventory?second edition in a sample of college students. Depression and Anxiety, 19(3), 187–189. 10.1002/da.20002 [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Young AH, & Cleare AJ (2017). Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatric Disease and Treatment, 13, 1245–1262. 10.2147/NDT.S114542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, Courtemanche J, Lavarenne AS, Marrelec G, Benali H, & Beauregard M (2013). Impact of meditation training on the default mode network during a restful state. Social Cognitive and Affective Neuroscience, 8(1), 4–14. 10.1093/scan/nsr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart—brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–88. 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research, 13. [Google Scholar]
- Valenza G, Citi L, Gentili C, Lanata A, Scilingo EP, & Barbieri R (2015). Characterization of Depressive States in Bipolar Patients Using Wearable Textile Technology and Instantaneous Heart Rate Variability Assessment. IEEE Journal of Biomedical and Health Informatics, 19(1), 263–274. 10.1109/JBHI.2014.2307584 [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M-A, Sanchez A, Josephy H, Baeken C, Brunoni AR, & De Raedt R (2017). Anodal tDCS over the right dorsolateral prefrontal cortex modulates cognitive processing of emotional information as a function of trait rumination in healthy volunteers. Biological Psychology, 123, 111–118. 10.1016/j.biopsycho.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma LM, Rottenberg J, & Kovacs M (2013). Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biological Psychology, 94(2), 272–281. 10.1016/j.biopsycho.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Bylsma LM, Jennings JR, George C, Baji I, Benák I, Dochnal R, Halas K, Kapornai K, Kiss E, Makai A, Varga H, Vetró Á, & Kovacs M (2016). Parasympathetic nervous system activity predicts mood repair use and its effectiveness among adolescents with and without histories of major depression. Journal of Abnormal Psychology, 125(3), 323–336. 10.1037/abn0000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, & Kovacs M (2013). The utility of combining RSA indices in depression prediction. Journal of Abnormal Psychology, 122(2), 314–321. 10.1037/a0032385 [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, & Kovacs M (2014). Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Development and Psychopathology, 26(4pt2), 1337–1352. 10.1017/S0954579414001060 [DOI] [PMC free article] [PubMed] [Google Scholar]


