Abstract
Background & Aims
Observational studies of predominantly white populations have found new-onset diabetes to be associated with increased risk of pancreatic cancer. We sought to determine whether this relationship applies to other races or ethnicities and to identify metabolic profiles associated with increased risk of pancreatic cancer.
Methods
We conducted a population-based cohort study of Asian, black, Hispanic and white patients from Kaiser Permanente Southern California from 2006 through 2016 (n=1,499,627). Patients with diabetes were identified based on glucose and hemoglobin A1c (HbA1c) measurements. We used Cox regression to assess the relationship between diabetes status and duration and pancreatic cancer. For patients with recent diagnoses of diabetes (1 year or less) we compared longitudinal changes in glucose, HbA1c, and weight, from time of diabetes diagnosis through 3 years prior to the diagnosis, in patients with vs without pancreatic cancer.
Results
We identified 2,002 incident cases of pancreatic cancer from nearly 7.5 million person-years of follow-up. Compared to patients without diabetes, individuals who received a recent diagnosis of diabetes had an almost 7-fold increase in risk of pancreatic cancer (relative risk, 6.91; 95% CI, 5.76–8.30). Among patients with a recent diagnosis of diabetes, those who developed pancreatic cancer had more rapid increases in levels of glucose (Δslope: cases, 37.47 mg/dL vs non-cases, 27.68 mg/dL) and HbA1c (Δslope: cases, 1.39% vs non-cases, 0.86%) in the month preceding the diagnosis of diabetes, and subtle weight loss in the prior years (slope: cases −0.18 kg/interval vs non-cases 0.33 kg/interval). These longitudinal changes in markers of metabolism were stronger for specific race and ethnic groups.
Conclusions
In a study of a large ethnically diverse population, we found risk of pancreatic cancer to be increased among patients with a diagnosis of diabetes in the past year among different races and ethnicities. Weight loss and rapid development of poor glycemic control were associated with increased risk of pancreatic cancer in multiple races.
Keywords: racial variation, type 2 diabetes, obesity, pancreas
INTRODUCTION
Pancreatic cancer is an aggressive malignancy that is often late diagnosed because it is generally asymptomatic at earlier stages.1 Understanding its etiology may improve our ability to identify high-risk patients and enhance earlier detection. While type 2 diabetes has been recognized as a risk factor for pancreatic cancer in epidemiologic studies,2 there is also evidence that diabetes may be a manifestation of the pancreatic tumor. Past studies have shown that there is a higher prevalence of new-onset diabetes among pancreatic cancer cases, and that glucose metabolism improves following tumor resection.3,4 Although still unclear, current literature suggests that adults with recent-onset diabetes have a higher pancreatic cancer risk compared to individuals with long-standing diabetes.2,5,6
The relationship between recent-onset diabetes and pancreatic malignancy has been explored in mainly case-control studies7 or within cohorts of primarily white individuals.8–11 Furthermore, many of the cohort studies consisted solely of diabetes patients and could only report a standardized incidence ratio because they lacked data on a non-diabetes comparison group.9–11 We recently demonstrated that African Americans and Latinos with recent-onset diabetes experienced elevated pancreatic cancer risk compared to those with long-standing diabetes in the Multiethnic Cohort.12 Other than these latest findings, however, research in ethnically diverse populations is quite limited, especially with granular details on metabolic parameters associated with diabetes (e.g. glucose, hemoglobin A1c [HbA1c], and weight).
To further investigate the relationship between diabetes duration and pancreatic cancer in a more representative sample, we conducted a retrospective cohort study of patients from Kaiser Permanente Southern California (KPSC). Our objective was to validate and assess the generalizability of previous findings by evaluating pancreatic cancer risk in a diverse population with comprehensive and longitudinal medical information from electronic health records. In addition, we aimed to establish a metabolic profile of diabetes patients to help characterize those who are at high risk of developing pancreatic cancer.
METHODS
Study Population
We conducted a retrospective cohort study of patients from KPSC, a large integrated health system throughout Southern California. KPSC’s diverse member population of over 4 million individuals is representative of the Southern California region.13 All patient data, including information on hospital visits, diagnosis codes, and laboratory tests, are stored in the electronic health record.
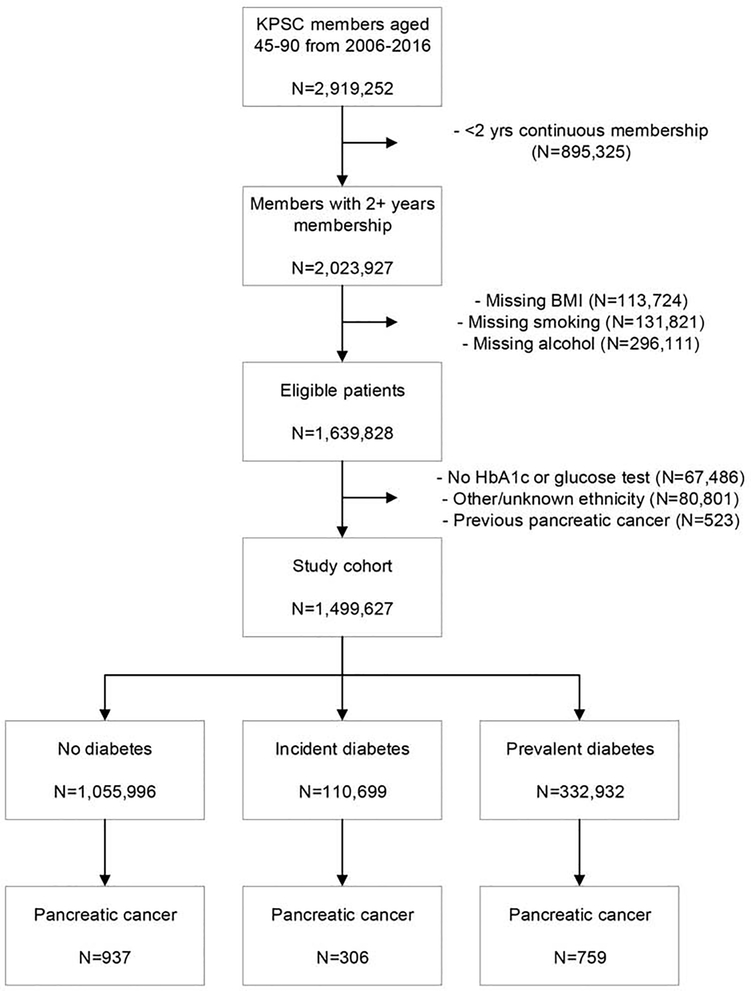
Patients were eligible for the study if they met the following inclusion criteria during 2006–2016: aged 45–90 years, ≥2 years of continuous membership, and available information for body mass index (BMI), smoking, and alcohol use. The two-year membership requirement was used to ensure that individuals were in the KPSC system long enough to establish an accurate medical history. Otherwise eligible patients were further excluded if they did not have a glucose (fasting, random or oral glucose tolerance) or HbA1c measurement (N=67,486), were not in the four major race/ethnicity groups (Asian, non-Hispanic black, Hispanic, non-Hispanic white) (N=80,801), or had a prior diagnosis of pancreatic cancer (N=523) (Figure 1). The date of cohort entry was the January 1st of the first year after the patient met all inclusion criteria (e.g. if a patient met the final inclusion criterion in December 2006, the cohort entry date would be January 1, 2007).
Figure 1:
Flowchart of study cohort
This study was approved by the KPSC Institutional Review Board (IRB).
Exposure Assessment
Individuals were identified as having diabetes if they had any of the following laboratory-based glycemic measurements according to American Diabetes Association (ADA) guidelines: HbA1c ≥6.5%, fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL or two-hour oral glucose tolerance test ≥200 mg/dL. The date of the earliest test within the diabetes range was considered the diabetes diagnosis date. Individuals were classified as prevalent diabetes if their diabetes diagnosis was on or before cohort entry and were categorized as incident diabetes if the diagnosis was after cohort entry. For incident diabetes patients, we evaluated diabetes duration as ≤1 year and >1 year using the time elapsed since diabetes diagnosis.
Assessment of other covariates
Covariate data were obtained from the most recent record prior to cohort entry. BMI was grouped into <25, 25–30, ≥30 kg/m2, smoking was categorized as never, former, and current, and alcohol was treated as a dichotomous variable. Pancreatitis (yes vs. no) was identified using International Classification of Diseases (ICD) codes (ICD-9: 577.0, 577.1; ICD-10: K85.x, K86.0, K86.1) and family history of pancreatic cancer (yes vs. no) was attained from the patient history files. Education information was obtained from the geocoding database13 and was categorized as high school or less, some college, and college graduate.
Outcome
Our primary outcome of interest was time from cohort entry to pancreatic cancer diagnosis. Patients in the final study cohort were censored at the first of the following: death, end of membership or end of study on December 31, 2016. Pancreatic cancer cases were identified from the internal KPSC cancer registry, which reports to the Surveillance, Epidemiology and End Result (SEER) program and meets its high reporting standards.14 Mortality information was obtained from inpatient records, the internal cancer registry and the California state death index.
Statistical Analyses
Baseline characteristics were compared across diabetes status using ANOVA for age and chi-square tests for all other variables. Pancreatic cancer incidence rates, age-standardized to the United States 2000 standard population and truncated to age 45+, were calculated for each group.
The relationship between diabetes and pancreatic cancer incidence was analyzed using Cox proportional hazards regression. Both diabetes status (prevalent, incident vs. none) and duration (incident ≤1 year, incident >1 year, vs. none) were treated as time-varying exposures. All models included age at cohort entry, gender, race/ethnicity, smoking status, BMI, alcohol intake, family history of pancreatic cancer, pancreatitis, and education as covariates.
Additional analyses were conducted within subgroups defined by gender, race/ethnicity, BMI, smoking status, alcohol intake and pancreatitis. Heterogeneity was evaluated using models with an interaction term for the exposure (diabetes status/duration) and the subgroup variable of interest.
Schoenfeld residuals were used to confirm no violation of the proportional hazards assumption, while Martingale and deviance residuals were used to evaluate model fit. All analyses were conducted using SAS 9.3 (Cary, NC).
Metabolic Profiling of Incident Diabetes Patients
To investigate which patients are at highest risk for pancreatic cancer, we evaluated metabolic profiles of incident diabetes patients at different timepoints of diabetes progression. Specifically, we examined fasting glucose, HbA1c and weight measurements from diabetes diagnosis up to three years prior in six-month intervals. For the time period containing the diabetes diagnosis, we included all measurements up to 30 days before and after diagnosis. If a patient had multiple measurements within a given interval, the average of those measurements was used.
Longitudinal changes in these metabolic markers were assessed using generalized estimating equations with the metabolic marker as a continuous outcome, time period as the exposure (to model the trend), age, gender and race as covariates, and patient as the clustering variable. Due to large increases in glucose and HbA1c during the period immediately prior to diabetes, the models for these markers included a spline term at this time interval (6 months to 1 month prior to diabetes) to model the change in trend. All models were run separately for patients with and without pancreatic cancer. Heterogeneity between cases and non-cases was evaluated using models with an interaction term for time period and case status. The models for glucose and HbA1c also included an interaction for the spline term and case status.
Furthermore, we ran all of the aforementioned models within race/ethnicity subgroups to evaluate whether the trends or changes in slope between cases and non-cases varied by race/ethnicity. Overall heterogeneity for the trend was evaluated using a model with two-way interactions for time period by case status, case status by race, and time period by race, and a three-way interaction for time period, case status and race. For glucose and HbA1c, we examined overall heterogeneity for the change in slope using two-way interactions for the spline term by case status, case status by race, and spline term by race, and a three-way interaction for the spline term, case status and race. The p-values for the three-way interactions were used to determine whether there was significant heterogeneity in the trend or change in slope by both case status and race/ethnicity.
Sensitivity Analyses
To assess the accuracy of the identification of incident diabetes, we re-ran all analyses dropping incident diabetes patients who did not have a prior metabolic test in the normal range (3.5%) or had a prescription for metformin within the past year (7.7%). In order to address the potential effects of diabetes treatment, we also re-ran the metabolic profile analyses dropping metabolic measurements taken within the 30 days after diabetes diagnosis. We also repeated the analyses using patient-day-weighted averages to account for multiple measurements in a given day. Given the ADA’s recommendation changes in 2010 to use HbA1c for diabetes screening15, we re-ran the metabolic analyses stratified by patients who were diagnosed with diabetes before (16.1%) and after (84.9%) 2010 to address potential differences in glycemic test ordering. Results were unchanged in all situations and thus we only present findings from the original models.
RESULTS
The final study cohort consisted of 1,499,627 patients at-risk for pancreatic cancer. The study population was on average 57.9 years old (standard deviation 10.9) and consisted of 55.2% females. Whites comprised nearly half of the cohort (45.7%), followed by Hispanics (32.5%), Asians (11.1%) and blacks (10.7%). From nearly 7.5 million person-years of follow-up, 2,002 persons developed pancreatic cancer, translating to an age-adjusted incidence rate of 30.7 cases per 100,000 person-years.
Among the entire cohort, 110,699 developed incident diabetes (7.4%), 332,932 had prevalent diabetes (22.2%) and 1,055,996 never had diabetes (70.4%). Age-adjusted incidence rates for pancreatic cancer were highest in incident diabetes patients (43.7 cases per 100,000 person-years), followed by prevalent diabetes patients (41.2 cases per 100,000 person-years) and lowest in non-diabetes patients (23.8 cases per 100,000 person-years). Prevalent and incident diabetes patients were more likely to be older, male, non-white, former smokers, and non-alcohol users. These individuals also tended to have higher BMI, lower education, and pancreatitis (Table 1). Within the race/ethnicity subgroups, blacks (26.5%), Hispanics (25.2%) and Asians (24.6%) had the highest proportion of prevalent diabetes while blacks (9.2%) and Asians (8.9%) had the greatest proportion of incident diabetes (Supplemental table 1).
Table 1:
Baseline characteristics of study cohort, by diabetes status
| Total (N=1,499,627) | No diabetes (N=1,055,996) | Incident diabetes (N=110,699) | Prevalent diabetes (N=332,932) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % |
| Pancreatic cancer cases | 2,002 | 0.1 | 937 | 0.1 | 306 | 0.3 | 759 | 0.2 |
| Person-years, total | 7,462,072.5 | 5,060,344.5 | 726,055.0 | 1,675,673.1 | ||||
| Follow-up time (years), mean (SD) | 5.0 (3.0) | 4.8 (2.9) | 6.6 (2.5) | 5.0 (3.0) | ||||
| Time to pancreatic cancer (years), mean (SD) | 3.8 (2.5) | 3.6 (2.5) | 4.3 (2.4) | 3.7 (2.5) | ||||
| Incidence rate, age-adjusted1 | 30.7 | 23.8 | 43.7 | 41.2 | ||||
| Age, mean (SD) | 57.9 (10.9) | 56.5 (10.5) | 59.5 (10.8) | 61.8 (11.2) | ||||
| Age group | ||||||||
| 45–50 | 433,225 | 28.9 | 353,854 | 33.5 | 23,417 | 21.2 | 55,954 | 16.8 |
| 50–54 | 249,754 | 16.7 | 187,661 | 17.8 | 19,124 | 17.3 | 42,969 | 12.9 |
| 55–59 | 227,267 | 15.2 | 158,721 | 15.0 | 18,244 | 16.5 | 50,302 | 15.1 |
| 60–64 | 191,088 | 12.7 | 125,114 | 11.9 | 15,673 | 14.2 | 50,301 | 15.1 |
| 65–69 | 153,534 | 10.2 | 93,519 | 8.9 | 12,945 | 11.7 | 47,070 | 14.1 |
| ≥70 | 244,759 | 16.3 | 137,127 | 13.0 | 21,296 | 19.2 | 86,336 | 25.9 |
| Gender | ||||||||
| Female | 827,460 | 55.2 | 608,694 | 57.6 | 59,145 | 53.4 | 159,621 | 47.9 |
| Male | 672,167 | 44.8 | 447,302 | 42.4 | 51,554 | 46.6 | 173,311 | 52.1 |
| Race/ethnicity | ||||||||
| Asian | 166,395 | 11.1 | 110,579 | 10.5 | 14,836 | 13.4 | 40,980 | 12.3 |
| Black | 160,228 | 10.7 | 103,112 | 9.8 | 14,730 | 13.3 | 42,386 | 12.7 |
| Hispanic | 487,185 | 32.5 | 327,726 | 31.0 | 36,892 | 33.3 | 122,567 | 36.8 |
| White | 685,819 | 45.7 | 514,579 | 48.7 | 44,241 | 40.0 | 126,999 | 38.2 |
| BMI kg/m2, mean (SD) | 29.2 (6.2) | 28.3 (5.7) | 31.2 (6.5) | 31.2 (6.8) | ||||
| BMI kg/m2 category | ||||||||
| <25 | 379,339 | 25.3 | 308,552 | 29.2 | 16,887 | 15.3 | 53,900 | 16.2 |
| 25–30 | 555,925 | 37.1 | 410,837 | 38.9 | 36,280 | 32.8 | 108,808 | 32.7 |
| ≥30 | 564,363 | 37.6 | 336,607 | 31.9 | 57,532 | 52.0 | 170,224 | 51.1 |
| Smoking status | ||||||||
| Never | 967,251 | 64.5 | 703,859 | 66.7 | 67,582 | 61.1 | 195,810 | 58.8 |
| Past | 392,214 | 26.2 | 253,045 | 24.0 | 30,909 | 27.9 | 108,260 | 32.5 |
| Current | 140,162 | 9.4 | 99,092 | 9.4 | 12,208 | 11.0 | 28,862 | 8.7 |
| Alcohol user | ||||||||
| No | 864,634 | 57.7 | 570,286 | 54.0 | 67,683 | 61.1 | 226,665 | 68.1 |
| Yes | 634,993 | 42.3 | 485,710 | 46.0 | 43,016 | 38.9 | 106,267 | 31.9 |
| Education2 | ||||||||
| High school or less | 586,579 | 39.1 | 388,368 | 36.8 | 47,785 | 43.2 | 150,426 | 45.2 |
| Some college | 523,205 | 34.9 | 370,140 | 35.1 | 40,980 | 37.0 | 112,085 | 33.7 |
| College graduate | 318,594 | 21.2 | 243,112 | 23.0 | 19,828 | 17.9 | 55,654 | 16.7 |
| Missing | 71,249 | 4.8 | 54,376 | 5.2 | 2,106 | 1.9 | 14,767 | 4.4 |
| Family history of pancreatic cancer | 16,066 | 1.1 | 12,068 | 1.1 | 1,063 | 1.0 | 2,935 | 0.9 |
| Pancreatitis | 18,418 | 1.2 | 8,510 | 0.8 | 1,403 | 1.3 | 8,505 | 2.6 |
Incidence rate per 100,000 person-years, age-standardized to US Census 2000 standard population, truncated to ages 45+.
Based on population geocoding files and not specific to individual patient.
All tests comparing distribution of risk factors across diabetes status had p-values <0.0001.
Pancreatic cancer risk was elevated three times for incident diabetes (relative risk [RR] 3.17, 95% CI 2.75–3.65) and roughly two times for prevalent diabetes (RR 1.85, 95% CI 1.67–2.05) compared to individuals without diabetes. When evaluating diabetes duration, patients with incident diabetes for ≤1 year and >1 year had almost seven times (RR 6.91, 95% CI 5.76–8.30) and two times (RR 1.96, 95% CI 1.62–2.38) the risk of pancreatic cancer, respectively, compared to individuals without diabetes. Similar patterns of associations for diabetes status/duration were present across all race/ethnicity subpopulations (Table 2), as well as within subgroups of gender, BMI, smoking status, and alcohol intake (Supplemental tables 2 & 3).
Table 2:
Association between diabetes status and duration and pancreatic cancer, among the entire cohort and within race/ethnicity subgroups
| Total (N=1,499,627) | Asian (N=166,395) | Black (N=160,228) | Hispanic (N=487,185) | White (N=685,819) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | N Cases | Person-years | RR (95% CI)2 | N Cases | Person-years | RR (95% CI)2 | N Cases | Person-years | RR (95% CI)2 | N Cases | Person-years | RR (95% CI)2 | N Cases | Person-years | RR (95% CI)2 | p3 |
| Diabetes status1 | 0.25 | |||||||||||||||
| None | 937 | 5,060,344.5 | 1 (ref) | 67 | 519,170.1 | 1 (ref) | 117 | 528,713.0 | 1 (ref) | 166 | 1,439,741.3 | 1 (ref) | 587 | 2,572,720.0 | 1 (ref) | |
| Incident | 306 | 726,055.0 | 3.17 (2.75–3.65) | 34 | 98,859.0 | 3.21 (2.04–5.03) | 37 | 100,617.6 | 2.82 (1.91–4.14) | 82 | 235,261.2 | 4.12 (3.10–5.47) | 153 | 291,317.3 | 2.90 (2.37–3.53) | |
| Prevalent | 759 | 1,675,673.1 | 1.85 (1.67–2.05) | 73 | 211,612.1 | 1.72 (1.22–2.43) | 116 | 230,597.3 | 1.74 (1.33–2.28) | 213 | 579,355.2 | 2.19 (1.77–2.71) | 357 | 654,108.5 | 1.77 (1.54–2.03) | |
| Diabetes duration1 | 0.09 | |||||||||||||||
| None | 937 | 5,060,344.5 | 1 (ref) | 67 | 519,170.1 | 1 (ref) | 117 | 528,713.0 | 1 (ref) | 166 | 1,439,741.3 | 1 (ref) | 587 | 2,572,720.0 | 1 (ref) | |
| Incident ≤1 yr | 175 | 102,711.5 | 6.91 (5.76–8.30) | 19 | 13,729.9 | 7.48 (4.17–13.41) | 20 | 13,540.9 | 6.01 (3.68–9.82) | 38 | 34,371.0 | 7.50 (5.09–11.06) | 98 | 41,069.8 | 6.89 (5.37–8.83) | |
| Incident >1 yr | 131 | 623,343.4 | 1.96 (1.62–2.38) | 15 | 85,129.1 | 2.25 (1.24–4.10) | 17 | 87,076.7 | 1.65 (0.97–2.80) | 44 | 200,890.2 | 2.73 (1.91–3.89) | 55 | 250,247.5 | 1.65 (1.24–2.19) | |
Incident diabetes status and duration treated as time-varying exposures.
From a Cox proportional hazards regression with age at cohort entry, gender, race, smoking, BMI, alcohol, pancreatitis, family history of pancreatic cancer and education as covariates.
P-values for heterogeneity across race/ethnicity.
Metabolic Profiling of Incident Diabetes Patients
Among the 110,699 incident diabetes patients, 306 individuals were diagnosed with pancreatic cancer, with an average time of 1.5 years (standard deviation 1.8 years) from diabetes to pancreatic cancer diagnosis. Approximately 75% of patients had glucose measurements at diabetes diagnosis and 25–38% had measurements in the preceding time intervals. For HbA1c, 68% had measurements around diabetes diagnosis and 10–14% had measurements in the periods before. For weight, around 90% had measurements at diabetes diagnosis and 65–80% had measurements in the prior intervals.
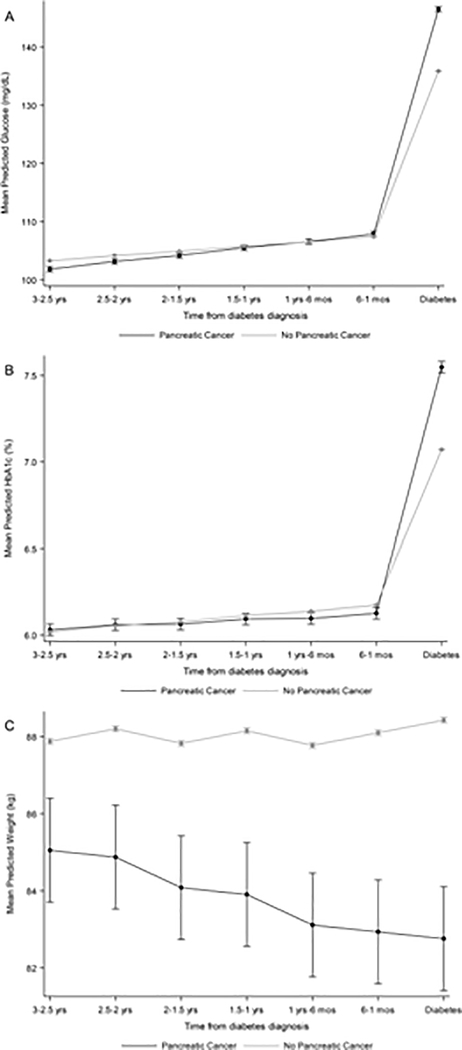
When evaluating changes in metabolic markers over time (Table 3), cases and non-cases had similar values and minor increases in fasting glucose in the three years to one month prior to diabetes diagnosis (cases: 1.19 mg/dL/interval; non-cases: 0.81 mg/dL/interval; p-heterogeneity=0.06). However, the change in slope immediately before diabetes diagnosis was much higher for cases compared to non-cases (cases: 37.47 mg/dL; non-cases: 27.68 mg/dL; p-heterogeneity<0.01; Figure 2a). This pattern was similar for HbA1c, where cases and non-cases had similar values and trends up to one month prior (cases: 0.03%/interval; non-cases: 0.04%/interval; p-heterogeneity=0.38), but cases had a much larger change in slope compared to non-cases during the interval right before diabetes (cases: 1.39%; non-cases: 0.86%; p-heterogeneity<0.001; Figure 2b). For weight, cases had a non-significant negative trend, while non-cases had a significant increasing trend over time (cases: −0.18 kg/interval; non-cases: 0.33 kg/interval; p-heterogeneity<0.001; Figure 2c). In contrast to glucose and HbA1c, differences in weight between cases and non-cases were observed starting from three years prior to diabetes (Figure 2).
Table 3:
Predicted trends for longitudinal measurements of fasting glucose, HbA1c and weight among patients with incident diabetes, by case status and race/ethnicity
| Total (N=110,699) | Asian (N=14,836) | Black (N=14,730) | Hispanic (N=36,892) | White (N=44,241) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic Cancer | No Pancreatic Cancer | Pancreatic Cancer | No Pancreatic Cancer | Pancreatic Cancer | No Pancreatic Cancer | Pancreatic Cancer | No Pancreatic Cancer | Pancreatic Cancer | No Pancreatic Cancer | |||||||
| Parameter1 | Estimate (CI)2 | Estimate (CI)2 | p3 | Estimate (CI)2 | Estimate (CI)2 | p3 | Estimate (CI)2 | Estimate (CI)2 | p3 | Estimate (CI)2 | Estimate (CI)2 | p3 | Estimate (CI)2 | Estimate (CI)2 | p3 | p4 |
| Glucose (mg/dL) | ||||||||||||||||
| Slope 3 yrs-1 mo | 1.19 (0.71–1.67) | 0.81 (0.78–0.84) | 0.06 | 1.70 (0.50–2.90) | 0.91 (0.84–0.97) | 0.27 | −0.20 (−1.55–1.16) | 0.88 (0.79–0.97) | 0.14 | 1.38 (0.34–2.43) | 1.01 (0.95–1.06) | 0.45 | 1.62 (0.90–2.33) | 0.80 (0.75–0.84) | 0.03 | 0.16 |
| Change in slope at 1 mo | 37.47 (31.51–43.43) | 27.68 (27.38–27.97) | <0.01 | 34.49 (18.57–50.42) | 18.98 (18.32–19.63) | 0.07 | 44.65 (24.36–64.95) | 27.46 (26.57–28.36) | 0.11 | 30.94 (20.87–41.01) | 29.99 (29.42–30.57) | 0.85 | 38.98 (30.53–47.43) | 28.72 (28.29–29.14) | 0.02 | 0.28 |
| HbA1c (%) | ||||||||||||||||
| Slope 3 yrs-1 mo | 0.03 (0.00–0.06) | 0.04 (0.04–0.04) | 0.38 | 0.03 (−0.12–0.19) | 0.03 (0.03–0.04) | 0.05 | 0.01 (−0.05–0.08) | 0.04 (0.03–0.04) | 0.41 | 0.01 (−0.05–0.07) | 0.04 (0.04–0.04) | 0.24 | 0.04 (0.01–0.08) | 0.04 (0.03–0.04) | 0.84 | 0.31 |
| Change in slope at 1 mo | 1.39 (1.12–1.67) | 0.86 (0.85–0.87) | <0.001 | 0.92 (0.43–1.41) | 0.62 (0.59–0.64) | 0.09 | 1.37 (0.74–2.00) | 0.93 (0.90–0.97) | 0.20 | 1.27 (0.81–1.74) | 1.01 (0.99–1.04) | 0.15 | 1.48 (1.05–1.91) | 0.79 (0.78–0.81) | <0.01 | 0.72 |
| Weight (kg) | ||||||||||||||||
| Slope 3 yrs-diagnosis | −0.18 (−0.44–0.08) | 0.33 (0.31–0.34) | <0.001 | −0.19 (−0.49–0.11) | 0.23 (0.20–0.26) | <0.01 | 0.24 (−0.39–0.87) | 0.40 (0.36–0.44) | 0.67 | −0.30 (−0.70–0.09) | 0.31 (0.28–0.33) | <0.01 | −0.16 (−0.61–0.28) | 0.35 (0.32–0.37) | 0.03 | 0.65 |
Metabolic markers were evaluated from diabetes diagnosis up to three years prior in six-month intervals.
From a GEE model with the metabolic characteristic as a continuous outcome variable (identity link), time period as the main exposure, age, gender and race as covariates, and patient as the clustering variable. Glucose and HbAlc models included a spline term at the 6–1 month time interval to model the change in slope. Models were run separately for cases and non-cases.
P-value for heterogeneity across cases and non-cases. Obtained from a GEE model with a time period*case status interaction term. Glucose and HbAlc models included an additional interaction term for change in slope*case status.
P-value for heterogeneity across cases status and race. Glucose and HbA1c models included an additional three-way interaction for change in slope*case status*race. Glucose and HbAlc models also included three-way interaction for change in slope.
Figure 2:
Predicted mean values of (a) fasting glucose, (b) HbA1c and (c) weight during the three years prior to diabetes, across pancreatic cancer status
Though there was no overall significant heterogeneity by race/ethnicity, patterns in the trends appeared to be stronger for specific subgroups (Table 3). For glucose, the difference between cases and non-cases for the change in slope was greatest among blacks and Asians, while there was hardly any difference among Hispanics (Supplemental figure 1). For HbA1c, whites and blacks had the largest differences between cases and non-cases for the change in slope (Supplemental figure 2). For weight, the negative trend for cases was strongest among Hispanics (Supplemental figure 3).
DISCUSSION
In this study, we evaluated the influence of diabetes status, diabetes duration, and metabolic profiles on pancreatic cancer risk within a large and diverse population-based cohort. We observed that patients with incident and prevalent diabetes had three and two times the risk, respectively, of pancreatic cancer compared with patients without diabetes. In addition, this risk was elevated nearly seven times for incident diabetes patients with ≤1 year of disease duration. Among incident diabetes patients, those who developed pancreatic cancer had greater increases in fasting glucose, HbA1c and weight loss in the time leading up to diabetes. The longitudinal changes in metabolic characteristics between cases and non-cases also appeared to be more pronounced for specific race/ethnicity groups.
Prior epidemiologic studies have found that diabetes is associated with a two times higher risk of pancreatic cancer, with higher risks observed for those with shorter disease durations. 2,6 Our main findings are generally consistent with past research, as both incident and prevalent diabetes patients had around a two to three times greater risk compared to non-diabetes patients. The elevated risk among incident diabetes patients was driven primarily by the seven-fold increased risk for those who had diabetes for ≤1 year, while those with longer disease durations had risks resembling that of prevalent diabetes patients. These results add support for the hypothesis that pancreatic cancer patients with diabetes for shorter periods may actually have a distinct form of diabetes that is induced by the pancreatic tumor.3,5
Importantly, we observed that these patterns of association were similar across race/ethnicity. Most of the prior literature on diabetes duration has been focused on white populations and only a sparse number of studies have examined this relationship among minorities.12,16,17 Despite this, our results are congruent with previous research showing that shorter durations of diabetes are also associated with higher pancreatic cancer risk among non-whites. To our knowledge, this is the first population-based cohort study to evaluate multiple race/ethnicity groups with sufficient power to detect a range of associations.
In our metabolic profiling of incident diabetes patients, we found that pancreatic cancer cases and non-cases had similar levels of fasting glucose and HbA1c in the time before diabetes, but cases had much higher increases in these glycemic markers in the final month preceding diabetes. Conversely, cases consistently had lower weight compared to non-cases, and both groups had subtle, yet opposite, trends in weight measurements leading up to diabetes. Interestingly, differences in weight between cases and non-cases predated the time when the glycemic markers started to differ across case status. Though the degree of weight loss across the two groups was minor, our results indicate that weight changes may occur earlier in the pathogenesis of pancreatic cancer.
These metabolic changes have been associated with pancreatic cancer in past studies of new-onset diabetes18–20 and have been identified as significant parameters in recent risk prediction models. 21,22 In particular, the Enriching New-onset Diabetes for Pancreatic Cancer model from the Mayo Clinic reported that increases in blood glucose and weight loss by diabetes diagnosis were predictive markers of pancreatic cancer risk.22 Additionally, the model developed in The Health Improvement Network in the United Kingdom found that higher levels of HbA1c and larger decreases in BMI at diabetes diagnosis were also significant predictors of pancreatic malignancy.21 As these previous studies were all conducted in mainly white populations, our study is the first to show that these metabolic changes can also be used to determine risk in heterogeneous populations with different lifestyles and genetic susceptibilities. The general consistency in findings across multiple racial/ethnic groups suggests that prediction models developed in predominantly white individuals21,22 would apply broadly to minority populations in the United States.
Our results may have important clinical implications for the early detection of pancreatic cancer among new-onset diabetes patients. Understanding these metabolic trends across cases and non-cases can help guide the development of prediction algorithms that utilize prior routine measurements of weight and glycemic markers. These models can also incorporate race/ethnicity-specific trends in their risk calculations, as some patterns seemed to be stronger in certain race/ethnicity subgroups. For instance, based on our findings, the formula for blacks and whites could have greater weight on HbA1c, while the model for Hispanics could have a larger effect from changes in adiposity. Finally, there appears to be a potential critical window for improved screening and intervention, given that the average time between diabetes and pancreatic cancer in our cohort was 1.5 years. When evaluating the 306 incident diabetes patients with pancreatic cancer, we observed that trends in metabolic markers began to shift around 1.5–2 years prior to pancreatic cancer diagnosis, roughly corresponding with the onset of diabetes (Supplemental table 4/Supplemental figure 4).
The major strengths of this study include the diverse patient population and the largest number of pancreatic cancer cases among all past cohort studies, which make our findings more robust and generalizable. Establishing a cohort from members of an integrated health system further assured that individuals had similar healthcare access and were less likely to be lost to follow-up. We also had more reliable and complete data by using the prospectively collected electronic health records. In particular, using glucose and HbA1c tests for both exclusion criteria and case ascertainment controlled for healthcare utilization and improved the accuracy of identifying diabetes patients, respectively. Though this excluded a large subset of patients, this was done to maximize the specificity of diabetes classification and to ensure that we could properly evaluate their glycemic profiles. Of note, we had considerable concordance with diabetes identified by ICD code (73%), and only <0.5% of eligible patients with diabetes ICD codes were missing glycemic measurements. Finally, this is the first study to evaluate longitudinal trends in metabolic markers across multiple race/ethnicity groups.
However, only a proportion of patients had available metabolic measurements in each time interval, so our findings for metabolic changes are based on a subset of patients that have more clinic visits. This could include a spectrum of patients from healthy individuals compliant with annual physicals to individuals with greater disease burden that necessitates more frequent encounters. The smaller subset appeared to limit the power in the race/ethnicity subgroup analyses, as the differences between cases and non-cases in the change in slope for glucose and HbA1c were not significant among non-white subgroups. However, these results became significant after combining the data for all non-white patients together. Furthermore, many patients were excluded due to missing data on BMI, smoking and alcohol. These were primarily patients whose memberships ended during the early years of the study period when the electronic health record system was being implemented in KPSC, so information on these risk factors was not available and could not be imputed. Lastly, we did not have much information on systemic insulin levels, another potential important metabolic biomarker,23 as this lab test is not frequently ordered by KPSC providers.
In this population-based cohort study, we illustrate that the elevated pancreatic cancer risk associated with new-onset diabetes is also present among multiple racial/ethnic minorities. Additionally, the metabolic profiles of these incident diabetes patients appear to have distinct patterns for certain race/ethnicity groups, suggesting that some biomarkers could have more clinical relevance or utility for specific populations. These findings offer more insight into the complex diabetes-pancreatic cancer relationship and may help guide decisions towards tailoring prevention techniques and methods for identifying patients most appropriate for screening.
Supplementary Material
Need to Know.
Background
Observational studies of predominantly white populations have found new-onset diabetes to be associated with increased risk of pancreatic cancer.
Findings
In a study of a large ethnically diverse population, we found risk of pancreatic cancer to be increased among patients with a diagnosis of diabetes in the past year among different races and ethnicities. Weight loss and poor glycemic control were associated with increased risk of pancreatic cancer in multiple races.
Implications for patient care
Patients with a recent diagnosis of diabetes should be evaluated for pancreatic cancer.
ACKNOWLEDGEMENTS
V. Wendy Setiawan is supported by the National Cancer Institute at the National Institutes of Health (RO1CA209798) and an American Cancer Society Research Scholar Grant (RSG-16-250-01-CPHPS). Brian Z. Huang is supported by T32 Training Grants from the National Cancer Institute at the National Institutes of Health (T32CA009142, T32CA229110). Research reported in this publication was supported in part by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health as an ancillary study of the Consortium for the Study of Chronic Pancreatitis and Pancreatic Cancer (CPDPC) (1U01DK108328-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant support:
V. Wendy Setiawan is supported by the National Cancer Institute at the National Institutes of Health (RO1CA209798) and an American Cancer Society Research Scholar Grant (RSG-16-250-01-CPHPS). Brian Z. Huang is supported by T32 Training Grants from the National Cancer Institute at the National Institutes of Health (T32CA009142, T32CA229110). Research reported in this publication was supported in part by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health as an ancillary study of the Consortium for the Study of Chronic Pancreatitis and Pancreatic Cancer (CPDPC) (1U01DK108328-01).
Abbreviations
- ADA
American Diabetes Association
- HbA1c
Hemoglobin A1c
- ICD
International Classification of Diseases
- KPSC
Kaiser Permanente Southern California
- SEER
Surveillance, Epidemiology and End Result
Footnotes
Disclosures: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 2015;26 Suppl 5:v56–68. [DOI] [PubMed] [Google Scholar]
- 2.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011;47:1928–1937. [DOI] [PubMed] [Google Scholar]
- 3.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen DK, Korc M, Petersen GM, et al. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017;66:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Cao G, Ma Q, et al. The bidirectional interation between pancreatic cancer and diabetes. World J Surg Oncol 2012;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J, You Y, Guo F, et al. Association of elevated risk of pancreatic cancer in diabetic patients: A systematic review and meta-analysis. Oncol Lett 2017;13:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol 2006;4:1366–1372; quiz 1301. [DOI] [PubMed] [Google Scholar]
- 9.Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control 1991;2:307–314. [DOI] [PubMed] [Google Scholar]
- 10.Chow WH, Gridley G, Nyren O, et al. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J Natl Cancer Inst 1995;87:930–931. [DOI] [PubMed] [Google Scholar]
- 11.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 1997;89:1360–1365. [DOI] [PubMed] [Google Scholar]
- 12.Setiawan VW, Stram DO, Porcel J, et al. Pancreatic Cancer Following Incident Diabetes in African Americans and Latinos: The Multiethnic Cohort. J Natl Cancer Inst June 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornbrook MC, Hart G, Ellis JL, et al. Building a virtual cancer research organization. J Natl Cancer Inst Monogr 2005:12–25. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Standards of medical care in diabetes−−2010. Diabetes Care 2010;33 Suppl 1:S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev 2006;15:1458–1463. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Tang H, Hassan MM, et al. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control 2011;22:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart PA, Kamada P, Rabe KG, et al. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas 2011;40:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Garcia Rodriguez LA, Malgerud L, et al. New-onset type 2 diabetes, elevated HbA1c, anti-diabetic medications, and risk of pancreatic cancer. Br J Cancer 2015;113:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munigala S, Singh A, Gelrud A, et al. Predictors for Pancreatic Cancer Diagnosis Following New-Onset Diabetes Mellitus. Clin Transl Gastroenterol 2015;6:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boursi B, Finkelman B, Giantonio BJ, et al. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients With New-Onset Diabetes. Gastroenterology 2017;152:840–850.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018;155:730–739.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872–2878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.