Abstract
In the Look AHEAD trial, randomization to Intensive Lifestyle Intervention (ILI) or Diabetes Support and Education (DSE) did not result in differences in cognitive outcomes. However, menopause and APOE genotype are factors that affect the response to this intervention. The effect of this intervention on a single cognitive assessment was examined in 3 groups of women: premenopausal or <5 years postmenopausal (N=594), within 5–10 years (n=388), and ≥10 years postmenopausal (n=963), and as a function of continuous years since menopause. The late postmenopausal group in the ILI had worse composite z-scores compared to those in the DSE, whereas the younger pre- or early postmenopausal women in the ILI had better composite z-scores than the DSE. A significant interaction between years since menopause and intervention arm, but not baseline age, was observed on executive function domains. ILI appeared only to benefit cognitive function among non-APOE4 carriers during pre- or early post menopause. These findings emphasize the importance of assessing menopause and APOE status to understand how weight loss impacts cognition.
Keywords: lifestyle intervention, menopause, cognition
INTRODUCTION
Mid-life obesity and type 2 diabetes (T2DM) increase the risk of Alzheimer’s disease (AD)(Anstey et al., 2011). However, it is not clear that obesity in late-life is associated with increased dementia risk. To the contrary, some epidemiology studies suggest that obesity may be protective against cognitive impairment and dementia in older adults (Bagger et al., 2004; Fitzpatrick et al., 2009). Moreover, unintentional weight loss in older individuals is associated with increased dementia incidence (LeBlanc et al., 2017).
Look AHEAD is a clinical trial that randomly assigned overweight or obese individuals with T2DM and cardiovascular disease (CVD) risk factors to an average of 9.8 years of Intensive Lifestyle Intervention (ILI) or Diabetes Support and Education (DSE). Participants entering the trial were aged 45 to 76. The mean length of intervention for ILI and DSE participants was 9.8 years. The timeline of LookAHEAD is illustrated in Figure 1. Cognition was assessed in the full cohort once: 10 to 13 years after enrollment (1 to 2 years after intervention cessation) in 3,751 participants (61% women). At this time, across both sexes, there were no overall differences between the intervention groups with respect to performance on a standard battery of cognitive function tests. However, based on pre-specified subgroup analyses, there was evidence that the intervention was beneficial for individuals who at baseline were free of CVD and not obese, but potentially harmful to those with Class III obesity or CVD (Espeland et al., 2017; Espeland et al., 2014; Rapp et al., 2017).
Figure 1:
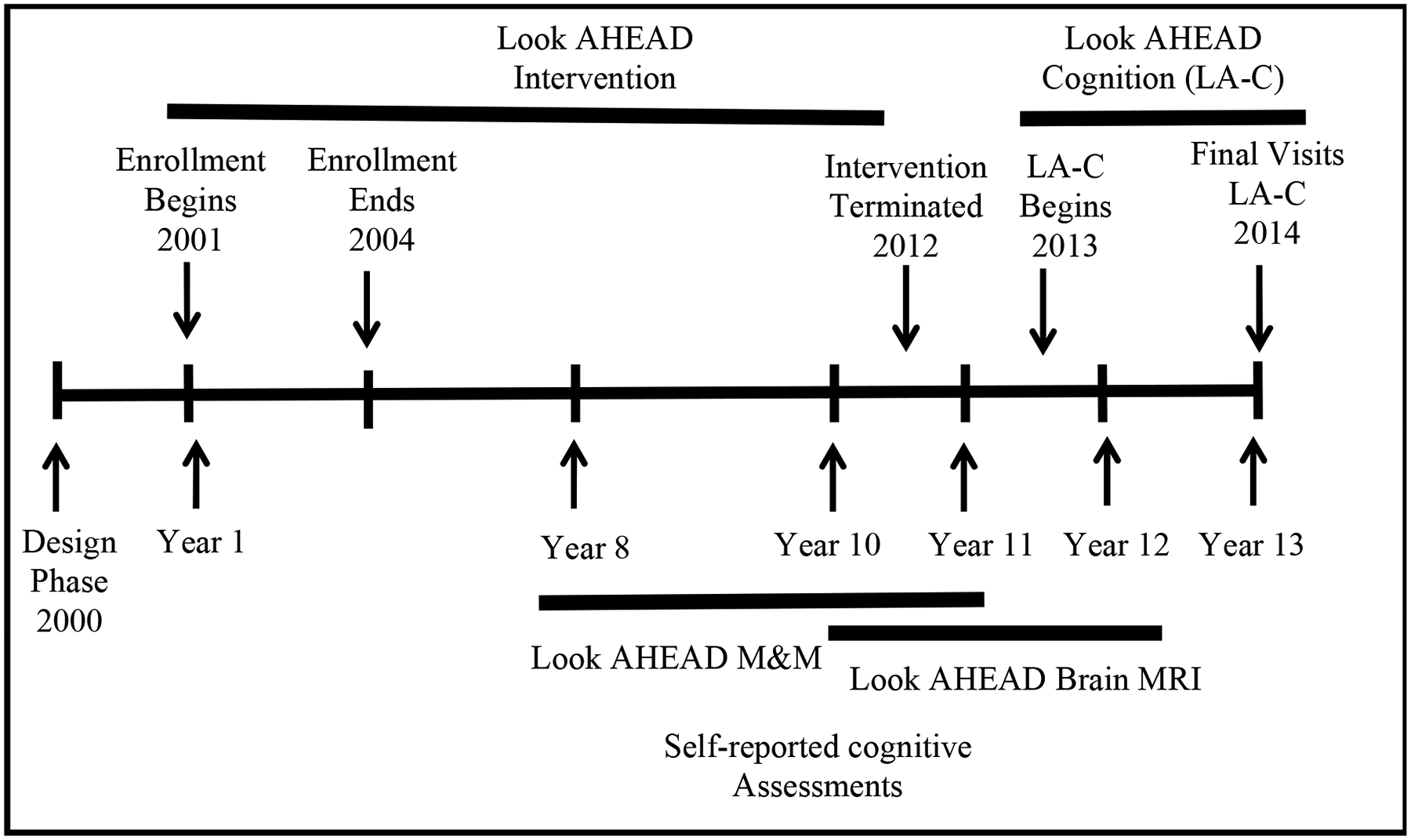
Look AHEAD study timeline
In women, AD dementia risk appears to be influenced by menopause, hormone replacement therapy (HRT), and APOE genotype. For example, brain glucose uptake is lower in older postmenopausal women compared to peri or premenopausal younger women(Mosconi et al., 2017) and in APOE4 carriers compared to non-carriers (Reiman et al., 2004). A recent meta-analysis found that women carrying an APOE4 allele had an increased risk of AD compared with men between the ages of 65 and 75 years (Neu et al., 2017). The majority of randomized clinical trials of HRT in women near the time of menopause have found no cognitive benefits(Espeland et al., 2013; Gleason et al., 2015; Henderson et al., 2016) and trials in older women (> 65 years) have reported harm (Espeland et al., 2004; Rapp et al., 2003). Larger physiological changes in metabolism and vascular health are observed in women who are more than 10 years post menopause compared to premenopausal or early post-menopausal (<6 years) women. This was exemplified in the differential vascular effects of HRT by menopausal groups. Oral estradiol therapy was associated with less progression of subclinical atherosclerosis than was placebo when therapy was initiated less than 6 years after menopause, but not when it was initiated 10 or more years after menopause (Hodis et al., 2016). Therefore, weight loss and T2DM interventions assessing cognitive outcomes should take into account the effect of time since menopause, APOE genotype, and HRT for providing cognitive benefits.
Preclinical studies identify the perimenopause to late menopause transition, a neuroendocrine state unique to the female, as a sex-specific risk factor for AD(Mosconi et al., 2017). In the female brain, estrogen activates glucose metabolism while simultaneously suppressing the ketogenic system thereby promoting brain reliance on glucose as its primary fuel to generate ATP (Yin et al., 2015). Both menopause and APOE4 associate with a shift in the preference for brain energy sources, from glucose to oxidation of fatty acids for brain energy utilization (Chouinard-Watkins and Plourde, 2014; Riedel et al., 2016). Therefore, weight loss in older postmenopausal women and in APOE4 carriers may deprive the brain of an important source of fuel: fat stored and released from adipocytes.
We hypothesized that randomization to the ILI would have beneficial effects on cognitive outcomes in women enrolled during the pre- and early postmenopausal women phase, and harmful when participants enter the trial in late post menopause. We also hypothesized that APOE genotype, HRT and baseline obesity modulated this interaction, with APOE4 allele and obesity attenuating any benefit from this intervention, whereas HRT would be associated with better cognitive outcomes.
METHODS
The design and methods of the Look AHEAD trial have been published previously (Ryan et al., 2003), as has its CONSORT diagram (2013). In brief, Look AHEAD was a single-blinded, randomized, controlled trial that recruited 5,145 individuals (during 2001 to 2004) who were overweight or obese and had T2DM and CVD risk factors. At enrollment, participants were 45–76 years of age and had a body mass index (BMI) >25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) <11%, systolic/diastolic blood pressure <160/<100 mmHg, and triglycerides <600 mg/dl. Local Institutional Review Boards approved protocols. All participants provided written informed consent.
Interventions
The ILI included diet modification and physical activity aimed at inducing and maintaining a weight loss of ≥7% of baseline weight (Wadden et al., 2006). ILI participants were assigned a daily calorie goal (1200–1800 kcal based on initial weight), with <30% of total calories from fat (<10% from saturated fat) and a minimum of 15% of total calories from protein. The physical activity goal was ≥175 minutes/week of activities similar in intensity to brisk walking. ILI participants met with trained staff weekly for the first 6 months and 3 times per month for the next 6 months, with a combination of group and individual contacts. During years 2–4, participants met with staff individually at least once a month, were contacted a second time each month by phone or e-mail and were offered a variety of centrally-approved group classes. Afterwards, ILI participants were encouraged to continue individual monthly sessions, and annual campaigns were used to promote maintenance of weight loss.
DSE participants were invited to attend three group sessions each year. These included standardized protocols on diet, physical activity, and social support (Wesche-Thobaben, 2011), with no specific diet, activity, or weight goals. Participants did not receive information on behavioral strategies for achieving adherence and were not weighed. Interventions were terminated September 2012. The mean [range] lengths of intervention for both ILI and DSE participants included in the analyses for this manuscript were 9.8 [8.4,11.1] years.
Menopause Status and Postmenopausal Hormone Therapy
At Look AHEAD enrollment, women were queried whether they had gone through menopause, either naturally or by surgery, and if so, their age when this had occurred during their follow-up visits. They were also queried about past prescription HRT use and all current prescriptions were reviewed.
Cognitive Function
Centrally trained and certified staff conducted standardized assessments of cognitive function in the full Look AHEAD cohort between August 2013 and December 2014. These took place 10–13 years after enrollment and form the basis of the results that we report. A subset of individuals had one or two earlier assessments as participants in the Look AHEAD Movement and Memory Study (4 clinics: years 8–11) and the Look AHEAD Brain MRI study (3 clinics: years 10–12).
The cognitive battery assessed several key cognitive functions that have previously been demonstrated to be related to obesity. Per protocol, the composite cognitive function score is the primary cognitive outcome from Look AHEAD (Espeland et al., 2014; Rapp et al., 2017). Tests (functions) included Trail Making Test-Parts A and B (TMT-A, TMT-B)(Reitan, 1958) (attention, executive function), Modified Stroop Color and Word Test (SCWT)(Houx et al., 1993; Stroop, 1935) (executive function), Digit Symbol-Coding Test (DSST) (Wechsler, 1981)(processing speed), Rey Auditory Verbal Learning Test (RAVLT) (Lezak et al., 2004) (learning, verbal memory), and Modified Mini-Mental State Exam (3MS) (Teng and Chui, 1987) (global cognitive functioning). Each neurocognitive test in this battery has been validated previously. A composite cognitive function score was formed by averaging standardized scores on each test, ordered so that higher scores reflected better performance (Espeland et al., 2014). Examiners were masked to intervention assignment.
Cognitive function was not formally assessed at baseline; however, participants completed the Beck Depression Inventory (BDI) and the Health Utilities Index (HUI), which contain questions about cognitive abilities (memory, decision-making, and problem-solving)(Espeland, M.A. et al., 2018). The assessment questions are included in Supplementary Table 1. These self-reported assessments were correlated with later objective assessments of cognitive function and we previously used these data to examine intervention effects on cognitive abilities throughout the trial. We dichotomized responses to these Likert scales as yes/no reporting any difficulty with each task at baseline.
Additional Study Procedures
Certified clinic staff, masked to intervention assignment, collected data. Calibrated digital scales were used throughout follow-up to obtain annual measures of weight (Ryan et al., 2003). Blood specimens were collected after a >12-hour fast; these were analyzed centrally for HbA1c. For participants who provided consent, TaqMan genotyping for the rs7412 and rs429358 single nucleotide polymorphisms was used to assign APOE allele carrier status (Elosua et al., 2003).
Statistical Analysis
The effect of menopause on the response to the intervention was not prespecified in the original protocol and was post hoc. However, before this analysis, we prespecified a comparison between the premenopausal or early menopausal group with the late menopausal group. Women were divided into 3 groups according to their menopausal status at the start of intervention delivery (i.e. Look AHEAD enrollment): premenopausal or <5 years postmenopausal, 5–10 years, and ≥10 years postmenopausal. We used chi-squared tests and one-way analyses of variance to examine unadjusted associations between menopausal status and various baseline participant characteristics and risk factors for cognitive deficits. We used linear regression modelling to assess differences between intervention groups and menopausal groups on cognitive testing outcomes, with covariate adjustment for education, race/ethnicity, baseline CVD risk factors (hypertension, history of CVD, and HbA1c%), and whether cognitive testing had been performed before (to control for potential learning effects): a subset of women who had enrolled in Look AHEAD ancillary studies had one or two previous assessments from ancillary study involvement. Formal tests of interactions were added to these models to assess the consistency of any intervention group differences by menopausal status. Models were subsequently fit with an additional adjustment for average weight loss over time. Adjusting for potentially confounding variables on cognitive outcome guarded against any imbalances among menopause groups. In addition to assessing the overall effects of menopausal status, the cognitive scores in the first group were contrasted with the third group in post hoc comparisons. To determine if there were differing effects of time since menopause on cognition vs. age on cognition, the effects of continuous years since menopause (with premenopausal women being coded as 0) and continuous baseline age were estimated separately for each cognitive outcome. Because time since menopause and age were highly correlated, we did not include both in models. Finally, for composite cognitive function, analyses were repeated as stratified by APOE4 carrier status, and self-reported current or pre-trial exposure to HRT.
RESULTS
Of the 3,063 women who enrolled in the Look AHEAD trial, 158 were excluded from analyses because they underwent gastric bypass surgery prior to cognitive assessment (Figure 2). An additional 355 were eliminated because data to determine menopausal status was missing. Finally, 605 additional women were eliminated because they were lost to follow-up or deceased prior to the cognitive assessments we report. Of those participants, 259 died prior to the cognitive assessment and 346 did not attend the cognitive assessment visit. This resulted in 1,945 women being included in our analyses (63% of the original cohort): 977 who had been assigned to the intensive lifestyle intervention and 968 who had been assigned to the control condition of diabetes support and education. Of these 1,945 women, genotyping was performed on 1,550 (80%).
Figure 2:
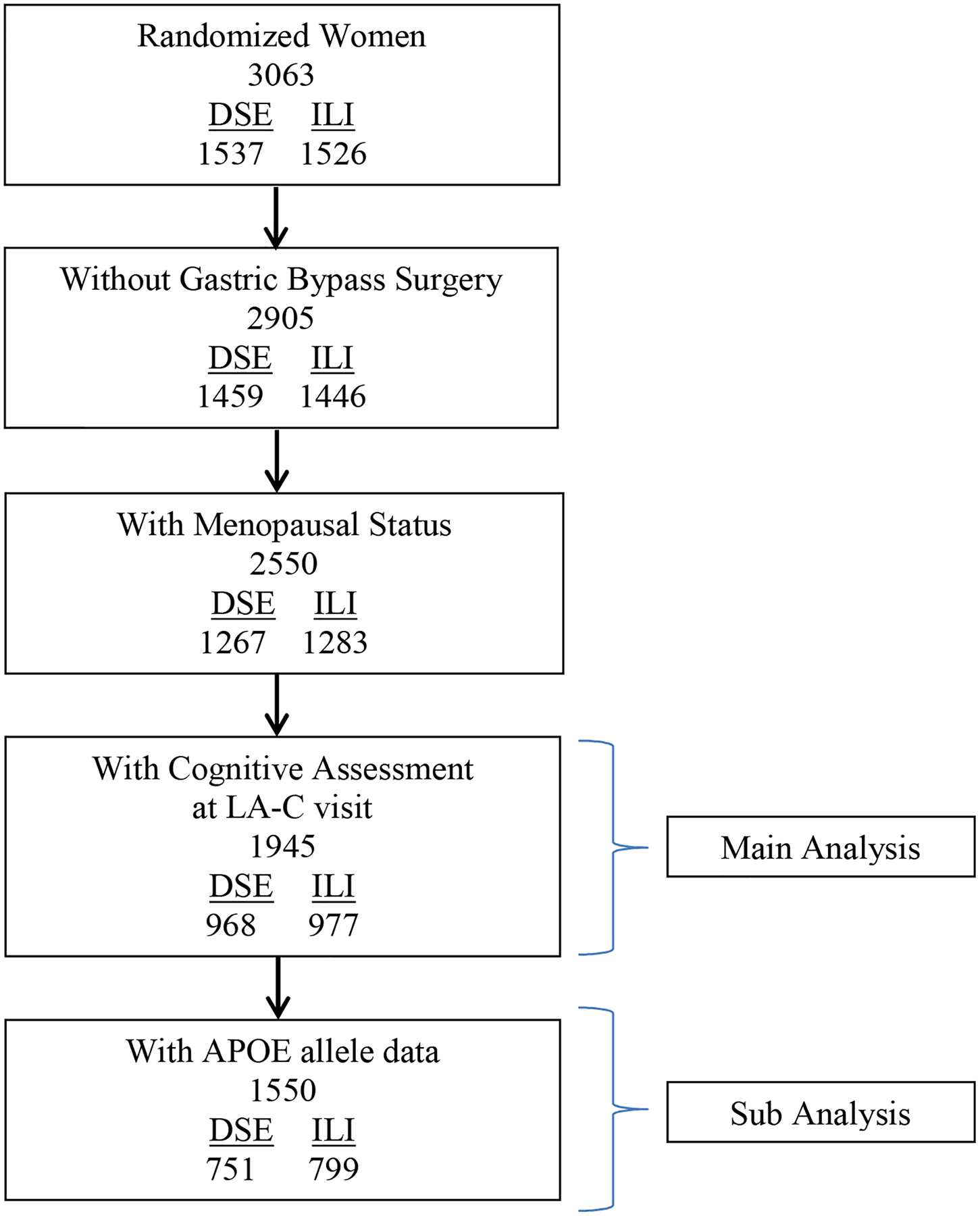
CONSORT diagram describing analytical cohort. LA-C: look AHEAD Continuation
The association of menopause with intervention assignment was examined two ways. First, women were divided into 3 groups premenopausal or <5 years postmenopausal (N=594), 5–10 years (n=388), and ≥10 years postmenopausal (n=963). Second, the association of continuous years since menopause with the response to the intervention was examined(n=1945). Table 1 describes women in these 3 groups at enrollment. While intervention assignment was equally distributed among these groups, many other risk factors for cognitive deficits varied among groups. These risk factors included longer duration of T2DM, more hypertension and greater % of participants with prior CVD history in late menopausal compared with the pre- or early menopausal groups at the time of enrollment. During an earlier assessment, self-reported scores on cognitive abilities (from BDI and HUI tests) were collected from almost all the participants. One out of the 1945 participants (0.05%) was missing the BDI decision-making item. For the HUI, 7 out of 1945 participants (0.36%)were missing the memory item, and 8 out of 1945 participants (0.41%) were missing the problem-solving item. There were no differences in these self-reported cognitive assessments by menopausal group or intervention group (Table 1).
Table 1:
Baseline characteristics of 1945 women with known baseline menopausal status and cognitive assessments, by baseline menopausal status: Mean (SD) or N (%).
| Menopausal Status at Baseline | p-value* | |||
|---|---|---|---|---|
| Pre- or <5 years
Postmenopausal N=594 |
5–10 years
Postmenopausal N=388 |
>10 years
Postmenopausal N=963 |
||
| Intervention arm | 0.2154 | |||
| DSE | 281 (47.3%) | 189 (48.7%) | 498 (51.7%) | |
| ILI | 313 (52.7%) | 199 (51.3%) | 465 (48.3%) | |
| Baseline Age | 52.1 (5.0) | 57.6 (4.0) | 61.3 (5.6) | <.0001 |
| Age at Cognitive Assessment | 64.4 (4.7) | 69.6 (3.8) | 73.2 (5.6) | <.0001 |
| BMI, kg/m2 | <.0001 | |||
| 25–29 | 74 (12.5%) | 47 (12.1%) | 173 (18.0%) | |
| 30–39 | 350 (58.9%) | 251 (64.7%) | 606 (62.9%) | |
| 40+ | 170 (28.6%) | 90 (23.2%) | 184 (19.1%) | |
| Parity | 509 (85.7%) | 334 (86.1%) | 879 (91.6%) | 0.0004 |
| Education | 0.0383 | |||
| <13 years | 132 (22.9%) | 101 (26.7%) | 260 (28.0%) | |
| 13–16 years | 238 (41.3%) | 140 (36.9%) | 389 (41.8%) | |
| >16 years | 207 (35.9%) | 138 (36.4%) | 281 (30.2%) | |
| Race/Ethnicity | 0.0021 | |||
| White | 298 (50.2%) | 210 (54.1%) | 504 (52.3%) | |
| Black | 110 (18.5%) | 68 (17.5%) | 205 (21.3%) | |
| Hispanic | 93 (15.7%) | 75 (19.3%) | 165 (17.1%) | |
| Other | 93 (15.7%) | 35 (9.0%) | 89 (9.2%) | |
| Ever used hormone therapy | 227 (38.3%) | 239 (62.9%) | 662 (69.6%) | <.0001 |
| Diabetes duration (years) | 5.9 (5.7) | 6.2 (6.6) | 6.8 (6.6) | 0.0096 |
| HbA1c% | 7.4 (1.3) | 7.2 (1.0) | 7.2 (1.1) | 0.0002 |
| Hypertension | 448 (75.4%) | 306 (78.9%) | 826 (85.8%) | <.0001 |
| Decision-making | 105 (17.7) | 67 (17.3) | 161 (16.7) | 0.89 |
| CVD history | 23 (3.9%) | 30 (7.7%) | 98 (10.2%) | <.0001 |
| APOE4 status | 0.4148 | |||
| Non-carrier | 354 (74.8%) | 227 (77.0%) | 610 (78.0%) | |
| Carrier – one copy | 107 (22.6%) | 61 (20.7%) | 162 (20.7%) | |
| Carrier – two copies | 12 (2.5%) | 7 (2.4%) | 10 (1.3%) | |
p-values are from chi-square tests of association for categorical variables, and one-way ANOVAs for continuous variables.
We hypothesized that randomization to the ILI would have beneficial effects on cognitive outcomes in women enrolled during the pre- and early postmenopausal women phase, and harmful when participants enter the trial in late post menopause. As shown in table 1, many baseline clinical and demographic characteristics (i.e. T2DM, hypertension, CVD, ethnicity, BMI, parity, education, age) differed between menopausal status groups. To address this imbalance, models were adjusted for education, race/ethnicity, hypertension, history of CVD, HbA1c%, and whether or not cognition had been assessed previously. Table 2 shows these adjusted mean cognitive function scores by intervention assignment and menopausal group. We did not adjust for age and time since menopause in the same model as these two variables were strongly correlated. Premenopausal or early postmenopausal women had better cognitive scores as compared to late postmenopausal women (all p<0.05). Compared with DSE participants, late postmenopausal women assigned to ILI lost more weight than pre- and early postmenopausal women. When comparing the premenopausal and late menopausal groups, significant interactions between menopausal group and intervention assignment were observed for the composite z-score (p=0.0238) and DSST (p=0.0055), and the interaction approached significance for the Stroop (p=0.0536). For each of these scores (Figure 3), pre- and early postmenopausal ILI women tended to outperform DSE women, while the reverse held for late postmenopausal women. For Stroop and trails B, lowers scores reflected greater improvements. Additional adjustment for the average percentage of weight changes for women from randomization up to the time of the cognitive assessment, i.e. a measure similar to the area under the curve traced by annual measures of percent weight changes, did not impact the model results (data not shown).
Table 2:
Adjusted* mean cognitive assessment scores by intervention assignment, and baseline menopausal status.
| Pre- or <5 years Post-menopausal | 5–10 years Post-menopausal | >10 years Post-menopausal | Relative Intervention Effects Mean (SE): ILI Minus DSE | p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DSE | ILI | DSE | ILI | DSE | ILI | Pre- or <5 years Post | 5–10 years Post | >10 years Post | Overall Interaction of Intervention by Menopause | Intervention Effect in Pre/<5 yrs. vs >10 yrs. | |
| Average % weight change | 2.07 (0.50) | 4.52 (0.50) | 1.28 (0.57) | 4.85 (0.57) | 2.10 (0.43) | 6.16 (0.43) | 2.45 (0.55) | 3.57 (0.68) | 4.06 (0.43) | 0.0675 | 0.0206 |
| Composite z-score | 0.10 (0.05) | 0.21 (0.05) | 0.001 (0.06) | −0.03 (0.06) | −0.23 (0.04) | −0.29 (0.04) | 0.10 (0.05) | −0.03 (0.07) | −0.06 (0.04) | 0.0636 | 0.0216 |
| 3MSE | 91.0 (0.48) | 91.5 (0.47) | 90.6 (0.54) | 90.1 (0.54) | 89.0 (0.41) | 89.1 (0.41) | 0.57 (0.52) | −0.47 (0.64) | 0.11 (0.41) | 0.4510 | 0.4864 |
| Stroop** | 31.3 (1.31) | 30.1 (1.30) | 33.1 (1.48) | 35.7 (1.48) | 37.5 (1.13) | 40.0 (1.12) | −1.19 (1.43) | 2.65 (1.76) | 2.46 (1.13) | 0.0956 | 0.0446 |
| DSST | 39.8 (0.77) | 41.7 (0.76) | 38.7 (0.86) | 38.3 (0.86) | 35.6 (0.66) | 34.6 (0.65) | 1.91 (0.83) | −0.35 (1.02) | −0.99 (0.65) | 0.0213 | 0.0061 |
| Trails (Part B)** | 118.6 (5.0) | 114.6 (4.9) | 121.4 (5.6) | 120.7 (5.6) | 138.0 (4.3) | 144.8 (4.2) | −0.96 (5.35) | −0.75 (6.60) | 6.76 (4.24) | 0.2631 | 0.1166 |
| AVLT Delayed | 8.72 (0.25) | 9.13 (0.24) | 8.00 (0.28) | 8.22 (0.28) | 7.66 (0.21) | 7.59 (0.21) | 0.41 (0.27) | 0.22 (0.33) | −0.06 (0.21) | 0.3700 | 0.1665 |
In addition to intervention and baseline menopausal status, models are adjusted for whether or not cognitive function had been assessed previously and the following baseline factors: education, race/ethnicity, CVD history, hypertension status, and HbA1c%. Adjustment for the average percent weight change across all follow-up visits did not affect the results (data not shown).
Higher scores reflect poorer performance
Bold p<0.05
3MSE: Modified Mini Mental State Examination
DSST: Digital Symbol Substitution Test
AVLT: Auditory-Verbal Learning Test
Figure 3:
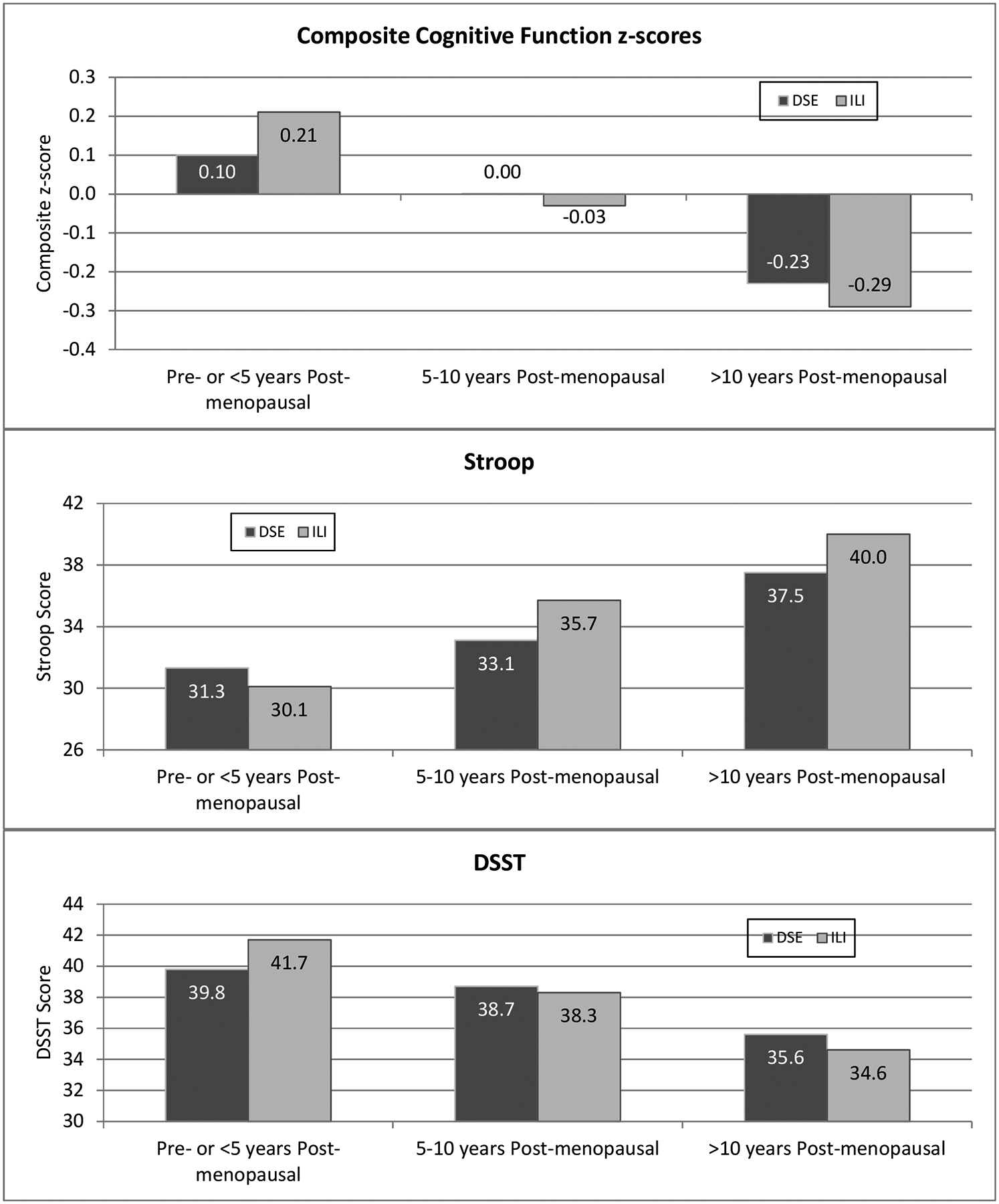
Adjusted mean scores by intervention assignment and menopausal group.
To compare the effect of menopause and age, the interaction between age and intervention arm on cognitive outcomes was compared in models based on continuous time since menopause vs models based on baseline age (Figure 4). The results indicate significant two-way interactions between years since menopause and intervention arm on the composite z-scores (p=0.035), Stroop (p=0.054), DSST (p=0.018) and Trails B (p=0.031). However, there were no significant interaction effects between baseline age and intervention on any of the cognitive outcomes. These results imply that time since menopause at enrollment better predicted the response to the intervention on some of the cognitive outcomes than the participants age at baseline. For example, a younger woman with a longer time since menopause at enrollment is less likely to benefit from a weight loss intervention on cognitive outcomes than an older woman with a more recent menopausal onset.
Figure 4:
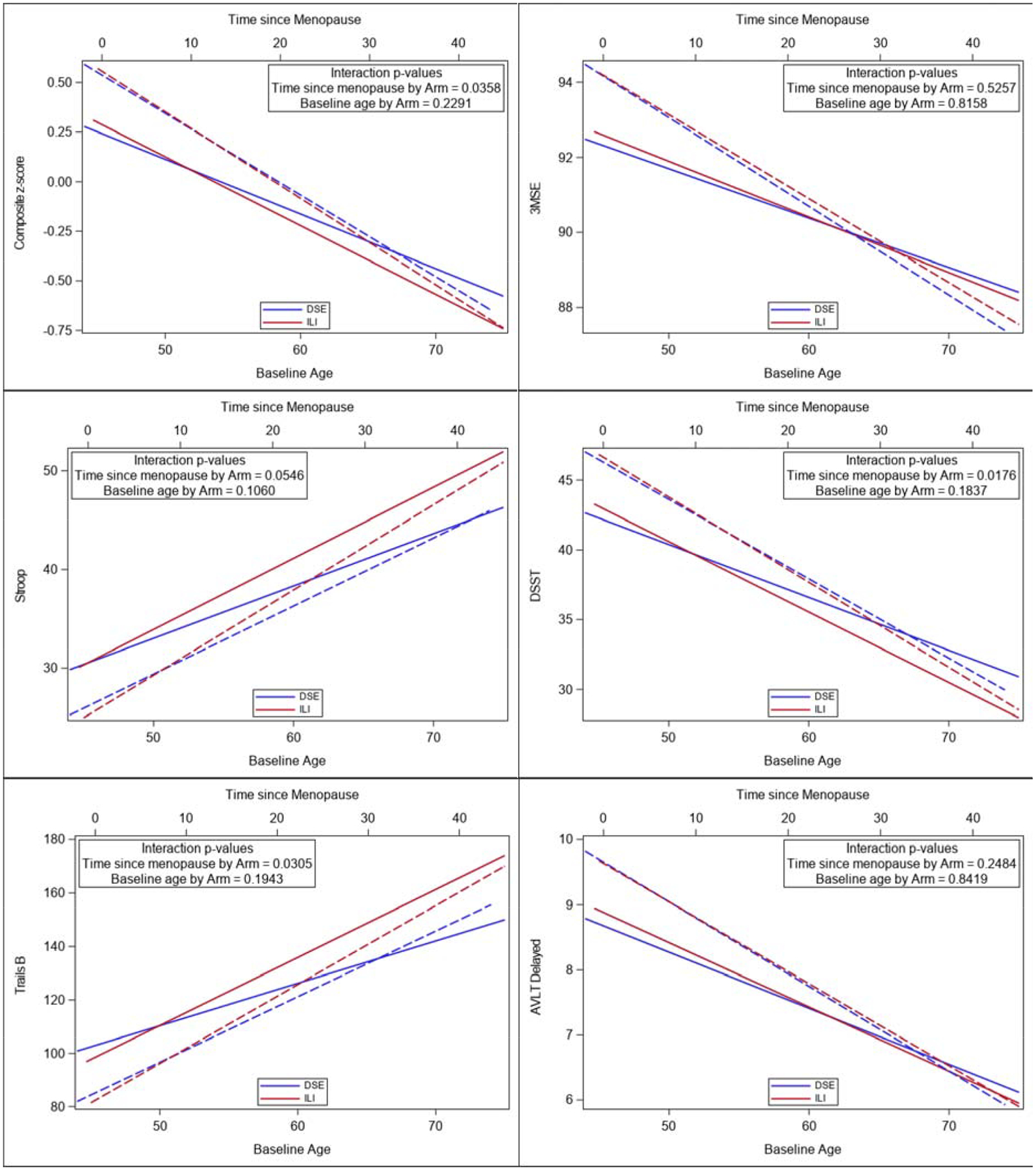
Comparison of baseline age vs time since menopause by intervention arm on cognitive scores. Sold lines depict Time since Menopause. Dashed lines depict Baseline Age.
We also hypothesized that APOE genotype, HRT and baseline obesity modulated the interaction between the intervention and menopause on cognitive outcomes. Table 3 examines the consistency of relative intervention effects among menopausal groups when stratified by APOE4 carrier status, history of HRT use, and baseline BMI. Included are tests for 3-way interactions to assess whether the patterns seen in Table 2 vary among these subgroups. Overall, pre- and early postmenopausal women significantly outperformed late menopausal women in every subgroup (all p<0.05). The pattern for any intervention-related benefits to wane and/or reverse from pre- and early post menopausal to late post menopausal was consistent across subgroups formed by HRT use and BMI, but less so for carriers of APOE4. Among noncarriers of APOE4, the intervention appeared to benefit cognitive function among pre- and early post menopausal women while the reverse held for late postmenopausal women (two-way interaction between menopause and intervention arm in APOE4 non-carriers, p=0.0017). However, the 3-way interaction did not reach statistical significance (p=0.0648). Among APOE4 carriers, there was little evidence for benefit or harm from the intervention. This pattern can be seen in the top panel of Figure 5.
Table 3:
Subgroup analyses - Adjusted* mean cognitive assessment composite z-scores by intervention assignment, and baseline menopausal status.
| Mean (SE) Relative Intervention Effects: ILI minus DSE | Mean (95% CI) Difference in Intervention Effects Between Pre/<5 yrs. vs >10 yrs.*** | ||||
|---|---|---|---|---|---|
| Pre- or <5 years Post-menopausal | 5–10 years Postmenopausal | >10 years Postmenopausal | p-value** | ||
| APOE4 | |||||
| Non APOE4 carrier | 0.15 (0.07) | −0.11 (0.09) | −0.14 (0.05) | 0.0040 | 0.29 (0.11, −0.46) |
| APOE4 carrier | −0.09 (0.12) | −0.14 (0.16) | 0.05 (0.10) | 0.5388 | −0.13 (−0.45, −0.18) |
| P = 0.0648 | |||||
| HRT | |||||
| Yes | 0.13 (0.09) | −0.10 (0.08) | −0.05 (0.05) | 0.1177 | 0.18 (−0.01, −0.38) |
| No | 0.08 (0.07) | 0.04 (0.11) | −0.05 (0.08) | 0.4775 | 0.13 (−0.08, −0.34) |
| P = 0.6302 | |||||
| BMI | |||||
| 25–29 | 0.23 (0.16) | 0.08 (0.19) | 0.10 (0.10) | 0.7610 | 0.13 (−0.24, −0.50) |
| 30–39 | 0.11 (0.07) | −0.09 (0.08) | −0.09 (0.05) | 0.0667 | 0.20 (0.02, −0.38) |
| 40+ | 0.04 (0.09) | 0.10 (0.13) | −0.08 (0.09) | 0.4614 | 0.12 (−0.14, −0.38) |
| P = 0.6710 | |||||
In addition to intervention and baseline menopausal status, models are adjusted for whether or not cognitive function had been assessed previously and the following baseline factors: education, race/ethnicity, CVD history, hypertension status, and HbA1c%.
Test for two-way interaction;
Test for three-way interaction and 95% confidence intervals
Figure 5:
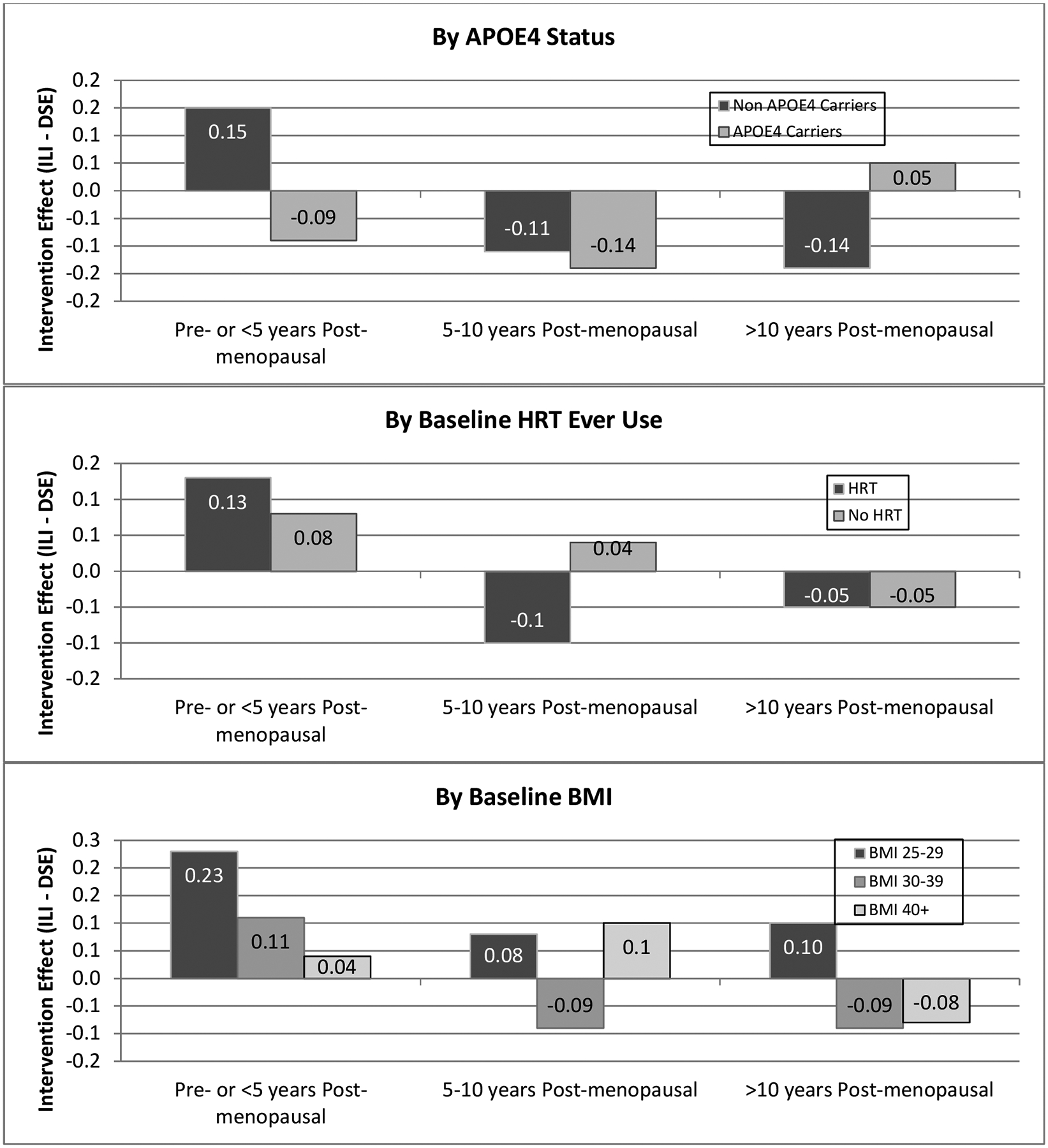
Intervention effects (ILI minus DSE) of composite cognitive function test scores by menopausal group, stratified by subgroups.
To address whether differential attrition (participants excluded due to missing data) could have biased our results, an inverse probability weighted analysis was used as described previously(Espeland et al., 2018; Weuve et al., 2012). First, a logistic regression model was fitted with the dependent variable being whether or not participants, based on their baseline characteristics, did not or did contribute to our current analysis. As recommended, a rich set of baseline predictors, including the self-reported cognitive measures above, for this modeling was used. The analysis was then repeated weighting data from individuals by the inverse of the probability from the logistic regression model. This allows for individuals who resemble those not contributing data to the analyses to have more weight, i.e. that they are “representing” not only themselves but others like themselves who have been lost. Overall, the estimates are very similar between the two approaches (Supplementary Table 2). The inferences tend to be slightly more significant with the inverse probability weighting. Thus, it appears that differential attrition served to attenuate, rather than exaggerate, the estimated relative intervention effects. It may be that individuals who were lost to follow-up were more likely to have risk factor profiles reflective of lower cognitive function and that by culling these individuals from the analytical data set, retention served to attenuate estimated differences.
DISCUSSION
In the Look AHEAD trial, randomization to 10 years of ILI vs. DSE was not associated with overall differences in cognitive function scores when assessed 1–2 years after the intervention ended. Similarly in both women and men, significant interactions with BMI and history of CVD were reported in which the less heavy and CVD-free individuals appeared to benefit while the heavier individuals with a history of CVD appeared to be harmed (Espeland et al., 2017). In this subgroup analysis, we observed a significant interaction between the onset of menopause and the intervention on cognitive scores. Older postmenopausal women had worse cognitive scores in two out of 5 cognitive outcomes in the ILI group compared with the DSE group. In contrast, younger pre- or early postmenopausal females had better cognitive scores in these outcomes in the ILI group compared with the DSE group. These effects were further modulated by APOE genotype. The positive effect of the intervention was only evident among APOE4 non-carriers. The response to intervention appears to be a function of menopausal state and not baseline age, as the interaction of years since menopause with the intervention on cognitive outcomes was significant while the interaction of baseline age with the intervention was not. In addition, the findings persisted after adjusting for aging associated CVD risk factors, such as hypertension, history of CVD events, and measures of glycemia.
Findings from this subgroup analysis suggests that executive function cognitive domains (assessed by Stroop, DSST and Trails part B) may be responsive to a weight loss intervention in group of younger pre- or early postmenopausal women with cardiovascular risk factors, obesity and T2DM. The gradual process of age-related executive function decline appears to begin in early to middle adulthood (Salthouse, 2009) and is accelerated during menopause (Halbreich et al., 1995), and among persons with obesity and T2DM (Saczynski et al., 2008; Zhao et al., 2015). Therefore, a weight loss intervention may counteract the effects of menopause and metabolic risk factors such as obesity and T2DM on this cognitive domain. In contrast, a weight loss intervention may not be an effective strategy for slowing memory cognitive decline associated with AD neuropathology which appears in older individuals and early on in those carrying the APOE4 allele (Hohman et al., 2017).
Why might time since menopause as opposed to baseline age affect the response to an intensive weight loss intervention? One explanation is the underlying differences in the health of the vasculature between younger or recently menopausal women and those who are older or further past the menopausal transition (Mendelsohn and Karas, 2005). In relatively healthy blood vessels (such as in pre or early menopausal woman), weight loss may offer protection by enhancing endothelial functions and blood flow (Bigornia et al., 2010). However, in vessels with substantial atherosclerotic plaque (more likely in an older woman), the beneficial response to weight loss may not only get blunted but also compounded with harm by limiting brain energy supply or affecting levels of neuroprotective hormones derived from adipose tissues. In the female brain, estrogen activates glucose metabolism while simultaneously suppressing the ketogenic system in brain thereby promoting brain reliance on glucose as its primary fuel to generate ATP (Yin et al., 2015). After menopause, there is evidence to support a shift in the preference for brain energy sources, from glucose to oxidation of fatty acids to generate ketones for brain energy utilization (Riedel et al., 2016). Since the AD brain utilizes fatty acids as an alternative source of fuel when glucose uptake is impaired (Cunnane et al., 2016), it is possible that weight loss in older women may compromise brain energy supply or alter neuroprotective hormones produced in adipocytes, such as leptin (Forny-Germano et al., 2019). Similar to menopause, several groups demonstrated that APOE4 carriers have abnormally low rates of glucose metabolism in several areas in the brain affected by AD (Mosconi et al., 2004; Reiman et al., 2004). If the APOE4 brain is a dual fuel dependent brain, being dependent upon glucose and ketone bodies (Riedel et al., 2016), then suppression of the ketogenic system in the APOE4 brain by weight loss would result in reduction in a critical fuel to generate ATP. Obesity in this regard may offer some brain protection to the APOE4 brains by supplying fatty acids as a source for brain energy consumption (Karmi et al., 2010).
The study has several limitations. First, objective assessment of cognitive function was not done at baseline so that we are unable to assess changes or to use baseline data as covariates. This reduces statistical power, however, we found significant differences at follow-up, indicating that the study had sufficient power. A second limitation is that there may have been differential follow-up that may bias our findings, e.g. if there was greater number of participants lost to follow-up in one intervention group compared to the other in a manner that biased the subgroup differences. While we cannot directly assess whether groups were balanced at baseline with respect to cognitive function scores, it is highly likely that balance was achieved given the large sample size and randomization, and the balance of known correlates of cognitive function. While Figure 2 demonstrates that overall rates of follow-up for the subgroups were comparable for the two intervention groups, there remains the possibility that there was differential between intervention groups in ways that might bias our findings for cognitive test scores. For example, the many baseline clinical demographic differences (such as T2DM, hypertension, CVD, ethnicity, BMI, parity, education) among groups with different time distances from menopause. A key is that, given the large sample size and the balanced attrition, randomization is expected to produce a balanced comparison between intervention groups within the menopause groups. The extensive covariate adjustment we performed in our analysis mitigates against this bias. We have also explored this using inverse propensity models and find no evidence of meaningful bias. Another limitation of this work is that it was not pre-specified in the original Look AHEAD protocol. Our work is hypothesis-driven and was pre-specified before analyses were begun as part of the internal review process of the Look AHEAD trial. Since age and time from menopause were highly correlated and not included in a single model, the interpretation of time from menopause effect is weakened as some of this effect could still relate to age. We acknowledge that this work should be considered as exploratory and requires further confirmation. Finally, the effect of HRT or baseline BMI on the response was not significant, a likely limitation of small sample size for a three-way interaction.
In conclusion, this analysis of Look AHEAD supports that intensive lifestyle interventions started before or early after the onset of menopause may have protective effects on measures of executive function within cognitive domains. On the contrary, intense lifestyle interventions in women who started the intervention many years after the onset of menopause appears to be harmful. In addition, weight loss may not provide cognitive benefit in APOE4 carriers. Future studies should directly evaluate the effect of a weight loss intervention on cognition in younger premenopausal group with obesity and T2DM. Studying the mechanisms of how menopause and APOE genotype influence the response to lifestyle interventions on cognition is warranted.
Supplementary Material
Highlights.
In women, dementia risk appears to be influenced by menopause and APOE genotype.
Older postmenopausal women had worse cognitive scores after an intensive weight loss program.
In contrast, younger pre- or early postmenopausal women had better cognitive scores.
The positive effect of the intervention was only evident among APOE4 non-carriers.
ACKNOWLEDGMENTS
Look AHEAD Research Group at End of Continuation
Clinical Sites
The Johns Hopkins University Frederick L. Brancati, MD, MHS1*; Jeanne M. Clark, MD, MPH1 (Co-Principal Investigators); Lee Swartz2; Jeanne Charleston, RN3; Lawrence Cheskin, MD3; Richard Rubin, PhD3*; Jean Arceci, RN; David Bolen; Danielle Diggins; Mia Johnson; Joyce Lambert; Sarah Longenecker; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun; Maria Sowers; Kathy Tyler
Pennington Biomedical Research Center George A. Bray, MD1; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Timothy Church, MD3; Catherine Champagne, PhD, RD; Valerie Myers, PhD; Jennifer Arceneaux, RN; Kristi Rau; Michelle Begnaud, LDN, RD, CDE; Barbara Cerniauskas, LDN, RD, CDE; Crystal Duncan, LPN; Helen Guay, LDN, LPC, RD; Carolyn Johnson, LPN, Lisa Jones; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Marisa Smith; Lauren Cox; Monica Lockett, LPN
The University of Alabama at Birmingham Cora E. Lewis, MD, MSPH1; Sheikilya Thomas, PhD,MPH2; Monika Safford, MD3; Stephen Glasser, MD3; Vicki DiLillo, PhD3; Gareth Dutton, PhD, Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Sara Hannum; Anne Hubbell, MS; Jane King, MLT; DeLavallade Lee; Andre Morgan; L. Christie Oden; Janet Wallace, MS; Cathy Roche, PhD, RN, BSN; Jackie Roche; Janet Turman
Harvard Center
Massachusetts General Hospital. David M. Nathan, MD1; Enrico Cagliero, MD3; Heather Turgeon, RN, BS, CDE2; Barbara Steiner, EdM2; Valerie Goldman, MS, RDN2; Linda Delahanty, MS, RDN3; Ellen Anderson, MS, RDN3; Laurie Bissett, MS, RDN; Christine Stevens, RN; Mary Larkin, RN; Kristen Dalton, BS, Roshni Singh, BS
Joslin Diabetes Center: Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth McKinney, BSN, RN; Barbara Fargnoli, MS, RD; Jeanne Spellman, BS, RD; Kari Galuski, RN; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; Ann McNamara, RN
University of Colorado Anschutz Medical Campus James O. Hill, PhD1; Marsha Miller, MS RD2; Holly Wyatt, MD3, Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Debbie Bochert; Gina Claxton-Malloy RD Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Loretta Rome, TRS; Terra Thompson, BA, Kirstie Craul, RD, CDE; Cecilia Wang, MD
Baylor College of Medicine John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Molly Gee, MEd, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Chu-Huang Chen, MD, PhD3; Peter Jones, MD3; Michele Burrington, RD, RN; Allyson Clark Gardner, MS, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Sarah Lee; Sarah Lane Liscum, RN, MPH; Susan Cantu-Lumbreras; Julieta Palencia, RN; Jennifer Schmidt; Jayne Thomas, RD; Carolyn White; Charlyne Wright, RN; Monica Alvarez, PCT
The University of Tennessee Health Science Center
University of Tennessee East. Karen C. Johnson, MD, MPH□; Karen L. Wilson, BSN□; Mace Coday, PhD3; Beate Griffin, RN, BS; Donna Valenski; Polly Edwards; Brenda Fonda; Kim Ward
University of Tennessee Downtown. Helmut Steinburg, MD3; Carolyn Gresham, BSN□; Moana Mosby, RN; Debra Clark, LPN; Donna Green RN; Abbas E. Kitabchi, PhD, MD (retired)
University of Minnesota Robert W. Jeffery, PhD1; Tricia Skarphol, MA2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott J. Crow, MD3; Manami Bhattacharya, BS; Cindy Bjerk, MS, RD; Kerrin Brelje, MPH, RD; Carolyne Campbell; Mary Ann Forseth, BA; Melanie Jaeb, MPH, RD; Philip Lacher, BBA; Patti Laqua, BS, RD; Birgitta I. Rice, MS, RPh, CHES; Ann D. Tucker, BA; Mary Susan Voeller, BA
St. Luke’s Roosevelt Hospital Center Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Carmen Pal, MD3; Lynn Allen, MD; Janet Crane, MA, RD, CDN; Lolline Chong, BS, RD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; Raashi Mamtani, MS
University of Pennsylvania Thomas A. Wadden, PhD1; Barbara J. Maschak-Carey, MSN, CDE2; Robert I. Berkowitz, MD3; Gary Foster, PhD3; Henry Glick, PhD3; Shiriki Kumanyika, PhD RD, MPH3; Yuliis Bell, BA; Raymond Carvajal, PsyD; Helen Chomentowski; Renee Davenport; Lucy Faulconbridge, PhD; Louise Hesson, MSN, CRNP; Sharon Leonard, RD; Monica Mullen, RD, MPH
University of Pittsburgh John M. Jakicic, PhD1; David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Daniel Edmundowicz, MD3; Lin Ewing, PhD, RN3; Andrea Hergenroeder, PhD, PT, CCS3; Mary L. Klem, PhD, MLIS3; Mary Korytkowski, MD3; Andrea Kriska, PhD3; Lewis H. Kuller, MD, DrPH3; Amy D. Rickman, PhD, RD, LDN3; Rose Salata, MD3; Monica E. Yamamoto, DrPH, RD, FADA3; Janet Bonk, RN, MPH; Susan Copelli, BS, CTR; Rebecca Danchenko, BS; Tammy DeBruce, BA; Barbara Elnyczky; David O. Garcia, PhD; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Diane Heidingsfelder, MS, RD, CDE, LDN; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Janet Krulia, RN, BSN, CDE; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Lisa Martich, BS, RD, LDN; Meghan McGuire, MS; Tracey Y. Murray, BS; Anna Peluso, MS; Karen Quirin; Jennifer Rush, MPH; Joan R. Ritchea; Linda Semler, MS, RD, LDN; Karen Vujevich, RN-BC, MSN, CRNP; Kathy Williams, RN, MHA; Donna L. Wolf, PhD
The Miriam Hospital/Brown Medical School Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio Helen P. Hazuda, PhD1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN
VA Puget Sound Health Care System / University of Washington Steven E. Kahn, MB, ChB1; Anne Kure, BS2; Edward J. Boyko, MD, MPH3; Edward Lipkin, MD, PhD3; Dace Trence, MD3; Subbulaxmi Trikudanathan, MD, MRCP, MMSc3; Elaine Tsai, MD3; Brenda Montgomery, RN, MS, CDE; Ivy Morgan-Taggart; Jolanta Socha, BS; Lonnese Taylor, RN, BS; Alan Wesley, BA
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Maria Cassidy-Begay, BSND, RND 2; Katie Toledo, MS, LPC2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Sara Michaels, MD3; Paul Bloomquist, MD3; Peter H. Bennett, MB, FRCP3; Bernadita Fallis, RN, RHIT, CCS; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Christina Morris, BA; Julie Nelson, RD; Carol Percy, RN, MS; Patricia Poorthunder; Sandra Sangster; Leigh A. Shovestull, RD, CDE; Miranda Smart; Janelia Smiley; Teddy Thomas, BS
University of Southern California Anne Peters, MD1; Siran Ghazarian, MD2; Elizabeth Beale, MD3; Kati Konersman, RD, CDE; Brenda Quintero-Varela; Edgar Ramirez; Gabriela Rios, RD; Gabriela Rodriguez, MA; Valerie Ruelas MSW, LCSW; Sara Serafin-Dokhan; Martha Walker, RD
Coordinating Center
Wake Forest University Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH1; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; David Lefkowitz, MD3; Patrick S. Reynolds, MD3; Denise Houston, PhD3; Mike E. Miller, PhD3; Laura D. Baker, PhD3; Nicholas Pajewski, PhD3; Stephen R. Rapp, PhD3; Stephen Kritchevsky, PhD3; Haiying Chen, PhD, MM3; Valerie Wilson, MD3; Delia S. West, PhD3; Ron Prineas, MD3; Tandaw Samdarshi, MD3; Amelia Hodges, BS, CCRP2; Karen Wall2; Carrie C. Williams, MA, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA; Tara D. Beckner; Valery S. Effoe, MD, MS; Melanie Franks, BBA; Katie Garcia, MS; Sarah A. Gaussoin, MS; Candace Goode; Michelle Gordon, MS; Lea Harvin, BS; Mary A. Hontz, BA; Don G. Hire, BS; Patricia Hogan, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Julia T. Rushing, MS; Debbie Steinberg, BS; Jennifer Walker, MS; Michael P. Walkup, MS;
Central Resources Centers
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories Santica M. Marcovina, PhD, ScD1; Jessica Hurting2; John J. Albers, PhD3, Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Charles Campbell 2; Zhu-Ming Zhang, MD3; Mary Barr; Susan Hensley; Julie Hu; Lisa Keasler; Yabing Li, MD
Hall-Foushee Communications, Inc.
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases Mary Evans, PhD; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD
National Heart, Lung, and Blood Institute Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR; Mario Stylianou, PhD
Centers for Disease Control and Prevention Edward W. Gregg, PhD; Ping Zhang, PhD
Funding and Support
Funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources. HNY was supported by the NIH grants R21AG056518, R01AG055770 and R01AG054434.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche, Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
1 Principal Investigator
2 Program Coordinator
3 Co-Investigator
All other Look AHEAD staffs are listed alphabetically by site.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts to report
ClinicalTrials.gov Identifier: NCT00017953 (Action For Health in Diabetes)
References
- 2013. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. New England Journal of Medicine 369(2), 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, Young J, 2011. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obesity reviews : an official journal of the International Association for the Study of Obesity 12(5), e426–437. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C, 2004. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obesity research 12(9), 1519–1526. [DOI] [PubMed] [Google Scholar]
- Bigornia SJ, Mott MM, Hess DT, Apovian CM, McDonnell ME, Duess M-A, Kluge MA, Fiscale AJ, Vita JA, Gokce N, 2010. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring, Md.) 18(4), 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard-Watkins R, Plourde M, 2014. Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients 6(10), 4452–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano CA, 2016. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci 1367(1), 12–20. [DOI] [PubMed] [Google Scholar]
- Elosua R, Demissie S, Cupples LA, Meigs JB, Wilson PW, Schaefer EJ, Corella D, Ordovas JM, 2003. Obesity modulates the association among APOE genotype, insulin, and glucose in men. Obes Res 11(12), 1502–1508. [DOI] [PubMed] [Google Scholar]
- Espeland, Carmichael O, Hayden K, Neiberg RH, Newman AB, Keller JN, Wadden TA, Rapp SR, Hill JO, Horton ES, Johnson KC, Wagenknecht L, Wing RR, 2018. Long-term Impact of Weight Loss Intervention on Changes in Cognitive Function: Exploratory Analyses from the Action for Health in Diabetes Randomized Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci 73(4), 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Dutton GR, Neiberg RH, Carmichael O, Hayden KM, Johnson KC, Jeffery RW, Baker LD, Cook DR, Kitzman DW, Rapp SR, Action for Health in Diabetes Research, G., 2018. Impact of a Multidomain Intensive Lifestyle Intervention on Complaints About Memory, Problem-Solving, and DecisionMaking Abilities: The Action for Health in Diabetes Randomized Controlled Clinical Trial. The journals of gerontology. Series A, Biological sciences and medical sciences 73(11), 1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Luchsinger JA, Baker LD, Neiberg R, Kahn SE, Arnold SE, Wing RR, Blackburn GL, Bray G, Evans M, Hazuda HP, Jeffery RW, Wilson VM, Clark JM, Coday M, Demos-McDermott K, Foreyt JP, Greenway F, Hill JO, Horton ES, Jakicic JM, Johnson KC, Knowler WC, Lewis CE, Nathan DM, Peters A, Pi-Sunyer X, Pownall H, Wadden TA, Rapp SR, 2017. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology 88(21), 2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Bray GA, Houston DK, Johnson KC, Kitabchi AE, Hergenroeder AL, Williamson J, Jakicic JM, van Dorsten B, Kritchevsky SB, 2014. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci 69(9), 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J, 2004. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama 291(24), 2959–2968. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, Vaughan L, Robinson J, Rapp SR, Goveas JS, Wactawski-Wende J, Stefanick ML, Li W, Resnick SM, 2013. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med 173(15), 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT Jr., Luchsinger JA, 2009. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 66(3), 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forny-Germano L, De Felice FG, Vieira M.N.d.N., 2019. The Role of Leptin and Adiponectin in ObesityAssociated Cognitive Decline and Alzheimer’s Disease. Frontiers in neuroscience 12, 1027–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S, 2015. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS medicine 12(6), e1001833; discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe S-H, 1995. Possible acceleration of age effects on cognition following menopause. Journal of Psychiatric Research 29(3), 153–163. [DOI] [PubMed] [Google Scholar]
- Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ, 2016. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology 87(7), 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP, 2016. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. New England Journal of Medicine 374(13), 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Tommet D, Marks S, Contreras J, Jones R, Mungas D, 2017. Evaluating Alzheimer’s disease biomarkers as mediators of age-related cognitive decline. Neurobiol Aging 58, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houx PJ, Jolles J, Vreeling FW, 1993. Stroop interference: aging effects assessed with the Stroop Color-Word Test. Experimental aging research 19(3), 209–224. [DOI] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Någren K, Solin O, Nuutila P, 2010. Increased Brain Fatty Acid Uptake in Metabolic Syndrome. Diabetes 59(9), 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ES, Rizzo JH, Pedula KL, Yaffe K, Ensrud KE, Cauley J, Cawthon PM, Cummings S, Hillier TA, 2017. Weight Trajectory over 20 Years and Likelihood of Mild Cognitive Impairment or Dementia Among Older Women. Journal of the American Geriatrics Society 65(3), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Fischer JS, 2004. Neuropsychological assessment. Oxford University Press, USA. [Google Scholar]
- Mendelsohn ME, Karas RH, 2005. Molecular and cellular basis of cardiovascular gender differences. Science 308(5728), 1583–1587. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S, Isaacson RS, de Leon MJ, Swerdlow RH, Brinton RD, 2017. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLOS ONE 12(10), e0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, Salmon E, Baron JC, De Cristofaro MT, Padovani A, Borroni B, Franceschi M, Bracco L, Pupi A, 2004. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 63(12), 2332–2340. [DOI] [PubMed] [Google Scholar]
- Neu SC, Pa J, Kukull W, et al. , 2017. Apolipoprotein e genotype and sex risk factors for alzheimer disease: A meta-analysis. JAMA Neurology 74(10), 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D, 2003. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama 289(20), 2663–2672. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Luchsinger JA, Baker LD, Blackburn GL, Hazuda HP, Demos-McDermott KE, Jeffery RW, Keller JN, McCaffery JM, Pajewski NM, Evans M, Wadden TA, Arnold SE, Espeland MA, 2017. Effect of a Long-Term Intensive Lifestyle Intervention on Cognitive Function: Action for Health in Diabetes Study. Journal of the American Geriatrics Society 65(5), 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J, 2004. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences 101(1), 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, 1958. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Perceptual and Motor Skills 8(3), 271–276. [Google Scholar]
- Riedel BC, Thompson PM, Brinton RD, 2016. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol 160, 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ, 2003. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled clinical trials 24(5), 610–628. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Jonsdottir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, Olafsdottir E, Harris TB, Gudnason V, Launer LJ, 2008. Cognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility--Reykjavik study. Am J Epidemiol 168(10), 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, 2009. When does age-related cognitive decline begin? Neurobiology of Aging 30(4), 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR, 1935. Studies of interference in serial verbal reactions. Journal of Experimental Psychology 18(6), 643. [Google Scholar]
- Teng EL, Chui HC, 1987. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry 48(8), 314–318. [PubMed] [Google Scholar]
- Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S, 2006. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 14(5), 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1981. Wais-r manual. New York: Psychological Corporation. [Google Scholar]
- Wesche-Thobaben JA, 2011. The development and description of the comparison group in the Look AHEAD trial. Clinical trials (London, England) 8(3), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, Mendes de Leon CF, 2012. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology 23(1), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yao J, Sancheti H, Feng T, Melcangi RC, Morgan TE, Finch CE, Pike CJ, Mack WJ, Cadenas E, Brinton RD, 2015. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging 36(7), 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Roberts RO, Ding D, Cha R, Guo Q, Meng H, Luo J, Machulda MM, Shane Pankratz V, Wang B, Christianson TJ, Aakre JA, Knopman DS, Boeve BF, Hong Z, Petersen RC, 2015. Diabetes is Associated with Worse Executive Function in Both Eastern and Western Populations: Shanghai Aging Study and Mayo Clinic Study of Aging. J Alzheimers Dis 47(1), 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


