Abstract
Objective:
This meta-analysis advances a framework to understand correspondence among units of analysis of the social processing construct within Research Domain Criteria (RDoC).
Method:
As requested for this special issue, eligible studies cited an RDoC-initiative paper or mentioned RDoC in the abstract, title or keywords, were empirical and peer-reviewed, and described a correlation or regression analysis (r, β, or Odds Ratio) between two different units of analysis in the social processing domain in youth. We examined the frequency (descriptive statistics) and magnitude of correspondence between unit-pairs (random-effects models), and predefined moderators (meta-regression).
Results:
Eight of twenty-eight possible unit-by-unit pairs were identified, with subjective-by-behavior units being the most common. Of those, only subjective-by-circuit had significant correspondence between units. Moderator analysis revealed that age and diagnosis of generalized anxiety disorder (GAD) moderated correspondence between subjective-by-circuit units of analysis, and that a diagnosis of autism spectrum disorder moderated correspondence between subjective-by-gene units of analysis. Younger ages and inclusion of either diagnostic group reduced correspondence.
Conclusions:
These findings indicate that the RDoC initiative has generated limited research within the social processing domain across units of analysis in youth to date. Moreover, National Institute of Mental Health (NIMH)-funded studies do not appear to be biased towards supporting the RDoC framework. However, the limited number of included studies precludes the generalizability of these findings and underscores the need for further research. Despite this, results suggest that the NIMH model for providing standard batteries of measurement tools may effectively reduce spurious correlations between subjective-by-behavior units of analysis.
In 2010, the National Institute of Mental Health (NIMH) adopted the Research Domain Criteria (RDoC) framework in an effort to advance mental health research by encouraging the translation of basic science findings to clinical applications and interventions (Insel et al., 2010; Sanislow et al., 2010). The RDoC Matrix was developed to provide a framework for examining constructs across units of analysis, such as genes or subjectively reported symptoms, within the same theoretical construct. However, the extent to which the RDoC framework has been adopted, whether there is correspondence between units of analysis within a construct, and whether third variables influence levels of correspondence between units of analysis have not been empirically examined. It is particularly important to do so in pediatric clinical populations, given that many forms of psychopathology emerge or and many domains are still undergoing development in childhood and adolescence (Cicchetti & Rogosch, 2002). This is especially true within the social processing domain as social skills and peer relationships become imperative for normative development in childhood and adolescence (Tudge & Winterhoff, 1993). Moreover, the onset of disorders associated with deficits in social processing often occurs in childhood (e.g. Autism Spectrum Disorder (ASD) at age 3; Short & Schopler, 1988), and adolescence (e.g. Social Anxiety Disorder at age 13; Beesdo et al., 2010). Thus, whether there will be correspondence between units of analysis within a given RDoC domain/construct/subconstruct remains to be evaluated in the social processing domain in youth. Likewise, given the heterogeneity in child clinical populations, examining the degree to which correspondence between units of analysis varies as a function of key study-level features (e.g., mean age, gender composition of participants, funding source) is also vital. Therefore, the goal of this meta-analysis is to quantify (1) the distribution of studies measuring different units of analysis that implement the RDoC framework within the social processing domain in children and adolescents, (2) the degree of correspondence across units of analysis within the social processing domain, and (3) if correspondence is influenced by other factors.
The RDoC Framework
The RDoC framework is comprised of six domains: social processing, negative valence systems, positive valence systems, arousal and regulatory systems, and cognitive systems. These domains are further comprised of constructs and subconstructs that are measured across eight units of analysis: genes, molecules, cells, circuits, physiology, behavior, subjective report, and paradigms. Although references to specific genes have recently been removed from the RDoC matrix, this analysis was conducted prior to that update; therefore the gene unit of analysis was included for completeness. The RDoC matrix was developed to provide researchers a framework for to examining multiple units of analysis, thereby linking findings from basic science units of analysis (gene, cell, molecule, physiology, circuit) to clinical units of analysis (behavior, subjective report) within a given subconstruct, construct, and domain.
The RDoC initiative resulted in two major departures from earlier research initiatives. First, by adopting the RDoC framework, the NIMH directed more funding into basic science, mechanistic, and clinical research with clear biological outcome measures. As a result, funding for psychosocial intervention research without clear biological outcome measures has decreased by 45% (Reardon, 2017; Teachman et al., 2018). Second, the RDoC framework encourages a dimensional investigation across units of analysis and diagnoses but within a domain/construct/subconstruct. As such, the RDoC initiative has influenced the allocation of funding and study design towards more biologically based research on mental health. This paper offers an empirical examination the effectiveness of promoting RDoC-style studies, the usefulness of the units of analysis within the RDoC matrix, and the correspondence between each unit within the RDoC domain.
To date there has not been a systematic examination of the level of correspondence between units of analysis, and whether the correspondence is in the expected direction. Namely, that heightened risk in one unit of analysis is associated with heightened risk in another unit of analysis. For example, the RDoC framework might suggest that deficits in understanding the mental state of others should be evident across multiple units of analysis (e.g., subjective, behavioral, physiological, and circuit-level measures). Moreover, whether the RDoC framework is being used to examine correspondence between units of analysis within the social processing domain/construct/subconstruct in youth who are at risk for or have emerging psychopathology has not specifically been tested.
The RDoC Social Processing Domain
According to the RDoC framework (Version 2), the social processing domain includes four constructs and corresponding subconstructs: affiliation and attachment, social communication (subconstructs: reception of facial communication, production of facial communication, reception of non-facial communication, and production of non-facial communication), perception and understanding of self (subconstructs: agency and self-knowledge), and perception of understanding of others (subconstructs: animacy perception, action perception, and understanding mental states; Supplemental Material Table 1). As summarized below, a substantial amount of research has been done to measure the constructs and subconstructs in the social processing domain for at least one unit of analysis in animal models and primarily adult humans. Yet, less is known about the correspondence between units of analysis or whether these relations extend to youth.
Affiliation and Attachment.
Specific cells in the medial preoptic area, bed nucleus of the stria terminalis, ventral tegmental area, and nucleus accumbens facilitate mother-infant attachment (Insel & Young, 2001). D2 and V1a receptor gene expression triggers a transition from avoidant behavior to maternal behavior after the birth of a child and has been associated with partner bonding (Insel & Young, 2001). Positive attachment styles, bonding, and affiliation at the molecular level are associated with oxytocin and arginine vasopressin levels (Young, Lim, Gingrich, & Insel, 2001). Differential engagement in neural circuits, including the substantia nigra, ventral tegmental area, and fusiform gyrus emerge when parents view their own child’s face versus unfamiliar children’s faces (Stoeckel, Palley, Gollub, Niemi, & Evins, 2014). Physiologically, low affiliation with peers, in the form of peer provocation, is related to increased heart rate reactivity and vagal regulation (Hessler & Fainsilber Katz, 2007). Attachment stress has been tested at the physiological level through cortisol and progesterone levels (Wirth & Schultheiss, 2006), at the behavioral level through the strange situation task (Ainsworth, 1964), and at the paradigm level using the Cyberball paradigm (Yaakobi & Williams, 2016). Moreover, subjective ratings of interpersonal attachment provide insight into perceptions of attachment and affiliations by one’s self or by others (Schwartz, Lindley, & Buboltz Jr, 2007). Additionally, subjective ratings have been used to examine how adolescent’s attachment orientations relate to their perceived ability to tell and detect lies and truths (Elaad et al., 2012).
Social Communication.
In the brain, the fusiform face area and surrounding portions of the superior temporal sulcus contain cells that exclusively respond to facial stimuli and face processing (Tsao, Freiwald, Tootell, & Livingstone, 2006). The serotonin transporter gene has been associated with deficits in social communication skills in ASD (Tordjman et al., 2001). Molecular studies have shown that D1, D2, and AVP 1a and 1b, oxytocin, estrogen, androgen, GABA, and serotoninergic receptors have roles in the reception and production of responses to facial and non-facial social stimuli (Skuse & Gallagher, 2009). Face processing has been measured within the circuit unit of analysis in the fusiform face area (Kanwisher, McDermott, & Chun, 1997), and within the physiological unit of analysis through the N170 event-related potential (ERP) in response to emotional faces (Leppänen, Moulson, Vogel-Farley, & Nelson, 2007). Non-facial social communication processing has also been modeled using real-time social interactions to uncover neural circuits, such as in the dorsal anterior cingulate (dACC) and insula during the anticipation of social evaluation, and in the amygdala during the receipt of social evaluation from purported peers, using the Virtual School Paradigm (Jarcho et al., 2016). Behavioral coding of the production of spontaneous facial expressions has been measured using the Facial Action Coding System (FACS; Sayette, Cohn, Wertz, Perrott, & Parrott, 2001). Various subjective report measures such as the Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012) assess production and processing of both facial and non-facial aspects of social communication.
Perception and Understanding of Self.
Perception and understanding of self also has been measured across many of the RDoC units of analysis and between the subconstructs of agency and self-knowledge. Von Economo cells in the frontoinsular cortex and dACC are engaged during self-reflective thinking (Allman et al., 2010). On a neural circuit level, the medial prefrontal cortex and the posterior cingulate cortex are engaged when making decisions about one’s own abilities, traits, and attitudes (Johnson et al., 2002). The P300 ERP has been used as a physiological index of attention to self-relevant stimuli (Gray, Ambady, Lowenthal, & Deldin, 2004). Behaviorally, tasks in which participants judge the agency of hand movements generated by either themselves or an experimenter have been used to assess delusions of control in schizophrenic patients (Daprati et al., 1997). These tasks are often corroborated by subjective-reports of perceptions about one’s own attitudes or experiences (Chapman, Chapman, & Raulin, 1978). Lastly, Paradigms, such as the Self-Referential Memory Paradigm, have been developed to test differences in remembering stimuli that are processed with reference to the self, compared to other semantic terms to assess self-knowledge (Harvey, Lee, Horan, Ochsner, & Green, 2011).
Perception and Understanding of Others.
Animacy and action perceptions as well as understanding mental states of others have also been measured using RDoC units of analysis. Broadly, single-nucleotide polymorphisms at the genetic level, such as at WFS1, OXTR, OXTm and AVOR1B, have been associated with perceiving and understanding empathy and actions of others (Baron-Cohen, Tager-Flusberg, & Lomardo, 2013). Specifically, action perception has been localized to mirror neuron cells (Braadbaart, Williams, & Waiter, 2013) within neural circuits, such as the inferior parietal cortex, supplementary motor area (SMA), and the premotor area (Jackson, Meltzoff, & Decety, 2006). Animacy perception is processed in the fusiform face area, superior temporal sulcus, and occipital face area (Schultz, Friston, O’Doherty, Wolpert, & Frith, 2005). Both animacy and action perception can be measured physiologically using Mu suppression (Braadbaart et al., 2013). Using a behavioral False Belief Task, neural circuit activation in the temporal pole, temporal sulcus, orbitofrontal cortex, and ACC has been implicated in developing an understanding of the mental states of others (Saxe, Carey, & Kanwisher, 2004). Subjective report measures have also been used to examine empathy (Muncer & Ling, 2006). In terms of action and animacy perception, the Point Light Displays of the Biological Motion Paradigm have been used to measure the ability to discern biological motion (Neri, Morrone, & Burr, 1998), whereas the Reading the Mind in the Eyes Paradigm tests the ability to understand other’s mental states (Baron-Cohen, S., & Cross, 1992).
In sum, a substantial body of research has measured each RDoC construct and subconstruct of the social processing domain within at least one unit of analysis. However, the RDoC initiative allows for the examination of correspondence between units of analysis. Additionally, much of the foundational research in the social processing domain has been conducted with adult and animal populations and examining parent-child and peer relationships. The extent to which individual studies have investigated correspondence between multiple units of analysis has not, to our knowledge, been empirically investigated in youth.
Plausible Moderators
In addition to quantifying the extent to which research in the social processing domain in youth has utilized the RDoC framework, as well as the degree of correspondence between units of analysis, another aim of this paper is to determine if other study-level factors influence the magnitude or direction of correspondence.
Age.
Age is an important moderator to investigate given that children and adolescents are actively developing social skills as they age, and specific skills come online at different points in development. For example, the ability to recognize familiar faces happens early in life (e.g., age 5–8 months; Legerstee, Anderson, & Schaffer, 1998), whereas understanding the goals and intentions of others occurs later (e.g., age 18 months; Meltzoff, 1995). Friendships begin to form at 18–36 months, yet continue to change and gain complexity throughout adolescence (Bagwell & Schmidt, 2013; Rubin, Bukowski, & Parker, 1998). Moreover, age-related changes in social processing in one unit of analysis might influence changes in other units of analysis. For example, subtle changes in brain development might precede the onset of behavioral changes in social decision-making (Arain et al., 2013).
Gender.
Gender is an important moderator to consider as it influences social development and psychopathology. For example, males and females respond differently to familiar and unfamiliar faces after the administration of arginine vasopressin (Thompson, George, Walton, Orr, & Benson, 2006). Specifically, males exhibit more neutral facial expressions and rate faces as less friendly, whereas females exhibit more affiliative facial expressions and rate faces as more friendly (Thompson et al., 2006). This suggests that gender differences in response to molecular units of analysis influence behavioral units of analysis. Moreover, early peer-friendships tend to be gender-matched, which exaggerates gender-stereotyped play behavior, which in turn, influences gender-specific social communication skills (Maccoby, 1999). Specifically, females tend to listen and take turns in conversation, whereas males tend to be more reactive and less verbose (Martin & Fabes, 2001; Mendelson, Gates, & Lerner, 2016). Gender differences also occur across different forms of psychopathology, with higher rates of depression and anxiety disorders in females, and higher rates of antisocial personality disorder and substance abuse in males (American Psychiatric Association. & American Psychiatric Association. DSM-5 Task Force., 2013). Together, these data suggest that gender influences the development of psychopathology which may be reflected at many different levels of analysis, and thereby differentially effect correspondence between units of analysis.
IQ.
Intelligence level (IQ) also moderates performance on goal-directed task-based behavior and influences social development. For example, adolescents who are more likely to respond with reactive aggression to social situations tend to have lower verbal IQ, whereas adolescents who exhibit proactive aggression in social situations tend to have higher verbal IQ (Arsenio, Adams, & Gold, 2009). Additionally, higher IQ is associated with better social cognition, perspective-taking, empathetic understanding, interpersonal social skills, verbal and nonverbal communication, and prosocial behavior; each of which is protective against certain forms of behavior common in psychopathology (Bennett, Farrington, & Huesmann, 2005). IQ is also highly heritable, and the heritability of IQ changes with age (Plomin & Spinath, 2004). Moreover, genes associated with IQ influence behavioral performance on cognitive, and social-emotional tasks (Jones et al., 2011; Plomin & Spinath, 2004). This suggests that IQ might have moderating effects on the correspondence between units of analysis, such as genes and behavior. Thus, examining IQ as a potential moderator of correspondence between units of analysis may be important.
Funding Agency.
Given that the NIMH has specifically directed more funding to “RDoC-informed research,” it is important to evaluate whether funding by the NIMH impacts reported levels of correspondence (i.e., the likelihood to report findings in support of RDoC). If differences in levels of correspondence exist between NIMH-funded studies compared to studies funded by non-NIMH agencies, this could suggest a bias in reporting or an improved design of NIMH-funded studies for explicitly testing correspondence between RDoC units of analysis. Such result would be useful for determining the impact of NIMH’s funding mission in promoting research consistent with the aims of RDoC and guiding decisions of future funding priorities by the NIMH. Additionally, by adding funding as a moderator of interest, we investigate how NIMH funding priorities may affect correspondence between different unit of analysis on the RDoC matrix which could help examine the usefulness of each unit pair within the RDoC matrix when correspondence is explicitly being tested.
Diagnosis.
Although RDoC shifts away from traditional DSM diagnostic boundaries, it is important to determine whether existing diagnostic categorization influences levels of correspondence between units of analysis. If it does then diagnosis should remain an important methodological consideration in research. Patients with different disorders may exhibit deficits within the social processing domain that are in opposite directions. For example, individuals with both antisocial personality disorder and ASD have deficits in empathy (Blair, 2013) and the ability to detect emotional facial and vocal expressions (Blair, Budhani, Colledge, & Scott, 2005), and demonstrate alterations in brain function and connectivity in the vmPFC and amygdala (Blair, 2013; Castelli, Frith, Happé, & Frith, 2002). However, the ability to take the perspective of others, or Theory of Mind (ToM), is sometimes impaired in ASD, yet intact in antisocial personality disorder (Jones, Happé, Gilbert, Burnett, & Viding, 2010). Thus, examining diagnosis as it relates to the correspondence between units of analysis might reveal important associations that correspond to specific patterns of psychopathology in youth.
Hypotheses and Aims
One of the primary goals of this study, as defined by the guidelines for this special issue, was to quantify the frequency with which studies specifically tested the correspondence between unit-by-unit analysis pairs within the social processing domain/constructs/subconstructs in children and adolescents. If the RDoC model is being tested as intended, and constructs are sufficiently well-specified, then many studies may have examined correspondence between units of analysis. Second, we performed a preliminary meta-analysis to test both the degree and direction of correspondence between units of analysis. It was hypothesized that correspondence between units of analysis will emerge within a domain/construct/subconstruct, such that deficits in one unit correspond to deficits in another. Lastly, when possible, we aimed to determine if age, gender, intelligence, funding source, and/or diagnosis has moderating effects on the degree or direction of correspondence between units of analysis.
Method
The methods and results reported in this manuscript are in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA; http://www.prisma-statement.org/) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009).
Information Sources and Literature Search
Four separate database searches were conducted using Google Scholar, Web of Science, PsycINFO, and PubMed covering the literature from July 2010 to December 2017, the range specified for this special issue. For each of the RDoC’s social processing domain subconstructs, keywords were generated to encompass all terms that could be used to describe that subconstruct (Supplemental Material Table 1). Keywords to identify articles explicitly focusing on children and adolescents were also included. For example, the subconstruct of reception of facial communication used the following Boolean string: (child OR adolescent OR teen OR school age OR youth) AND (facial communication OR affect recognition OR facial recognition OR facial characterization OR facial expression OR emotion identification OR joint attention OR implicit social perception). Each subconstruct’s set of keywords was searched independently within each database to find articles that applied specifically to a single subconstruct. This was done to enable tests of correspondence between units of analysis within a single subconstruct, as well as collapsed across constructs and domains. Restrictions were applied when available within a database to include only peer-reviewed and empirical articles in English.
Inclusion/Exclusion Criteria
The following inclusion criteria were used, as required across all articles in this special issue, to identify eligible studies: (1) cite at least one of the three original papers which defined the RDoC initiative in 2010 (Cuthbert & Insel, 2010; Insel et al., 2010; Sanislow et al., 2010) or include “RDoC” or “Research Domain Criteria” in the title, abstract, or keywords of the manuscript; (2) human studies of children and adolescents ages 2–18 years; (3) specifically examine the social processing domain in the RDoC framework based on keywords described in Supplemental Material Table 1; (4) empirical research; (5) peer-reviewed; (6) full-text article available in English; (7) measure at least two units of analysis (based on Version 2 of the RDoC matrix); and (8) test correspondence between two different units of analysis using a correlation or regression analysis (r, β, or Odds Ratio [OR]).
Study Selection
The initial literature searches identified 28,384 articles in Google Scholar, 385 in Web of Science, 0 in PsycINFO, and 166 in PubMed. Among these, 26,074 duplicate papers were identified and removed, yielding 2,795 articles of interest. The large number of duplicates was the result of conducting a separate search for each subconstruct. In so doing, there was a high degree of overlap in the articles each search identified. Abstracts for non-duplicate articles were screened for eligibility criteria by two independent undergraduate- or graduate-level reviewers, with 20.5% overlap. Reliability among undergraduate coders was fair (ICC(2,1)= .622, p < .001); thus, a graduate-level coder reviewed all of the inclusion and exclusion criteria again with the undergraduate coders and had them re-review all of their decisions until there was 100% consensus on all decisions. Then, a graduate student coder independently screened all abstracts that were included by one or both undergraduate coders to ensure 100% agreement. This resulted in 158 articles that were eligible for full-text review. The full-text review included a discussion amongst all coders (2 undergraduate and 3 graduate-level) for each article that matched specific inclusion/exclusion criteria to determine if all of the inclusion criteria were met. Discrepancies were resolved by consensus with senior co-authors (M.D.L & J.M.J). This resulted in 33 articles that were eligible for subsequent analyses (Figure 1). The most common reasons for rejection at this stage were better fit with another RDoC domain (N = 57), no mention of RDoC (N= 31), not an empirical article (N=30), or failure to test for correspondence across units of analysis (N = 10).
Figure 1:
PRISMA Flowchat
Data Collection Processes
Statistics were extracted for all included articles by three independent Ph.D.-level graduate student coders with 100% overlap. For each article, we extracted the Pearson product-moment correlation (r) value, the standardized β, or OR across two units of analysis, the names of the two compared measures, their corresponding unit of analysis, and the sample size. Units of analysis, such as “subjective” or “behavior” were defined using the guidelines published on the NIMH RDoC Matrix website under “units of analysis”. For example, measures of electroencephalography (EEG), electromyography (EMG), or cortisol were considered “physiology” units, whereas measures of parent- or child-reports on the youth’s behavior were considered “subjective” units (Supplemental Material Table 2). Additionally, the direction of each measure was coded either in the “positive direction,” whereby higher values on the measure corresponded to more normative outcomes, or a “negative direction,” whereby more negative values represent more severe pathological outcomes. For example, higher scores on the Child Behavior Checklist (CBCL; Achenbach, 2011), a subjective measure, reflect more psychopathology. Thus, the direction would be coded as negative (Patriquin, Lorenzi, Scarpa, Calkins, & Bell, 2015). Conversely, more time spent looking at faces, a behavioral eye-tracking measure, reflects a more normative behavior. Thus, the direction would be coded as positive. Once the direction of each measure was determined, the expected direction of the correlation, as hypothesized by the author or as inferred by well-accepted norms (see examples above), was calculated based on the combined directions of each measure. Then, in Comprehensive Meta-Analysis, Version 3 (CMA-3; Borenstein, Hedges, Higgins, & Rothstein, 2015), the effect sizes were computed to compare the expected direction of the correlation with the actual value of the observed correlation. Significant “positive” effect sizes indicated correspondence between the two measures in the expected direction, significant “negative” effect sizes indicated disagreement between the two measures, meaning the correlation was not in the expected direction. Non-significant effect sizes indicated no relation (neither correspondence nor disagreement) between the two measures.
The circuit unit of analysis consisted of six functional magnetic resonance imaging (fMRI)-based studies. Within fMRI studies, the direction of brain activation in relation to psychopathology may or may not be known and often varies based on region and task. In fact, four studies reported at least one correlation without an explicit hypothesis of the expected direction of activation. Three studies exclusively reported correlations in the absence of directional hypotheses. The absence of specific hypotheses may reflect the fact that the utilization of developmental social neuroscience to study psychopathology in youth is in its infancy. In order to accommodate this, the circuit unit of analysis was coded in two ways. The first approach to coding of the direction of the circuit unit of analysis was identical to the method described above for all other units of analysis. This method examined explicitly-stated hypotheses by the authors that might vary based on the task and the region measured in their study. In this approach, any correlation that had a hypothesized direction was coded as such, and any correlation that did not have a hypothesized direction was coded as “positive”. Because not all correlation values had explicitly hypothesized direction, a second atheoretical approach was also conducted. In this coding method, all increases in brain activation were coded as “positive” no matter the region, the task, or the hypothesis. By utilizing a single direction for all circuit measures, correspondence between brain activation can be made uniformly across other units of analysis to determine how the level of activation/size/structural integrity corresponds with other units of analysis, rather than a proposed more “normative vs. pathological” pattern of activation corresponds with other units of analysis.
Predefined moderators of interest included average participant age (in years), gender (ratio of males to females), funding agency name (NIMH vs. other funding agency), and diagnosis (dummy coded for all DSM-5 categorical diagnoses; for example, diagnosis of ASD was dummy coded as either 0 for non-ASD samples, or 1 for ASD samples). Full-scale IQ, verbal and non-verbal IQ values were extracted but could not be analyzed due to an insufficient number of studies.
Intraclass correlation coefficients (ICC) were calculated for all extracted data to determine the reliability between coders. The absolute agreement between coders was excellent across all 33 articles (ICC(2,1)>0.826 on all coded variables; Table 1). Corresponding authors were contacted for five articles (Aoki et al., 2017; Bal & Lord, 2015; Blanken et al., 2016; Herrington, Miller, Pandey, & Schultz, 2016; Van Goozen et al., 2016) because additional data was needed to generate effect sizes for extracted correlations. We obtained data for all but one of the articles. As a result, only correlation values with sufficient data from that article (Van Goozen et al., 2016) were included in this study.
Table 1:
Intra-Class Correlation Analysis to assess agreement between three coders for data extraction.
| Measure | N | Cronbach’s Alpha | ICC(2,1) | P-value |
|---|---|---|---|---|
| Age | 372 | 0.878 | 0.848 | <.001 |
| FSIQ | 53 | 0.999 | 0.999 | <.001 |
| VIQ | 17 | 0.999 | 0.998 | <.001 |
| NVIQ | 35 | 1 | 1 | <.001 |
| Sample Size | 345 | 1 | 1 | <.001 |
| Correlation Value | 370 | 0.999 | 0.999 | <.001 |
| Effect Direction | 416 | 0.826 | 0.824 | <.001 |
Data Items and Analysis
Data extracted from included articles were entered into CMA. Effect sizes were calculated using the correlation (r), β, or OR, and sample size for each unit-by-unit correspondence value in each study. Then, in order to link effect sizes to each study, the weighted average effect sizes for each unit-by-unit pair (i.e., subjective-by-behavior, etc.) were used in the model when multiple correlations between a unit pair were present in a single study. This method accounts for the lack of independence of correspondence measure samples within a single study. Additionally, between-study samples were confirmed to not include overlapping samples. Data extracted for the single longitudinal study included (Chawarska, Macari, Powell, DiNicola, & Shic, 2016) was handled in the same manner, condensing temporal information into one average correlation value per unit-by-unit pair.
The distribution of studies was assessed based on the type of unit-by-unit pairs measured as well as the subconstruct and construct measured within each study. The number of studies with correlations for each RDoC unit-by-unit of analysis can be found in Table 2.
Table 2.
Distribution of applicable constructs, subconstructs, and Units of Analysis of Included Articles
| Citation | Literature Search Constructs | Literature Search Subconstructs | Total Units of Analysis | Unit-by-Unit Pair(s) |
|---|---|---|---|---|
| (Blanken et al., 2016) | Social Communication | Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication | 2 | Subjective × Behavior |
| (Briggs-Gowan et al., 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Subjective × Behavior |
| (Chawarska et al., 2016) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Subjective × Behavior |
| (Cohn et al., 2015) | Affiliation and Attachment, Social Communication | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication | 2 | Circuit × Subjective |
| (Dadds, Moul, Hawes, Mendoza Diaz, & Brennan, 2015) | Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 3 | Gene × Physiology, Gene × Subjective |
| (Gatzke-Kopp et al., 2015) | Social Communication, Perception and Understanding of Others | Production of Non-Facial Communication, Production of Facial Communication, Reception of Facial Communication, Action Perception | 2 | Physiology × Subjective |
| (Van Goozen et al., 2016) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 4 | Gene × Physiology, Gene × Behavior, Gene × Subjective, Physiology × Subjective, Subjective × Behavior |
| (Henderson et al., 2014) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Circuit × Subjective |
| (Herrington et al., 2016) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 3 | Circuit × Subjective, Subjective × Behavior |
| (Ho et al., 2016) | Affiliation and Attachment, Social Communication, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Animacy Perception, Action Perception | 2 | Circuit × Behavior |
| (Bal & Lord, 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception | 2 | Subjective × Behavior |
| (Jarcho et al., 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Action Perception | 2 | Circuit × Behavior |
| (Kim et al., 2013) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Subjective × Behavior |
| (Kohls et al., 2014) | Social Communication | Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication | 2 | Circuit × Subjective |
| (Kujawa et al., 2014) | Affiliation and Attachment, Social Communication, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Action Perception, Understanding Mental States | 3 | Physiology × Behavior, Physiology × Subjective, Subjective × Behavior |
| (Luking et al., 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Subjective × Behavior |
| (Luking et al., 2016) | Affiliation and Attachment, Social Communication | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, | 2 | Subjective × Behavior |
| (Martin, Hamshere, Stergiakouli, O’Donovan, & Thapar, 2014) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Gene × Subjective |
| (Nelson et al., 2015) | Affiliation and Attachment, Social Communication | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication | 2 | Physiology × Subjective |
| (Parish-Morris et al., 2013) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Subjective × Behavior |
| (Patriquin, Lorenzi, Scarpa, Calkins, & Bell, 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Physiology × Subjective |
| (Thomas et al., 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Gene × Subjective |
| (Tseng et al., 2015) | Affiliation and Attachment, Social Communication, Perception and Understanding of Self, Perception and Understanding of Others | Affiliation and Attachment, Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Agency, Self-Knowledge, Animacy Perception, Action Perception, Understanding Mental States | 2 | Circuit × Subjective |
| (van den Bulk et al., 2014) | Social Communication, Perception and Understanding of Others | Production of Non-Facial Communication, Reception of Non Facial Communication, Production of Facial Communication, Reception of Facial Communication, Animacy Perception | 2 | Circuit × Subjective |
| Aoki et al 2017 | Social Communication | Production of Facial Communication | 2 | Subjective × Circuit |
| Baribeau et al 2017 | Social Communication, Perception and Understanding of Self | Production of Facial Communication, Agency | 3 | Subjective × Gene, Behavior × Gene, Subjective × Behavior |
| Björnsdotter et all 2016 | Social Communication, Perception and Understanding of Others | Production of Non-Facial Communication, Action Perception | 2 | Subjective × Circuit |
| Germine et al 2016 | Social Communication | Reception of Facial Communication | 2 | Behavior × Gene |
| Lloyd-Fox et al 2017 | Social Communication, Perception and Understanding of Self | Production of Facial Communication | 2 | Subjective × Circuit |
| McQuade et al 2017a | Affiliation and Attachment | Affiliation and Attachment, Self-Knowledge | 3 | Behavior × Physiology, Subjective × Physiology, Subjective × Behavior |
| McQuade et al 2017b | Affiliation and Attachment | Affiliation and Attachment | 2 | Subjective × Physiology |
| Parellada et al 2017 | Social Communication, Affiliation and Attachment | Affiliation and Attachment, Production of Facial Communication, Self-Knowledge | 2 | Subjective × Circuit |
| Rudra et al 2016 | Social Communication, Affiliation and Attachment | Reception of Facial Communication | 2 | Subjective × Behavior |
Note. Included here is the final set of included articles in the review, including the constructs, subconstructs, number of units of analysis, and types of units of analysis for each.
Correspondence between each unit-by-unit pair was assessed using random-effects models for each pair of unit-by-unit analysis (Schmidt, Oh, & Hayes, 2009) that had a least 2 studies (Pigott, 2012). Potential publication bias was assessed using the tandem method (Ferguson & Brannick, 2012). The first criterion is the Egger’s regression test, wherein significant findings suggest publication bias (Egger & Smith, 1998). The second criterion is the Trim and Fill method which evaluates an asymmetrical funnel plot to test unbalanced plots, then “trims” the studies responsible for the observed asymmetry, and assigns new effect sizes to correct for suspected publication bias. If the effect is no longer significant, then publication bias is suspected (Duval & Tweedie, 2000). The third criterion is Orwin’s Fail Safe N, wherein publication bias is suspected if the number of non-significant missing studies (i.e., file-drawer articles) needed to reduce the combined effects to a trivial level (+/− .10) is lower than the number of studies in the analysis (Ferguson & Brannick, 2012). Publication bias was deemed “probable” if indicated by all three criteria, “possible” if one or two criteria were met, and “unlikely” if no evidence of bias was found.
Moderator analyses were conducted within each unit-by-unit pair when heterogeneity existed across the studies, and there were a sufficient number of studies to have reasonable ability to interpret the results (Sánchez‐Meca & Marín‐Martínez, 1998). Heterogeneity within each unit-by-unit comparison was assessed with I2 which provides a percentage of the amount of variance that is attributable to between-study variation (Huedo-Medina, Sánchez-Meca, Marín-Martínez, & Botella, 2006). Moderator analyses were conducted if there was a significant I2 with a cutoff of 25% (Huedo-Medina et al., 2006), using meta-regression for the continuous moderators (i.e., age, gender ratio, and IQ) and analogue-to-ANOVA for the categorical moderators (i.e., funding agency and diagnosis).
Results
Distribution of RDoC-based Studies
Thirty-three studies were identified that matched the inclusion criteria. Eight unit-by-unit pairs were identified across studies: subjective-by-behavior (n=14), subjective-by-circuit (n=10), subjective-by-physiology (n=7), subjective-by-gene (n=5), behavior-by-circuit (n=2), physiology-by-gene (n=2), behavior-by-physiology (n=2), and behavior-by-gene (n=3). None of the other possible unit-by-unit pairs were represented in included studies. Twenty-seven studies included two units of analysis, five studies included three units of analysis, and one study included four units of analysis. Moreover, a majority number of studies (n=14) included subjective-by-behavior units, and all studies included the subjective unit of analysis. Additionally, ten of the thirty-three studies included more than one diagnostic group.
Articles were not specific to a single subconstruct or construct. For example, although a set of search terms for each construct and subconstruct were entered in an independent search to identify articles that specifically assessed a single construct or subconstruct, all ten searches (see Supplemental Material Table 1) yielded the same 11 papers. In fact, only four studies were uniquely identified by a single search (Table 2). Given this unexpected lack of specificity for constructs and subconstructs within a single article, we were unable to conduct analyses on the correspondence between units of analysis within a single construct or subconstruct. Instead, we examined correspondence between units of analysis across the entire social processing domain with hopes that this preliminary analysis would encourage future research.
Correspondence between Unit-by-unit Pairs within the Social Processing Domain
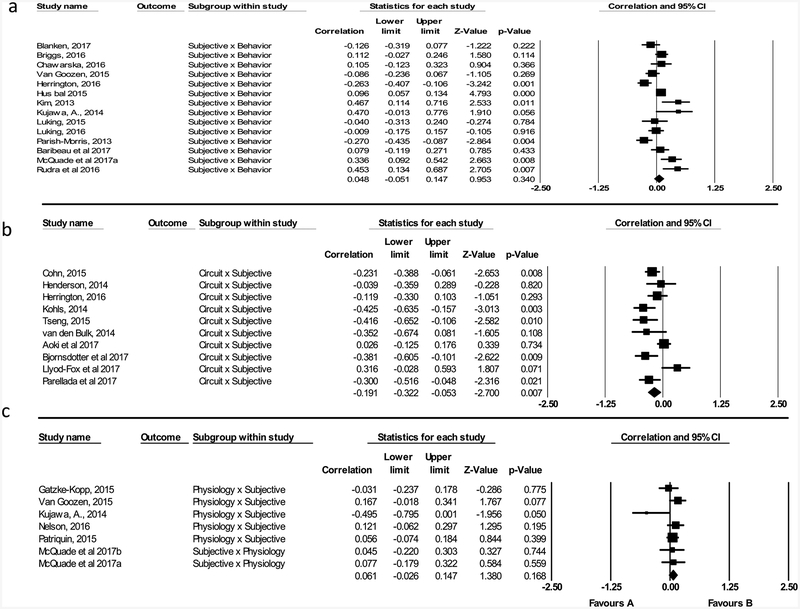
Subjective-by-Behavior.
Fourteen studies reported associations between subjective reported symptoms and behavioral performance on tasks. Effect sizes ranged in magnitude from r = −0.270 to 0.470 (positive effects indicate a correlation between more severe subjective-reported symptoms and worse performance on behavioral tasks; negative effects indicate more severe subjective-reported symptoms correlated with better performance on behavioral tasks). However, the relation between subjective report and behavior was not significant (r= 0.048, [95% CI: −0.051, 0.147], Z = 0.953, p= 0.340; Figure 2a). There was significant heterogeneity in effect sizes (I2 = 77.562); thus, exploratory moderator analyses were conducted (see Moderator Analyses below).
Figure 2:
Random Effects models for (a) Subjective-by-Behavior, (b) Subjective-by-Circuit, (c) Subjective-by-Physiology
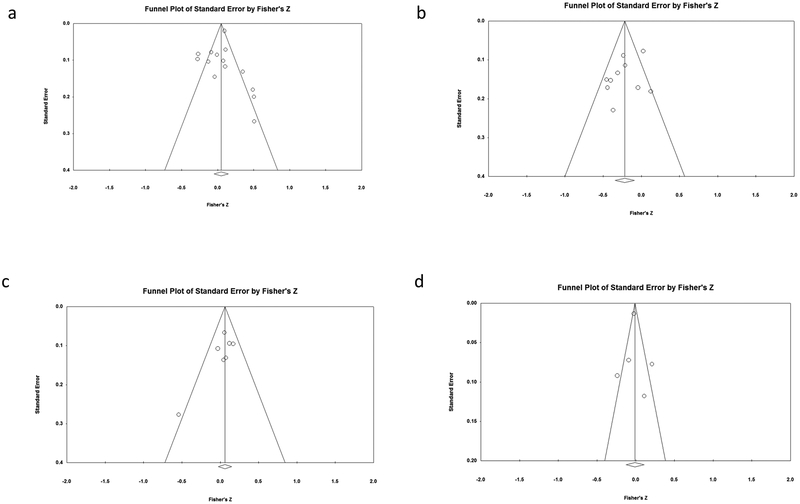
Publication bias was not evident based on the Egger’s regression method bias (p = 0.775; see Figure 3a for funnel plot). Using Duval and Tweedie’s Trim and Fill method, our sample of effect sizes is missing two studies to the left of mean. When accounting for these studies to correct for suspected publication bias, the aggregated effect size decreased from r = 0.04 to 0.02, though still not significantly different from zero. In conclusion, it is possible that publication bias may be present, but it appears to have had little impact. Orwin’s fail-safe N was not applicable because the model of subjective by behavior was not significant (Ferguson & Brannick, 2012). Thus, publication bias is unlikely to be problematic for the subjective-by-behavior unit pair model.
Figure 3:
Publication Bias Funnel Plot for (a) Subjective-by-Behavior, (b) Subjective-by-Circuit, (c) Subjective-by-Physiology, (d) Subjective-by-Gene
Subjective-by-Circuit.
Ten studies reported associations between subjective symptoms and neural circuits. Using the hypothesis-driven method of coding (method 1), effect sizes ranged from r = −0.425 to 0.125 (positive effects indicate a correlation between less severe subjectively-reported symptoms and more normative neural circuits; negative effects indicate more severe subjectively-reported symptoms and more normative neural circuits). The relation between subjective report and circuit was significant (r = −0.214, [95% CI: −0.330, −0.091], Z = −3.386, p = 0.001). There was significant heterogeneity among effect sizes (I2 = 55.829); thus, moderator analyses were conducted.
Using the hypothesis-driven method of coding (method 1), publication bias was not evident according to the Egger’s regression method bias (p = 0.200; see Figure 3b for funnel plot). Using Duval and Tweedie’s Trim and Fill method, one study was missing to the right of the mean. While the adjusted effect size correcting for suspected publication bias increased from r = −0.214 to −0.191, it was still significantly different from zero. Therefore, it is possible that publication bias may be present, but it appears to have had little impact. Orwin’s fail-safe N was 9, which is less than the total number of studies (N = 10). Taken together, these analyses suggest that publication bias is unlikely to be problematic.
Using the atheoretical method (method 2), the magnitude of effect sizes ranged from r = −0.425 to 0.316 (positive effects indicate a correlation between less severe subjectively-reported symptoms and increased brain activation or volume size or structural integrity; negative effects indicate more severe subjectively reported symptoms and decreased brain activation or volume size or structural integrity). The relation between subjective report and circuit was significant (r = −0.191, [95% CI: −0.322, −0.053], Z = −2.700, p= 0.007; Figure 2b). There was significant heterogeneity among effect sizes (I2 = 64.614); thus, moderator analyses were conducted (see Moderator Analyses below).
Using the atheoretical method (method 2), publication bias was not evident according to the Egger’s regression method bias (p=0.354). Using Duval and Tweedie’s Trim and Fill method, two studies were missing to the right of the mean. When accounting for this study to correct for suspected publication bias, the aggregated effect size increased from r = −0.191 to −0.132, and it was no longer significantly different from zero. This suggests publication bias may be present and the true effect size may be larger than detected and nonsignificant. However, Orwin’s fail-safe N was 7, which is less than the total number of studies (N = 10). Taken together, these analyses suggest that publication bias is possible.
Subjective-by-Physiology.
Seven studies reported associations between subjective reported symptoms and physiological arousal. Effect sizes ranged in magnitude from r = −0.495 to 0.167 (positive effects indicate a correlation between worse subjective-reported symptoms and increased physiological arousal; negative effects indicate a worse subjective-reported symptoms and decreased physiological arousal). The relation between subjective report and physiology was not significant (r = 0.061, [95% CI: −0.026, 0.147], Z = 1.380, p = 0.168; Figure 2c). There was not a significant heterogeneity in effect sizes (I2 = 16.431); thus, moderator analyses were not conducted.
Publication bias was not evident according to Egger’s regression method bias (p = 0.129; see Figure 3c for funnel plot). Using Duval and Tweedie’s Trim and Fill method, our sample of effect sizes is missing one study to the right of mean. When accounting for this study to correct for suspected publication bias, the aggregated effect size increased from r = 0.061 to 0.075 though still not significantly different from zero. In conclusion, publication bias may be present in our data, but appears to have had little impact. Orwin’s fail-safe N was not applicable because the model of subjective-by-physiology was not significant (Ferguson & Brannick, 2012). Thus, publication bias is unlikely to be problematic for the subjective-by-physiology unit pair model.
Subjective-by-Gene.
Four studies reported associations between subjective reported symptoms and genetic loading for psychopathology. Effect sizes ranged in magnitude from r= −.233 to 0.208 (positive effects indicate a correlation between worse subjective-reported symptoms and increased genetic loading for psychopathology; negative effects indicating a worse subjective-reported symptoms and decreased genetic loading for psychopathology). The relation between subjective report and genes was not significant (r = −0.009, [95% CI: −0.126, 0.108], Z = −0.153, p = 0.879; Figure 2d). There was significant heterogeneity in effect sizes (I2 = 76.079); thus, a moderator analysis was conducted.
Publication bias was not evident according to the Egger’s regression method bias (p = 0.872; see Figure 3d for funnel plot). Using the Duval and Tweedie’s Trim and Fill method, no studies were missing to the left of the mean or the right of the mean. Orwin’s fail-safe N was not applicable because the model of subjective-by-gene was not significant (Ferguson & Brannick, 2012). Thus, publication bias is unlikely for the subjective-by-gene unit pair model.
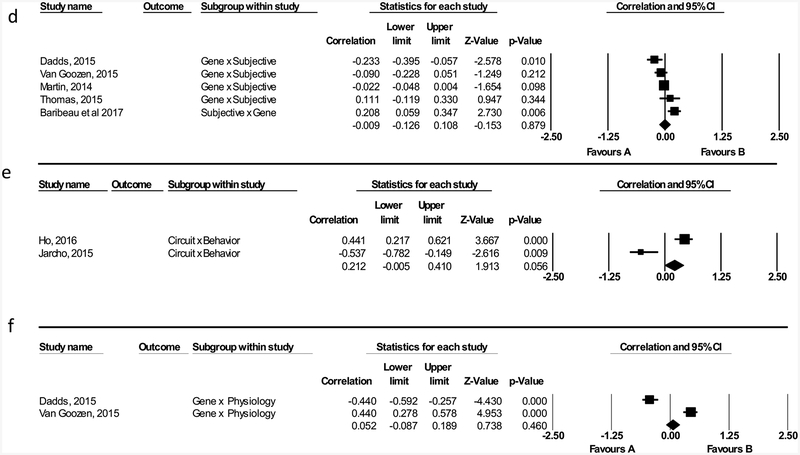
Behavior-by-Circuit.
Two studies reported associations between performance on behavioral tasks and brain activation. Effect sizes ranged in magnitude from r= −0.537 to 0.441 (positive effects indicate a correlation between worse performance on behavioral tasks and increased brain activation; negative effects indicate a worse behavioral tasks and decreased brain activation). The relation between performance on behavioral tasks and neural circuit activation was not significant (r = −0.047, [95% CI: −0.800, 0.042], Z = −0.764, p = 0.931; Figure 2e). There was significant heterogeneity in effect sizes (I2 = 93.987); however, moderator analyses, and publication bias analysis were not performed due to the small number of studies (n = 2).
Physiology-by-Gene.
Two studies reported associations between physiological arousal and genetic loading for psychopathology. Effect sizes ranged in magnitude from r = −0.440 to 0.440 (positive effects indicate a correlation between increased physiological arousal and increased genetic loading for psychopathology; negative effects indicating increased physiological arousal and decreased genetic loading for psychopathology). The relation between physiological arousal and genes was not significant (r= 0.001, [95% CI: −0.728, 0.729], Z = −0.003, p= 0.998; Figure 2f). There was significant heterogeneity in effect sizes (I2 = 97.707); however, moderator analyses, and publication bias analysis were not performed due to the small number of studies (n = 2).
Moderator Analyses
Subjective-by-Behavior.
Mean age of participants (β = −0.002, SE = 0.025, [95% CI: −0.060, 0.056], p = .932), gender ratio (β = −0.439, SE = 0.386, [95% CI: −1.313, 0.435], p = .285), diagnosis of ASD (Q[2] = 0.506, p = 0.776), diagnosis of ADHD (Q[2] = 0.212, p = 0.899), inclusion of a neurotypical sample (Q[1] = 0.316, p = 0.574), and funding source (NIMH versus other funding agency; Q[1] = 0.499, p = 0.480) did not moderate the relation between subjectively reported symptoms and behavioral performance. All other moderators could not be examined because insufficient studies included moderators of interest.
Subjective-by-Circuit.
Using the hypothesis-driven method of coding (method 1), diagnosis of GAD (Q[1] = 4.287, p = 0.038) moderated the relations between subjectively reported symptoms and neural circuits, such that studies including participants with GAD did not have correspondence between subjective reported symptoms and neural circuits (r= −0.046, [95% CI: −0.224, 0.136], Z = −0.494, p= 0.621), and studies without participants with GAD did have correspondence between subjectively reported symptoms and neural circuits (r= −0.269, [95% CI: −0.376, −0.156], Z = −4.542, p< 0.001). The mean age of participants (β = −0.030, SE = 0.013, [95% CI: −0.062, 0.0009], p = 0.055), gender ratio (β = −0.083, SE = 0.360, [95% CI: −0.935, 0.768], p 0.824), diagnosis of ASD (Q[1] = 0.195, p = 0.659), diagnosis of SAD (Q[1] = 0.523, p = 0.470), diagnosis of MDD (Q[1] = 0.105, p = 0.746), diagnosis of ADHD (Q[1] = 0.623, p = 0.430), funding source (NIH versus other funding agency; Q[1] = 0.019, p = 0.892), and funding source (NIMH versus other funding agency; Q[1] = 5.026, p = 0.081) did not moderate the relation between subjectively reported symptoms and neural circuits. All other moderators could not be examined because insufficient studies included moderators of interest.
Using the atheoretical method (method 2), the mean age of participants (β = −0.040, SE = 0.014, [95% CI: −0.072, −0.007], p = 0.023) moderated the relations between subjectively reported symptoms and brain activation, such that older ages were indicative of greater correspondence between subjectively reported symptoms and brain activation. Gender ratio (β = −0.146, SE = 0.422, [95% CI: −1.144, 0.852], p = 0.739), diagnosis of ASD (Q[1] = 0.550, p = 0.458), diagnosis of GAD (Q[1] = 2.439, p = 0.118), diagnosis of SAD (Q[1] = 0.277, p = 0.598), diagnosis of MDD (Q[1] = 0.025, p = 0.873), diagnosis of ADHD (Q[1] = 0.128, p = 0.720), funding source (NIH versus other funding agency; Q[1] = 0.068, p = 0.794), funding source (NIMH versus other funding agency; Q[1] = 0.406, p = 0.524) did not moderate the relation between subjectively reported symptoms and brain activation. All other moderators could not be examined because insufficient studies included moderators of interest.
Subjective-by-Gene.
There were not enough studies to conduct meta-regression analysis for age or gender ratio of male participants. Diagnosis of ASD (Q[1] = 6.776, p = 0.030) did moderate the relations between subjective reported symptoms and genes. However, effects were not significant for the ASD sample (r = −0.110, k = 2, [95% CI: −0.307, 0.097], Z = −1.044, p = 0.297), or for the non-ASD samples (r = −0.011, k = 2, [95% CI: −0.201, 0.181], Z = −0.110, p=0.913). Inclusion of a normative sample (Q[1] = 0.053, p = 0.820) did not moderate the relations between subjective reported symptoms and genes. All other moderators could not be examined because insufficient studies included moderators of interest.
Discussion
This study is the first meta-analysis to directly measure: 1) whether researchers are conducting RDoC-based studies in youth that examine correspondence between at least two different units of analysis in the social processing domain and corresponding constructs/subconstructs; 2) if these studies demonstrate correspondence between RDoC units of analysis; and 3) whether study-level variables (i.e., diagnoses of participants and funding source) influence these relations. In doing so, we found relatively few RDoC-based studies, most examining correspondence between subjective-report measures and other units of analysis, with many unit pairs yet to be examined. Additionally, only the subjective-by-circuit unit pair showed correspondence. Age and diagnosis of GAD moderated correspondence for the subjective-by-circuit unit pair, and a diagnosis of ASD moderated correspondence for the subjective-by-gene unit pair, but no other study-level variables significantly moderated correspondence between any unit pair.
Distribution of RDoC-Based Studies
Among studies that met the inclusion criteria, four interesting trends emerged in the distribution of units of analysis and constructs/subconstructs. First, the distribution of specific unit-by-unit pairs was highly skewed. Specifically, all studies included the subjective unit of analysis. It is possible that subjective reports are more common because they are relatively easy to administer, inexpensive, efficient, and provide insights into the patient’s actual experiences, the perceptions of those experiences by involved others, or observed primary symptoms. Thus, while research may explicitly follow the RDoC framework, correspondence is most frequently tested between a limited range of units of analysis. The disproportionate use of subjective report and the lack of specificity regarding subconstructs or constructs raises questions about the practicality of testing correspondence between certain units of analysis within and across the social processing matrix. For example, subjective reports often take the form of a questionnaire. Questionnaires typically quantify psychological functions (e.g., social motivation, social communication, and social awareness) that fail to map on to specific subconstructs, constructs, or even domains. For instance, a global measure of social functioning may capture peer-based social skills (social processes), anxiety and withdrawal symptoms (negative valence system), and reward in response to social feedback (positive valence systems). Thus, while subjective measures are used most frequently in RDoC-based research for the study of social processes in youth, currently available questionnaires may be poorly mapped to specific subconstructs/constructs/domains. Conversely, the cellular unit of analysis may be better able to be tested in a subconstruct-specific manner. For example, activation of “face cells” in the fusiform face area of the brain largely map on to the subconstruct of reception of facial information (Kanwisher et al., 1997). Thus, it is unlikely that every unit of analysis is equally well-suited to measuring each social processing subconstruct.
Second, studies examining biological underpinnings of psychopathology across units of analysis were most represented by circuit and physiology measures’ correspondence with subjective reported symptoms, and only two studies examine correspondence between multiple biological units of analysis. In the context of the broader literature it is unclear whether this pattern of examination of correspondence between units is a departure from pre-RDoC style research. Additionally, only six of the studies examined more than two units of analysis, suggesting that studies explicitly testing social processing in youth using the RDoC framework may not be augmenting their study designs with increasing units of analysis to look at social processing within the entire RDoC matrix.
Third, ten of the thirty-three studies included more than one diagnostic group which may be more common than the broader literature. Thus, this trend may reflect the move toward a more dimensional approach to examining social processing using the RDoC framework.
Fourth, studies did not conform to a single construct or subconstruct within the social processing domain. Eleven of twenty-four studies in our literature search were retrieved using keywords for each of the distinct subconstructs. This calls into question the specificity with which researchers have approached RDoC-based research in the social processing domain to date. It may be that distinguishing subconstructs within the social processing domain may be more difficult compared to other RDoC domains. For instance, when examining a participant’s ability to understand the mental states of others, the task design likely relies on their ability to perceive the other person as an entity (animacy perception), perceive that persons’ emotional expressions (reception of facial information), and ultimately formulate an idea of what that person is thinking about (understand the mental states of others). Consequently, it might be hard to meaningfully disentangle one subconstruct from other subconstructs within the social processing domain.
Correspondence between Unit-by-unit Pairs
Correspondence was assessed across all available unit pairs; however, given the limited number of studies within each unit pair, results should be interpreted with caution in order to draw conclusions about the correspondence between each unit pair. Nevertheless, we provide our interpretations for each unit pair in order to provide future directions for research within each unit pair and across the social processing domain broadly.
Subjective-by-Behavior.
Although they were the most commonly-studied pair, subjective report and behavior units did not correspond. These results suggest that subjective report measures and behavioral measures of social processing are not associated with each other. This means that a child’s subjective beliefs about their social processing skills or a parent’s beliefs about their child’s social processing skills are inconsistent with the child’s actual performance on social processing tasks. This could reflect the challenge of having insight into one’s own social processing abilities particularly in the presence of a social processing deficit (Capps, Sigman, & Yirmiya, 1995; Lerner, Calhoun, Mikami, & De Los Reyes, 2012; Lombardo & Baron-Cohen, 2011). Likewise, parent-reports may lack accuracy due to a restricted range of observations of their child in social situations. Alternatively, personal attribution biases may skew one’s self-report about their social abilities because they experience their behavior as negative or positive, regardless of actual performance (De Los Reyes, Augenstein, Wang, et al., 2015; De Los Reyes & Kazdin, 2005; Rappaport, Pagliaccio, Pine, Klein, & Jarcho, 2017). Another possible explanation for the lack of agreement between these measures could be that the behavioral tasks are not ecologically valid and thus fail to capture the social deficits that youth report experiencing in their daily lives (De Los Reyes, Augenstein, Aldao, et al., 2015; De Los Reyes & Kazdin, 2005). All of these possible explanations provide important information about the validity of both subjective reports and behavioral measures of social processing that should be considered in future studies.
The lack of correspondence between subjective report and behavior might also be due to a lack of consistency in constructs measured within each individual correlation extracted from an article. In this sample of articles, many subjective report measures did not measure social processing but were correlated with a social processing task. For example, three of the studies assessed how subjective report measures of internalizing and externalizing symptoms correlated with a behavioral measure of social and non-social reward functioning (Kujawa, Arfer, Klein, & Proudfit, 2014; Luking, Pagliaccio, Luby, & Barch, 2015, 2016). Thus, these correlations do not reflect a one-to-one representation at multiple units of analysis within the same construct or domain. This heterogeneity may have contributed to the lack of correspondence. Future studies examining correspondence within a single construct are needed to elucidate the nature of correspondence between subjective and behavioral units of analysis.
Subjective-by-Circuit.
The only significant correspondence across units of analysis was found between subjective report and circuits. In the hypothesis-driven approach, more severe subjective reports of symptoms were associated with decreased circuit measures. Two studies did not measure functional activity but instead structural integrity of white matter and volumetric assessment of grey matter. Of the studies examining functional brain activation, regions included have broadly been described as interconnected networks that support social processing. Given the complexity of social processing, these networks include brain regions implicated in numerous aspects of social processing, from basic perception of faces (fusiform gyrus; Kanwisher et al., 1997) to higher-order self-awareness (posterior cingulate cortex; Northoff & Bermpohl, 2004) and emotional salience detection (amygdala; Anderson & Phelps, 2001). This suggests that independent of tasks, regions, or constructs, deactivation in the social processing network is consistently associated with more social processing deficits. This could have far-reaching implications, not only for hypothesizing directions of correlations in future studies but also for the development of interventions aimed at increasing activation in the social processing network in youth. For instance, future research might specifically examine the extent to which psychosocial interventions increase engagement of the social processing network.
However, there was variability in subjective reports assessed and only a small number of studies were included in this model. Subjective measures were not directly compared to circuit measures of social processing within the same construct or domain. Some subjective report measures captured constructs such as internalizing symptoms (Henderson et al., 2014; van den Bulk et al., 2014), social anxiety (Herrington et al., 2016; van den Bulk et al., 2014), callous-unemotional traits (Cohn et al., 2015) and ASD symptoms (Aoki et al., 2017; Bjornsdotter, Wang, Pelphrey, & Kaiser, 2016; Kohls et al., 2014; Lloyd-Fox et al., 2017; Parellada et al., 2017). Despite their variability, these subjective measures largely mapped onto internalizing symptoms or deficits in social processing capacity, making them more homogenous than other unit pairs. Therefore, subjective-by-circuit correspondence might reflect an absence of engagement of the social processing network that is correlated specifically with social deficits and internalizing symptoms in youth rather than the same type of social deficit measured across both units of analysis. The extent to which specific type of social deficit measured using subjective reports correlates with brain activation in response to a task that measured the exact same particular social deficit remains unclear and should be a topic of future research.
An unexpected limitation of this study was the large number of studies with circuit-based measures that failed to specify a directional hypothesis. Nevertheless, the strength of correspondence using the hypothesis-driven approach was stronger than the atheoretical approach suggesting specific hypothesis regarding direction of association of circuit measures enhance correspondence between brain function and subjectively reported symptoms. Our results underscore the value of specifying a priori hypotheses in neuroimaging research. However, we found correspondence between subjective report and brain activation within the social processing domain in youth using both an atheoretical method, in which we coded for level of activation rather than pathology, and a hypothesis-driven method, in which we used the expected direction of activation predicted by the authors. The results suggest that decreased activation in the social processing circuit is associated with more severe subjectively reported symptomology regardless of region or task. However, this is in contradiction to some previous work that shows differential engagement in regions and networks that are task-dependent, construct-dependent, and deficit-specific (Dalton et al., 2005; Herrington et al., 2016; Kleinhans et al., 2010). For example, relative to their typically-developing peers, youth with ASD exhibit greater activation in the precuneous and less activation in the fusiform gyrus while matching emotional faces to labels (Wang, Dapretto, Hariri, Sigman, & Bookheimer, 2004). Therefore, it is critical that future circuit-by-subjective studies (1) explicitly state their hypothesized direction of activation, (2) measure the same regions of interest, and (3) test their hypothesis in populations with varying types of social deficits in order to better understand whether increased brain activation is more normative or more pathological for the given construct under investigation.
Non-significant unit pairs.
Subjective-by-physiology, subjective-by-gene, behavior-by-circuit, and physiology-by-gene measures of social processing showed no associations with each other. This lack of correspondence could be due to a couple of factors. One possibility is that there may truly be no correspondence across units. For instance, one’s own, or one’s parents’, subjective beliefs about one’s social processing skills may truly be unrelated with known physiological and genetic markers of social processing. This is supported by previous research that has shown it to be difficult for individuals, or their parents, to have insights into their social processing abilities, particularly when deficits in social processing are present (Capps et al., 1995; Lerner et al., 2012; Lombardo & Baron-Cohen, 2011). Another possibility is that physiological or genetic measures may be related to discrete social processing skills rather than social interactions in their entirety. To determine if this is true, future studies should compare across more discrete social processing skills and units of analysis to reveal more correspondence between units of analysis within social processing in youth. To this end, there was variability in the types of construct measured between the subjective-by-physiology and subjective-by-gene units of analyses which could have contributed to the lack of correspondence between these unit pairs. As for the behavior-by-circuits and physiology-by-gene unit pairs, both had only two studies to represent the pair. As such, it is likely premature to make inferences about correspondence between these unit pairs in the present literature. Moreover, the genes unit of analysis was recently removed from the RDoC matrix in order to emphasize more robust evidence of association using genome wide association studies. Therefore, it is likely that our results highlighting the lack of literature examining correspondence of gene units is indicative of the current state of the field in genomic research.
Publication Bias
Publication bias was unlikely in the subjective-by-gene unit pair. Publication bias analyses could not be conducted for the behavior-by-circuit or physiology-by-gene unit pairs due to an insufficient number of studies.
Publication bias was possible in the subjective-by-behavior unit pair suggesting that effect sizes may be smaller than detected. Of the unit pairs, subjective-by-behavior studies were the most abundant in this meta-analysis, potentially because they are among the least expensive and least time-consuming studies to conduct. As such, it is possible that non-significant findings are less likely to be published and instead tasks are tweaked to produce more robust results prior to publication thus resulting in possible missing non-significant studies. Moreover, there are a paucity of journals that encourage the publication of null results. Thus, our results demonstrate the need for greater publication of null results, particularly for studies examining the correspondence between subjectively reported symptoms and behavior.
Publication bias was possible in the subjective-by-circuit (using both the hypothesis-driven and atheoretical methods), and subjective-by-physiology unit pairs such that effect size may be larger than detected. Notably, there are significant task demands associated with the circuit and physiology methodologies, especially for youth (i.e., requirement to stay very still for a long time when undergoing an fMRI or during an EEG). The potential bias could therefore reflect the presence of a distinct pattern of association between subjective-by-circuit and subjective-by-physiology units of analyses for those that can meet task demands relative to those who cannot do so (e.g., younger populations, more cognitively- or behaviorally-impaired populations). This effectively reduces the representation of these groups who cannot meet task demands in the literature. Thus, there may be differences in the direction or strength of the relations between circuit activation and subjectively reported symptoms as a function of these populations. Specifically, it is possible that populations with more severe deficits in social functioning may also not be as likely to be included in neuroimaging studies and potentially diminishing observed effect sizes.
Another factor that could be contributing to the observed publication bias within the circuit-by-subjective unit of analysis, specifically, could be heterogeneity in circuit measures and analytic procedures/pipelines used. Within the circuit units of analysis, six studies measured blood-oxygen-level (BOLD) task-related activation, one study measured resting state functional connectivity, one study measured structural integrity of white matter, and one study examined volumetric size of grey matter, which all measure different aspects of neural circuits that are not comparable. Moreover, five of the ten studies reported correlations as part of post-hoc analyses. Thus, associations between subjective-by-circuit units of analysis might be true for whole-brain analysis BOLD measures in particular. Other analytic techniques, such as functional connectivity, multivariate analysis, dynamic causal modeling, or diffusion tensor imaging might yield different associations between subjective-by-circuit units of analysis and should be a topic of future studies (Carp, 2012). Regardless, the possible publication bias suggests that correspondence between the subjective-report measures and brain activation should be interpreted with these pipeline specifics in mind. Future studies addressing under sampled populations and variations in analytic techniques might help clarify the generalizability of the observed pattern of association between subjective-by-circuit units broadly.
Moderators
Age and gender ratio.
Age significantly moderated the relationship between subjectively reported symptoms and circuit measures using the atheoretical method and was trending toward reaching significance using the hypothesis-driven method, which may reflect our relatively low-power for detecting smaller effect sizes given the number of included studies. Studies that included older adolescents showed greater correspondence between units than studies that included younger participants. Thus, it is likely that development of social processing skills continues throughout adolescence and stabilizes into early adulthood. This is consistent with literature suggesting the continued development of brain regions is positively associated with social processing into early adulthood (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Boehme et al., 2017; Monahan, Guyer, Silk, Fitzwater, & Steinberg, 2016; Raznahan et al., 2010). Gender ratio did not moderate relations between subjectively reported symptoms and circuit measures of social processing. Moreover, neither age nor gender ratio moderated relations between subjectively reported symptoms and behavioral performance on tasks of social processing. These data suggest, regardless of age or gender, the relation between one’s perceptions about one’s social processing and one’s behavioral performance on social processing tasks is consistent. Thus, despite known differences in behavior measures of social processing with respect to age (La Greca & Prinstein, 1999) and in behavior (Eagly, 2013) as well as differences in circuit measures of social processing with respect to gender (Groschwitz, Plener, Groen, Bonenberger, & Abler, 2016; Lee et al., 2013; Owens, Allen, & Spangler, 2010), age and gender do not appear to influence the strength of relations between these units of analysis. However, studies examining the subjective-by-behavior unit pair did not show correspondence; therefore, conclusions about the moderating effects of age and gender on this unit pair should be updated as the literature expands in order to fully determine if they indeed have no effect on correspondence.
Funding.
Funding by the NIMH or the NIH versus other agencies did not moderate correspondence between subjective-by-behavior, subjective-by-circuit, or subjective-by-gene unit pairs. Thus, despite the NIMH’s explicit goal of funding studies designed to assess RDoC correspondence, these studies do not find greater correspondence between units of analysis than studies funded by any other agencies. However, it is possible that only a limited number of studies funded under the RDoC initiative have been published given that the RDoC framework was only established in 2010. Therefore, future examination of the literature when RDoC study publications are more abundant should be conducted to replicate our null results.
Diagnosis.
Diagnosis could only be examined within the subjective-by-behavior, subjective-by-circuit, and subjective-by-gene units of analysis, and only for a limited number of diagnoses for each unit pair due to an insufficient number of studies in other unit pairs and diagnoses. For the subjective-by-circuit using the hypothesis-driven method only, GAD moderated correspondence, such that studies including participants with GAD did not correspond and studies without participants with GAD did indeed correspond. It is possible that participants with GAD are in fact demonstrating elevated anxiety symptoms while participating in studies, and therefore may be engaging brain regions associated with anxious internal responses rather than external task stimuli, thereby reducing correspondence. Alternatively, it is possible that heterogeneity in informants (e.g., parent vs. self) of subjectively reported symptoms may contribute to reduced correspondence in GAD samples, a finding that has consistently been found in the anxiety literature (De, Reyes, Bunnell, & Beidel, 2013; Niditch & Varela, 2011; Pereira et al., 2015). For the subjective-by-gene unit pair, an ASD diagnosis moderated correspondence, such that studies without participants with ASD showed greater correspondence. One possibility is that circuit-level processing of social information may be encoded differently or may be impaired in ASD, and therefore could impact social insight. Likewise, individuals with ASD may overestimate social skills when subjectively reporting symptoms which is consistent with previous literature in ASD (Lerner et al., 2012). Alternatively, it may be that there is simply greater heterogeneity in social processing skills in ASD compared to other populations (Kim, Macari, Koller, & Chawarska, 2016; Pelphrey, Shultz, Hudac, & Wyk, 2011). No other diagnoses moderated correspondence between the subjective-by-behavior, subjective-by-circuit, and subjective-by-gene units of analysis. This might indicate that using a traditional diagnostic model for examining social processing in youth might not be an important factor to consider for designing a study which is more consistent with RDoC-based study designs. However, many diagnoses could not be examined due to the small number of studies meeting inclusionary criteria in each unit pair. Therefore, future studies should examine the correspondence between units of analysis within other diagnostic groups influence relations between units of analysis.
Limitations
Though this paper illustrates interesting findings regarding a lack of correspondence between many RDoC units of analysis, it also highlights the paucity of studies directly and explicitly testing correspondence between units of analysis within the social processing domain in youth. As a result, there is limited in power for interpreting null findings of correspondence, publication bias, and moderator analysis between unit pairs. Moreover, we are also underpowered to examine many moderators of interest such as informant report, additional diagnoses, analytic method, unit of analysis modality (i.e., functional brain activation vs. white matter integrity), and construct and subconstruct effects on correspondence. Additionally, based on the guidelines put forth by the special issue, we were not able to include all studies examining correspondence between units of analysis (i.e., prior to 2010 or not explicitly testing the RDoC framework). Therefore the generalizability of our results are limited. Moreover, the PsycINFO database did not yield any results based on our search criteria despite multiple checks to ensure accurate search parameters which was unexpected. Thus, future studies may wish to employ a broader array of related search criteria (beyond those called for in the present Special Issue) to increase representation across databases.
Conclusion and Future Directions
Despite the shift towards funding research that supports the RDoC initiative, the seven years (2010–2017) since the initiative was launched have yielded a limited number of studies that explicitly conducted multi-unit research within the social processing domain in youth. Of these studies, the units of analysis were highly skewed towards subjective measures. Subjective measures cut across a broad range of social processing constructs and subconstructs which likely contributed to the lack of construct specificity within studies. Future studies should therefore target individual subconstructs in order to more explicitly test correspondence within RDoC. However, one must consider the utility of examining correspondence across units of analysis within a given subconstruct or construct in the social processing in youth. For example, neural response to the presentation of emotional faces specifically maps on to the reception of facial communication subconstruct. However, an adolescent’s response to peer rejection reflects complex processes that likely extend across subconstructs and constructs, including reception of facial communication, animacy perception, understanding the mental states of others and affiliation and attachment. Thus, the specificity gained by a narrow focus on a single subconstruct comes at the cost of understanding mechanisms engaged in ecologically-valid contexts. Conversely, it is unclear whether units with the capacity for greater specificity can account for the complexity of subconstructs in the social processing domain. For example, it is unlikely that the interaction between specific molecular processes and specific physiological responses will adequately explain how understanding mental states develops.
To better understand the nature and validity of correspondence between subjective reports and neural circuits, future studies need to clarify two points. First, fMRI-based studies should explicitly state the expected direction of activation for all reported regions in order to understand the nature of correspondence between subjective report and brain activation. This will clarify whether there are differences in the direction of brain activation vary in based on task design or region, or, if indeed decreased activation in the social processing network broadly is more pathological independent of region, task, or subjectively reported deficit. Second, studies should also focus on a larger range of subjectively reported social processing deficits, and measure the same social processing construct across both units of analysis, to see if correspondence between subjective-by-circuit units of analysis can generalize beyond ASD-like social deficits and internalizing symptoms.
This meta-analysis constrained the search parameters to studies that explicitly sought to examine RDoC-based research questions by referencing one of the three original RDoC papers or studies that directly mention RDoC in the title, abstract, or keywords as specified in the guidelines for all articles in this special issue. However, there are many studies that similarly examine correspondence between units of analysis that are not explicitly RDoC-based (Faja, Clarkson, & Webb, 2016; Jackson, Nelson, & Proudfit, 2014; Lerner, De Los Reyes, Drabick, Gerber, & Gadow, 2017; Vanhalst, Gibb, & Prinstein, 2015). As such, while the present meta-analysis can be said to provide a snapshot of the present literature that is testing associations across units of analysis and which are explicitly testing claims of RDoC; it cannot be said to represent the full literature regarding these associations in youth populations (e.g., it excludes earlier studies as well as contemporaneous studies that do not explicitly cite and reference the original RDoC paper), and generalizability of the study findings is limited. Thus, a future meta-analysis may seek to more fully capture the patterns of association across units in the current literature.
Lastly, future studies should also examine correspondence of RDoC units of analysis across domains rather than within a single domain. This strategy might provide greater insight into relations between various aspects of social processing in normative and abnormal child development given that many phenotypic presentations of social processing deficits crosscut RDoC domains. For example, those with social anxiety disorder experience deficits in the social processing domain (social information processing), the arousal and regulatory domain (panic responses), the cognitive domain (negative attribution bias), the positive valence system (prediction error related to peer feedback), and the negative valence domain (anxiety and threat response). Therefore, to better understand how the RDoC framework and units of analysis can be leveraged to enhance our understanding of normative and pathological development, examining the intersection of these domains might be the next step in connecting research across units of analysis.
Supplementary Material
Conflicts of Interest and Source of Funding:
None of the authors have any conflicts of interest to disclose. Matthew D. Lerner, Ph.D was supported by NIMH R01 MH110585, the Simons Foundation (SFARI# 381283), and a NARSAD Young Investigator Award (#24890) during the preparation of this manuscript. Johanna Jarcho, Ph.D. was supported by the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) Young Investigator Award: Ellen Schapiro & Gerald Axelbaum Investigator during the preparation of this manuscript. The authors would also like to acknowledge Dorothy McGee and Kristy Lee for their work conducting the literature for this manuscript.
Contributor Information
Tessa Clarkson, Department of Psychology, Temple University, Philadelphia, PA
Erin Kang, Department of Psychology, Stony Brook University, Stony Brook, NY
Nicole Capriola-Hall, Department of Psychology, University of Alabama, Tuscaloosa, AL
Matthew D. Lerner, Department of Psychology, Stony Brook University, Stony Brook, NY
Johanna Jarcho, Department of Psychology, Temple University, Philadelphia, PA
Mitchell J. Prinstein, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC.
References
- Achenbach TM (2011). Child behavior checklist In Encyclopedia of clinical neuropsychology (pp. 546–552). Springer. [Google Scholar]
- Ainsworth MD (1964). Patterns of attachment behavior shown by the infant in interaction with his mother. Merrill-Palmer Quarterly, 10(1), 51–58. 10.2307/23082925 [DOI] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, … Hof PR (2010). The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Structure and Function, 214(5–6), 495–517. 10.1007/s00429-010-0254-0 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., A. A., & American Psychiatric Association. DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders : DSM-5. : American Psychiatric Publishing. 10.1016/B978-1-4377-2242-0.00016-X [DOI]
- Anderson AK, & Phelps EA (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411(6835), 305–309. 10.1038/35077083 [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, & Damasio AR (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience, 2(11). Retrieved from http://neurosci.nature.com [DOI] [PubMed] [Google Scholar]
- Aoki Y, Yoncheva YN, Chen B, Nath T, Sharp D, Lazar M, … Di Martino A (2017). Association of White Matter Structure With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. JAMA Psychiatry, 74(11), 1120–1128. 10.1001/jamapsychiatry.2017.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, … Sharma S (2013). Maturation of the adolescent brain Arain M. Neuropsychiatric Disease and Treatment, 9, 449–461. 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenio WF, Adams E, & Gold J (2009). Social Information processing, moral reasoning, and emotion attributions: Relations with adolescents’ reactive and proactive aggression. Child Development, 80(6), 1739–1755. 10.1111/j.1467-8624.2009.01365.x [DOI] [PubMed] [Google Scholar]
- Bagwell CL, & Schmidt ME (2013). Friendships in childhood and adolescence. Guilford Press. [Google Scholar]
- Bal VH, & Lord C (2015). Replication of Standardized ADOS Domain Scores in the Simons Simplex Collection. Autism Research, 8(5), 583–592. 10.1002/aur.1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Dupuis A, Paton TA, Scherer SW, Schachar RJ, Arnold PD, … Anagnostou E (2017). Oxytocin Receptor Polymorphisms are Differentially Associated with Social Abilities across Neurodevelopmental Disorders. Scientific Reports, 7(1), 1–11. 10.1038/s41598-017-10821-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, & Cross P (1992). Reading the eyes: Evidence for the role of perception in the development of a theory of mind. Mind & Language, 7(1), 172–186. [Google Scholar]
- Baron-Cohen S, Tager-Flusberg H, & Lomardo MV (2013). Understanding Other Minds: Perspectives from developmental social neuroscience. Oxford University Press; 10.1093/acprof:oso/9780199692972.001.0001 [DOI] [Google Scholar]
- Beesdo K, Jacobi F, Hoyer J, Low NCP, Höfler M, & Wittchen HU (2010). Pain associated with specific anxiety and depressive disorders in a nationally representative population sample. Social Psychiatry and Psychiatric Epidemiology, 45(1), 89–104. 10.1007/s00127-009-0045-1 [DOI] [PubMed] [Google Scholar]
- Bennett S, Farrington DP, & Huesmann LR (2005). Explaining gender differences in crime and violence: The importance of social cognitive skills. Aggression and Violent Behavior, 10(3), 263–288. 10.1016/j.avb.2004.07.001 [DOI] [Google Scholar]
- Bjornsdotter M, Wang N, Pelphrey K, & Kaiser MD (2016). Evaluation of quantified social perception circuit activity as a neurobiological marker of autism spectrum disorder. JAMA Psychiatry, 73(6), 614–621. 10.1001/jamapsychiatry.2016.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2013). The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience, 14(11), 786–799. 10.1038/nrn3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Budhani S, Colledge E, & Scott S (2005). Deafness to fear in boys with psychopathic tendencies. Journal of Child Psychology and Psychiatry and Allied Disciplines, 46(3), 327–336. 10.1111/j.1469-7610.2004.00356.x [DOI] [PubMed] [Google Scholar]
- Blanken LME, White T, Mous SE, Basten M, Muetzel RL, Jaddoe VWV, … Tiemeier H (2016). Cognitive functioning in children with internalising, externalising and dysregulation problems : a population-based study. European Child & Adolescent Psychiatry. 10.1007/s00787-016-0903-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme R, Lorenz RC, Gleich T, Romund L, Pelz P, Golde S, … Beck A (2017). Reversal learning strategy in adolescence is associated with prefrontal cortex activation. European Journal of Neuroscience, 45(1), 129–137. 10.1111/ejn.13401 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, & Rothstein HR (2015). Biostat: comprehensive meta-analysis (version 3)[software]. Englewood: Biostat. [Google Scholar]
- Braadbaart L, Williams JHG, & Waiter GD (2013). Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? International Journal of Psychophysiology, 89(1), 99–105. 10.1016/j.ijpsycho.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Pollak SD, Grasso D, Voss J, Mian ND, Zobel E, … Pine DS (2015). Attention bias and anxiety in young children exposed to family violence. Journal of Child Psychology and Psychiatry, 56(11), 1194–1201. 10.1111/jcpp.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capps L, Sigman M, & Yirmiya N (1995). Self-competence and emotional understanding in high-functioning children with autism. Development and Psychopathology, 7(01), 137 10.1017/S0954579400006386 [DOI] [Google Scholar]
- Carp J (2012). The secret lives of experiments: Methods reporting in the fMRI literature. NeuroImage, 63(1), 289–300. 10.1016/j.neuroimage.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, & Frith U (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain, 125(8), 1839–1849. 10.1093/brain/awf189 [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, & Raulin MJ (1978). Perceptual aberration scale. Journal of Abnormal Psychology, 87, 299–407. 10.1037/t20156-000 [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Powell K, DiNicola L, & Shic F (2016). Enhanced Social Attention in Female Infant Siblings at Risk for Autism. Journal of the American Academy of Child & Adolescent Psychiatry, 55(3), 188–195.e1. 10.1016/j.jaac.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (2002). A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology, 70(1), 6–20. 10.1037/0022-006X.70.1.6 [DOI] [PubMed] [Google Scholar]
- Cohn MD, Pape LE, Schmaal L, van den Brink W, van Wingen G, Vermeiren RRJM, … Popma A (2015). Differential relations between juvenile psychopathic traits and resting state network connectivity. Human Brain Mapping, 36(6), 2396–2405. 10.1002/hbm.22779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, & Gruber C (2012). Social responsiveness scale (SRS). [Google Scholar]
- Cuthbert BN, & Insel TR (2010). Toward New Approaches to Psychotic Disorders: The NIMH Research Domain Criteria Project. Schizophrenia Bulletin, 36(6), 1061–1062. 10.1093/schbul/sbq108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, Moul C, Hawes DJ, Mendoza Diaz A, & Brennan J (2015). Individual Differences in Childhood Behavior Disorders Associated With Epigenetic Modulation of the Cortisol Receptor Gene. Child Development, 86(5), 1311–1320. 10.1111/cdev.12391 [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, … Davidson RJ (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519–526. 10.1038/nn1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daprati E, Franck N, Georgieff N, Proust J, Pacherie E, Dalery J, & Jeannerod M (1997). Looking for the agent: an investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition, 65(1), 71–86. 10.1016/S0010-0277(97)00039-5 [DOI] [PubMed] [Google Scholar]
- De A, Reyes L, Bunnell BE, & Beidel DC (2013). Informant Discrepancies in Adult Social Anxiety Disorder Assessments: Links With Contextual Variations in Observed Behavior HHS Public Access. J Abnorm Psychol, 122(2), 376–386. 10.1037/a0031150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, Augenstein TM, Aldao A, Thomas S. a, Daruwala S, Kline K, & Regan T (2015). Implementing psychophysiology in clinical assessments of adolescent social anxiety: use of rater judgments based on graphical representations of psychophysiology. Journal of Clinical Child and Adolescent Psychology : The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 44(2), 264–279. 10.1080/15374416.2013.859080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DAG, Burgers DE, & Rabinowitz J (2015). The validity of the multi-informant approach to assessing child and adolescent mental health. Psychological Bulletin, 141(4), 858–900. 10.1037/a0038498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, & Kazdin AE (2005). Week 3 Informant discrepancies in the assessment of childhood psychopathology: a critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131(4), 483–509. 10.1037/0033-2909.131.4.483 [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Eagly AH (2013). Sex differences in social behavior: A social-role interpretation. Psychology Press. [Google Scholar]
- Egger M, & Smith GD (1998). Bias in location and selection of studies. BMJ (Clinical Research Ed.), 316(7124), 61–66. 10.1136/bmj.316.7124.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaad E, Lavy S, Cohenca D, Berholz E, Thee P, & Ben-Gigi Y (2012). Lies, truths, and attachment orientations in late adolescence. Personality and Individual Differences, 52(6), 670–673. 10.1016/j.paid.2011.12.018 [DOI] [Google Scholar]
- Faja S, Clarkson T, & Webb SJ (2016). Neural and behavioral suppression of interfering flankers by children with and without autism spectrum disorder. Neuropsychologia, 93(May), 251–261. 10.1016/j.neuropsychologia.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CJ, & Brannick MT (2012). Publication bias in psychological science: Prevalence, methods for identifying and controlling, and implications for the use of meta-analyses. Psychological Methods, 17(1), 120–128. 10.1037/a0024445 [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Greenberg M, & Bierman K (2015). Children’s Parasympathetic Reactivity to Specific Emotions Moderates Response to Intervention for Early-Onset Aggression. Journal of Clinical Child & Adolescent Psychology, 44(2), 291–304. 10.1080/15374416.2013.862801 [DOI] [PubMed] [Google Scholar]
- Germine L, Robinson EB, Smoller JW, Calkins ME, Moore TM, Hakonarson H, … Gur RE (2016). Association between polygenic risk for schizophrenia, neurocognition and social cognition across development. Translational Psychiatry, 6(10), e924 10.1038/tp.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray HM, Ambady N, Lowenthal WT, & Deldin P (2004). P300 as an index of attention to self-relevant stimuli. Journal of Experimental Social Psychology, 40(2), 216–224. 10.1016/S0022-1031(03)00092-1 [DOI] [Google Scholar]
- Groschwitz RC, Plener PL, Groen G, Bonenberger M, & Abler B (2016). Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: An fMRI study. Psychiatry Research: Neuroimaging, 255, 43–49. 10.1016/j.pscychresns.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Harvey P, Lee J, Horan WP, Ochsner K, & Green MF (2011). Do patients with schizophrenia benefit from a self-referential memory bias? Schizophrenia Research, 127(1–3), 171–177. 10.1016/j.schres.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Vallejo AI, Ely BA, Kang G, Krain Roy A, Pine DS, … Gabbay V (2014). The neural correlates of emotional face-processing in adolescent depression: a dimensional approach focusing on anhedonia and illness severity. Psychiatry Research: Neuroimaging, 224(3), 234–241. 10.1016/j.pscychresns.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Miller JS, Pandey J, & Schultz RT (2016). Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. Social Cognitive and Affective Neuroscience, 11(6), 907–914. 10.1093/scan/nsw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler DM, & Fainsilber Katz L (2007). Children’s emotion regulation: Self-report and physiological response to peer provocation. Developmental Psychology, 43(1), 27–38. 10.1037/0012-1649.43.1.27 [DOI] [PubMed] [Google Scholar]
- Ho TC, Zhang S, Sacchet MD, Weng H, Connolly CG, Henje Blom E, … Yang TT (2016). Fusiform Gyrus Dysfunction is Associated with Perceptual Processing Efficiency to Emotional Faces in Adolescent Depression: A Model-Based Approach. Frontiers in Psychology, 7(February), 1–14. 10.3389/fpsyg.2016.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, & Botella J (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Insel TR, & Young LJ (2001). The neurobiology of attachment. Nature Reviews Neuroscience, 2(2), 129–136. 10.1038/35053579 [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, & Proudfit GH (2014). In an Uncertain World, Errors Are More Aversive: Evidence From the Error-Related Negativity. Emotion (Washington, D.C.), 14(5), 6–10. 10.1037/emo0000020 [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, & Decety J (2006). Neural circuits involved in imitation and perspective-taking. NeuroImage, 31(1), 429–439. 10.1016/j.neuroimage.2005.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Davis MM, Shechner T, Degnan KA, Henderson HA, Stoddard J, … Nelson EE (2016). Early-Childhood Social Reticence Predicts Brain Function in Preadolescent Youths During Distinct Forms of Peer Evaluation. Psychological Science, 27(6), 821–835. 10.1177/0956797616638319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Tanofsky-Kraff M, Nelson EE, Engel SG, Vannucci A, Field SE, … Yanovski JA (2015). Neural activation during anticipated peer evaluation and laboratory meal intake in overweight girls with and without loss of control eating. NeuroImage, 108, 343–353. 10.1016/j.neuroimage.2014.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, & Prigatano GP (2002). Neural correlates of self-reflection. Brain : A Journal of Neurology, 125(Pt 8), 1808–1814. 10.1093/brain/awf181 [DOI] [PubMed] [Google Scholar]
- Jones AP, Happé FGE, Gilbert F, Burnett S, & Viding E (2010). Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 51(11), 1188–1197. 10.1111/j.1469-7610.2010.02280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CRG, Pickles A, Falcaro M, Marsden AJS, Happé F, Scott SK, … Charman T (2011). A multimodal approach to emotion recognition ability in autism spectrum disorders. Journal of Child Psychology and Psychiatry, 52(3), 275–285. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 17(11), 4302–4311. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9151747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Arizpe J, Rosen B, Razdan V, Haring C, Jenkins S, … Leibenluft E (2013). Impaired fixation to eyes during facial emotion labelling in children with bipolar disorder or severe mood dysregulation. Journal of Psychiatry & Neuroscience, 38(6), 407–416. 10.1503/jpn.120232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Macari S, Koller J, & Chawarska K (2016). Examining the phenotypic heterogeneity of early autism spectrum disorder: subtypes and short-term outcomes. Journal of Child Psychology and Psychiatry, 57(1), 93–102. 10.1111/jcpp.12448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, & Aylward E (2010). Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia, 48(12), 3665–3670. 10.1016/j.neuropsychologia.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Thönessen H, Bartley GK, Grossheinrich N, Fink GR, Herpertz-Dahlmann B, & Konrad K (2014). Differentiating neural reward responsiveness in autism versus ADHD. Developmental Cognitive Neuroscience, 10, 104–116. 10.1016/j.dcn.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Arfer KB, Klein DN, & Proudfit GH (2014). Developmental Cognitive Neuroscience Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Accident Analysis and Prevention, 10, 140–147. 10.1016/j.dcn.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Greca AM, & Prinstein MJ (1999). Peer group In Developmental issues in the clinical treatment of children. (pp. 171–198). Needham Heights, MA, US: Allyn & Bacon. [Google Scholar]
- Lee MR, Cacic K, Demers CH, Haroon M, Heishman S, Hommer DW, … Salmeron BJ (2013). Gender differences in neural-behavioral response to self-observation during a novel fMRI social stress task. 10.1016/j.neuropsychologia.2013.11.022 [DOI] [PMC free article] [PubMed]
- Legerstee M, Anderson D, & Schaffer A (1998). Five- and eight-month-old infants recognize their faces and voices as familiar and social stimuli. Child Development, 69(1), 37–50. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9499555 [PubMed] [Google Scholar]
- Leppänen JM, Moulson MC, Vogel-Farley VK, & Nelson C. a. (2007). An ERP Study of Emotional Face Processing in the Adult and Infant Brain. Child Development, 78(1), 232–245. 10.1111/j.1467-8624.2007.00994.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MD, Calhoun CD, Mikami AY, & De Los Reyes A (2012). Understanding parent-child social informant discrepancy in youth with high functioning autism spectrum disorders. Journal of Autism and Developmental DisordersLerner, [DOI] [PubMed] [Google Scholar]; Calhoun MD, Mikami CD, Y. A, & De Los Reyes A (2012). Understanding Parent-Child Social Informant Discrepancy in Youth with High Functioning Autism Spectrum Disorders. Journal of Autism and D, 42(12), 2680–2692. 10.1007/s10803-012-1525-9 [DOI] [PubMed] [Google Scholar]
- Lerner MD, De Los Reyes A, Drabick DAG, Gerber AH, & Gadow KD (2017). Informant discrepancy defines discrete, clinically useful autism spectrum disorder subgroups. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(7), 829–839. 10.1111/jcpp.12730 [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Pasco G, Gliga T, Jones EJH, Murphy DGM, … Johnson MH (2017). Cortical responses before 6 months of life associate with later autism. European Journal of Neuroscience, 47(6), 736–749. 10.1111/ejn.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, & Baron-Cohen S (2011). The role of the self in mindblindness in autism. Consciousness and Cognition, 20(1), 130–140. 10.1016/j.concog.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2015). Child Gain Approach and Loss Avoidance Behavior: Relationships With Depression Risk, Negative Mood, and Anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry, 54(8), 643–651. 10.1016/j.jaac.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Do losses loom larger for children than adults? Emotion, 16(3), 338–348. 10.1037/emo0000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby EE (1999). The two sexes: Growing up apart, coming together (Vol. 4). Harvard University Press. [Google Scholar]
- Martin CL, & Fabes RA (2001). The stability and consequences of young children’s same-sex peer interactions. Developmental Psychology, 37(3), 431–446. 10.1037//0012-1649.37.3.431 [DOI] [PubMed] [Google Scholar]
- Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, & Thapar A (2014). Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biological Psychiatry, 76(8), 664–671. 10.1016/j.biopsych.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade JD, & Breaux RP (2017a). Are Elevations in ADHD Symptoms Associated with Physiological Reactivity and Emotion Dysregulation in Children? Journal of Abnormal Child Psychology, 45(6), 1091–1103. 10.1007/s10802-016-0227-8 [DOI] [PubMed] [Google Scholar]
- McQuade JD, Penzel TE, Silk JS, & Lee KH (2017b). Parasympathetic Nervous System Reactivity Moderates Associations Between Children’s Executive Functioning and Social and Academic Competence. Journal of Abnormal Child Psychology, 45(7), 1355–1367. 10.1007/s10802-016-0246-5 [DOI] [PubMed] [Google Scholar]
- Meltzoff AN (1995). Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Developmental Psychology, 31(5), 838–850. 10.1037/0012-1649.31.5.838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JL, Gates JA, & Lerner MD (2016). Friendship in School-Age Boys With Autism Spectrum Disorders: A Meta-Analytic Summary and Developmental, Process-Based Model. Psychological Bulletin. 10.1037/bul0000041 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Monahan KC, Guyer AE, Silk J, Fitzwater T, & Steinberg L (2016). Integration of developmental neuroscience and contextual approaches to the study of adolescent psychopathology. Developmental Psychopathology, 1–46. [Google Scholar]
- Muncer SJ, & Ling J (2006). Personality and individual differences - Psychometric analysis of the Empathy Quotient (EQ) scale. Personality and Individual Differences, 40, 1111–1119. 10.1016/j.paid.2005.09.020 [DOI] [Google Scholar]
- Neri P, Morrone MC, & Burr DC (1998). Seeing biological motion. Nature, 395(6705), 894–896. 10.1038/27661 [DOI] [PubMed] [Google Scholar]
- Niditch LA, & Varela RE (2011). Mother-child disagreement in reports of child anxiety: Effects of child age and maternal anxiety. Journal of Anxiety Disorders, 25(3), 450–455. 10.1016/j.janxdis.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, & Bermpohl F (2004). Cortical midline structures and the self. Trends in Cognitive Sciences, 8(3), 102–107. 10.1016/j.tics.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Owens TE, Allen MD, & Spangler DL (2010). An fMRI study of self-reflection about body image: Sex differences. 10.1016/j.paid.2010.02.012 [DOI] [Google Scholar]
- Parellada M, Pina-Camacho L, Moreno C, Aleman Y, Krebs MO, Desco M, … Janssen J (2017). Insular pathology in young people with high-functioning autism and first-episode psychosis. Psychological Medicine, 47(14), 2472–2482. 10.1017/S0033291717000988 [DOI] [PubMed] [Google Scholar]
- Parish-Morris J, Chevallier C, Tonge N, Letzen J, Pandey J, & Schultz RT (2013). Visual attention to dynamic faces and objects is linked to face processing skills: A combined study of children with autism and controls. Frontiers in Psychology, 4(APR), 1–7. 10.3389/fpsyg.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, Calkins SD, & Bell MA (2015). Broad implications for respiratory sinus arrhythmia development: Associations with childhood symptoms of psychopathology in a community sample. Developmental Psychobiology, 57(1), 120–130. 10.1002/dev.21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, & Wyk B. C. Vander. (2011). Constraining Heterogeneity: The Social Brain and its Development in Autism Spectrum Disorder. J Child Psychol Psychiatry, 52(6), 631–644. 10.1111/j.1469-7610.2010.02349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AI, Muris P, Barros L, Goes R, Marques T, & Russo V (2015). Agreement and discrepancy between mother and child in the evaluation of children???s anxiety symptoms and anxiety life interference. European Child and Adolescent Psychiatry, 24(3), 327–337. 10.1007/s00787-014-0583-2 [DOI] [PubMed] [Google Scholar]
- Pigott TD (2012). Advances in Meta-Analysis. Boston, MA: Springer US; 10.1007/978-1-4614-2278-5 [DOI] [Google Scholar]
- Plomin R, & Spinath FM (2004a). Intelligence: Genetics, Genes, and Genomics. Journal of Personality and Social Psychology, 86(1), 112–129. 10.1037/0022-3514.86.1.112 [DOI] [PubMed] [Google Scholar]
- Plomin R, & Spinath FM (2004b). Intelligence: Genetics, Genes, and Genomics. Journal of Personality and Social Psychology, 86(1), 112–129. 10.1037/0022-3514.86.1.112 [DOI] [PubMed] [Google Scholar]
- Rappaport BI, Pagliaccio D, Pine DS, Klein DN, & Jarcho JM (2017). Discriminant validity, diagnostic utility, and parent-child agreement on the Screen for Child Anxiety Related Emotional Disorders (SCARED) in treatment- and non-treatment-seeking youth. Journal of Anxiety Disorders 10.1016/j.janxdis.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, … Giedd JN (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences, 107(39), 16988–16993. 10.1073/pnas.1006025107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon S (2017). US mental-health agency?s push for basic research has slashed support for clinical trials. Nature, 546(7658), 339–339. 10.1038/546338a [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski WM, & Parker JG (1998). Peer Interactions, Relationships, and Groups Handbook of Child Psychology. John Wiley & Sons, Inc. 10.1002/9780470147658.chpsy0310 [DOI] [Google Scholar]
- Rudra A, Ram JR, Loucas T, Belmonte MK, & Chakrabarti B (2016). Bengali translation and characterisation of four cognitive and trait measures for autism spectrum conditions in India. Molecular Autism, 7(1), 1–8. 10.1186/s13229-016-0111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Meca J, & Marín‐Martínez F (1998). Testing continuous moderators in meta‐analysis: A comparison of procedures. British Journal of Mathematical and Statistical Psychology, 51(2), 311–326. [Google Scholar]
- Sanislow C. a, Pine DS, Quinn KJ, Kozak MJ, Garvey M. a, Heinssen RK, … Cuthbert BN (2010). Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology, 119(4), 631–639. 10.1037/a0020909 [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, & Kanwisher N (2004). Understanding Other Minds: Linking Developmental Psychology and Functional Neuroimaging. Annual Review of Psychology, 55(1), 87–124. 10.1146/annurev.psych.55.090902.142044 [DOI] [PubMed] [Google Scholar]
- Sayette MA, Cohn JF, Wertz JM, Perrott MA, & Parrott DJ (2001). A psychometric evaluation of the facial action coding system for assessing spontaneous expression. Journal of Nonverbal Behavior, 25(3), 167–185. 10.1023/A:1010671109788 [DOI] [Google Scholar]
- Schmidt FL, Oh I-S, & Hayes TL (2009). Fixed-versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. British Journal of Mathematical and Statistical Psychology, 62(1), 97–128. 10.1348/000711007X255327 [DOI] [PubMed] [Google Scholar]
- Schultz J, Friston KJ, O’Doherty J, Wolpert DM, & Frith CD (2005). Activation in posterior superior temporal sulcus parallels parameter inducing the percept of animacy. Neuron, 45(4), 625–635. 10.1016/j.neuron.2004.12.052 [DOI] [PubMed] [Google Scholar]
- Schwartz JP, Lindley LD, & Buboltz WC Jr (2007). Adult attachment orientations: Relation to affiliation motivation. Counselling Psychology Quarterly, 20(3), 253–265. 10.1080/09515070701308480 [DOI] [Google Scholar]
- Short AB, & Schopler E (1988). Factors relating to age of onset in autism. Journal of Autism and Developmental Disorders, 18(2), 207–216. [DOI] [PubMed] [Google Scholar]
- Skuse DH, & Gallagher L (2009). Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Sciences. 10.1016/j.tics.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Palley LS, Gollub RL, Niemi SM, & Evins AE (2014). Patterns of brain activation when mothers view their own child and dog: An fMRI study. PLoS ONE, 9(10). 10.1371/journal.pone.0107205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachman BA, McKay D, Barch DM, Prinstein MJ, Hollon SD, & Chambless DL (2018). How Psychosocial Research Can Help the National Institute of Mental Health Achieve Its Grand Challenge to Reduce the Burden of Mental Illnesses and Psychological Disorders. American Psychologist 10.1037/amp0000361 [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, & Benson J (2006). Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences of the United States of America, 103(20), 7889–7894. 10.1073/pnas.0600406103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, … Anderson GM (2001). Role of the serotonin transporter gene in the behavioral expression of autism. Mol Psychiatry, 6(4), 434–439. 10.1038/sj.mp.4000873 [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, & Livingstone MS (2006). A cortical region consisting entirely of face-selective cells. Science (New York, N.Y.), 311(5761), 670–674. 10.1126/science.1119983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WL, Thomas LA, Harkins E, Pine DS, Leibenluft E, & Brotman MA (2015). Neural correlates of masked and unmasked face emotion processing in youth with severe mood dysregulation. Social Cognitive and Affective Neuroscience, 11(1), 78–88. 10.1093/scan/nsv087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudge JRH, & Winterhoff PA (1993). Vygotsky, Piaget, and Bandura: Perspectives on the Relations between the Social World and Cognitive Development. Human Development, 36(2), 61–81. 10.1159/000277297 [DOI] [Google Scholar]
- van den Bulk BG, Meens PHF, van Lang NDJ, de Voogd EL, van der Wee N. J. a, Rombouts S. a R. B., … Vermeiren RRJM (2014). Amygdala activation during emotional face processing in adolescents with affective disorders: the role of underlying depression and anxiety symptoms. Frontiers in Human Neuroscience, 8(June), 393 10.3389/fnhum.2014.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goozen SHM, Langley K, Northover C, Hubble K, Rubia K, Schepman K, … Thapar A (2016). Identifying mechanisms that underlie links between COMT genotype and aggression in male adolescents with ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(4), 472–480. 10.1111/jcpp.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhalst J, Gibb BE, & Prinstein MJ (2015). Lonely adolescents exhibit heightened sensitivity for facial cues of emotion. 10.1080/02699931.2015.1092420 [DOI] [PMC free article] [PubMed]
- Wang AT, Dapretto M, Hariri AR, Sigman M, & Bookheimer SY (2004). Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 43(4), 481–490. 10.1097/00004583-200404000-00015 [DOI] [PubMed] [Google Scholar]
- Wirth MM, & Schultheiss OC (2006). Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Hormones and Behavior, 50(5), 786–795. 10.1016/j.yhbeh.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Yaakobi E, & Williams KD (2016). Ostracism and attachment orientation: Avoidants are less affected in both individualistic and collectivistic cultures. British Journal of Social Psychology, 55(1), 162–181. 10.1111/bjso.12122 [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, & Insel TR (2001). Cellular Mechanisms of Social Attachment. Hormones and Behavior, 40(2), 133–138. 10.1006/hbeh.2001.1691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.