Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects multiple organs, including the central nervous system. Neuropsychiatric SLE (NPSLE) is a severe and potentially fatal condition. Several factors including autoantibodies have been implicated in the pathogenesis of NPSLE. However, definitive biomarkers of NPSLE are yet to be identified owing to the complexity of this disease. This is a major barrier to accurate and timely diagnosis of NPSLE. Studies have identified several autoantibodies associated with NPSLE;some of these autoantibodies are well investigated and regarded as symptom-specific. In this review, we discuss recent advances in our understanding of the manifestations and pathogenesis of NPSLE. In addition, we describe representative symptom-specific autoantibodies that are considered to be closely associated with the pathogenesis of NPSLE.
Keywords: autoantibody, biomarker, neuropsychiatric systemic lupus erythematosus, pathogenesis, symptom-specific
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects multiple organs, including the central nervous system (CNS) 1). SLE is characterized by the loss of immune tolerance to nuclear antigens due to development of autoantibodies and immune complex formation;the resultant complement activation results in cell destruction and tissue injury 1, 2). Patients with SLE show considerable variability with respect to symptoms. In particular, neuropsychiatric (NP) SLE is a severe and potentially fatal condition that is characterized by CNS manifestations 1, 2). In 1999, the American College of Rheumatology Research Committee developed standard nomenclature and case definitions for 19 manifestations of NPSLE (Table 1) 3). NP manifestations usually occur in the early stage of SLE and 39%-50% of patients exhibit SLE symptoms. The reported prevalence of NPSLE varies widely from 4%-91%;this may be attributable to variability in clinical presentations, different selection criteria, and heterogeneity among study populations 4-6). In a recent 3-year prospective study of 370 SLE patients with no history of CNS involvement (excluding non-specific minor CNS complaints and peripheral nervous system symptoms), the prevalence of major CNS events was 4.3% with an estimated incidence of 7.8 events/100 person-years 7). In spite of recent advances, the diagnosis of NPSLE is typically challenging, due in part to the absence of specific and reliable laboratory or imaging biomarkers 2, 8). The diagnosis of NPSLE requires exclusion of other causes such as infection, concurrent disease, metabolic abnormalities, or drug adverse events 1, 2, 5, 6, 8). Therefore, investigation of disease-specific or symptom-specific autoantibodies observed in NPSLE is a key imperative to facilitate timely and accurate diagnosis. Here, we review recent NPSLE studies that focused on the pathogenesis of NPSLE and the autoantibodies associated with NPSLE manifestations, especially symptom-specific autoantibodies.
Table 1.
Neuropsychiatric syndromes according to the criteria proposed by the American College of Rheumatology (Reference 3)
| Central NPSLE | Peripheral NPSLE |
| Aseptic meningitis1 | Guillain-Barre syndrome |
| Cerebrovascular disease1 | Autonomic neuropathy |
| Demyelinating syndrome1 | Mononeuropathy |
| Headache1 | Myasthenia gravis |
| Movement disorder1 | Cranial neuropathy |
| Myelopathy1 | Plexopathy |
| Seizure disorders1 | Polyneuropathy |
| Acute confusional state2 | |
| Anxiety disorder2 | |
| Cognitive dysfunction2 | |
| Mood disorder2 | |
| Psychosis2 |
1. Focal NPSLE, 2. Diffuse NPSLE
NPSLE : neuropsychiatric systemic lupus erythematosus
2. Pathogenesis of NPSLE
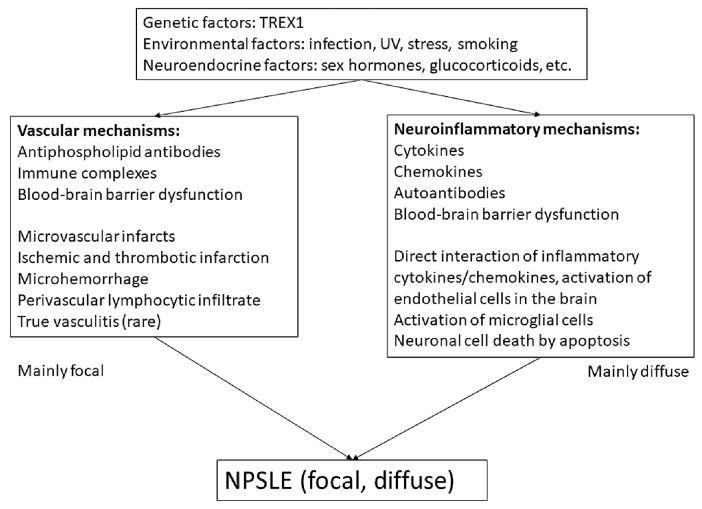
The pathogenesis of NPSLE is highly complex, with detailed mechanisms yet to be elucidated 1, 2, 6). The pathogenic pathways suggested thus far include blood-brain barrier (BBB) dysfunction, vascular occlusion, neuroendocrine-immune imbalance, and tissue and neuronal damage caused by autoantibodies and proinflammatory cytokines [interleukin (IL)-1, IL-6, IL-8, IL-10, IL-17, type 1 interferons, tumor necrosis factor, colony-stimulating and macrophage-stimulating factors] 1, 2, 6, 8, 9). Predisposing genetic factors, neuroendocrine factors, and environmental factors are also thought to be important. Figure 1 illustrates the currently proposed pathogenetic mechanisms of NPSLE 1, 2, 6).
Fig. 1.
Pathogenic mechanisms of neuropsychiatric systemic lupus erythematosus (NPSLE)
Abbreviations : UV, ultraviolet
2.1 Predisposing factors
Predisposing genetic factors are believed to play an important role in SLE pathogenesis 2, 6). In NPSLE patients, mutations in TREX1, which encodes three-prime repair exonuclease 1 (DNAse III), were shown to be associated with neurological involvement. Polymorphisms in TREX1 were found to be associated with neurological involvement in European patients with SLE 10). Loss-of function mutations in TREX1 augment the production of type 1 interferons in mice and lead to early-onset cerebral NPSLE 11, 12). In addition, HLA-DRB1*04 and rs10181656(G) alleles were shown to be associated with ischemic cardiovascular disease (CVD) in Caucasian patients with SLE 13, 14). More recently, Rullo et al. reviewed recent advances in our understanding of the genetic basis of SLE, including genetic variants in recently identified SLE-associated loci, the immunological pathways affected by these gene products, and the disease manifestations linked to these loci 15). In addition, environmental factors and neuroendocrine factors play an important role in the development of SLE. In previous studies, silica exposure, smoking, oral contraceptives, postmenopausal hormone therapy, and endometriosis were found to be risk factors for SLE 16). Indeed, sex steroid hormones (17β-estradiol, testosterone, prolactin, progesterone, dehydroepiandrosterone) reportedly impact the immune response and the severity of disease in SLE;this also explains the sex disparity in SLE. Other environmental factors, such as ultraviolet radiation, vitamin D, infection (e.g., Epstein-Barr virus), vaccination, heavy metals, solvents, and pesticides are considered as risk factors for SLE 6, 16).
2.2 Mechanisms of NPSLE
Recent reports have identified two major mechanisms for the development of NPSLE, i.e., vascular mechanisms and neuroinflammatory mechanisms (Figure 1) 1, 2, 6). Among the vascular mechanisms, vasculopathy is implicated in CNS damage in patients with NPSLE;autopsy studies have shown pathological findings of multi-focal microinfarcts, small-vessel noninflammatory vasculopathy and occlusion, embolism, cortical atrophy, and microhemorrhages 1, 17, 18). Anti-phospholipid antibodies (aPL) and deposition of immune complexes are likely to be associated with these conditions 1, 18-20). Injury to large and small blood vessels mediated by aPL initiates vascular damage, finally resulting in focal, and in part, diffuse neuropsychiatric events (seizures, cognitive dysfunction, etc.). The second mechanism involves autoimmune inflammation mediated by autoantibodies, resulting in increased permeability of the BBB, intrathecal formation of immune complexes, and production of inflammatory mediators (IFN-α, IL-6, IL-8, IP-10, MCP-1, etc.) 20, 21). Direct CNS tissue injury caused by excitatory amino acid toxicity, oxidative stress, plasminogen activator inhibitor 1 (PAI-1), and matrix metalloproteinase 9 (MMP9) activity have also been suggested 20, 22, 23). These processes can cause CNS damage by activation of microglial cells and induction of neuronal cell death by apoptosis 1, 6), leading to mainly diffuse NSPLE symptoms, such as acute confusional state and psychosis 1, 20). As previously described, BBB dysfunction plays an important role in the pathogenesis of NSPLE. Normally, the brain is immunologically privileged and is sheltered from foreign substances in the circulation. The BBB limits the entry of soluble molecules and cells into brain parenchyma and regulates both uptake into and efflux out of the brain 1, 20, 24). Although the precise mechanism of BBB dysfunction is still unclear, permeability of the BBB can be affected by both SLE factors (immune complex deposition, cytokine/chemokines) and non-SLE factors (smoking or hypertension) that induce endothelial dysfunction in brain vasculature 1, 8, 20, 25). In this regard, autoantibodies reacting with neuronal cells or those that have been reported as specific for each NPSLE symptom (from the circulation or intrathecal production) might be associated with BBB dysfunction. Here, we review the representative autoantibodies that are potentially associated with the pathogenesis of NPSLE.
3. Autoantibodies potentially associated with specific NPSLE symptoms
Table 2 shows the representative autoantibodies that have been recently described as potentially associated with NPSLE pathogenesis. More than 100 autoantibodies have been described in patients with SLE or NPSLE 26);however, none of these have been definitively implicated in the complex process of NPSLE pathogenesis. Therefore, extensive research is ongoing to establish distinct pathogenic roles for each autoantibody.
Table 2.
Representative autoantibodies associated with NPSLE
| Target of autoantibodies
(autoantibodies) |
Serum/CSF | Prevalence in
SLE patients |
Associated NPSLE symptoms |
| Phospholipid:
β2-glycoprotein 1 and cardiolipin (aCL-Ab) |
Serum, CSF | Up to 45% | Focal NPSLE (CVD, seizures, chorea)
Diffuse NPSLE (cognitive dysfunction, psychosis, depression, headache) |
| Ribosomal P protein (anti-ribosmal P Ab) | Serum, CSF | 6%-46% | Elevated titers in active SLE
Diffuse NPSLE (psychosis, depression) |
| NMDA receptor subtype 2 (anti-NMDA/NR2 Ab) | Serum, CSF | 30%-40% | Diffuse NPSLE (depression cognitive dysfunction) |
| MAP-2 (anti-MAP-2 Ab) | Serum, CSF | 17%, 33.3% (CSF) | Focal NPSLE (seizures, chorea, sensory neuropathy)
Diffuse NPSLE (psychosis, headache) |
| U1 ribonucleoprotein (Anti-U1RNP Ab) | Serum, CSF | 18% (CSF) | NPSLE in general |
| Structural endothelial proteins (AECA) | Serum | 17-75% | Psychosis, depression |
| TPI (anti-TPI Ab) | Serum, CSF | 30%-40% | Focal NPSLE (aseptic meningitis)
Less frequent in acute confusional state |
| GAPDH (anti-GAPDH Ab) | Serum | 47% | Increased intracranial pressure, cognitive dysfunction |
AECA : anti-endothelial cell antibody ; aCL : anti-cardiolipin ; CVD : cerebrovascular disease ; CSF : cerebrospinal fluid ; GAPDH : glyceraldehyde-3-phosphate dehydrogenase ; MAP-2 : microtubule-associated protein 2 ; NMDA/ NR2 : N-methyl-D-aspartate receptor 2 ; NPSLE : neuropsychiatric systemic lupus erythematosus ; U1-RNP : U1 ri bonucleoprotein ; TPI : triosephosphate isomerase
3.1 Anti-phospholipid antibodies (aPL)
The aPL antibody family targets proteins associated with anionic phospholipids in the plasma membrane that regulate the blood clotting cascade; subsequent activation of procoagulants promotes thrombosis and cerebral infarction 27). Anti-cardiolipin (aCL), anti-β2GP1 antibodies, and lupus anticoagulant (LAC) are the most widely investigated autoantibodies targeting phospholipids. These have been recognized as major risk factors for NPSLE and are believed to contribute to the development of thrombosis and other NPSLE symptoms, such as seizures, stroke, chorea, movement disorders, cognitive dysfunction, and myelopathy 28-32). In vitro studies have demonstrated direct binding of aPL with CNS cells;in addition, intrathecal passive transfer of IgG isolated from aPL-positive patients was shown to induce cognitive dysfunction in mice 33). At least, exacerbation of procoagulant state by aPL is believed to be associated with focal NPSLE causing intravascular thrombosis and cerebral ischemia 20).
3.2 Anti-ribosomal P protein antibodies (anti-ribo P)
Anti-ribo P are specific autoantibodies occurring in up to 46% of patients;target epitopes are located in the C-terminal end of three highly conserved phosphorylated proteins, P0, P1, and P2, which are present in the 60S subunit of ribosomes 6, 34). Many retrospective studies have suggested an association between elevated serum or CSF levels of anti-ribo P and NPSLE manifestations; however, the results have been contested 35-37). Recent longitudinal studies and prospective studies have shown their association with lupus psychosis 38-41). In a study by Hanly et al., anti-ribo P was found to be a predictor of psychosis 42). In a mouse model, anti-ribo P recognized neurons in the hippocampus, cingulate, and primary olfactory piriform cortex, and induced long-term depressive-like behavior when introduced into cerebral ventricles 43). Matus et al. reported that anti-ribo P from psychiatric lupus induced a rapid and sustained increase in calcium reflux and apoptosis in rat neurons expressing cell-surface P-antigen protein P331. The death of these neurons in specific brain regions (such as hippocampus) was found to affect the memory and emotional behavior of rats 44). Direct evidence of a pathogenic role for these antibodies in humans is still lacking;nevertheless, experimental data and prospective studies support the role of anti-ribo P in the causation of diffuse NPSLE.
3.3 Anti-N-methyl-D-aspartate receptor antibodies (anti-NMDA)
Anti-NMDA antibodies occur in 30%-40% of patients with SLE;these have been demonstrated as a subset of double-stranded DNA (dsDNA) antibodies that cross-react with NMDA receptors, specifically with the NMDA receptor subunit 2 (NR2) 45). NMDA receptors are widely distributed in the brain and localized within glutamatergic synapses;a particularly high density is observed in the amygdala and hippocampus, which modulate cognitive function, emotional processes, and memory 46, 47). Activation of NMDA receptors is critical in learning and memory;however, prolonged stimulation can cause apoptotic death of neuronal cells. The potential pathogenic role of anti-NMDA detected in CSF has been demonstrated in both in vitro and in vivo studies 48, 49). Interestingly, no neuronal damage was observed if the BBB remained intact;however, several pathological changes were detected when the BBB was disrupted 46-48). Correlation between CSF anti-NMDA and diffuse NPSLE manifestations has also been reported 50). Furthermore, it has been suggested that anti-NR2/dsDNA antibodies may also help distinguish SLE patients with central diffuse NPSLE manifestations from patients with peripheral manifestations;the pathogenic factors and mechanisms underlying these manifestations are probably different 51). Then, how do these autoantibodies gain access to the brain in SLE? Yoshio et al. have reported that anti-NR2/dsDNA antibodies from SLE patients activate endothelial cells and induce expression of surface molecules [intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1)] as well as the production of IL-6 and IL-8 52). Hirohata et al. also reported that the severity of BBB damage plays a critical role in the development of diffuse NPSLE (acute confusional state) through the accelerated entry of larger amounts of anti-NR2 antibodies into the CNS 53). These results indicate that activation of BBB endothelial cells by anti-NMDA may cause inflammation, disrupt the BBB, and promote entry of autoantibodies into the CSF in patients with SLE.
3.4 Anti-microtubule-associated protein 2 antibodies (anti-MAP-2)
MAP-2 is one of the abundant groups of cytoskeletal components predominantly expressed in neurons 54). MAP-2 regulates the nucleation and stabilization of microtubules, organelle transport protein kinases that are involved in signal transduction 55). In a study by Yamada et al., 33.3% of NPSLE patients tested positive for anti-MAP-2 in the CSF 54). Williams also reported that 17% of SLE patients had anti-MAP-2 in contrast to 4% of neurologic injury/disease control patients 56). Patients who tested positive for anti-MAP-2 exhibited neuropsychiatric symptoms (psychosis, seizures, neuropathy, and cerebritis). Moreover, both anti-ribo P titers and IL-6 levels in CSF were significantly higher in NPSLE patients having anti-MAP-2, indicating some association between them 54).
3.5 Anti-U1 ribonucleoprotein antibodies (anti-U1RNP)
Anti-U1RNP reacts with proteins that are associated with U1 RNA and form U1 small nuclear ribonucleoprotein (snRNP);these are detectable in 25%-47% of SLE patients 57). The snRNP are RNA-protein complexes that are abundant in the nucleus;these are involved in the nuclear processing of pre-mRNA along with other proteins comprising the spliceosome 58). In a study by Sato et al., anti-U1RNP in CSF, but not that in serum, was associated with NPSLE and mixed connective tissue disease 59). Recent studies have shown that anti-RNP as well as anti-Sm antibodies were less likely to be produced within the CNS;this was assessed by calculating anti-RNP and anti-Sm indices in the CSF (indices of each reflect the intrathecal production of these antibodies) 60). Nevertheless, the detailed role of anti-U1RNP should be further investigated.
3.6 Anti-endothelial cell antibodies (AECA)
AECAs target a heterogenous group of antigens including structural endothelial proteins (ranging from 10 to 200 kDa) as well as adhesion molecules to endothelial cells;these are found in a variety of diseases that are characterized by vessel wall damage 61). The reported prevalence of AECA in SLE patients ranges from 17% to 75%. Conti et al. found an association of serum AECA with psychosis and depression in NPSLE 62). AECA can activate endothelial cells by inducing the expression of adhesion molecules (ICAM-1 and VCAM-1) and stimulating the production of cytokines (IL-1, IL-8) and chemokines [such as monocyte-chemotactic protein 1 (MCP-1)]. AECA can also enhance the production of tissue factor and von Willebrand factor, promoting thrombosis. However, low specificity due to the lack of a standardized detection method and possible presence of natural AECAs (seen in a small percentage of healthy individuals and showing low affinity for their target antigens) limit the use of AECAs as diagnostic and prognostic markers 61, 62).
3.7 Anti-triosephosphate isomerase antibodies (anti-TPI)
Triosephosphate isomerase (TPI) is an important glycolytic enzyme that catalyzes the interconversion of dihydroxyacetone phosphate and D-glyceraldehyde-3-phosphate 63). TPI is mediated solely by glycolysis in red blood cells and in brain cells, and its deficiency is associated with hemolytic anemia and neurological disorders. TPI is involved in the stability of neuronal microtubules 63-66). Watanabe et al. have suggested some association with the pathogenesis of NPSLE 64-66). Furthermore, in our previous study, anti-TPI-TPI immune complex was detected in the CSF of anti-TPI-positive NPSLE patients 65). In a lupus model mouse, anti-TPI was detected in MRL/lpr mice;in addition, anti-TPI was shown to bind to brain tissue in the meninges, choroid plexus, hippocampus, and periventricular lesions using anti-TPI-producing hybridoma inoculation into the brain hemisphere 66). Recently, we reported a higher frequency of aseptic meningitis in anti-TPI-positive NPSLE patients;in addition, serum anti-TPI index showed a positive correlation with serum IgG levels 8). These results indicate that anti-TPI may be associated with NPSLE, mainly focal NPSLE (aseptic meningitis);the underlying mechanism may involve disruption of the BBB via formation of immune complexes in the CNS. Indeed, the underlying mechanism of aseptic meningitis in SLE patients is still unclear. A possible mechanism can be considered along with meningeal inflammation similar to that proposed for multiple sclerosis 67): immune cells first pass through the meninges via the bloodstream to the choroid plexus, as a part of immune surveillance. The interaction of autoimmune myelin-specific T cells with myelin-loaded antigen-presenting cells can induce T cell reactivation and production of inflammatory cytokines/chemokines in the meninges. The additional inflammatory cells compromise local BBB integrity, which finally triggers immune cell infiltration into the CNS 8, 67). In any case, further investigation is needed to clarify the pathogenic role of anti-TPI in NPSLE.
3.8 Anti-glyceraldehyde-3-phosphate dehydrogenase antibodies (anti-GAPDH)
GAPDH is a glycolytic enzyme;however, more recent evidence indicates that mammalian GAPDH performs a number of other functions 68). Its activity contributes to membrane fusion, microtubule bundling, phosphotransferase activity, nuclear RNA export, DNA replication and repair, and further, to neuronal cell death 68, 69). Takasaki et al. first reported that anti-GAPDH is one of the elements of proliferating cell nuclear antigens, specifically those reactive with serum from SLE patients 69). Furthermore, Delunardo et al. demonstrated the reaction of anti-GAPDH with neuronal cells and its association with cognitive dysfunction in patients with SLE 70). Recently, Sun et al. also reported a positive correlation of serum anti-GAPDH with SLEDAI-2K, ESR, IgG, and IgM;in addition, anti-GAPDH showed an association with increased intracranial pressure, indicating its potential role in the induction of brain tissue damage 71). Although the direct effect of anti-GAPDH is yet to be elucidated, it may serve as a useful biomarker for cerebrovascular damage in NPSLE patients.
4. Conclusion
Neuropsychiatric sequelae are among the main causes of morbidity and mortality in patients with SLE, but they are the least well-understood aspect of the disease. Appropriate evaluation and accurate classification of NP manifestations is an important focus of NPSLE treatment and research. However, its complex pathogenesis and polymorphic phenotype hampers the identification of pertinent, robust biomarkers. Further investigation of biomarkers (such as disease-specific or symptom-specific autoantibodies and cytokine/chemokine expression profiles) is required to increase our knowledge and improve the management of NPSLE.
Conflict of interest
The authors declare there is no conflict of interest.
References
- 1.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus:pathogenesis and biomarkers. Nat Rev Neurol, 10: 579-596, 2014. doi: 10.1038/nrneurol.2014.148. [DOI] [PubMed] [Google Scholar]
- 2.Magro-Checa C, Zirkzee EJ, Huizinga TW, Steup-Beekman GM. Management of Neuropsychiatric Systemic Lupus Erythematosus:Current Approaches and Future Perspectives. Drugs, 76: 459-483, 2016. doi: 10.1007/s40265-015-0534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.[No authors listed]. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum, 42: 599-608, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus:an international inception cohort study. Arthritis Rheum, 56: 265-273, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol, 6: 358-367, 2010. doi: 10.1038/nrrheum.2010.62. [DOI] [PubMed] [Google Scholar]
- 6.Govoni M, Bortoluzzi A, Padovan M, et al. The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J Autoimmun, 74: 41-72, 2016. doi: 10.1016/j.jaut.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Kampylafka EI, Alexopoulos H, Kosmidis ML, et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus:a 3-year prospective study of 370 patients. PLoS One, 8: e55843, 2013. doi: 10.1371/journal.pone.0055843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato S, Yashiro M, Asano T, Kobayashi H, Watanabe H, Migita K. Association of anti-triosephosphate isomerase antibodies with aseptic meningitis in patients with neuropsychiatric systemic lupus erythematosus. Clin Rheumatol, 36: 1655-1659, 2017. doi: 10.1007/s10067-017-3653-2. [DOI] [PubMed] [Google Scholar]
- 9.McGlasson S, Wiseman S, Wardlaw J, Dhaun N, Hunt DPJ. Neurological Disease in Lupus: Toward a Personalized Medicine Approach. Front Immunol, 9: 1146, 2018. doi: 10.3389/fimmu.2018.01146. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namjou B, Kothari PH, Kelly JA, et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun, 12: 270-279, 2011. doi: 10.1038/gene.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellyard JI, Jerjen R, Martin JL, et al. Identification of a pathogenic variant in TREX1 in early-onset cerebral systemic lupus erythematosus by Whole-exome sequencing. Arthritis Rheumatol, 66: 3382-3386, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell, 134: 587-598, 2008. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundström E, Gustafsson JT, Jönsen A, et al. HLA-DRB1*04/*13 alleles are associated with vascular disease and antiphospholipid antibodies in systemic lupus erythematosus. Ann Rheum Dis, 72: 1018-1025, 2013. doi: 10.1136/annrheumdis-2012-201760. [DOI] [PubMed] [Google Scholar]
- 14.Svenungsson E, Gustafsson J, Leonard D, et al. A STAT4 risk allele is associated with ischaemic cerebrovascular events and anti-phospholipid antibodies in systemic lupus erythematosus. Ann Rheum Dis, 69: 834-840, 2010. doi: 10.1136/ard.2009.115535. [DOI] [PubMed] [Google Scholar]
- 15.Rullo OJ, Tsao BP. Recent insights into the genetic basis of systemic lupus erythematosus. Ann Rheum Dis, 72 Suppl 2:ii56-61, 2013. doi: 10.1136/annrheumdis-2012-202351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol, 28: 497-505, 2016. doi: 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhiannon JJ. Systemic lupus erythematosus involving the nervous system:presentation, pathogenesis, and management. Clin Rev Allergy Immunol, 34: 356-360, 2008. doi: 10.1007/s12016-007-8052-z. [DOI] [PubMed] [Google Scholar]
- 18.Hanly JG, Walsh NM, Sangalang V. Brain pathology in systemic lupus erythematosus. J Rheumatol, 19: 732-741, 1992. [PubMed] [Google Scholar]
- 19.Mehta N, Uchino K, Fakhran S, et al. Platelet C4d is associated with acute ischemic stroke and stroke severity. Stroke, 39: 3236-3241, 2008. doi: 10.1161/STROKEAHA.108.514687. [DOI] [PubMed] [Google Scholar]
- 20.Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol, 10: 338-347, 2014. doi: 10.1038/nrrheum.2014.15. [DOI] [PubMed] [Google Scholar]
- 21.Yoshio T, Okamoto H, Kurasawa K, Dei Y, Hirohata S, Minota S. IL-6, IL-8, IP-10, MCP-1 and G-CSF are significantly increased in cerebrospinal fluid but not in sera of patients with central neuropsychiatric lupus erythematosus. Lupus, 25: 997-1003, 2016. doi: 10.1177/0961203316629556. [DOI] [PubMed] [Google Scholar]
- 22.Ainiala H, Hietaharju A, Dastidar P, et al. Increased serum matrix metalloproteinase 9 levels in systemic lupus erythematosus patients with neuropsychiatric manifestations and brain magnetic resonance imaging abnormalities. Arthritis Rheum, 50: 858-865, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kwieciński J, Kłak M, Trysberg E, Blennow K, Tarkowski A, Jin T. Relationship between elevated cerebrospinal fluid levels of plasminogen activator inhibitor 1 and neuronal destruction in patients with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum, 60: 2094-2101, 2009. doi: 10.1002/art.24603. [DOI] [PubMed] [Google Scholar]
- 24.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis, 37: 13-25, 2010. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Gelb S, Stock AD, Anzi S, Putterman C, Ben-Zvi A. Mechanisms of neuropsychiatric lupus:The relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. J Autoimmun, 91: 34-44, 2018. doi: 10.1016/j.jaut.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus:more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum, 34: 501-537, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Harris EN, Pierangeli S. Antiphospholipid antibodies and cerebral lupus. Ann N Y Acad Sci, 823: 270-278, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Sanna G, Bertolaccini ML, Cuadrado MJ. Neuropsychiatric manifestations in systemic lupus erythematosus:prevalence and association with antiphospholipid antibodies. J Rheumatol, 30: 985-992, 2003. [PubMed] [Google Scholar]
- 29.Hanly JG. Attribution in the assessment of nervous system disease in SLE. Rheumatology (Oxford), 54: 755-756, 2015. doi: 10.1093/rheumatology/keu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade RM, Alarcón GS, González LA, et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV). Ann Rheum Dis, 67: 829-834, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mok MY, Chan EY, Fong DY, Leung KF, Wong WS, Lau CS. Antiphospholipid antibody profiles and their clinical associations in Chinese patients with systemic lupus erythematosus. J Rheumatol, 32: 622-628, 2005. [PubMed] [Google Scholar]
- 32.McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology, 64: 297-303, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Katzav A, Ben-Ziv T, Blank M, Pick CG, Shoenfeld Y, Chapman J. Antibody-specific behavioral effects: intracerebroventricular injection of antiphospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J Neuroimmunol, 272: 10-15, 2014. doi: 10.1016/j.jneuroim.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Mahler M, Kessenbrock K, Raats J, Williams R, Fritzler MJ, Blüthner M. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. J Mol Med (Berl), 81: 194-204, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Nojima Y, Minota S, Yamada A, Takaku F, Aotsuka S, Yokohari R. Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann Rheum Dis, 51: 1053-1055, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddouk S, Marzouk S, Jallouli M, et al. Clinical and diagnostic value of ribosomal P autoantibodies in systemic lupus erythematosus. Rheumatology (Oxford), 48: 953-957, 2009. doi: 10.1093/rheumatology/kep142. [DOI] [PubMed] [Google Scholar]
- 37.Karassa FB, Afeltra A, Ambrozic A, et al. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus:an international meta-analysis. Arthritis Rheum, 54: 312-324, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Bonfa E, Golombek SJ, Kaufman LD, et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med, 317: 265-271, 1987. [DOI] [PubMed] [Google Scholar]
- 39.West SG, Emlen W, Wener MH, Kotzin BL. Neuropsychiatric lupus erythematosus:a 10-year prospective study on the value of diagnostic tests. Am J Med, 99: 153-163, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe T, Sato T, Uchiumi T, Arakawa M. Neuropsychiatric manifestations in patients with systemic lupus erythematosus:diagnostic and predictive value of longitudinal examination of anti-ribosomal P antibody. Lupus, 5: 178-183, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Briani C, Lucchetta M, Ghirardello A, et al. Neurolupus is associated with anti-ribosomal P protein antibodies:an inception cohort study. J Autoimmun, 32: 79-84, 2009. doi: 10.1016/j.jaut.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Hanly JG, Urowitz MB, Siannis F, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis:results from an international inception cohort study. Arthritis Rheum, 58: 843-853, 2008. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum, 56: 938-948, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Matus S, Burgos PV, Bravo-Zehnder M, et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J Exp Med, 204: 3221-3234, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauvsnes MB, Omdal R. Systemic lupus erythematosus, the brain, and anti-NR2 antibodies. J Neurol, 259: 622-629, 2012. doi: 10.1007/s00415-011-6232-5. [DOI] [PubMed] [Google Scholar]
- 46.Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity;antibody impairs memory. Immunity, 21: 179-188, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior:antibodies alter emotion. Proc Natl Acad Sci U S A, 103: 678-683, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med, 7: 1189-1193, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Husebye ES, Sthoeger ZM, Dayan M, et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis, 64: 1210-1213, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum, 58: 1130-1115, 2008. doi: 10.1002/art.23399. [DOI] [PubMed] [Google Scholar]
- 51.Fragoso-Loyo H, Cabiedes J, Orozco-Narváez A, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS One, 3: e3347, 2008. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshio T, Okamoto H, Hirohata S, Minota S. IgG anti-NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum, 65: 457-463, 2013. doi: 10.1002/art.37745. [DOI] [PubMed] [Google Scholar]
- 53.Hirohata S, Arinuma Y, Yanagida T, Yoshio T. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther, 16: R77, 2014. doi: 10.1186/ar4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada Y, Nozawa K, Nakano S, et al. Antibodies to microtubule-associated protein-2 in the cerebrospinal fluid are a useful diagnostic biomarker for neuropsychiatric systemic lupus erythematosus. Mod Rheumatol, 26: 562-568, 2016. doi: 10.3109/14397595.2015.1123345. [DOI] [PubMed] [Google Scholar]
- 55.Sánchez C, Díaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol, 61: 133-168, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Williams RC Jr, Sugiura K, Tan EM. Antibodies to microtubule-associated protein 2 in patients with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum, 50: 1239-1247, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Migliorini P, Pratesi F, Tommasi C, Anzilotti C. The immune response to citrullinated antigens in autoimmune diseases. Autoimmun Rev, 4: 561-564, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Dema B, Charles N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies (Basel), 5 pii:E2, 2016. doi: 10.3390/antib5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato T, Fujii T, Yokoyama T, et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum, 62: 3730-3740, 2010. doi: 10.1002/art.27700. [DOI] [PubMed] [Google Scholar]
- 60.Hirohata S, Sakuma Y, Yanagida T, Yoshio T. Association of cerebrospinal fluid anti-Sm antibodies with acute confusional state in systemic lupus erythematosus. Arthritis Res Ther, 16: 450, 2014. doi: 10.1186/s13075-014-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perricone C, Pendolino M, Olivieri M, Conti F, Valesini G, Alessandri C. Neuropsychiatric manifestations associated with anti-endothelial cell antibodies in systemic lupus erythematosus. Isr Med Assoc J, 17: 171-178, 2015. [PubMed] [Google Scholar]
- 62.Conti F, Alessandri C, Bompane D, et al. Autoantibody profile in systemic lupus erythematosus with psychiatric manifestations:a role for anti-endothelial-cell antibodies. Arthritis Res Ther, 6: R366-372, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orosz F, Wágner G, Liliom K, et al. Enhanced association of mutant triosephosphate isomerase to red cell membranes and to brain microtubules. Proc Natl Acad Sci U S A, 97: 1026-1031, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe H, Seino T, Sato Y. Antibodies to triosephosphate isomerase in patients with neuropsychiatric lupus. Biochem Biophys Res Commun, 321: 949-953, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Sasajima T, Watanabe H, Sato S, Sato Y, Ohira H. Anti-triosephosphate isomerase antibodies in cerebrospinal fluid are associated with neuropsychiatric lupus. J Neuroimmunol, 181: 150-156, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Sato S, Watanabe H, Shio K, Kobayashi H, Ohira H. Association of anti-triosephosphate isomerase antibody and MRL/MpJ-Faslpr mouse. J Neuroimmunol, 226: 110-115, 2010. doi: 10.1016/j.jneuroim.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 67.Russi AE, Brown MA. The meninges:new therapeutic targets for multiple sclerosis. Transl Res, 165: 255-269, 2015. doi:10.1016/j.trsl.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sirover MA. Role of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in normal cell function and in cell pathology. J Cell Biochem, 66: 133-140, 1997. [PubMed] [Google Scholar]
- 69.Takasaki Y, Kaneda K, Matsushita M, et al. Glyceraldehyde 3-phosphate dehydrogenase is a novel autoantigen leading autoimmune responses to proliferating cell nuclear antigen multiprotein complexes in lupus patients. Int Immunol, 16: 1295-1304, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Delunardo F, Soldati D, Bellisario V, et al. Anti-GAPDH Autoantibodies as a Pathogenic Determinant and Potential Biomarker of Neuropsychiatric Diseases. Arthritis Rheumatol, 68: 2708-2716, 2016. doi: 10.1002/art.39750. [DOI] [PubMed] [Google Scholar]
- 71.Sun J, Li X, Zhou H, et al. Anti-GAPDH Autoantibody Is Associated with Increased Disease Activity and Intracranial Pressure in Systemic Lupus Erythematosus. J Immunol Res, 2019: 7430780, 2019. doi: 10.1155/2019/7430780. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]