Abstract
Patients are avoiding hospitals for fear of contracting severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). We are witnessing a re-emergence of rare complications of myocardial infarctions (MI) due to delayed revascularization. Herein, we describe a case of hemorrhagic pericarditis from thrombolytics administered to a patient with late presenting MI. (Level of Difficulty: Beginner.)
Key Words: complication, COVID-19, myocardial infarction, STEMI
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; MI, myocardial infarction; SARS- CoV-2, severe acute respiratory syndrome-coronavirus-2; STEMI, ST-segment elevation myocardial infarction
Graphical abstract
Patients are avoiding hospitals for fear of contracting severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). We are witnessing a re-emergence…
Presentation
A 69-year-old male presented to an outside hospital with exertional chest pain of unknown duration. His vital signs on admission were: blood pressure 99/71 mm Hg; heart rate was 66 beats/min; his oxygen saturation was 97% on room air.
Learning Objectives
-
•
To recognize the re-emergence of rare complications of MI delayed presentation and management during the COVID-19 pandemic.
-
•
To acknowledge patient fears of acquiring SARS CoV-2 infection and subsequent delay in seeking timely medical care for MI.
Physical examination of his general appearance revealed he was in distress due to pain; his jugular vein distention was 2 cm above the clavicle; his chest was clear bilaterally; he had regular heart sounds of S1/S2, audible with no murmurs, and his extremities were warm with trace edema bilaterally.
Medical history
His history included hypertension, hyperlipidemia, abdominal aortic aneurysm of 2.7 cm, uncontrolled diabetes, and tobacco use of 1 pack-year.
Investigation
Electrocardiography revealed a posterior ST-segment elevation myocardial infarction (STEMI) (Figure 1) for which he received tenecteplase, clopidogrel, and aspirin. He was airlifted to the authors’ hospital for coronary angiography due to ongoing chest pain and persistent ST-segment elevation. Angiography revealed a left-dominant system with a culprit 100% thrombotic occlusion of the left circumflex coronary artery and a nonculprit 90% proximally diseased left anterior descending artery (Figures 2 and 3, Videos 1 and 2). Although the present authors were able to wire past the lesion, balloon angioplasty was unsuccessful due to extensive thrombus burden.
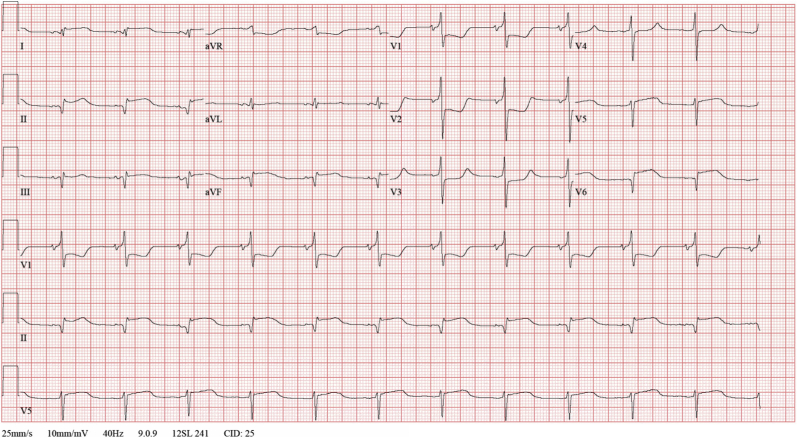
Figure 1.
12-Lead Electrocardiography
A 12-lead electrocardiogram shows 4-mm horizontal ST-segment depression in leads V1 to V3, a tall broad R-wave (>30 ms) in leads V1 to V3, and an upright T-wave in lead V3. A 2-mm ST-segment elevation is observable in II, aVF, and V6. This is compatible with inferior-lateral-posterior ST-segment elevation myocardial infarction.
Figure 2.
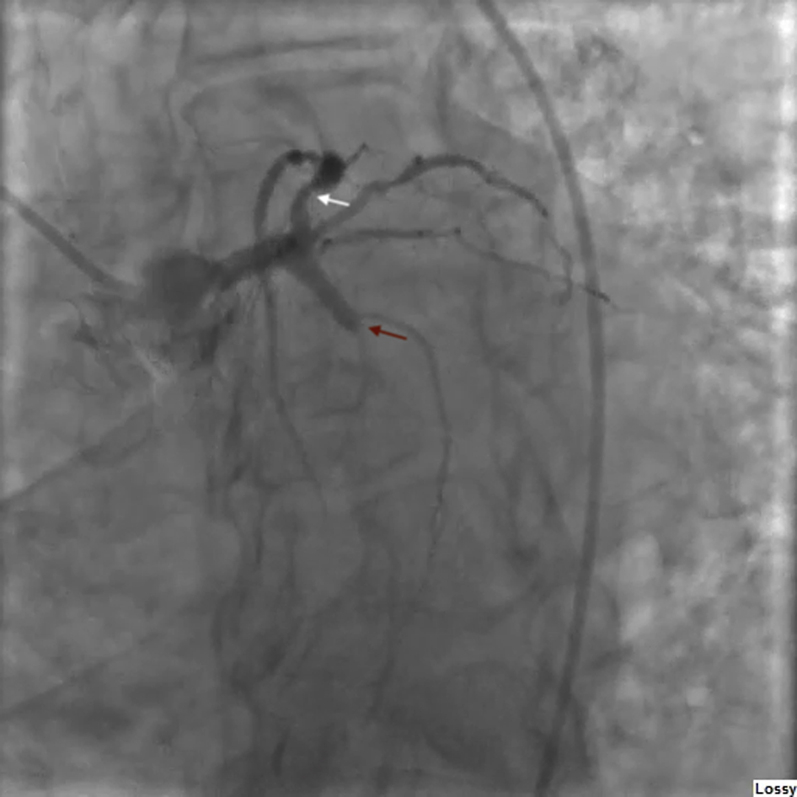
Left Anterior Oblique Caudal Coronary Angiogram
Caudal left anterior oblique coronary angiogram shows a culprit 100% occlusion of the left circumflex coronary artery (red arrow) and a nonculprit left anterior descending artery with 90% proximal stenosis (white arrow).
Figure 3.
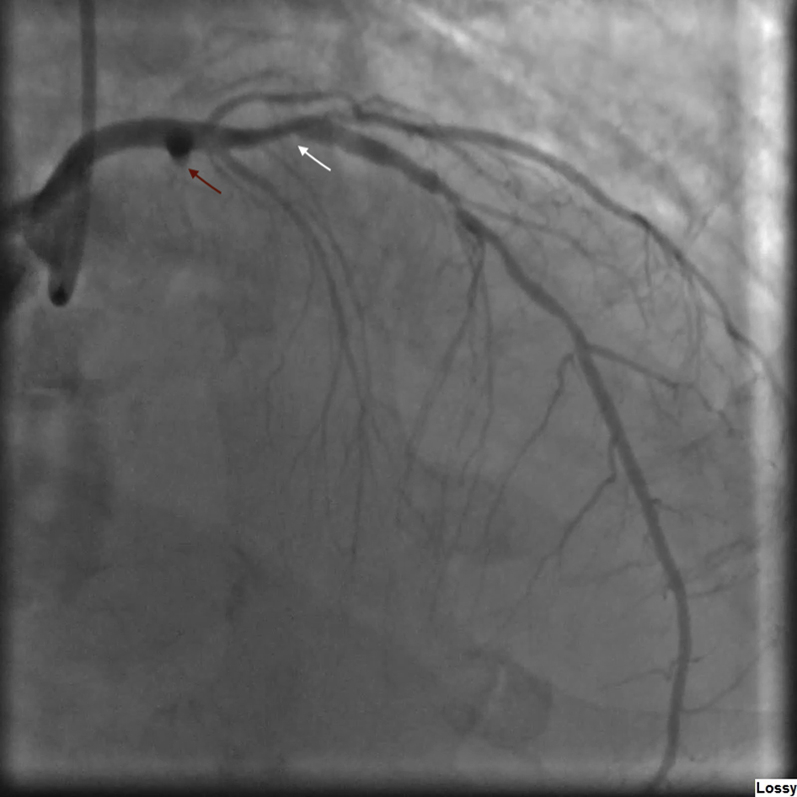
Right Anterior Oblique Coronary Angiogram
Coronary angiogram right anterior oblique view shows a culprit 100% occlusion of the left circumflex coronary artery (red arrow) and a nonculprit left anterior descending artery with 90% proximal stenosis (white arrow). LAO = left anterior oblique.
Online Video 1.
Coronary angiogram, LAO caudal view shows a culprit 100% occlusion of the left circumflex coronary artery and a nonculprit left anterior descending artery with 90% proximal stenosis. LAO = left anterior oblique.
Online Video 2.
Coronary angiogram, RAO view shows a culprit 100% occlusion of the left circumflex coronary artery and a non-culprit left anterior descending artery with 90% proximal stenosis. RAO = right anterior oblique.
Management
An intra-aortic balloon pump was inserted, and he was transferred to the coronary care unit for medical management of a STEMI. Transthoracic echocardiography revealed a left ventricular ejection fraction of 25% (Video 3) and a small circumferential pericardial effusion with visible thrombus (Figure 4, Video 4). On further inquiry about the duration of symptoms, he admitted having chest pain for the last 6 days but had been avoiding seeking medical care due to the ongoing pandemic. Hemorrhagic pericarditis was diagnosed after treatment of the myocardial infarction (MI) with tenecteplase. His STEMI was managed with aspirin, 81 mg daily, and ticagrelor, 90 mg twice daily. Anticoagulation was avoided, and the intra-aortic balloon pump was removed the next day. He tested negative for coronavirus disease-2019 (COVID-19).
Online Video 3.
2D transthoracic echocardiogram. Apical four-chamber view showing a 1.9-cm circumferential pericardial effusion and left ventricular ejection fraction of 25%.
Figure 4.

2-Dimensional Transthoracic Echocardiogram
Subcostal view shows a small pericardial effusion with visible thrombus (arrow), suggestive of hemorrhagic pericarditis. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
Online Video 4.
2D Transthoracic echocardiogram. Subcostal view shows a 1.9-cm circumferential pericardial effusion with visible thrombus, suggestive of hemorrhagic pericarditis.
Discussion
The COVID-19 syndrome triggered a global pandemic with more than 3 million confirmed cases to date. Hospital systems worldwide have instituted emergency protocols to limit the spread of this pandemic, and some have transitioned to fibrinolytic agents for initial management of MI, including STEMI (1). Although STEMI presentations are on the decline because patients are afraid of hospital contact (2, 3, 4), delayed revascularization poses a challenge due to re-emergence of rare MI-related complications.
A study conducted in China reported that patients with STEMI delayed seeking help out of fear of COVID-19 infection (2). Italy also revealed a decrease in MI admissions for the last several months compared to the same time frame in the previous year (3). Moroni et al. (5) described complications of STEMI in 3 Italian patients who avoided the hospital because of COVID-19 infection. One U.S. study observed a 38% reduction of STEMI activations during the early phase of the pandemic (4). Multiple studies corroborate the fact that public fear of contracting severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is leading to a decline in timely presentation of MI patients (2, 3, 4). This phenomenon has been reported before in the SARS-CoV-1, and H1N1 pandemics of 2003 and 2009, respectively (6). The present patient reported avoiding early medical care due to a fear of acquiring COVID-19 infection in the hospital. One study suggested a significant increase in mortality during ongoing pandemic that is not fully explained by COVID-19 deaths alone (3). This raise the question of whether some patients died from undiagnosed MI.
The incidence of MI-related complications increases with delayed presentation and management (7). In addition to the common sequalae of MI (i.e., arrhythmia and heart failure), rare complications such as papillary muscle rupture, ventricular septal rupture, and pericarditis can develop from delayed management (8). Streptokinase trials from the early 1990s revealed that the incidence of early postinfarction pericarditis (first 2 to 5 days after the MI) was 20%, with a decline to 6% after thrombolytic therapy, and 4% after primary PCI. Generally, a pericardial effusion is not present in this group of patients (8). Late pericarditis (i.e., Dressler’s syndrome) develops 1 week after MI and has an incidence of 3% in the pre-revascularization era that has currently decreased to 0.1% (8). Diagnosis can be made from symptoms, diffuse ST elevations on electrocardiography, and a pericardial effusion on echocardiography (8). The risk of Dressler’s syndrome is increased after early post-MI pericarditis (8). Risk factors include late presentation (>6 h) and primary PCI failure (odds ratio: 2.8), both of which are expressions of reduced myocardial salvage or failed revascularization (8). This patient’s course was complicated by hemorrhagic pericardial effusion, likely from thrombolytic treatments and an underlying inflamed pericardium. Hemorrhagic pericarditis has been described in case reports in patients with large transmural MI receiving thrombolytic therapy as early as 2 h after the institution of therapy (9). This complication usually evolves into tamponade with hemodynamic instability requiring pericardiocentesis (9). Fortunately, this patient’s small pericardial effusion did not require invasive therapy and was managed with avoidance of anticoagulation during his stay in the coronary care unit.
Prompt coronary vascular reperfusion has decreased the incidence of rare MI complications and has reduced mortality (7). Before the COVID-19 pandemic, approximately 13% of STEMIs in the United States were managed with fibrinolytic-focused reperfusion strategies, mostly in geographically isolated areas (1). In the COVID-19 era, the American College of Cardiology still recommends PCI as the standard of care for STEMI (10). However, emergency departments are facing delays in patient triage, and similarly, cardiac catheterization laboratory activations are slowed due to extra steps required to ensure safety of the staff (2). In centers capable of performing PCI, immediate fibrinolytic administration in the emergency department may mitigate system-based delays. However, hemorrhagic pericarditis could represent a caveat to this eminent and necessary strategy, especially in cases of delayed MI.
Follow-up
Echocardiography after 6 days showed resolution of hemorrhagic pericardial effusion. The present patient successfully underwent a staged PCI of the left anterior descending artery lesion on the 10th day and was transferred to the cardiology ward before discharge on hospital day 19.
Conclusions
With the progression of this pandemic, management of MI with thrombolytics or delayed reperfusion strategies may show a re-emergence of rare complications such as post-MI pericarditis. This will be further exacerbated by patients who avoid medical care due to fear of contracting SARS-CoV-2 infection. Hemorrhagic pericarditis is a rare entity precipitated by thrombolytic drugs in late-presenting MI, which may lead to deleterious outcomes. Health care providers should continue educating patients to recognize life-threatening cardiovascular symptoms and seek timely care to avoid serious complications.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Daniels M.J., Cohen M.G., Bavry A.A., Kumbhani D.J. Reperfusion of STEMI in the COVID-19 Era---business as usual? Circulation. 2020;141:1948–1950. doi: 10.1161/CIRCULATIONAHA.120.047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam C.C.F., Cheung K.S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Filippo O., D’Ascenzo F., Angelini F. Reduced rate of hospital admissions for ACS during covid-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moroni F., Gramegna M., Ajello S., Baldetti L., Vilca L.M. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. J Am Coll Cardiol Case Rep. 2020;2:1620–1624. doi: 10.1016/j.jaccas.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung N.C.Y., Lau J.T.F., Choi K.C., Griffiths S. Population responses during the pandemic phase of the influenza A(H1N1)pdm09 epidemic, Hong Kong, China. Emerg Infect Dis. 2017;23:813–815. doi: 10.3201/eid2305.160768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel M.R., Calhoon J.H., Dehmer G.J. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 appropriate use criteria for coronary revascularization in patients with acute coronary syndromes: a report of the American College of Cardiology appropriate use criteria task force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:570–591. doi: 10.1016/j.jacc.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Imazio M., Negro A., Belli R. Frequency and prognostic significance of pericarditis following acute myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol. 2009;103:1525–1529. doi: 10.1016/j.amjcard.2009.01.366. [DOI] [PubMed] [Google Scholar]
- 9.Renkin J., De Bruyne B., Benit E., Joris J.M., Carlier M., Col J. Cardiac tamponade early after thrombolysis for acute myocardial infarction: a rare but not reported hemorrhagic complication. J Am Coll Cardiol. 1991;17:280–285. doi: 10.1016/0735-1097(91)90739-v. [DOI] [PubMed] [Google Scholar]
- 10.Mahmud E., Dauerman H.L., Welt F.G. Management of acute myocardial infarction during the COVID-19 pandemic. Catheter Cardiovasc Interv. 2020 Apr 20 doi: 10.1002/ccd.28946. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]