Highlights
-
•
We report the presence of SARS-CoV-2 in the nasopharyngeal swab of a laryngectomee.
-
•
Viral nasal priming might modulate the systemic inflammatory response.
-
•
Smoking exposure is a risk factor for SARS-CoV-2 infection and multiple malignancies.
Letter to the Editor:
The COrona VIrus Disease - 19 (COVID-19) is a novel airborne viral disease that appeared in Wuhan, China, in December 2019, and nearly 5 million cases and more than 350.000 deaths have been reported so far worldwide [1]. The immunopathogenesis has not been fully elucidated but, after the penetration into the upper airways, the coronavirus SARS-CoV-2 exploits ACE-2 and TMPRSS2 receptors for entry and subsequent systemic propagation while eliciting a T helper 1 (Th1) cell-polarized response [2], [3]. From preliminary evidence, cancer patients seem to be more susceptible because of an immunosuppressive state induced by the tumor itself and by the treatments (surgery or chemotherapy) administered; in addition, a possible higher risk for severe events was shown in the cancer population [4]. However, high-quality evidence regarding predictive/prognostic factors in the COVID-19 cancer cohort is still scant and many issues need to be solved. For instance, the interplays between tobacco smoking exposure, a well-known carcinogen and inducer of ACE-2 in the airways, and the cytokine storm found in a minority of COVID-19 patients are still being investigated [5].
We would like to describe the clinical course of a COVID-19 laryngectomized patient who gives us the chance to discuss several insights on COVID-19 pathophysiology and the still poorly understood relationships between immunity and infection in head and cancer patients.
Case description
On April 5, 2020 a 77-year-old male former smoker (50 pack-years), with arterial hypertension and chronic renal failure, presented to the Emergency Department (ED) of our hospital because of shortness of breath and fever (38.1°). His medical history was remarkable for multiple epithelial malignancies that were all notably associated with tobacco exposure. In 1998, he received partial laryngectomy for a supraglottic pT2N0 squamous cell carcinoma; in 2008, he underwent total laryngectomy for a second primary pT4N1M0 laryngeal cancer. Furthermore, he underwent right nephroureterectomy in 2017 and enlarged cystoprostatectomy and chemotherapy in 2018 for metachronous urothelial carcinoma. A week before the admission, he underwent an ultrasound-guided biopsy of a large retroperitoneal mass suspicious for tumor recurrence.
On admission, PaO2 was 69 mmHg and laboratory tests revealed an increase in lymphocyte count, C-reactive protein, and D-dimer; chest tomography showed bilateral patchy ground-glass opacities (Fig. 1 ). Despite permanent tracheostomy, naso- and oropharyngeal swabs were (improperly?) collected at the ED and they resulted positive for SARS-CoV-2. A combination of lopinavir/ritonavir, hydroxychloroquine, enoxaparin, and linezolid was started but, because of rapidly worsening respiratory failure, and the finding of severe and multifactorial acute kidney injury with severe metabolic acidosis, he was admitted to the intensive care unit on day 1. After ventilatory support for 5 days, he finally could be transferred to the infectious disease ward and on day 7, an endotracheal sample confirmed the persistence of SARS-CoV-2. On day 18, there was still a low positivity limited to the nucleocapsid protein in the endotracheal sample. On day 22, thanks to a substantial improvement in its conditions, he could be finally transferred to a long-term care facility where he is still recovering.
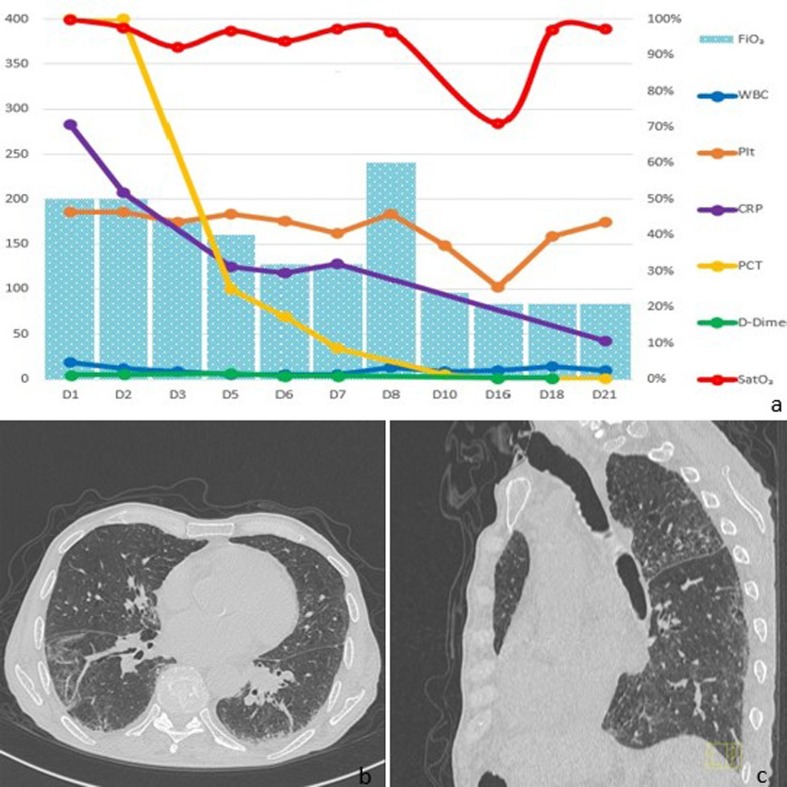
Fig. 1.
In (a) the temporal trends of main inflammatory parameters are shown, as well as the Peripheral Blood Oxygen Saturation (SpO2, red line) and the Fraction of Inspired Oxygen (FiO2, blue columns). In (b) and (c), axial and sagittal computed tomography scan views show bilateral ground-glass opacities and the permanent separation of the upper and lower airways. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussion and conclusion
We believe that three major lessons can be learned from the present case. First, in a typical example of what Slaughter and colleagues called “field cancerization” in 1953, the history of multiple smoke-related malignancies of the airways and the urothelial tract supports a general failure of the patient’s immune surveillance system which might have, in addition, favored the infection and its clinical course [6]. However, the evidence supporting such a relationship is still uncertain while it must be underscored that the delay or the interruption of oncological care may have more deleterious effects than the pandemic itself [7]. Secondly, smoke-induced pulmonary inflammation and ACE-2 overexpression, old age, and the direct penetration into the lower airways because of permanent tracheostomy may have all justified an increased susceptibility to the infection and the rapid and severe clinical deterioration [3], [5]. Finally, despite the upper airways are functionally excluded from the lungs after total laryngectomy, the finding of SARS-CoV-2 in nasopharyngeal swabs can suggest a possible hematogenous spread from a primary pulmonary infection. The two receptors required for SARS-CoV-2 infection were shown to be co-expressed in type II pneumocytes, ileal enterocytes, and nasal epithelial cells [8]. The apparently innocent nasal localization in this patient must not go unnoticed: in a bidirectional unified airway model, the type of local immune response in the nasal mucosa has the chance to drive the systemic cytokine response and the priming of the lung immunity [9], [10]. In addition, there is experimental evidence that nasal exposure to non-SARS-CoV-2 coronavirus is capable to prepare the lower airways for a faster and enhanced innate and adaptive response to the pathogen, with a final overall reduction in terms of both morbidity and mortality in infected mice [10]. A better understanding of the interplays between this novel coronavirus and the immune system, along with the tumor- and treatment-associated immunomodulatory effects on the course of the infection are urgently needed. Only in this way, we will able to derive evidence-based and practical indications for the optimal management of cancer patients during this pandemic.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard, Available at covid19.who.int [data accessed on 2020/5/18].
- 2.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason R.J. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respiratory J. 2020;55:2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slaughter D.P., Southwick H.W., Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Hartemink K.J., van Akkooi A.C., Houwink A.P., Voest E.E. Patients with cancer in the era of 2019 novel coronavirus disease. Eur J Cancer. 2020;132:125–126. doi: 10.1016/j.ejca.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler C., Allon S.J., Nyquist S.K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is enriched in specific subsets across tissues. Cell. 2020 doi: 10.2139/ssrn.3555145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111(6):1171–1183. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- 10.Hua X., Vijay R., Channappanavar R., Athmer J., Meyerholz D.K., Pagedar N. Nasal priming by a murine coronavirus provides protective immunity against lethal heterologous virus pneumonia. JCI Insight. 2018;3(11) doi: 10.1172/jci.insight.99025. [DOI] [PMC free article] [PubMed] [Google Scholar]