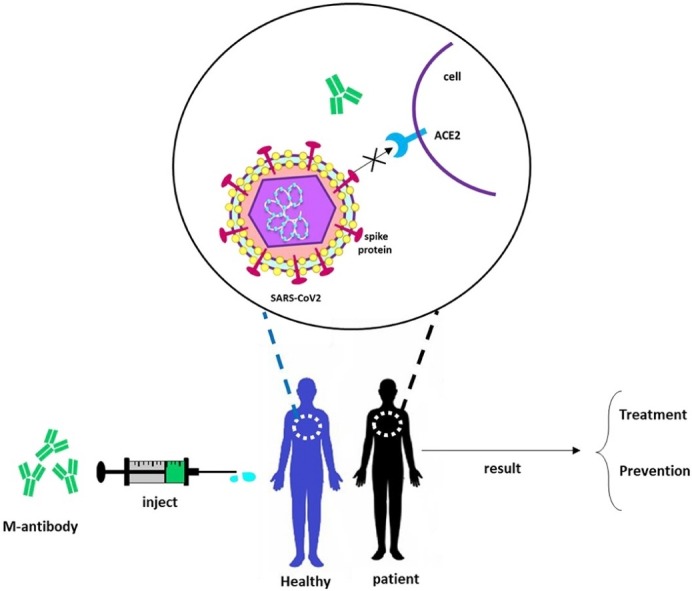
Graphical abstract
Keywords: COVID-19, SARS-CoV, Treatment, Monoclonal antibodies
Highlights
-
•
Human neutralizing antibodies can block COVID-19 infection.
-
•
Most monoclonal antibodies inhibit binding S protein to ACE2 receptor.
-
•
Researchers are trying to develop treatments based on antibodies to block and/or neutralize SARS-CoV-2.
-
•
The genetic and structural similarity of the virus to SARS-CoV helps for designing new methods for treatment of COVID-19.
Abstract
Coronavirus disease 2019 (COVID-19) is expanding rapidly, which made it as one of top priorities for scientists to develop novel treatment strategies. Researchers are racing to develop treatments based on antibodies to block and/or neutralize the coronavirus in affected patients. Initially, the genetic and structural similarity of the virus to severe acute respiratory syndrome coronavirus (SARS-CoV) created the potential for understanding disease pathogenesis. Researchers have published reports of specific monoclonal antibodies against to COVID-19 (B38, H4, 47D11) and hope that this method is effective. As well as studies on patients who are plasma therapy, the patient's condition shows improvement. The evidence for these studies is very promising and demonstrates the potential of monoclonal antibody therapy as a therapeutic approach and prevention of covid-19 infection.
1. Introduction
At the end of 2019, a novel coronavirus disease (COVID-19), also called as Severe Acute Respiratory Syndrome CoronaVirus-2 (SARS-CoV-2), appeared in Wuhan, China [1]. It has spread to other countries very soon, rapidly increased on a pandemic scale [[2], [3], [4]]. Coronaviruses (CoVs) are found in avian and mammalian species [5], which have a wide range from a common cold virus to more severe diseases such as SARS-CoV and Middle East respiratory syndrome (MERS)-CoV [6]. It is believed that they are originated from bats, but the exact source of SARS-CoV-2, animal reservoirs, and enzymatic transmission patterns have not fully understood yet [7]. Coronaviruses, resemble each other in morphology and chemical structure, are enveloped viruses, spherical or pleomorphic enveloped particles containing single-stranded (positive-sense) RNA associated with a nucleoprotein within a capsid and comprised of spike (s) protein [6].
There are several efforts to design effecting drugs to treat COVID-19 infection, but no vaccine or curative drug has been found to treat the disease so far [8]. Major researches have focused on identifying anti-viral molecules that target S proteins, which play an important function in virus entry and viral replication cycle in the host cell [6]. The genetic and structural similarities of the virus with SARS-CoV created a potential for understanding the pathogenesis of the this infection [9]. As monoclonal antibodies could neutralize other coronaviruses biotherapy of COVID-19 could also be as of interest [10,11]. Effective treatment options for SARS-CoV-2 could be based on the use of broad-spectrum antiviral drugs (BSA), or by using specific therapeutic molecules that can directly disrupt each stage of the viral life cycle, or receptor proteins located at the host cellular deactivated [9],(24), [25]. They include fusion inhibitor peptide, neutralizing antibodies against SARS-CoV-2, anti-ACE2 (Angiotensin-converting enzyme 2) monoclonal antibodies, and protease inhibitors [12],(13), [14].
2. Characteristics of SARS-CoV-2
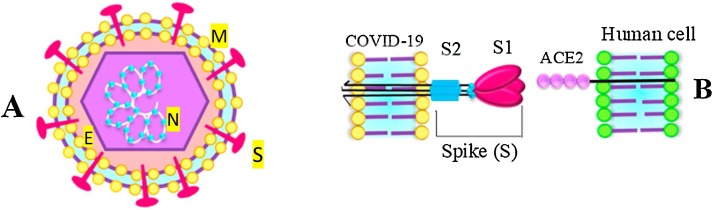
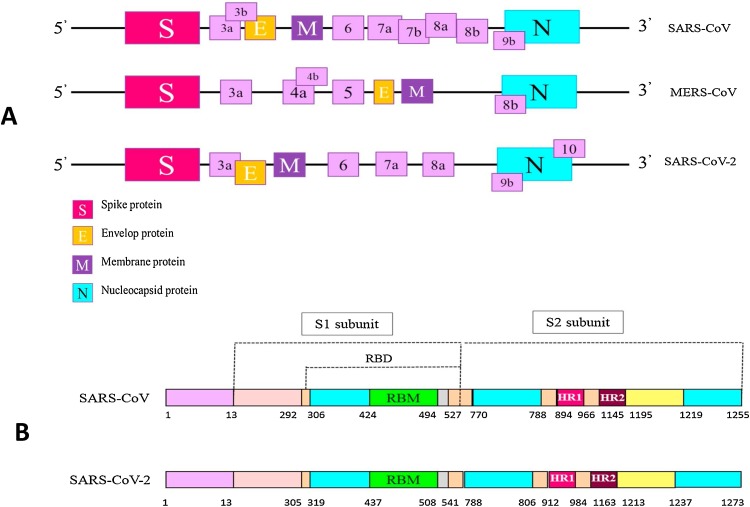
The virus genome encodes structural and non-structural proteins. The most important structural proteins of the virus include the spike (S), membrane (M) and envelop (E) and nucleic capsid (N) proteins (Fig. 1 ) [9]. CoVs infection begins through the interaction of spike protein and receptor recognition by host cells [6,9]. The S protein has two functional subunits that mediate cell attachment (the S1 subunit, existing of four core domains S1A through S1D) and fusion of the viral and cellular membrane (the S2 subunit). As shown in Fig. 2 , SARS-CoV-2 and SARS have a high genetic similarity to each other [15,16]. The spike proteins of SARS-CoV-2 and SARS-CoV are 77.5 % identical by primary amino acid sequence. Considering the importance of spike protein in viral fusion and antigen receptor similarity between SARS-CoV and SARS-CoV-2 (including ACE2 for SARS and SARS-CoV-2, DPP4 for MERS) [6,9,17], pathogenesis of COVID-19 could be better understood, which could be helpful in designing therapeutic strategy.
Fig. 1.
(A) The novel coronavirus structure. (B) Spike protein consists of two subunits S1 and S2. Studies have shown that COVID-19 through ACE2 receptor binds to human epithelial cells. ACE2 is used as its receiver to the host. The connection between the amplitude of the receptor binding in the spike protein and the cell receptor mediated by membrane fusion and the onset of the virus life cycle.
Fig. 2.
(A) Genome sequencing and codon positions of structural proteins in the novel Coronavirus, SARS-CoV and MERS-CoV. (B) Compare and evaluate the structure of Spike protein and position RBD, receptor-binding domain; RBM, receptor-binding motif; HR1 / 2, heptad repeat 1/2 in SARS-CoV and COVID-19.
3. Monoclonal antibody therapy
Immunotherapy as an effective method for clinical treatment of infectious diseases is also proposed [18]. Based on the available evidence and previous experience in the treatment of other viral infections such as influenza, SARS, MERS, and Ebola, early prescribing of stimulant plasma or immunoglobulin overuse of patients with significant antibody titers can lead to reduce the mortality rate [17],(18, 21), [22]. The use of monoclonal antibodies is a new outlook in the prevention of infectious diseases. Monoclonal antibodies are used to bind to one specific substance in the body. This binding is very versatile and can mimic, block, or cause changes to enact precise mechanisms, and provide an effective therapeutic intervention with a very specific treatment for diseases [19]. Passive immunization antibodies that can detect epitope region from foreign particles of the virus can reduce virus proliferation and disease severity [20]. As of the similarities between the SARS-CoV-2 and SARS-CoV, several studies have suggested the use of SARS antiviral monoclonal antibodies in patients with the SARS-CoV-2 (Table 1, Table 2 ; anti-SARS antibodies. Most monoclonal antibodies have been identified to identify the S1 fragment of SARS-CoV and receptor Binding Domain (RBD) in subunit S1 is the most important goal for SARS-CoV-2 [21], as monoclonal antibodies can block the interaction of RBD and its ACE2 receptor [22]. Some monoclonal antibodies recognize the epitopes in unit S2 of SARS-CoV and suggest that other mechanisms may play a role in neutralization [23]. The combination of monoclonal antibodies targeting S-proteins in SARS-CoV detects different epitopes in laboratory and in vivo cells that can be potentially effective at the viral level; for example, CR3022 alone did not show neutralization, but a mixture of CR3022 and CR3014 showed neutralization [24].
Table 1.
Neutralizing monoclonal antibodies targeting S1 fragment of SARS-CoV.
| M-antibody | Target Region | Virus binding and virus blocking | Identification Method |
Reference |
|---|---|---|---|---|
| 80R | S1 domain 426−492 | Antibody is bound to amino acid residues 426−492 on S1 segment of SARS-CoV. The interaction of S1 subunit protein with the ACE2 receptor is blocked. | Phage display | [13,34] |
| CR3014 | S1 domain 318−510 | Antibody is bound to amino acid residues 318−510 on S1 segment of SARS-CoV. The interaction of S1 subunit protein with the ACE2 receptor is blocked. | Phage display | [22,35] |
| CR3022 | S1 domain 318−510 | Antibody is bound to amino acid residues 318−510 on S1 segment of SARS-CoV. The interaction of S1 subunit protein with the ACE2 receptor is blocked. | Phage display | [24] |
| 68 | S1 domain 130−150 | Antibody is bound to amino acid residues 130−150 of SARS- CoV. | HuMAb-Mouse | [36,37] |
| 201 | S1 domain 490−510 | Antibody is bound to amino acid residues 490−510 on S1 segment of SARS-CoV. The interaction of S1 subunit protein with the ACE2 receptor is blocked. | HuMAb-Mouse | [[36], [37], [38]] |
| 4D4 | S1 domain 12−261 N-terminal of RBD |
Antibody is bound to amino acid residues 12−261 of SARS-CoV & N-terminal of RBD. Inhibiting the post-interaction in viral influence in vitro. | – | [39] |
| M396 | S protein | Antibody is bound to amino acid residues 482−491 on S1 segment of SARS-CoV. The interaction of S subunit protein using CDR (complementary determining region) loops H1, H2, H3, and L3 with the ACE2 receptor is blocked. | Phage display | [37] |
| S230 | S protein | Antibody is bound to SARS-CoV. The interaction of S1 subunit protein with the ACE2 receptor is blocked. | EBV Transformed Human B cell |
[40,41] |
| F26G18 | S1 domain 460−476 | Antibody is bound to amino acid residues 460−476 on S1 segment of SARS-CoV. The interaction of S1 subunit (RBD) protein with the ACE2 receptor is blocked. | – | [40] |
| F26G19 | S1 domain 359−362, 391−392, 424−427, 486−492 | Antibody is bound to S1 domain 359−362, 391−392, 424−427,486−492 of SARSCoV. The interaction of S1 subunit (RBD) protein with the ACE2 receptor is blocked. | – | [40] |
Table 2.
Neutralizing monoclonal antibodies targeting S2 fragment of SARS-CoV.
| M-antibody | Target Region | Virus binding and virus blocking | Identification Method |
Reference |
|---|---|---|---|---|
| 1A9 | S2 domain HR1, HR2 | Antibody is bound to the Heptad repeat (HR) loops including heptad repeat (HR1, HR2) domain on S2 segment of SARS-CoV. The interaction of S2 subunit protein (amino acid residues 1111−1130) with host cell receptor is blocked. | – | [42,43] |
| B1 | S2 domain 1023−1189 | Antibody is bound to amino acid residues 1023−1189 on S2 segment of SARS-CoV. The interaction of S2 subunit protein with the ACE2 receptor is blocked. | Phage display | [23] |
| 1F8 | S2 domain HR1 | Antibody is bound to the HR1 domain on S2 segment of SARS-CoV. The interaction of S2 subunit protein with the ACE2 receptor is blocked. | Xeno-Mouse | [39] |
| 5E9 | S2 domain HR2 | Antibody is bound to the HR2 domain on S2 segment of SARS-CoV. The interaction of S2 subunit protein with the ACE2 receptor is blocked. | Xeno-Mouse | [39] |
4. Human neutralizing antibodies block SARS-CoV-2
The results of the new studies are very promising; the researchers proposed neutralizing antibodies that block COVID-19. B38, H4, 47D11 are new antibodies that have shown excellent results in neutralizing the novel coronavirus infection.
4.1. 47D11
This antibody was discovered by Wang et al., using an ELISA-(cross) reactivity approach, assessing antibody-containing supernatant derived from immunized transgenic H2L2 mice. 47D11 was found to bind to SARS-CoV-2 and SARS-CoV, and to potently inhibit the virus' infection of Vero cells, a type of cell line. The chimeric 47D11 H2L2 antibody was reformatted and expressed as a fully human IgG1 isotope antibody for further study. Using ELISA 47D11 was shown to target the S1B receptor-binding domain (RBD) of SARS-S and SARS2-S and inhibits the binding of S protein to the human-ACE2 receptor. This study showed that 47D11 neutralizes SARS-CoV and SARS-CoV-2 through a yet unknown mechanism that is different from 86 receptor binding interference [25].
4.2. B38, H4
The report on four human-origin monoclonal antibodies (B5, B38, H2, and H4) from a convalescent patient showed that all four antibodies bound to SARS-CoV-2 receptor-binding domain (RBD), but not to SARS-CoV RBD. Evaluation of the ability of each antibody to inhibit binding between RBD and ACE showed that B38 and H4 have complete com-petition with ACE2 for binding to RBD. In contrast, B5 dis-played partial competition, while H2 did not compete with ACE2 for RBD binding [26].
5. Challenges in monoclonal antibody therapy
Although this method has promising results in neutralizing infection, large-scale production of monoclonal antibodies is intensive, expensive, and time-consuming, especially against emerging pathogens [27]. Monoclonal antibody sequences that are effective against SARS-CoV can be cloned and expressed in appropriate expression systems such as mammals, yeasts or plants [28]. Expression systems in plants can be used for the rapid production of monoclonal antibodies in a short time and at reasonable cost [29], which could be one of the most important benefits of epidemic conditions.
6. Neutralizing antibodies (NAbs) responses to SARS-CoV-2
In a study of 175 COVID-19 recovered patients with mild symptoms, SARS-CoV-2-specific NAbs were detected at the convalescent phase of infection from day 10–15 after the onset of the disease and remained thereafter. The titers of NAbs were variable in different patients. Plasma NAbs titers in elderly and middle-age patients had significantly higher. Plasma C-reactive protein (CRP) levels were positive correlated with NAbs titer. The NAbs titer negative correlated with the lymphocyte counts of patients at the time of admission; it could suggest that other immune responses, including T cells or cytokines, may contribute to the recovery of these patients. One of the important practical results of this study was the highly variable levels of NAbs in the patients of COVID-19. It could indicate that convalescent plasma and serum from recovered donors should be titrated before use in passive antibody therapy; an easy task that can be performed using the PsV neutralization assay [30].
7. Clinical statues patients’ treatment with COVID-19 with convalescent plasma
Plasma therapy, including neutralizing monoclonal antibodies, is one of the treatment strategies which have been investigated in several studies with promising results. Evaluation of computed tomography (CT) scan of patients with an acute conditions has shown that the viral load has decreased within a few days of treatment with plasma congestion, while the clinical conditions of the patients were also improved [31].
8. Future prospective
Since no effective vaccine or drug has been developed to treat and combat the COVID-19 yet, the current approach for management focuses on supportive care. Passive antibody therapy could be a way to limit the progress of COVID-19 pandemic [32]. The current knowledge about neutralizing antibodies provides useful information for passive antibody therapy and vaccine development against SARS-CoV-2. However, the effect of antibodies in protection against pulmonary SARS-CoV should be considered with precautions, while some patients with SARS died, showed strong responses of neutralizing antibody and accumulation in lung inflammation, which can be due to acute injury fatal lung [33]. It is to be hoped understanding the mechanisms of neutralizing monoclonal antibodies performance will provide valuable implications for antibodies in treatment of SARS-CoV-2 in the near future.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momtazmanesh S., Ochs H.D., Uddin L.Q., Perc M., Routes J.M., Nuno Vieira D. All together to Fight Novel Coronavirus Disease (COVID-19) Am. J. Trop. Med. Hygiene. 2020;102(6):1181–1183. doi: 10.4269/ajtmh.20-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanaei S., Rezaei N. COVID-19: Developing from an outbreak to a pandemic. Arch. Med. Res. 2020 doi: 10.1101/2020.03.14.988345. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miłek J., Blicharz-Domańska K. Coronaviruses in avian species–review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018;62(3):249–255. doi: 10.2478/jvetres-2018-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus – a perspective. Expert Rev. Clin. Immunol. 2020;16(5):465–470. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceccarelli M., Berretta M., Rullo E.V., Nunnari G., Cacopardo B. Editorial–Differences and similarities between Severe Acute Respiratory Syndrome (SARS)-CoronaVirus (CoV) and SARS-CoV-2. Would a rose by another name smell as sweet? Eur. Rev. Med. Pharmacol. Sci. 2020;24:2781–2783. doi: 10.26355/eurrev_202003_20551. [DOI] [PubMed] [Google Scholar]
- 10.Ju B., Zhang Q., Ge X., Wang R., Yu J., Shan S. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 doi: 10.1038/s41586-020-2380-z. May 26. [DOI] [PubMed] [Google Scholar]
- 11.Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84(106560):1–6. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badani H., Garry R.F., Wimley W.C. Peptide entry inhibitors of enveloped viruses: the importance of interfacial hydrophobicity. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2014;1838(9):2180–2197. doi: 10.1016/j.bbamem.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson J., Schiffer C., Lee S.-K., Swanstrom R. Springer; 2009. Viral Protease Inhibitors. Antiviral Strategies; pp. 85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. S1198-743X(20)30171-30173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutino‐Moguel M.T., Eades C., Rezvani K., Armstrong‐James D. Immunotherapy for infectious diseases in haematological immunocompromise. Br. J. Haematol. 2017;177(3):348–356. doi: 10.1111/bjh.14595. [DOI] [PubMed] [Google Scholar]
- 19.Lu R.-M., Hwang Y.-C., Liu I.-J., Lee C.-C., Tsai H.-Z., Li H.-J. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020;27(1):1–30. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker L.M., Burton D.R. Passive immunotherapy of viral infections:’super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018;18(5):297. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279(5):3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Brink E.N., ter Meulen J., Cox F., Jongeneelen M.A., Thijsse A., Throsby M. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79(3):1635–1644. doi: 10.1128/JVI.79.3.1635-1644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan J., Yan X., Guo X., Cao W., Han W., Qi C. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005;333(1):186–193. doi: 10.1016/j.bbrc.2005.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ter Meulen J., Van Den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3(7) doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C., Li W., Drabek D., Okba N.M., van Haperen R., Osterhaus A.D. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11(1):1–6. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020 doi: 10.1126/science.abc2241. May 13. pii: eabc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparrow E., Friede M., Sheikh M., Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017;95(3):235. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheikhshahrokh A., Ranjbar R., Saeidi E., Dehkordi F.S., Heiat M., Ghasemi-Dehkordi P. Frontier therapeutics and vaccine strategies for SARS-CoV-2 (COVID-19): a review. Iran. J. Public Health. 2020;49:18–29. doi: 10.18502/ijph.v49iS1.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg Y., Sack M., Montefiori D., Forthal D., Mao L., Hernandez-Abanto S. Rapid high-level production of functional HIV broadly neutralizing monoclonal antibodies in transient plant expression systems. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S. 2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. [Google Scholar]
- 31.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casadevall A., Pirofski L.-a. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 2005;79(10):5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ter Meulen J., Bakker A.B., van den Brink E.N., Weverling G.J., Martina B.E., Haagmans B.L. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenough T.C., Babcock G.J., Roberts A., Hernandez H.J., Thomas W.D., Jr, Coccia J.A. Development and characterization of a severe acute respiratory syndrome—associated coronavirus—neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J. Infect. Dis. 2005;191(4):507–514. doi: 10.1086/427242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coughlin M., Lou G., Martinez O., Masterman S.K., Olsen O.A., Moksa A.A. Generation and characterization of human monoclonal neutralizing antibodies with distinct binding and sequence features against SARS coronavirus using XenoMouse®. Virology. 2007;361(1):93–102. doi: 10.1016/j.virol.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elshabrawy H.A., Coughlin M.M., Baker S.C., Prabhakar B.S. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry J.D., Hay K., Rini J.M., Yu M., Wang L., Plummer F.A. Taylor & Francis; 2010. Neutralizing Epitopes of the SARS-CoV S-Protein Cluster Independent of Repertoire, Antigen Structure or mAb Technology. MAbs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. 2007;104(29):12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lip K.-M., Shen S., Yang X., Keng C.-T., Zhang A., Oh H.-L.J. Monoclonal antibodies targeting the HR2 domain and the region immediately upstream of the HR2 of the S protein neutralize in vitro infection of severe acute respiratory syndrome coronavirus. J. Virol. 2006;80(2):941–950. doi: 10.1128/JVI.80.2.941-950.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng O.-W., Keng C.-T., Leung C.S.-W., Peiris J.M., Poon L.L.M. Substitution at aspartic acid 1128 in the SARS coronavirus spike glycoprotein mediates escape from a S2 domain-targeting neutralizing monoclonal antibody. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102415. [DOI] [PMC free article] [PubMed] [Google Scholar]