Abstract
Introduction
To explore the prevalence of dysphonia in European patients with mild-to-moderate COVID-19 and the clinical features of dysphonic patients.
Methods
The clinical and epidemiological data of 702 patients with mild-to-moderate COVID-19 were collected from 19 European Hospitals. The following data were extracted: age, sex, ethnicity, tobacco consumption, comorbidities, general, and otolaryngological symptoms. Dysphonia and otolaryngological symptoms were self-assessed through a 4-point scale. The prevalence of dysphonia, as part of the COVID-19 symptoms, was assessed. The outcomes were compared between dysphonic and nondysphonic patients. The association between dysphonia severity and outcomes was studied through Bayesian analysis.
Results
A total of 188 patients were dysphonic, accounting for 26.8% of cases. Females developed more frequently dysphonia than males (P = 0.022). The proportion of smokers was significantly higher in the dysphonic group (P = 0.042). The prevalence of the following symptoms was higher in dysphonic patients compared with nondysphonic patients: cough, chest pain, sticky sputum, arthralgia, diarrhea, headache, fatigue, nausea, and vomiting. The severity of dyspnea, dysphagia, ear pain, face pain, throat pain, and nasal obstruction was higher in dysphonic group compared with nondysphonic group. There were significant associations between the severity of dysphonia, dysphagia, and cough.
Conclusion
Dysphonia may be encountered in a quarter of patients with mild-to-moderate COVID-19 and should be considered as a symptom list of the infection. Dysphonic COVID-19 patients are more symptomatic than nondysphonic individuals. Future studies are needed to investigate the relevance of dysphonia in the COVID-19 clinical presentation.
Key Words: Dysphonia, Covid-19, Coronavirus, Voice, Symptoms, Clinical, Findings, ENT
INTRODUCTION
Since the first reported cases from Wuhan, the coronavirus disease 2019 (COVID-19) has spread rapidly worldwide.1 As of April 25, a total of 2,719,897 individuals have been diagnosed through laboratory testing, with 187,705 corresponding deaths.2 According to Asian studies, the clinical presentation of COVID-19 mainly includes fever, fatigue, cough, and anorexia.3 , 4 However, recent European epidemiological studies suggested that European mild-to-moderate COVID-19 patients could present a different clinical picture than Asian.5 In Europe, the most prevalent symptoms are headache, total loss of smell, nasal obstruction and cough.5 , 6 Thus, the otolaryngological symptoms are more prevalent than previously presumed in Asia. Throughout a recent epidemiological study,7 we observed the occurrence of dysphonia in some COVID-19 patients, with a minority reporting aphonia over the clinical course of the disease.
The aim of this study was to investigate the occurrence of dysphonia in mild-to-moderate COVID-19 patients.
METHODS
Five European Ethics Committees approved the study protocol (HAP2020-011; CHUSP20032020; EpiCURA-2020-2303, CHUC,P20/30-24/03-B325-2020; J.Bordet Institute: CE3137). The patient informed consent was obtained electronically.
Setting & patients
From March 22 to April 24, 2020, the data of hospitalized or home-managed COVID-19 patients were collected by otolaryngologists and nonotolaryngologist physicians from 19 European Hospitals (Paris, Marseille, Milan, Verona, Naples, Genova, Florence, Foggia, Forli, Sevilla, Santiago de Compostela, San Sebastian, Mons, Brussels, Charleroi, and Saint-Ghislain).
The diagnosis of Covid-19 infection was based on the WHO interim guidance.8 Precisely, nasal or throat swabs were collected in suspected patients and the virus was identified through reverse transcription polymerase chain reaction (RT-PCR) analysis. The details of the viral RNA extraction and analyses are available in a previous paper.7
The mild-to-moderate COVID-19 patients were identified through different ways. First, the database of the laboratories of the hospitals provided a list of hospitalized or home-managed patients. Second, many patients were referred by the family physician or specialists from hospital departments (eg, Emergency, Internal Medicine, or Otolaryngology Departments). A public call of University of Mons allowed an increase in the recruitment of patients. Third, many infected health care workers were included.
Patients who were hospitalized in intensive care units were not included due to their health status and the inability to answer to the questions. Patients who reported dysphonia prior to the infection were not included in the present analysis. Patients with a history of head & neck trauma, (chemo) radiotherapy, benign or malignant laryngeal lesions, or head and neck cancer surgery were identified through the epidemiological questionnaire and carefully excluded.
Regarding the inclusion and exclusion criteria, the patients included in the present study were defined as mild-to-moderate COVID-19 patients.
Epidemiological & clinical outcomes
Due to the sanitary situation, the data were collected through an electronic patient-reported outcome questionnaire. Home-managed patients were contacted over the phone. Physicians completed the questionnaire in the patient's room for hospitalized patients through an electronic device. For these patients, physicians had access to the patient medical records to complete some data. The professional Survey Monkey (San Mateo, California, USA) was used for the patient reported outcome questionnaire.
The following epidemiological and clinical data were considered: age, sex, ethnicity, tobacco consumption, comorbidities, general, and otolaryngological symptoms. The general symptoms included headache, asthenia, myalgia, arthralgia, cough, loss of appetite, dyspnea, fever, diarrhea, nausea/vomiting, abdominal pain, and chest pain. The otolaryngological symptoms included nasal obstruction, rhinorrhea, postnasal drip, sore throat, face pain, dysphonia, ear pain, dysphagia, and sticky throat mucus. Otolaryngological symptoms were assessed through a 4-point scale, ranging from 0 (=no symptom) to 4 (=very severe symptom). The prevalence rates of total or partial loss of smell and dysgeusia were evaluated through questions from the smell and taste component of the National Health and Nutrition Examination Survey (NHNES).8 NHNES is a population survey that monitors the health of U.S. citizens through a nationally representative sample of 5,000 persons on a yearly basis.8
Statistical methods
The statistical analysis was realized through Bayesian networks. The details about the qualitative and quantitative models of the network analysis were reported in a previous study.7 The outcomes were compared between dysphonic and nondysphonic patients. The relationships between dysphonia, epidemiological data and general and otolaryngological symptoms were studied. A P - value <0.05 was considered as significant.
RESULTS
The data of 702 patients were collected. Among them, 188 reported dysphonia (26.8%) as a COVID-19 symptom while 514 patients did not report dysphonia during the clinical course of the disease. Seven dysphonic patients reported aphonia throughout the clinical course of the disease (3.7%). The characteristics of patient groups are described in Table 1 . There were 70.8% of females, the proportion of females reaching 76.6% in the dysphonic group. The female proportion was significantly higher in the dysphonic group compared to the nondysphonic group (P = 0.022). The majority of patients were Caucasian. The percentage of smokers was significantly higher in dysphonic group compared with nondysphonic group (P = 0.042). The proportion of allergic patients was similar in both groups. The most prevalent comorbidities were allergic rhinitis, hypertension, and gastroesophageal reflux disease.
TABLE 1.
Epidemiological Characteristics of Dysphonic and Nondysphonic Patients
| Characteristic | Patients | Dysphonic | Nondysphonic |
|---|---|---|---|
| (n = 702) | (n = 188) | (n = 514) | |
| Age | |||
| Median (SD) - yo | 40.3 ± 11.8 | 42.0 ± 11.9 | 39.6 ± 11.7 |
| Gender (n - %) | |||
| Male | 206 (29.2) | 44 (23.4) | 162 (31.5) |
| Female | 496 (70.8) | 144 (76.6) | 352 (68.5) |
| Ethnicity (n - %) | |||
| European | 640 (91.2) | 172 (91.5) | 469 (91.2) |
| Asian | 11 (1.6) | 2 (1.1) | 9 (1.8) |
| Black African | 14 (2.0) | 1 (0.5) | 13 (2.5) |
| North African | 26 (3.7) | 10 (5.3) | 16 (3.1) |
| North American | 1 (0.1) | 0 (0) | 1 (0.2) |
| South American | 5 (0.1) | 2 (1.1) | 3 (0.6) |
| Oceanian | 0 (0) | 0 (0) | 0 (0) |
| Mixing | 4 (0.6) | 1 (0.5) | 3 (0.6) |
| Addictions (n - %) | |||
| Nonsmoker | 597 (85.0) | 148 (78.2) | 450 (87.5) |
| Mild smoker (1–10 cigarettes daily) | 83 (11.8) | 19 (10.1) | 64 (12.5) |
| Moderate smoker (11–20 cigarettes daily) | 18 (2.6) | 9 (4.8) | 9 (1.8) |
| Heavy smoker (>20 cigarettes daily) | 4 (0.6) | 2 (1.1) | 2 (0.4) |
| Allergic patients | 126 (17.9) | 37 (19.7) | 89 (17.3) |
| Comorbidities | |||
| Allergic rhinitis | 75 (10.7) | 23 (12.2) | 52 (10.1) |
| High blood pressure | 60 (8.5) | 15 (8.0) | 45 (8.8) |
| GERD | 53 (7.5) | 15 (8.0) | 38 (7.4) |
| Asthma | 42 (6.0) | 13 (6.9) | 29 (5.6) |
| Hypothyroidism (treated) | 42 (6.0) | 12 (6.4) | 30 (5.8) |
| Depression | 23 (3.3) | 9 (4.8) | 14 (2.7) |
| CRS with or without polyps | 11 (1.6) | 5 (2.7) | 6 (1.2) |
| Heart problems | 8 (1.1) | 2 (1.1) | 6 (1.2) |
| Diabetes (insulin-dependent) | 8 (1.1) | 4 (2.1) | 4 (0.8) |
| Autoimmune diseases | 3 (0.4) | 1 (0.5) | 2 (0.4) |
| Respiratory insufficiency | 3 (0.4) | 2 (1.1) | 1 (0.2) |
| Hypothyroidism (untreated) | 2 (0.3) | 0 (0) | 2 (0.4) |
| Renal failure | 0 (0) | 0 (0) | 0 (0) |
| Hepatic insufficiency | 0 (0) | 0 (0) | 0 (0) |
| Neurological diseases | 0 (0) | 0 (0) | 0 (0) |
The percentages are in brackets. Abbreviations: CRS, chronic rhinosinusitis; GERD, gastroesophageal reflux disease; SD, standard deviation.
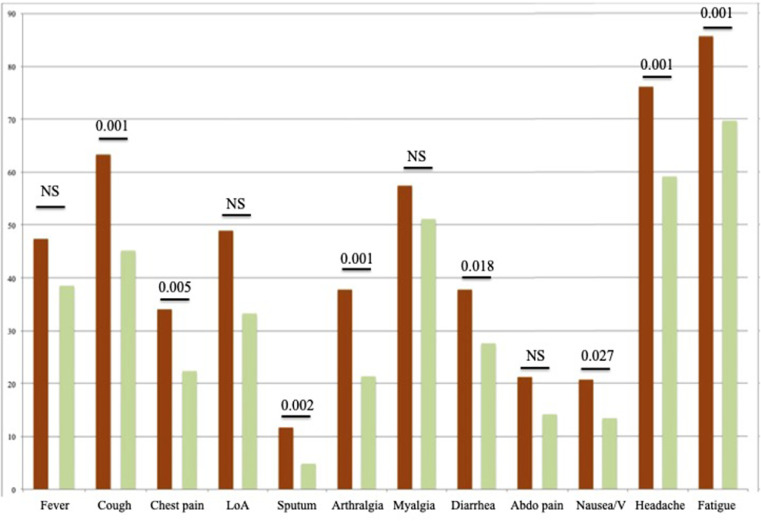
The prevalence of general symptoms in both groups is available in Table 2 . Patients with dysphonia had a higher proportion of systemic symptoms compared with nondysphonic patients, including cough, chest pain, sticky sputum, arthralgia, diarrhea, headache, fatigue, nausea, and vomiting (Figure 1 ).
TABLE 2.
Prevalence of General Symptoms in Dysphonic and Nondysphonic Patients
| Symptoms | Patients | Dysphonic | Nondysphonic |
|---|---|---|---|
| (n = 702) | (n = 188) | (n = 514) | |
| Fever | 287 (40.9) | 89 (47.3) | 198 (38.5) |
| Cough | 351 (50.0) | 119 (63.3) | 232 (45.1) |
| Chest pain | 179 (25.5) | 64 (34.0) | 115 (22.4) |
| Loss of appetite | 263 (37.5) | 92 (48.9) | 171 (333) |
| Sticky Sputum | 47 (6.7) | 22 (11.7) | 25 (4.9) |
| Arthralgia | 181 (25.8) | 71 (37.8) | 110 (21.4) |
| Myalgia | 371 (52.9) | 108 (57.5) | 263 (51.2) |
| Diarrhea | 213 (30.3) | 71 (37.8) | 142 (27.6) |
| Abdominal pain | 113 (16.1) | 40 (21.3) | 73 (14.2) |
| Nausea/vomiting | 108 (15.4) | 39 (20.7) | 69 (13.4) |
| Headache | 447 (63.7) | 143 (76.1) | 304 (59.1) |
| Asthenia | 519 (73.9) | 161 (85.6) | 358 (69.6) |
The percentages are in brackets.
FIGURE 1.
The Proportions of general symptoms in dysphonic and nondysphonic patients.
The ordinate axis consists of the prevalence of symptoms (percentages). Dysphonic Patients are represented in orange, nondysphonic are in green. Abbreviations: Abdo pain, Abdominal pain; LoA, loss of appetite; V, vomiting.
Symptoms are described in Table 3 . According to NHNES questions, the total and partial losses of smell concerned 128 (68.1%) and 20 (10.6%) dysphonic patients, respectively. In the group of nondysphonic patients, there were 369 (71.8%) and 66 (12.8%) individuals with total and partial losses of smell, respectively. As reported in Table 3, dysphonic patients had higher proportions of dyspnea, dysphagia, ear pain, face pain, throat pain, and nasal obstruction compared with nondysphonic individuals. The severity of dysphonia was significantly associated with the severity of dysphagia. A significant positive association was found between dysphonia and cough.
TABLE 3.
Severity of Ear, Nose, and Throat Symptoms in Patients With or Without Dysphonia
| Ear, nose & throat | 0 = No Problem |
1 = Very Mild Problem |
2 = Mild/Slight Problem |
3 = Moderate Problem |
4 = Severe Problem |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Dysphonic | Nondysphonic | Dysphonic | Nondysphonic | Dysphonic | Nondysphonic | Dysphonic | Nondysphonic | Dysphonic | Nondysphonic | P-value |
| Nasal obstruction | 48 (25.5) | 205 (39.9) | 50 (26.6) | 146 (28.4) | 51 (27.1) | 87 (16.9) | 26 (13.8) | 57 (11.1) | 13 (6.9) | 19 (3.7) | 0.008 |
| Rhinorrhea | 74 (39.4) | 240 (46.7) | 68 (36.2) | 179 (34.8) | 28 (14.9) | 67 (13.0) | 15 (8.0) | 27 (5.3) | 3 (1.6) | 1 (0.2) | NS |
| Postnasal drip | 95 (50.5) | 309 (60.1) | 50 (27.6) | 112 (21.8) | 23 (12.2) | 67 (13.0) | 18 (9.6) | 24 (4.7) | 2 (1.1) | 2 (0.4) | NS |
| Throat pain | 76 (40.4) | 301 (58.6) | 53 (28.2) | 141 (27.4) | 39 (20.7) | 47 (9.1) | 12 (6.4) | 22 (4.3) | 8 (4.3) | 3 (0.6) | <0.001 |
| Facial pain | 98 (52.1) | 343 (66.7) | 28 (14.9) | 75 (14.6) | 22 (11.7) | 47 (9.1) | 31 (16.5) | 39 (7.6) | 9 (4.8) | 10 (2.0) | <0.001 |
| Ear pain | 114 (60.6) | 409 (79.6) | 47 (25.0) | 74 (14.4) | 15 (8.0) | 20 (3.9) | 9 (4.8) | 11 (2.1) | 3 (1.6) | 0 (0) | <0.001 |
| Dysphagia | 131 (69.7) | 453 (88.1) | 35 (18.6) | 43 (8.4) | 13 (6.9) | 13 (2.5) | 8 (4.3) | 5 (1.0) | 1 (0.5) | 0 (0) | <0.001 |
| Dyspnea | 76 (40.4) | 306 (59.5) | 52 (27.7) | 124 (24.1) | 29 (15.4) | 55 (10.7) | 21 (11.2) | 19 (3.7) | 10 (5.3) | 10 (2.0) | <0.001 |
The patients assessed their symptom severity through a 5-point scale (0 to 4).
DISCUSSION
The spread of COVID-19 in Europe led to the emergence of an otolaryngological clinical picture of the infection, which is mainly associated with loss of smell and taste. However, many other otolaryngological symptoms could be prevalent in COVID-19. The self-reported dysphonia could concern a quarter of COVID-19 patients.
Dysphonia is encountered in less than 20% of common viral infections such as common cold or flu.9 , 10 Naturally, the prevalence of dysphonia in COVID-19 remains lower compared with other common symptoms, such as total loss of smell and taste. However, the place of dysphonia in the diagnosis of the disease and, regarding our results, as part of a more severe clinical presentation of the disease have to be investigated in future studies. The realization of multidimensional investigation of voice in COVID-19 is still challenging regarding the risk of contamination related to the videolaryngostroboscopic examination. Physicians have to keep in mind that COVID-19 patients may develop dysphonia throughout the clinical course of disease as in common viral infection of the upper aerodigestive tract mucosa.
Dysphonia could be likely related to laryngeal involvement by the airway inflammatory process and may be caused by vocal fold edema or inflammation. However, the etiology of dysphonia in COVID-19 patients needs to be investigated. An ongoing study conducted in the Anatomy Department of University of Mons observed that vocal folds were associated with a high expression of angiotensin-converting enzyme 2 (ACE2), which is the COVID-19 receptor (Figure 2 ) (No published data). These preliminary data could strengthen the corditis etiology of dysphonia related to COVID-19; ACE2 being present in epithelia of the lung, small intestine, vascular endothelium, and oral cavity.11 , 12 Studies about the expression of ACE2 receptor by laryngeal cells would provide interesting findings to clarify the pathogenesis of dysphonia in patients affected by COVID-19. Moreover, regarding the ACE2 expression in lung, abdominal, and chest muscles,13 it could be conceivable that muscle or lung virus-related impairments may lead to dysphonia. As for the ACE2 expression in laryngeal tissue, this hypothesis has to be investigated in future studies.
FIGURE 2.
Angiotensin converting enzyme-2 (ACE2) expression in vocal folds.
The immunohistochemistry analysis found a high expression of ACE2 in vocal fold tissues of healthy human (ongoing study - unpublished data). Ampliation: 1/100.
Another issue that could be studied concerns the impact of gender in the voice-related presentation of COVID-19. Indeed, female could be most at risk of dysphonia in COVID-19, which could involve gender-related difference in the inflammatory process.14 The future studies have to be conducted in the context of potential hormonal and gender differences in the expression of ACE2, in the inflammatory process and, therefore, the clinical presentation of the disease.7 , 15
This study is a preliminary epidemiological study investigating potential association between COVID-19 and the development of dysphonia. Many limitations have to be addressed. First, the lack of video-laryngostroboscopical examination of dysphonic patients limits us in the characterization of the dysphonia. Due to the sanitary situation, it is however discouraged to perform such examinations in COVID-19 patients. Second, many confounding factors might have occurred such as the occurrence of co-infection by COVID-19 and other virus (influenza), potentiating the risk to develop inflammation in the upper aerodigestive tract mucosa and dysphonia, the stress of patients and the stress-related risk to develop laryngopharyngeal reflux, and the occurrence of seasonal allergies with laryngopharyngeal symptoms.
CONCLUSION
Dysphonia was encountered in 26.8% of patients with mild-to-moderate COVID-19 and could be added to the symptom list of COVID-19. Dysphonia may appear in patients with a more severe clinical COVID-19 presentation. Only 3.7% of patients reported severe dysphonia characterized by aphonia. Future studies are needed to investigate the relevance of dysphonia in the COVID-19 clinical presentation.
Acknowledgments
FRMH for the grant. UMONS Staff and authorities for the support. All physicians who contributed to the study.
Footnotes
Financial disclosure: Nothing to disclose.
Conflict of interest: Authors have no conflict of interest.
References
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Data. https://covid19.who.intConsulted April 25, 8:00 P.M.
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Yu X, Zhao H, et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Int Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015;125:1102–1106. doi: 10.1002/lary.24999. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JE, Dolin R, Blaser MJ. Nineth Edition. Elsevier; 2019. Principles and Practice of Infectious Diseases-Elsevier. [Google Scholar]
- 10.Mossad SB, Macknin ML, Medendorp SV, et al. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann Intern Med. 1996;125:81–88. doi: 10.7326/0003-4819-125-2-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004 Jun;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li MY, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamekh M, Deny M, Romano M, et al. Differential susceptibility to infectious respiratory diseases between males and females linked to sex-specific innate immune inflammatory response. Front Immunol. 2017 Dec 13;8:1806. doi: 10.3389/fimmu.2017.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Vignera S, Cannarella R, Condorelli RA, et al. Sex-specific SARS-CoV-2 Mortality: among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082948. pii: E2948. [DOI] [PMC free article] [PubMed] [Google Scholar]