Abstract
Bleeding and delayed healing of gastric ulcer are well-recognized in patients following Clopidorgrel treatment. Our previous studies have shown that endoplasmic reticulum stress (ER) is involved in Clopidogrel-induced gastric mucosal damage through activating p38 mitogen-activated protein kinases (MAPK) pathway. This present study aims to further investigate the role of MAP kinase phosphatase 5 (MKP-5), a MKP known to dephosphorylate and inactivate p38/MAPK, in Clopidogrel-induced gastric mucosal injury and the underlying mechanisms. It shows that MKP-5 is down-regulated at both mRNA and protein levels in the gastric mucosa from bleeding patients who took Clopidogrel over one year. In vitro study using human gastric epithelial cell line GES-1 demonstrates that exposure to Clopidorgrel (1.0-2.0 mM) increases phosphorylation of p38/MAPK and decreases MKP-5 expression simultaneously. Overexpression of MKP-5 promotes GES-1 cell proliferation and reduces apoptosis following Clopidogrel exposure. Interestingly, overexpression of MKP-5 also attenuates Clopidorgrel-induced tight junction (TJ) destruction by down-regulating expression of ER stress-related protein C/EBP homologous protein (CHOP) and tribbles pseudokinase 3 (TRIB3). These three effects, increased proliferation, reduced apoptosis and attenuated TJ destruction, are regulated through inhibited phosphorylation of p38/MAPK signaling pathway. We conclude that MKP-5 is down-regulated in Clopidogrel-induced gastric mucosa injury in vivo and in vitro via phosphorylation and activation of p38/MAPK signaling pathway. Overexpression of MKP-5 reverses Clopidogrel-induced gastric mucosal injury. These findings imply that MKP-5 may be a potential therapeutic target in Clopidogrel-induced gastric mucosal injury and bleeding.
Keywords: Clopidogrel, MKP-5, p38/MAPK, gastric bleeding, apoptosis
Introduction
Clopidogrel is an alternative antiplatelet agent that inhibits adenosine diphosphate (ADP)-induced platelet aggregation. It is widely used in patients with acute coronary syndromes (ACS) and those undergoing percutaneous coronary intervention (PCI) [1].
Ticagrel and Prasugrel once were preferred by European and US guidelines due to their stronger platelet inhibition over Clopidogrel for ACS patients with or without PCI [2-5]. However, Ticagrel or Prasugrel in triple therapy is now not recommended due to their significantly increased bleeding in patients who underwent PCI compared with use of Clopidogrel [4,6-8]. Therefore, use of Clopidogrel remains irreplaceable in these patients. Although Clopidogrel usually does not induce gastric mucosal injury in health volunteers, studies show that it is not safe for patients with high risk of peptic ulcer [9,10]. The underlying mechanism has not been completely understood.
The MAPKs are negatively regulated by the MAP phosphatases (MKPs) through direct dephosphorylation [11]. The MKPs constitute one group of the dual-specificity phosphatases (DUSPs) that exhibit the capability to dephosphorylate MAPKs on regulatory threonine and tyrosine residues [12-14]. MKP-5 which is also called DUSP10 is a MAP kinase phosphatase known to dephosphorylate and negatively regulate p38/MAPK and JNK/MAPK in human embryonic kidney and prostate epithelial cells [15]. By GO analysis of the whole gene expression profile, we identified that MKP-5 mRNA is downexpressed under ER stress when gastric epithelial cells are exposed to Clopidogrel [16]. However, relationship among MKP-5 and p38/MAPK signal pathway when gastric epithelial cells exposure to Clopidogrel remains to be determined.
ER is a multifunctional organelle that plays critical roles in multiple biological processes including synthesizing proteins, monitoring protein folding and trafficking. If the ER can not resolve cell stress, it will cause unfolded or misfolded proteins to accumulate in the ER lumen, leading to ER stress, which is involved in signaling pathways, including inflammation and cell apoptosis or death [17]. Apoptosis is activated by a series of downstream transcriptional effectors of ER, such as transcriptional activation of pro-apoptotic transcription factor C/EBP homologous protein (CHOP), tribbles pseudokinase 3 (TRIB3), nuclear protein 1 (NUPR1), and eukaryotic translation initiation factor 2 subunit 1 (EIF2S1) [18]. Our previous studies have shown that Clopidogrel can lead to ER stress and gastric epithelial cell apoptosis through activation of the p38/MAPK signaling pathway and its downstream pro-apoptotic transcription factor TRIB3 and CHOP [16]. The increased mucosal epithelial apoptosis further leads to disruption of gastric mucosal tight junction [19] therefore loss of gastric mucosa integrity.
The present study aims to further elucidate intracellular signaling molecule(s) and potential therapeutic target(s) in Clopidogrel-induced gastric mucosal injury by analyzing the effect of this drug on p38/MAPK phosphorylation in biopsy specimens and expression of tight junction proteins in vitro GES-1 cell line. We further investigate whether MKP-5 regulated gastric mucosal epithelial cells apoptosis through p38/MAPK mediated ER stress, which provided a potential therapeutic target for prevention and treatment of Clopidogrel-induced gastric side effects.
Materials and methods
Chemicals and solutions
Clopidogrel (purity 97.95%), purchased from MedChen Express (New Jersey, MCE, USA) (Catalog No. HY-17459; CAS No. 120202-66-6), was dissolved in DMSO. Final concentration of DMSO in working culture medium was restricted to be less than 0.1% (v/v). We used the 24 h IC50 concentration (1.5 mmol/L) of Clopidogrel for follow-up study [19].
Human tissue specimens
Use of biopsy specimen in this study was approved by the local ethics committee at Zhongda Hospital affiliated to Southeast University (Nanjing, China, approval # 2018ZDSYLL052-PO1), in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained for experimentation with human subjects. The privacy rights of human subjects are always observed. This study did not have special requirement for gender and age. Human tissue specimens were collected and divided into two groups: In group one, 17 specimens were collected from random gastric ulcer bleeding patients without history of oral antiplatelet and anticoagulant use, who underwent gastroduodenoscopy in the department of gastroenterology, Zhongda Hospital affiliated to Southeast University. In group two, 23 specimens were collected from gastric ulcer bleeding patients who were at the time taking Clopidogrel for more than one year. None of these patients in both groups had history of any cancers with chemotherapy or radiotherapy.
Cell culture
Human gastric epithelial cell line GES-1 was obtained from the Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM (Carlsbad, GIBCO, USA) supplemented with 10% FBS (Carlsbad, GIBCO, USA) and maintained in a 37°C incubator with 5% CO2.
MKP-5 stable transfection
GES-1 cells were transfected with a pEGFP-N1entry plasmid containing a full-length of human MKP-5 cDNA or control plasmid (GenePharma, Shanghai, China). Transfected cells were obtained after selecting with G418 (1,000 μg/ml) for at least three passages. Three clones with stable MKP-5 overexpression, at least three-fold, were used in subsequent experiments.
Quantitative real-time reverse transcription PCR (qRT-PCR)
Total RNA was prepared by using TRIzol reagent (Invitrogen, CA, USA) and was reversely-transcribed into cDNA via reverse Transcription Kit (Takara, Dalian, China). Real-time PCR was performed with SYBR Green (Takara, Dalian China). β-actin was used as reference. MKP-5 primers: forward TGAAGCACACTCGGATGACC; reverse: CCTCGAACTCTAGCAACTGCC; β-actin primers: forward GCACAGAGCCTCGCCTT; reverse: GTTGTCGACGACGAGCG. The performance of RT-qPCR was conducted on ABI 7500 system (Applied Bio-systems, MA, USA). Thermal cycle was as follows: 95°C for 30 sec, 95°C for 5 sec for 40 cycles, and 60°C for 35 sec.
Cell counting kit-8 (CCK8) assay
GES-1 cells (5×103/well) were grown on 96-well plates in triplicates, and were cultured in 100 µL DMEM-HG (Hyclone, Logan, UT, USA) containing 10% FBS for 2 days. CCK8 was used to evaluate cell proliferation according to the manufacturer’s instructions (Dojindo, Laboratories, Kumamoto, Japan). Briefly, 10 µL CCK8 solution was added to each plate, and cells were incubated for 3 h at 37°C. Cell viability was measured at 450 nm. All experiments were performed in triplicate and repeated at least two times.
Flow cytometry analysis
For quantification of the apoptotic cells, Annexin V (AV) and Propidium iodide (PI) apoptosis detection kit (R&D, Minneapolis, MN, USA) was utilized according to the manufacturer’s protocol described as previous study [19].
Terminal deoxynucleotidyl transferase (TUNEL) assay
The GES-1 cells were grown on a coverslip and created groups. Cells were fixed in 4% paraformaldehyde solution for 1 h at room temperature after they reached 60% fusion on the coverslip. They were incubated in permeabilization solution (0.1% Triton-X-100 in 0.1% sodium citrate), and the apoptotic cells were labeled with a TUNEL kit from Roche (Mannheim, Germany) according to the manufacturer’s instructions. All images were taken by FV1000 (Olympus, Japan). The apoptotic (TUNEL-positive) cells were quantified using the Image J.
Immunohistochemistry (IHC)
Immunohistochemistry was performed using a Dako Real Envision Detection System (Dako, Glostrup, Denmark). Slides were incubated with a primary antibody specific to MKP-5 (Sigma-Aldrich, St. Louis, MO, USA), followed by incubation with a biotinylated secondary antibody and enzyme conjugate (Dako). They were stained with 3, 3’-diaminobenzidine and counterstained with hematoxylin. All sections were scored by the semi-quantitative H-score approach and validated independently by two experienced pathologists.
Western blot analysis
Proteins were extracted by a RIPA lysis buffer (Solarbio, CN) containing protease and phosphatase inhibitor cocktail (Roche, Basel, Switzerland) and quantified using a BCA protein quantification kit (Thermo, USA). Total protein lysates were separated by SDS-PAGE gels and then transferred onto PVDF membranes (Whatman, Germany). The PVDF membranes were blocked with 1% BSA solution for 1h at room temperature and incubated with specific primary antibodies at 4°C overnight followed by the secondary antibody incubation at room temperature for 1 h. Specific antibody against β-actin, MKP-5, p-p38, p-pJNK, p-pERK, Occludin, ZO-1, CHOP and TRIB3 were purchased from Cell Signaling Technology (CST, Beverley, MA, USA). All primary antibodies were diluted at 1:1500, and the corresponding secondary antibodies were diluted at 1:5000.
Transmission and scanning electron microscope study
Transmission electron microscopic (TEM) images were achieved using a Hitachi HD2300A microscope for the size of the magnetic particles as previously described [16]. For scanning electron microscopy (SEM), GES-1 cells after treatment were grown on silicon chip in six-well plates. The cultures were placed in 5% CO2 at 37°C for 4 h in a total volume of 2 ml. Then the silicon chips were removed and washed twice with PBS. The cells were fixed first with 2.5% glutaraldehyde for > 2 h, and then with 2% osmic acid. They were analyzed with a Hitachi S2520 scanning electron microscope.
Statistics
Statistical analysis was performed by use of SPSS 19.0 (IBM, New York, NY, USA). All results were described as mean ± SD. Independent samples t-test was used in analyzing the difference of means between two groups. One-way analysis of variance was used in comparing the difference among three or more groups. P < 0.05 was considered statistically significant.
Results
MKP-5 expression is significantly down-regulated in gastric mucosa tissues from bleeding patients with long-term Clopidogrel use
As shown in Figure 1 from our previous study [16], using whole gene expression profile, we identified that MKP-5 was down-regulated by Clopidogrel in gastric mucosal epithelial cell line GES-1. To investigate the MKP-5 expression in gastric bleeding patients taking Clopidogrel over one year, we performed real-time PCR and immunohistochemistry in human gastric mucosal biopsy specimens. Results showed that MKP-5 is under-expressed both at mRNA and protein levels in gastric mucosal biopsy specimens (P < 0.01) (Figure 2A and 2B), confirming findings in our previous whole gene expression profile.
Figure 1.
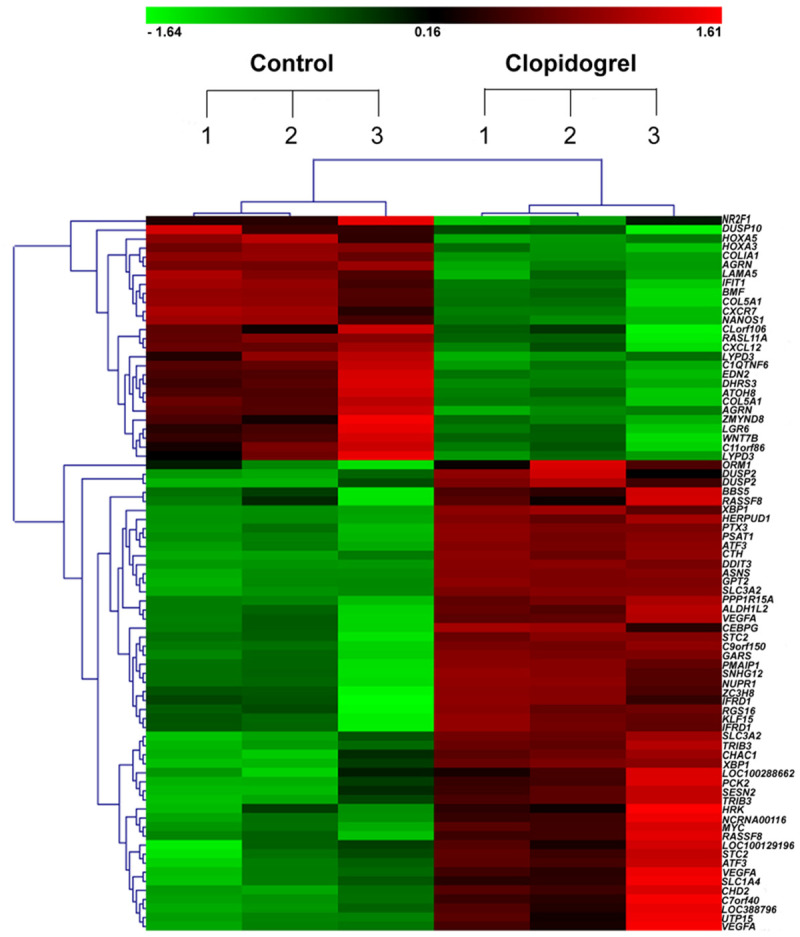
Hierarchical cluster analysis of 79 differentially expressed genes in 6 GES-1 samples. Three normal and three Clopidogrel-treated GES-1 cell samples. Each column represents one sample, where red denotes an increase in gene expression and green denotes a decrease in gene expression as compared with the other group. The brighter the color, the higher the gene expression level.
Figure 2.
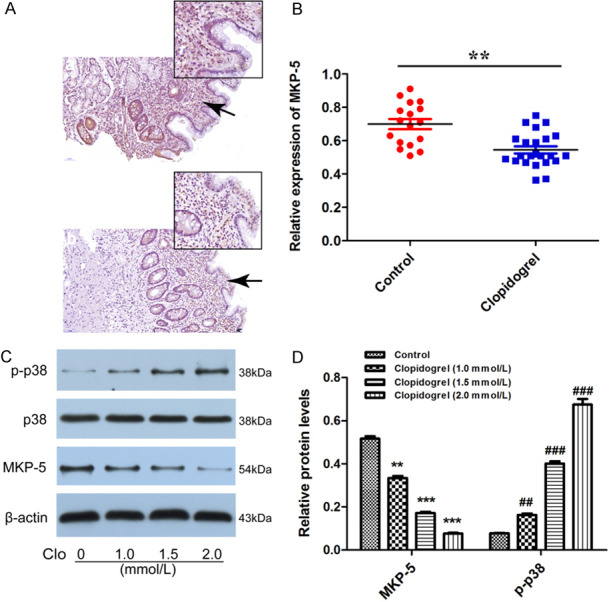
Expression of MKP-5 and phosphorylated p38/MAPK in Clopidogrel-treated gastric mucosal tissues and cells. A and B. MKP-5 expression was down-expressed at both protein and mRNA levels in gastric mucosal biopsy specimens that were validated by immunohistochemistry and qRT-PCR. **P < 0.01 vs. control. Scale bar = 50 μm. C and D. Western blot analysis showed MKP-5 was decreased and negatively correlated of Clopidogrel. As MKP-5 decreased, the level of phosphorylated p38/MAPK was upregulated. **P, ##P < 0.01 and ***P, ###P < 0.001 vs. control.
Clopidogrel down-regulates MKP-5 and activates the p38/MAPK signaling pathway in GES-1 cells
We then investigated the expression of MKP-5 and p38/MAPK in GES-1 cells exposed to Clopidogrel in vitro. Dimopoulou M’s in vitro experiment mimicking Clopidogrel disintegration in stomach demonstrated that the concentration of Clopidogrel was saturated after 15 minutes and was about 150 ug/ml (0.36 mmo/L), and the rest of Clopidogrel was present in stomach as precipitate [20]. In vivo, a previous study has shown that the highest concentration of Clopidogrel obtained in serum after administration of 75 mg/d of Clopidogrel for 24 h was 0.04 µmol/l [21]. Unfortunately, the real concentration of Clopidogrel in gastric mucosa remains unknown in literature due to ethical reason. In our previous study we performed MTT assay to optimize the concentration in vitro studies. We found that the IC50 of Clopidogrel was calculated as 10.14 ± 1.72, 1.57 ± 0.58 and 0.39 ± 0.16 mM for GES-1 cells treated for 12, 24, or 36 h, respectively [19]. Therefore, in the next Western-blot study, we chose 1.0, 1.5 and 2.0 mmol/L of Clopidogrel to treat GES-1 cells for 24 h. We found MKP-5 protein level was decreased after Clopidogrel treatment and inversely correlated with Clopidogrel concentration. Unexpectedly, the level of phosphorylated p38/MAPK was upregulated (Figure 2C and 2D). These results suggest that MKP-5 is present and functional in human gastric mucosa epithelium cells and treatment of Clopidogrel inhibits dephosphorylation of p38/MAPK. Increased phosphorylation of p38/MAPK signaling may lead to initiation of a series of apoptosis-related progress.
MKP-5 overexpression inhibits the apoptosis of GES-1 cells induced by Clopidogrel
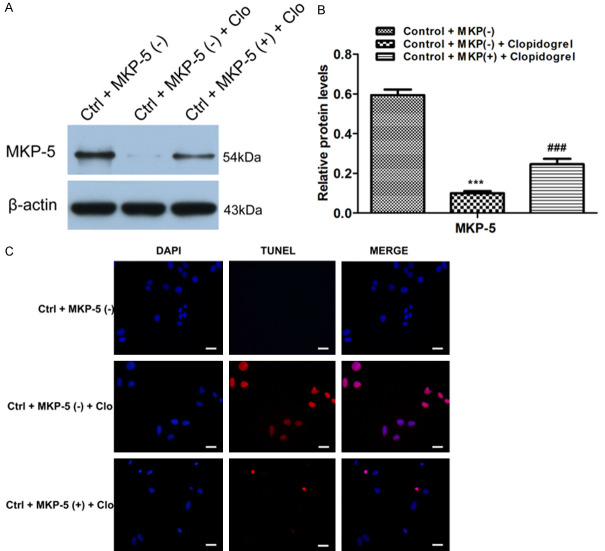
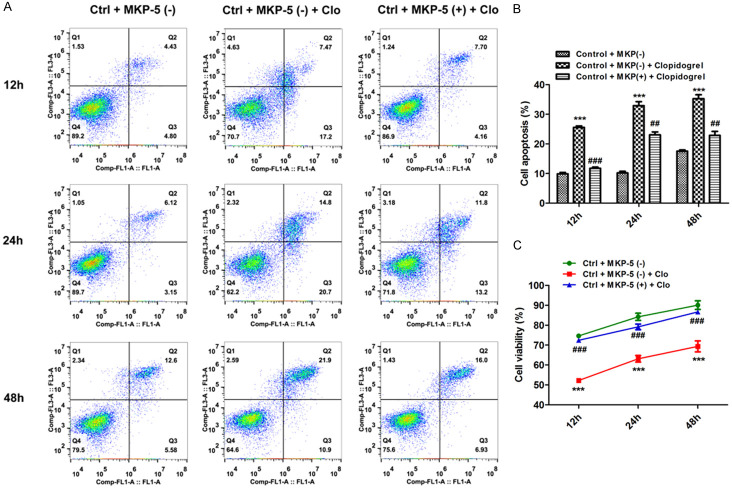
To further investigate the effect of MKP-5 in apoptosis of GES-1 cells, we used MKP-5 overexpress plasmid. After GES-1 cells were transfected with MKP-5 overexpression or control blank plasmid, respectively, the transfection efficiency was assessed by qRT-PCR and western blotting (Figure 3). Results showed that MKP-5 overexpression inhibits Clopidogrel-induced MKP-5 downregulation (Figure 4A and 4B). TUNEL assay further demonstrated that percentage of apoptotic cells is significantly lower in MKP-5 overexpressed cells than that in control GES-1 cells treated by Clopidogrel (Figure 4C). This result is further confirmed by both flow cytometry (Figure 5A and 5B) and CCK8 assay (Figure 5C).
Figure 3.
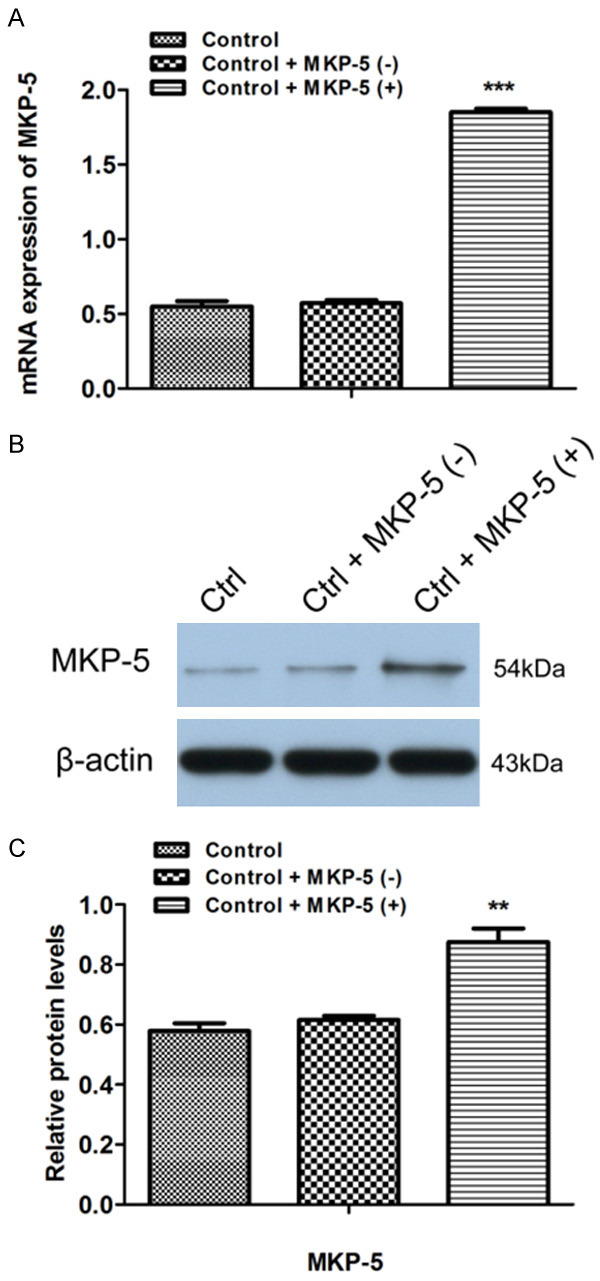
Construction of MKP-5 overexpression plasmid. After the GES-1 cells were transfected with MKP-5 overexpressing and blank plasmid, respectively, the transfection efficiency was assessed by qRT-PCR (A) and Western blotting (B and C). **P < 0.01 and ***P < 0.001 vs. control + MKP-5 (-).
Figure 4.
Effects of MKP-5 on Clopidogrel-induced GES-1 cells apoptosis by TUNEL assay. A and B. Western blot results revealed that MKP-5 overexpression inhibited Clopidogrel-induced downregulation of MKP-5. ***P < 0.001 vs. control + MKP-5 (-); ###P < 0.001 vs. control + MKP-5 (-) + Clopidogrel. C. TUNEL assay showed that the proportion of apoptotic cells induced by Clopidogrel was lower in MKP-5 overexpressed cells than that in blank plasmid group. Scale bar = 100 μm.
Figure 5.
Effects of MKP-5 on Clopidogrel-induced GES-1 cells apoptosis by flow cytometry and CCK8 assay. A and B. MKP-5 overexpression inhibited Clopidogrel-induced gastric mucosal cell apoptosis. ***P < 0.001 vs. control + MKP-5 (-); ##P < 0.01 and ###P < 0.001 vs. control + MKP-5 (-) + Clopidogrel. C. CCK-8 assay revealed that MKP-5 overexpression accelerated proliferation of gastric mucosal cells upon exposure to Clopidogrel; ###P < 0.001 vs. control + MKP-5 (-) + Clopidogrel.
MKP-5 overexpression attenuates Clopidogrel-induced TJs disruption in GES-1 cells
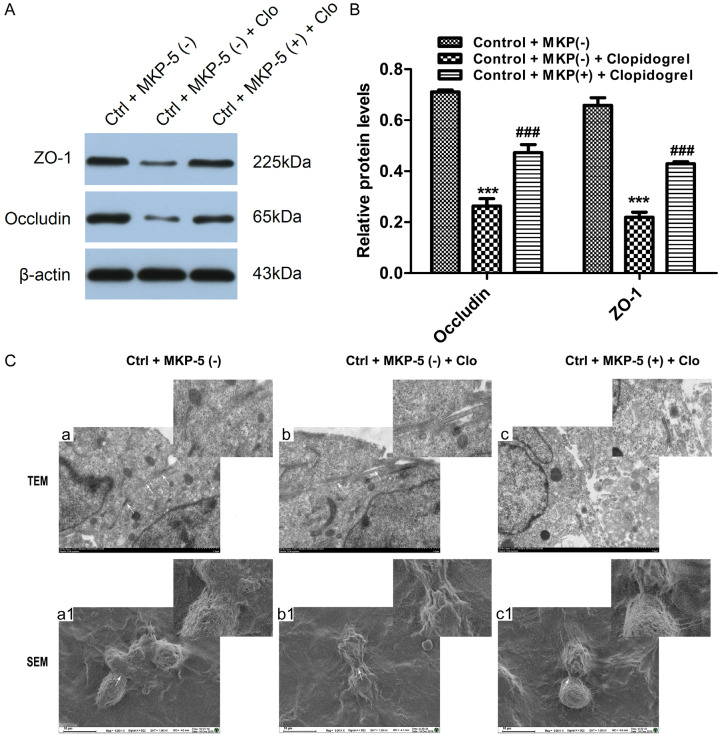
We next investigated whether MKP-5 affects TJs or not. As shown in Figure 6A and 6B, the amount of TJ protein Occludin and ZO-1 was significantly decreased after treatment with Clopidogrel. However, this effect was attenuated by MKP-5 overexpression. By transmission and scanning electron microscopy, we found that TJs are tight and intact in normal GES-1 cells. Clopidogrel severely disrupts TJ ultrastructure, characterized by decreased electron-dense materials in the TJs and absence of TJ membrane fusion, widened intercellular gaps and tracer extravasation. Intriguingly, MKP-5 overexpression markedly alleviates these effects (Figure 6Ca-c). Scanning electron microscopy showed normal GES-1 cells are connected to each other by tight and adherence junction complex, featuring continuous strands with almost no strand breaks. By contrast, Clopidogrel treated cells showed TJ complex with discontinuous network of tight junction strands. In the MKP-5 overexpression group, however, TJ strands were almost continuous with a few minor fractures (Figure 6Ca1-c1).
Figure 6.
Effects of MKP-5 on TJs in GES-1 cells. A and B. Western blot analysis showed expression of TJ protein Occludin and ZO-1. ***P < 0.001 vs. control + MKP-5 (-); ###P < 0.001 vs. control + MKP-5 (-) + Clopidogrel. C. TEM a: The TJ structures were extremely tight and intact. TEM b: After Clopidogrel treated, the TJ was severely disrupted, characterized by decreased electron-dense materials and absence of TJ membrane fusion, further widened gaps and tracer extravasation. TEM c: MKP-5 overexpression markedly alleviated the TJ disruption. White arrowheads indicate typical changes in TJ structure. Scale bar = 1 μm. SEM a1: The normal GES-1 cells were connected to each other by the tight and adherence junction complex, featuring continuous strands with almost no strand breaks. SEM b1: Clopidogrel-treated cells showed a complex but strongly discontinuous network of tight junction strands. SEM c1: In the MKP-5 overexpression group, tight junction strands were almost continuously restored with only a few minor fractures. White arrowheads indicate typical changes in TJ structure. Scale bar = 10 μm.
MKP-5 overexpression inhibits Clopidogrel-induced phosphorylation of p38/MAPK and ER stress
With above results that Clopidogrel-induced GES-1 cell apoptosis is mainly due to ER-stress involving p38/MAPK signaling pathway. Our previous GO analysis showed that MKP-5 is involved in Clopidogrel-induced ER stress [16]. We then studied whether MKP-5 interacts with p38, JNK or ERK/MAPK phosphorylation and affects the ER stress. In these experiments, overexpression of MKP-5 decreased the amount of phosphorylated p38/MAPK induced by Clopidogrel, but did not affect phosphorylation of either JNK or ERK (Figure 7A and 7B). ER stress-related transcription factors CHOP and TRIB3 were both significantly down-regulated by MKP-5 overexpression (Figure 7C and 7D).
Figure 7.
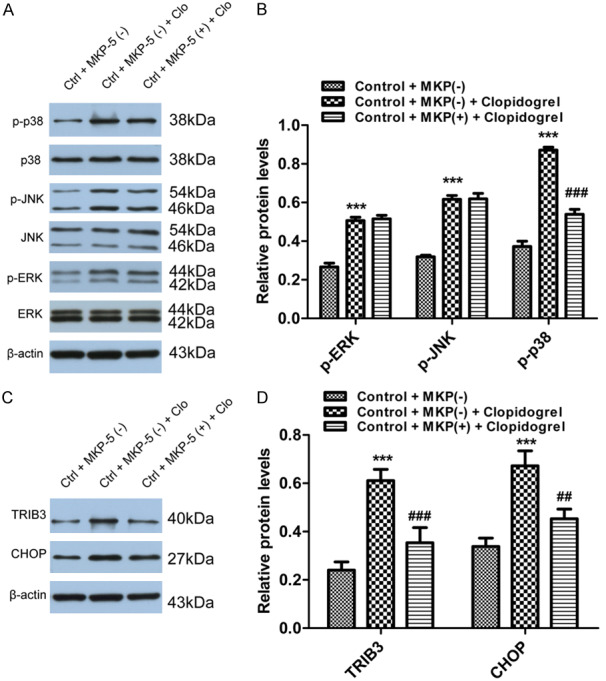
Effects of MKP-5 on MAPK signaling pathway and ER stress. A and B. MKP-5 overexpression decreased phosphorylated p38/MAPK induced by Clopidogrel, but no effect on either JNK or ERK pathway. ***P < 0.001 vs. control + MKP-5 (-); ###P < 0.001 vs. control + MKP-5 (-) + Clopidogrel. C and D. CHOP and TRIB3 were both significantly down-regulated by MKP-5 overexpression. ***P < 0.001 vs. control + MKP-5 (-); ##P < 0.01 and ###P < 0.001 vs. control + MKP-5 (-) + Clopidogrel.
Discussion
Clopidogrel is widely used in cardiovascular disease, but bleeding in certain patients is concerning for its clinical use [22]. Understanding of the underlying mechanism for gastric peptic ulcer related to Clopidogrel is critical to avoid this side effect.
Gastric mucosal integrity is critically dependent on the balance between cell proliferation (renewal) and cell death by apoptosis. Recent studies have shown that tyrosine kinases play a pivotal role in maintaining this balance [23-26]. Previous study by Luo [23] in rats suggested that Clopidogrel delays gastric ulcer healing by attenuating gastric epithelial cell proliferation via phosphorylation activation of the ERK/MAPK pathway. However, our studies show that Clopidogrel could upregulate all three MAPK signaling pathways: p38, ERK and JNK/MAPK in human gastric epithelial cell lines [16,19] injuring the gastric mucosal epithelial cells. These findings are consistent with Chang’s [27] study which showed that all three MAPK signal transduction pathway are upregulated in ethanol-induced gastric ulcers in rats. These discrepancies might be due to certain difference by species, but possibility of other unknown factors remains to be studied. Besides of these observations, we also found in our previous studies that p38/MAPK inhibitor SB203580 could specifically alleviate the Clopidogrel-induced apoptosis [16] and TJs destruction [19]. We therefore hypothesize that p38/MAPK might play a key role in Clopidogrel-induced gastric mucosal injury.
We subsequently used Agilent whole gene expression profiling analysis to test above hypothesis. The pathway and Go analysis displayed that the MKP-5 gene expression was significantly down-regulated in Clopidogrel-induced MAPKs’ phosphorylation and ER stress, supporting that p38/MAPK pathway does play a role in Clopidogrel-induced gastric mucosal injury. It’s known that MAPKs’ phosphorylation status and activity are regulated by MKPs. MKPs dephosphorylate Thr-X-Tyr motif of an activated MAPK and thereby restrict the magnitude and duration of kinase activity [28]. However, it remains unknown which particular MKP among all known 11 members might be involved in Clopidogrel-induced p38/MAPK dephosphorylation. Our finding for the first time indicates that MKP-5 plays critical role in this process. There are 11 different MKPs, and the MKP-1 is the most investigated phosphatase among them. MKP-1 now is reported to suppress p38 and JNK/MAPK activity, and is a suppressor of inflammation in vivo [29,30]. Enhanced mRNA and protein expression of MKP-1 were found to be higher in the human gastric adenocarcinoma tissues [31] and rats portal hypertensive (PHT) gastric mucosa tissues [32-34]. However, MKP-5 which found in both cytoplasm and nucleus, is the less known member of the MKPs family. Until now there has been few reports of MKP-5 in digestive diseases. Consistent with the chip results, our present study firstly confirmed that Clopidogrel-exposed human gastric mucosa and in vitro cell lines did significantly reduce the MKP-5 expression level. Meanwhile, we also found the expression of MKP-5 was negatively correlated with the degree of phosphorylation p38/MAPK.
To further confirm the effect of MKP-5 on p38/MAPK dephosphorylation, we used MKP-5 overexpression plasmid to see which downstream signaling molecule is dephosphorylated. Result shows MKP-5 overexpression specifically dephosphorylates and inactivates p38/MAPK, but not JNK or ERK/MAPK in Clopidogrel-treated GES-1 cells. This is consistent with other observations in colon cancer in which overexpression of MKP-5 promotes cell proliferation [35]. In the same above article, Nomura reported that overexpression of MKP-5 affects all three pathways, p38, JNK/MAPK and ERK/MAPK, which seems different from our observation. Whether this reflects a dose-dependent manner due to plasmid concentration or is simply due to different cell lines used remains to be determined [35-37]. These results suggest that Clopidogrel can induce down-regulation of MKP-5 expression, therefore decreasing dephosphorylation of p38/MAPK, eventually leading to cell apoptosis.
Our previous study has demonstrated that Clopidogrel-induced phosphorylation of p38/MAPK could increase the expression of the ER stress transcription factor TRIB3 and CHOP, thereby inducing GES-1 cells apoptosis [16]. Go analysis in our previous study [16] and other studies [38] revealed that MKP-5 is involved in ER stress-induced cell apoptosis. Here by MKP-5 overexpression plasmid, we confirmed that MKP-5 overexpression down regulates Clopidogrel-induced ER stress through inactivating p38/MAPK. However, response to ER stress is an intricate signaling network with multiple transcription factors, molecular chaperones and signaling pathways involved [39]. Subsequent studies on the complicated signaling pathways triggered by Clopidogrel are currently under further investigation.
The protective gastric epithelial barrier comprises three components: The compact epithelial cell lining, the specialized mucus covering and the bicarbonate ions. TJs exist among the gastric epithelial cell layer, which is the most critical in binding the epithelial cell layers and thus separating physiologically distinct compartments, restricting luminal antigens [40]. So the apoptosis of gastric mucosal epithelial cells directly leads to the TJs collapse, further inducing inflammation and ulceration. Indeed, as represented in Figure 6, with the inhibition of GES-1 cells apoptosis after MKP-5 overexpression, the expression of tight junction protein Occludin and ZO-1 were significantly increased and the disruption of the TJs were also partially repaired, suggesting that MKP-5 contributes to protective mechanism in response to injury in gastric mucosa.
Conclusion
In conclusion, the present study revealed that MKP-5 is downexpressed in gastric mucosa exposed to Clopidogrel. Overexpression of MKP-5 inhibits Clopidogrel-induced gastric epithelial cells damage. This protective effect is due to inhibition of ER stress-induced apoptosis via MKP-5-mediated p38/MAPK dephosphorylation in gastric epithelial cells. These findings help us better understand the critical role of MKP-5-p38/MAPK signal pathway, apoptosis and gastric intercellular TJ proteins in Clopidogrel-induced gastric mucosal injury, implying potential therapeutic target in this side effects.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (NO. 81600415&81600429&81570503), Postgraduate Research and Practice Innovati on Program of Jiangsu Province (No. KYCX18_0178). Southeast University’s basic scientific research support (NO. 3290004407) and the Nanjing Municipal Science and Technology Bureau (NO. 201715012). We wish to thank Dr. Guoqing Wang, PhD and Dr. FenGan Ding, PhD, Department of Pathology, Zhongda Hospital affiliated to Southeast University, Nanjing, China, for their excellent technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Xie HG, Zou JJ, Hu ZY, Zhang JJ, Ye F, Chen SL. Individual variability in the disposition of and response to clopidogrel: pharmacogenomics and beyond. Pharmacol Ther. 2011;129:267–289. doi: 10.1016/j.pharmthera.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 4.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134:e123–155. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 7.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 8.Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;138:527–536. doi: 10.1161/CIRCULATIONAHA.118.034722. [DOI] [PubMed] [Google Scholar]
- 9.Chan FK, Ching JY, Hung LC, Wong VW, Leung VK, Kung NN, Hui AJ, Wu JC, Leung WK, Lee VW, Lee KK, Lee YT, Lau JY, To KF, Chan HL, Chung SC, Sung JJ. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352:238–244. doi: 10.1056/NEJMoa042087. [DOI] [PubMed] [Google Scholar]
- 10.Lai KC, Chu KM, Hui WM, Wong BC, Hung WK, Loo CK, Hu WH, Chan AO, Kwok KF, Fung TT, Wong J, Lam SK. Esomeprazole with aspirin versus clopidogrel for prevention of recurrent gastrointestinal ulcer complications. Clin Gastroenterol Hepatol. 2006;4:860–865. doi: 10.1016/j.cgh.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Lawan A, Shi H, Gatzke F, Bennett AM. Diversity and specificity of the mitogen-activated protein kinase phosphatase-1 functions. Cell Mol Life Sci. 2013;70:223–237. doi: 10.1007/s00018-012-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soulsby M, Bennett AM. Physiological signaling specificity by protein tyrosine phosphatases. Physiology (Bethesda) 2009;24:281–289. doi: 10.1152/physiol.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 14.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 15.Nunes-Xavier C, Roma-Mateo C, Rios P, Tarrega C, Cejudo-Marin R, Tabernero L, Pulido R. Dual-specificity MAP kinase phosphatases as targets of cancer treatment. Anticancer Agents Med Chem. 2011;11:109–132. doi: 10.2174/187152011794941190. [DOI] [PubMed] [Google Scholar]
- 16.Wu HL, Duan ZT, Jiang ZD, Cao WJ, Wang ZB, Hu KW, Gao X, Wang SK, He BS, Zhang ZY, Xie HG. Increased endoplasmic reticulum stress response is involved in clopidogrel-induced apoptosis of gastric epithelial cells. PLoS One. 2013;8:e74381. doi: 10.1371/journal.pone.0074381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang EB, Kwon IS, Koo JH, Kim EJ, Kim CH, Lee J, Yang CH, Lee YI, Cho IH, Cho JY. Treadmill exercise represses neuronal cell death and inflammation during Abeta-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis. 2013;18:1332–1347. doi: 10.1007/s10495-013-0884-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymol. 2008;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu HL, Gao X, Jiang ZD, Duan ZT, Wang SK, He BS, Zhang ZY, Xie HG. Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 MAPK activation. Toxicology. 2013;304:41–48. doi: 10.1016/j.tox.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Dimopoulou M, Mourouti CS, Vertzoni M, Symillides M, Reppas C. In-vitro evaluation of performance of solid immediate release dosage forms of weak bases in upper gastrointestinal lumen: experience with miconazole and clopidogrel salts. J Pharm Pharmacol. 2016;68:579–587. doi: 10.1111/jphp.12406. [DOI] [PubMed] [Google Scholar]
- 21.Franca CN, Pinheiro LF, Izar MC, Brunialti MK, Salomao R, Bianco HT, Kasmas SH, Barbosa SP, de Nucci G, Fonseca FA. Endothelial progenitor cell mobilization and platelet microparticle release are influenced by clopidogrel plasma levels in stable coronary artery disease. Circ J. 2012;76:729–736. doi: 10.1253/circj.cj-11-1145. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM, Harrington RA, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Hlatky MA, Kaul S, Lindner JR, Moliterno DJ, Mukherjee D, Schofield RS, Rosenson RS, Stein JH, Weitz HH, Wesley DJ American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118:1894–1909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 23.Luo JC, Huo TI, Hou MC, Lin HY, Li CP, Lin HC, Chang FY, Lee FY. Clopidogrel delays gastric ulcer healing in rats. Eur J Pharmacol. 2012;695:112–119. doi: 10.1016/j.ejphar.2012.07.054. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Kao HW, Robinson D, Kung HJ, Wu CW, Chen HC. Tyrosine kinases and gastric cancer. Oncogene. 2000;19:5680–5689. doi: 10.1038/sj.onc.1203924. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Saini N, Parris AB, Zhao M, Yang X. Ganetespib induces G2/M cell cycle arrest and apoptosis in gastric cancer cells through targeting of receptor tyrosine kinase signaling. Int J Oncol. 2017;51:967–974. doi: 10.3892/ijo.2017.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi Y, Toyomasu Y, Saravanaperumal SA, Bardsley MR, Smestad JA, Lorincz A, Eisenman ST, Cipriani G, Nelson Holte MH, Al Khazal FJ, Syed SA, Gajdos GB, Choi KM, Stoltz GJ, Miller KE, Kendrick ML, Rubin BP, Gibbons SJ, Bharucha AE, Linden DR, Maher LJ 3rd, Farrugia G, Ordog T. Hyperglycemia increases interstitial cells of Cajal via MAPK1 and MAPK3 signaling to ETV1 and KIT, leading to rapid gastric emptying. Gastroenterology. 2017;153:521–535. e520. doi: 10.1053/j.gastro.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang X, Luo F, Jiang W, Zhu L, Gao J, He H, Wei T, Gong S, Yan T. Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-kappaB pathway in vivo and in vitro. Int Immunopharmacol. 2015;28:604–615. doi: 10.1016/j.intimp.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 2013;280:489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turpeinen T, Nieminen R, Moilanen E, Korhonen R. Mitogen-activated protein kinase phosphatase-1 negatively regulates the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in A549 human lung epithelial cells. J Pharmacol Exp Ther. 2010;333:310–318. doi: 10.1124/jpet.109.157438. [DOI] [PubMed] [Google Scholar]
- 30.Korhonen R, Turpeinen T, Taimi V, Nieminen R, Goulas A, Moilanen E. Attenuation of the acute inflammatory response by dual specificity phosphatase 1 by inhibition of p38 MAP kinase. Mol Immunol. 2011;48:2059–2068. doi: 10.1016/j.molimm.2011.06.439. [DOI] [PubMed] [Google Scholar]
- 31.Bang YJ, Kwon JH, Kang SH, Kim JW, Yang YC. Increased MAPK activity and MKP-1 overexpression in human gastric adenocarcinoma. Biochem Biophys Res Commun. 1998;250:43–47. doi: 10.1006/bbrc.1998.9256. [DOI] [PubMed] [Google Scholar]
- 32.Kawanaka H, Tomikawa M, Jones MK, Pai R, Szabo IL, Sugimachi K, Sarfeh IJ, Tarnawski AS. Portal hypertensive gastric mucosa has reduced activation of MAP kinase (ERK2) in response to alcohol injury: a key to impaired healing? FASEB J. 2001;15:574–576. doi: 10.1096/fj.00-0450fje. [DOI] [PubMed] [Google Scholar]
- 33.Kawanaka H, Tomikawa M, Baatar D, Jones MK, Pai R, Szabo IL, Sugimachi K, Sarfeh IJ, Tarnawski AS. Despite activation of EGF-receptor-ERK signaling pathway, epithelial proliferation is impaired in portal hypertensive gastric mucosa: relevance of MKP-1, c-fos, c-myc, and cyclin D1 expression. Life Sci. 2001;69:3019–3033. doi: 10.1016/s0024-3205(01)01409-6. [DOI] [PubMed] [Google Scholar]
- 34.Kawanaka H, Tomikawa M, Jones MK, Szabo IL, Pai R, Baatar D, Tsugawa K, Sugimachi K, Sarfeh IJ, Tarnawski AS. Defective mitogen-activated protein kinase (ERK2) signaling in gastric mucosa of portal hypertensive rats: potential therapeutic implications. Hepatology. 2001;34:990–999. doi: 10.1053/jhep.2001.28507. [DOI] [PubMed] [Google Scholar]
- 35.Nomura M, Shiiba K, Katagiri C, Kasugai I, Masuda K, Sato I, Sato M, Kakugawa Y, Nomura E, Hayashi K, Nakamura Y, Nagata T, Otsuka T, Katakura R, Yamashita Y, Sato M, Tanuma N, Shima H. Novel function of MKP-5/DUSP10, a phosphatase of stress-activated kinases, on ERK-dependent gene expression, and upregulation of its gene expression in colon carcinomas. Oncol Rep. 2012;28:931–936. doi: 10.3892/or.2012.1862. [DOI] [PubMed] [Google Scholar]
- 36.Tanoue T, Moriguchi T, Nishida E. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J Biol Chem. 1999;274:19949–19956. doi: 10.1074/jbc.274.28.19949. [DOI] [PubMed] [Google Scholar]
- 37.Theodosiou A, Smith A, Gillieron C, Arkinstall S, Ashworth A. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene. 1999;18:6981–6988. doi: 10.1038/sj.onc.1203185. [DOI] [PubMed] [Google Scholar]
- 38.Song Z, Ma J, Lu Y, Zhou C, Zhao T, Ai X, Wei X, Lin J, Wang W, Yan W, Jiao P. The protective role of the MKP-5-JNK/P38 pathway in glucolipotoxicity-induced islet beta-cell dysfunction and apoptosis. Exp Cell Res. 2019;382:111467. doi: 10.1016/j.yexcr.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Di S, Fan C, Ma Z, Li M, Guo K, Han D, Li X, Mu D, Yan X. PERK/eIF-2alpha/CHOP pathway dependent ROS generation mediates butein-induced non-small-cell lung cancer apoptosis and G2/M phase arrest. Int J Biol Sci. 2019;15:1637–1653. doi: 10.7150/ijbs.33790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]