Abstract
Circular RNAs (circRNAs), a new star noncoding RNA (ncRNA), show stability, conservation, abundance, and tissue and stage specificity. They act as key regulators of biological processes. They target the mRNAs of many other different genes or signaling pathways, and closely link associated genes into regulatory networks. Growing evidence has demonstrated that circRNAs may play an important role in the carcinogenesis, progression and chemoradiation resistance of many cancers including head and neck cancers (HNC). CircRNA, like other ncRNA, such as miRNA, lncRNA, usually is considered to be non-protein coding transcript. However, recent studies indicated that abnormal translation of circRNAs may be involved in human diseases. In this review, we collected the origin, classification, characteristics, function of circRNAs, exosmal circRNAs, and then synthesize current study results to highlight aberration of circRNAs in various types of HNC, and try to clarify the molecular mechanisms of circRNAs affecting the pathogenesis and progression of HNC, as well as pay particular attention to provide a new avenue to the diagnosis and treatment strategy for HNC.
Keywords: Circular RNA, head and neck cancer, protein translation, biomarker, therapeutic target
Introduction
Head and neck cancers (HNC) are one of the most common malignancies worldwide with diverse biological behaviors. Its pathogenesis may be associated with three major etiological factors, including environmental factors, genetic factors, and Epstein-Barr virus or human papillomavirus infection [1]. Determination of the molecular mechanisms of HNC carcinogenesis and progression may enable the discovery of early diagnostic biomarkers and effective therapeutic targets. Recent advances have reported that deregulation of circular RNA (circRNA) might play critical role in the development of HNC [2-4], and provided a new clue as to the understanding of tumor biology and therapeutic strategy of HNC.
CircRNA is a type of single-stranded RNA which, unlike the better-known linear RNA, forms a covalently closed continuous loop without 5’ caps and 3’ tails [5,6]. This feature confers numerous properties to circRNAs, many of which have gradually been identified and having been paid more and more attention. CircRNA is a rising star in the field of RNA molecular biology in recent years. In fact, circRNA was first found in viroid as early as 1970s [7], and was first found in human cells in 1990s in the study of delete in colorectal carcinoma gene [8], but its research has been annihilated for more than 20 years. Most RNA is considered linear, so circRNA is considered to be a genetic accident or an experimental artificial product. Until 2012, Salzman et al. [9] discovered a large amount of circRNA expression in human cells; then, in 2013, two important studies on circRNA acting as a molecular sponge [10,11], suggesting that these circRNA molecules may play an important role in organisms. With the rapid development of high-throughput sequencing technology and bioinformatics, a large number of circRNA molecules have been found in different organisms, and the golden age of rapid research of circRNA is coming.
Emerging evidence has demonstrated that circRNAs show abnormal expression in human diseases, including central nervous system diseases, cardiovascular diseases and cancers [6], indicating their marked potential in the prediction and prognosis of diseases and clinical treatment. Certainly, some circRNAs have also been shown to be deregulated in distinct HNC [3]. The important roles of circRNAs as miRNA sponge [11,12], gene transcription and expression regulators [12], and RNA-binding proteins (RBP) sponges [13] are gradually uncovered. Furthermore, numerous of studies have confirmed the function of circRNAs in tumor cell proliferation, migration and invasion, apoptosis, angiogenesis, deterioration and recurrence of cancer, which may potentially serve as a novel biomarker and therapeutic target for cancer prevention and treatment [12,14,15].
CircRNAs in animals are a large class of particularly stable RNAs produced by circularization of specific exons or intron. Most circRNAs were not associated with translating ribosomes, therefore, circRNAs were deemed to be noncoding. However, recent findings revealed that some circRNAs could generate proteins in vivo [16-18]. Additionally, some exosomal circRNAs have been found and gradually used in the diagnosis and treatment of diseases, especially cancers. These new findings expand the landscape of circRNA applications and enhance the recognition of its function.
In the present review, we summarize the biological properties of circRNAs and the known molecular mechanisms, as well as their functions, especially those related to human tumors including HNC.
Biogenesis of circRNAs
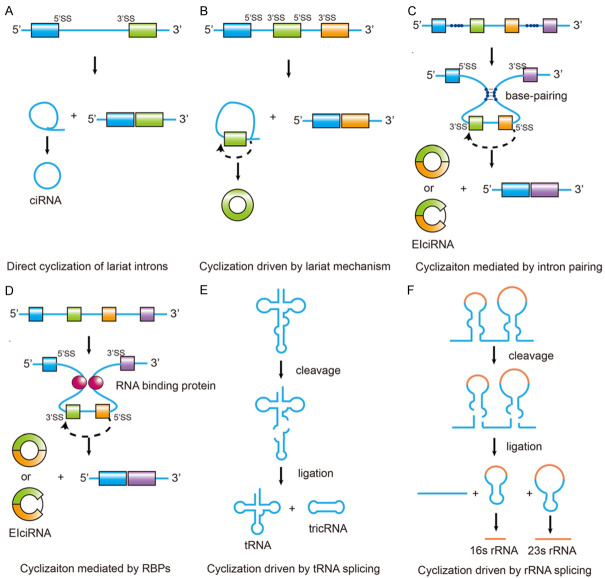
Most circRNAs in organisms are synthesized by linear RNA precursor, which is accomplished by a variety of noncanonical splicing models. The process named backsplicing, where downstream exons are spliced to upstream exons in reverse order [19]. Currently, 6 models have been proposed for the formation of circRNAs [5,20-24] (Table 1): (1) direct circularization of lariat introns, the 3’ downstream of the lariat intron is trimmed to form a circular intronic RNA (ciRNA); (2) lariat-driven circularization (exon skipping), the exon-skipping event during alternative splicing promotes the 3’ splice site (SS) of the exon to covalently splice to the 5’SS; (3) intron-pairing driven circularization (direct backsplicing), intron pairing brings the appropriate splice signals within proximity of each other, which promotes circularization; (4) circularization mediated by RBPs and trans-acting factors, RBPs bring the appropriate splice signals within proximity of each other, which promotes circularization; (5) circularization driven by tRNA splicing; (6) circularization driven by rRNA splicing. Three of them are speculative models of backsplicing of exonic circRNA (ecircRNA) and exonic-intronic circRNA (EIciRNA), and other three are speculative patterns of ciRNAs processing maturation mechanism. The representative models of origin of circRNAs are diagrammed in Figure 1.
Table 1.
Related properties of circRNAs
| Categories | Properties |
|---|---|
| Classification | exonic circRNA (ecircRNA) |
| intronic RNA (ciRNA) | |
| exonic-intronic circRNA (EIciRNA) | |
| circRNA produced by circularization of viral RNA genome, tRNA, rRNA and snRNA | |
| antisense circRNA originated from antisense transcripts | |
| Biogenesis | exon-skipping or lariat-driven circularization |
| direct back-splicing or intron-pairing-driven circularization | |
| RNA-binding-protein-driven circularization | |
| direct circularization of lariat introns | |
| circularization driven by tRNA splicing | |
| circularization driven by rRNA splicing | |
| Features | high abundance and incredible diversity |
| high stability | |
| evolutionary conservation | |
| tissue-specific expression | |
| specificity related with developmental-stage or -age expression | |
| competing endogenous RNAs or miRNA mediated activities | |
| ecircRNA mainly exists in the cytoplasm or exosomes, while EIciRNA and ciRNA mainly exist in the nucleus | |
| Biofunctions | miRNA sponges |
| RBP sponges | |
| gene transcription and expression regulators | |
| protein/peptide translators | |
| others |
Figure 1.
Zhang et al. Representative models for the production of circRNA [25]. A. Direct circularization of lariat introns. Canonical linear splicing generates a lariat structure. The 3’ downstream of the lariat intron is trimmed to form a circular intronic RNA (ciRNA). B. Circularization driven by lariat mechanism. The exon-skipping event during alternative splicing promotes the 3’ splice site (3’SS) of the exon to covalently splice to the 5’SS. C. Circularization mediated by intron pairing. Intron pairing brings the appropriate splice signals within proximity of each other, which promotes circularization. D. Circularization mediated by RNA-binding proteins (RBPs). RBPs bring the appropriate splice signals within proximity of each other, which promotes circularization. E. Circularization driven by tRNA splicing. F. Circularization driven by rRNA splicing.
Classification of circRNAs
CircRNAs can be classified into five groups according to its biogenesis pattern [5,24-27] (Table 1): (1) ecircRNA, composed of only exons; (2) retained-intron circRNA or EIciRNA, composed of introns at the region between exons; (3) ciRNA, composed of only introns; (4) circRNA produced by circularization of viral RNA genome, tRNA, rRNA and snRNA; (5) antisense circRNA originated from antisense transcripts. Among circRNAs, ecircRNAs are the most, accounting for more than 80% of identified circRNAs. In addition, circRNA can be divided into intragenic circRNA and intergenic circRNA according to the parental gene location of circRNA.
Features of circRNAs
Increasing evidence revealed several highlighted characteristics of circRNAs [9,12,23,27] (Table 1): (1) abundance and diversity: more than 20,000 different circRNAs have been identified in eukaryotes; (2) stability: circRNAs presented with more stable property than linear mRNAs due to their covalently closed loop structures which confer them resistant to RNase R; (3) conservation: circRNAs are highly conserved in different species, such as humans, mice, nematodes, zebrafish, drosophila, protists, and plants; (4) location: ecircRNA mainly exists in the cytoplasm or exosomes, while EIciRNA and ciRNA mainly exist in the nucleus; (5) specificity: circRNAs often exhibit tissue and developmental-stage specific expression; (6) some circRNAs contain miRNA binding sites and can competitively attenuate endogenous miRNA-mediated activities; (7) the sequence conservativeness of ecircRNA was higher than that of ciRNA and intergenic circRNA.
Biofunctions of circRNAs
At present circRNA has several putative functions as follows [16,24,28] (Table 1 and Figure 2): (1) miRNA sponges, circRNAs can bind to miRNA as RNA sponge and increase downstream gene expression by regulating miRNA activities; (2) gene transcription and expression regulators, circRNAs that are considered as a type of alternative splicing isoforms may play a key role in regulating gene expression, leading to cancer related dysregulation. EIciRNAs and ciRNAs may regulate transcription and expression in the nucleus while ecircRNAs in the cytoplasm; (3) RBP sponges, circRNAs may bind to and sequester RBPs via their conserved seed matches, resulting in the formation of large RNA-protein complexes and affecting translation; (4) protein/peptide translators; (5) other undiscovered roles.
Figure 2.
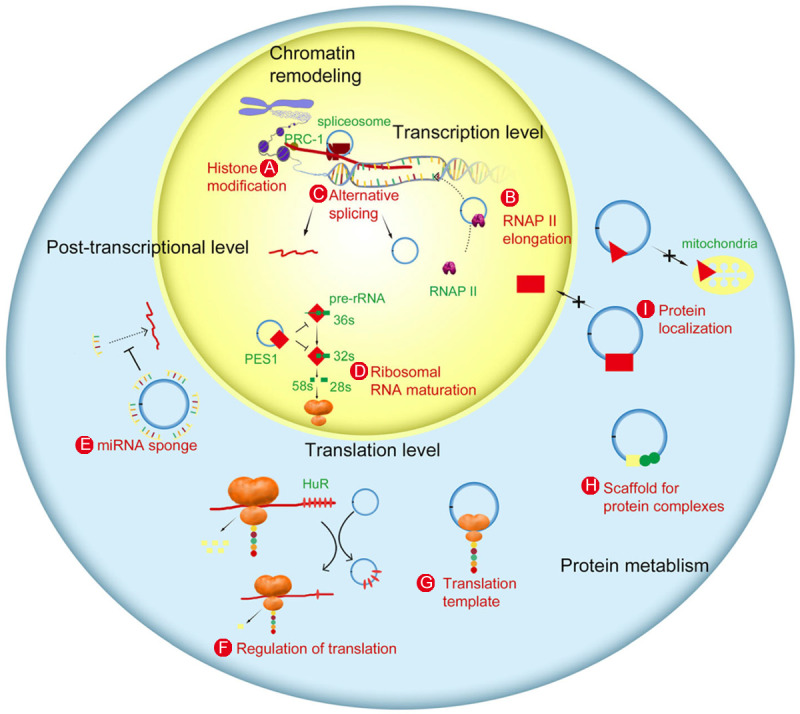
Zhang et al. The biological functions of circRNA [25]. CircRNAs can impact genetic output at almost every stage of a gene’s life cycle-from epigenetic regulation to transcriptional and posttranscriptional control to translational control. A. Histone modification; B. RNAP II elongation; C. Alternative splicing; D. RNA maturation; E. miRNA sponge; F. Translation regulation; G. Translation; H. Scaffold for proteins; I. Protein localization.
Here, we focus on interpreting the protein translation potential of circRNA. In general, circRNA is considered untranslatable. The first natural circRNA found able to encode protein is the genome of hepatitis virus back to 1980’s [29]. Raising studies have demonstrated the potential of circRNAs in proteins translation [30]. Several circRNAs with protein coding ability were listed in Table 2. Chen et al. [31] showed that the eukaryotic ribosome can initiate translation mechanism on circRNA when the circRNA structure contains internal ribosome entry site. It has been confirmed that circ-ZNF609 can be translated into protein functioning in myogenesis [32]. CircMbl3 is found to be translated in a splicing-dependent but cap-independent way in fly head extracts [33]. N6-methyladenosine can promote the initiation of protein translation from circRNA in human cells, and a single N6-methyladenosine residue in circRNA is sufficient to drive the translation, and suggests a role of circRNA-derived proteins in cellular responses to environmental stress [16]. Additionally, study on human glioma cell lines U251 and U373 has displayed a novel protein (FBXW7-185aa) encoded from circ-FBXW7, which contributes to inhibit glioma tumorigenesis [34]; and other two novel tumor suppressive proteins (SHPRH-146aa and PINT87aa) encoded respectively by circ-SHPRH and circPINTexon2 have been identified in glioblastoma [17,35]. Moreover, computational analysis on sequencing of human transcriptomes has revealed the universal existence of circRNAs with coding potential, which provide a new direct for the functional studies of circRNAs. Meng et al. [36] have introduced an integrated tool to detect circRNAs with protein coding potential from high-throughput sequencing data, thus facilitating the investigation of circRNA translation as well as novel functions of circRNAs and circRNA-derived proteins. Until now, some circRNA-related databases were gradually constructed. For example, the circRNADb database (http://reprod.njmu.edu.cn/circrnadb/circRNADb.php) reveals in more detail whether endogenous circRNA can encode functional proteins in mammalian cells [37]; the circBank database (http://www.circbank.cn) exhibits 140783 circRNAs have protein coding potential. On the other hand, Bartsch et al. [38] had detailed a sucrose gradient-based method to evaluate the coding potential of candidate circRNAs (or any transcript of interest) and its association with the translation machinery. Collectively, recent advances have proved the protein coding ability of circRNAs and abnormal translation of circRNAs may contribute to human diseases including tumors. The protein coding potency of circRNA will expand its territory for application and improve its clinical value.
Table 2.
CircRNAs with protein translation function
Exosomal circRNAs
Exosomes are nanoscale extracellular vesicles of endocytic origin secreted by most types of cells and circulate in bodily fluids such as blood, urine, saliva, and breast milk [39]. Exosomes have emerged as critical mediators of intercellular communication in both physiological and pathological processes including cancer progression [40,41]. Li et al. [42] first reported the enrichment and stability of abundant circRNAs in exosomes compared to the progenitor cells by using RNA-sequence analyses. Recently, circRNAs have also been reported to be enriched and stable in saliva, plasma, and even in some exosomes derived from serum, urine and tumor [42,43], suggesting the potential of circRNAs as biomarkers. Moreover, based on the characteristics of exosomes, exosomes as therapeutic vectors began to be used in the treatment of tumors [44], suggesting the possibility of circRNA as a therapeutic target. Going forward, the application prospects of exosomal circRNAs are bright.
Using high-throughput sequencing and qRT-PCR, Yang et al. [45] identified three differentially expressed (DE) exosomal circRNAs including has_circ_007293, has_circ_031752 and has_circ_020135 in serum from patients with papillary thyroid carcinoma (PTC) compared with a benign thyroid goiter. Li et al. [46] found that exosomal circ-IARS expression in pancreatic ductal adenocarcinoma (PDAC) tissues and in plasma exosomes of patients with metastatic disease was positively correlated with liver metastasis, vascular invasion, and tumor-node-metastasis (TNM) stage and negatively correlated with postoperative survival time. Li et al. [47] also found that exosomal circ-PDE8A expression in plasma was associated with progression and prognosis in PDAC patients and circ-PDE8A promotes the invasive growth of PDAC cells via miRNA-338/MACC1/MET pathway. In addition, circPTGR1 was upregulated in serum exosomes from hepatocellular carcinoma (HCC) patients and was associated with the clinical stage and prognosis. Next experiments uncovered that exosomal circPTGR1 promotes HCC metastasis via miRNA449a/MET pathway [48]. Zhang et al. [49] found that exosomal circRNA ciRS-133 derived from gastric cancer could promote white adipose browning through targeting the miRNA-133/PRDM16 pathway. The above results imply that the presence of circRNAs in exosomes may be important indicator for early diagnosis and prognostic prediction in many cancers. Currently, accumulating evidence suggests that exosomal circRNAs can modulate cellular proliferation, invasion, migration, tumor metastasis and drug resistance through certain mechanisms such as targeting signaling pathways or sponging miRNAs [39,46,48-50] (Table 3). Up to now, the exoRBase database (http://www.exoRBase.org) was constructed and contains 58 330 circRNAs, 15 501 lncRNAs and 18 333 mRNAs in human blood exosomes [51]. Although only a few of circRNAs have established functional roles or clinical applications, exosomal circRNAs are a novel frontier in cancer research. More and more exosomal circRNAs have been identified and applied for cancer drug delivery system, cancer therapy and clinical treatment evaluation [52-54].
Table 3.
Exosomal circRNAs in cancers
| CircRNA | Location | Cancer | Mechanism | Function | Reference |
|---|---|---|---|---|---|
| has_circ_007293 | serum | PTC | - | - | [45] |
| has_circ_031752 | serum | PTC | - | - | [45] |
| has_circ_020135 | serum | PTC | - | - | [45] |
| circ-IARS | Plasma | PADC | Targets miRNA-122, ZO-1, RhoA, RhoA-GTP, and F-actin | Promotes tumor metastasis, increases endothelial monolayer permeability | [46] |
| circ-PDE8A | Plasma | PADC | Targets miRNA-338/MACC1/MET pathway | Promotes cell invasive growth | [47] |
| circPTGR1 | Serum | HCC | Targets miRNA449a/MET pathway | Promotes tumor metastasis | [48] |
| ciRS-133 | Plasma | GC | Targets miRNA-133/PRDM16 pathway | Promotes white adipose browning | [49] |
Note: -, Not retrieved. PTC: Papillary thyroid carcinoma; PADC: Pancreatic ductal adenocarcinoma; HCC: Hepatocellular carcinoma; GC: Gastric cancer.
CircRNAs and head and neck cancers
Recent studies have indicated that circRNAs may have vital roles during the development and progression of multiform types of cancers including HNC [12,55]. CircRNAs have become a novel area of interest in the early diagnosis and therapy of cancers due to their abundance, high stability and notable regulatory functions, and the enrichment and stability of exosomal circRNAs in body fluids, and the association with tumor chemoradiation. Herein, we review the relationship between circRNAs and HNC, and summarize the roles of circRNAs and their possible biological mechanisms in different HNC.
CircRNAs and nasopharyngeal carcinoma
Using the qRT-PCR, Shuai et al. [56] examined the expression level of circRNA_0000285 in 150 nasopharyngeal carcinoma (NPC) and 100 adjacent tissues, 150 serum samples from NPC patients and 100 serum samples from healthy controls. They found that circRNA_0000285 was significantly increased in NPC tissues and serum samples from patients with NPC, and significantly associated with tumor size, differentiation, cervical lymph node metastasis, distant metastasis and TNM stage. Additionally, univariate and multivariate analyses indicated that circRNA_0000285 may be a novel prognostic biomarker for NPC. Using the human circRNA microarray, Chen et al. [57] found that circRNA_000543 expression was upregulated in NPC tissues, and in tissues from patients with poorer overall survival. The following investigation demonstrated that circRNA_000543 knockdown sensitized NPC cells by targeting miRNA-9/platelet-derived growth factor receptor B axis. So circRNA_000543 may be a potential therapeutic target for NPC. Ke et al. [58] found that circHIPK3 was highly expressed in NPC tissues and cell lines and circHIPK3 depletion dramatically repressed tumor growth and metastasis in vivo. Next experiments revealed that circHIPK3 facilitated NPC progression through protecting ELF3 from miRNA-4288-mediated silencing. In brief, NPC and its associated circRNAs are shown in Table 4.
Table 4.
Nasopharyngeal carcinoma and its associated circRNAs
| CircRNA | Sample | Expression | Mechanism | Function | Reference |
|---|---|---|---|---|---|
| circRNA_000543 | Tissue | Up | Sponges miRNA-9, targets PDGFRB | Radioresistant, poorer overall survival | [57] |
| circRNA_0000285 | Tissue and serum | Up | - | Associated with tumor size, differentiation, metastasis, and TNM stage, independent prognostic factor, radiosensitivity | [56] |
| circHIPK3 | Tissue and cell | Up | Sponges miRNA-4288, targets ELF3 | Promotes cell proliferation and invasion | [58] |
On the other hand, researchers have identified EBV-encoded circRNAs, such as EBV circBARTs and ebv_circ_RPMS1. They deemed that EBV circBARTs might contribute to viral oncogenesis and ebv_circ_RPMS1 may be a novel viral regulator of host and/or viral gene expression [59,60]. The circRNAs originated from EBV and their functions and potential clinical applications in NPC need to be explored in the future.
CircRNAs and laryngeal cancer
Using microarray analysis, Xuan et al. [4] showed that 698 circRNAs were DE in laryngeal squamous cell carcinoma (LSCC) tissues, including 302 upregulated and 396 downregulated circRNA transcripts. Using qRT-PCR method, high expression of hsa_circ_100855 and low expression of hsa_circ_104912 are associated with T3-4 stage, lymph node metastasis, and advanced clinical stage of LSCC. Based on the microarray and bioinformatics analyses, Fan et al. [61] identified 506 DE circRNAs from LSCC and normal laryngeal mucosa tissues, and predicted that hsa_circ_0044520 and hsa_circ_0044529 play important regulatory roles by sponging hsa-miRNA-4726-5p and hsa-miRNA-4640-5p in the tumorigenesis of LSCC. Combined with qPCR methods, Gao et al. [62] confirmed that 382 circRNAs were DE in miRNA-145-5p overexpressed LSCC cells and revealed that miRNA-145-5p may be a core of the competing endogenous RNA (ceRNA) network to inhibit the LSCC progression. Lu et al. [63] detected 29 circRNAs were significantly upregulated and 19 circRNAs were significantly downregulated in the LSCC tissues by RNA-sequencing, then qRT-PCR validation result indicated that hsa_circ:chr20:31876585-31897648 may be a novel promising tumor suppresser in LSCC. Wu et al. [64] revealed that circRNA hg19_circ_0005033 promotes proliferation, migration and invasion of CD133+CD44+ laryngeal cancer (LC) stem cells, implicating circRNAs are associated with cancer immunity regulation. Zhang et al. [65] found that LSCC patients with high TNM stages, poorly differentiated tumors, lymph node metastases and poor prognosis had high ciRS-7 (CDR1as) expression level but low miRNA-7 level. In vitro and in vivo studies demonstrated that CDR1as is an oncogene, which promotes LSCC progression by regulating miRNA-7 signal, and suggested that CDR1as/miRNA-7 pathway was a key pathway in LSCC progression. Subsequent reports also shed light on the roles and mechanisms of several circRNAs in LC. Hsa_circ_0023028 functions as an miRNA-194-5p sponge to promote the proliferation, migration and invasion of LC cells [66]; upregulation of circFLNA contributes to LSCC migration by targeting circFLNA-miRNA-486-3p-FLNA axis [67]; circMYLK serves as an oncogene to promote cancer progression via miRNA-195/cyclin D1 axis in LSCC [68]. Collectively, LC and its associated circRNAs are displayed in Table 5.
Table 5.
Laryngeal cancer and its associated circRNAs
| CircRNA | Sample | Expression | Mechanism | Function | Reference |
|---|---|---|---|---|---|
| hsa_circRNA_100855 | Tissue | Up | - | Associated with T3-4 stage, lymph node metastasis, and later clinical stage | [4] |
| hsa_circRNA_104912 | Tissue | Down | - | Associated with T3-4 stage, lymph node metastasis, poor differentiation, and later clinical stage | [4] |
| hsa_circ_0044520 | Tissue | Up | Sponges hsa-miRNA-4726-5p | Collagen synthesis | [61] |
| hsa_circ_0044529 | Tissue | Up | Sponges hsa-miRNA-4640-5p | Collagen synthesis | [61] |
| hg19_circ_0005033 | Cell | Up | - | Proliferation, migration, invasion; chemotherapy resistance | [64] |
| ciRS-7(CDR1as) | Tissue and cell | Up | Sponges miRNA-7, targets ki-67, CCNE1 and PIK3CD | Promotes tumor growth | [65] |
| hsa_circ_0023028 | Tissue and cell | Up | Sponges miRNA-194-5p | Promotes cell proliferation, migration, and invasion | [66] |
| circFLNA | Tissue and cell | Up | Sponges miRNA-486-3p, targets cyclin D1 | Promotes cell migration, poor survival | [67] |
| circMYLK | Tissue and cell | Up | Sponges miRNA-195 | Promotes cell proliferation and cell cycle transition | [68] |
Taken together, aforesaid studies indicate that circRNAs may play important roles in the development and progression of LSCC and may be helpful for the diagnosis and prognosis of this disease. These results may provide a potential therapeutic target for the treatment of LSCC.
CircRNAs and oral cancer
Using high-throughput sequencing technology or circRNA microarray analysis for human oral squamous cell carcinoma (OSCC), many circRNAs were DE between OSCC tissues and adjacent tissues [69-71]. Subsequently, a series of works confirmed some circRNAs play key roles in tumor development and progression of oral cancer (Table 6). CircRNA_100290 serves as a ceRNA to counteract miRNA-378a-mediated GLUT1 suppression, thus promoting glycolysis and cell proliferation in OSCC [72]. CircRNA_0109291 regulates cell growth and migration in OSCC and is associated with prognosis of OSCC patients [73]. Moreover, circDOCK1 regulates BIRC3 expression through competitively binding to miRNA-196a-5p as a ceRNA, and participates in the process of apoptosis of OSCC cells [74]. Li et al. [69] explored the regulatory role of the hsa_circ_0008309-miRNA-136-5p/hsa-miRNA-382-5P-ATXN1 network in OSCC and identified that hsa_circ_0008309 may inhibit miRNA-136-5p and miRNA-382-5p expression and increase ATXN1 expression in the OSCC cell lines. Su et al. [71] determined that upregulation of hsa_circ_0007059 suppresses cell growth, migration and invasion, and facilitates apoptosis of OSCC cells. Meanwhile, hsa_circ_0007059 was determined to affect malignant behavior via AKT/mTOR signaling pathway. Sun et al. [75] showed that hsa_circ_001242 was significantly downregulated in OSCC and may act as a potential novel biomarker for the diagnosis and treatment of OSCC. Su et al. [70] found that hsa_circ_0005379 expression is significantly lower in OSCC tissue compared to paired non-cancerous tissue and is associated with tumor size and differentiation. Next experiments manifested that hsa_circ_0005379 may be regulate OSCC malignancy through the EGFR pathway and may be a new therapeutic target for OSCC.
Table 6.
Oral cancer and its associated circRNAs
| CircRNA | Sample | Expression | Mechanism | Function | Reference |
|---|---|---|---|---|---|
| has_circRNA_100290 | Tissue | Up | Sponges miRNA-378a, targets GLUT1 | Promotes glycolysis and cell proliferation | [72] |
| circRNA_0109291 | Tissue and cell | Up | - | Promotes cell proliferation, migration and apoptosis, poorer prognosis | [73] |
| circDOCK1 | Cell and tissue | Up | Sponges miRNA-196a-5p, targets BIRC3 | Suppresses cell apoptosis | [74] |
| hsa_circ_0008309 | Tissue and cell | Down | Sponges miRNA-136-5P and miRNA-382-5P, targets ATXN1 | Correlated with pathological differentiation | [69] |
| hsa_circ_0001874 | Saliva | Up | - | Correlated with TNM stage and tumor grade | [76] |
| hsa_circ_0001971 | Saliva | Up | - | Correlated with TNM stage | [76] |
| hsa_circ_0007059 | Tissue and cell | Down | Targets AKT/mTOR pathway | Promotes cell growth, migration, invasion, and suppresses cell apoptosis | [71] |
| hsa_circ_001242 | Tissue and cell | Down | - | Negatively correlated with tumor size and T stage | [75] |
| hsa_circ_0005379 | Tissue and cell | Down | Targets EGFR pathway | Associated with tumor size and differentiation; drug sensitivity | [70] |
Additionally, Zhao et al. [76] obtained 32 dysregulated cricRNAs in the saliva from the OSCC patients by microarray. Among these DE circRNAs, the expression levels of salivary hsa_circ_0001874 and hsa_circ_0001971 were correlated with the TNM stage, severity of oral mucosal lesions and outcome of surgical treatment. This result indicated the potential of salivary hsa_circ_0001874 and hsa_circ_0001971 as new biomarkers for the diagnosis of OSCC.
CircRNAs and tongue cancer
By high-throughput sequencing, Qiu et al. [77] revealed 322 DE circRNAs in tongue squamous cell carcinoma (TSCC) tissue. Then RT-PCR results showed that circRNA expression in TSCC tissue was higher than that in adjacent tissue. Bioinformatics analyses indicated that the DE circRNAs might promote the development and progression of TSCC.
CircRNAs and hypopharyngeal cancer
Cao et al. [78] discovered that 2392 circRNAs are DE between hypopharyngeal squamous cell carcinoma (HSCC) and adjacent normal tissues by microarray. Of the circRNAs, 1304 are upregulated, including hsa_circ_0024108, hsa_circ_0058106 and hsa_circ_0058107, while 1088 are downregulated, including hsa_circ_0001189, hsa_circ_0002260 and hsa_circ_0036722. The functions of these circRNAs in HSCC have not been well characterized. Feng et al. [79] demonstrated that 173 circRNAs were DE between HSCC and adjacent normal tissues by circRNA sequencing, including 71 upregulated and 102 downregulated circRNAs. And they also demonstrated that a ceRNA subnetwork, consisting of two circRNAs (hsa_circ_0008287 and hsa_circ_0005027) and one miRNA (has-miRNA-548c-3p), which significantly affects both ErbB and Hippo signaling pathways (Table 7).
Table 7.
Hypopharyngeal cancer and its associated circRNAs
CircRNAs and thyroid cancer
Compared with normal thyroid tissues, 88 significantly upregulated circRNAs and 10 downregulated circRNAs were found in PTC tissues. Compared with benign thyroid lesions, 129 circRNAs and 226 circRNAs were significantly upregulated and downregulated in PTC tissues. Further overlap analysis suggested that hsa_circRNA_100395/miRNA-141-3p/miRNA-200a-3p axis may be involved in the pathogenesis of PTC [80]. Based on circRNA, miRNA and mRNA databases, Liu et al. [81] constructed a circRNA-miRNA-hubgene subnetwork of PTC including the 2 DE circRNAs, 3 DE miRNAs, and 4 DE mRNAs. These results indicated ceRNAs are the key regulator in the pathogenesis of PTC. However, this hypothesis needs further verification. Next, Ren et al. [82] have also found 206 up- and 177 downregulated circRNAs in PTC tissues by microarray. Their study suggested that PTC-related hsa_circRNA_047771 and hsa_circRNA_007148 may serve as potential diagnostic biomarkers and prognostic predictors for PTC patients. Furthermore, a series of studies have identified that a number of thyroid cancer-related circRNAs play a critical role in PTC pathogenesis and progression. Circ_0067934 could improve the development of PTC by promoting epithelial-mesenchymal-transition (EMT) and PI3K/AKT signaling pathways [83]. CircRNA_102171 overexpression promotes PTC progression through activating Wnt/β-catenin pathway in a CTNNBIP1-dependent way [84]. Decreased expression of hsa_circ_0137287 predicts aggressive clinicopathologic characteristics in PTC [85]. CircRNA circZFR exerted oncogenic roles via regulating miRNA-1261/C8orf4 axis in PTC, which suggested circZFR might be a potential therapeutic target [86]. CircRNA circ-ITCH suppresses PTC progression through miRNA-22-3p/CBL/β-catenin pathway [87]. CircBACH2 was highly expressed in PTC tissues and PTC cell lines, and the circBACH2/miRNA-139-5p/LMO4 axis could be targeted as a potential treatment strategy for PTC [88]. Moreover, growing evidence shows that certain circRNAs act as a crucial ceRNA contribute to the tumorigenesis and progression of PTC, such as circ_0039411, circ_0058124, circ_0025033, circ_0008274, circNUP214, circ_0004458 and circRNA_NEK6 [14,89-94]. In summary, thyroid cancer and its associated circRNAs are exhibited in Table 8.
Table 8.
Thyroid cancer and its associated circRNAs
| CircRNA | Sample | Expression | Mechanism | Function | Reference |
|---|---|---|---|---|---|
| circ_0067934 | Tissue and cell | Up | Targets EMT and PI3K/AKT pathways | Poor prognosis, promotes cell proliferation, migration, and invasion and inhibits apoptosis | [83] |
| circRNA_102171 | Tissue and cell | Up | Targets CTNNBIP1-dependent Wnt/β-catenin pathway | Promotes cell proliferation, migration and invasion while inhibits apoptosis | [84] |
| hsa_circ_0137287 | Tissue | Down | - | Correlated with aggressive clinicopathologic characteristics | [85] |
| circZFR | Tissue | Up | Sponges miRNA-1261, targets C8orf4 | Promotes cell proliferation, migration and invasion | [86] |
| circ-ITCH | Tissue | Down | Sponges miRNA-22-3p, targets CBL/β-catenin pathway | Promotes cell proliferation and invasion and suppresses apoptosis | [87] |
| circBACH2 | Tissue and cell | Up | Sponges miRNA-139-5p, targets LMO4 | Promotes cell proliferation, migration, and invasion | [88] |
| circ_0039411 | Tissue and cell | Up | Sponges miRNA-1179 and miRNA-1205 | Promotes tumorigenesis and progression | [93] |
| circ_0058124 | Tissue and cell | Up | Sponges miRNA-218-5p, targets NUMB | Promotes tumorigenesis and invasiveness | [94] |
| circ_0025033 | Tissue and cell | Up | Sponges miRNA-1231 and miRNA-1304 | Promotes cell proliferation and invasion | [92] |
| circ_0008274 | Tissue and cell | Up | Targets AMPK/mTOR pathway | Promotes cell proliferation and invasion | [14] |
| circNUP214 | Tissue and cell | Up | Sponges miRNA-145, targets ZEB2 | Promotes cell proliferation, invasion, migration, and tumorigenesis | [91] |
| circ_0004458 | Tissue and cell | Up | Sponges miRNA-885-5p, targets RAC1 | Promotes cell proliferation and suppresses cell cycle arrest and apoptosis | [90] |
| circRNA_NEK6 | Tissue and cell | Up | Sponges miRNA-370-3p, targets FZD8 and Wnt pathway | Promotes cell growth and invasion | [89] |
CircRNAs and esophageal cancer
Firstly, profiling and bioinformatics analyses revealed many DE circRNAs in esophageal cancer (EC) [95-97], these results indicated that dysregulated circRNAs were involved in the tumorigenesis and progression of EC. Secondly, the circRNA-mediated interacted networks of esophageal squamous cell carcinoma (ESCC) were built, and the construction of network can facilitate a better understanding of circRNA-related mechanisms in ESCC [2,96,98]. Finally, several dysregulated circRNAs were confirmed that they play important roles in ESCC. Li et al. [99] reported that cir-ITCH expression was usually low in ESCC compared to the peritumoral tissue and cir-ITCH acted as sponge of miRNA-7, miRNA-17 and miRNA-214. Their reports also indicate that cir-ITCH may have an inhibitory effect on ESCC by regulating the Wnt/β-catenin pathway. Study by Xia et al. has verified circ_0067934 is upregulated in ESCC tissue and promotes ESCC cell proliferation [100]. Dysregulation of circRNA_100876 expression leads to poor prognosis in ESCC by accelerating cell proliferation and metastasis [101]. CircRNA ciRS-7 was significantly upregulated in the ESCC tissues and cells, and can trigger the growth, migration, invasion and metastasis of ESCC cells via miRNA-7/KLF4 & NF-κB signals, or miRNA-7/HOXB13 & NF-κB signaling pathways or miRNA-876-5p/MAGE-A family axis [102-104]. Further functional experiments indicated targeted inhibition of ciRS-7 might be a potential approach for ESCC treatment. Studies have also identified that upregulated circ_0000337 and circ_0006168 promote cell proliferation, migration and invasion of ESCC [105,106]. Wang et al. [107] determined that circ0043898 is presented as tumor inhibitor and could be a candidate biomarker in the therapeutic target and diagnosis of EC. Furthermore, recent works revealed that upregulated circ-TTC17 while downregulated circ-SMAD7 play a key role in affecting the proliferation and migration of ESCC cells [108,109]. Additionally, He et al. [109] determined that circVRK1 was downregulated in ESCC tissues and cell lines, and identified that circVRK1 suppressed ESCC progression by regulating miRNA-624-3p/PTEN axis and PI3K/AKT signaling pathway. Altogether, above findings suggest that the deregulated expression of circRNAs is involved in the development and progression of EC through certain molecular mechanisms including miRNA sponges and regulation of pathways (Table 9).
Table 9.
Esophageal cancer and its associated circRNAs
| CircRNA | Sample | Expression | Mechanism | Function | Reference |
|---|---|---|---|---|---|
| cir-ITCH | Tissue | Down | Sponges miRNA-7, miRNA-17, miRNA-214, targets Wnt/β-catenin pathway | Inhibits tumor progression | [99] |
| has_circ_0067934 | Tissue | Up | - | Poor differentiation and lower TNM stage, promotes proliferation and migration | [100] |
| circRNA_100876 | Tissue and cell | Up | - | Poor prognosis, promotes cell proliferation and metastasis | [101] |
| ciRS-7 | Tissue and cell | Up | Sponges miRNA-7, miRNA-876-5p, targets KLF4, HOXB13, MAGE-A, and NF-κB pathways | Promotes cell growth, migration, invasion, and metastasis | [102-104] |
| hsa_circ_0000337 | Tissue and cell | Up | Sponges miRNA-670-5p | Promotes cell proliferation, migration and invasion | [105] |
| hsa_circ_0006168 | Tissue and cell | Up | Sponges miRNA-100, targets rapamycin (mTOR) | Promotes cell proliferation, migration and invasion | [106] |
| circ0043898 | Tissue | Down | Targets histone H3 and BMI1 | Inhibits cell proliferation, migration and invasion and induces cell apoptosis and death | [107] |
| circVRK1 | Tissue and cell | Down | Sponges miRNA-624-3p, targets PTEN/PI3K/AKT pathway | Tumor suppression, radioresistance | [110] |
| circ-TTC17 | Tissue, cell and plasma | Up | Sponges miRNAs | Promotes cell proliferation and migration | [108] |
| circ-SMAD7 | Plasma and tissue | Down | - | Promotes cell proliferation and migration | [109] |
CircRNAs in chemoradiation resistance of HNC
As we know, the emergence of chemoradiation resistance can lead to treatment failure and poor prognosis. At present, studies have found altered circRNA expression in radioresistant or chemoresistant tumors, such as EC, breast cancer, glioma, osteosarcoma, colorectal cancer, cervical cancer, prostate cancer, pancreatic cancer and non-small cell lung cancer, etc. [95,111-120]. These results indicate possible involvement of these dysregulated circRNAs in the development of chemoradiation resistance. Through biological analysis, some circRNAs have been found to influence the chemoradiation resistance of cancer cells by regulating specific genes or signaling pathways [121-126].
Previous studies have also reported the deregulation of circRNAs were closely associated with chemoradiation resistance of HNC. Su et al. [95] first detected 57 upregulated circRNAs and 17 downregulated circRNAs in human radioresistant EC cell line, this suggested aberrant expression of circRNAs might be associated with the radioresistance of EC. Shuai et al. [56] found that circRNA_0000285 expression was significantly increased in patients with radioresistant NPC compared with patients with radiosensitive NPC. Chen et al. [57] found that circRNA_000543 expression was higher in radioresistant NPC samples than in radiosensitive NPC samples. Further investigations demonstrated that circRNA_000543 can target miRNA-9/platelet-derived growth factor receptor B axis. These works indicated that circRNA_0000285 and circRNA_000543 may be involved in NPC radiosensitivity. Wu et al. [64] revealed DE circRNAs in CD133+CD44+ LC stem cells, and identified that circRNA hg19_circ_0005033 promotes cisplatin-chemotherapy resistance of LC stem cells. Su et al. [70] found that hsa_circ_0005379 expression level was significantly downregulated in OSCC tissue, while overexpression of hsa_circ_0005379 could significantly enhance the sensitivity of OSCC to the cetuximab drug in vitro and in vivo.
Although there is still very little study regarding circRNAs and chemoradiation resistance in HNC, it has a great potential that circRNAs can be used as novel biomarkers to predict the efficiency of chemoradiation and prognosis or recurrence in radiation or chemotherapy-resistant cancers. Moreover, some circRNAs will become the novel therapeutic targets for HNC patients with chemoradiation resistance.
Conclusions
Overall, the data elucidate the physiological and pathological roles of numerous circRNAs in human diseases, especially tumors. However, there remain a number of circRNAs to be investigated and specific molecular mechanisms to be explored. Since 2012, prostate cancer antigen 3 has be recognized as the first lncRNA biomarker by the FDA approval for clinical diagnosis of prostate cancer, efforts are being made to find more effective tumor markers. Compared with lncRNAs and miRNAs, circRNAs has the advantage of high stability, which can be regarded as promising clinical biomarkers for diagnosis, prognosis and therapy. In addition, since the first report of abundant presence of circRNAs in exosomes and the detection of exosomal circRNAs from cancer cells, the studies of circRNAs are coming into a new sight. Exosomal circRNAs may participate in the processes of cell growth, angiogenesis and EMT and be applied for targeted therapy of cancers. Exosomal circRNAs are likely to be regarded as a new class of exosome-based cancer biomarkers and to play potential biological function in cancer development and progression. Hence, the accurate detection of exosomal circRNAs and the effective development of treatment vector of exosomal circRNAs are two urgent programs for cancer patients.
To date, increasing studies have demonstrated that certain circRNAs were translated in vivo, and the coding potency of circRNAs may attribute to human diseases. However, we need overwhelming evidence to uncover the protein coding function of circRNAs, and to better understand the biological mechanism of circRNAs in related diseases, including tumors. This discovery gives circRNAs a new function and provides a new direction for research of circRNA in the future.
In conclusion, although the roles of circRNAs in HNC have just begun to be revealed, the potential application of circRNAs as diagnostic biomarkers and therapeutic targets will bring a board prospect for the biotherapy of HNC patients.
Acknowledgements
This work was supported by the Natural Science Fund of Fujian Province China, (No. 2017J01374).
Disclosure of conflict of interest
None.
References
- 1.Pezzuto F, Buonaguro L, Caponigro F, Ionna F, Starita N, Annunziata C, Buonaguro FM, Tornesello ML. Update on head and neck cancer: current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology. 2015;89:125–136. doi: 10.1159/000381717. [DOI] [PubMed] [Google Scholar]
- 2.Jiang C, Xu D, You Z, Xu K, Tian W. Dysregulated circRNAs and ceRNA network in esophageal squamous cell carcinoma. Front Biosci. 2019;24:277–290. doi: 10.2741/4717. [DOI] [PubMed] [Google Scholar]
- 3.Wang WL, Yang Z, Zhang YJ, Lu P, Ni YK, Sun CF, Liu FY. Competing endogenous RNA analysis reveals the regulatory potency of circRNA_036186 in HNSCC. Int J Oncol. 2018;53:1529–1543. doi: 10.3892/ijo.2018.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xuan L, Qu L, Zhou H, Wang P, Yu H, Wu T, Wang X, Li Q, Tian L, Liu M, Sun Y. Circular RNA: a novel biomarker for progressive laryngeal cancer. Am J Transl Res. 2016;8:932–939. [PMC free article] [PubMed] [Google Scholar]
- 5.Eger N, Schoppe L, Schuster S, Laufs U, Boeckel JN. Circular RNA splicing. Adv Exp Med Biol. 2018;1087:41–52. doi: 10.1007/978-981-13-1426-1_4. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao KY, Sun HS, Tsai SJ. Circular RNA-new member of noncoding RNA with novel functions. Exp Biol Med. 2017;242:1136–1141. doi: 10.1177/1535370217708978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 9.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 11.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 12.Han YN, Xia SQ, Zhang YY, Zheng JH, Li W. Circular RNAs: a novel type of biomarker and genetic tools in cancer. Oncotarget. 2017;8:64551–64563. doi: 10.18632/oncotarget.18350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: an important part of circRNA maintenance and function. J Neurosci Res. 2020;98:87–97. doi: 10.1002/jnr.24356. [DOI] [PubMed] [Google Scholar]
- 14.Zhou GK, Zhang GY, Yuan ZN, Pei R, Liu DM. Has_circ_0008274 promotes cell proliferation and invasion involving AMPK/mTOR signaling pathway in papillary thyroid carcinoma. Eur Rev Med Pharmacol Sci. 2018;22:8772–8780. doi: 10.26355/eurrev_201812_16644. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Cao Q, Liu J, Zhang J, Li B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer. 2019;18:16. doi: 10.1186/s12943-018-0936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begum S, Yiu A, Stebbing J, Castellano L. Novel tumour suppressive protein encoded by circular RNA, circ-SHPRH, in glioblastomas. Oncogene. 2018;37:4055–4057. doi: 10.1038/s41388-018-0230-3. [DOI] [PubMed] [Google Scholar]
- 18.Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. CircRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Xue W, Li X, Zhang J, Chen S, Zhang JL, Yang L, Chen LL. The biogenesis of nascent circular RNAs. Cell Rep. 2016;15:611–624. doi: 10.1016/j.celrep.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Holdt LM, Kohlmaier A, Teupser D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell Mol Life Sci. 2018;75:1071–1098. doi: 10.1007/s00018-017-2688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Liang W, Zhang P, Chen J, Qian H, Zhang X, Xu W. Circular RNAs: emerging cancer biomarkers and targets. J Exp Clin Cancer Res. 2017;36:152. doi: 10.1186/s13046-017-0624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, Li H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017;14:992–999. doi: 10.1080/15476286.2016.1220473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Lu T, Wang Q, Liu J, Jiao W. Circular RNAs: crucial regulators in the human body. Oncol Rep. 2018;40:3119–3135. doi: 10.3892/or.2018.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kos A, Dijkema R, Arnberg AC, van der Meide PH, Schellekens H. The hepatitis delta (δ) virus possesses a circular RNA. Nature. 1986;323:558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- 30.Granados-Riveron JT, Aquino-Jarquin G. The complexity of the translation ability of circRNAs. Biochim Biophys Acta. 2016;1859:1245–1251. doi: 10.1016/j.bbagrm.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 32.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I. Circ-ZNF609 Is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S. Translation of circRNAs. Mol Cell. 2017;66:9–21. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, Huang N, Yang X, Zhao K, Zhou H, Huang S, Xie B, Zhang N. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110:304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P, Liu H, Xu J, Xiao F, Zhou H, Yang X, Huang N, Liu J, He K, Xie K, Zhang G, Huang S, Zhang N. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9:4475. doi: 10.1038/s41467-018-06862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng X, Chen Q, Zhang P, Chen M. CircPro: an integrated tool for the identification of circRNAs with protein-coding potential. Bioinformatics. 2017;33:3314–3316. doi: 10.1093/bioinformatics/btx446. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Han P, Zhou T, Guo X, Song X, Li Y. CircRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci Rep. 2016;6:34985. doi: 10.1038/srep34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartsch D, Zirkel A, Kurian L. Characterization of circular RNAs (circRNA) associated with the translation machinery. Methods Mol Biol. 2018;1724:159–166. doi: 10.1007/978-1-4939-7562-4_13. [DOI] [PubMed] [Google Scholar]
- 39.Boriachek K, Islam MN, Moller A, Salomon C, Nguyen NT, Hossain MSA, Yamauchi Y, Shiddiky MJA. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. 2018;14 doi: 10.1002/smll.201702153. [DOI] [PubMed] [Google Scholar]
- 40.Groza M, Zimta AA, Irimie A, Achimas-Cadariu P, Cenariu D, Stanta G, Berindan-Neagoe I. Recent advancements in the study of breast cancer exosomes as mediators of intratumoral communication. J Cell Physiol. 2020;235:691–705. doi: 10.1002/jcp.29096. [DOI] [PubMed] [Google Scholar]
- 41.Yang XX, Sun C, Wang L, Guo XL. New insight into isolation, identification techniques and medical applications of exosomes. J Control Release. 2019;308:119–129. doi: 10.1016/j.jconrel.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fanale D, Taverna S, Russo A, Bazan V. Circular RNA in exosomes. Adv Exp Med Biol. 2018;1087:109–117. doi: 10.1007/978-981-13-1426-1_9. [DOI] [PubMed] [Google Scholar]
- 44.Xie X, Wu H, Li M, Chen X, Xu X, Ni W, Lu C, Ni R, Bao B, Xiao M. Progress in the application of exosomes as therapeutic vectors in tumor-targeted therapy. Cytotherapy. 2019;21:509–524. doi: 10.1016/j.jcyt.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Yang C, Wei Y, Yu L, Xiao Y. Identification of altered circular RNA expression in serum exosomes from patients with papillary thyroid carcinoma by high-throughput sequencing. Med Sci Monit. 2019;25:2785–2791. doi: 10.12659/MSM.915658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, Liu H, Bi H, Liu X, Li X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res. 2018;37:177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Yanfang W, Li J, Jiang P, Peng T, Chen K, Zhao X, Zhang Y, Zhen P, Zhu J, Li X. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi: 10.1016/j.canlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Liu W, Zou Y, Wang G, Deng Y, Luo J, Zhang Y, Li H, Zhang Q, Yang Y, Chen G. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine. 2019;40:432–445. doi: 10.1016/j.ebiom.2018.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng T, Yang H, Sun W, Wang X, Zhu K, Fan Q, Li J, Ying G, Ba Y. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. 2019;144:2501–2515. doi: 10.1002/ijc.31977. [DOI] [PubMed] [Google Scholar]
- 50.Dai X, Chen C, Yang Q, Xue J, Chen X, Sun B, Luo F, Liu X, Xiao T, Xu H, Sun Q, Zhang A, Liu Q. Exosomal circRNA_100284 from arsenite-transformed cells, via microRNA-217 regulation of EZH2, is involved in the malignant transformation of human hepatic cells by accelerating the cell cycle and promoting cell proliferation. Cell Death Dis. 2018;9:454. doi: 10.1038/s41419-018-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y, Zheng Q, Li Y, Wang P, He X, Huang S. ExoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46:D106–D112. doi: 10.1093/nar/gkx891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenner AW, Su GH, Momen-Heravi F. Isolation of extracellular vesicles for cancer diagnosis and functional studies. Methods Mol Biol. 2019;1882:229–237. doi: 10.1007/978-1-4939-8879-2_21. [DOI] [PubMed] [Google Scholar]
- 53.Ketabat F, Pundir M, Mohabatpour F, Lobanova L, Koutsopoulos S, Hadjiiski L, Chen X, Papagerakis P, Papagerakis S. Controlled drug delivery systems for oral cancer treatment-current status and future perspectives. Pharmaceutics. 2019;11:302. doi: 10.3390/pharmaceutics11070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theodoraki MN, Yerneni S, Gooding WE, Ohr J, Clump DA, Bauman JE, Ferris RL, Whiteside TL. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology. 2019;8:e1593805. doi: 10.1080/2162402X.2019.1593805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao X, Cai Y, Xu J. Circular RNAs: biogenesis, mechanism, and function in human cancers. Int J Mol Sci. 2019;20:3926. doi: 10.3390/ijms20163926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shuai M, Hong J, Huang D, Zhang X, Tian Y. Upregulation of circRNA_0000285 serves as a prognostic biomarker for nasopharyngeal carcinoma and is involved in radiosensitivity. Oncol Lett. 2018;16:6495–6501. doi: 10.3892/ol.2018.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L, Zhou H, Guan Z. CircRNA_000543 knockdown sensitizes nasopharyngeal carcinoma to irradiation by targeting miR-9/platelet-derived growth factor receptor B axis. Biochem Biophys Res Commun. 2019;512:786–792. doi: 10.1016/j.bbrc.2019.03.126. [DOI] [PubMed] [Google Scholar]
- 58.Ke Z, Xie F, Zheng C, Chen D. CircHIPK3 promotes proliferation and invasion in nasopharyngeal carcinoma by abrogating miR-4288-induced ELF3 inhibition. J Cell Physiol. 2019;234:1699–1706. doi: 10.1002/jcp.27041. [DOI] [PubMed] [Google Scholar]
- 59.Huang JT, Chen JN, Gong LP, Bi YH, Liang J, Zhou L, He D, Shao CK. Identification of virus-encoded circular RNA. Virology. 2019;529:144–151. doi: 10.1016/j.virol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Toptan T, Abere B, Nalesnik MA, Swerdlow SH, Ranganathan S, Lee N, Shair KH, Moore PS, Chang Y. Circular DNA tumor viruses make circular RNAs. Proc Natl Acad Sci U S A. 2018;115:E8737–E8745. doi: 10.1073/pnas.1811728115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan Y, Xia X, Zhu Y, Diao W, Zhu X, Gao Z, Chen X. Circular RNA expression profile in laryngeal squamous cell carcinoma revealed by microarray. Cell Physiol Biochem. 2018;50:342–352. doi: 10.1159/000494010. [DOI] [PubMed] [Google Scholar]
- 62.Gao W, Zhang Y, Niu M, Bo Y, Li H, Xue X, Lu Y, Zheng X, Tang Y, Cui J, He L, Thorne RF, Wang B, Wu Y. Identification of miR-145-5p-centered competing endogenous RNA network in laryngeal squamous cell carcinoma. Proteomics. 2019;19:e1900020. doi: 10.1002/pmic.201900020. [DOI] [PubMed] [Google Scholar]
- 63.Lu C, Shi X, Wang AY, Tao Y, Wang Z, Huang C, Qiao Y, Hu H, Liu L. RNA-Seq profiling of circular RNAs in human laryngeal squamous cell carcinomas. Mol Cancer. 2018;17:86. doi: 10.1186/s12943-018-0833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Zhang Y, Niu M, Shi Y, Liu H, Yang D, Li F, Lu Y, Bo Y, Zhang R, Li Z, Luo H, Cui J, Sang J, Xiang C, Gao W, Wen S. Whole-transcriptome analysis of CD133+CD144+ cancer stem cells derived from human laryngeal squamous cell carcinoma cells. Cell Physiol Biochem. 2018;47:1696–1710. doi: 10.1159/000490992. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Hu H, Zhao Y, Zhao Y. CDR1as is overexpressed in laryngeal squamous cell carcinoma to promote the tumour’s progression via miR-7 signals. Cell Prolif. 2018;51:e12521. doi: 10.1111/cpr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Su X, Zhu C, Zhou J. Knockdown of hsa_circ_0023028 inhibits cell proliferation, migration, and invasion in laryngeal cancer by sponging miR-194-5p. Biosci Rep. 2019;39:BSR20190177. doi: 10.1042/BSR20190177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang JX, Liu Y, Jia XJ, Liu SX, Dong JH, Ren XM, Xu O, Zhang HZ, Duan HJ, Shan CG. Upregulation of circFLNA contributes to laryngeal squamous cell carcinoma migration by circFLNA-miR-486-3p-FLNA axis. Cancer Cell Int. 2019;19:196. doi: 10.1186/s12935-019-0924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duan X, Shen N, Chen J, Wang J, Zhu Q, Zhai Z. Circular RNA MYLK serves as an oncogene to promote cancer progression via microRNA-195/cyclin D1 axis in laryngeal squamous cell carcinoma. Biosci Rep. 2019;39:BSR20190227. doi: 10.1042/BSR20190227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B, Wang F, Li X, Sun S, Shen Y, Yang H. Hsa_circ_0008309 may be a potential biomarker for oral squamous cell carcinoma. Dis Markers. 2018;2018:7496890. doi: 10.1155/2018/7496890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su W, Wang Y, Wang F, Sun S, Li M, Shen Y, Yang H. Hsa_circ_0005379 regulates malignant behavior of oral squamous cell carcinoma through the EGFR pathway. BMC Cancer. 2019;19:400. doi: 10.1186/s12885-019-5593-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Su W, Wang Y, Wang F, Zhang B, Zhang H, Shen Y, Yang H. Circular RNA hsa_circ_0007059 indicates prognosis and influences malignant behavior via AKT/mTOR in oral squamous cell carcinoma. J Cell Physiol. 2019;234:15156–15166. doi: 10.1002/jcp.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Yu J, Tian H, Shan Z, Liu W, Pan Z, Ren J. Circle RNA hsa_circRNA_100290 serves as a ceRNA for miR-378a to regulate oral squamous cell carcinoma cells growth via Glucose transporter-1 (GLUT1) and glycolysis. J Cell Physiol. 2019;234:19130–19140. doi: 10.1002/jcp.28692. [DOI] [PubMed] [Google Scholar]
- 73.Ouyang SB, Wang J, Zhao SY, Zhang XH, Liao L. CircRNA_0109291 regulates cell growth and migration in oral squamous cell carcinoma and its clinical significance. Iran J Basic Med Sci. 2018;21:1186–1191. doi: 10.22038/IJBMS.2018.30347.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Wei Y, Yan Y, Wang H, Yang J, Zheng Z, Zha J, Bo P, Tang Y, Guo X, Chen W, Zhu X, Ge L. CircDOCK1 suppresses cell apoptosis via inhibition of miR196a5p by targeting BIRC3 in OSCC. Oncol Rep. 2018;39:951–966. doi: 10.3892/or.2017.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun S, Li B, Wang Y, Li X, Wang P, Wang F, Zhang W, Yang H. Clinical significance of the decreased expression of hsa_circ_001242 in oral squamous cell carcinoma. Dis Markers. 2018;2018:6514795. doi: 10.1155/2018/6514795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao SY, Wang J, Ouyang SB, Huang ZK, Liao L. Salivary circular RNAs hsa_circ_ 0001874 and hsa_circ_0001971 as novel biomarkers for the diagnosis of oral squamous cell carcinoma. Cell Physiol Biochem. 2018;47:2511–2521. doi: 10.1159/000491624. [DOI] [PubMed] [Google Scholar]
- 77.Qiu X, Ke X, Ma H, Han L, Chen Q, Zhang S, Da P, Wu H. Profiling and bioinformatics analyses reveal differential expression of circular RNA in tongue cancer revealed by high-throughput sequencing. J Cell Biochem. 2019;120:4102–4112. doi: 10.1002/jcb.27695. [DOI] [PubMed] [Google Scholar]
- 78.Cao S, Wei D, Li X, Zhou J, Li W, Qian Y, Wang Z, Li G, Pan X, Lei D. Novel circular RNA expression profiles reflect progression of patients with hypopharyngeal squamous cell carcinoma. Oncotarget. 2017;8:45367–45379. doi: 10.18632/oncotarget.17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng C, Li Y, Lin Y, Cao X, Li D, Zhang H, He X. CircRNA-associated ceRNA network reveals ErbB and Hippo signaling pathways in hypopharyngeal cancer. Int J Mol Med. 2019;43:127–142. doi: 10.3892/ijmm.2018.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng N, Shi L, Zhang Q, Hu Y, Wang N, Ye H. Microarray profiling of circular RNAs in human papillary thyroid carcinoma. PLoS One. 2017;12:e0170287. doi: 10.1371/journal.pone.0170287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Q, Pan LZ, Hu M, Ma JY. Molecular network-based identification of circular RNA-associated ceRNA network in papillary thyroid cancer. Pathol Oncol Res. 2020;26:1293–1299. doi: 10.1007/s12253-019-00697-y. [DOI] [PubMed] [Google Scholar]
- 82.Ren H, Liu Z, Liu S, Zhou X, Wang H, Xu J, Wang D, Yuan G. Profile and clinical implication of circular RNAs in human papillary thyroid carcinoma. PeerJ. 2018;6:e5363. doi: 10.7717/peerj.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, Yan X, Zhang H, Zhan X. CircRNA circ_0067934 overexpression correlates with poor prognosis and promotes thyroid carcinoma progression. Med Sci Monit. 2019;25:1342–1349. doi: 10.12659/MSM.913463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bi W, Huang J, Nie C, Liu B, He G, Han J, Pang R, Ding Z, Xu J, Zhang J. CircRNA circRNA_102171 promotes papillary thyroid cancer progression through modulating CTNNBIP1-dependent activation of beta-catenin pathway. J Exp Clin Cancer Res. 2018;37:275. doi: 10.1186/s13046-018-0936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lan X, Cao J, Xu J, Chen C, Zheng C, Wang J, Zhu X, Zhu X, Ge M. Decreased expression of hsa_circ_0137287 predicts aggressive clinicopathologic characteristics in papillary thyroid carcinoma. J Clin Lab Anal. 2018;32:e22573. doi: 10.1002/jcla.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei H, Pan L, Tao D, Li R. Circular RNA circZFR contributes to papillary thyroid cancer cell proliferation and invasion by sponging miR-1261 and facilitating C8orf4 expression. Biochem Biophys Res Commun. 2018;503:56–61. doi: 10.1016/j.bbrc.2018.05.174. [DOI] [PubMed] [Google Scholar]
- 87.Wang M, Chen B, Ru Z, Cong L. CircRNA circ-ITCH suppresses papillary thyroid cancer progression through miR-22-3p/CBL/beta-catenin pathway. Biochem Biophys Res Commun. 2018;504:283–288. doi: 10.1016/j.bbrc.2018.08.175. [DOI] [PubMed] [Google Scholar]
- 88.Cai X, Zhao Z, Dong J, Lv Q, Yun B, Liu J, Shen Y, Kang J, Li J. Circular RNA circBACH2 plays a role in papillary thyroid carcinoma by sponging miR-139-5p and regulating LMO4 expression. Cell Death Dis. 2019;10:184. doi: 10.1038/s41419-019-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen F, Feng Z, Zhu J, Liu P, Yang C, Huang R, Deng Z. Emerging roles of circRNA_NEK6 targeting miR-370-3p in the proliferation and invasion of thyroid cancer via Wnt signaling pathway. Cancer Biol Ther. 2018;19:1139–1152. doi: 10.1080/15384047.2018.1480888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jin X, Wang Z, Pang W, Zhou J, Liang Y, Yang J, Yang L, Zhang Q. Upregulated hsa_circ_0004458 contributes to progression of papillary thyroid carcinoma by inhibition of miR-885-5p and activation of RAC1. Med Sci Monit. 2018;24:5488–5500. doi: 10.12659/MSM.911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Tian Y, Hu Y, Yang Z, Zhang L, Luo J. CircNUP214 sponges miR-145 to promote the expression of ZEB2 in thyroid cancer cells. Biochem Biophys Res Commun. 2018;507:168–172. doi: 10.1016/j.bbrc.2018.10.200. [DOI] [PubMed] [Google Scholar]
- 92.Pan Y, Xu T, Liu Y, Li W, Zhang W. Upregulated circular RNA circ_0025033 promotes papillary thyroid cancer cell proliferation and invasion via sponging miR-1231 and miR-1304. Biochem Biophys Res Commun. 2019;510:334–338. doi: 10.1016/j.bbrc.2019.01.108. [DOI] [PubMed] [Google Scholar]
- 93.Yang Y, Ding L, Li Y, Xuan C. Hsa_circ_0039411 promotes tumorigenesis and progression of papillary thyroid cancer by miR-1179/ABCA9 and miR-1205/MTA1 signaling pathways. J Cell Physiol. 2020;235:1321–1329. doi: 10.1002/jcp.29048. [DOI] [PubMed] [Google Scholar]
- 94.Yao Y, Chen X, Yang H, Chen W, Qian Y, Yan Z, Liao T, Yao W, Wu W, Yu T, Chen Y, Zhang Y. Hsa_circ_0058124 promotes papillary thyroid cancer tumorigenesis and invasiveness through the NOTCH3/GATAD2A axis. J Exp Clin Cancer Res. 2019;38:318. doi: 10.1186/s13046-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su H, Lin F, Deng X, Shen L, Fang Y, Fei Z, Zhao L, Zhang X, Pan H, Xie D, Jin X, Xie C. Profiling and bioinformatics analyses reveal differential circular RNA expression in radioresistant esophageal cancer cells. J Transl Med. 2016;14:225. doi: 10.1186/s12967-016-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun J, Yuan X, Li X, Wang D, Shan T, Wang W, Wan Q, Wang X, Yan J, Gao S. Comparative transcriptome analysis of the global circular RNAs expression profiles between SHEE and SHEEC cell lines. Am J Transl Res. 2017;9:5169–5179. [PMC free article] [PubMed] [Google Scholar]
- 97.Shi P, Sun J, He B, Song H, Li Z, Kong W, Wang J, Wang J, Xue H. Profiles of differentially expressed circRNAs in esophageal and breast cancer. Cancer Manag Res. 2018;10:2207–2221. doi: 10.2147/CMAR.S167863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo L, Zheng L, Zhao Y, Wang Q. Profiling and bioinformatic analyses indicate differential circRNA and miRNA/isomiR expression and interactions. Biomed Res Int. 2018;2018:8518563. doi: 10.1155/2018/8518563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia W, Qiu M, Chen R, Wang S, Leng X, Wang J, Xu Y, Hu J, Dong G, Xu PL, Yin R. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci Rep. 2016;6:35576. doi: 10.1038/srep35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao S, Chen G, Yan L, Li L, Huang X. Contribution of dysregulated circRNA_100876 to proliferation and metastasis of esophageal squamous cell carcinoma. Onco Targets Ther. 2018;11:7385–7394. doi: 10.2147/OTT.S177524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang H, Wei L, Qin T, Yang N, Li Z, Xu Z. Circular RNA ciRS-7 triggers the migration and invasion of esophageal squamous cell carcinoma via miR-7/KLF4 and NF-kappaB signals. Cancer Biol Ther. 2019;20:73–80. doi: 10.1080/15384047.2018.1507254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li RC, Ke S, Meng FK, Lu J, Zou XJ, He ZG, Wang WF, Fang MH. CiRS-7 promotes growth and metastasis of esophageal squamous cell carcinoma via regulation of miR-7/HOXB13. Cell Death Dis. 2018;9:838. doi: 10.1038/s41419-018-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sang M, Meng L, Sang Y, Liu S, Ding P, Ju Y, Liu F, Gu L, Lian Y, Li J, Wu Y, Zhang X, Shan B. Circular RNA ciRS-7 accelerates ESCC progression through acting as a miR-876-5p sponge to enhance MAGE-A family expression. Cancer Lett. 2018;426:37–46. doi: 10.1016/j.canlet.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 105.Song H, Xu D, Shi P, He B, Li Z, Ji Y, Agbeko CK, Wang J. Upregulated circ RNA hsa_circ_0000337 promotes cell proliferation, migration, and invasion of esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:1997–2006. doi: 10.2147/CMAR.S195546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi Y, Guo Z, Fang N, Jiang W, Fan Y, He Y, Ma Z, Chen Y. Hsa_circ_0006168 sponges miR-100 and regulates mTOR to promote the proliferation, migration and invasion of esophageal squamous cell carcinoma. Biomed Pharmacother. 2019;117:109151. doi: 10.1016/j.biopha.2019.109151. [DOI] [PubMed] [Google Scholar]
- 107.Wang W, Ma J, Lu J, Fang D, Xiong X, Yang X, Xie T. Circ0043898 acts as a tumor inhibitor and performs regulatory effect on the inhibition of esophageal carcinoma. Cancer Biol Ther. 2018;19:1117–1127. doi: 10.1080/15384047.2018.1480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Q, Zhang Q, Sun H, Tang W, Yang L, Xu Z, Liu Z, Jin H, Cao X. Circ-TTC17 promotes proliferation and migration of esophageal squamous cell carcinoma. Dig Dis Sci. 2019;64:751–758. doi: 10.1007/s10620-018-5382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, Wang Q, Zhu D, Rong J, Shi W, Cao X. Up-regulation of circ-SMAD7 inhibits tumor proliferation and migration in esophageal squamous cell carcinoma. Biomed Pharmacother. 2019;111:596–601. doi: 10.1016/j.biopha.2018.12.116. [DOI] [PubMed] [Google Scholar]
- 110.He Y, Mingyan E, Wang C, Liu G, Shi M, Liu S. CircVRK1 regulates tumor progression and radioresistance in esophageal squamous cell carcinoma by regulating miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int J Biol Macromol. 2019;125:116–123. doi: 10.1016/j.ijbiomac.2018.11.273. [DOI] [PubMed] [Google Scholar]
- 111.Gao D, Zhang X, Liu B, Meng D, Fang K, Guo Z, Li L. Screening circular RNA related to chemotherapeutic resistance in breast cancer. Epigenomics. 2017;9:1175–1188. doi: 10.2217/epi-2017-0055. [DOI] [PubMed] [Google Scholar]
- 112.Xiong W, Ai YQ, Li YF, Ye Q, Chen ZT, Qin JY, Liu QY, Wang H, Ju YH, Li WH, Li YF. Microarray analysis of circular RNA expression profile associated with 5-fluorouracil-based chemoradiation resistance in colorectal cancer cells. Biomed Res Int. 2017;2017:8421614. doi: 10.1155/2017/8421614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kun-Peng Z, Xiao-Long M, Lei Z, Chun-Lin Z, Jian-Ping H, Tai-Cheng Z. Screening circular RNA related to chemotherapeutic resistance in osteosarcoma by RNA sequencing. Epigenomics. 2018;10:1327–1346. doi: 10.2217/epi-2018-0023. [DOI] [PubMed] [Google Scholar]
- 114.Xu N, Chen S, Liu Y, Li W, Liu Z, Bian X, Ling C, Jiang M. Profiles and bioinformatics analysis of differentially expressed circrnas in taxol-resistant non-small cell lung cancer cells. Cell Physiol Biochem. 2018;48:2046–2060. doi: 10.1159/000492543. [DOI] [PubMed] [Google Scholar]
- 115.Yu D, Li Y, Ming Z, Wang H, Dong Z, Qiu L, Wang T. Comprehensive circular RNA expression profile in radiation-treated HeLa cells and analysis of radioresistance-related circRNAs. PeerJ. 2018;6:e5011. doi: 10.7717/peerj.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abu N, Hon KW, Jeyaraman S, Yahaya A, Abdullah NM, Mustangin M, Sulaiman SA, Jamal R, Ab-Mutalib NS. Identification of differentially expressed circular RNAs in chemoresistant colorectal cancer. Epigenomics. 2019;11:875–884. doi: 10.2217/epi-2019-0042. [DOI] [PubMed] [Google Scholar]
- 117.Chen T, Luo J, Gu Y, Huang J, Luo Q, Yang Y. Comprehensive analysis of circular RNA profiling in AZD9291-resistant non-small cell lung cancer cell lines. Thorac Cancer. 2019;10:930–941. doi: 10.1111/1759-7714.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greene J, Baird AM, Casey O, Brady L, Blackshields G, Lim M, O’Brien O, Gray SG, McDermott R, Finn SP. Circular RNAs are differentially expressed in prostate cancer and are potentially associated with resistance to enzalutamide. Sci Rep. 2019;9:10739. doi: 10.1038/s41598-019-47189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao M, Xu J, Zhong S, Liu Y, Xiao H, Geng L, Liu H. Expression profiles and potential functions of circular RNAs in extracellular vesicles isolated from radioresistant glioma cells. Oncol Rep. 2019;41:1893–1900. doi: 10.3892/or.2019.6972. [DOI] [PubMed] [Google Scholar]
- 120.Shao F, Huang M, Meng F, Huang Q. Circular RNA signature predicts gemcitabine resistance of pancreatic ductal adenocarcinoma. Front Pharmacol. 2018;9:584. doi: 10.3389/fphar.2018.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y, Dong Y, Zhao L, Su L, Luo J. Circular RNAMTO1 suppresses breast cancer cell viability and reverses monastrol resistance through regulating the TRAF4/Eg5 axis. Int J Oncol. 2018;53:1752–1762. doi: 10.3892/ijo.2018.4485. [DOI] [PubMed] [Google Scholar]
- 122.Chi BJ, Zhao DM, Liu L, Yin XZ, Wang FF, Bi S, Gui SL, Zhou SB, Qin WB, Wu DM, Wang SQ. Downregulation of hsa_circ_0000285 serves as a prognostic biomarker for bladder cancer and is involved in cisplatin resistance. Neoplasma. 2019;66:197–202. doi: 10.4149/neo_2018_180318N185. [DOI] [PubMed] [Google Scholar]
- 123.Huang X, Li Z, Zhang Q, Wang W, Li B, Wang L, Xu Z, Zeng A, Zhang X, Zhang X, He Z, Li Q, Sun G, Wang S, Li Q, Wang L, Zhang L, Xu H, Xu Z. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18:71. doi: 10.1186/s12943-019-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sang Y, Chen B, Song X, Li Y, Liang Y, Han D, Zhang N, Zhang H, Liu Y, Chen T, Li C, Wang L, Zhao W, Yang Q. circRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther. 2019;27:1638–1652. doi: 10.1016/j.ymthe.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shang J, Chen WM, Wang ZH, Wei TN, Chen ZZ, Wu WB. CircPAN3 mediates drug resistance in acute myeloid leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp Hematol. 2019;70:42–54. e43. doi: 10.1016/j.exphem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y, Zhang J, Li J, Gui R, Nie X, Huang R. CircRNA_014511 affects the radiosensitivity of bone marrow mesenchymal stem cells by binding to miR-29b-2-5p. Bosn J Basic Med Sci. 2019;19:155–163. doi: 10.17305/bjbms.2019.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]