Abstract
In light-sensitive drug delivery systems, more and more nanoparticles were applied to load various drug molecules. However, few studies focused on their biomedical effects such as the regulation of heme oxygenase-1 (HO-1) expression and reactive oxygen species (ROS) generation which could influence cellular redox reaction. In the present article, through review of literature, analysis of high-throughput sequencing database, the mechanisms of drug delivery based on organic (poly-lactic-co-glycolic acid (PLGA) and polyethylene glycol (PEG)) and inorganic (Au, ZnO, SiO2 and TiO2) nanoparticles were introduced briefly. Besides, it was also expounded that nuclear factor-erythroid 2 (NF-E2)-related factor 2/BTB domain and CNC homolog 1 (Nrf2/Bach1) might be involved in the regulation of HO-1 and the quantum effect of photon altered ROS generation. The exogenous nanoparticles certainly have various biomedical effects that even affect the pathogenesis of some diseases like atopic dermatitis. Thus, biomedical effect of nanoparticles depends on HO-1/ROS needs further research.
Keywords: Drug delivery system, nanoparticles, HO-1, ROS, Nrf2, atopic dermatitis, microRNA
Introduction
In response to disease progression or certain functions/biorhythms of the organism, drug delivery systems (DDS) could release active molecules at theaccurate site with an appropriate rate is particularly appealing. Light-sensitive DDS is receiving increasing attention owing to the possibility of developing materials which sensitive to innocuous electromagnetic radiation (mainly in the ultraviolet (UV), visible and near-infrared range), and applied at well delimited sites. Thus, biocompatible materials sensitive to certain physiological variables or external physicochemical stimuli can be used for achieving this aim. Nanoparticles (NPs), owing to their unique physical/chemical properties, have gained tremendous attention for their potential biomedical and biological applications in light-sensitive DDS [1]. For example, silica NPs (SiO2-NPs), generally considered as biocompatible, and have been developed into DDS [2], as biosensors for intracellular pathogens [3,4] and as MRI contrast enhancers [5]. However, because nanomaterials can enter the human body through several ports, the widespread use and increased human exposure in NPs have raised concerns about their potentially toxic effect [6]. Like in the previous study, the biphasic effect of silver NP was observed in HepG2 cells, accelerating cell proliferation through p38-MAPK activation at low doses [7], but with cytotoxic effect at high concentrations [8]. Furthermore, various studies have demonstrated that NPs might result in thrombus formation, lung inflammation, oxidative stress and mechanical irritation of skin [1].
Among these, oxidative stress, which has lots of detrimental effects at high level such as damaging cellular structures and even leading to cell death through oxidation of macromolecules [9-12]. In Fernandez study [13], it has been shown that in liver, the PIBCA (poly-isobutyl cyanoacrylate, biodegradable) or PS (polystyrene, not biodegradable) NPs can induce oxidative stress locally via a depletion of reduced glutathione (GSH) and oxidized glutathione (GSSG) levels, as well as inhibition of superoxide dismutase (SOD) activity and a slight increase in catalase activity. Thus, it has to be stressed that long-term studies are needed to prove the safe use of NPs because chronic depletion of the anti-oxidant defense will lead to severe health problems.
As well know, abundant intracellular ROS can trigger the cellular defense process such as induction of antioxidative enzymes. HO-1, an important cellular antioxidative enzyme, could be induced by pharmacological agents as well as environmental elements like UV radiation and other cellular stress [14]. Abundant evidence has demonstrated that the common pathway for regulating HO-1 mainly depends on several redox-dependent transcriptional activators, such as Nrf2/Kelch-like ECH-associated protein 1 (Keap1), NF-κB, and AP-1, along with the transcriptional repressor Bach1 [15]. Notably, Bach1, as a repressor, has a stronger affinity than Nrf2 with the antioxidant response element (ARE) of HO-1. Only Bach1 was exported from nuclear, Nrf2 could bind to the HO-1 promoter and activate gene expression [16].
In present review, our knowledge in this field mainly comes from drug delivery (pharmaceutical research) and toxicology (xenobiotics) studies. To address the problems depicted earlier, we try to analyze the basis of the light-sensitive DDS and illustrate the mechanism of regulation of HO-1/ROS according to some selected experimental results. In addition, the properties, biocompatibility and action mechanism of NPs are also briefly introduced. Finally, some suspicious points about the NPs regulation of HO-1/ROS would be discussed in detail. Nanomaterials are used more and more widely and their biological effects should not be ignored. Thus, summarizing the advantages and disadvantages of nanomaterials is a benefit and significant for further research about them.
The mechanism of organic NPs in light-sensitive DDS
The DDS based on organic NPs has various manners to deliver drugs. A key mechanism is based on the photodynamic effects of the photosensitizer (PS). In brief, 400-690 nm light irradiation transforms the PS to the singlet state. Through inter-system crossing, in the irradiated area, PS then relaxes to triplet state which could generate ROS [17,18]. Although the generated ROS is very aggressive [19,20], it is also required for cleaving chemical bonds in DDS. Thus in common DDS nanostructures, PS might be encapsulated for converting luminous energy to ROS to trigger drug release. For example, in vitro, fullerene C60 could generate ROS after 532 nm light irradiation, conjugated ROS-sensitive thioketal bond would be ruptured and allowed 43% loaded doxorubicin to be released (Figure 1) [21].
Figure 1.
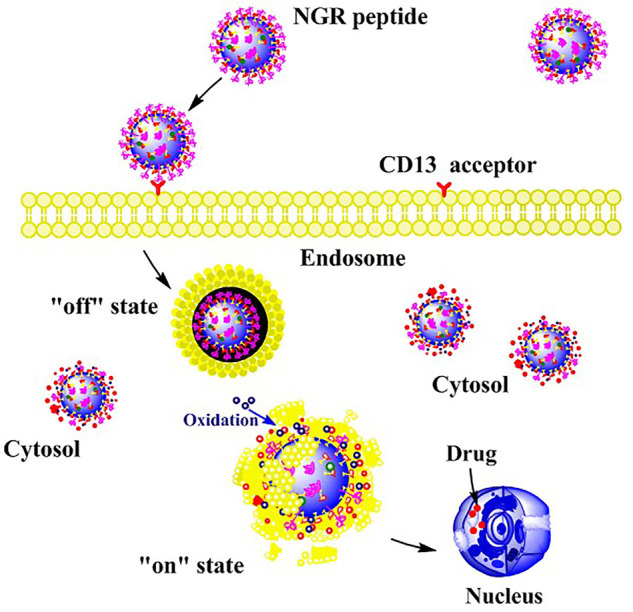
Schematic diagram light-sensitive DDS based on fullerene C60 nanoparticles. Fullerene C60-based nanoparticles modified with NGR peptide were taken up by CD13-expressing tumor cells. Under the acidic conditions of the endosome, these nanoparticles existed in an “off” state that could not release doxorubicin. However, in vivo, they were converted to “on” state when triggered by light exposure of a specific wavelength (532 nm).
However, because of scattering and light absorption of lipids and water, shortwave radiation (<700 nm) can’t penetrate deeper than 1 cm in tissues [22,23]. Thus, the innocuous near-infrared (NIR) light (650~900 nm) has been used for triggering drug delivery in deeper living tissues [24-26]. Although NIR is more penetrative, it has not enough energy to excite PS to an arousal state. In this context, up-conversion nanoparticles (UCNP), a NIR-to-UV/visible light switch was applied in DDS since it could convert several absorbed low-energy photons to emit higher-energy ones [27,28]. In Chen study [29], a DDS comprises PEG, was also linked with UCNPs make 75% drug release following 10 mins exposure to 980 nm radiation in vitro. It was also reported that in vivo, intravenous injection of DDS (AB3-NPs) to medullary thyroid cancer cell TT-xenografted mice inhibited tumor growth more effectively than the control group. In conclusion, the mechanism of the photochemical activatable system which enables remotely controlled drug release comprised of organic NPs commonly generates ROS and material whose chemical bonds are susceptible to light or ROS [29].
Liposomes, consist of concentric bilayers of phospholipids and/or other amphiphilic molecules, forming nano-sized vesicles by aqueous compartments. Despite liposomes were widely used in DDS, the optimal system based on liposomes is still a challenge. In most cases, the liposome-DDS trigger drug delivery mainly depends on passive diffusion, so that the required local drug concentrations for the optimum therapeutic effect might not be reached [30]. Therefore, it is imperative to find new approaches that could trigger rapid drug release upon the liposome arrival to the desired site [31]. Most attention was focused on the liposome structure destabilization caused by external energy despite the mechanism are complicated. External energy could influence the photo-polymerization of membrane lipids, induce photo-isomerization, photo-oxidation (by ROS) or photo-deprotection [32]. For example, liposomes with plasmenylcholine could convert to hexagonal phase from lamellar phase under 630-820 nm light [33]. ROS from PS induced the photo-oxidation of the plasmalogen vinyl ether linkage and then the plasmenylcholine was decomposed to lysolipids and fatty aldehydes, following phase transition and the leakage of the intraliposomal content [30,34]. The character and quantity of PS determine the rate of photo-initiated drug delivery. For example, bacteriochlorophyll α, a NIR sensitizer, could lead to 100% calcein release in less than 20 min after 800 nm irradiation. The observed release rate was twofold faster than the control DDS without PS [33]. Recently, biotechnology of photochemical activated DDS has basically mature. Even different NPs and PS molecules could generate ROS in vivo, the specific mechanism is still unclear [21,29]. Certainly, there are also other manners for light-sensitive DDS, such as light-sensitive chemistry/bonds, but there were few studies indicated that such materials could regulate HO-1 or ROS.
The organic NPs regulation of HO-1/ROS
As we have known, in DDS based on organic NPs, the ROS generation would be due to the irradiated PS. When ROS in cells was increased, various pathways migh regulate HO-1 expression. However, Yadav [35] has discovered an interesting phenomenon in the rat model with a traumatic injury. Liposome-encapsulated hemoglobin (LEH), an artificial oxygen carrier to address post-hemorrhage oxygen deficit, was post-inserted into the conjugated HDAS-PEG2K to generate HDAS-PEG2K-LEH. After treating with HDAS-PEG2K-LEH injection over 4 weeks, immunohistochemical staining for rat HO-1 did not show an induced expression of HO-1 in lung, liver, spleen, and kidney. It might suggest that neither liposome nor PEG2K could regulate the expression of HO-1 in these organs. In addition, Abdel [36] also confirmed that in the cavernous tissue of aged rats, after receiving lipofectamine, the gene expression and enzyme activity of HO-1 had an insignificant difference. It may just because liposomes are biocompatible and in Yadav and Abdel studies, there was no PS, the key factor for ROS generation.
However, also as the main nanomaterials, Poly-D, L-lactide-co-glycolide (PLGA) may have a different effect on HO-1 expression. In Giovanni study, two different melatonin NP carriers, PLGA and PLGA-PEG, were prepared to be used in experimental animal model of sepsis. After treating, oxidative stress was measured in tissue homogenates by detecting HO-1 expression, total thiol groups and lipid hydroperoxides (LOOH). In all the examined organs, all the melatonin formulations restored total thiol group levels insignificantly. Both melatonin NP formulation significantly decreased LOOH levels when compared with sepsis vehicle animals. The PLGA-PEG-melatonin was able to reduced LOOH levels more significantly in the heart, lung, and liver when compared with PLGA. Meanwhile no significant changes were observed between two NP formulations in the kidney. Interestingly, HO-1 expression was differently affected following treatment with various melatonin formulations. The PLGA-PEG-melatonin formulation was more effective in inducing HO-1 protein compared with free melatonin, with the exception of the kidney. A similar phenomenon was also discovered in Amin study [37]. Anthocyanins, an unstable hydrophilic drug, was encapsulated in biodegradable NP formulation based on PLGA and PEG as stabilizer. In SH-SY5Y cell lines, the drug with PLGA-PEG had a stronger ability for scavenging free radical and abrogating ROS generation via the p38-MAPK/JNK pathways accompanied by induction of endogenous Nrf2 and HO-1. However, there was another point suggested that formulation with PEG has a stronger effect just because it could allow the drugs to avoid the macrophages uptake. However, in Yadav’s study, there was no difference in HO-1 expression between HDAS-PEG2K-LEH and the control group. Therefore, the regulation of HO-1/ROS from PLGA or PEG is complicated and needs further study. Moreover, though liposome is the widely applied organic nanomaterial, few studies have verified it can regulate ROS generation or HO-1 expression. Oppositely, in Yusuf study [38], liposomal encapsulation of Ag-NP could suppress the Ag-NP-induced ROS and enhance its cytotoxicity against THP1-differentiated macrophages and unexpectedly suppressed reduced glutathione (GSH) levels (P<0.05) resulting in a redox imbalance.
The mechanism of inorganic NPs in light-sensitive DDS
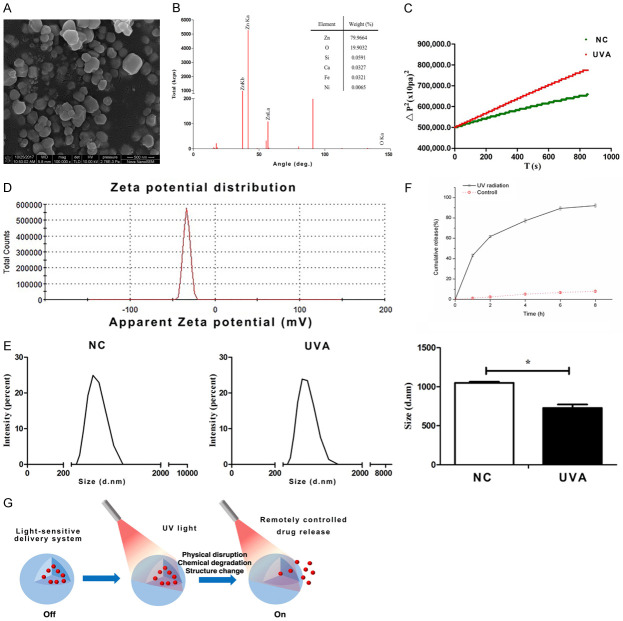
In addition to organic materials, some inorganic nanomaterials were also used to light-sensitive DDS. On the one hand, the electron-rich photothermal agents (e.g., gold NPs [39], carbon nanotubes [40] and graphene nanosheets [41,42]) can transduce NIR to vibrational energy, result in local hyperthermia which could dismantle a carrier with thermosensitive components [43]. On the other hand, some nanomaterials release drugs via changing hydrophilia (Figure 2). In our studies, we detected that ZnO could combine with AKBA (anti-oxidative drugs) originally due to its hydrophobicity. After UVA irradiation (200 KJ/m2), the hydrophilia of ZnO-NPs was increased (the contact angle was decreased) and resulted in drug release. It was also confirmed that in an aqueous solution, after UVA irradiation, the diameter of ZnO-NPs was significantly decreased. Perhaps because of the increased hydrophilia, ZnO could be more dispersed in aqueous. In one word, all results determined the AKBA was released from ZnO-NPs mainly due to the changed hydrophilia.
Figure 2.
UVA-controlled ZnO DDS. A. SEM-image of ZnO-NPs. B. Analysis of element composition by FTIR. C. The contact angle of ZnO. D. Zeta potential distribution of ZnO. E. The diameter of ZnO-NPs. F. The UV-controlled release behavior of AKBA. G. The schematic of the UVA-sensitive drug release system.
Furthermore, some other NPs (SiO2, TiO2) could be readily modified with chemical groups which could generate new functionalities for application in DDS [2,44]. For example, SiO2-NPs (70 nm) were covalently conjugated with the photoactive o-nitrobenzyl bromide on surface via amino groups [45]. Thus, drugs with phosphate, hydroxy or carboxylic groups could be attached to the o-nitrobenzyl bromide covalently. Once nanocomposites undergo 310 nm irradiation, the o-nitrobenzyl bromide groups convert to o-nitrobenzaldehyde, the covalent bond would be cleaved and lead to drug delivery. In addition, another synthesis manner of nanocomposite used azobenzene chains as either impellers or nanovalves. In Lu study [46], it was demonstrated that once cancer cells contact the DDS with 413 nm irradiation, the drug molecular (camptothecin) would be released via activating the trans-to-cis photoisomerization of the azobenzene moieties. TiO2 (diameter, 1-100 nm), could also be modified for application in DDS because of the absorption of UV. Song [47] hasreported that amphiphilic TiO2 nanotubes were used to provide a highly controllable drug release system based on a hydrophobic cap (monolayer of OPDA) which can be removed by UV-induced chain scission (Figure 3). The most significant bio-character of inorganic NPs is their mini volume and penetratingability, enabling external control of the intracellular drug delivery.
Figure 3.
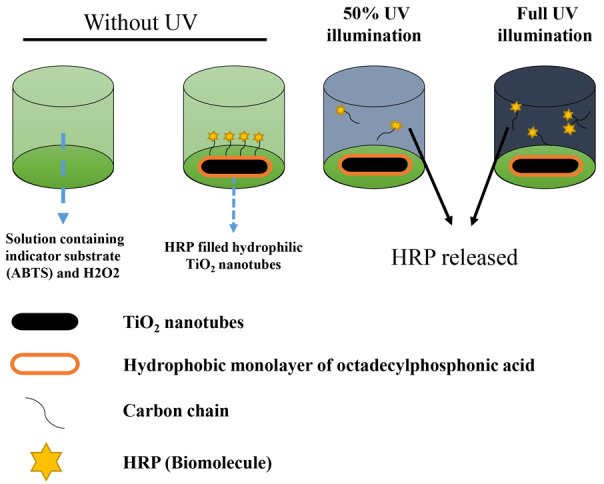
The schematic diagram of TiO2 drug delivery system after UV illumination.
The inorganic particles regulation of HO-1/ROS
Au
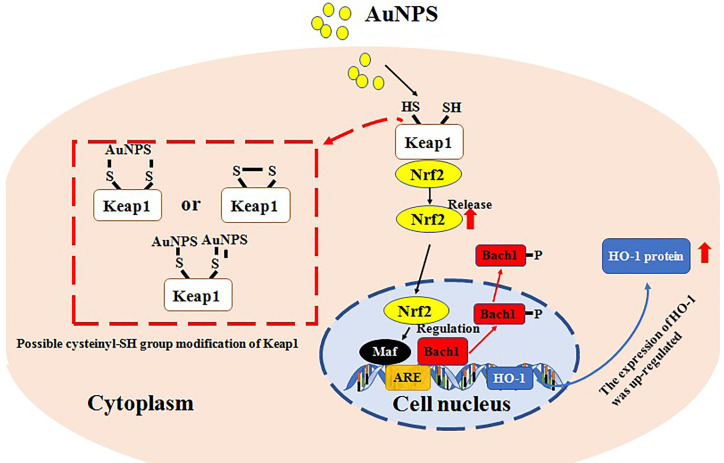
Although the mechanism of inorganic NPs participate in DDS is various and complicated, abundance studies have identified once NPs are contacting with cells or ingested by cells, the expression of HO-1 and generation of ROS might be regulated. According to Lai study [48], in human vascular endothelial cells (ECs), gold NPs (Au-NPs) could induce HO-1 mRNA and protein expression in a concentration- and time-dependent manner via Nrf2 and Bach1. It is noteworthy that the Au-NPs enhanced Nrf2 protein levels in nuclear, not via inducing Nrf2 expression but accelerated the cytosolic Nrf2 translocation to the nucleus, and, concomitantly, Bach1 exited the nucleus followed by the increased tyrosine phosphorylation. By chromatin immunoprecipitation assay, it was revealed that the translocated Nrf2 bound to ARE which located in the E2 enhancer region of the HO-1 gene promoter. Meanwhile, it was revealed Au-NPs might not promote intracellular ROS production or endoplasmic reticulum stress in the ECs. It suggested some NPs not always regulate HO-1 or ROS simultaneously (Figure 4). Interestingly, in Chakraborty study [49], an opposite phenomenon was detected. In osteosarcoma cell line, via thorough mechanistic study, size-dependent apoptotic activity of Au-NPs was confirmed. Increasing concentrations of Au-NPs of 46 nm size, enhanced the rate of ROS-induced apoptosis in osteosarcoma cells by disrupting their mitochondrial membrane potential. Considerably higher cell death was observed for 46 and 60 nm Au-NPs compared to 38 nm at all concentrations of 200, 400 and 800 ng/mL. Therefore, size-dependent apoptotic of NPs should be pay attention to and reflection.
Figure 4.
The proposed mechanism of action of the AuNPs in causing hO-1 expression in human vascular endothelial cells. Abbreviations: are, antioxidant-response element; AuNPs, gold nanoparticles; DTT, dithiothreitol; gsh, glutathione; lMB, leptomycin B; Nac, N-acetylcysteine.
ZnO
Similarly, it has been identified ZnO was connected with HO-1 regulation. In Yan study [50], human coronary artery endothelial cells (HCAECs) were exposed to ZnO-NPs for 24 h directly and the level of HO-1 in supernatants of culture media was significantly increased. Furthermore, in Fukui study [51], it was demonstrated that both in vivo (rat lung) and in vitro (A549 cells), ZnO-NPs could release zinc ion which might induce strong oxidative stress. In Osmond M’s GEO database (GSE 60159), compared with the control group, it was detected that HO-1 was significantly increased (5.63 fold) in primary human hepatic stellate cells treated with ZnO-NPs (30 ug/ml, 24 h) (Table 1). It was just determined ZnO can alter HO-1 expression and oxidative stress. Whether through Nrf2/Bach1 or influence ROS generation is still unknown. However, in Li study [52], the results indicated that ZnO-NPs could significantly inhibit human multiple myeloma cell proliferation and cell death in a time- and dose-dependent manner in vitro, and this outcome can be confirmed by cell morphology and apoptosis assay. Meanwhile, the results also showed that ZnO NPs could effectively increase ROS production and decrease ATP levels in human multiple myeloma cells.
Table 1.
The top 10 differentially expressed genes in primary human hepatic stellate cells treated with ZnO-NPs
| Gene.symbol | Adj.P.Val | P.Value | LogFC |
|---|---|---|---|
| HSPA6 | 0.000137 | 9.93e-09 | 7.17 |
| MT1M | 0.000137 | 1.16e-08 | 6.72 |
| HMOX1 | 0.000137 | 3.01e-08 | 5.63 |
| IL11 | 0.000137 | 3.11e-08 | 5.55 |
| DNAJA4 | 0.000137 | 4.30e-08 | 5.47 |
| CNOT8 | 0.000137 | 6.54e-08 | -4.81 |
| MANEA | 0.000137 | 6.82e-08 | -4.99 |
| IFIT3 | 0.000137 | 5.47e-08 | -5.06 |
| SOCS5 | 0.000137 | 5.07e-08 | -5.33 |
| RECK | 0.000137 | 7.05e-08 | -5.36 |
SiO2
SiO2-NPs, generally considered as biocompatible materials, have been much investigated for their toxicity at high doses, such as cardiovascular system impairment [53], liver fibrosis [54], and granuloma formation in the liver and spleen of rats [55]. In vitro, using the HUVEC cell line model, SiO2-NPs were detected to decrease cell viability and induce ROS generation [56]. However, in Jennifer study [57], it was identified SiO2-NPs at low concentration (up to 0.5 mg/ml) had a beneficial effect on normal human facial skin fibroblasts in terms of increasing their proliferation and metabolic activity. The low dose SiO2 treatment could induce the translocation of Nrf2 from cytoplasm to nucleus followed by overexpression of HO-1. In addition, it was also observed that in A549 cells, SiO2-NPs exposure could activate the Nrf2/ARE signaling pathway via the up-regulation of PERK (PKR-like endoplasmic reticulum-regulated kinase). While in Nrf2-/- mice, SiO2-NPs exposure would increase the level of ROS. These results suggested that SiO2 with various concentrations have different biological effects and Nrf2 might protect against oxidative stress induced by SiO2-NPs through PERK/Nrf2/ARE/HO-1 pathway.
TiO2
TiO2 NPs, also widely used in light-sensitive DDS, its possible toxic mechanisms might be related to oxidative stress and/or inflammatory responses directly or indirectly [58]. Recently, it was suggested that the photocatalytic activity of TiO2 NPs has been a major concern owing to fear of the ROS generation [59]. During mitochondrial respiration in the lung tissue of rats, TiO2 NPs could decrease nicotinamide adenine dinucleotide (NADH) levels accompanied with ROS generation (hydroxyl radicals, superoxide radicals, and singlet oxygen) [60]. Previous studies have also indicated that TiO2 NPs caused liver and kidney function damages by increasing ROS generation and decreasing antioxidant capacity [61,62]. Furthermore, in mice, long term TiO2 NPs exposure was accompanied by a decrease of glutathione content and an increase ROS generation [63-65]. In Niu study [66], it was also detected that the protein expression of Nrf2, HO-1, NQO1 (NAD(P)H dehydrogenase quinone 1) and GCLC (glutamate-cysteine ligase catalytic subunit) were all reduced in heart, liver, and kidney of mice after exposing to TiO2 NPs. All results suggested chronic TiO2 NPs exposure might result in the accumulation of ROS and down-regulated expression of anti-oxidative genes.
In context, it was known that both in organic- and inorganic-based DDS, HO-1 would be regulated mainly through Nrf-2/Bach1. Nevertheless, the altered HO-1 level and ROS generation are often accompanying even the mechanism of ROS generation is unknown and ROS accumulation was really detected. ROS generation and partitioning are novel examples of quantum biology. In vivo, according to the studies of reduced flavoenzymes/molecular oxygen [67], quantum biology [68] and radical pair mechanism (RPM) [69], the control of ROS generation might be related with coherent spin dynamics in a radical pair (RP) reaction [70]. In brief, a coherent singlet-triplet intersystem crossing was driven by the RP spin dynamic which is governed by internal magnetic interactions such as electron-nuclear hyperfine interactions (HFIs) and applied magnetic fields (light and radiofrequency). External magnetic fields might alter RP spin dynamics and the generation of ROS through the Zeeman resonance effect. In reduced flavoenzymes, an electron transfer activates molecular oxygen (O2) and then a triplet-born, spin-correlated RP will be created between flavin semiquinone (FADH•) and superoxide (O2 •-) (Figure 5) [71,72]. We hypothesize that in light-control DDS, the quantum effect of photon might alter the RP spin dynamic and regulate ROS generation. Furthermore, the strength, frequency, amplitude, and orientation could also alter the relative ROS product yields in vitro [70]. Thus, the photo-sources with different energy would have various effects on ROS generation.
Figure 5.
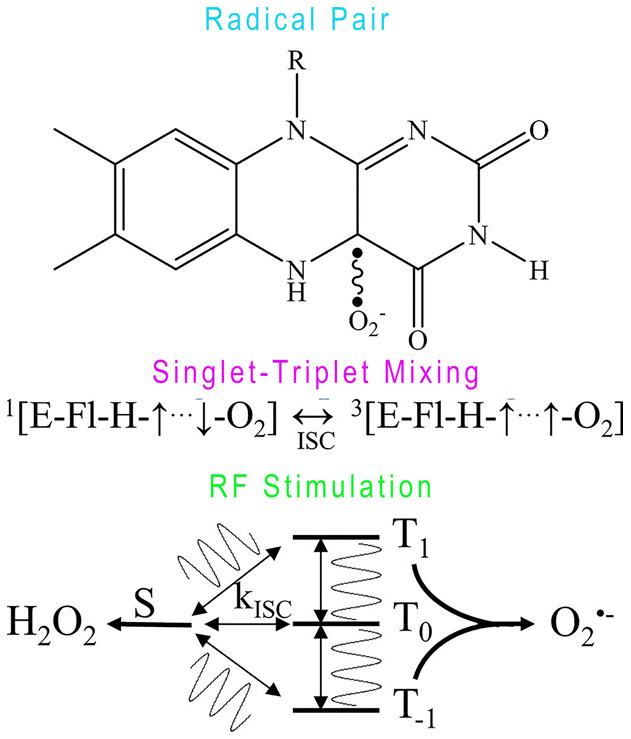
The schematic of ROS generation. Flavin semiquinone (FADH•) and superoxide (O2 •-) spin-correlated radical pair (top). The radical pairs undergo intersystem crossing (ISC) between singlet (S) and triplet states (T-1,0,1) at rates (kISC) determined by electron-nuclear hyperfine and Zeeman interactions (middle). An applied RF oscillating magnetic field tuned at Zeeman resonance (1.4 MHz at 50 μT magnetic field), modifies kISC (bottom) and ultimately affects the relative amount of singlet (H2O2) and triplet (O2 •-) reaction products. Resonance effect on ROS product yields is a key manifestation of quantum biology.
Traditional pathophysiology-based DDS leaves few options for controlling the rate and extent of drug release at target sites. In contrast, light-control systems may enable drugs to undergo spatiotemporal delivery with remote control. The NPs can certainly penetrate the human body via the lungs, intestines, and skin. However, thus far, most studies of nanomaterials ignored their biological effect or affirmed their various cytotoxicity unilaterally. Abundance evidence suuested NPs could also be of benefit for some diseases. In present review, the regulation of HO-1/ROS from NPs was summarized. Interestingly, the mechanisms of various diseases were also associated with the level of HO-1/ROS. For example, in allergic diseases, oxidative stress and a deregulated immune system are the main pathogenesis [73]. Ample evidence has demonstrated that HO-1 was a significant antioxidant enzyme in disease process [74] and regulated the bio-functions of mast cells [75,76], helper T cells [77,78], dendritic cells [79] and regulatory T cells [80] in allergy. In Marit Ilves study [81], it was revealed that in the mouse model of allergic dermatitis, ZnO-NP was able to reach into the deep layers of allergic skin and diminish the local inflammation through inducing the infiltration of T cells and macrophages along with downregulating expression of skin cytokines (interleukin-6 (IL-6), IL-10, tumor necrosis factor (TNF) and interferon-γ (IFN-γ)). Although HO-1 was not detected in this study, the infiltration of T cells and mast cells [75], and the expression of cytokines might be regulated by HO-1 [82]. Nevertheless, the mechanism of HO-1 regulated by ZnO-NPs in the pathogenesis of AD was just a hypothesis need further explored. Thus, the pharmacokinetic behavior of different types of nanoparticles requires detailed investigation and a database of health risks associated with different nanoparticles at various concentrations (e.g. Target organs, tissue or cells) should be created [1].
Conclusion
In our review, the mechanism of organic NPs regulated HO-1 is still unclear while it was concluded that the inorganic NPs might regulate HO-1 through Nrf2 or Bach1 translocation. However, the theory of NPs induced Nrf2 translocation was still unknown. MicroRNAs (miRNAs), a class of small (about 22-nucleotide) noncoding RNAs, control gene expression through mRNA degradation or translation repression via 3’-untranslated region (3’-UTRs) of target mRNA [83]. In addition, the miRNA profile is sensitive accompanied by dysregulation when the external stimulus appeared. Owing to the abundant count and the particular mechanism of regulation, miRNA may participate in Nrf2 translocation after NPs exposure. Furthermore, the level of glutathione and the activity of SOD were well-known associated with ROS generation. More and more kinds of NPs were applied in light-control DDS. Additional studies are needed to test the toxicology of NPs for various pharmacological drugs in diverse disease models. The most important significance of this review is reminding other researchers that some nanoparticles could also have some underlying benefit for some diseases. Therefore, when a material with a new structure was made and used. A rounded analysis is necessary.
Acknowledgements
The authors thank Prof. JW Bartsch (University Marburg, Germany) and CP for proofreading the manuscript. This work was supported by the Key projects of Chongqing Municipal Science and Technology Commission (cstc2017jcyjbx0044) and Fundamental Research Funds for the Central Universities (2019CDQYGD038).
Disclosure of conflict of interest
None.
Abbreviations
- DDS
drug delivery systems
- NP
Nanoparticle
- GSH
glutathione
- GSSG
oxidized glutathione
- SOD
superoxide dismutase
- ROS
radical oxygen species
- Nrf2
nuclear factor erythroid 2-related factor 2
- Bach1
BTB and CNC homolog 1
- Keap1
Kelch-like ECH-associated protein 1
- PS
photosensitizer
- NIR
near-infrared
- UCNP
up-conversion nanoparticles
- LEH
Liposome-encapsulated hemoglobin
- PLGA
Poly-D, L-lactide-co-glycolide
- LOOH
lipid hydroperoxides
- ECs
endothelial cells
- HCAECs
human coronary artery endothelial cells
- PERK
PKR-like endoplasmic reticulum-regulated kinase
- GCLC
glutamate-cysteine ligase catalytic subunit
- RPM
radical pair mechanism
- RP
radical pair
- HFIs
hyperfine interactions
- FADH•
flavin semiquinone
- ISC
intersystem crossing
- 3’-UTRs
3’-untranslated region
References
- 1.Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J Nanobiotechnology. 2004;2:12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YC, Huang XC, Luo YL, Chang YC, Hsieh YZ, Hsu HY. Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systems. Sci Technol Adv Mater. 2013;14:044407. doi: 10.1088/1468-6996/14/4/044407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstead AL, Li B. Nanomedicine as an emerging approach against intracellular pathogens. Int J Nanomedicine. 2011;6:3281–93. doi: 10.2147/IJN.S27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens DL, Lee BY, Xue M, Thomas CR, Meng H, Ferris D, Nel AE, Zink JI, Horwitz MA. Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrob Agents Chemother. 2012;56:2535–45. doi: 10.1128/AAC.06049-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostevsek N, Abramovic I, Hudoklin S, Kreft ME, Sersa I, Sepe A, Vidmar J, Sturm S, Spreitzer M, Scancar J, Kobe S, Zuzek Rozman K. Hybrid FePt/SiO2/Au nanoparticles as a theranostic tool: in vitro photo-thermal treatment and MRI imaging. Nanoscale. 2018;10:1308–1321. doi: 10.1039/c7nr07810b. [DOI] [PubMed] [Google Scholar]
- 6.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164:1665–8. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 7.Jiao ZH, Li M, Feng YX, Shi JC, Zhang J, Shao B. Hormesis effects of silver nanoparticles at non-cytotoxic doses to human hepatoma cells. PLoS One. 2014;9:e102564. doi: 10.1371/journal.pone.0102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima R, Seabra AB, Duran N. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 2012;32:867–79. doi: 10.1002/jat.2780. [DOI] [PubMed] [Google Scholar]
- 9.Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28:1563–74. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 10.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorn ME, Hasselbalch HC. The role of reactive oxygen species in myelofibrosis and related neoplasms. Mediators Inflamm. 2015;2015:648090. doi: 10.1155/2015/648090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Urrusuno R, Fattal E, Feger J, Couvreur P, Therond P. Evaluation of hepatic antioxidant systems after intravenous administration of polymeric nanoparticles. Biomaterials. 1997;18:511–7. doi: 10.1016/s0142-9612(96)00178-0. [DOI] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 15.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem. 2010;285:153–62. doi: 10.1074/jbc.M109.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future. Chem Rec. 2017;17:775–802. doi: 10.1002/tcr.201600121. [DOI] [PubMed] [Google Scholar]
- 18.Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR. Photoantimicrobials-are we afraid of the light? Lancet Infect Dis. 2017;17:e49–e55. doi: 10.1016/S1473-3099(16)30268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Hu C, Ran W, Meng J, Yin Q, Li Y. Recent progress in light-triggered nanotheranostics for cancer treatment. Theranostics. 2016;6:948–68. doi: 10.7150/thno.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:R61–109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Wang B, Wang L, Lu T, Fu Y, Zhang H, Zhang Z. Fullerene (C60)-based tumor-targeting nanoparticles with “off-on” state for enhanced treatment of cancer. J Control Release. 2016;235:245–258. doi: 10.1016/j.jconrel.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Juzenas P, Juzeniene A, Kaalhus O, Iani V, Moan J. Noninvasive fluorescence excitation spectroscopy during application of 5-aminolevulinic acid in vivo. Photochem Photobiol Sci. 2002;1:745–8. doi: 10.1039/b203459j. [DOI] [PubMed] [Google Scholar]
- 23.Klohs J, Wunder A, Licha K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res Cardiol. 2008;103:144–51. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 24.Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett. 2002;323:207–10. doi: 10.1016/s0304-3940(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 25.Nagasaki T, Shinkai S. The concept of molecular machinery is useful for design of stimuli-responsive gene delivery systems in the mammalian cell. J Incl Phenom Macro. 2007;58:205–219. [Google Scholar]
- 26.Bisby RH, Mead C, Morgan CG. Wavelength-programmed solute release from photosensitive liposomes. Biochem Biophys Res Commun. 2000;276:169–73. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 27.Lu F, Yang L, Ding Y, Zhu JJ. Highly emissive Nd3+-sensitized multilayered upconversion nanoparticles for efficient 795 nm operated photodynamic therapy. Adv Funct Mater. 2016;26:4778–4785. [Google Scholar]
- 28.Gao W, Wang Z, Lv L, Yin D, Chen D, Han Z, Ma Y, Zhang M, Yang M, Gu Y. Photodynamic therapy induced enhancement of tumor vasculature permeability using an upconversion nanoconstruct for improved intratumoral nanoparticle delivery in deep tissues. Theranostics. 2016;6:1131–44. doi: 10.7150/thno.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Jaskula-Sztul R, Esquibel CR, Lou I, Zheng Q, Dammalapati A, Harrison A, Eliceiri KW, Tang W, Chen H, Gong S. Neuroendocrine tumor-targeted upconversion nanoparticle-based micelles for simultaneous NIR-controlled combination chemotherapy and photodynamic therapy, and fluorescence imaging. Adv Funct Mater. 2017;27:1604671. doi: 10.1002/adfm.201604671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv Drug Deliv Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim JD, Bae SK, Kim JC, Lee EO. Surface modifications of liposomes for recognition and response to environmental stimuli. Surf Sci Series. 2003;109:555–577. [Google Scholar]
- 32.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–60. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 33.Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. Triggerable plasmalogen liposomes: improvement of system efficiency. Biochim Biophys Acta. 1996;1279:25–34. doi: 10.1016/0005-2736(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 34.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv Drug Deliv Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 35.Yadav VR, Nag O, Awasthi V. Biological evaluation of liposome-encapsulated hemoglobin surface-modified with a novel PEGylated nonphospholipid amphiphile. Artif Organs. 2014;38:625–33. doi: 10.1111/aor.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel Aziz MT, Mostafa T, Atta H, Mahfouz S, Wassef M, Fouad H, Kamel M, Rashed L, Sabry D, Mouhamed O. Effect of HO-1 cDNA-liposome complex transfer on erectile signalling of aged rats. Andrologia. 2009;41:176–83. doi: 10.1111/j.1439-0272.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- 37.Amin FU, Shah SA, Badshah H, Khan M, Kim MO. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Abeta1-42-induced oxidative stress. J Nanobiotechnology. 2017;15:12. doi: 10.1186/s12951-016-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf A, Casey A. Liposomal encapsulation of silver nanoparticles (AgNP) improved nanoparticle uptake and induced redox imbalance to activate caspase-dependent apoptosis. Apoptosis. 2020;25:120–134. doi: 10.1007/s10495-019-01584-2. [DOI] [PubMed] [Google Scholar]
- 39.Song J, Pu L, Zhou J, Duan B, Duan H. Biodegradable theranostic plasmonic vesicles of amphiphilic gold nanorods. ACS Nano. 2013;7:9947–60. doi: 10.1021/nn403846v. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Wang C, Wang X, Wang X, Cheng L, Li Y, Liu Z. Mesoporous silica coated single-walled carbon nanotubes as a multifunctional light-responsive platform for cancer combination therapy. Adv Funct Mater. 2015;25:384–392. [Google Scholar]
- 41.Hu SH, Fang RH, Chen YW, Liao BJ, Chen IW, Chen SY. Photoresponsive protein-graphene-protein hybrid capsules with dual targeted heat-triggered drug delivery approach for enhanced tumor therapy. Adv Funct Mater. 2014;24:4144–4155. [Google Scholar]
- 42.Shim G, Kim MG, Park JY, Oh YK. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv Drug Deliv Rev. 2016;105:205–227. doi: 10.1016/j.addr.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Kim HS, Lee DY. Photothermal therapy with gold nanoparticles as an anticancer medication. J Pharm Investig. 2016;47:19–26. [Google Scholar]
- 44.Slowing I, Trewyn B, Giri S, Lin V. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. 2007;17:1225–1236. [Google Scholar]
- 45.Wu C, Chen C, Lai J, Chen J, Mu X, Zheng J, Zhao Y. Molecule-scale controlled-release system based on light-responsive silica nanoparticles. Chem Commun (Camb) 2008:2662–4. doi: 10.1039/b804886j. [DOI] [PubMed] [Google Scholar]
- 46.Lu J, Choi E, Tamanoi F, Zink JI. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008;4:421–6. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song YY, Schmidt-Stein F, Bauer S, Schmuki P. Amphiphilic TiO2 nanotube arrays: an actively controllable drug delivery system. J Am Chem Soc. 2009;131:4230–2. doi: 10.1021/ja810130h. [DOI] [PubMed] [Google Scholar]
- 48.Lai TH, Shieh JM, Tsou CJ, Wu WB. Gold nanoparticles induce heme oxygenase-1 expression through Nrf2 activation and Bach1 export in human vascular endothelial cells. Int J Nanomedicine. 2015;10:5925–39. doi: 10.2147/IJN.S88514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty A, Das A, Raha S, Barui A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J Photochem Photobiol B. 2020;203:111778. doi: 10.1016/j.jphotobiol.2020.111778. [DOI] [PubMed] [Google Scholar]
- 50.Yan Z, Wang W, Wu Y, Wang W, Li B, Liang N, Wu W. Zinc oxide nanoparticle-induced atherosclerotic alterations in vitro and in vivo. Int J Nanomedicine. 2017;12:4433–4442. doi: 10.2147/IJN.S134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukui H, Horie M, Endoh S, Kato H, Fujita K, Nishio K, Komaba LK, Maru J, Miyauhi A, Nakamura A, Kinugasa S, Yoshida Y, Hagihara Y, Iwahashi H. Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem Biol Interact. 2012;198:29–37. doi: 10.1016/j.cbi.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Guo D, Yin X, Ding S, Shen M, Zhang R, Wang Y, Xu R. Zinc oxide nanoparticles induce human multiple myeloma cell death via reactive oxygen species and Cyt-C/Apaf-1/Caspase-9/Caspase-3 signaling pathway in vitro. Biomed Pharmacother. 2020;122:109712. doi: 10.1016/j.biopha.2019.109712. [DOI] [PubMed] [Google Scholar]
- 53.Du Z, Zhao D, Jing L, Cui G, Jin M, Li Y, Liu X, Liu Y, Du H, Guo C, Zhou X, Sun Z. Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc Toxicol. 2013;13:194–207. doi: 10.1007/s12012-013-9198-y. [DOI] [PubMed] [Google Scholar]
- 54.van der Zande M, Vandebriel RJ, Groot MJ, Kramer E, Herrera Rivera ZE, Rasmussen K, Ossenkoppele JS, Tromp P, Gremmer ER, Peters RJ, Hendriksen PJ, Marvin HJ, Hoogenboom RL, Peijnenburg AA, Bouwmeester H. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part Fibre Toxicol. 2014;11:8. doi: 10.1186/1743-8977-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanov S, Zhuravsky S, Yukina G, Tomson V, Korolev D, Galagudza M. In vivo toxicity of intravenously administered silica and silicon nanoparticles. Materials. 2012;5:1873–1889. [Google Scholar]
- 56.Duan J, Yu Y, Li Y, Yu Y, Li Y, Zhou X, Huang P, Sun Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS One. 2013;8:e62087. doi: 10.1371/journal.pone.0062087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mytych J, Wnuk M, Rattan SI. Low doses of nanodiamonds and silica nanoparticles have beneficial hormetic effects in normal human skin fibroblasts in culture. Chemosphere. 2016;148:307–15. doi: 10.1016/j.chemosphere.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 58.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jovanovic B. Review of titanium dioxide nanoparticle phototoxicity: developing a phototoxicity ratio to correct the endpoint values of toxicity tests. Environ Toxicol Chem. 2015;34:1070–7. doi: 10.1002/etc.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freyre-Fonseca V, Delgado-Buenrostro NL, Gutierrez-Cirlos EB, Calderon-Torres CM, Cabellos-Avelar T, Sanchez-Perez Y, Pinzon E, Torres I, Molina-Jijon E, Zazueta C, Pedraza-Chaverri J, Garcia-Cuellar CM, Chirino YI. Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicol Lett. 2011;202:111–9. doi: 10.1016/j.toxlet.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 61.Zhang R, Niu Y, Li Y, Zhao C, Song B, Li Y, Zhou Y. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ Toxicol Chem. 2010;30:52–60. doi: 10.1016/j.etap.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Shi Z, Niu Y, Wang Q, Shi L, Guo H, Liu Y, Zhu Y, Liu S, Liu C, Chen X, Zhang R. Reduction of DNA damage induced by titanium dioxide nanoparticles through Nrf2 in vitro and in vivo. J Hazard Mater. 2015;298:310–9. doi: 10.1016/j.jhazmat.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 63.Hwang YJ, Jeung YS, Seo MH, Yoon JY, Kim DY, Park JW, Han JH, Jeong SH. Asian dust and titanium dioxide particles-induced inflammation and oxidative DNA damage in C57BL/6 mice. Inhal Toxicol. 2010;22:1127–33. doi: 10.3109/08958378.2010.528805. [DOI] [PubMed] [Google Scholar]
- 64.Cui Y, Liu H, Zhou M, Duan Y, Li N, Gong X, Hu R, Hong M, Hong F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res A. 2011;96:221–9. doi: 10.1002/jbm.a.32976. [DOI] [PubMed] [Google Scholar]
- 65.Winter M, Beer HD, Hornung V, Kramer U, Schins RP, Forster I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5:326–40. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- 66.Niu L, Shao M, Liu Y, Hu J, Li R, Xie H, Zhou L, Shi L, Zhang R, Niu Y. Reduction of oxidative damages induced by titanium dioxide nanoparticles correlates with induction of the Nrf2 pathway by GSPE supplementation in mice. Chem Biol Interact. 2017;275:133–144. doi: 10.1016/j.cbi.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 67.Massey V. Activation of molecular oxygen by flavins and flavoproteins. Chem Biol Interact. 1994;269:22459–62. [PubMed] [Google Scholar]
- 68.Brookes JC. Quantum effects in biology: golden rule in enzymes, olfaction, photosynthesis and magnetodetection. Proc Math Phys Eng Sci. 2017;473:20160822. doi: 10.1098/rspa.2016.0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Renger G. Light induced oxidative water splitting in photosynthesis: energetics, kinetics and mechanism. J Photochem Photobiol B. 2011;104:35–43. doi: 10.1016/j.jphotobiol.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 70.Usselman RJ, Chavarriaga C, Castello PR, Procopio M, Ritz T, Dratz EA, Singel DJ, Martino CF. The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Sci Rep. 2016;6:38543. doi: 10.1038/srep38543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–54. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Usselman RJ, Hill I, Singel DJ, Martino CF. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS One. 2014;9:e93065. doi: 10.1371/journal.pone.0093065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 74.Almolki A, Taille C, Martin GF, Jose PJ, Zedda C, Conti M, Megret J, Henin D, Aubier M, Boczkowski J. Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L26–34. doi: 10.1152/ajplung.00237.2003. [DOI] [PubMed] [Google Scholar]
- 75.Takamiya R, Murakami M, Kajimura M, Goda N, Makino N, Takamiya Y, Yamaguchi T, Ishimura Y, Hozumi N, Suematsu M. Stabilization of mast cells by heme oxygenase-1: an anti-inflammatory role. Am J Physiol Heart Circ Physiol. 2002;283:H861–70. doi: 10.1152/ajpheart.00740.2001. [DOI] [PubMed] [Google Scholar]
- 76.Ndisang JF, Gai P, Berni L, Mirabella C, Baronti R, Mannaioni PF, Masini E. Modulation of the immunological response of guinea pig mast cells by carbon monoxide. Immunopharmacology. 1999;43:65–73. doi: 10.1016/s0162-3109(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 77.Xia ZW, Zhong WW, Meyrowitz JS, Zhang ZL. The role of heme oxygenase-1 in T cell-mediated immunity: the all encompassing enzyme. Curr Pharm Des. 2008;14:454–64. doi: 10.2174/138161208783597326. [DOI] [PubMed] [Google Scholar]
- 78.Choi BM, Oh GS, Lee JW, Mok JY, Kim DK, Jeong SI, Jang SI. Prenylated chalcone from sophora flavescens suppresses Th2 chemokine expression induced by cytokines via heme oxygenase-1 in human keratinocytes. Arch Pharm Res. 2010;33:753–60. doi: 10.1007/s12272-010-0515-8. [DOI] [PubMed] [Google Scholar]
- 79.Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 80.Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–6. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 81.Ilves M, Palomaki J, Vippola M, Lehto M, Savolainen K, Savinko T, Alenius H. Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part Fibre Toxicol. 2014;11:38. doi: 10.1186/s12989-014-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–35. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 83.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]