Abstract
Tartrate-resistant acid phosphatase (ACP5) could regulate cancer cell proliferation; however, its role in hepatocellular carcinoma (HCC) remains largely unknown. Here, we investigated the function of ACP5 in HCC and examined the underlying molecular mechanisms. The expression of ACP5 was evaluated by immunohistochemistry and quantitative reverse transcription and polymerase chain reaction (qRT-PCR) in a series of HCC tissues. The effects of ACP5 silencing on cell proliferation, cell cycle, apoptosis, migration, and invasion of HCC were assessed in vitro by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, clonogenic assays, flow cytometry, and transwell assays. The results find that ACP5 is overexpressed in HCC tissues compared with adjacent normal tissues. Knockdown of ACP5 inhibits the proliferation, migration and invasion of HCC cell lines. Furthermore, silencing of ACP5 induces cell cycle G2/M phase arrest and increases apoptosis of HCC cell lines. ACP5 provides potential novel targets for the treatment of HCC.
Keywords: Hepatocellular carcinoma, ACP5, proliferation, cell cycle, apoptosis, invasion
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and it is one of the leading causes of death among malignancies in China [1,2]. Despite advances in diagnostic and treatment modalities, the prognosis for HCC has not been significantly improved, and the 5-year survival rate for patients with HCC remains poor, which is largely attributable to the high rates of distant metastasis [3]. Thus, there is an urgent need to develop new strategies to HCC treatment.
Tartrate-resistant acid phosphatase (ACP5) has been received considerable attention [4,5]. Accumulating evidence has recently demonstrated that ACP5 is one of the most common overexpressed oncogenes in a multitude of human solid tumors [6-8]. An increasing number of ACP5 have been identified to be differentially expressed in many human cancers and to be implicated in tumor progression and metastasis. However, the role of ACP5 in hepatocellular carcinogenesis remains uncertain. In this study, we investigated the expression of ACP5 in human HCC using qRT-PCR, Western blot, and immunohistochemistry, and further explored the potential functions of ACP5 in HCC progression.
Materials and methods
HCC tissue samples
From 2005 to 2014, tumor samples and normal pericarcinomatous tissues were collected from 92 HCC patients receiving surgery at the Affiliated Baiyun Hospital of Guizhou Medical University (Guizhou, China). These tissue samples were conserved in liquid nitrogen after collection or prepared in paraffin sections. No systemic or local treatment had been received before operation. Both tumor and nontumor tissues were histologically confirmed. All the tissue samples were obtained with informed consent from all the patients. This study was approved by the Institute Research Ethics Committee of Guizhou Medical University, Guizhou, China.
Cell lines
HCC cell lines MHCC97L, Huh7, HepG2, HCCLM3, SMMC-7721, MHCC97H and normal liver cell lines HL-7702 were from the Tumor Cell Bank of Chinese Academy of Sciences. All the cell lines were grown in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 Ag/AL streptomycin, and 100 Ag/AL penicillin (pH 7.2-7.4) in a humidified incubator containing 5% CO2 at 37°C.
Immunohistochemistry
For each patient sample, three paraffin sections of 5 μm were prepared, one for hematoxylin and eosin (HE) staining and the other two for immunohistochemical staining. Phosphate buffer saline (PBS) instead of primary antibodies was used for negative control, and the breast cancer tissue was used for positive control. Sections were dewaxed using xylene, followed by hydration with ethanol solutions and addition of EDTA for antigen retrieval. Later, sections were blocked with normal goat serum for 30 min to eliminate non-specific binding. Sections were incubated with primary antibody against ACP5 (Abcam, Cambridge, UK). Sections were then incubated with biotin-labeled secondary antibodies for 30 min at room temperature, followed by staining with diaminobenzidine (DAB). Finally, the sections were counterstained with hematoxylin. The result of staining was determined by two doctors who did not know the clinical condition of patients. The proportions of positive cells of 0, 1-5, 6-25, 26-75, and 76-100% were assigned with scores of 0, 1, 2, 3, and 4, respectively. Scores of 0-2 were considered as negative expression, and scores of 3-4 were considered as positive expression.
Quantitative RT-PCR
Total RNA of tissues or cultured cells was isolated using TRIzol reagent (Invitrogen, Life Technologies, CA, USA). Reverse transcription was performed using a First-strand cDNA Synthesis System (Invitrogen, Life Technologies, CA, USA). qRT-PCR was carried out using Power SYBR® Green PCR Master Mix (ABI, USA) on the 7500 real time PCR system (ABI, life technology). The GAPDH was used as a loading control for each specific gene. Each experiment was performed three times and each sample was tested in triplicate. The sequences of human ACP5 primers were 5’-TGCAAGATGAGAATGGCGTG-3’ (sense) and 5’-CAAAGCCACCCAGTGAGTCT-3’ (antisense). The primers for human GAPDH were 5’-GCACCGTCAAGGCTGAGAAC-3’ (sense) and 5’-TGGTGAAGACGCCAGTGGA-3’ (antisense).
Western blot analysis
Whole cells were lysed on ice in a lysis buffer (RIPA, Beyotime, Shanghai, China) with a protease inhibitor mixture cocktail (Roche, Switzerland). After centrifugation at 12,000 rpm for 30 min at 4°C, the protein concentrations of supernatants in samples were measured by the bovine serum albumin (BCA) protein assay (Thermo scientific, Rockford, IL, USA). Equal amounts of protein (30 μg) were separated by 10-12% NUPAGE Bis-Tris Gel (Invitrogen, Life Technologies, CA, USA) electrophoresis (constant voltage: 120 mv) and transferred onto polyvinylidene fluoride (PVDF, 0.45 μm) membranes (constant current: 350 mA for 70/120 min). After being blocked by Tris-buffered saline and Tween 20 (TBST) buffer containing 5% non-fat powder milk for 2 h, the membranes were incubated with primary antibodies overnight on ice. After washing the membranes several times in TBST while agitating, the detection was performed using the appropriate secondary horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit antibody (Cell Signaling Technology, Inc., MA, USA). Immunoreactive bands on the blots were visualized with enhanced chemiluminescence (ECL) reagent kit (Beit Haemek, Israel).
Short hairpin RNA transfection of human HCC cell line
Human ACP5 shRNA (5’-GGCTATCTGCGCTTCCACTAT-3’) and control-shRNA were synthesized by GenePharma (shanghai, china). Cells were transfected with sh ACP5 or control-shRNA using Lipofectamine 2000 (Invitrogen, Life Technologies, CA, USA) according to the manufacturer’s instructions.
MTT assays
The proliferation of cells was evaluated by the MTT assay. Cells were plated on a 96-well plate at 3 × 103 cells/well and were allowed to grow for different times. The growth rate was determined by the cell number and counted in triplicate every day by MTT assay. Briefly, cells were incubated with 50 μL of 0.2% MTT for 4 h at 37°C in a 5% CO2 incubator. Following MTT incubation, 150 μL of 100% Dimethyl sulfoxide (DMSO) was added to dissolve the crystals. Viable cells were counted every day by reading the absorbance at 490 nm using a 96-plate reader BP800 (Dynex Technologies, USA).
Clonogenic assays
Clonogenic assay was conducted to examine the effect of ACP5-shRNA on cell growth in HCC cell lines. 4 × 105 cells were plated in a 6-well plate. After 24 h of transfection, the cells were trypsinized, and 1,000 single viable cells were plated in three 6-well plates. The cells were then incubated for 14 days at 37°C in the condition of 5% CO2. Colonies were stained with 0.1% crystal violet, washed with water, and counted ten random fields manually. The colonies containing at least 100 cells were scored. The surviving fraction in ACP5-shRNA transfected cells was normalized to untreated control cells with respect to clonogenic efficiency.
Wound healing assay
Wound healing assay was adopted to test the migration ability of HCC cells. In our study, cells were digested after transfection by specific shRNA and control shRNA to human ACP5 for 24 h on 6-well plates, and 2 × 105 cells were plated in 24-well plates. When cell confluence reached approximately 100%, the old medium was removed and the monolayer was wounded by scratching with a 10-μL sterile pipette tip lengthwise along the chamber, then cells were washed three times with PBS and cultured with serum-free medium at 37°C. Images of cells migrating into the wound were photographed at 0 h and 48 h using an inverted microscope (Olympus, Tokyo, Japan).
Cell migration and invasion assay
The invasion and migration abilities of HCC cells in vitro were evaluated by Matrigel coated Transwell and Transwell inserts (BD Biosciences, San Diego, CA, USA). A total of 5 × 104 cells in 100 mL serum-free medium were added to the upper chamber, and 600 mL medium containing 10% FBS was added into the lower chamber. The cells were left to invade the Matrigel for the appropriate time, the non-invading cells on the upper surface of the membrane were removed by wiping, and the invading cells were fixed and stained with 0.05% crystal violet. The number of invading or migrating cells was counted under a microscope in five predetermined fields for each membrane at × 400 magnification.
Cell cycle analysis and apoptosis assay
Cells were digested after transfection by specific shRNA and control shRNA to human ACP5, washed with ice-cold PBS once and fixed in 70% ethanol. Fixed cells were washed in PBS, prior to incubation with 1 mg/mL RNase A (Invitrogen, CA, USA) for 20 min at 37°C, washed in PBS and incubated with 0.1 mg/mL propidium iodide (Sigma-Aldrich, USA) for 20 min on ice. Intensities of fluorescence signals of treatments were determined by Apoptosis assay kits (Invitrogen, CA, USA) on a FACS Calibur Flow Cytometer (Becton-Dickinson, Franklin-Lakes, NJ, USA).
Statistical analysis
For continuous variables, data were expressed as mean ± standard deviation (SD). The difference of ACP5 mRNA or protein expression between tumor tissues and adjacent normal tissues was evaluated using Student’s t-test in GraphPad Prism 5.0 Software (GraphPad Software, Inc., La Jolla, CA, USA). All statistical tests were two-tailed and statistical significance was assumed for P < 0.05.
Results
ACP5 expression levels are significantly upregulated in human HCC
qRT-PCR was performed to detect the expression of ACP5 mRNA in 92 paired HCC tissues and corresponding nonneoplastic liver tissues. ACP5 expression is significantly upregulated in HCC tissues compared with the related normal pericarcinomatous tissues (Figure 1A). Immunohistochemical staining results show that ACP5 expression in HCC specimens is significantly upregulated compared to adjacent non-tumoral liver tissues (Figure 1B). ACP5 overexpression is observed in 66 of 92 (71.74%), and HCC specimens when compared with the non-malignant group (34 of 92, 36.96%).
Figure 1.
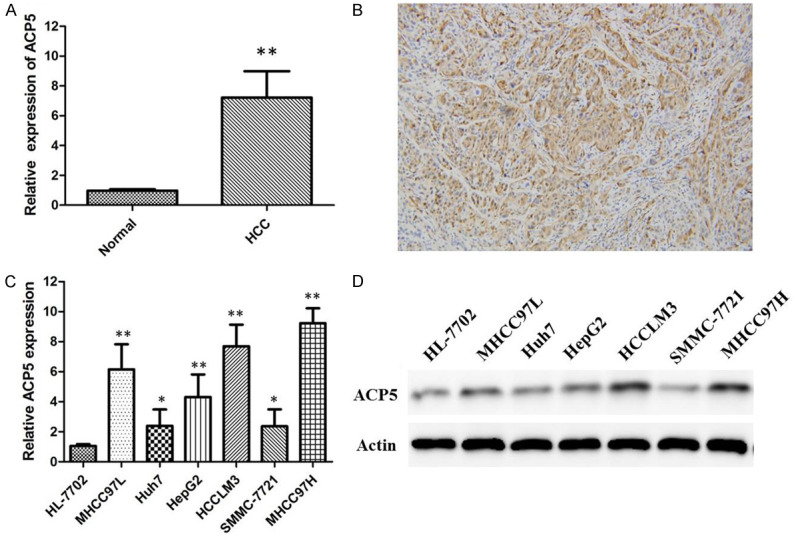
Changes of ACP5 expression in HCC tissues and cell lines. ACP5 mRNA expression levels in 92 paired HCC tissues and corresponding nonneoplastic liver tissues expressed as relative expression normalised to the expression of GAPDH (A); Immunohistochemical staining of ACP5 in HCC tissues. Original magnification, × 200 (B); ACP5 mRNA (C) and protein (D) expression levels in a series of human HCC cell lines including MHCC97L, Huh7, HepG2, HCCLM3, SMMC-7721 and MHCC97H.
ACP5 is up-regulated in HCC cell lines and associated directly with the ability of cell proliferation and migration of HCC cell lines
Then, we detected the mRNA and protein expression of ACP5 in a series of human HCC cell lines, including MHCC97L, Huh7, HepG2, HCCLM3, SMMC-7721 and MHCC97H by qRT-PCR and western blot analysis, respectively. Our results indicate that HCCLM3 and MHCC97H cells (high metastatic potential) show the higher expression of ACP5, in relation to Huh7 (Figure 1C) and SMMC7721 cells (Figure 1D) (low metastatic potential). Thus, we use MHCC97H and HCCLM3 cells as the models to investigate the effect of ACP5 on HCC progression.
To further assess the biological function of ACP5 in HCC, we established two stable cell lines (denoted as MHCC97H-shACP5 and HCCLM3-shACP5) after lentiviral infection with LV-shACP5. As shown in Figure 2, ACP5 expression is distinctly decreased at mRNA and protein levels in MHCC97H-shACP5 and HCCLM3-shACP5 compared with control-shRNA cells, indicating that the specific shRNA of ACP5 effectively suppresses the expression of ACP5 in HCC cell lines.
Figure 2.
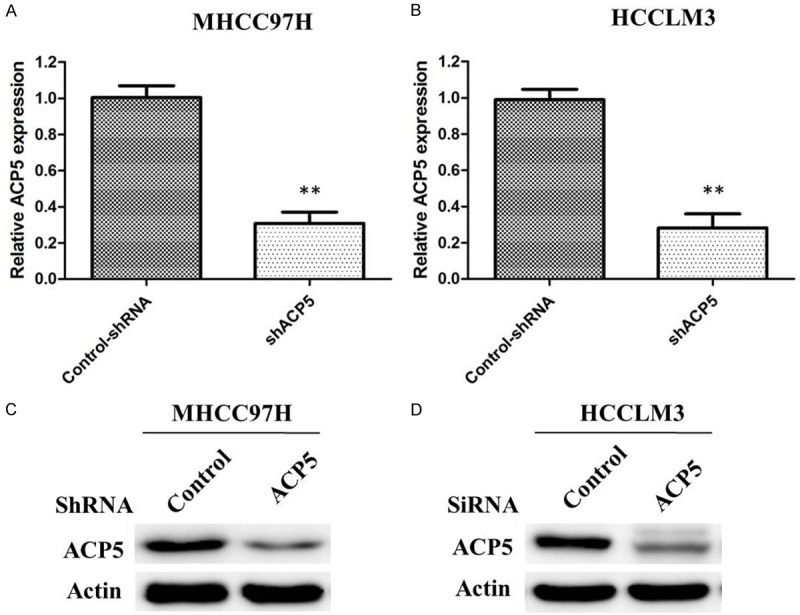
Efficency of ACP5 knockdown in MHCC97H and HCCLM3 cells. Cells were infected with ACP5 shRNA or control shRNA, and ACP5 mRNA expression was analyzed by qRT-PCR in both MHCC97H cells (A) and HCCLM3 cells (B); Cells were infected with ACP5 shRNA or control shRNA, and ACP5 protein expression was analyzed by western blot in both MHCC97H cells (C) and HCCLM3 cells (D).
We measured the effects of ACP5 expression levels on HCC cell proliferation by MTT and Clonogenic assays. It is shown that ACP5 knockdown is associated with significantly decreased cell viability of MHCC97H (Figure 3A) and HCCLM3 (Figure 3B) cells compared with cells transfected with control-shRNA. Furthermore, ACP5 knockdown in MHCC97H (Figure 3C) and HCCLM3 (Figure 3D) cells consistently reduces the colony formation ability compared with control-shRNA cells (P < 0.01), suggesting that ACP5 may act as an oncogene involved in the promotion of HCC cell proliferation.
Figure 3.
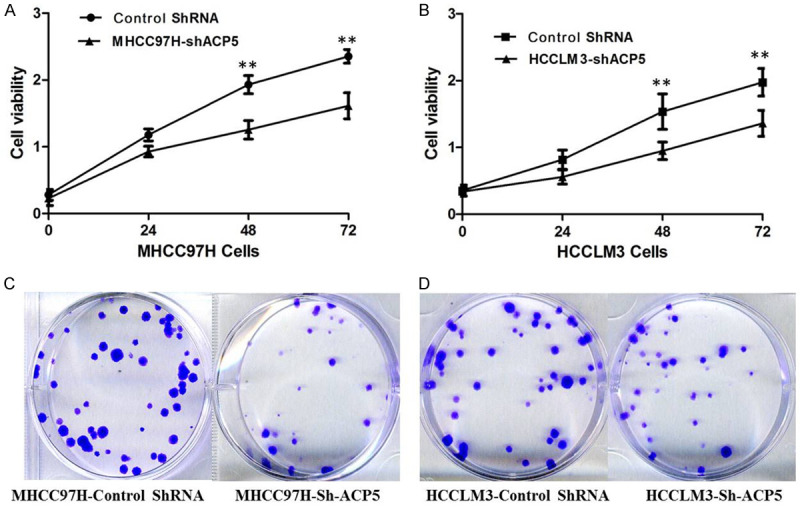
ACP5 knockdown inhibits cell proliferation. Proliferation of MHCC97H (A) and HCCLM3 cells (B) was measured by MTT assay; Colony formation in MHCC97H (C) and HCCLM3 cells (D) was measured by colony-formation assay.
Next, we carried out scratch wound-healing assay and matrigel invasion assay to evaluate whether ACP5 regulated the ability of migration and invasion of HCC cells. We find that ACP5 depletion markedly diminishes wound-healing capacity in MHCC97H (Figure 4A) and HCCLM3 (Figure 4B) cells. Furthermore, ACP5 knockdown impaires cell invasion through Matrigel in MHCC97H-shACP5 (Figure 4C) and HCCLM3-shACP5 (Figure 4D) cells (P < 0.05), suggesting that ACP5 promotes migration and invasion in HCC cells in vitro.
Figure 4.
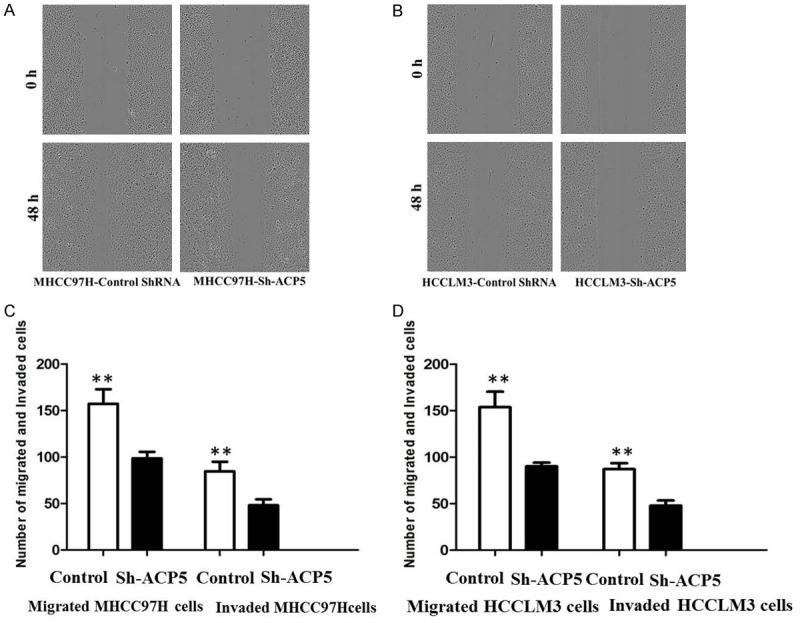
ACP5 knockdown inhibits the migration and invasion of MHCC97H and HCCLM3 cells. Cell migration was measured by Wound healing assays in MHCC97H (A) and HCCLM3 cells (B); Cell migration and invasion were measured by T ranswell assays MHCC97H (C) and HCCLM3 cells (D).
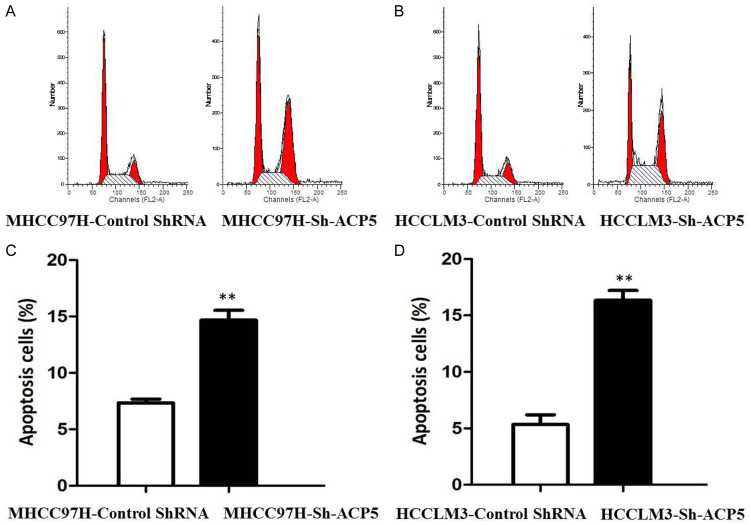
Downregulation of ACP5 expression induces cell cycle G2/M phase arrest and increases apoptosis
Additionally, flow cytometry was used to examine cell-cycle distribution. Compared with control cells, ACP5 knockdown increases the percentage of MHCC97H (Figure 5A) and HCCLM3 (Figure 5B) cells in G2/M phase (P < 0.05). Furthermore, the apoptosis was also determined by flow cytometry. Compared with HCCLM3 and MHCC97H cells, the apoptotic rates of MHCC97H-shACP5 (Figure 5C) and HCCLM3-shACP5 (Figure 5D) cells are significantly increased by 14.67% and 16.33%, respectively (P < 0.01). Thus, the down-regulation of ACP5 expression by shRNA induces cell-cycle G2/M phase arrest and increases apoptosis in HCCLM3 and MHCC97H cells.
Figure 5.
Down-regulation of ACP5 expression by shRNA induces cell-cycle G2/M phase arrest and increases apoptosis in MHCC97H and HCCLM3 cells. Flow cytometry was used to detect cell cycle in MHCC97H (A) and HCCLM3 cells (B); Flow cytometry was also used to measure apoptosis in MHCC97H (C) and HCCLM3 cells (D).
Discussion
ACP5 has been shown to play important roles in many developmental processes, including cancers [9,10]. In recent years, researchers have found that ACP5 is abnormally expressed in various tumors which is related to the increased cell proliferation and invasion, and the reduced apoptosis. Although ACP5 has been proved to function as an oncogene in many malignances, the relationship between ACP5 and HCC has not been fully elucidated. In the present study, we employed immunohistochemistry, qRT-PCT and western blot to accurately detect the expression level of ACP5 in HCC tissues. Besides, we used two HCC cell lines to explore the possible regulatory function of ACP5 in the tumorigenesis of HCC. Here, we describe that the protein and mRNA ACP5 levels are highly expressed in most human primary HCC tissues, whereas it is lowly expressed in adjacent normal liver tissues, suggesting the important roles of ACP5 in human HCC tumorigenesis.
Western blot and qRT-PCR analyses show that ACP5 expression levels are significantly increased in six HCC cell lines, especially in HCCLM3 and MHCC97H cells. HCCLM3 and MHCC97H cell lines exhibit much higher metastatic ability, revealing that ACP5 may promote the tumor metastasis. Therefore, we selected HCCLM3 and MHCC97H cell lines to further investigate the involvement of ACP5 in HCC progression. Further, we carefully evaluated the direct effect of ACP5 on the ability of cell proliferation, migration, and invasion. Inhibition of ACP5 is found to suppress the proliferation and colony forming capability of HCCLM3 and MHCC97H cells compared with control-shRNA cells. Combining with the previous reports, our observation further confirms the oncogenic roles of ACP5 in HCC. The downregulation of ACP5 leads to growth inhibition of HCC cells, which might be correlated with cell arrest in G2/M phase of cell cycle and apoptosis enhancement. In previous studies, it was found that ACP5 is predictive of peritoneal dissemination in patients with gastric cancer, and might play a crucial role in the establishment of peritoneal dissemination [11]. Another study also reported that ACP5, confers spontaneous metastasis in vivo, engages a key pathway governing metastasis, and is prognostic in human primary melanomas [12]. Previous studies indicated that ACP5 promotes the invasion and distal metastases of melanoma and breast cancer cells [13,14]. Our wound healing assays and transwell migration/invasion systems suggest that inhibition of ACP5 expression in HCCLM3 and MHCC97H cells by shRNA transfection inhibits the wound healing, migration and invasion of HCC cells. Thus, ACP5 might be a potential target for HCC therapy.
In conclusion, we find that ACP5 expression is upregulated in the majority of HCC clinical tissue specimens. Our results also indicate that inhibition of ACP5 suppresses cell proferation, invasion and migration in HCC cell lines. Down-regulation of ACP5 expression induces cell cycle G2/M phase arrest and increases apoptosis. These findings provide information which for facilitating the development of a novel therapeutic approach against HCC.
Acknowledgements
This work was supported by grants from the International S&T Cooperation Program of China (Grant No. 2015DFG31850) and Tianjin Science and Technology Plan Project (Grant No. 14RCGFSY00147), Tianjin Clinical Research Center for Organ Transplantation Project (15ZXLCSY00070), Key projects of Tianjin Health Industry (16KG108) and Tianjin Science and technology plan projec (19ZXDBSY00010).
Disclosure of conflict of interest
None.
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Adams LM, Warburton MJ, Hayman AR. Human breast cancer cell lines and tissues express tartrate-resistant acid phosphatase (TRAP) Cell Biol Int. 2007;31:191–195. doi: 10.1016/j.cellbi.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Honig A, Rieger L, Kapp M, Krockenberger M, Eck M, Dietl J, Kämmerer U. Increased tartrate-resistant acid phosphatase (TRAP) expression in malignant breast, ovarian and melanoma tissue: an investigational study. BMC Cancer. 2006;6:199. doi: 10.1186/1471-2407-6-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capeller B, Caffier H, Sutterlin MW, Dietl J. Evaluation of tartrate-resistant acid phosphatase (TRAP) 5b as serum marker of bone metastases in human breast cancer. Anticancer Res. 2003;23:1011–1015. [PubMed] [Google Scholar]
- 7.Chao TY, Yu JC, Ku CH, Chen MM, Lee SH, Janckila AJ, Yam LT. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res. 2005;11:544–550. [PubMed] [Google Scholar]
- 8.Xia L, Huang W, Tian D, Chen Z, Zhang L, Li Y, Hu H, Liu J, Chen Z, Tang G, Dou J, Sha S, Xu B, Liu C, Ma J, Zhang S, Li M, Fan D, Nie Y, Wu K. ACP5, a direct transcriptional target of FoxM1, promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Oncogene. 2014;33:1395–1406. doi: 10.1038/onc.2013.90. [DOI] [PubMed] [Google Scholar]
- 9.Lyubimova NV, Pashkov MV, Tyulyandin SA, Gol’dberg VE, Kushlinskii NE. Tartrateresistant acid phosphatase as a marker of bone metastases in patients with breast cancer and prostate cancer. Bull Exp Biol Med. 2004;138:77–79. doi: 10.1023/b:bebm.0000046945.95479.d6. [DOI] [PubMed] [Google Scholar]
- 10.Zenger S, He W, Ek-Rylander B, Vassiliou D, Wedin R, Bauer H, Andersson G. Differential expression of tartrate-resistant acid phosphatase isoforms 5a and 5b by tumor and stromal cells in human metastatic bone disease. Clin Exp Metastasis. 2011;28:65–73. doi: 10.1007/s10585-010-9358-4. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura M, Tanaka K, Toiyama Y, Okugawa Y, Okigami M, Yasuda H, Saigusa S, Ohi M, Inoue Y, Uchida K, Mohri Y, Kusunoki M. Clinical significance of tartrate-resistant acid phosphatase type-5 expression in human gastriccancer. Anticancer Res. 2014;34:3425–3429. [PubMed] [Google Scholar]
- 12.Scott KL, Nogueira C, Heffernan TP, van Doorn R, Dhakal S, Hanna JA, Min C, Jaskelioff M, Xiao Y, Wu CJ, Cameron LA, Perry SR, Zeid R, Feinberg T, Kim M, Vande Woude G, Granter SR, Bosenberg M, Chu GC, DePinho RA, Rimm DL, Chin L. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell. 2011;20:92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YY, Janckila AJ, Ku CH, Yu CP, Yu JC, Lee SH, Liu HY, Yam LT, Chao TY. Serum tartrate-resistant acid phosphatase 5b activity as a prognostic marker of survival in breast cancer with bone metastasis. BMC Cancer. 2010;10:158. doi: 10.1186/1471-2407-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reithmeier A, Panizza E, Krumpel M, Orre LM, Branca RM, Lehtiö J, Ek-Rylander B, Andersson G. Tartrate-resistant acid phosphatase (TRAP/ACP5) promotes metastasis-related properties via TGFβ2/TβR and CD44 in MDA-MB-231 breast cancer cells. BMC Cancer. 2017;17:650. doi: 10.1186/s12885-017-3616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]