Abstract
Cardiovascular diseases (CVDs) have been the leading cause of death in United States. While tremendous progress has been made for treating CVDs over the year, the high prevalence and substantial medical costs requires the necessity for novel methods for the early diagnosis and treatment monitoring of CVDs. Macrophages are a promising target due to its crucial role in the progress of CVDs (atherosclerosis, myocardial infarction and inflammatory cardiomyopathies). Positron emission tomography (PET) is a noninvasive imaging technique with high sensitivity and provides quantitive functional information of the macrophages in CVDs. Although 18F-FDG can be taken up by active macrophages, the PET imaging tracer is non-specific and susceptible to blood glucose levels. Thus, developing more specific PET tracers will help us understand the role of macrophages in CVDs. Moreover, macrophage-targeted PET imaging will further improve the diagnosis, treatment monitoring, and outcome prediction for patients with CVDs. In this review, we summarize various targets-based tracers for the PET imaging of macrophages in CVDs and highlight research gaps to advise future directions.
Keywords: Macrophages, positron emission tomography (PET), cardiovascular diseases (CVDs), atherosclerosis, myocardial infarction, cardiac sarcoidosis, myocarditis, pericarditis
Introduction
Cardiovascular diseases (CVDs) account for an immense health and economic burden in the United States and worldwide [1]. According to a report from the American Heart Association (AHA) in 2016, more than 121.5 million individuals are afflicted with CVDs. It is estimated that the annual direct and indirect costs of CVDs are $351.2 billion. CVDs are the leading cause of death in the United States [2].
Macrophages have been implicated in several CVDs, including the most prevalent CVDs with high morbidity and mortality such as atherosclerosis, myocardial infarction and inflammatory cardiomyopathies [3,4]. In atherogenesis, macrophages are involved in the lesion initiation stage and advanced progression [5-7]. Apolipoprotein B-containing lipoproteins (apoB-LPs) accumulate at vascular intima and undergo a series of modifications, which triggers the recruitment of monocytes from the spleen and bone marrow. The infiltrated monocytes then differentiate into macrophages and take up the modified lipoprotein, thereby becoming “foam cells” [8,9]. As the lesion expands, the lumen becomes narrow and may induce an ischemic event, such as angina pectoris [10]. During the subsequent progression, under endoplasmic reticulum stress, the macrophage derived foam cells undergo apoptosis and necrosis, which contributes to the formation of necrotic cores [11]. Enzymes secreted by macrophages, such as matrix metalloproteinases (MMPs), further erode the fibrous cap and makes plaque vulnerable to rupture and thrombosis [12]. Once the artery is completely occluded by in situ plaque, or thrombus derived from plaque rupture, the acute ischemic events (myocardial infarction or stroke) is triggered [13]. Following myocardial infarction, monocyte-derived macrophages infiltrate the infarcted heart within 24 hours. During the early phase (days 0-3), the infiltrating macrophages primarily secrete pro-inflammatory cytokines (TNF-α, IL-β, IL-6) and matrix proteases (MMPs) to clear dying cell debris. After approximately 5-7 days, these macrophages switch from a pro-inflammatory state to a pro-reparative state, secreting TGFβ1 and IL-10 to promote healing and decrease inflammation [14-16]. Macrophages have also been implicated in other inflammatory cardiovascular disease, such as cardiac sarcoidosis [17], myocarditis [18], peri/endocarditis [19] and vasculitis [20]. As such, macrophage tracking is important to aid early diagnosis, monitoring of disease activity and progression, treatment evaluation, and outcome prediction in CVDs.
Traditional imaging techniques such as computed tomography (CT) or magnetic resonance (MR) provide anatomical information but supply limited functional information. Positron emission tomography (PET) is an important nuclear imaging technique that can fill in this void [21]. Compared with other functional imaging modalities, such as optical fluorescence or bioluminescence, PET provides limitless penetration, quantitative accuracy, high sensitivity at picomolar level, and is easily translated to the clinic [21-23]. However, PET is limited in its morphological delineation ability due to low spatial resolution. Thus, hybrid PET/CT or PET/MR imaging has been increasingly applied preclinically and clinically to acquire both functional and anatomical information [24]. With the support of a cyclotron, short half-life isotope (11C, 18F, 68Ga) and long half-life isotope (89Zr, 64Cu) can meet nearly any labelling requirement of small molecules, large antibodies, or nanoparticles [25-27]. Therefore, PET imaging incorporated applications hold excellent potential for non-invasively tracking macrophages in CVDs.
Currently, the glucose metabolism-based tracer, 18F-FDG, is the most commonly-investigated PET tracer for imaging macrophages in CVDs. To date, previous reviews have excellently summarized relevant studies on FDG-based macrophages imaging in CVDs [28-31]; While 18F-FDG is readily available and has been widely employed in both the preclinical and clinical setting, some limitations still exist. First, it is a non-specific probe that can accumulate in other metabolically active cells and introduces background signal [32]. Moreover, 18F-FDG is affected by blood glucose levels, insulin levels and drug interference, which limits application in diabetic patients with hyperglycemia [33]; Additionally, FDG imaging of the macrophages in heart requires advanced patient preparation (fasting overnight or high fat diet) to suppress physiological signal from myocardial uptake. However, these methods are not always successful and feasible, particularly to MI patients or those in a poor physical condition. Standard methods to make a repeatable and effective suppression are still lacking, increasingly the likelihood of inaccurate quantification [30,33,34]. Thus, the development of specific and convenient PET tracers is still required.
Herein, we systematically review the currently available PET tracers other than 18F-FDG for the imaging of macrophages and summarize their applications in CVDs. These tracers can be generally classified into metabolism or proliferation based, chemokine receptor-targeted, somatostatin receptor-targeted, translocator protein targeted, mannose receptor-targeted, secreted enzymes-targeted, nanoparticle-based, antibody-based, and other potential targets. Some potential tracers currently applied in other diseases will also be discussed. We also discuss the current research limitations and highlight future directions. We believe the macrophage targeted PET imaging will deepen our understanding of macrophages’ function in CVDs, as well as facilitate early diagnosis, risk stratify and treatment monitoring, and provide benefits to CVDs patients in the future.
Metabolism & proliferation based PET imaging
Amino acid metabolism: 11C-methionine
L-[methyl-11C] methionine (11C-methionine, 11C-MET) is a radioisotope-labeled amino acid that is take up by more metabolically active cells and has been used in PET oncology applications [35]. Morooka M and colleagues first investigated the uptake of 11C-methionine in myocardial infarction/reperfusion patients. They found the highest accumulation of 11C-methionine in the infarcted area, whereas 201Tl and 18F-FDG uptake decreased (Figure 1A) [36]. A similar phenomenon was also reported in a rat infarction/reperfusion model using 14C-methionine. The tracer uptake closely corresponded to macrophage infiltration based on histology at day 3-7 [37]. Cellular uptake assay indicated the uptake of isotope-labeled methionine in pro-inflammatory M1 macrophages was higher than other cells and that the PET signal could be modulated with anti-integrin treatment [38].
Figure 1.
A. 11C-methionine PET, 18F-FDG PET and 201Tl SPECT imaging in an 43-years-old acute myocardial infarction patient. Infarction zone (red arrow) in anterior wall exhibited decreased uptake on 201Tl and 18F-FDG images, but relatively increased uptake on 11C-methionine images; B. 11C-methionine and 18F-FDG PET imaging in an experimental autoimmune myocarditis (EAM) rat, colocalization of focal myocardial uptake of both tracers is shown. 11C-methionine uptake was also observed in extracardiac areas (asterisk) and liver (arrowheads); C. 68Ga-Pentixafor PET/MRI imaging of atherosclerotic carotid plaques. The lesions were semi-quantitatively classified into four groups based on the cross-sectional luminal obstructive features on T2-weighted MRI, including Non-eccentric (non-significant luminal obstruction), Mild (≤10%), moderately (>10%), severely (≥25%) the white arrow indicate the arterial regions of interest; D. CCR5 targeted 64Cu-DOTA-vMIP-II PET imaging in a atherosclerotic mice model at 2 and 4 weeks. 64Cu-DOTA-vMIP-II significantly accumulated at the site of injury lesions, whereas the sham site had only weak accumulation. Adapted with permission from [36,39,63,70].
Maya et al. compared the distribution of 11C-methionine and 18F-FDG in a rat autoimmune myocarditis model, which revealed by histology that both tracers colocalized at inflammatory lesions. An excellent positive correlation also existed between 11C-methionine and 18F-FDG. However, the contrast of 11C-methionine was lower than 18F-FDG for discriminating inflammatory and noninflammatory lesions (Figure 1B) [39] (Table 1).
Table 1.
Summary of metabolism/proliferation based, chemokine receptor-targeted, SSTR-targeted probes illustrated in the present review
| Target | Disease | Preclinical/clinical | Radiotracer |
|---|---|---|---|
| Amino acid metabolism | Myocardial infarction | Clinical | 11C-methionine [36,38] |
| Preclinical | 14C-methionine [37] 11C-methionine [38] | ||
| Myocarditis | Preclinical | 11C-methionine [39] | |
| Choline metabolism | Atherosclerosis | Preclinical | 11C-Choline [42]; 18F-FCH [43] |
| Clinical | 11C-Choline [41]; 18F-FCH [44,45] | ||
| Proliferation | Atherosclerosis | Preclinical/clinical | 18F-FLT [47] |
| Abdominal aortic aneurysms | Preclinical | 18F-FLT [48] | |
| Chemokine receptors | |||
| CXCR4 | Myocardial infarction | Preclinical | 68Ga-pentixafor [57] |
| Clinical | 68Ga-pentixafor [57-59,158] | ||
| Atherosclerosis | Preclinical | 68Ga-pentixafor [60] | |
| Clinical | 68Ga-pentixafor [60-65] | ||
| CCR2 | Cardiac injury/Myocardial infarction | Preclinical | 68Ga-DOTA-ECL1i [69] |
| CCR5 | Atherosclerosis | Preclinical | 64Cu-DOTA-vMIP-II [70] |
| Vascular injury/Atherosclerosis | Preclinical | 64Cu-DOTA-DAPTA-comb [71]; 64Cu-DOTA-Vmip-II-comb [72] | |
| Somatostatin receptors (SSTR) | Myocardial infarction | Preclinical | 68Ga-DOTA-TATE and 68Ga-citrate [90] |
| Clinical | 68Ga-DOTA-TOC [86] | ||
| Sarcoidosis/Myocarditis/Pericarditis | Clinical | 68Ga-DOTA-TOC [84-87]; 68Ga-DOTA-NOC [88] | |
| 68Ga-DOTA-TATE [89] | |||
| Atherosclerosis | Preclinical | 68Ga-DOTA-TATE [75]; 68Ga-DOTA-NOC [79]; 18F-FDR-NOC [79] | |
| Clinical | 68Ga-DOTA-TATE [76-78,82,83]; 64Cu-DOTA-TATE [80,81] | ||
| 68Ga-DOTA-TOC [80] |
Choline metabolism: 11C-choline and 18F-FCH
Cholines are involved in the metabolism of cell membrane lipids and is regulated by choline kinase, which is upregulated in tumor cells and activated macrophages [40]. In contrast to 18F-FGD, radiolabeled choline or fluorocholine is not significantly taken up by myocardium. Currently, 11C-choline and 18F-FCH have garnered the most attention in imaging of macrophages of CVDs. Kato et al. retrospectively analyzed the uptake of 11C-choline in plaques from 93 elderly prostate cancer patients. They found that 95% of patients have positive PET results and that the tracer uptake rarely colocalized with calcification [41]. This finding was subsequently supported by mouse model study, which found a 2.6-fold higher uptake in inflamed plaque than the healthy vessel wall using ex vivo autoradiography [42].
Compared to the short half-life of 11C, 18F-fluorocholine (18F-FCH) is more suitable in the clinical setting for choline-based imaging. Matter et al. found 18F-FCH uptake at murine plaque correlated well with Oil staining and macrophages staining with better sensitivity compared to 18F-FDG [43]. A small retrospective study including five patients showed that 17 negative lesions were verified among the 31 detected vessel wall alterations. 16 of the 17 lesions were further identified as solely calcified lesions, indicating relatively stable plaque uptake of 18F-FCH [44]. Voo S and co-workers performed a prospective clinical study on the feasibility of 18F-FCH to identify vulnerable plaques. Ten stroke patients with over 70% carotid artery stenosis were involved in their study. 18F-FCH uptake in ipsilateral symptomatic carotid plaques significantly correlated with CD68+ macrophage staining in carotid endarterectomy specimens. Importantly, compared to the contralateral asymptomatic carotid arteries, ipsilateral symptomatic plaques exhibited higher uptake, indicating 18F-FCH is promising for identifying vulnerable plaques [45] (Table 1).
Proliferation: 18F-FLT
3’-deoxy-3’-[18F]fluorothymidine (18F-FLT) is a classic PET tracer traditionally used for visualizing tumor proliferation [46]. In 2015, Ye et al. investigated the possibility of 18F-FLT PET imaging for monitoring macrophages proliferation in atherosclerotic mice, a rabbit model, and patients. The PET results revealed an increased 18F-FLT signal in atherosclerotic lesions in animal models and patients. In vivo results were further verified by autoradiography and flow cytometry. Apart from plaques, significant 18F-FLT uptake was also observed in spleen and bone marrow, which may due to the proliferation of hematopoietic stem and progenitor cells. Interestingly, they further found the signal correlated with the duration of a high cholesterol diet, and the signal was reduced by fluorouracil, a proliferation inhibitor [47]. Since macrophage proliferation is closely related to the progression of plaque, 18F-FLT PET imaging may serve as a tool for monitoring plaque progression and therapy. Recently, Gandhi R and colleagues also investigated the feasibility of 18F-FLT PET imaging in abdominal aortic aneurysm, By using an AngII-induced mice model, they confirmed the uptake of 18F-FLT in 14-day aortae was greater than that in 28-day and control, and the uptake also correlated positively with aortic volume [48] (Table 1).
Chemokine receptors
Chemokines exert chemotactic effects on cells via binding with its receptor. Chemokine family can be divided into the major (CC, CXC) and the minor (C and CX3C) groups based on the N-terminal cysteine residues [49,50]. Among the various chemokine receptors, CXCR4, CCR2, and CCR5 garnered prominent attention in recent years due to their high expression on monocytes/macrophages and closely related leukocytes as well as their crucial roles in CVDs progression [51-54].
CXCR4
CXCR4-CXCL12 is involved in leukocyte recruitment to the injured region in CVDs. The inhibition of CXCR4-CXCL12 (SDF-1) has shown benefits for acute myocardial infarction and atherosclerosis [52]. Driven by CXCR4 targeted PET imaging in tumor [55,56], Thackeray and colleagues evaluated 68Ga-pentixafor PET imaging in myocardial infarction disease. In a murine model, significantly increased signal was observed in the infarct region, which could also be blocked by a CXCR4 antagonist and attenuated by long-term enalapril treatment. The autoradiography results colocalized with the presence of macrophages and granulocytes in histology, indicating this PET signal originates from recruited inflammatory cells. Interestingly, the authors found variable degree of 68Ga-pentixafor uptake in MI patients and the signal correlated with the bone marrow uptake [57]. At the same time, Lapa et al. reported the uptake of 68Ga-pentixafor in 3 of 7 AMI patients (5-10 days after MI) [58]. They further verified 17/22 positive imaging in another study (patients on 2-13 days after MI), in which the 68Ga-pentixafor PET signal not only negatively correlated with the time point of imaging, but also has a correlation with scar volumes assessed by CMR. In addition, the tracer signal could be observed up to 13 days after MI [59].
In 2017, 68Ga-pentixafor was first evaluated in atherosclerotic plaques in a rabbit model and eight patients. The authors demonstrated high tracer uptakes in plaques of rabbit model by PET/MR. The signal was reduced by 40% with pre-injection of CXCR4 inhibitor. Ex vivo analysis showed 125I-pentixafor uptake on autoradiography colocalized with the macrophage-rich region in plaque and correlated with the expression of CXCR4. Importantly, they further identified intense tracer uptake in two carotid atherosclerotic patients with over 50% carotid stenosis. The additional immunohistochemistry staining of human plaques showed that CXCR4 mostly located in the macrophages-rich area but not in lymphocytes or endothelial-rich areas [60]. The next year, two groups respectively investigated the relationship of 68Ga-pentixafor uptake and cardiovascular risks with a larger cohort of patients. Weiberg et al. retrospectively analyzed 51 patients who underwent 68Ga-pentixafor PET/CT imaging. They found that the tracer uptake was associated with cardiovascular risk factors and increased with the number of risk factors (age, arterial hypertension, hypercholesterolemia, history of smoking, prior cardiovascular events) [61]. Li and colleagues performed 68Ga-pentixafor PET/MR imaging in 38 patients, which revealed those with high uptake of tracer (mean TBRmax>1.7) exhibited a higher incidence of hypertension, hypercholesterolemia, diabetes, and history of cardiovascular disease. These results illustrate the potential of 68Ga-pentixafor in the characterization of atherosclerosis [62]. By taking advantage of MR in delineation of plaques, the same group involved 72 additional patients and divided them into four groups (non-eccentric, mild, moderately and severely) according to the degree of carotid plaque. They found the 68Ga-pentixafor uptake was absent in the non-eccentric group but significantly increased among the other three groups. However, no statistical differences exist between the other three groups. Additionally, they correlated the MRI results with histological evidence and found high expression of CXCR4 within inflamed atheroma and pre-atheroma, but not the fibroatheroma with low macrophages. This indicated that 68Ga-pentixafor PET imaging can recognize plaques with inflammatory activity (Figure 1C) [63]. They further proved 68Ga-pentixafor PET imaging could identify more lesions than 18F-FDG PET, with a weak correlation between the two probes [64]. Using the motion-corrected technique, Derlin et al. showed 68Ga-pentixafor PET/CT imaging could identify small coronary atherosclerotic plaques in patients after acute myocardial infarction [65] (Table 1).
CCR2
In 2016, a seven amino acid peptide (LGTFLKC) with excellent inhibition property to CCR2 was identified and named extracellular loop 1 inverso (ECL1i) by the authors [66]. Subsequently, it was developed into a PET imaging tracer by Liu et al. for in vivo imaging of CCR2+ cells in an ischemia-reperfusion injury model after lung transplantation [67] and a LPS-induced lung inflammation model [68]. In 2019, CCR2 targeted PET imaging was first investigated in cardiac injury by Heo and colleagues. The investigators used 68Ga to radiolabel the ECL1i peptide (68Ga-DOTA-ECL1i), which showed high tracer uptake at cardiac injury lesions in a cardiomyocyte ablation model as well as in a myocardial infarction/reperfusion (I/R) model. However, in a CCR knock-out mice model, minimal signal was detected. To further support the specificity, they performed autoradiography with MI patients’ heart tissue (acute and chronic). The human specimens exhibited heterogeneous radioactive signal levels, which could also be blocked. the signal was correlated with the number of CD68+ macrophages and with CD68+/CCR2+ cells, but not with CD68+/CCR2- cells, implying the tracer could specifically recognize CCR2+ macrophages and monocytes [69] (Table 1).
CCR5
In 2013, Liu and colleagues radiolabeled a viral macrophage inflammatory protein II (vMIP-II) with 64Cu (64Cu-DOTA-vMIP-II) and used PET imaging to monitor the distribution in a vascular injury-accelerated atherosclerosis model. They found 3-fold accumulation at the injury site in the model compared to the sham site (Figure 1D). Compared with 18F-FDG, the tracer displayed better accumulation at injury sites. The PET signal could also be blocked by eight chemokines. Among them, CCR5 and CXCR4 antagonists exhibited the most pronounced blocking effects [70]. Subsequently, to improve tracer accumulation, the authors designed two CCR5 targeted nanoparticle probes, 64Cu-DOTA-Vmip-II-comb and 64Cu-DOTA-DAPTA-comb. These nano-based tracers displayed higher uptake and contrast ratios when compared to previous tracers without nanoparticle conjugation [71,72] (Table 1).
Somatostatin receptor (SSTR)
Somatostatin (SST) is a cyclic polypeptide with broad inhibitory effects on the release of other secretory proteins. SST receptors (SSTRs) are G-protein-coupled receptors with five subtypes, which are expressed on immune cells and neuroendocrine tumors [73]. Thus, PET imaging probes targeting SSTRs have been previously developed and used for diagnosing neuroendocrine tumor [74]. Since 2010, SSTR-targeted tracers have been employed for cardiovascular imaging.
68Ga-DOTA-TATE is a radiotracer selectively binding to the SSTR2. The accumulation of 68Ga-DOTA-TATE at atherosclerotic plaques was reported in a mouse model. The autoradiography result displayed the tracer uptake co-localized with the macrophage-rich plaques from histology, indicating 68Ga-DOTA-TATE is suitable for imaging of macrophages in plaques [75]. Three retrospective clinical studies demonstrated the detectability of 68Ga-DOTA-TATE in human plaques in coronary or large arteries. All of the studies found that individuals with prior cardiovascular events exhibited higher tracer uptake in plaque [76-78]. Rinne et al. compared the three SSTR-targeted tracers 68Ga-DOTA-TATE, 68Ga-DOTA-NOC and 18F-FDR-NOC in atherosclerotic mice model. They found that 68Ga-DOTA-TATE and 68Ga-DOTA-NOC were superior for imaging plaque than 18F-FDR-NOC [79]. In addition, another group investigated a long half-life isotope radiolabeled tracer 64Cu-DOTA-TATE. They found that, compared with 68Ga-DOTA-TOC, 64Cu-DOTA-TATE had enhanced accumulation within plaque in large arteries. The 64Cu-DOTA-TATE uptake was associated with cardiovascular risk factors [80] and CD163 gene expression, which has been reported to be highly expressed on activated M2 macrophages [81]. In 2017, a prospective clinical study named “VISION” involved 42 atherosclerotic patients systematically investigated 68Ga-DOTA-TATE for detecting inflamed plaque. In this study, the authors demonstrated 68Ga-DOTA-TATE could specifically bind with the SST2 receptor, which also colocalized with CD68+ macrophages. 68Ga-DOTA-TATE was better able to discriminate high-risk and low-risk coronary lesion compared to 18F-FDG [82]. However, another prospective study involving 20 patients, questioned the ability of 68Ga-DOTA-TATE PET imaging to discriminate the symptomatic and asymptomatic carotid plaques, citing the lack of SST2 expression on excised human plaques [83].
SSTR2-targeted PET imaging in a cardiac sarcoidosis patient was first reported by Reiter et al. (Figure 2A) [84]. The same group further carried out two clinical studies to investigate SSTR targeted PET/CT imaging for detecting myocardial inflammation compared to cardiac magnetic resonance (CMR). Patients with cardiac sarcoidosis (CS) [85] or peri-/myocarditis, sub-acute myocardial infarction (MI) [86] were involved. Based on segment analysis, PET and CMR displayed high concordant results (CS: 96.1%; Peri-/myocarditis or MI: 85.3%), except a small portion of patients or segments different between the two modalities. Although it is hard to make a clear conclusion due to the lack of enough histopathologic data, it indicated that the combination of the two modalities (PET/MR) might be an optimal diagnostic approach. Another similar pilot study involving 17 histologically proven CS-positive patients revealed that only one patient displayed positive PET/CT results compared to the five positive patients verified by CMR. This discrepancy was attributed to treatment at the time of PET imaging, implying 68Ga-DOTA-TOC PET/CT is helpful to monitor disease activity. This technique would also be particularly advantageous for individuals with contraindications to CMR, such as patients with pacemaker [87]. However, given the small cohort of patients, the value of 68Ga-DOTA-TOC PET imaging requires further evaluation. Gormsen and co-workers performed a comparative study of 68Ga-DOTA-NOC and 18F-FDG in 19 suspected CS patients. They found that 68Ga-DOTA-NOC PET imaging displayed higher diagnostic accuracy and better interobserver agreement than FDG-PET. The inferiority of FDG imaging may be ascribed to the incomplete suppression of physiological myocardial uptake by fasting [88]. In contrast, another recent study suggested decreased sensitivity of 68Ga-DOTA-TATE in detecting CS inflammation compared with FDG. The authors also found weak SSTR2 expression in all three sarcoid heart specimens examined by immunostaining [89]. MI mice study indicated that neither 68Ga-citrate or 68Ga-DOTA-TATE is superior to FDG PET imaging for detecting post-MI inflammation [90]. Heterogeneous results have raised more questions on the diagnostic ability of SSTR2-targeted PET imaging of macrophages in inflammatory cardiomyopathies that must be reconciled in future studies (Table 2).
Figure 2.
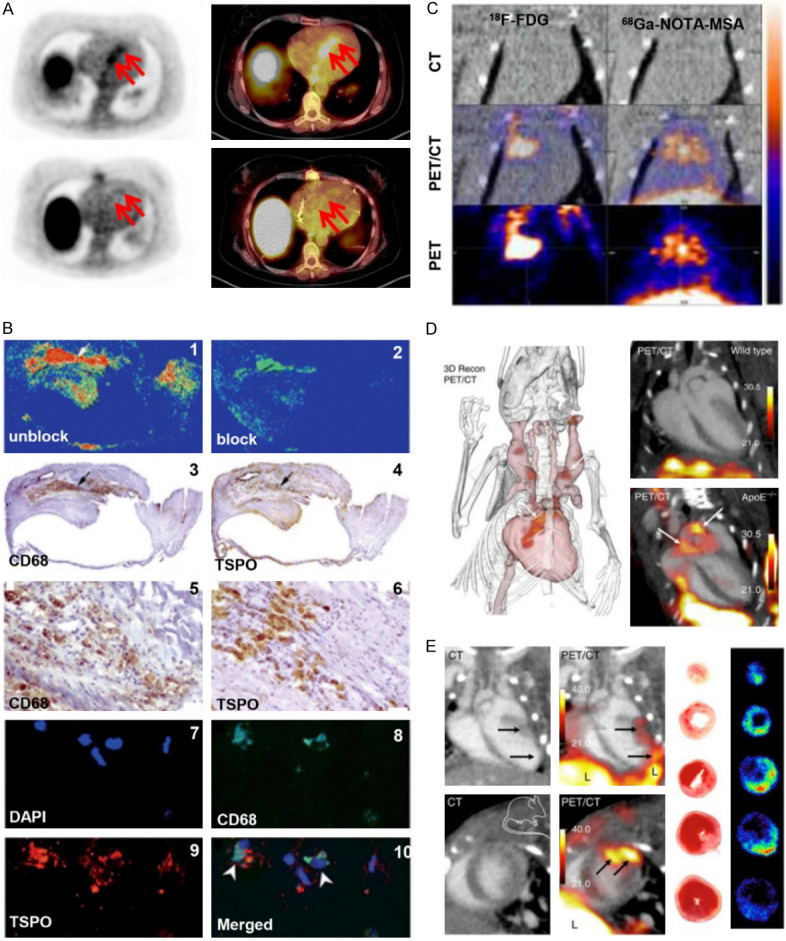
A. SSTR-targeted 68Ga-DOTA-TOC PET/CT imaging in a 54-year-old patient with cardiac sarcoidosis. The red arrow in upper images indicate the inflammatory area. The lower images are 68Ga-DOTA-TOC PET/CT imaging after 10-months systemic treatment. B. The verifying for the specificity of TSPO-targeted tracer PK11195, in atherosclerotic samples from patients who underwent carotid endarterectomy. Representative autoradiography, immunohistochemistry and immunofluorescence microscopy. The autoradiography showed that 3H-PK11195 binding with plaques and unlabeled PK11195 could block its binding (1-2). The immunohistochemistry showed the positive area of macrophage marker CD68 (3 and 5) and TSPO (4 and 6) respectively. 7-10 were immunofluorescence staining of DAPI, CD68. TSPO and merged image respectively. C. MR-targeted 68Ga-NOTA-MSA PET imaging of macrophages compared with 18F-FDG PET imaging in myocarditis model. D. 18F-Macroflor PET/CT imaging of macrophages in aortic plaques of mice with atherosclerosis. Representative images of PET/CT in wild type mice (upper) and atherosclerotic mice model (lower); Three-dimensional image of PET/CT in atherosclerotic mice model. PET signal was in red (arrows); E. 18F-Macroflor PET/CT imaging of macrophages in murine myocardial infarction model. Long axis view (upper row) and short axis view (lower row); Ex vivo autoradiography and it corresponding TTC staining of infarct heart at day 2 post-MI. Adapted with permission from [84,96,108,139].
Table 2.
Summary of TSPO, MR, MMP, MPO targeted or nanoparticle-based probes illustrated in the present review
| Target | Disease | Preclinical/clinical | Radiotracer |
|---|---|---|---|
| Translocator protein (TSPO) | Atherosclerosis/vascular injury | Preclinical | 11C-PK11195 [94]; 18F-FEDAA1106 [97]; 18F-PBR06 [98] |
| 18F-FEMPA [99]; 18F-GE-180 [100] | |||
| Clinical | 11C-PK11195 [96] | ||
| Abdominal aortic aneurysms | Clinical | 11C-PK11195 [95] | |
| Myocardial infarction | Preclinical/clinical | 18F-GE-180 [101] | |
| Myocarditis | Preclinical | 18F-PBR28 [102]; 18F-CB251 [102] | |
| Mannose receptor (MR) | Atherosclerosis | Preclinical | 18F-FDM [104]; 68Ga-NOTA-MSA [105]; 68Ga-MMR [106] |
| 68Ga-anti-MMR Nb [107] | |||
| Myocarditis | Preclinical | 68Ga-NOTA-MSA [108] | |
| Secreted enzymes | |||
| MMPs | Myocardial infarction | Preclinical | 68Ga-DOTA-MMP-2/9 targeted peptide [114] |
| Atherosclerosis | Preclinical | 68Ga-DOTA-TCTP-1 [116] | |
| MPO | Myocardial infarction | Preclinical | 18F-MAPP [117]; 11C-AZD3241 [124] |
| Nanoparticles (NPs) | |||
| Modified dextran | Atherosclerosis | Preclinical | 64Cu-TNP [132]; 89Zr-DNP [133] |
| High density lipoprotein | Atherosclerosis | Preclinical | 89Zr-AI-HDL and 89Zr-PL-HDL [134] |
| Hyaluronan | Atherosclerosis | Preclinical | 89Zr-HA-NP [135] |
| Dendrimers | Atherosclerosis | Preclinical | 64Cu-(LyP-1)4-dendrimer [136] |
| Porphysome | Myocardial infarction | Preclinical | 64Cu-folate-porphysome [137] |
| Mesoporous silica | Atherosclerosis | Preclinical | 18F-DBCO-MSNs [138] |
| Polyglucose | Atherosclerosis/MI | Preclinical | 18F-Macroflor [139] |
Translocator protein (TSPO)
The 18 kDa-protein translocator protein (TSPO), previously known as peripheral benzodiazepine receptor (PBR), is localized on the outer mitochondrial membrane. TSPO is involved in several essential cellular functions, such as cholesterol transport or steroidogenesis [91,92]. TSPO is expressed in both M1 and M2 macrophages, and some studies reported a higher expression in M2 macrophages [93].
The first generation TSPO-targeted PET tracer, 11C-PK11195 has shown higher accumulation at inflamed plaques compared to non-inflamed plaques in atherosclerotic mice model, but a comparable uptake in healthy artery walls may limit in vivo imaging applications [94]. Another study did not observed any aortic uptake in asymptomatic abdominal aortic aneurysms patients either [95]. However, a subsequent clinical study demonstrated the feasibility of 11C-PK11195 PET for imaging of intraplaque macrophages. Histologic staining on patient carotid plaque verified the tracer specificity since TSPO and CD68 co-localized with tracer uptake on autoradiography (Figure 2B). Moreover, they suggested the higher 11C-PK11195 target-to background ratio (TBR) combined with lower CT attenuation could distinguish recently symptomatic and asymptomatic plaques in patients [96].
The nonspecific binding and short half-life of 11C prompted the design of new generation tracers. To date, 18F-FEDAA1106, 18F-PBR06, 18F-FEMPA and 18F-GE-180 have been investigated in inflammatory vascular models. 18F-FEDAA1106 showed significant uptake in vascular injury regions and displayed higher specificity to discriminate inflamed and non-inflamed vasculature compared to 18F-FDG [97]. 18F-PBR06 PET imaging revealed a higher uptake in atherosclerotic plaques of 32 weeks than that of 22 weeks, implying the potential for evaluating plaque progression [98]. Hellberg et al. reported that both 18F-FEDAA1106 [99] and 18F-GE-180 [100] accumulated in atherosclerotic plaques and the signal correlated well with the distribution of macrophages. However, comparable uptake in healthy vessel walls, attributed to the ubiquitous nature of TPSO expression, limits in vivo PET imaging applications. Clinical translation may be possible since interspecies differences exist.
The third generation TSPO-targeted tracer 18F-GE-180 was investigated in myocardial infarction. In MI mice model, elevated 18F-GE-180 signal could be observed at 1 weeks after infarction and was localized to CD68+ macrophages. After interval decline at 4 weeks, the signal then increased in remote myocardium where less inflammatory cells exist at 8 weeks, implying the complexity of TSPO signal which could also reflect mitochondrial impairment. Interestingly, obvious 11C-PK11195 PET signal was further verified on both heart and brain in a patient after MI, suggesting the immune activation may exist at the heart-brain axis [101]. Another two TSPO tracers have been investigated in rat myocarditis model. In this study, 18F-PBR28 displayed no significant elevation in the model, but 18F-CB251 did [102] (Table 2).
Mannose receptor (CD206)
A highly effective endocytic receptor, the mannose receptor (MR) expression has been reported on macrophages, dendritic cells and nonvascular endothelium [103]. Consensus is that MR is generally expressed on M2 macrophages. Staining of atherosclerotic coronary sections from patients that have suffered sudden cardiac death revealed intense MR positive M2 macrophages in the unstable plaques, which featured with thin-cap fibroatheromas, but almost no MR expression in stable plaques [104].
Mannose is a C2-epimeric sugar molecule with a chemical structure similar to glucose; the only difference is the opposite orientation of oxygen and hydroxyl groups on the second carbon atom. Mannose binds on macrophages via MRs and enters cells via glucose transporters (GLUTs) [104]. Tahara et al. found the uptake of 18F-labeled mannose (18F-FDM) in rabbit plaque is comparable to 18F-FDG and was proportional to macrophage density in tissue. More importantly, using six sets of cell experiments, the authors demonstrated the specific binding of FDM to MR and a macrophage uptake increase of more than 35% for 18F-FDM compared to 18F-FDG [104]. Mannosylated human serum albumin (MSA), a 6-8 nm protein, binds with macrophages via terminal mannose residues. It was previously developed for imaging of sentinel lymph nodes, but Kim and colleagues recently used 68Ga radiolabeled NOTA-MSA for plaque imaging in a rabbit model. The 68Ga-NOTA-MSA uptake in plaque was significantly higher than in healthy arteries and was comparable to 18F-FDG. Cell uptake and blocking experiments verified the binding specificity to M2 macrophages [105].
Senders et al. evaluated a MR targeted nanobody, which was previously used in tumor PET imaging, in atherosclerotic murine and rabbit models after radiolabeling with 64Cu or 68Ga. In the mice model, the 64Cu-MMR showed significant accumulation in the aortic root and arch. Autoradiography and histologic staining for macrophages proved the tracer specificity. Subsequent 64Cu-MMR PET/MR imaging in a rabbit model indicated tracer uptake positively correlated with vessel wall area, but not with the FDG uptake. To meet clinical demands, short-lived 68Ga was also used for radiolabeling. Compared with 4-month results, the tracer uptake, vessel wall area and vascular permeability progressively increased at 8 months in rabbit models. 68Ga-MMR autoradiography also positively correlated with CD206 expression, indicating 68Ga-MMR PET/MR has potential to reflect disease progression and the amount of CD206+ macrophages [106]. Another MR targeted nanobody (68Ga-anti-MMR Nb) has also been studied in ApoE (-/-) atherosclerotic mice model. The positive PET results matched with autoradiographs and Sudan-IV-staining. Immunofluorescence staining revealed MR was predominantly expressed in macrophages located in the fibrous cap layer and shoulder region of the plaques [107].
MR-targeted PET imaging was also investigated in myocarditis disease. The infiltration of MR-positive macrophages was confirmed in a rat myocarditis model and using human histological samples (Figure 2C). The left ventricle uptake of 68Ga-NOTA-MSA in myocarditis was 1.8-fold higher compared with the control group, which could be reduced by cyclosporine-A treatment. Cell and tissue block experiments demonstrated the specificity of 68Ga-NOTA-MSA. Compared with echocardiography, the 68Ga-NOTA-MSA PET imaging displayed early diagnosis efficiency for detecting myocarditis [108] (Table 2).
Macrophages enzymes
Metalloproteinases (MMPs)
Macrophages releases proteolytic matrix metalloproteinases (MMPs) that degrade the cardiac extracellular matrix or erode fibrotic plaque caps and as a result are involved in the cardiac remodeling or plaque rupture process in CVDs. Among them, type 2 and 9 MMPs have been investigated the most [12,109,110]. Molecular imaging of macrophages using MMPs targeted probes have received considerable attention over the recent decade.
While molecular imaging probes have been developed, their application as PET imaging tracers has been relatively unexplored. In 2005, Su and colleagues reported a SPECT probe, 99mTc-RP805, which could specifically bind to the activated catalytic domain of MMPs and displayed a fivefold uptake increase in the infarcted area and a twofold increase in the remote region of MI mice heart [111]. Another study from this group revealed that regional uptake of 99mTc-RP805 was highly concordant with ex vivo MMP-2 activity and the SPECT signal predicted late left ventricular remodeling [112]. Another activatable cell-penetrating peptide probe (ACPP) was described for imaging MMP activity in MI. 177Lu and 125I were chelated to the ends of this peptide. Once MMPs cleaved the middle of ACPP, the 125I-peptide would be washed out and the 177Lu-peptide remained. The authors observed a ten-fold uptake in the infarct myocardium compared to the remote region. However, it still requires an in vivo imaging investigation [113]. To develop an MMP-targeted PET probe, Kiugel et al. selected a peptide using a phage display technique. The peptide displayed a robust performance for targeting MMP-2/9 in a tumor model. Then it was radiolabeled with 68Ga for PET imaging in a myocardial infarction rat model. Ex vivo autoradiography showed higher accumulation in the infarcted area compared to the remote area. Moreover, the tracer uptake also correlated with CD68+ macrophages 7 days after MI. However, high blood signal, tracer instability, and slow clearance limit in vivo PET imaging applications [114].
MMP-targeted SPECT imaging probes for atherosclerosis have been summarized in a previous review by Lenglet et al. [115]. Recently, Kiugel et al. published a PET study for imaging of MMP in atherosclerotic plaques. However, they also observed low blood clearance and tracer instability that would limit applications. In an atherosclerotic murine model, a 1.8-fold uptake of 68Ga-DOTA-TCTP-1 in plaque was detected ex vivo, but it was not discernable with in vivo PET imaging, which may be ascribed to a low target-to-background ratio [116] (Table 2).
Myeloperoxidase (MPO)
Myeloperoxidase (MPO) is an important enzyme associated with oxidative stress and is secreted by neutrophils and pro-inflammatory M1 macrophages but not the anti-inflammatory M2 macrophages [117]. MPO is involved in early plaque formation since it impairs cholesterol transport. By oxidizing low-density lipoproteins (LDL) MPO aids in foam cell formation [118]. It has also been reported that MPO contributes to cardiac remodeling and heart failure following myocardial infarction [119]. As such, MPO is an exciting target for imaging macrophages in CVDs.
To date, there are limited MPO-targeted PET probes, though several MRI and SPECT imaging studies have been conducted. Chen et al. designed an MPO-Gd (bis-5HT-DTPA-Gd) probe for MR imaging and subsequently demonstrated its feasibility for imaging MPO activity in myocardial infarction and atherosclerotic plaque models [120-122]. Wu et al. radiolabeled bis-5HT-DTPA with 111In for SPECT imaging in an atherosclerotic mice model. The probe showed high uptake in the aortic wall of atherosclerotic mice model, but the tracer elimination was significantly slower compared to WT mice. This may result from the increase in size after self-oligomerization of the probe induced by MPO oxidization [123].
The lower sensitivity and potential toxicity limit the application of these MR or SPECT tracers in a clinical setting. In 2015, the MPO inhibitor-based PET tracer 11C-AZD3241 was found to enter the monkey brain [124]. However, the clinical application of the probe was still limited since the tracer only visualized the presence of MPO but not the activity and the short half-life of 11C. To overcome these limitations, Wang et al. synthesized a 18F-labeled PET tracer, 18F-MAPP, which displayed high specificity and sensitivity, nontoxic, proper half-life, and crossed the blood-brain barrier. Similar to the mechanism of MPO-Gd, MPO could oxidize 18F-MAPP and causes free radicals to bind to proteins and local retention of the radiotracer; thus the probe reflected MPO activity. The authors also demonstrated the feasibility and specificity of 18F-MAPP in a mice myocardial infarction model and found the signal-background ratio was twice as high as MPO-Gd or its SPECT analogs, results promising for clinical application [117] (Table 2).
Nanoparticles (NPs) based PET imaging
Recently, PET imaging with NPs has garnered significant interest in CVDs [125]. As a multi-functional platform, nanoparticles could amplify the PET signal as well as carry therapeutic drugs, thus exhibit additional theranostic merits [126-128].
NPs based PET imaging of macrophages generally exploits the high endocytosis or micropinocytosis activity of macrophages [129,130]. However, whether NPs can be taken up by macrophages at an injury site is complicated and is related to multiple factors including size, shape, surface properties, and opsonization. The general consensus is that NPs diameter between 10 and 300 nm are preferable for macrophages uptake at diseased sites. If much larger (over 1000 nm) or smaller (under 8 nm), NPs tend to accumulate in the liver and lungs or are cleared by the kidneys, respectively [131]. The choice of isotope for PET imaging depends on the circulation time of the NPs. Long-circulating nanomaterials require isotopes with long half-lifes, such as 89Zr or 64Cu. The clinical feasibility of short half-life isotopes such as 18F or 68Ga are more suitable to NPs that are rapily cleared [131].
In 2008, Nahrendorf et al. synthesized a 64Cu-labeled trimodal (PET/MRI/optical) NP. The 64Cu-TNP had a monocrystalline iron oxide NP MION as the core and was modified with a surface dextran coating. In ApoE-deficient mice models, 64Cu-TNP displayed significant accumulation in the aortic root and arch, which correlated with MR and optical imaging results. Ex vivo autoradiography and Oil Red O staining corroborated that the sites where 64Cu-TNP accumulated were macrophage-rich plaques. Flow cytometry of cellular suspensions from atherosclerotic plaque revealed macrophages contribute to 73.9% of overall signal. Compared with 18F-FDG, 64Cu-TNP PET signal was slightly higher and persisted longer [132]. Based on this work, this group further developed a core-free dextran nanoparticles (DNP) using clinically approved components and modified them with a 89Zr chelator and a near-infrared fluorochrome (VT680) for PET/NIR imaging of atherosclerotic plaque. High uptake of 89Zr-DNP was observsed in macrophage-rich plaques in a murine model. Moreover, the signal decreased using a siRNA targeted to CCR2, which is a monocytes/macrophages recruitment marker [133]. Perez-Medina et al. developed a discoidal high-density lipoprotein (HDL) nanoparticle. After labeling with 89Zr or using near-infrared fluorescence, the HDL-NP was found to migrate to macrophage-rich plaques in three atherosclerotic models (mice, rabbit and pig). Since HDL is a natural material removes cholesterol from plaque, HDL-NP has promise for clinical translation [134]. Another biocompatible material used for in vivo imaging is hyaluronan (HA), which is a component of extracellular matrix and has diverse biological activities. Recently, 89Zr/Cy7 labeled hyaluronan nanoparticles (HA-NPs) showed 30% higher accumulation in the aortas of a atherosclerotic model mice compared to controls using PET/MR imaging. The Cy7-HA-NPs displayed a 6 to 40-fold uptake increase in aortic macrophages compared to normal tissue macrophages. In addition, the HA-NPs exhibited immune modulation effects that inhibited disease progression [135]. LyP-1 is a cyclic 9-amino acid peptide that binds with the p32 protein on activated macrophages. Seo et al. improved the dectection of macrophages using LyP-1 decorated dendritic NPs compared to 18F labeled single LyP-1 imaging [136]. Ni et al. described a 64Cu-folate-porphysome, a bilayered nanovesicle self-assembled from phospholipid-porphyrin conjugates, for tracking macrophages after myocardial infarction. After decoration with folate on the surface and radiolabeled with 64Cu, the PET results showed that 64Cu-folate-porphysome accumulated at infarct sites on days 2 and 7. However, 64Cu-porphysome without folate modification did not [137].
Compared with 89Zr and 64Cu, 18F is more suitable in the clinic since there is less radiation exposure from a short half-life. 18F radiolabel NPs have recently drawn attention in PET imaging. Based on a pre-targeting strategy, Jeong et al. investigated the feasibility of 18F-labeledmesoporous silica NPs (DBCO-MSNs) for tracking macrophages migration in atherosclerosis model and tumor model. DBCO-MSNs and macrophages were incubated in vitro and then injected into model mice. After 1-8 days, [18F]2 was intravenously injected for DBCO-MSNs labeling. After PET imaging, atherosclerotic mice injected with DBCO-MSNs-RAW cells showed higher SUVs in aortas than the mice injected with normal RAW cells, which were supported by autoradiography and immunostaining. These results indicate pre-targeting with DBCO-MSNs is feasible for tracking macrophages in an atherosclerotic model [138]. Keliher and colleagues reported small modified-polyglucose NPs (18F-Macroflor, 5.0 nm diameter), which displayed a high avidity for macrophage. Since the size of NPs were below the renal elimination threshold, they were eliminated from the kidneys and had a short circulating time (only 21.7 min in healthy non-human primate). The small size and short circulating time made Macroflor suitablefor labeling with short half-life isotopes such as 18F. The 18F-Macroflor was then successfully applied for imaging of macrophage-rich plaques in mice and ribbit models (Figure 2D) and injured heart tissue in a myocardial infarction mice model (Figure 2E). Flow cytometry gating on isolated cells from aortic or infarcted tissues verified the NP was primarily taken up by macrophages but not by neutrophils or lymphocytes. The clinically suitable radioisotope, natural material, and facile click labeling made the 18F-Macroflor highly promising for clinical translation [139] (Table 2).
Other potential targets
Inducible nitric oxide synthase (iNOS) is a crucial enzyme that generates large amounts of NO when induced by inflammatory stimuli. Studies have shown that iNOS is highly upregulated in activated M1 macrophages and can therefore be exploited for targeted macrophage imaging [140]. 18F-NOS was developed in 2009 and has successfully been used for PET imaging of iNOS in models of lung inflammation [141,142]. Moreover, increased 18F-NOS was reported in patients with organ rejection after heart transplantation surgery. The myocardial 18F-NOS uptake also correlated with iNOS staining in heart tissue [143].
Formyl peptide receptor 1 (Fpr1) belongs to the G-protein-coupled receptor family. Expression of Fpr1 has been reported on activated macrophages and other leukocytes. A previous study labeled a Fpr1 specific binding peptide (cinnamoyl-F-(D)L-F-(D)L-F) with 64Cu for PET imaging of infiltrating macrophages in osteoarthritic rats. The diseased knee displayed approximately a 6-fold increase compared to its contralateral healthy knee. The tissue and cell lines staining indicated the expression of Fpr1 expressed on macrophages and synovial membranes [144].
Complement receptor of the immunoglobulin superfamily (CRIg) is expressed on tissue-resident macrophages and has an affinity for C3b and iC3b, which can clear probes tagged with C3b and iC3b [145]. Zhang et al. reported a nanobody targeting CRIg for SPECT imaging. 99mTc-NbV4m119 accumulated in arthritic lesions of the inflamed murine paws. Immunofluorescence staining verified the co-localization of CRIg and CD68+ macrophages in inflamed knee synovium tissue [146].
Several CD markers on macrophages have been identified, which also hold potential to detect macrophages in CVDs using PET imaging. However, to date, most probes targeted these CD markers were antibody-based and mainly investigated in tumor models or other inflammatory related diseases. We will highlight all of these studies irrespective of the modality and disease type. The targets included CD80, CD163, CD11b, CD169, CD68 and F4/80.
The co-stimulatory molecules CD80 and CD86 were found on M1 macrophages in human atherosclerotic plaque and more so in vulnerable plaques [147]. Muller et al. synthesized 11C-AM7, a CD80-specific radiotracer, and showed 3-fold higher binding to vulnerable human plaques compared to stable plaques ex vivo [147]. The 11C-AM7 PET imaging was further proved in a shear stress-induced atherosclerosis mouse model [148]. CD163 is a scavenger receptor for removing plasma hemoglobin during intravascular hemolysis. It has been found to be highly expressed on monocytes and macrophages, particularly M2 macrophages. Eichendorff et al. radiolabeled a CD163 antibody (ED2) with 68Ga and found that it could specifically bind to the CD163 receptor in vitro. Additionally, 68Ga-ED2 accumulated in macrophage-rich tissues of collagen-induced arthritis rat [149]. CD11b, also known as Mac-1, is expressed on macrophage and other leucocytes. Over the years, several radiolabeled anti-CD11b antibody tracers have been investigated in tumor or inflammatory models, including 64Cu-NOTA-αCD11b-mAb in breast and melanoma tumor [150], 89Zr-anti-CD11b Ab in glioblastoma [151], 64Cu-αCD11b in chronic ear inflammation [152], 89Zr-α-CD11b in inflammatory bowel disease (IBD) [153], and 99mTc-MAG3-anti-CD11b in atherosclerosis [154]. 99mTc-SER-4 is a SPECT probe targeting CD169 (sialoadhesin, Sn) and was recently developed to monitor macrophages in chronic transplant rejection [155]. As a pan-macrophages marker in murine model, an F4/80 receptor targeted antibody was applied for imaging tumor-associated macrophages by labeling with 111In. In a murine breast cancer model, the 111In-anti-F4/80-A3-1 uptake was prominate in the tumor and spleen and decreased after clodronate treatment [156]. CD68 is another scavenger receptor on macrophages and is involved in the uptake of modified low-density lipoproteins thereby promoting the formation of foam cells. Thus, an anti-CD68 PET tracer (64Cu-CD68-Fc) may help monitor plaque development [157] (Table 3).
Table 3.
Summary of other potential targets illustrated in the present review
| Target | Disease | Preclinical/clinical | Radiotracer |
|---|---|---|---|
| iNOS | Acute lung inflammation | Clinical | 18F-NOS [141,142] |
| Heart transplantation | Clinical | 18F-NOS [143] | |
| Fpr1 | Osteoarthritis | Preclinical | cFLFLF-PEG-64Cu [144] |
| CRIg | Rheumatoid arthritis | Preclinical | 99mTc-NbV4m119 [146] |
| CD80 | Atherosclerosis | Preclinical | 11C-AM7 [147,148] |
| CD163 | Collagen-induced arthritis | Preclinical | 68Ga-ED2 [149] |
| CD11b | Cancer | Preclinical | 64Cu-NOTA-αCD11b-mAb [150]; 89Zr-anti-CD11b Ab [151] |
| Inflamed ear/IBD/Atherosclerosis | Preclinical | 64Cu-αCD11b [152]; 89Zr-α-CD11b [153]; 99mTc-anti-CD11b [154] | |
| CD169 | Allograft rejection | Preclinical | 99mTc-SER-4 [155] |
| CD68 | Atherosclerosis | Preclinical | 64Cu-CD68-Fc [157] |
| F4/80 | Breast tumor | Preclinical | 111In-anti-F4/80-A3-1 [156] |
Conclusions and future perspectives
Since the cardiovascular system so vital and well protected, biopsies are risky and challenging. Thus, noninvasive PET imaging has immense potential to provide diagnostic information, particularly when integrated with the soft-tissue contrast of MRI. In vivo tracking of macrophages will benefit both basic research and clinical decision making for CVDs. Revealing macrophages function in CVDs may permit early detection of atherosclerosis or discrimination of unstable plaques. In turn, this can decrease risk of MI or stroke, facilitate the diagnosis of inflammatory cardiomyopathies, and enable monitoring of anti-inflammatory therapies in CVDs.
Over the past 15 years, over 50 macrophage-targeted probes have been described and studied. Among them, metabolism or proliferation-based tracers, including 18F-FDG, 11C-methionine, 11C-Choline, 18F-FCH and 18F-FLT, are nonspecific radiotracers, which seem inferior to specific target-based probes. However, in view of current positive clinical results, the clinical value for diagnosis and therapy monitoring using these probes should not be ignored despite their nonspecific nature.
Additional clinical and comparative studies are required the probes currently developed, particularly those with promising animal study results. To date, including the metabolism- and proliferation-based tracers, 13 probes in have been clinically investigated, including 68Ga-pentixafor, 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE, 64Cu-DOTA-TATE, 11C-PK11195, 18F-GE-180 and 18F-NOS. Unfortunately, most of them involved a small number of patients, were single-center, and retrospective studies. More multi-center and prospective clinical studies would help determine the clinical feasibility and effectiveness of these tracers. Moreover, comparative studies are still lacking. While difficult to compare because different targets may reflect various aspects of macrophage function or the nonspecifity of FDG, additional characteristic information of each tracer would be provide. Furthermore, there are still several available tracers that have not been fully exploited in CVDs. Antibody-based probes are an example that have been developed for PET and SPECT imaging in cancer and other inflammatory diseases but not investigated in CVDs. Thus, antibody probes may hold promising for CVDs in the future.
Next generation probes can be developed by modifying the chemical structure those that already exist. Current probes are not all perfect; thus, modifying the chemical structure to balance the hydrophile or lipophilia may improve probes’ performance. Alternatively, developing new targets and novel tracers are still appealing. Each target reflects just one of macrophage functions and not all patients may respond effectively to one tracer. Previous studies have reported positive imaging in some patients but not in the others, which may be ascribed to different receptor expression levels among individuals. Hence, novel targets and tracers with high specificity and generally expression are helpful. Apart from diagnosis, tracers for therapy or theranostic are another promising direction. Early diagnosis would aid in the early prevention of CVDs. Therapy or theranostic tracers would help drug release for modulating macrophages polarization and also treatment monitoring. To this end, NPs are an excellent choice due to the versatile properties that facilitate drug loading and imaging applications.
Target specificity is an essential aspect of imaging studies. Most of studies discussed in this review generally used a comparison between immunohistochemical staining for CD68+ and autoradiography to verify the tracers’ specificity for macrophages. While this strategy is useful, it is not comprehensive. This comparison does not provide information about the contribution from other cells to the signal. While finding an absolutely pure target is challenging, the specificity of a tracer clarifies what the signal is truly reflecting. Flow cytometric gating on cells derived from target tissues has been applied in some reports, which may helpful to overcome this concern.
Distinguishing macrophage subtypes with precision is challenging. Generally, two or three cell markers are required to verify M1 or M2 macrophages using flow cytometry, which is difficult for with single-targeted PET imaging. Additionally, M1 and M2 macrophage phenotypes are not stable and can change in response to environmental signals. Although some targets are reported to be preferably expressed on M2 or M1 macrophages, the specificity is not entirely clear, which makes imaging of macrophage subtypes difficult.
PET imaging of macrophages have been designed to target and image one aspect of macrophages. PET imaging of macrophages in CVDs have distinct advantages for patient diagnosis and can inform treatment decisions. Future work will help us understand the role of macrophages in CVDs and ultimately benefit patients.
Acknowledgements
This study was funded by The National Natural Science Foundation of China (Grant Nos. 91959208, 81971646), The University of Wisconsin-Madison, The National Institutes of Health (P30CA014520).
Disclosure of conflict of interest
None.
References
- 1.Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, Mente A, Yusuf S. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. 2017;121:677–694. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 5.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–537. doi: 10.1038/s41590-018-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 11.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JL. Metalloproteinases in atherosclerosis. Eur J Pharmacol. 2017;816:93–106. doi: 10.1016/j.ejphar.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke SA, Dunne A, Monaghan MG. The role of macrophages in the infarcted myocardium: orchestrators of ECM remodeling. Front Cardiovasc Med. 2019;6:101. doi: 10.3389/fcvm.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (part 4) J Am Coll Cardiol. 2018;72:2213–2230. doi: 10.1016/j.jacc.2018.08.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JW, Giannarelli C, Rahman A, Randolph GJ, Kovacic JC. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (part 1) J Am Coll Cardiol. 2018;72:2166–2180. doi: 10.1016/j.jacc.2018.08.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serei VD, Fyfe B. The many faces of cardiac sarcoidosis. Am J Clin Pathol. 2020;153:294–302. doi: 10.1093/ajcp/aqz169. [DOI] [PubMed] [Google Scholar]
- 18.Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937. [DOI] [PubMed] [Google Scholar]
- 19.Iung B, Duval X. Infective endocarditis: innovations in the management of an old disease. Nat Rev Cardiol. 2019;16:623–635. doi: 10.1038/s41569-019-0215-0. [DOI] [PubMed] [Google Scholar]
- 20.Jiemy WF, Heeringa P, Kamps J, van der Laken CJ, Slart R, Brouwer E. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging of macrophages in large vessel vasculitis: current status and future prospects. Autoimmun Rev. 2018;17:715–726. doi: 10.1016/j.autrev.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Dobrucki LW, Sinusas AJ. PET and SPECT in cardiovascular molecular imaging. Nat Rev Cardiol. 2010;7:38–47. doi: 10.1038/nrcardio.2009.201. [DOI] [PubMed] [Google Scholar]
- 22.Jivraj N, Phinikaridou A, Shah AM, Botnar RM. Molecular imaging of myocardial infarction. Basic Res Cardiol. 2014;109:397. doi: 10.1007/s00395-013-0397-2. [DOI] [PubMed] [Google Scholar]
- 23.Garcia EV. Physical attributes, limitations, and future potential for PET and SPECT. J Nucl Cardiol. 2012;19(Suppl 1):S19–29. doi: 10.1007/s12350-011-9488-3. [DOI] [PubMed] [Google Scholar]
- 24.Wilk B, Wisenberg G, Dharmakumar R, Thiessen JD, Goldhawk DE, Prato FS. Hybrid PET/MR imaging in myocardial inflammation post-myocardial infarction. J Nucl Cardiol. 2019 doi: 10.1007/s12350-019-01973-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimlott SL, Sutherland A. Molecular tracers for the PET and SPECT imaging of disease. Chem Soc Rev. 2011;40:149–162. doi: 10.1039/b922628c. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Adv Drug Deliv Rev. 2010;62:1031–1051. doi: 10.1016/j.addr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Conti PS. Target-specific delivery of peptide-based probes for PET imaging. Adv Drug Deliv Rev. 2010;62:1005–1022. doi: 10.1016/j.addr.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Kircher M, Lapa C. Novel noninvasive nuclear medicine imaging techniques for cardiac inflammation. Curr Cardiovasc Imaging Rep. 2017;10:6. doi: 10.1007/s12410-017-9400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millar BC, Prendergast BD, Alavi A, Moore JE. 18FDG-positron emission tomography (PET) has a role to play in the diagnosis and therapy of infective endocarditis and cardiac device infection. Int J Cardiol. 2013;167:1724–1736. doi: 10.1016/j.ijcard.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Blomberg BA, Hoilund-Carlsen PF. [(1)(8)F]-fluorodeoxyglucose PET imaging of atherosclerosis. PET Clin. 2015;10:1–7. doi: 10.1016/j.cpet.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Ali A, Tawakol A. FDG PET/CT imaging of carotid atherosclerosis. Neuroimaging Clin N Am. 2016;26:45–54. doi: 10.1016/j.nic.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Bengel FM. Imaging of post-infarct inflammation: moving forward toward clinical application. Circ Cardiovasc Imaging. 2016;9:e004713. doi: 10.1161/CIRCIMAGING.116.004713. [DOI] [PubMed] [Google Scholar]
- 33.Fox JJ, Strauss HW. One step closer to imaging vulnerable plaque in the coronary arteries. J Nucl Med. 2009;50:497–500. doi: 10.2967/jnumed.108.056325. [DOI] [PubMed] [Google Scholar]
- 34.Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I. Variability and uncertainty of 18F-FDG PET imaging protocols for assessing inflammation in atherosclerosis: suggestions for improvement. J Nucl Med. 2015;56:552–559. doi: 10.2967/jnumed.114.142596. [DOI] [PubMed] [Google Scholar]
- 35.Leung K. Molecular imaging and contrast agent database (MICAD) Bethesda (MD): National Center for Biotechnology Information (US); 2004. l-[methyl-(11)C]Methionine. [PubMed] [Google Scholar]
- 36.Morooka M, Kubota K, Kadowaki H, Ito K, Okazaki O, Kashida M, Mitsumoto T, Iwata R, Ohtomo K, Hiroe M. 11C-methionine PET of acute myocardial infarction. J Nucl Med. 2009;50:1283–1287. doi: 10.2967/jnumed.108.061341. [DOI] [PubMed] [Google Scholar]
- 37.Taki J, Wakabayashi H, Inaki A, Imanaka-Yoshida K, Hiroe M, Ogawa K, Morooka M, Kubota K, Shiba K, Yoshida T, Kinuya S. 14C-Methionine uptake as a potential marker of inflammatory processes after myocardial ischemia and reperfusion. J Nucl Med. 2013;54:431–436. doi: 10.2967/jnumed.112.112060. [DOI] [PubMed] [Google Scholar]
- 38.Thackeray JT, Bankstahl JP, Wang Y, Wollert KC, Bengel FM. Targeting amino acid metabolism for molecular imaging of inflammation early after myocardial infarction. Theranostics. 2016;6:1768–1779. doi: 10.7150/thno.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maya Y, Werner RA, Schutz C, Wakabayashi H, Samnick S, Lapa C, Zechmeister C, Jahns R, Jahns V, Higuchi T. 11C-Methionine PET of myocardial inflammation in a rat model of experimental autoimmune myocarditis. J Nucl Med. 2016;57:1985–1990. doi: 10.2967/jnumed.116.174045. [DOI] [PubMed] [Google Scholar]
- 40.DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, Reiman R, Price DT, Coleman RE. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805–1814. [PubMed] [Google Scholar]
- 41.Kato K, Schober O, Ikeda M, Schafers M, Ishigaki T, Kies P, Naganawa S, Stegger L. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:1622–1628. doi: 10.1007/s00259-009-1152-7. [DOI] [PubMed] [Google Scholar]
- 42.Laitinen IE, Luoto P, Nagren K, Marjamaki PM, Silvola JM, Hellberg S, Laine VJ, Yla-Herttuala S, Knuuti J, Roivainen A. Uptake of 11C-choline in mouse atherosclerotic plaques. J Nucl Med. 2010;51:798–802. doi: 10.2967/jnumed.109.071704. [DOI] [PubMed] [Google Scholar]
- 43.Matter CM, Wyss MT, Meier P, Spath N, von Lukowicz T, Lohmann C, Weber B, Ramirez de Molina A, Lacal JC, Ametamey SM, von Schulthess GK, Luscher TF, Kaufmann PA, Buck A. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscler Thromb Vasc Biol. 2006;26:584–589. doi: 10.1161/01.ATV.0000200106.34016.18. [DOI] [PubMed] [Google Scholar]
- 44.Bucerius J, Schmaljohann J, Bohm I, Palmedo H, Guhlke S, Tiemann K, Schild HH, Biersack HJ, Manka C. Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans--first results. Eur J Nucl Med Mol Imaging. 2008;35:815–820. doi: 10.1007/s00259-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 45.Vöö S, Kwee RM, Sluimer JC, Schreuder FH, Wierts R, Bauwens M, Heeneman S, Cleutjens JP, van Oostenbrugge RJ, Daemen JW, Daemen MJ, Mottaghy FM, Kooi ME. Imaging intraplaque inflammation in carotid atherosclerosis with 18F-fluorocholine positron emission tomography-computed tomography: prospective study on vulnerable atheroma with immunohistochemical validation. Circ Cardiovasc Imaging. 2016;9:e004467. doi: 10.1161/CIRCIMAGING.115.004467. [DOI] [PubMed] [Google Scholar]
- 46.Leung K. Molecular imaging and contrast agent database (MICAD) Bethesda (MD): National Center for Biotechnology Information (US); 2004. 3’-Deoxy-3’’-[(18)F]fluorothymidine. [PubMed] [Google Scholar]
- 47.Ye YX, Calcagno C, Binderup T, Courties G, Keliher EJ, Wojtkiewicz GR, Iwamoto Y, Tang J, Perez-Medina C, Mani V, Ishino S, Johnbeck CB, Knigge U, Fayad ZA, Libby P, Weissleder R, Tawakol A, Dubey S, Belanger AP, Di Carli MF, Swirski FK, Kjaer A, Mulder WJ, Nahrendorf M. Imaging macrophage and hematopoietic progenitor proliferation in atherosclerosis. Circ Res. 2015;117:835–845. doi: 10.1161/CIRCRESAHA.115.307024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gandhi R, Cawthorne C, Craggs LJL, Wright JD, Domarkas J, He P, Koch-Paszkowski J, Shires M, Scarsbrook AF, Archibald SJ, Tsoumpas C, Bailey MA. Cell proliferation detected using [(18)F]FLT PET/CT as an early marker of abdominal aortic aneurysm. J Nucl Cardiol. 2019 doi: 10.1007/s12350-019-01946-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajagopalan L, Rajarathnam K. Structural basis of chemokine receptor function--a model for binding affinity and ligand selectivity. Biosci Rep. 2006;26:325–339. doi: 10.1007/s10540-006-9025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz MK, Wells TN. New therapeutics that modulate chemokine networks. Nat Rev Drug Discov. 2002;1:347–358. doi: 10.1038/nrd795. [DOI] [PubMed] [Google Scholar]
- 51.van der Vorst EP, Doring Y, Weber C. Chemokines and their receptors in atherosclerosis. J Mol Med (Berl) 2015;93:963–971. doi: 10.1007/s00109-015-1317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212. doi: 10.3389/fphys.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110:174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol. 2010;88:41–55. doi: 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- 55.Wester HJ, Keller U, Schottelius M, Beer A, Philipp-Abbrederis K, Hoffmann F, Simecek J, Gerngross C, Lassmann M, Herrmann K, Pellegata N, Rudelius M, Kessler H, Schwaiger M. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5:618–630. doi: 10.7150/thno.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kircher M, Herhaus P, Schottelius M, Buck AK, Werner RA, Wester HJ, Keller U, Lapa C. CXCR4-directed theranostics in oncology and inflammation. Ann Nucl Med. 2018;32:503–511. doi: 10.1007/s12149-018-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thackeray JT, Derlin T, Haghikia A, Napp LC, Wang Y, Ross TL, Schafer A, Tillmanns J, Wester HJ, Wollert KC, Bauersachs J, Bengel FM. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc Imaging. 2015;8:1417–1426. doi: 10.1016/j.jcmg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Lapa C, Reiter T, Werner RA, Ertl G, Wester HJ, Buck AK, Bauer WR, Herrmann K. [(68)Ga]Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 expression after myocardial infarction. JACC Cardiovasc Imaging. 2015;8:1466–1468. doi: 10.1016/j.jcmg.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Reiter T, Kircher M, Schirbel A, Werner RA, Kropf S, Ertl G, Buck AK, Wester HJ, Bauer WR, Lapa C. Imaging of C-X-C Motif chemokine receptor CXCR4 expression after myocardial infarction with [(68)Ga]pentixafor-PET/CT in correlation with cardiac MRI. JACC Cardiovasc Imaging. 2018;11:1541–1543. doi: 10.1016/j.jcmg.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Hyafil F, Pelisek J, Laitinen I, Schottelius M, Mohring M, Doring Y, van der Vorst EP, Kallmayer M, Steiger K, Poschenrieder A, Notni J, Fischer J, Baumgartner C, Rischpler C, Nekolla SG, Weber C, Eckstein HH, Wester HJ, Schwaiger M. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with the radiotracer (68)Ga-Pentixafor for PET. J Nucl Med. 2017;58:499–506. doi: 10.2967/jnumed.116.179663. [DOI] [PubMed] [Google Scholar]
- 61.Weiberg D, Thackeray JT, Daum G, Sohns JM, Kropf S, Wester HJ, Ross TL, Bengel FM, Derlin T. Clinical molecular imaging of chemokine receptor CXCR4 expression in atherosclerotic plaque using (68)Ga-Pentixafor PET: correlation with cardiovascular risk factors and calcified plaque burden. J Nucl Med. 2018;59:266–272. doi: 10.2967/jnumed.117.196485. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Heber D, Leike T, Beitzke D, Lu X, Zhang X, Wei Y, Mitterhauser M, Wadsak W, Kropf S, Wester HJ, Loewe C, Hacker M, Haug AR. [68Ga]Pentixafor-PET/MRI for the detection of Chemokine receptor 4 expression in atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2018;45:558–566. doi: 10.1007/s00259-017-3831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Yu W, Wollenweber T, Lu X, Wei Y, Beitzke D, Wadsak W, Kropf S, Wester HJ, Haug AR, Zhang X, Hacker M. [(68)Ga]Pentixafor PET/MR imaging of chemokine receptor 4 expression in the human carotid artery. Eur J Nucl Med Mol Imaging. 2019;46:1616–1625. doi: 10.1007/s00259-019-04322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kircher M, Tran-Gia J, Kemmer L, Zhang X, Schirbel A, Werner RA, Buck AK, Wester HJ, Hacker M, Lapa C, Li X. Imaging inflammation in atherosclerosis with CXCR4-directed (68)Ga-Pentixafor PET/CT - correlation with (18)F-FDG PET/CT. J Nucl Med. 2020;61:751–756. doi: 10.2967/jnumed.119.234484. [DOI] [PubMed] [Google Scholar]
- 65.Derlin T, Sedding DG, Dutzmann J, Haghikia A, Konig T, Napp LC, Schutze C, Owsianski-Hille N, Wester HJ, Kropf S, Thackeray JT, Bankstahl JP, Geworski L, Ross TL, Bauersachs J, Bengel FM. Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [(68)Ga]pentixafor PET/CT. Eur J Nucl Med Mol Imaging. 2018;45:1934–1944. doi: 10.1007/s00259-018-4076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Auvynet C, Baudesson de Chanville C, Hermand P, Dorgham K, Piesse C, Pouchy C, Carlier L, Poupel L, Barthelemy S, Felouzis V, Lacombe C, Sagan S, Chemtob S, Quiniou C, Salomon B, Deterre P, Sennlaub F, Combadiere C. ECL1i, d(LGTFLKC), a novel, small peptide that specifically inhibits CCL2-dependent migration. FASEB J. 2016;30:2370–2381. doi: 10.1096/fj.201500116. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Li W, Luehmann HP, Zhao Y, Detering L, Sultan DH, Hsiao HM, Krupnick AS, Gelman AE, Combadiere C, Gropler RJ, Brody SL, Kreisel D. Noninvasive Imaging of CCR2(+) cells in ischemia-reperfusion injury after lung transplantation. Am J Transplant. 2016;16:3016–3023. doi: 10.1111/ajt.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Gunsten SP, Sultan DH, Luehmann HP, Zhao Y, Blackwell TS, Bollermann-Nowlis Z, Pan JH, Byers DE, Atkinson JJ, Kreisel D, Holtzman MJ, Gropler RJ, Combadiere C, Brody SL. PET-based imaging of chemokine receptor 2 in experimental and disease-related lung inflammation. Radiology. 2017;283:758–768. doi: 10.1148/radiol.2016161409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heo GS, Kopecky B, Sultan D, Ou M, Feng G, Bajpai G, Zhang X, Luehmann H, Detering L, Su Y, Leuschner F, Combadiere C, Kreisel D, Gropler RJ, Brody SL, Liu Y, Lavine KJ. Molecular imaging visualizes recruitment of inflammatory monocytes and macrophages to the injured heart. Circ Res. 2019;124:881–890. doi: 10.1161/CIRCRESAHA.118.314030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Pierce R, Luehmann HP, Sharp TL, Welch MJ. PET imaging of chemokine receptors in vascular injury-accelerated atherosclerosis. J Nucl Med. 2013;54:1135–1141. doi: 10.2967/jnumed.112.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK, Gropler RJ, Hawker CJ, Liu Y. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J Nucl Med. 2014;55:629–634. doi: 10.2967/jnumed.113.132001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luehmann HP, Detering L, Fors BP, Pressly ED, Woodard PK, Randolph GJ, Gropler RJ, Hawker CJ, Liu Y. PET/CT imaging of chemokine receptors in inflammatory atherosclerosis using targeted nanoparticles. J Nucl Med. 2016;57:1124–1129. doi: 10.2967/jnumed.115.166751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 74.Leung K. Molecular imaging and contrast agent database (MICAD) Bethesda (MD): National Center for Biotechnology Information (US); 2004. (68)Ga-1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid-Cpa-cyclo(d-Cys-amino-Phe-hydroorotic acid-D-4-amino-Phe(carbamoyl)-Lys-Thr-Cys)-D-Tyr-NH2 (JR11) [PubMed] [Google Scholar]
- 75.Li X, Bauer W, Kreissl MC, Weirather J, Bauer E, Israel I, Richter D, Riehl G, Buck A, Samnick S. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis. 2013;230:33–39. doi: 10.1016/j.atherosclerosis.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 76.Rominger A, Saam T, Vogl E, Ubleis C, la Fougere C, Forster S, Haug A, Cumming P, Reiser MF, Nikolaou K, Bartenstein P, Hacker M. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med. 2010;51:193–197. doi: 10.2967/jnumed.109.070672. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, Bauer W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2:52. doi: 10.1186/2191-219X-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mojtahedi A, Alavi A, Thamake S, Amerinia R, Ranganathan D, Tworowska I, Delpassand ES. Assessment of vulnerable atherosclerotic and fibrotic plaques in coronary arteries using (68)Ga-DOTATATE PET/CT. Am J Nucl Med Mol Imaging. 2015;5:65–71. [PMC free article] [PubMed] [Google Scholar]
- 79.Rinne P, Hellberg S, Kiugel M, Virta J, Li XG, Kakela M, Helariutta K, Luoto P, Liljenback H, Hakovirta H, Gardberg M, Airaksinen AJ, Knuuti J, Saraste A, Roivainen A. Comparison of somatostatin receptor 2-targeting PET tracers in the detection of mouse atherosclerotic plaques. Mol Imaging Biol. 2016;18:99–108. doi: 10.1007/s11307-015-0873-1. [DOI] [PubMed] [Google Scholar]
- 80.Malmberg C, Ripa RS, Johnbeck CB, Knigge U, Langer SW, Mortensen J, Oturai P, Loft A, Hag AM, Kjaer A. 64Cu-DOTATATE for noninvasive assessment of atherosclerosis in large arteries and its correlation with risk factors: head-to-head comparison with 68Ga-DOTATOC in 60 patients. J Nucl Med. 2015;56:1895–1900. doi: 10.2967/jnumed.115.161216. [DOI] [PubMed] [Google Scholar]
- 81.Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Hojgaard L, Ripa RS, Kjaer A. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol. 2015;35:1696–1703. doi: 10.1161/ATVBAHA.114.305067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, Starks LT, Martin-Garrido A, Manavaki R, Yu E, Kuc RE, Grassi L, Kreuzhuber R, Kostadima MA, Frontini M, Kirkpatrick PJ, Coughlin PA, Gopalan D, Fryer TD, Buscombe JR, Groves AM, Ouwehand WH, Bennett MR, Warburton EA, Davenport AP, Rudd JH. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J Am Coll Cardiol. 2017;69:1774–1791. doi: 10.1016/j.jacc.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wan MYS, Endozo R, Michopoulou S, Shortman R, Rodriguez-Justo M, Menezes L, Yusuf S, Richards T, Wild D, Waser B, Reubi JC, Groves A. PET/CT imaging of unstable carotid plaque with (68)Ga-labeled somatostatin receptor ligand. J Nucl Med. 2017;58:774–780. doi: 10.2967/jnumed.116.181438. [DOI] [PubMed] [Google Scholar]
- 84.Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J. 2015;36:2404. doi: 10.1093/eurheartj/ehv278. [DOI] [PubMed] [Google Scholar]
- 85.Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, Pizarro C, Skowasch D, Thomas L, Schlesinger-Irsch U, Thomas D, Bundschuh RA, Bauer WR, Gartner FC. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget. 2016;7:77807–77814. doi: 10.18632/oncotarget.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, Buck AK, Ertl G, Bauer WR. Imaging of myocardial inflammation with somatostatin receptor based PET/CT - A comparison to cardiac MRI. Int J Cardiol. 2015;194:44–49. doi: 10.1016/j.ijcard.2015.05.073. [DOI] [PubMed] [Google Scholar]
- 87.Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, Grohe C, Nickenig G, Skowasch D. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail. 2018;5:249–261. doi: 10.1002/ehf2.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016;6:52. doi: 10.1186/s13550-016-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bravo PE, Bajaj N, Padera RF, Morgan V, Hainer J, Bibbo CF, Harrington M, Park MA, Hyun H, Robertson M, Lakdawala NK, Groarke J, Stewart GC, Dorbala S, Blankstein R, Di Carli MF. Feasibility of somatostatin receptor-targeted imaging for detection of myocardial inflammation: a pilot study. J Nucl Cardiol. 2019 doi: 10.1007/s12350-019-01782-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thackeray JT, Bankstahl JP, Wang Y, Korf-Klingebiel M, Walte A, Wittneben A, Wollert KC, Bengel FM. Targeting post-infarct inflammation by PET imaging: comparison of (68)Ga-citrate and (68)Ga-DOTATATE with (18)F-FDG in a mouse model. Eur J Nucl Med Mol Imaging. 2015;42:317–327. doi: 10.1007/s00259-014-2884-6. [DOI] [PubMed] [Google Scholar]
- 91.Leung K. Molecular imaging and contrast agent database (MICAD) Bethesda (MD): National Center for Biotechnology Information (US); 2004. 1-(2-Chlorophenyl)-N-[(11)C]methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide. [PubMed] [Google Scholar]
- 92.Denora N, Natile G. An updated view of translocator protein (TSPO) Int J Mol Sci. 2017;18:2640. doi: 10.3390/ijms18122640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukherjee S, Sonanini D, Maurer A, Daldrup-Link HE. The Yin and Yang of imaging tumor associated macrophages with PET and MRI. Theranostics. 2019;9:7730–7748. doi: 10.7150/thno.37306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laitinen I, Marjamaki P, Nagren K, Laine VJ, Wilson I, Leppanen P, Yla-Herttuala S, Roivainen A, Knuuti J. Uptake of inflammatory cell marker [11C]PK11195 into mouse atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2009;36:73–80. doi: 10.1007/s00259-008-0919-6. [DOI] [PubMed] [Google Scholar]
- 95.Tegler G, Sorensen J, Ericson K, Bjorck M, Wanhainen A. 4D-PET/CT with [(11)C]-PK11195 and [(11)C]-(D)-deprenyl does not identify the chronic inflammation in asymptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2013;45:351–356. doi: 10.1016/j.ejvs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, Davies AH, Rimoldi OE, Camici PG. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2012;33:1902–1910. doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 97.Cuhlmann S, Gsell W, Van der Heiden K, Habib J, Tremoleda JL, Khalil M, Turkheimer F, Meens MJ, Kwak BR, Bird J, Davenport AP, Clark J, Haskard D, Krams R, Jones H, Evans PC. In vivo mapping of vascular inflammation using the translocator protein tracer 18F-FEDAA1106. Mol Imaging. 2014:13. doi: 10.2310/7290.2014.00014. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H, Xiao J, Zhou J, Tan H, Hu Y, Mao W, Fu Z, Lin Q, Shi H, Cheng D. 18F-PBR06 PET/CT imaging for evaluating atherosclerotic plaques linked to macrophage infiltration. Nucl Med Commun. 2019;40:370–376. doi: 10.1097/MNM.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 99.Hellberg S, Silvola JMU, Kiugel M, Liljenback H, Savisto N, Li XG, Thiele A, Lehmann L, Heinrich T, Vollmer S, Hakovirta H, Laine VJO, Yla-Herttuala S, Knuuti J, Roivainen A, Saraste A. 18-kDa translocator protein ligand (18)F-FEMPA: biodistribution and uptake into atherosclerotic plaques in mice. J Nucl Cardiol. 2017;24:862–871. doi: 10.1007/s12350-016-0527-y. [DOI] [PubMed] [Google Scholar]
- 100.Hellberg S, Liljenback H, Eskola O, Morisson-Iveson V, Morrison M, Trigg W, Saukko P, Yla-Herttuala S, Knuuti J, Saraste A, Roivainen A. Positron emission tomography imaging of macrophages in atherosclerosis with (18)F-GE-180, a radiotracer for translocator protein (TSPO) Contrast Media Mol Imaging. 2018;2018:9186902. doi: 10.1155/2018/9186902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, Bauersachs J, Wollert KC, Bengel FM. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol. 2018;71:263–275. doi: 10.1016/j.jacc.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 102.Kim GR, Paeng JC, Jung JH, Moon BS, Lopalco A, Denora N, Lee BC, Kim SE. Assessment of TSPO in a rat experimental autoimmune myocarditis model: a comparison study between [(18)F]fluoromethyl-PBR28 and [(18)F]CB251. Int J Mol Sci. 2018;19:276. doi: 10.3390/ijms19010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 104.Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, Tahara A, Constantinescu CC, Zhou J, Boersma HH, Imaizumi T, Nakano M, Finn A, Fayad Z, Virmani R, Fuster V, Bosca L, Narula J. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 105.Kim EJ, Kim S, Seo HS, Lee YJ, Eo JS, Jeong JM, Lee B, Kim JY, Park YM, Jeong M. Novel PET Imaging of atherosclerosis with 68Ga-labeled NOTA-neomannosylated human serum albumin. J Nucl Med. 2016;57:1792–1797. doi: 10.2967/jnumed.116.172650. [DOI] [PubMed] [Google Scholar]
- 106.Senders ML, Hernot S, Carlucci G, van de Voort JC, Fay F, Calcagno C, Tang J, Alaarg A, Zhao Y, Ishino S, Palmisano A, Boeykens G, Meerwaldt AE, Sanchez-Gaytan BL, Baxter S, Zendman L, Lobatto ME, Karakatsanis NA, Robson PM, Broisat A, Raes G, Lewis JS, Tsimikas S, Reiner T, Fayad ZA, Devoogdt N, Mulder WJM, Perez-Medina C. Nanobody-facilitated multiparametric PET/MRI phenotyping of atherosclerosis. JACC Cardiovasc Imaging. 2019;12:2015–2026. doi: 10.1016/j.jcmg.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Varasteh Z, Mohanta S, Li Y, Lopez Armbruster N, Braeuer M, Nekolla SG, Habenicht A, Sager HB, Raes G, Weber W, Hernot S, Schwaiger M. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using (68)Ga-NOTA-anti-MMR nanobody: non-invasive imaging of atherosclerotic plaques. EJNMMI Res. 2019;9:5. doi: 10.1186/s13550-019-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee SP, Im HJ, Kang S, Chung SJ, Cho YS, Kang H, Park HS, Hwang DW, Park JB, Paeng JC, Cheon GJ, Lee YS, Jeong JM, Kim YJ. Noninvasive imaging of myocardial inflammation in myocarditis using (68)Ga-tagged mannosylated human serum albumin positron emission tomography. Theranostics. 2017;7:413–424. doi: 10.7150/thno.15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lindsey ML. Assigning matrix metalloproteinase roles in ischaemic cardiac remodelling. Nat Rev Cardiol. 2018;15:471–479. doi: 10.1038/s41569-018-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lebel R, Lepage M. A comprehensive review on controls in molecular imaging: lessons from MMP-2 imaging. Contrast Media Mol Imaging. 2014;9:187–210. doi: 10.1002/cmmi.1555. [DOI] [PubMed] [Google Scholar]
- 111.Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, Dione DP, Cavaliere P, Chow C, Bourke BN, Hu XY, Azure M, Yalamanchili P, Liu R, Cheesman EH, Robinson S, Edwards DS, Sinusas AJ. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
- 112.Sahul ZH, Mukherjee R, Song J, McAteer J, Stroud RE, Dione DP, Staib L, Papademetris X, Dobrucki LW, Duncan JS, Spinale FG, Sinusas AJ. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: relationship to myocardial dysfunction. Circ Cardiovasc Imaging. 2011;4:381–391. doi: 10.1161/CIRCIMAGING.110.961854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Duijnhoven SM, Robillard MS, Hermann S, Kuhlmann MT, Schafers M, Nicolay K, Grull H. Imaging of MMP activity in postischemic cardiac remodeling using radiolabeled MMP-2/9 activatable peptide probes. Mol Pharm. 2014;11:1415–1423. doi: 10.1021/mp400569k. [DOI] [PubMed] [Google Scholar]
- 114.Kiugel M, Kyto V, Saanijoki T, Liljenback H, Metsala O, Stahle M, Tuomela J, Li XG, Saukko P, Knuuti J, Roivainen A, Saraste A. Evaluation of (68)Ga-labeled peptide tracer for detection of gelatinase expression after myocardial infarction in rat. J Nucl Cardiol. 2018;25:1114–1123. doi: 10.1007/s12350-016-0744-4. [DOI] [PubMed] [Google Scholar]
- 115.Lenglet S, Thomas A, Chaurand P, Galan K, Mach F, Montecucco F. Molecular imaging of matrix metalloproteinases in atherosclerotic plaques. Thromb Haemost. 2012;107:409–416. doi: 10.1160/TH11-10-0717. [DOI] [PubMed] [Google Scholar]
- 116.Kiugel M, Hellberg S, Kakela M, Liljenback H, Saanijoki T, Li XG, Tuomela J, Knuuti J, Saraste A, Roivainen A. Evaluation of [(68)Ga]Ga-DOTA-TCTP-1 for the detection of metalloproteinase 2/9 expression in mouse atherosclerotic plaques. Molecules. 2018;23:3168. doi: 10.3390/molecules23123168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang C, Keliher E, Zeller MWG, Wojtkiewicz GR, Aguirre AD, Buckbinder L, Kim HY, Chen J, Maresca K, Ahmed MS, Motlagh NJ, Nahrendorf M, Chen JW. An activatable PET imaging radioprobe is a dynamic reporter of myeloperoxidase activity in vivo. Proc Natl Acad Sci U S A. 2019;116:11966–11971. doi: 10.1073/pnas.1818434116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ali M, Pulli B, Chen JW. Molecular imaging of macrophage enzyme activity in cardiac inflammation. Curr Cardiovasc Imaging Rep. 2014;7:9258. doi: 10.1007/s12410-014-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mollenhauer M, Friedrichs K, Lange M, Gesenberg J, Remane L, Kerkenpass C, Krause J, Schneider J, Ravekes T, Maass M, Halbach M, Peinkofer G, Saric T, Mehrkens D, Adam M, Deuschl FG, Lau D, Geertz B, Manchanda K, Eschenhagen T, Kubala L, Rudolph TK, Wu Y, Tang WHW, Hazen SL, Baldus S, Klinke A, Rudolph V. Myeloperoxidase mediates postischemic arrhythmogenic ventricular remodeling. Circ Res. 2017;121:56–70. doi: 10.1161/CIRCRESAHA.117.310870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nahrendorf M, Sosnovik D, Chen JW, Panizzi P, Figueiredo JL, Aikawa E, Libby P, Swirski FK, Weissleder R. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation. 2008;117:1153–1160. doi: 10.1161/CIRCULATIONAHA.107.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ronald JA, Chen JW, Chen Y, Hamilton AM, Rodriguez E, Reynolds F, Hegele RA, Rogers KA, Querol M, Bogdanov A, Weissleder R, Rutt BK. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009;120:592–599. doi: 10.1161/CIRCULATIONAHA.108.813998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rashid I, Maghzal GJ, Chen YC, Cheng D, Talib J, Newington D, Ren M, Vajandar SK, Searle A, Maluenda A, Lindstedt EL, Jabbour A, Kettle AJ, Bongers A, Power C, Michaelsson E, Peter K, Stocker R. Myeloperoxidase is a potential molecular imaging and therapeutic target for the identification and stabilization of high-risk atherosclerotic plaque. Eur Heart J. 2018;39:3301–3310. doi: 10.1093/eurheartj/ehy419. [DOI] [PubMed] [Google Scholar]
- 123.Wu MC, Ho HI, Lee TW, Wu HL, Lo JM. In vivo examination of 111In-bis-5HT-DTPA to target myeloperoxidase in atherosclerotic ApoE knockout mice. J Drug Target. 2012;20:605–614. doi: 10.3109/1061186X.2012.702768. [DOI] [PubMed] [Google Scholar]
- 124.Johnstrom P, Bergman L, Varnas K, Malmquist J, Halldin C, Farde L. Development of rapid multistep carbon-11 radiosynthesis of the myeloperoxidase inhibitor AZD3241 to assess brain exposure by PET microdosing. Nucl Med Biol. 2015;42:555–560. doi: 10.1016/j.nucmedbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 125.Stendahl JC, Sinusas AJ. Nanoparticles for cardiovascular imaging and therapeutic delivery, part 1: compositions and features. J Nucl Med. 2015;56:1469–1475. doi: 10.2967/jnumed.115.160994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCarthy JR. Multifunctional agents for concurrent imaging and therapy in cardiovascular disease. Adv Drug Deliv Rev. 2010;62:1023–1030. doi: 10.1016/j.addr.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 130.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 131.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13:125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 132.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112:755–761. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perez-Medina C, Binderup T, Lobatto ME, Tang J, Calcagno C, Giesen L, Wessel CH, Witjes J, Ishino S, Baxter S, Zhao Y, Ramachandran S, Eldib M, Sanchez-Gaytan BL, Robson PM, Bini J, Granada JF, Fish KM, Stroes ES, Duivenvoorden R, Tsimikas S, Lewis JS, Reiner T, Fuster V, Kjaer A, Fisher EA, Fayad ZA, Mulder WJ. In vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc Imaging. 2016;9:950–961. doi: 10.1016/j.jcmg.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beldman TJ, Senders ML, Alaarg A, Perez-Medina C, Tang J, Zhao Y, Fay F, Deichmoller J, Born B, Desclos E, van der Wel NN, Hoebe RA, Kohen F, Kartvelishvily E, Neeman M, Reiner T, Calcagno C, Fayad ZA, de Winther MPJ, Lutgens E, Mulder WJM, Kluza E. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano. 2017;11:5785–5799. doi: 10.1021/acsnano.7b01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seo JW, Baek H, Mahakian LM, Kusunose J, Hamzah J, Ruoslahti E, Ferrara KW. (64)Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjug Chem. 2014;25:231–239. doi: 10.1021/bc400347s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ni NC, Jin CS, Cui L, Shao Z, Wu J, Li SH, Weisel RD, Zheng G, Li RK. Non-invasive macrophage tracking using novel porphysome nanoparticles in the post-myocardial infarction murine heart. Mol Imaging Biol. 2016;18:557–568. doi: 10.1007/s11307-015-0922-9. [DOI] [PubMed] [Google Scholar]
- 138.Jeong HJ, Yoo RJ, Kim JK, Kim MH, Park SH, Kim H, Lim JW, Do SH, Lee KC, Lee YJ, Kim DW. Macrophage cell tracking PET imaging using mesoporous silica nanoparticles via in vivo bioorthogonal F-18 labeling. Biomaterials. 2019;199:32–39. doi: 10.1016/j.biomaterials.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 139.Keliher EJ, Ye YX, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, Groenen H, Fay F, Perez-Medina C, Calcagno C, Carlucci G, Reiner T, Sun Y, Courties G, Iwamoto Y, Kim HY, Wang C, Chen JW, Swirski FK, Wey HY, Hooker J, Fayad ZA, Mulder WJ, Weissleder R, Nahrendorf M. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat Commun. 2017;8:14064. doi: 10.1038/ncomms14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anavi S, Tirosh O. iNOS as a metabolic enzyme under stress conditions. Free Radic Biol Med. 2020;146:16–35. doi: 10.1016/j.freeradbiomed.2019.10.411. [DOI] [PubMed] [Google Scholar]
- 141.Zhou D, Lee H, Rothfuss JM, Chen DL, Ponde DE, Welch MJ, Mach RH. Design and synthesis of 2-amino-4-methylpyridine analogues as inhibitors for inducible nitric oxide synthase and in vivo evaluation of [18F]6-(2-fluoropropyl)-4-methyl-pyridin-2-amine as a potential PET tracer for inducible nitric oxide synthase. J Med Chem. 2009;52:2443–2453. doi: 10.1021/jm801556h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang HJ, Isakow W, Byers DE, Engle JT, Griffin EA, Kemp D, Brody SL, Gropler RJ, Miller JP, Chu W, Zhou D, Pierce RA, Castro M, Mach RH, Chen DL. Imaging pulmonary inducible nitric oxide synthase expression with PET. J Nucl Med. 2015;56:76–81. doi: 10.2967/jnumed.114.146381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herrero P, Laforest R, Shoghi K, Zhou D, Ewald G, Pfeifer J, Duncavage E, Krupp K, Mach R, Gropler R. Feasibility and dosimetry studies for 18F-NOS as a potential PET radiopharmaceutical for inducible nitric oxide synthase in humans. J Nucl Med. 2012;53:994–1001. doi: 10.2967/jnumed.111.088518. [DOI] [PubMed] [Google Scholar]
- 144.Yang X, Chordia MD, Du X, Graves JL, Zhang Y, Park YS, Guo Y, Pan D, Cui Q. Targeting formyl peptide receptor 1 of activated macrophages to monitor inflammation of experimental osteoarthritis in rat. J Orthop Res. 2016;34:1529–1538. doi: 10.1002/jor.23148. [DOI] [PubMed] [Google Scholar]
- 145.Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 146.Zheng F, Put S, Bouwens L, Lahoutte T, Matthys P, Muyldermans S, De Baetselier P, Devoogdt N, Raes G, Schoonooghe S. Molecular imaging with macrophage CRIg-targeting nanobodies for early and preclinical diagnosis in a mouse model of rheumatoid arthritis. J Nucl Med. 2014;55:824–829. doi: 10.2967/jnumed.113.130617. [DOI] [PubMed] [Google Scholar]
- 147.Muller A, Mu L, Meletta R, Beck K, Rancic Z, Drandarov K, Kaufmann PA, Ametamey SM, Schibli R, Borel N, Kramer SD. Towards non-invasive imaging of vulnerable atherosclerotic plaques by targeting co-stimulatory molecules. Int J Cardiol. 2014;174:503–515. doi: 10.1016/j.ijcard.2014.04.071. [DOI] [PubMed] [Google Scholar]
- 148.Meletta R, Steier L, Borel N, Mu L, Keller C, Chiotellis A, Russo E, Halin C, Ametamey SM, Schibli R, Kramer SD, Muller Herde A. CD80 is upregulated in a mouse model with shear stress-induced atherosclerosis and allows for evaluating CD80-targeting PET tracers. Mol Imaging Biol. 2017;19:90–99. doi: 10.1007/s11307-016-0987-0. [DOI] [PubMed] [Google Scholar]
- 149.Eichendorff S, Svendsen P, Bender D, Keiding S, Christensen EI, Deleuran B, Moestrup SK. Biodistribution and PET imaging of a novel [68Ga]-anti-CD163-antibody conjugate in rats with collagen-induced arthritis and in controls. Mol Imaging Biol. 2015;17:87–93. doi: 10.1007/s11307-014-0768-6. [DOI] [PubMed] [Google Scholar]
- 150.Hoffmann SHL, Reck DI, Maurer A, Fehrenbacher B, Sceneay JE, Poxleitner M, Oz HH, Ehrlichmann W, Reischl G, Fuchs K, Schaller M, Hartl D, Kneilling M, Moller A, Pichler BJ, Griessinger CM. Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer. Theranostics. 2019;9:5869–5885. doi: 10.7150/thno.33275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nigam S, McCarl L, Kumar R, Edinger RS, Kurland BF, Anderson CJ, Panigrahy A, Kohanbash G, Edwards WB. Preclinical immunoPET imaging of glioblastoma-infiltrating myeloid cells using zirconium-89 labeled anti-CD11b antibody. Mol Imaging Biol. 2019 doi: 10.1007/s11307-019-01427-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cao Q, Huang Q, Mohan C, Li C. Small-animal PET/CT imaging of local and systemic immune response using (64)Cu-alphaCD11b. J Nucl Med. 2019;60:1317–1324. doi: 10.2967/jnumed.118.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dmochowska N, Tieu W, Keller MD, Wardill HR, Mavrangelos C, Campaniello MA, Takhar P, Hughes PA. Immuno-PET of innate immune markers CD11b and IL-1beta detects inflammation in murine colitis. J Nucl Med. 2019;60:858–863. doi: 10.2967/jnumed.118.219287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu G, Hu Y, Xiao J, Li X, Li Y, Tan H, Zhao Y, Cheng D, Shi H. 99mTc-labelled anti-CD11b SPECT/CT imaging allows detection of plaque destabilization tightly linked to inflammation. Sci Rep. 2016;6:20900. doi: 10.1038/srep20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Neill AS, Terry SY, Brown K, Meader L, Wong AM, Cooper JD, Crocker PR, Wong W, Mullen GE. Non-invasive molecular imaging of inflammatory macrophages in allograft rejection. EJNMMI Res. 2015;5:69. doi: 10.1186/s13550-015-0146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Terry SY, Boerman OC, Gerrits D, Franssen GM, Metselaar JM, Lehmann S, Oyen WJ, Gerdes CA, Abiraj K. 111In-anti-F4/80-A3-1 antibody: a novel tracer to image macrophages. Eur J Nucl Med Mol Imaging. 2015;42:1430–1438. doi: 10.1007/s00259-015-3084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bigalke B, Phinikaridou A, Andia ME, Cooper MS, Schuster A, Wurster T, Onthank D, Munch G, Blower P, Gawaz M, Nagel E, Botnar RM. PET/CT and MR imaging biomarker of lipid-rich plaques using [64Cu]-labeled scavenger receptor (CD68-Fc) Int J Cardiol. 2014;177:287–291. doi: 10.1016/j.ijcard.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 158.Rischpler C, Nekolla SG, Kossmann H, Dirschinger RJ, Schottelius M, Hyafil F, Wester HJ, Laugwitz KL, Schwaiger M. Upregulated myocardial CXCR4-expression after myocardial infarction assessed by simultaneous GA-68 pentixafor PET/MRI. J Nucl Cardiol. 2016;23:131–133. doi: 10.1007/s12350-015-0347-5. [DOI] [PubMed] [Google Scholar]



