Abstract
Graphene-family nanomaterials (GFNs) have been widely used in cancer therapy, tissue engineering, antibacterial and biological imaging due to their optical, thermal, and drug absorption properties. When used as drug and gene nanocarrier, the major limitations are aggregation, biocompatibility, and inappropriate release of drugs or genes. To overcome these problems, researchers have developed a variety of functionalization processes. In this review, we grouped the functionalization according to the decoration molecules, putting particular emphasis on the gene delivery. Organic and inorganic materials resulted as the major sets to introduce functional sections onto graphene oxide (GO). We also classified the target molecules used in the GO delivery system, as well as introduced other strategies to increase the delivery efficacy such as controlled release and magnetic targeting.
Keywords: Graphene oxide, nanocarrier, gene delivery, target delivery
Introduction
Nanotechnology is one of the most rapidly developing fields. Many different nanomaterials, which possess unique and extraordinary physiological and chemical properties, are currently undergoing preclinical/clinical testing, including dendrimers, liposomes, polymers, metallic nanoparticles (NPs), carbon nanomaterials, and viral NPs. All of them have distinct advantages and disadvantages in terms of functionality, physiochemical properties, biodistribution, pharmacokinetic behavior, immunogenicity, and toxicity [1,2]. Among those, graphene is a type of carbon nanomaterials widely used for in nanomedicine. The theoretical existence of graphene was discussed 60 years ago by Slonczewski and Weiss [3]. Later on (in 2004) single sheets of graphene were isolated through mechanical exfoliation by Novoselov (repeated peeling, scotch-tape technique) [4].
Due to its optical, thermal, mechanical, and electrical properties, graphene is applied for conducting polymers, battery electrodes, printable inks, antibacterial papers [5-7]. To further exploit the pristine graphene, Graphene-family nanomaterials (GFNs) including few-layer-graphene (FLG), ultrathin graphite, graphene quantum dots (GQDs), graphene oxide (GO), reduced graphene oxide (rGO), and graphene nanosheets (GNS) have been developed [6,8,9]. In this way, GFNs are analogous to carbon nanotubes (CNTs), which can vary in wall number, diameter, length, and surface chemistry [6]. Compared to pristine graphene, other GFNs exhibit distinct dispersion/aggregation behaviors, biocompatibility, and other advantages due to their different surface properties [10-12]. In 2008, Sun et al. developed the pegylated GO (PEG-GO) that is soluble in buffers and serum without agglomeration [13]. In 2012, Sasidharan and colleagues revealed that carboxyl functioned graphene has a better hemocompatibility [11]. Moreover, Mendonca et al. found that the toxic effects of rGO are peripheral and transitory in the short-term analysis after systemic administration [14]. A consensus on the toxicity of GFNs impacting the body at different levels such as organs, blood, cells and subcellular structures, has not yet been reached [15]; nonetheless, researchers have reached a standard view on the toxicity of graphene being dependent on their shape, dose, size, time and functionalization [16].
The interaction between GFNs and biological molecules has been addressed by previous studies [6]. In 2008, Liu et al. used PEG and nano-graphene oxide to obtain a delivery material that can absorb the hydrophobic aromatic molecules camptothecin (CPT) analog SN38 [17]. Since then, GFNs have been intensively explored as nanocarriers to be applied in gene delivery drugs, bioimaging, and tissue engineering [18]. Gene therapy mainly depends on ensuring the successful transfer of the therapeutic gene to the targeted cell [19]. The major limitations of gene therapy are poor cellular uptake, degradation by nucleases and rapid renal clearance following systemic administration. The decoration of GFNs prevents target drug or gene aggregation, minimizes its side-effects, controls release at proper time and location in chemotherapy. In this paper, we reviewed the studies on GFNs used in drug and gene delivery published over the recent two years. These functional moieties were summed up into several categories. Furthermore, we presented strategies to ameliorate the delivery efficacy.
Functionalization of graphene used in the delivery
GO that has excellent process ability has become a promising functional nanoreinforcing material for various biomedical applications. Employing the covalent or noncovalent method named “graft” or “load”, GO can be modified with other nanoparticles (NPs) or biomolecules to expand its biomedical applications [20]. Nanohybrids offers several advantages due to the unique properties of each counterpart. In 2017 and 2018, there were nearly 200 papers about GO used as nanocarrier that classified the decoration of GO into certain types (Figure 1). Organic and inorganic are the two major sets. We sorted the organic function into linear polymers, nonlinear polymers, polysaccharides, amino acids-protein-aptamer (APA), and nonpolymers. These categories are listed and censused in Figure 1, except for the nonpolymers that were less frequently used. The representative literature of the first three categories are listed in Table 1. The subgroup was censused in separate categories. Besides, the subgroups, which had passed the in vivo antitumor assay, were red labeled; blue stars indicated that the gene delivery was successful.
Figure 1.
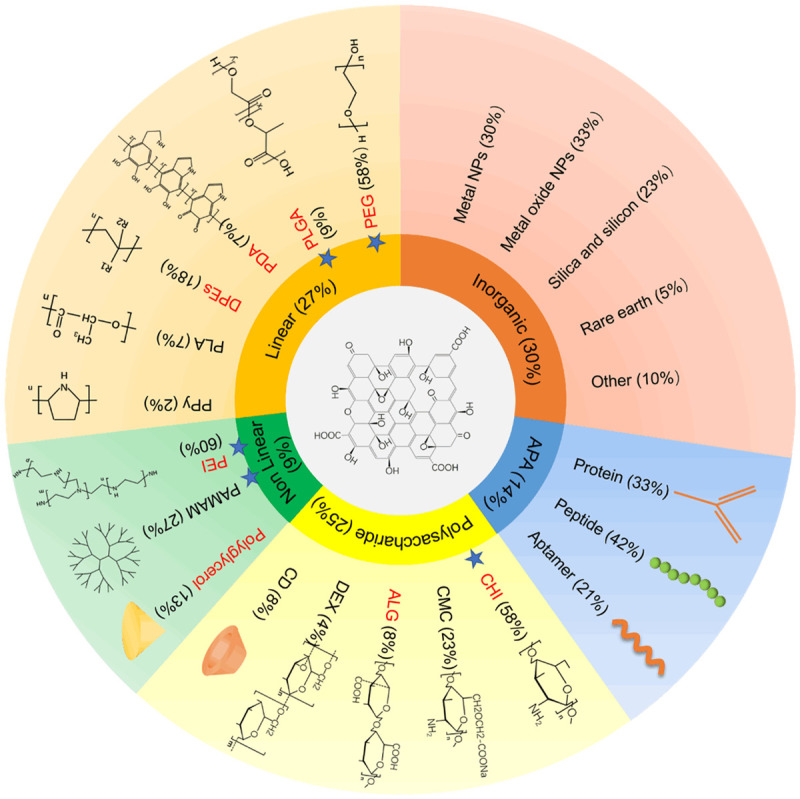
Functionalization of GO. Organic and inorganic were the two major sets. Organic set was further divided into linear polymers, nonlinear polymers, polysaccharides, amino acids-protein-aptamer (APA), and nonpolymers (not show). The subgroups which had passed the in vivo antitumor assay were red labeled. Blue stars indicated the gene delivery have been realized. Inorganic and APA have not undergone these screen cause of its complexity.
Table 1.
The representative literature of first three categories of organic subset were listed
| Classification | Modified materials | Nanocomposites | Loaded drugs | In vitro/in vivo model | Results | references |
|---|---|---|---|---|---|---|
| Linear polymers | PEG, polyethylene glycol | FA/PEGylated GO nanosheet | HDAC1/K-Ras siRNAs | MIA PaCa-2 tumor bearing mice | Irradiation prompt the siRNA release; FA/GO/(HDAC1+K-Ras) siRNA showed 52% reduction of tumor volume | [24] |
| PLGA, poly (D, L-lactic-co-glycolic acid) | GO/PP-SS-DOX, GO/PEG-PLGA-doxorubicin | PEG-PLGA-SS-DOX | B16 tumor bearing mice | Redox-sensitive PP-SS-DOX specifically release DOX in cancer cells, result in higher inhibition rate than free DOX | [29] | |
| PDA, polydopamine | rGO-PDA | Ara, cytarabine hydrochloride | HeLa cells tumor bearing mice | Greater inhibition of cancer cell and tumor growth than free Ara when combine the chemotherapy and phototherapy | [34] | |
| PVP, polyvinylpyrrolidone | PVP-rGO/Bi2S3 | DOX | BEL-7402 tumor bearing mice | Greater inhibition of cancer cell and tumor growth than free DOX when combine the chemotherapy and phototherapy | [37] | |
| Nonlinear polymers | PEI, polyethylenimine | GO-PEG-PEI | Cas9/sgRNA (EGFP and CXCR-4) | AGS.EGFP cells | Transfection efficacy is about 39% | [179] |
| PAMAM, polyamidoamine | GPD, GO-PEG-PAMAM | EPAC1 siRNA tagged with Cy5 | HUVEC cells MDA-MB-231 | Higher transfection capability than Lipofectamine 2000; A maximum transfection efficiency of 56.5%; lower cell invasion | [57] | |
| hPG, hyperbranched polyglycerol | nanographene sheets-hPG | DOX | HeLa cells tumor bearing mice | Efficiently inhibit tumor growth | [59] | |
| Polysaccharide | CHI, chitosan | GC-GO, galactosylated chitosan-GO | DOX | HepG2 tumor bearing mice | GC-GO-DOX shown higher inhibition rate than free DOX | [64] |
| CMC, carboxymethyl cellulose | CMC-GO nanocomposite hydrogel beads | DOX | SW480 cells | GO-CMC/DOX complexes shown lower cytotoxicity under the same conditions than free DOX | [65] | |
| CD, cyclodextrins | GO-HP-β-CD, GO-hydrophilic hydroxypropyl-β-CD | DEX, dexamethasone | No anticancer assay | [71] | ||
| ALG, alginate | GO-ALG | 5-FU, 5-fluorouracil | HT-29 tumor bearing mice | Mice treated with GO-ALG/5-FU showed prolonged survival time and inhibited tumor growth | [73] |
Organic decoration
Linear polymers
Polyethylene glycol (PEG) is the most frequently used linear polymer. Biocompatible neutrally charged PEG leads to high aqueous solubility and stability in physiological solutions including serum [21,22]. In 2008, Liu et al. used PEG and GO and obtain a delivery material that can absorb the hydrophobic aromatic molecules camptothecin (CPT) analog SN38 [17]. Ribonuclease A (RNase A) and protein kinase A (PKA) were also successfully loaded on PEGylated GO [23]. Moreover, Yin et al. used the PEGylated GO as a vehicle to co-deliver HDAC1 and K-Ras siRNAs into MIA PaCa-2 cells in BALB/c mice [24].
PLGA (poly (D, L-lactic-co-glycolic acid)) was initially used as clinical suture material due to its excellent biocompatibility and tunable rate of in vivo biodegradation. PLGA-based micro/nanoparticles can be used for the delivery of macromolecules, such as protein or various types of nucleic acid [25]. GO/PLGA nanofibers are formed by electrospinning technique, where human embryonic kidney 293 cells or mesenchymal stem cells (MSCs) can be successfully transfected by pGFP-GO/PLGA [26]. Besides, 5-iodo-2-deoxyuridine (IUdR) or 5-fluorouracil can be loaded on PLGA functionalized GO, which can further improve the properties of the particles (fits function, magnetic targeting property and MRI ability) [27,28]. DOX is released from GO/PP-SS-DOX (conjugat mPEG-PLGA (PP) with DOX via disulfide bond) nanohybrids in cancerous cells due to the reductive environment [29], while bone morphogenetic protein-2 (BMP-2) is delivered using GO-PLGA as microcarrier in bone tissue engineering [30].
Polydopamine (PDA) was used for surface modification or to steady the nanocarrier due to its excellent attachment property [31]. PDA doped graphene nanohybrids are used in bioimaging when absorbing DNA [32], and in drug delivery, while enwrapping the mesoporous silica nanoparticles [33]. For example, antitumor assay in vivo was conducted in HeLa bearing mice treated with combined chemotherapy and photothermal therapy [34]. Yet, so far, no gene delivery has been reported in the PDA-GO delivery system.
Most of the other linear polymers were the derivatives of polyethylene (DPEs), among which polyvinylpyrrolidone (PVP) was most frequently used. PVP is a nonionic, nontoxic and biocompatible polymer surfactant that could also serve as a biocompatible stabilizer of GO in the physiological environment [35]. PVP functionalized GO has been identified as nanocarriers for SN-38 [36]. PVP-rGO/Bi2S3 nanocomposite has a high storage capacity for DOX and simultaneously displays perfect photothermal conversion efficiency in the NIR region [37]. The DPEs also include polyethylene glycosylated [38], poly acrylic acid (PAA) [39,40], poly methacrylic acid (PMAA) [41]. In addition, other polymers like poly lactic acid (PLA) [42] and polypyrrole (PPy) [43].
Nonlinear polymers
Nonlinear polymers such as hyperbranched polyethylenimine (PEI), polyamidoamine (PAMAM), polyglycerol (PG) and cyclodextrins (CDs) have prominent spatial structure and are treated as self-carrier. Cationic polymers such as PEI can condense plasmid DNA and RNA into stable complexes via electrostatic interactions [44]. Wang et al. compared the intracellular delivery efficiency of GO-PEI and GO-PEG in Raw264.7 cells and found that GO-PEI has better aggregating features in the cytoplasm [45]. Thus, PEG and PEI dual-functionalized GO are more suitable to be used as a gene nanocarrier. Moreover, GO-PEG-PEI (also named as RGPP) exhibited various delivery abilities for both siRNA and large size plasmids in 11 cell lines including human, mouse, cancer and normal [46]. Furthermore, GO-PEG-PEI loaded with plasmid-based Stat3 siRNA can suppress malignant melanoma growth in mice [47,48]. When loaded with miR-7b overexpression plasmid or siRNA-targeting Ckip-1 increases the bone formation [49,50]. Moreover, Yue and his team have constructed the GO-PEG-PEI nanocarrier for the delivery of high-molecular-weight Cas9/single-guide RNA (sgRNA) complexes and efficiently executed the function of gene editing in human AGS cells [51]. Most of the studies on the GO-PEI gene delivery system have focused on the cell level, except for an earlier report on cardiac repair in vivo [24,52]. Although relatively fewer studies have addressed the drug delivery; in these, the antitumor assays were performed in vivo via GO-PEI drug delivery system [53].
Dendrimers are highly branched nearly spherical and symmetrical macromolecules. Poly (amidoamine) (PAMAM) are the most commonly used dendrimers, in which interior cavities are used to encapsulate hydrophobic or hydrophilic drugs, while their terminal functionalized outside surface change the physicochemical, reactivity, dynamics, and biological properties [54]. In 2012, graphene-oleate-PAMAM or GO-PAMAM were used to deliver the plasmid DNA of EGFP into HeLa and MG-63 cells [55]. DOX and MMP-9 shRNA plasmid co-delivery can also be performed by GO-PAMAM leading to higher cytotoxicity of MCF-7 cells [56]. Yadav et al. have developed a PEG and PAMAM modified GO (GPD), which, when coated with EPAC1 siRNA inhibited cell migration and decreased invasion of MDA-MB-231 cells [57]. Moreover, PAMAM functioned graphene nanostars loaded with metalloproteinase 9 (MMP-9) overexpression plasmid reduced hepatic fibrosis of artificial liver cirrhosis mice [58]. Besides, graphene nanostars, which are new kind of GFNs, after being linked to PAMAM-G5 and loaded with the plasmid encoding for metalloproteinase 9, could significantly reduce hepatic injury and improve liver restoration in mice with liver cirrhosis [58]. However, no in vivo antitumor assays have been tested using GO-PAMAM nanocarrier.
Tu et al. have obtained graphene derivatives with the property of pH-triggered surface charge conversion, and near-infrared irradiation (NIR) controlled DOX release by using hyperbranched polyglycerolamine and 2,3-dimethylmaleic anhydride (DA) to modify graphene. This nanohybrid showed high antitumor efficacy in tumor bearing-mice [59]. The ring form polymer cyclodextrins (CDs) will be presented in the polysaccharide subgroup.
Polysaccharide
Polysaccharides are a class of commonly used biomacromolecules, most of which are naturally derived [60]. Chitosan, derived from a deacetylated form of chitin, is extensively used in the fabrication of drug carriers or other biomedical materials due to its biocompatible, antibacterial, and biodegradable properties [61]. In 2011, Chitosan functionalized GO was first obtained via a facile amidation process and was used to successfully deliver camptothecin (CPT) or pDNA (pRL-CMV) into human cancer cell lines [62]. MDR1 siRNA could be transfected by chitosan functionalized GO and could efficiently knockdown the MDR1 mRNA and its translation product P-gp expression levels in MCF-7/Dox cells [63]. Wang et al. have measured the in vivo antitumor efficacy of galactosylated chitosan decorate GO [64].
Cellulose, which is another polymer of glucose, is also used in drug delivery systems. Carboxymethyl cellulose (CMC) and hydroxyethyl cellulose (HEC), for example, are frequently used to decorate GO. DOX loaded CMC/graphene quantum dot (GQD) nanocomposite hydrogel film or CMC/GO has a pH-dependent release profile and strong cytotoxicity to K562 or SW480 cells [65,66]. Drug loading and release behavior of folic acid (FA) on rGO-HEC nanohybrids have been also investigated [67].
Cyclodextrins (CDs) belongs to a family of macrocyclic compounds consisting of several glucose subunits with a relatively hydrophobic central cavity and a hydrophilic outer surface [68]. Thus, CDs are widely used in drug delivery by hosting the poorly soluble drugs and forming a biocompatible complex. CDs functionalized GO as a scaffold with or without further decoration could deliver DOX, CPT, Dexamethasone (DEX) or SN38 (7-ethyl-10-hydroxycamptothecin) to alter the cell growth [69-72]. Yet, so far, the antitumor efficacy of those CDs has not been tested in vivo. As far as other polysaccharides are concerned, GO-alginate (ALG) possesses pH-controlled 5-fluorouracil (5-FU) release and high antitumor efficacy [73]. The cytotoxicity of Dextran (DEX) conjugated GO loaded with curcumin (CUR) to 4T1 and MCF-7 nucleolin over-expressed cancer cells have been detected [74].
Hydrogel-based drug delivery systems facilitate controlled and adjustable drug release [75]. The most commonly used hydrogels are polysaccharide-based materials. These include CMC [65], Sterculia gum and carbopol polymers [76], sterculia gum-polyacrylamide hydrogel [77], gum tragacanth [78], poly (ethylene glycol) dimethacrylate [79], polyacrylamide hydrogels [80], chitosan [81], chitosan-g-poly (N-isopropylacrylamide) (CPN) [82]. Also, tripeptide hydrogels (not polysaccharide) [83] have been studied to modified GO. Among them, CMC [65], chitosan [81] and CPN [82] have been testing for cancer therapy. Especially, GOFA-DOX/HACPN have been tested in nude mice implanted with MCF-7/LUC cell [82].
Compared to hydrogels, nanogels can be used as a more feasible delivery system due to their small size [84]. So far, the in vitro anticancer assay of alginate [85], isopropylacrylamide [86], salep, and isopropylacrylamide [87] modified GO nanogels loaded with DOX have been performed. Aerogels based on GO and polyvinyl alcohol (PVA) can be potentially used for dermal delivery and treatment of trauma bleeding [88]. The release of 5-FU from the hybrid aerogels of chitosan, CMC and GO has also been addressed by previous studies [89]. Polyacrylamide/graphene oxide/gelatin/sodium alginate (PAM/GO/Gel/SA) composite hydrogel and accelerate peripheral nerve regeneration [90]. These hydrogel-graphene nanocarriers have a near-infrared (NIR) or pH-dependent release. Nevertheless, there are no reports on the delivery of RNA or DNA being reported via hydrogel-graphene nanocarrier.
Amino acid-protein-aptamer
Protein and amino acid functionalization are a commonly used tools for GO. Ginsenoside Rh2 (Rh2) can be loaded on the Arg or Lys functionalized pristine graphene [91]. Collagen-coated 3D graphene foams itself without additional load, stimulating the differentiation of DA neurons from MSC [92]. FA-BSA decorated GO load with DOX secure an increased effective drug concentration to MCF-7 and A549 cells [93]. Gelatin [90] and tripeptide [83] hydrogels decorated GO are used for nerve regeneration or drug delivery. Moreover, the Caspase-3 specific peptide probe is tagged with FAM and further anchored to GO. Thus, acquired a caspase-3 activated imaging agent can detect cell apoptosis [94]. Moreover, some peptides or antibodies (such as octaarginine (R8) and anti-HER2 antibody [95]) that are treated as target molecules are addressed in the latter part.
Aptamers are oligonucleotide molecules that can bind to targets with similar affinities and specificities to antibodies. This affinity is applied in biosensor and biomedicine [96]. Most of the fluorophore-tagged aptamers are attached to GO via π-π stacking interactions where they display a weak fluorescence signal due to efficient quenching; the fluorescence is activated when nanocomposites entry the cells and aptamer bind to higher affinity target molecules. This tactic has been used in Cytochrome c (Cyt c) [94], cancerrelated microRNA (miRNA) [97] and tumor exosomes detection [98]. Thrombin binding aptamer (TBA) undergoes a conformational change when potassium (K+) is added, which thus far has been used in potassium detection when combined with FRET-based sensors [99]. The affinity of aptamer molecules applied in targeted delivery is discussed later on.
Nonpolymers
Researchers have used the nonpolymeric organic materials to improve the functional properties of GO. Ma et al. have used soy phosphatidylcholine (SPC) membrane to encapsulate DOX-loaded NGO (NGO/DOX) and to increase its stability and biocompatibility. Administration of DOX-loaded NGO in mice, can inhibit tumor growth and prolong the survival time [100]. Moreover, fluorinated graphene, which possesses better properties in molecular sensing, was used to deliver DOX and CPT to Hela and oral epithelial cells [101]; while adipic dihydrazide (ADH) and heparin (Hep) modified GO were used to deliver DOX to HepG2 and MCF-7 cells, and have a decreased DOX release in heart, lung and kidney [102]. GO-DOPA-maleimide-c (RGDfC) synthesized via Thiol-Maleimide ‘Click’ Chemistry was previously applied to the targeted DOX chemotherapy and photothermal to MDA-MB-231 and HeLa cells [103]. Sedghi et al. have used 3-aminopropyltriethoxysilane (APTES, Si(OEt)3) as a crosslink between electrospun nanofibers and GO [104]. Also, pegylated phospholipids could be used as a link to anchor the functional molecules to GO [105].
Inorganic NPs decoration
Inorganic NPs, which possess high surface to volume ratio, controllable shape and size, facile surface modification, stability and unique optical and magnetic properties [106] include metal NPs (Au, Ag, Cu, Pt and Ti), metal oxide NPs (Fe3O4, ZnO and TiO2), Rare-Earth NPs (Gd, Ce, Eu), as well as other inorganic materials (SiO2 and so on) [107-109]. Organic-inorganic nanohybrid offers numerous advantages by combining the unique properties of organic and inorganic counterparts. In 2012, Huang et al. established a grapheneinorganic nanohybrid, which has been currently applied in electronics, optics, electrochemical energy conversion and storage, solar energy harvesting, and so on [8]. This paper reviewed graphene-inorganic nanohybrids used as nanocarriers.
Metal NPs
In 2013, PEI-functionalized GO-encapsulating gold NPs (GOPEI-AuNPs) were applied to deliver DNA into HeLa cells as a novel pDNA gene vector and its transfection efficiency was higher than PEI-functionalized GO [110]. GO sheet attached to Au@PANI (polyaniline) core-shell nanoparticles exhibited NIR/pH-responsive DOX release property and excellent NIR photothermal transduction efficiency, leading to synergistic therapeutic efficacy in tumor-bearing mice [111]. Moreover, Usman et al. have doped GO with rare earth metal Gd (Gadolinium) and protocatechuic acid via hydrogen bonding and π-π interactions, then coated the nanomaterial with AuNPs through electrostatic interactions, thus fabricating the GAGPAu that can be used as both PA nanocarrier and magnetic resonance imaging (MRI) contrast agent [112]. Graphene-gold composites could also deliver miRNA-101 or miRNA-122 into cancer cells [113,114]. Most of the reports used gold as MRI or SERS (Surface-Enhanced Raman Spectroscopy) contrast agent, while GO as a load moiety. In graphene-silver composites, the silver section can also provide antibacterial activity [115], while Habiba et al. revealed that the photodynamic therapy of Ag-GQDs is enhanced by Ag compared to GQDs [116]. Cu-crosslinked carboxymethylcellulose/naproxen/graphene quantum dot nanocomposite has been reported as an oral drug delivery nanocarrier [117]. GO coated titanium as a bone implant, was always used to deliver drug resulting in cell differentiation [118]. Rare earth paramagnetic metal Gd based NPs are used as MRI contrast agents [119].
Metal oxide NPs
Metal oxide NPs include iron oxide, titanium oxide, and zinc oxide. Mostly used magnetic nanoparticles include iron oxide and its derivatives (such as CoFe2O4 [115]) [120]. Iron oxide nanoparticles (IONP) have been extensively studied in biomedical applications, mainly as magnetic guided cell signaling due to their high magnetic properties [121]. When combined with GO or GQDs, IONP has been applied for MRI imaging [27,122], magnetic hyperthermia therapy [123,124], and magnetic-guided behavior [27,125]. Moreover, magnetic hyperthermia of IONP facilitated the temperature-dependent drug release of GO [124]. TiO2-based nanohybrid has drawn significant attention in the photocatalysis field [126]. Hybrid nanocomposite Pr-TiO2/NGO, which consists of graphene oxide nanosheets and TiO2 nanocrystals doped with rare earth metal praseodymium (Pr), combined the photocatalytic activity therapy, photothermal therapy and anticancer drug DOX, thus leading to significant inhibition of HeLa cell growth [127]. Mesoporous zinc oxide (ZnO) scaffolds coated with drop-cast graphene oxide (GO) forming a delayed-release biolayer system could be used in bone tissue engineering [128].
Other inorganic NPs
Mesoporous silica nanoparticles (MSNs) possess high surface area available for drug loading [129]. GO or GQDs coated drug-loaded MSNs could break up because of NIR-induced thermal effect, thus achieving controlled drug release [43,130]. There are different modes between silica and GO, such as GQDs that can be incorporated into the cavity of MSNs hollow. This controlled release drug delivery platform has been proved in tumor-bearing mice [131].
Except for those inorganic NPs mentioned above, ZnS QD is a diagnostic agent candidate due to its optical characteristics. Zeng et al. obtained a theranostic nanoparticle by combining InP/ZnS (core/shell) QDs to GO loaded with miR-122, which can be used for targeted imaging and induction of apoptosis of drug-resistant hepatoma cells in tumor-bearing mice [132]. Similarly, Diaz-Diestra et al. combined ZnS:Mn to DOX leaded GO obtaining a theranostic platform [133].
Hydroxyapatite (HAP) had biocompatibility, bioactivity, and osteoconductivity, which have been extensively used in the medical field, especially for bone repair or regeneration and drug delivery applications. GO-HAP composites, apart from bone regeneration, could deliver absorbing ibuprofen (IBU) [134], 5-FU [135]. Cheang et al. have used GO-HAP nanocomposites to deliver a plasmid that expressed suicide gene herpes simplex virus thymidine kinase (HSV-TK) to inhibit the proliferation of cancer cells [136].
Optimization strategies for improving the delivery efficacy
Although dozens of new drugs appear each year, almost all of them face the challenge of effectively targeting the diseased tissue and cells. To increase the delivery efficacy and reduce the side-effects, there appear some efficient strategies. Among them, target agent modification is a feasible strategy for NPs-based drugs [137]. Various target agents have been used to improve GO-based nanocarrier [138]. Trigger controlled delivery, which dependent on tumor microenvironment, specifically gives the drug efficacy to designated cells [139]. Here we presented several targeting agents and trigger controlled systems that have been used in optimizing the GO-based platform (Figure 2). The representative literatures which have compare the target-effect are listed in Table 2.
Figure 2.
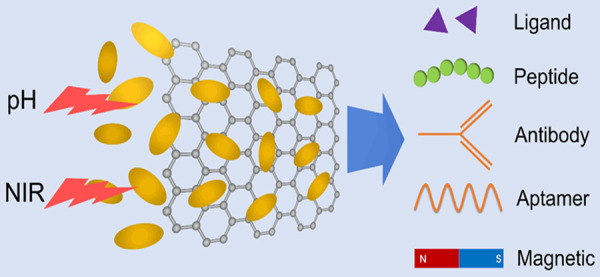
Optimizing strategies to improve the delivery efficacy. Photothermal and acidic condition were the commonly used controlled release stimuli. Target molecules include ligand, protein, peptide and aptamer. Magnetic targeting was a powerful way for inorganic NPs and GO nanohybrid.
Table 2.
The representative target molecules used in GO-based nanoplatform
| Target molecules | Targets | Nanocomposites | Loaded drugs | In vitro/in vivo model | Results | references |
|---|---|---|---|---|---|---|
| FA | Folate binding protein on the cell membrane | GO/PP-SS-DOX, GO/PEG-PLGA-DOX | PEG-PLGA-SS-DOX | B16 tumor bearing mice | The tumor growth inhibition rate of non-targeting and the targeting nanohybrids was 60.70% and 81.78% respectively. | [29] |
| HA | CD44 on the cell membrane | HA-rGO | indocyanine green | KB cells tumor bearing mice | The targeting nanohybrids almost completely inhibited the tumor growth, while poorer effect using non-targeting nanohybrids. | [153] |
| GA | Protein Kinase C of mitochondrion | GA-GO | DOX | HepG2 tumor bearing mice | The average tumor weights of GA-GO@DOX, GO@DOX, and control groups were 0.59, 0.70, and 1.29 g after 21 d treatment, respectively. | [155] |
| antibodies of VEGF | VEGF | PTX-GHP-VEGF | PTX, paclitaxel | SW-13 tumor bearing mice | The survival rate of mice in the PTX-GHP-VEGF+NIR-treated group was 100%, and dramatically higher than nontarget group. | [161] |
| P-L-Arg | glycosaminoglycan receptors on the cell membrane | P-L-Arg-Au@GO | miRNA-101 | MCF7, MDA and HU-02 | Au@GO-PEG-(P-L-Arg) displayed more toxicity on MCF7 cell line in comparison to Au@GO-PEG. | [113] |
| AS1411 aptamer | nucleolin on the cell membrane | GO-DEX-Apt-CUR | CUR, curcumin | 4T1, MCF-7 and CHO | GO-DEX-Apt-CUR are significantly more cytotoxic compared with GO-DEX-CUR in MCF-7 or 4T1 cells. | [74] |
Ligand
The most commonly used target agent is folate acid (FA). The overexpression of the folate acid receptor has been found in many the cancer cells [140]. Targeting efficacies of FA that modifies GO NPs have been proven in vitro and in vivo. So far, different particles coated with FA have been tested, including GO carrying DOX, colchicine (COLC) [141], paclitaxel [142], curcumin [143], camptothecin [144], copper complex (regard as a substitution cis-dichlorodiamineplatinum) [145], and siRNA [24]. Mice bearing HELA cells [100], HepG2 cells [141], PaCa-2 cells [24], B16 cells [29], MCF-7 cells [82] or EAT (Ehrlich Ascites Tumor) cells [146] have been used as models in previous studies. Among these in vivo assays, two showed a significant inhibitory effect of tumor growth [29,100].
Hyaluronic acid (HA) is extensively used as a major ligand that binds to the CD44 (hyaluronan) receptor with high affinity. Similar to FA receptor, CD44 receptor is overexpressed in many cancer tissue [147]. HA modified GO or GQDs have been used to deliver DOX [148], Histamine dihydrochloride (HDC) [149], 5-Fluorouracil (5-FU) [150], SNX-2112 [151], miR-21 peptide nucleic acid (PNA) probes [152] in vitro or in vivo. Anti-tumor assays have been identified in HELA cells [148], Panc-1 cells [150], MDA-MB-231 bearing mice [152]. However, there is still no chemotherapy research comparing the differences of in vivo anti-tumor efficacy of GO-based material with or without HA; nonetheless, Miao et al. obtained remarkable results in photothermal therapy in 2015 [153].
In GO-based nanocarrier, Triphenylphosphonium (TPP) [59,154], Glycyrrhetinic acid (GA) [155], Hypericin [156] are used to target mitochondria. The alteration of mitochondria functions is associated with malignant transformation, and this provide targets for novel cancer therapeutics [157]. Among them, GA is believed to target the hepatocellular carcinoma (HCC) cells and exhibit a certain degree of targeting property to the SMMC-7721 [158]. Other drug delivery systems use these mitochondria target molecules, offering enhanced drug efficacy via preferential mitochondria accumulation to push the cells to apoptosis stage.
Protein
The antibody of marker molecules which overexpressed on the cancer cells (CMMs) can direct the nanohybrid to cancer cells [159]. P-glycoprotein (P-gp) is overexpressed in cancer cells when they acquire multi-drug resistance (MDR) [160]. Zeng et al. have synthesized GPMQNs (graphene-P-gp loaded with miR-122-InP@ZnS quantum dots nanocomposites) which utilize P-gp antibody to target the tumor in nude mice [132]. The monoclonal antibodies have been used against vascular endothelial growth factor (VEGF) to modify GO and to increase the delivery efficacy to VEGF overexpressed cancer cells [161]. Epidermal growth factor receptor (EGFR) is highly expressed on the surface of tumor cells. Cetuximab (CET, an EGFR monoclonal antibody) modified GO may inhibit the growth of xenograft tumors with implanted CT-26 cells [162]. Moreover, the antibody of heparin sulfate proteoglycan glypican-3 (GPC3), a potential molecular target for hepatocellular carcinoma (HCC), is used to decorate rGO [163]. Anti-HER2, an antibody of a transmembrane tyrosine kinase receptor overexpressed in 25%-30% of human breast cancer, is used in S180 cells bearing mice [95]. Proteins, such as transferrin (TF) or lactoferrin (LF), could guide the DOX-loaded GO NPs to melanoma cells or glioma cells, respectively through their receptors [164,165].
Peptide and aptamer
Cell-penetrating peptides (CPPs) and cell-targeting peptides (CTPs) facilitate the selective delivery of chemotherapeutic drugs [166,167]. These peptides, including poly-L-arginine (P-L-Arg) [113], Arg-Gly-Asp (RGD) [168], c (RGDfC) [103], c (RGDyK) [169], octaarginine (R8) [95] are used to enhance the selectivity of GO nanohybrid. CPPs and CTPs decorated GO loaded with miRNA or drug have been already tested in vitro and in vivo. The chimeric peptide (MPG-2H1) has been designed to facilitate the endocytosis of pDNA loaded GQDs [170].
Kim et al. have fabricated a fibroblast activation protein (FAP) activated promelittin decorated rGO nanosheet DOX delivery system, which displayed a better antitumor efficacy via tumor microenvironment targeted therapy in vivo. Moreover, Melittin used in this system is a nonspecific cytolytic peptide that attacks all lipid membranes. FAP is a serine protease that uniquely cleaves the Pro-Xxx amino acid bond; FAP has been reported to be selectively overexpressed on the membranes of cancer-associated fibroblasts (CAFs) [105].
AS1411 aptamer, which is an ssDNA aptamer that can be recognized by nucleolin and can improve the intracellular uptake, assists CUR-loaded GO to target 4T1 and MCF-7 cells [74]. ST-3 aptamer facilitates entry of GO into the biofilm, making the antibacterial activity of ST-3-GO superior to that of its component molecules [171].
Trigger controlled delivery system
Trigger controlled drug release, and the applications have been developed in a new drug delivery system to provide enhanced efficiency or more beneficial therapy [172]. GO-based hydrogels were discussed in previous section. These delivery platforms could prolong and sustain drug release. Dynamic bonding interactions between GO and its cargo have shown the controlled response to external stimuli such as changes in pH or temperature. The photothermal effect has been suggested for cancer therapy and controlled drug release due to the induced heating of graphene via absorption of NIR light [173,174]. Many drugs, such as DOX [133], curcumin (Cur) [143], colchicine (COLC) [141], 5-FU [73] have shown much faster release in acidic conditions (just as tumors) than in neutral conditions. NIR or pH is the capital stimulus in the controlled drug release of GO-based nanocarriers. There was a different example with alginate (ALG) dissolved in the alkaline environment of the colon, where 5-FU loaded on GO-ALG had a specific release into the colon via oral administration, thus significantly inhibiting the tumor growth [73].
Glutathione (GSH)-triggered release has shown to be successful using GPMQNs (graphene oxide InP@ZnS QDs) as nanocarrier [132]. Fibroblast activation protein (FAP) has been used to activate a cytolytic peptide (detail in previous section) [105]. The particular target microenvironment offered an opportunity to design stimuli-responsive drug delivery systems for controlled release. IONP and GO nanohybrids can be guided by using an external magnetic field [175]. Lu et al. have developed cetuximab (CET) and magnetic dual-target delivery system and combined chemotherapy with photothermal therapy [162].
Conclusion and perspective
GFNs have been widely used for cancer and bacteria treatment, tissue engineering, and biological imaging. Most of these applications rely on the excellent absorption property of GO-based materials. Many efforts have been made to improve GO-based materials as gene and drug nanocarriers via various decorations. In the present study, we grouped the functionalization according to the decoration molecules, placing a higher emphasis on the used nucleic acid deliveries (Figure 1). Organic and inorganic materials are the major sets used to introduce functional sections onto GO. The representative literature of the first three categories of the organic subgroup is listed in Table 1. In terms of cancer treatment efficiency, there is no significant difference in antitumor inhibition rate when using GO delivery nanoplatforms and free drugs. However, it will be different if combined with other strategies such as: phototherapy, target delivery, controlled release and so on.
Controlled and targeted release restricts the nanocarrier delivery efficacy and clinical application. GO-based nanoplatform offer some feasible strategies to improve the drug efficacy. Photothermal and pH-controlled release are excellent characteristics of GO nanocarrier [59,73]. The most common target molecules used in the GO delivery system are shown in Figure 2. Most of the target molecules specifically have an affinity to targets of cancer cells. Cleavable chemical bonds, under the catalytic of a particular enzyme or other molecules in cancer microenvironment, could explicitly give an accumulation or activation of drugs [29,105]. Besides, macrophages or monocytes, due to their tumor-tropic migratory property and strong phagocytic capacity, can serve as a cellular carrier to enhance the delivery efficacy, also known as biomimetic delivery systems (BDS). GO-based nanomaterials combine to macrophage-mediated delivery display appreciable tumor inhibition rate [176].
Though chemotherapy is successfully conducted in tumor-bearing mice, most of the gene therapy remained at the cell level except in Paul’s work on cardiac repair [52], and Yin’s work on antitumor [24]. Both plasmid DNA and small RNA can be delivered via GO-based nanocarrier. In vivo gene delivery was, in most cases, carried out for tissue engineering and bioimaging [177,178]. In conclusion, GO-based nanomaterials still have a long way to go to be applied in clinical work, especially in gene delivery. We believe that target delivery and controlled release, as optimization strategies, are certain fields to be further explored.
Acknowledgements
The present study was supported by the Wenzhou Public Welfare Science and Technology Foundation (Y20160336), Zhejiang Province Natural Sciences Foundation (LQ17H050002), Natural Science Foundation of Inner Mongolia Autonomous Region of China (2017BS0805, 2018BS08014), Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT-20-B22), Research Program of Science and Technology at Universities of Inner Mongolia Autonomous Region (NJZZ18186), Research Funds of Baotou Medical College (BYJJ-YF 201616, BSJJ201707, BSJJ201708).
Disclosure of conflict of interest
None.
References
- 1.Wen AM, Rambhia PH, French RH, Steinmetz NF. Design rules for nanomedical engineering: from physical virology to the applications of virus-based materials in medicine. J Biol Phys. 2013;39:301–325. doi: 10.1007/s10867-013-9314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics-application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660–677. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slonczewski JC, Weiss PR. Band structure of graphite. Physical Review. 1958;109:272–279. [Google Scholar]
- 4.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 5.Geim AK. Graphene: status and prospects. Science. 2009;324:1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez VC, Jachak A, Hurt RH, Kane AB. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol. 2012;25:15–34. doi: 10.1021/tx200339h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song S, Shen H, Wang Y, Chu X, Xie J, Zhou N, Shen J. Biomedical application of graphene: from drug delivery, tumor therapy, to theranostics. Colloids Surf B Biointerfaces. 2020;185:110596. doi: 10.1016/j.colsurfb.2019.110596. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Qi X, Boey F, Zhang H. Graphene-based composites. Chem Soc Rev. 2012;41:666–686. doi: 10.1039/c1cs15078b. [DOI] [PubMed] [Google Scholar]
- 9.Panwar N, Soehartono AM, Chan KK, Zeng S, Xu G, Qu J, Coquet P, Yong KT, Chen X. Nanocarbons for biology and medicine: sensing, imaging, and drug delivery. Chem Rev. 2019;119:9559–9656. doi: 10.1021/acs.chemrev.9b00099. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Wang Z, White JC, Xing B. Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol. 2014;48:9995–10009. doi: 10.1021/es5022679. [DOI] [PubMed] [Google Scholar]
- 11.Sasidharan A, Panchakarla LS, Sadanandan AR, Ashokan A, Chandran P, Girish CM, Menon D, Nair SV, Rao CN, Koyakutty M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small. 2012;8:1251–1263. doi: 10.1002/smll.201102393. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan S, Kim JH. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int J Nanomedicine. 2016;11:1927–1945. doi: 10.2147/IJN.S105264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1:203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendonca MC, Soares ES, de Jesus MB, Ceragioli HJ, Irazusta SP, Batista AG, Vinolo MA, Maróstica Júnior MR, da Cruz-Höfling MA. Reduced graphene oxide: nanotoxicological profile in rats. J Nanobiotechnology. 2016;14:13. doi: 10.1186/s12951-016-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou L, Song B, Liang H, Liu J, Feng X, Deng B, Sun T, Shao L. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol. 2016;13:57. doi: 10.1186/s12989-016-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalwani G, D’Agati M, Khan AM, Sitharaman B. Toxicology of graphene-based nanomaterials. Adv Drug Deliv Rev. 2016;105:109–144. doi: 10.1016/j.addr.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Ding R, Zhao X, Li Y, Qu L, Pei H, Yildirimer L, Wu Z, Zhang W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov Today. 2017;22:1302–1317. doi: 10.1016/j.drudis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Ibraheem D, Elaissari A, Fessi H. Gene therapy and DNA delivery systems. Int J Pharm. 2014;459:70–83. doi: 10.1016/j.ijpharm.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Zhu S, Yan L, Zhao F, Zhao Y. Graphene-based smart platforms for combined cancer therapy. Adv Mater. 2018;31:e1800662. doi: 10.1002/adma.201800662. [DOI] [PubMed] [Google Scholar]
- 21.Jeon SI, Lee JH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide: I. simplified theory. J Colloid Interface Sci. 1991;142:149–158. [Google Scholar]
- 22.Bottini M, Rosato N, Bottini N. PEG-modified carbon nanotubes in biomedicine: current status and challenges ahead. Biomacromolecules. 2011;12:3381–3393. doi: 10.1021/bm201020h. [DOI] [PubMed] [Google Scholar]
- 23.Shen H, Liu M, He H, Zhang L, Huang J, Chong Y, Dai J, Zhang Z. PEGylated graphene oxide-mediated protein delivery for cell function regulation. ACS Appl Mater Interfaces. 2012;4:6317–6323. doi: 10.1021/am3019367. [DOI] [PubMed] [Google Scholar]
- 24.Yin F, Hu K, Chen Y, Yu M, Wang D, Wang Q, Yong KT, Lu F, Liang Y, Li Z. SiRNA delivery with pegylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics. 2017;7:1133–1148. doi: 10.7150/thno.17841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding D, Zhu Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C Mater Biol Appl. 2018;92:1041–1060. doi: 10.1016/j.msec.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Shen H, Song S, Zhang L, Chen W, Dai J, Zhang Z. Graphene oxide incorporated PLGA nanofibrous scaffold for solid phase gene delivery into mesenchymal stem cells. J Nanosci Nanotechnol. 2018;18:2286–2293. doi: 10.1166/jnn.2018.14362. [DOI] [PubMed] [Google Scholar]
- 27.Shirvalilou S, Khoei S, Khoee S, Raoufi NJ, Karimi MR, Shakeri-Zadeh A. Development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: in vitro and in vivo evaluations. Chem Biol Interact. 2018;295:97–108. doi: 10.1016/j.cbi.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi Gazestani A, Khoei S, Khoee S, Emamgholizadeh Minaei S, Motevalian M. In vivo evaluation of the combination effect of near-infrared laser and 5-fluorouracil-loaded PLGA-coated magnetite nanographene oxide. Artif Cells Nanomed Biotechnol. 2018;46:25–33. doi: 10.1080/21691401.2018.1450265. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, Wu J, Jiang W, Liu R, Li Z, Luan Y. Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater Sci Eng C Mater Biol Appl. 2018;89:15–24. doi: 10.1016/j.msec.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Fu C, Yang X, Tan S, Song L. Enhancing cell proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts by BMP-2 delivery in graphene oxide-incorporated PLGA/HA biodegradable microcarriers. Sci Rep. 2017;7:12549. doi: 10.1038/s41598-017-12935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Q, Zhou F, Liu W. Bioinspired catecholic chemistry for surface modification. Chem Soc Rev. 2011;40:4244–4258. doi: 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- 32.Meng Y, Liu P, Zhou W, Ding J, Liu J. Bioorthogonal DNA adsorption on polydopamine nanoparticles mediated by metal coordination for highly robust sensing in serum and living cells. ACS Nano. 2018;12:9070–9080. doi: 10.1021/acsnano.8b03019. [DOI] [PubMed] [Google Scholar]
- 33.Tran AV, Shim K, Vo Thi TT, Kook JK, An SSA, Lee SW. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 2018;1:397–413. doi: 10.1016/j.actbio.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Nan X, Shi W, Sun Y, Su H, He Y, Liu X, Zhang Z, Ge D. Polydopamine-functionalized nanographene oxide: a versatile nanocarrier for chemotherapy and photothermal therapy. Nanotechnology. 2017;28:295102. doi: 10.1088/1361-6528/aa761b. [DOI] [PubMed] [Google Scholar]
- 35.Wajid AS, Das S, Irin F, Ahmed T, Shelburne JL, Parviz D, Fullerton RJ, Jankowski AF, Hedden RC, Green MJ. Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production. Carbon. 2012;50:526–534. [Google Scholar]
- 36.Karki N, Tiwari H, Pal M, Chaurasia A, Bal R, Joshi P, Sahoo NG. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: a comparative study. Colloids Surf B Biointerfaces. 2018;169:265–272. doi: 10.1016/j.colsurfb.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Dou R, Du Z, Bao T, Dong X, Zheng X, Yu M, Yin W, Dong B, Yan L, Gu Z. The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near-infrared light-responsive nanovehicle for chemo-photothermal therapy of cancer. Nanoscale. 2016;8:11531–11542. doi: 10.1039/c6nr01543c. [DOI] [PubMed] [Google Scholar]
- 38.Thapa RK, Byeon JH, Choi HG, Yong CS, Kim JO. PEGylated lipid bilayer-wrapped nano-graphene oxides for synergistic co-delivery of doxorubicin and rapamycin to prevent drug resistance in cancers. Nanotechnology. 2017;28:295101. doi: 10.1088/1361-6528/aa7997. [DOI] [PubMed] [Google Scholar]
- 39.Ganguly S, Ray D, Das P, Maity PP, Mondal S, Aswal VK, Dhara S, Das NC. Mechanically robust dual responsive water dispersible-graphene based conductive elastomeric hydrogel for tunable pulsatile drug release. Ultrason Sonochem. 2018;42:212–227. doi: 10.1016/j.ultsonch.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Consiglio G, Di Pietro P, D’Urso L, Forte G, Grasso G, Sgarlata C, Cossement D, Snyders R, Satriano C. Surface tailoring of polyacrylate-grafted graphene oxide for controlled interactions at the biointerface. J Colloid Interface Sci. 2017;506:532–542. doi: 10.1016/j.jcis.2017.07.080. [DOI] [PubMed] [Google Scholar]
- 41.Abbasian M, Roudi MM, Mahmoodzadeh F, Eskandani M, Jaymand M. Chitosan-grafted-poly (methacrylic acid)/graphene oxide nanocomposite as a pH-responsive de novo cancer chemotherapy nanosystem. Int J Biol Macromol. 2018;118:1871–1879. doi: 10.1016/j.ijbiomac.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 42.Samadi S, Moradkhani M, Beheshti H, Irani M, Aliabadi M. Fabrication of chitosan/poly(lactic acid)/graphene oxide/TiO2 composite nanofibrous scaffolds for sustained delivery of doxorubicin and treatment of lung cancer. Int J Biol Macromol. 2017;110:416–424. doi: 10.1016/j.ijbiomac.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Shou D, Chen C, Mao H, Kong Y, Tao Y. Core-shell structured polypyrrole/mesoporous SiO2 nanocomposite capped with graphene quantum dots as gatekeeper for irradiation-controlled release of methotrexate. Mater Sci Eng C Mater Biol Appl. 2017;81:206–212. doi: 10.1016/j.msec.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly (ethylene imine) and its derivatives. J Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 45.Wang B, Su X, Liang J, Yang L, Hu Q, Shan X, Wan J, Hu Z. Synthesis of polymer-functionalized nanoscale graphene oxide with different surface charge and its cellular uptake, biosafety and immune responses in Raw264.7 macrophages. Mater Sci Eng C Mater Biol Appl. 2018;90:514–522. doi: 10.1016/j.msec.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Xie J, Li T, Mai Z, Wang L, Wang X, Chen T. Gene delivery ability of polyethylenimine and polyethylene glycol dual-functionalized nanographene oxide in 11 different cell lines. R Soc Open Sci. 2017;4:170822. doi: 10.1098/rsos.170822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin D, Li Y, Lin H, Guo B, Du Y, Li X, Jia H, Zhao X, Tang J, Zhang L. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology. 2013;24:105102–105113. doi: 10.1088/0957-4484/24/10/105102. [DOI] [PubMed] [Google Scholar]
- 48.Yin D, Li Y, Guo B, Liu Z, Xu Y, Wang X, Du Y, Xu L, Meng Y, Zhao X, Zhang L. Plasmid-based Stat3 siRNA delivered by functional graphene oxide suppresses mouse malignant melanoma cell growth. Oncol Res. 2016;23:229–236. doi: 10.3727/096504016X14550280421449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Zhou Q, Song W, Wu K, Zhang Y, Zhao Y. Dual-functionalized graphene oxide based siRNA delivery system for implant surface biomodification with enhanced osteogenesis. ACS Appl Mater Interfaces. 2017;9:34722–34735. doi: 10.1021/acsami.7b12079. [DOI] [PubMed] [Google Scholar]
- 50.Dou C, Ding N, Luo F, Hou T, Cao Z, Bai Y, Liu C, Xu J, Dong S. Graphene-based microRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv Sci (Weinh) 2018;5:1700578. doi: 10.1002/advs.201700578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yue H, Zhou X, Cheng M, Xing D. Graphene oxide-mediated Cas9/sgRNA delivery for efficient genome editing. Nanoscale. 2018;10:1063–1071. doi: 10.1039/c7nr07999k. [DOI] [PubMed] [Google Scholar]
- 52.Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VT, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulzar A, Xu J, Xu L, Yang P, He F, Yang D, An G, Ansari MB. Redox-responsive UCNPs-DPA conjugated NGO-PEG-BPEI-DOX for imaging-guided PTT and chemotherapy for cancer treatment. Dalton Trans. 2018;47:3921–3930. doi: 10.1039/c7dt04093h. [DOI] [PubMed] [Google Scholar]
- 54.Araujo RV, Santos SDS, Igne Ferreira E, Giarolla J. New advances in general biomedical applications of PAMAM dendrimers. Molecules. 2018;23:27. doi: 10.3390/molecules23112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Ma D, Tang H, Tan L, Xie Q, Zhang Y, Ma M, Yao S. Polyamidoamine dendrimer and oleic acid-functionalized graphene as biocompatible and efficient gene delivery vectors. ACS Appl Mater Interfaces. 2014;6:8173–8183. doi: 10.1021/am500812h. [DOI] [PubMed] [Google Scholar]
- 56.Gu Y, Guo Y, Wang C, Xu J, Wu J, Kirk TB, Ma D, Xue W. A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mater Sci Eng C Mater Biol Appl. 2017;70:572–585. doi: 10.1016/j.msec.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Yadav N, Kumar N, Prasad P, Shirbhate S, Sehrawat S, Lochab B. Stable dispersions of covalently tethered polymer improved graphene oxide nanoconjugates as an effective vector for siRNA delivery. ACS Appl Mater Interfaces. 2018;10:14577–14593. doi: 10.1021/acsami.8b03477. [DOI] [PubMed] [Google Scholar]
- 58.Melgar-Lesmes P, Luquero A, Parra-Robert M, Mora A, Ribera J, Edelman ER, Jimenez W. Graphene-dendrimer nanostars for targeted macrophage overexpression of metalloproteinase 9 and hepatic fibrosis precision therapy. Nano Lett. 2018;18:5839–5845. doi: 10.1021/acs.nanolett.8b02498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tu Z, Qiao H, Yan Y, Guday G, Chen W, Adeli M, Haag R. Directed graphene-based nanoplatforms for hyperthermia: overcoming multiple drug resistance. Angew Chem Int Ed Engl. 2018;57:11198–11202. doi: 10.1002/anie.201804291. [DOI] [PubMed] [Google Scholar]
- 60.Seidi F, Jenjob R, Phakkeeree T, Crespy D. Saccharides, oligosaccharides, and polysaccharides nanoparticles for biomedical applications. J Control Release. 2018;284:188–212. doi: 10.1016/j.jconrel.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 61.Ali A, Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int J Biol Macromol. 2018;109:273–286. doi: 10.1016/j.ijbiomac.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 62.Bao H, Pan Y, Ping Y, Sahoo NG, Wu T, Li L, Li J, Gan LH. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small. 2011;7:1569–1578. doi: 10.1002/smll.201100191. [DOI] [PubMed] [Google Scholar]
- 63.Cao X, Feng F, Wang Y, Yang X, Duan H, Chen Y. Folic acid-conjugated graphene oxide as a transporter of chemotherapeutic drug and siRNA for reversal of cancer drug resistance. J Nanopart Res. 2013;15:1965–1976. [Google Scholar]
- 64.Wang C, Zhang Z, Chen B, Gu L, Li Y, Yu S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J Colloid Interface Sci. 2018;516:332–341. doi: 10.1016/j.jcis.2018.01.073. [DOI] [PubMed] [Google Scholar]
- 65.Rasoulzadeh M, Namazi H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr Polym. 2017;168:320–326. doi: 10.1016/j.carbpol.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Javanbakht S, Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater Sci Eng C Mater Biol Appl. 2018;87:50–59. doi: 10.1016/j.msec.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Mianehrow H, Afshari R, Mazinani S, Sharif F, Abdouss M. Introducing a highly dispersed reduced graphene oxide nano-biohybrid employing chitosan/hydroxyethyl cellulose for controlled drug delivery. Int J Pharm. 2016;509:400–407. doi: 10.1016/j.ijpharm.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Gentili A. Cyclodextrin-based sorbents for solid phase extraction. J Chromatogr A. 2020;1609:460654. doi: 10.1016/j.chroma.2019.460654. [DOI] [PubMed] [Google Scholar]
- 69.Einafshar E, Asl AH, Nia AH, Mohammadi M, Malekzadeh A, Ramezani M. New cyclodextrin-based nanocarriers for drug delivery and phototherapy using an irinotecan metabolite. Carbohydr Polym. 2018;194:103–110. doi: 10.1016/j.carbpol.2018.03.102. [DOI] [PubMed] [Google Scholar]
- 70.Borandeh S, Abdolmaleki A, Abolmaali SS, Tamaddon AM. Synthesis, structural and in-vitro characterization of beta-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: a versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr Polym. 2018;201:151–161. doi: 10.1016/j.carbpol.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 71.Xiao Y, Zhang M, Fan Y, Zhang Q, Wang Y, Yuan W, Zhou N, Che J. Novel controlled drug release system engineered with inclusion complexes based on carboxylic graphene. Colloids Surf B Biointerfaces. 2018;175:18–25. doi: 10.1016/j.colsurfb.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 72.Zhang YM, Cao Y, Yang Y, Chen JT, Liu Y. A small-sized graphene oxide supramolecular assembly for targeted delivery of camptothecin. Chem Commun (Camb) 2014;50:13066–13069. doi: 10.1039/c4cc04533e. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B, Yan Y, Shen Q, Ma D, Huang L, Cai X, Tan S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater Sci Eng C Mater Biol Appl. 2017;79:185–190. doi: 10.1016/j.msec.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 74.Alibolandi M, Mohammadi M, Taghdisi SM, Ramezani M, Abnous K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr Polym. 2017;155:218–229. doi: 10.1016/j.carbpol.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 75.Sepantafar M, Maheronnaghsh R, Mohammadi H, Radmanesh F, Hasani-Sadrabadi MM, Ebrahimi M, Baharvand H. Engineered hydrogels in cancer therapy and diagnosis. Trends Biotechnol. 2017;35:1074–1087. doi: 10.1016/j.tibtech.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 76.Singh B, Singh B. Modification of sterculia gum polysaccharide via network formation by radiation induced crosslinking polymerization for biomedical applications. Int J Biol Macromol. 2018;116:91–99. doi: 10.1016/j.ijbiomac.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 77.Singh B, Singh B. Influence of graphene-oxide nanosheets impregnation on properties of sterculia gum-polyacrylamide hydrogel formed by radiation induced polymerization. Int J Biol Macromol. 2017;99:699–712. doi: 10.1016/j.ijbiomac.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 78.Rahmani Z, Sahraei R, Ghaemy M. Preparation of spherical porous hydrogel beads based on ion-crosslinked gum tragacanth and graphene oxide: study of drug delivery behavior. Carbohydr Polym. 2018;194:34–42. doi: 10.1016/j.carbpol.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Teodorescu F, Oz Y, Queniat G, Abderrahmani A, Foulon C, Lecoeur M, Sanyal R, Sanyal A, Boukherroub R, Szunerits S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J Control Release. 2017;246:164–173. doi: 10.1016/j.jconrel.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 80.Jo H, Sim M, Kim S, Yang S, Yoo Y, Park JH, Yoon TH, Kim MG, Lee JY. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017;48:100–109. doi: 10.1016/j.actbio.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 81.Saeednia L, Yao L, Berndt M, Cluff K, Asmatulu R. Structural and biological properties of thermosensitive chitosan-graphene hybrid hydrogels for sustained drug delivery applications. J Biomed Mater Res A. 2017;105:2381–2390. doi: 10.1002/jbm.a.36096. [DOI] [PubMed] [Google Scholar]
- 82.Fong YT, Chen CH, Chen JP. Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials (Basel) 2017;7:388. doi: 10.3390/nano7110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iglesias D, Melle-Franco M, Kurbasic M, Melchionna M, Abrami M, Grassi M, Prato M, Marchesan S. Oxidized nanocarbons-tripeptide supramolecular hydrogels: shape matters! ACS Nano. 2018;12:5530–5538. doi: 10.1021/acsnano.8b01182. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Chen Q, Zhou S. Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem Soc Rev. 2018;47:4198–4232. doi: 10.1039/c7cs00399d. [DOI] [PubMed] [Google Scholar]
- 85.Xu X, Wang J, Wang Y, Zhao L, Li Y, Liu C. Formation of graphene oxide-hybridized nanogels for combinative anticancer therapy. Nanomedicine. 2018;14:2387–2395. doi: 10.1016/j.nano.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Ji P, Zhang W, Ai S, Zhang Y, Liu J, Liu J, He P, Li Y. Hybridization of graphene oxide into nanogels to acquire higher photothemal effects for therapeutic delivery. Nanotechnology. 2018;30:115701. doi: 10.1088/1361-6528/aaf8e4. [DOI] [PubMed] [Google Scholar]
- 87.Bardajee GR, Hooshyar Z, Farsi M, Mobini A, Sang G. Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release. Mater Sci Eng C Mater Biol Appl. 2017;72:558–565. doi: 10.1016/j.msec.2016.11.109. [DOI] [PubMed] [Google Scholar]
- 88.Mellado C, Figueroa T, Baez R, Castillo R, Melendrez M, Schulz B, Fernandez K. Development of graphene oxide composite aerogel with proanthocyanidins with hemostatic properties as a delivery system. ACS Appl Mater Interfaces. 2018;10:7717–7729. doi: 10.1021/acsami.7b16084. [DOI] [PubMed] [Google Scholar]
- 89.Wang R, Shou D, Lv O, Kong Y, Deng L, Shen J. pH-controlled drug delivery with hybrid aerogel of chitosan, carboxymethyl cellulose and graphene oxide as the carrier. Int J Biol Macromol. 2017;103:248–253. doi: 10.1016/j.ijbiomac.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y, Wang Y, Niu C, Zhang L, Li G, Yang Y. Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J Biomed Mater Res A. 2018;106:1951–1964. doi: 10.1002/jbm.a.36393. [DOI] [PubMed] [Google Scholar]
- 91.Zare-Zardini H, Taheri-Kafrani A, Amiri A, Bordbar AK. New generation of drug delivery systems based on ginsenoside Rh2-, Lysine- and Arginine-treated highly porous graphene for improving anticancer activity. Sci Rep. 2018;8:586. doi: 10.1038/s41598-017-18938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tasnim N, Thakur V, Chattopadhyay M, Joddar B. The efficacy of graphene foams for culturing mesenchymal stem cells and their differentiation into dopaminergic neurons. Stem Cells Int. 2018;2018:3410168. doi: 10.1155/2018/3410168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma N, Liu J, He W, Li Z, Luan Y, Song Y, Garg S. Folic acid-grafted bovine serum albumin decorated graphene oxide: an efficient drug carrier for targeted cancer therapy. J Colloid Interface Sci. 2017;490:598–607. doi: 10.1016/j.jcis.2016.11.097. [DOI] [PubMed] [Google Scholar]
- 94.Liu C, Hu YL, Deng WJ, Pan QS, Yi JT, Chen TT, Chu X. A graphene oxide nanosensor enables the co-delivery of aptamer and peptide probes for fluorescence imaging of a cascade reaction in apoptotic signaling. Analyst. 2017;143:208–214. doi: 10.1039/c7an01515a. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Sun Q, Cui C, Li J, Wang Y. Anti-HER2 functionalized graphene oxide as survivin-siRNA delivery carrier inhibits breast carcinoma growth in vitro and in vivo. Drug Des Devel Ther. 2018;12:2841–2855. doi: 10.2147/DDDT.S169430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang T, Chen C, Larcher LM, Barrero RA, Veedu RN. Three decades of nucleic acid aptamer technologies: lessons learned, progress and opportunities on aptamer development. Biotechnol Adv. 2019;37:28–50. doi: 10.1016/j.biotechadv.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 97.Cao Y, Dong H, Yang Z, Zhong X, Chen Y, Dai W, Zhang X. Aptamer-conjugated graphene quantum dots/porphyrin derivative theranostic agent for intracellular cancer-related microRNA detection and fluorescence-guided photothermal/photodynamic synergetic therapy. ACS Appl Mater Interfaces. 2017;9:159–166. doi: 10.1021/acsami.6b13150. [DOI] [PubMed] [Google Scholar]
- 98.Jin D, Yang F, Zhang Y, Liu L, Zhou Y, Wang F, Zhang GJ. ExoAPP: exosome-oriented, aptamer nanoprobe-enabled surface proteins profiling and detection. Anal Chem. 2018;90:14402–14411. doi: 10.1021/acs.analchem.8b03959. [DOI] [PubMed] [Google Scholar]
- 99.Datta D, Sarkar K, Mukherjee S, Meshik X, Stroscio MA, Dutta M. Graphene oxide and DNA aptamer based sub-nanomolar potassium detecting optical nanosensor. Nanotechnology. 2017;28:325502–325510. doi: 10.1088/1361-6528/aa79e0. [DOI] [PubMed] [Google Scholar]
- 100.Ma K, Fu D, Liu Y, Rui D, Yu D, Guo Z, Cui C, Wang L, Xu J, Mao C. Cancer cell targeting, controlled drug release and intracellular fate of biomimetic membrane-encapsulated drug-loaded nano-graphene oxide nanohybrids. J Mater Chem B. 2018;6:5080–5090. doi: 10.1039/C8TB00804C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gong P, Zhao Q, Dai D, Zhang S, Tian Z, Sun L, Ren J, Liu Z. Functionalized ultrasmall fluorinated graphene with high NIR absorbance for controlled delivery of mixed anticancer drugs. Chemistry. 2017;23:17531–17541. doi: 10.1002/chem.201702917. [DOI] [PubMed] [Google Scholar]
- 102.Zhang B, Yang X, Wang Y, Zhai G. Heparin modified graphene oxide for pH-sensitive sustained release of doxorubicin hydrochloride. Mater Sci Eng C Mater Biol Appl. 2017;75:198–206. doi: 10.1016/j.msec.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 103.Oz Y, Barras A, Sanyal R, Boukherroub R, Szunerits S, Sanyal A. Functionalization of reduced graphene oxide via thiol-maleimide “click” chemistry: facile fabrication of targeted drug delivery vehicles. ACS Appl Mater Interfaces. 2017;9:34194–34203. doi: 10.1021/acsami.7b08433. [DOI] [PubMed] [Google Scholar]
- 104.Sedghi R, Shaabani A, Mohammadi Z, Samadi FY, Isaei E. Biocompatible electrospinning chitosan nanofibers: a novel delivery system with superior local cancer therapy. Carbohydr Polym. 2017;159:1–10. doi: 10.1016/j.carbpol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 105.Kim MG, Shon Y, Kim J, Oh YK. Selective activation of anticancer chemotherapy by cancer-associated fibroblasts in the tumor microenvironment. J Natl Cancer Inst. 2017;109:10. doi: 10.1093/jnci/djw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haque ST, Chowdhury EH. Recent progress in delivery of therapeutic and imaging agents utilizing organic-inorganic hybrid nanoparticles. Curr Drug Deliv. 2018;15:485–496. doi: 10.2174/1567201814666171120114034. [DOI] [PubMed] [Google Scholar]
- 107.Khan AAP, Khan A, Asiri AM, Ashraf GM, Alhogbia BG. Graphene oxide based metallic nanoparticles and their some biological and environmental application. Curr Drug Metab. 2017;18:1020–1029. doi: 10.2174/1389200218666171016100507. [DOI] [PubMed] [Google Scholar]
- 108.Gera S, Sampathi S, Dodoala S. Role of nanoparticles in drug delivery and regenerative therapy for bone diseases. Curr Drug Deliv. 2017;14:904–916. doi: 10.2174/1567201813666161230142123. [DOI] [PubMed] [Google Scholar]
- 109.Bouzigues C, Gacoin T, Alexandrou A. Biological applications of rare-earth based nanoparticles. ACS Nano. 2011;5:8488–8505. doi: 10.1021/nn202378b. [DOI] [PubMed] [Google Scholar]
- 110.Xu C, Yang D, Mei L, Lu B, Chen L, Li Q, Zhu H, Wang T. Encapsulating gold nanoparticles or nanorods in graphene oxide shells as a novel gene vector. ACS Appl Mater Interfaces. 2013;5:2715–2724. doi: 10.1021/am400212j. [DOI] [PubMed] [Google Scholar]
- 111.Chen H, Liu Z, Li S, Su C, Qiu X, Zhong H, Guo Z. Fabrication of graphene and AuNP core polyaniline shell nanocomposites as multifunctional theranostic platforms for SERS real-time monitoring and chemo-photothermal therapy. Theranostics. 2016;6:1096–1104. doi: 10.7150/thno.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Usman MS, Hussein MZ, Kura AU, Fakurazi S, Masarudin MJ, Ahmad Saad FF. Graphene oxide as a nanocarrier for a theranostics delivery system of protocatechuic acid and gadolinium/gold nanoparticles. Molecules. 2018;23:16. doi: 10.3390/molecules23020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Assali A, Akhavan O, Adeli M, Razzazan S, Dinarvand R, Zanganeh S, Soleimani M, Dinarvand M, Atyabi F. Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomedicine. 2018;14:1891–1903. doi: 10.1016/j.nano.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 114.Yuan Y, Zhang Y, Liu B, Wu H, Kang Y, Li M, Zeng X, He N, Zhang G. The effects of multifunctional MiR-122-loaded graphene-gold composites on drug-resistant liver cancer. J Nanobiotechnology. 2015;13:12. doi: 10.1186/s12951-015-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kooti M, Sedeh AN, Motamedi H, Rezatofighi SE. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl Microbiol Biotechnol. 2018;102:3607–3621. doi: 10.1007/s00253-018-8880-1. [DOI] [PubMed] [Google Scholar]
- 116.Habiba K, Encarnacion-Rosado J, Garcia-Pabon K, Villalobos-Santos JC, Makarov VI, Avalos JA, Weiner BR, Morell G. Improving cytotoxicity against cancer cells by chemo-photodynamic combined modalities using silver-graphene quantum dots nanocomposites. Int J Nanomedicine. 2016;11:107–119. doi: 10.2147/IJN.S95440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Javanbakht S, Nazari N, Rakhshaei R, Namazi H. Cu-crosslinked carboxymethylcellulose/naproxen/graphene quantum dot nanocomposite hydrogel beads for naproxen oral delivery. Carbohydr Polym. 2018;195:453–459. doi: 10.1016/j.carbpol.2018.04.103. [DOI] [PubMed] [Google Scholar]
- 118.Ren L, Pan S, Li H, Li Y, He L, Zhang S, Che J, Niu Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci Rep. 2018;8:15143. doi: 10.1038/s41598-018-33353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Usman MS, Hussein MZ, Fakurazi S, Ahmad Saad FF. Gadolinium-based layered double hydroxide and graphene oxide nano-carriers for magnetic resonance imaging and drug delivery. Chem Cent J. 2017;11:47. doi: 10.1186/s13065-017-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madni A, Noreen S, Maqbool I, Rehman F, Batool A, Kashif PM, Rehman M, Tahir N, Khan MI. Graphene-based nanocomposites: synthesis and their theranostic applications. J Drug Target. 2018;26:858–883. doi: 10.1080/1061186X.2018.1437920. [DOI] [PubMed] [Google Scholar]
- 121.Kwon HJ, Shin K, Soh M, Chang H, Kim J, Lee J, Ko G, Kim BH, Kim D, Hyeon T. Large-scale synthesis and medical applications of uniform-sized metal oxide nanoparticles. Adv Mater. 2018;30:e1704290. doi: 10.1002/adma.201704290. [DOI] [PubMed] [Google Scholar]
- 122.Su X, Chan C, Shi J, Tsang MK, Pan Y, Cheng C, Gerile O, Yang M. A graphene quantum dot@Fe3O4@SiO2 based nanoprobe for drug delivery sensing and dual-modal fluorescence and MRI imaging in cancer cells. Biosens Bioelectron. 2017;92:489–495. doi: 10.1016/j.bios.2016.10.076. [DOI] [PubMed] [Google Scholar]
- 123.Yao X, Niu X, Ma K, Huang P, Grothe J, Kaskel S, Zhu Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small. 2017;13:11. doi: 10.1002/smll.201602225. [DOI] [PubMed] [Google Scholar]
- 124.Rodrigues RO, Baldi G, Doumett S, Garcia-Hevia L, Gallo J, Bañobre-López M, Dražić G, Calhelha RC, Ferreira ICFR, Lima R, Gomes HT, Silva AMT. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater Sci Eng C Mater Biol Appl. 2018;93:206–217. doi: 10.1016/j.msec.2018.07.060. [DOI] [PubMed] [Google Scholar]
- 125.Fu G, Zhu L, Yang K, Zhuang R, Xie J, Zhang F. Diffusion-weighted magnetic resonance imaging for therapy response monitoring and early treatment prediction of photothermal therapy. ACS Appl Mater Interfaces. 2016;8:5137–5147. doi: 10.1021/acsami.5b11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang L, Zhao X. Wet-chemical preparation of TiO(2)-based composites with different morphologies and photocatalytic properties. Nanomaterials (Basel) 2017;7:310. doi: 10.3390/nano7100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jang H, Choi MH, Yim Y, Kim YK, Min DH. Dual-wavelength irradiation and dox delivery for -cancer cell ablation with photocatalytic Pr doped TiO2/NGO -hybrid nanocomposite. Adv Healthc Mater. 2015;4:1833–1840. doi: 10.1002/adhm.201500157. [DOI] [PubMed] [Google Scholar]
- 128.Laurenti M, Lamberti A, Genchi GG, Roppolo I, Canavese G, Vitale Brovarone C, Ciofani G, Cauda V. Graphene oxide finely tunes the bioactivity and drug-delivery of mesoporous ZnO scaffolds. ACS Appl Mater Interfaces. 2018;11:449–456. doi: 10.1021/acsami.8b20728. [DOI] [PubMed] [Google Scholar]
- 129.Tzur-Balter A, Shatsberg Z, Beckerman M, Segal E, Artzi N. Mechanism of erosion of nanostructured porous silicon drug carriers in neoplastic tissues. Nat Commun. 2015;6:6208–6215. doi: 10.1038/ncomms7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jang Y, Kim S, Lee S, Yoon CM, Lee I, Jang J. Graphene oxide wrapped SiO2/TiO2 hollow nanoparticles loaded with photosensitizer for photothermal and photodynamic combination therapy. Chemistry. 2017;23:3719–3727. doi: 10.1002/chem.201605112. [DOI] [PubMed] [Google Scholar]
- 131.Yang D, Yao X, Dong J, Wang N, Du Y, Sun S, Gao L, Zhong Y, Qian C, Hong H. Design and investigation of core/shell GQDs/hMSN nanoparticles as an enhanced drug delivery platform in triple-negative breast cancer. Bioconjug Chem. 2018;29:2776–2785. doi: 10.1021/acs.bioconjchem.8b00399. [DOI] [PubMed] [Google Scholar]
- 132.Zeng X, Yuan Y, Wang T, Wang H, Hu X, Fu Z, Zhang G, Liu B, Lu G. Targeted imaging and induction of apoptosis of drug-resistant hepatoma cells by miR-122-loaded graphene-InP nanocompounds. J Nanobiotechnology. 2017;15:9. doi: 10.1186/s12951-016-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diaz-Diestra D, Thapa B, Badillo-Diaz D, Beltran-Huarac J, Morell G, Weiner BR. Graphene oxide/ZnS: Mn nanocomposite functionalized with folic acid as a nontoxic and effective theranostic platform for breast cancer treatment. Nanomaterials (Basel) 2018;8:18. doi: 10.3390/nano8070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yao C, Zhu J, Xie A, Shen Y, Li H, Zheng B, Wei Y. Graphene oxide and creatine phosphate disodium dual template-directed synthesis of GO/hydroxyapatite and its application in drug delivery. Mater Sci Eng C Mater Biol Appl. 2017;73:709–715. doi: 10.1016/j.msec.2016.11.083. [DOI] [PubMed] [Google Scholar]
- 135.Yang Y, Wang Y, Zhu M, Chen Y, Xiao Y, Shen Y, Xie A. RGO/AuNR/HA-5FU nanocomposite with multi-stage release behavior and efficient antitumor activity for synergistic therapy. Biomater Sci. 2017;5:990–1000. doi: 10.1039/c7bm00007c. [DOI] [PubMed] [Google Scholar]
- 136.Cheang TY, Lei YY, Zhang ZQ, Zhou HY, Ye RY, Lin Y, Wang S. Graphene oxide-hydroxyapatite nanocomposites effectively deliver HSV-TK suicide gene to inhibit human breast cancer growth. J Biomater Appl. 2018;33:216–226. doi: 10.1177/0885328218788242. [DOI] [PubMed] [Google Scholar]
- 137.Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:23. doi: 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu CC, Zhao JJ, Zhang R, Li H, Chen B, Zhang LL, Yang H. Multifunctionalization of graphene and graphene oxide for controlled release and targeted delivery of anticancer drugs. Am J Transl Res. 2017;9:5197–5219. [PMC free article] [PubMed] [Google Scholar]
- 139.Thakkar S, Sharma D, Kalia K, Tekade RK. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: a review. Acta Biomater. 2020;101:43–68. doi: 10.1016/j.actbio.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 140.Yoo HS, Park TG. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J Control Release. 2004;100:247–256. doi: 10.1016/j.jconrel.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 141.Zhang G, Du R, Qian J, Zheng X, Tian X, Cai D, He J, Wu Y, Huang W, Wang Y, Zhang X, Zhong K, Zou D, Wu Z. A tailored nanosheet decorated with a metallized dendrimer for angiography and magnetic resonance imaging-guided combined chemotherapy. Nanoscale. 2017;10:488–498. doi: 10.1039/c7nr07957e. [DOI] [PubMed] [Google Scholar]
- 142.Kansara V, Patil R, Tripathi R, Jha PK, Bahadur P, Tiwari S. Functionalized graphene nanosheets with improved dispersion stability and superior paclitaxel loading capacity. Colloids Surf B Biointerfaces. 2019;173:421–428. doi: 10.1016/j.colsurfb.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 143.Malekmohammadi S, Hadadzadeh H, Farrokhpour H, Amirghofran Z. Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: a new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter. 2018;14:2400–2410. doi: 10.1039/c7sm02248d. [DOI] [PubMed] [Google Scholar]
- 144.Deb A, R V. Natural and synthetic polymer for graphene oxide mediated anticancer drug delivery-a comparative study. Int J Biol Macromol. 2018;107:2320–2333. doi: 10.1016/j.ijbiomac.2017.10.119. [DOI] [PubMed] [Google Scholar]
- 145.Li G, Yang Y, Zhou R, Meng F, Li X. Functionalized graphene oxide as a nanocarrier of new copper (II) complexes for targeted therapy on nasopharyngeal carcinoma. Eur J Pharm Sci. 2018;123:249–259. doi: 10.1016/j.ejps.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 146.Chauhan G, Chopra V, Tyagi A, Rath G, Sharma RK, Goyal AK. “Gold nanoparticles composite-folic acid conjugated graphene oxide nanohybrids” for targeted chemo-thermal cancer ablation: in vitro screening and in vivo studies. Eur J Pharm Sci. 2017;96:351–361. doi: 10.1016/j.ejps.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 147.Platt VM, Szoka FC Jr. Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5:474–486. doi: 10.1021/mp800024g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shao L, Zhang R, Lu J, Zhao C, Deng X, Wu Y. Mesoporous silica coated polydopamine functionalized reduced graphene oxide for synergistic targeted chemo-photothermal therapy. ACS Appl Mater Interfaces. 2017;9:1226–1236. doi: 10.1021/acsami.6b11209. [DOI] [PubMed] [Google Scholar]
- 149.Cherukula K, Nurunnabi M, Jeong YY, Lee YK, Park IK. A targeted graphene nanoplatform carrying histamine dihydrochloride for effective inhibition of leukemia-induced immunosuppression. J Biomater Sci Polym Ed. 2018;29:734–749. doi: 10.1080/09205063.2017.1390382. [DOI] [PubMed] [Google Scholar]
- 150.Nigam Joshi P, Agawane S, Athalye MC, Jadhav V, Sarkar D, Prakash R. Multifunctional inulin tethered silver-graphene quantum dots nanotheranostic module for pancreatic cancer therapy. Mater Sci Eng C Mater Biol Appl. 2017;78:1203–1211. doi: 10.1016/j.msec.2017.03.176. [DOI] [PubMed] [Google Scholar]
- 151.Liu X, Cheng X, Wang F, Feng L, Wang Y, Zheng Y, Guo R. Targeted delivery of SNX-2112 by polysaccharide-modified graphene oxide nanocomposites for treatment of lung cancer. Carbohydr Polym. 2018;185:85–95. doi: 10.1016/j.carbpol.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 152.Hwang DW, Kim HY, Li F, Park JY, Kim D, Park JH, Han HS, Byun JW, Lee YS, Jeong JM, Char K, Lee DS. In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy. Biomaterials. 2017;121:144–154. doi: 10.1016/j.biomaterials.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 153.Miao W, Shim G, Kim G, Lee S, Lee HJ, Kim YB, Byun Y, Oh YK. Image-guided synergistic photothermal therapy using photoresponsive imaging agent-loaded graphene-based nanosheets. J Control Release. 2015;211:28–36. doi: 10.1016/j.jconrel.2015.05.280. [DOI] [PubMed] [Google Scholar]
- 154.Guo M, Xiang HJ, Wang Y, Zhang QL, An L, Yang SP, Ma Y, Wang Y, Liu JG. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem Commun (Camb) 2017;53:3253–3256. doi: 10.1039/c7cc00670e. [DOI] [PubMed] [Google Scholar]
- 155.Zhang C, Liu Z, Zheng Y, Geng Y, Han C, Shi Y, Sun H, Zhang C, Chen Y, Zhang L, Guo Q, Yang L, Zhou X, Kong L. Glycyrrhetinic acid functionalized graphene oxide for mitochondria targeting and cancer treatment in vivo. Small. 2017;14:1703306. doi: 10.1002/smll.201703306. [DOI] [PubMed] [Google Scholar]
- 156.Han C, Zhang C, Ma T, Zhang C, Luo J, Xu X, Zhao H, Chen Y, Kong L. Hypericin-functionalized graphene oxide for enhanced mitochondria-targeting and synergistic anticancer effect. Acta Biomater. 2018;77:268–281. doi: 10.1016/j.actbio.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 157.Roth KG, Mambetsariev I, Kulkarni P, Salgia R. The mitochondrion as an emerging therapeutic target in cancer. Trends Mol Med. 2020;26:119–134. doi: 10.1016/j.molmed.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu F, Yang D, Liu Y, Cao Q, Sun Y, Wang Q, Tang H. Improving dispersive property, biocompatibility and targeting gene transfection of graphene oxide by covalent attachment of polyamidoamine dendrimer and glycyrrhetinic acid. Colloids Surf B Biointerfaces. 2018;171:622–628. doi: 10.1016/j.colsurfb.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 159.Mahalingaiah PK, Ciurlionis R, Durbin KR, Yeager RL, Philip BK, Bawa B, Mantena SR, Enright BP, Liguori MJ, Van Vleet TR. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110–125. doi: 10.1016/j.pharmthera.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 160.Chen X, Zhang M, Liu LX. The overexpression of multidrug resistance-associated proteins and gankyrin contribute to arsenic trioxide resistance in liver and gastric cancer cells. Oncol Rep. 2009;22:73–80. [PubMed] [Google Scholar]
- 161.Deng W, Qiu J, Wang S, Yuan Z, Jia Y, Tan H, Lu J, Zheng R. Development of biocompatible and VEGF-targeted paclitaxel nanodrugs on albumin and graphene oxide dual-carrier for photothermal-triggered drug delivery in vitro and in vivo. Int J Nanomedicine. 2018;13:439–453. doi: 10.2147/IJN.S150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu YJ, Lin PY, Huang PH, Kuo CY, Shalumon KT, Chen MY, Chen JP. Magnetic graphene oxide for dual targeted delivery of doxorubicin and photothermal therapy. Nanomaterials (Basel) 2018;8:20. doi: 10.3390/nano8040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iannazzo D, Pistone A, Salamo M, Galvagno S, Romeo R, Giofre SV, Branca C, Visalli G, Di Pietro A. Graphene quantum dots for cancer targeted drug delivery. Int J Pharm. 2017;518:185–192. doi: 10.1016/j.ijpharm.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 164.Zhao B, Dong K, Lin M, Dong G, Shan S, Lawson T, Yan L, Zhang W, Shi B, Chou S, Baker MS, Liu Y. A transferrin triggered pathway for highly targeted delivery of graphene-based nanodrugs to treat choroidal melanoma. Adv Healthc Mater. 2018;7:e1800377. doi: 10.1002/adhm.201800377. [DOI] [PubMed] [Google Scholar]
- 165.Song MM, Xu HL, Liang JX, Xiang HH, Liu R, Shen YX. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery. Mater Sci Eng C Mater Biol Appl. 2017;77:904–911. doi: 10.1016/j.msec.2017.03.309. [DOI] [PubMed] [Google Scholar]
- 166.Kalmouni M, Al-Hosani S, Magzoub M. Cancer targeting peptides. Cell Mol Life Sci. 2019;76:2171–2183. doi: 10.1007/s00018-019-03061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mousavizadeh A, Jabbari A, Akrami M, Bardania H. Cell targeting peptides as smart ligands for targeting of therapeutic or diagnostic agents: a systematic review. Colloids Surf B Biointerfaces. 2017;158:507–517. doi: 10.1016/j.colsurfb.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 168.Guo Y, Xu H, Li Y, Wu F, Li Y, Bao Y, Yan X, Huang Z, Xu P. Hyaluronic acid and Arg-Gly-Asp peptide modified Graphene oxide with dual receptor-targeting function for cancer therapy. J Biomater Appl. 2017;32:54–65. doi: 10.1177/0885328217712110. [DOI] [PubMed] [Google Scholar]
- 169.Yang Y, Chen S, Liu L, Li S, Zeng Q, Zhao X, Li H, Zhang Z, Bouchard LS, Liu M, Zhou X. Increasing cancer therapy efficiency through targeting and localized light activation. ACS Appl Mater Interfaces. 2017;9:23400–23408. doi: 10.1021/acsami.7b05463. [DOI] [PubMed] [Google Scholar]
- 170.Ghafary SM, Nikkhah M, Hatamie S, Hosseinkhani S. Simultaneous gene delivery and tracking through preparation of photo-luminescent nanoparticles based on graphene quantum dots and chimeric peptides. Sci Rep. 2017;7:9552. doi: 10.1038/s41598-017-09890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mao B, Cheng L, Wang S, Zhou J, Deng L. Combat biofilm by bacteriostatic aptamer-functionalized graphene oxide. Biotechnol Appl Biochem. 2018;65:355–361. doi: 10.1002/bab.1631. [DOI] [PubMed] [Google Scholar]
- 172.Ding C, Li Z. A review of drug release mechanisms from nanocarrier systems. Mater Sci Eng C Mater Biol Appl. 2017;76:1440–1453. doi: 10.1016/j.msec.2017.03.130. [DOI] [PubMed] [Google Scholar]
- 173.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 174.Kurapati R, Raichur AM. Near-infrared light-responsive graphene oxide composite multilayer capsules: a novel route for remote controlled drug delivery. Chem Commun (Camb) 2013;49:734–736. doi: 10.1039/c2cc38417e. [DOI] [PubMed] [Google Scholar]
- 175.Alegret N, Criado A, Prato M. Recent advances of graphene-based hybrids with magnetic nanoparticles for biomedical applications. Curr Med Chem. 2017;24:529–536. doi: 10.2174/0929867323666161216144218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qiang L, Cai Z, Jiang W, Liu J, Tai Z, Li G, Gong C, Gao S, Gao Y. A novel macrophage-mediated biomimetic delivery system with NIR-triggered release for prostate cancer therapy. J Nanobiotechnology. 2019;17:83. doi: 10.1186/s12951-019-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Baek S, Oh J, Song J, Choi H, Yoo J, Park GY, Han J, Chang Y, Park H, Kim H, Cho SG, Kim BS, Kim J. Generation of integration-free induced neurons using graphene oxide-polyethylenimine. Small. 2017;13:10. doi: 10.1002/smll.201601993. [DOI] [PubMed] [Google Scholar]
- 178.Wang F, Zhang B, Zhou L, Shi Y, Li Z, Xia Y, Tian J. Imaging dendrimer-grafted graphene oxide mediated anti-miR-21 delivery with an activatable luciferase reporter. ACS Appl Mater Interfaces. 2016;8:9014–9021. doi: 10.1021/acsami.6b02662. [DOI] [PubMed] [Google Scholar]
- 179.Yue H, Zhou X, Cheng M, Xing D. Graphene oxide-mediated Cas9/sgRNA delivery for efficient genome editing. Nanoscale. 2017;10:1063–1071. doi: 10.1039/c7nr07999k. [DOI] [PubMed] [Google Scholar]


