Abstract
Ellagic acid (EA), a polyphenolic compound from pomegranate fruit extracts, has been reported to possess anti-proliferation, pro-apoptosis, and anti-invasion effects on many cancers. However, its effect on melanoma is yet to be clarified. In the present study, we investigated the anti-cancer effects of EA on melanoma cells in vitro and in vivo. The results indicated that 40 µM of EA significantly inhibited the proliferation, migration, and invasion of WM115 and A375 cells. The EA treatment significantly decreased the expression of p-EGFR and Vimentin, but increased the expression of E-cadherin in both cell lines. We further found that EGFR activation significantly abolished the effect of EA on WM115 and A375 cells. Moreover, EA treatment impaired in vivo tumorigenesis of A375 cells. Moreover, elevated pEGFR expression was an independent detrimental factor for melanoma patients. Taken together, our study provided evidence that EA treatment inhibits the migration, invasion and proliferation of melanoma cells via EGFR signaling pathway. These findings strongly suggested that EA might be useful for the development of new therapeutic strategies at melanoma.
Keywords: Ellagic acid, melanoma, proliferation, migration, invasion, EGFR
Introduction
Melanoma is a highly lethal cancer, and the number of newly diagnosed cases of melanoma in the world is as high as 80,000 every year. The incidence of melanoma is annually increasing at a rate of 4.1%, and it is faster than any other malignancies. From the distribution of melanoma in various parts of the world, environmental and genetic factors play significant roles in the pathogeny of Melanoma. Many therapeutic approaches may improve patients’ survival, including surgical resection, immunotherapy, biotherapy, radiation, or chemotherapy [1,2]. As the understanding of the molecular biology of melanoma is further deepened, some specific drugs targeting melanoma have been developed and undergone clinical trials.
Several natural products, including fruit and vegetable components, could prevent the development of various types of cancer. Ellagic acid (EA) is a kind of natural polyphenol compound with a wide range of pharmacological effect. At present, many reports have showed that EA inhibited the activity of tyrosinase and blocked the formation of melanin. The anticancer activity of EA has been studied and the results showed that EA can significantly reduce the growth rate of tumor cells and promote their apoptosis. It has been also reported that EA may exert effect on melanoma cells.
Receptor tyrosine kinase epidermal growth factor receptor (EGFR) belongs to the ErbB receptor family, which also includes three other members, ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4) [3]. EGFR is usually strongly activated in various tumorigenic processes [4], which also participates in the development and metastasis of melanoma [5,6]. Bankfalvi et al found that over-expression of EGFR in tumor cells enhanced the adhesion of the tumor cells to the extracellular matrix and promoted distant metastasis of tumor cells [7]. High positive rate of EGFR is detected at advanced TNM stages, indicating that over-expression of EGFR not only promotes the proliferation of tumor cells, but also is closely related to the invasion and metastasis of tumors [8,9].
However, the molecular mechanism involved in the effect of EA on melanoma is still far from elucidation. We hypothesized that EA could inhibit the proliferation and invasion of melanoma cells via targeting EGFR. First of all, we first examined the effect of EA on cellular behaviors, the expression levels of EGFR and EMT makers in melanoma cells. Thereafter, we silenced EGFR expression in melanoma cells, and measured the effect of EA on cellular functions and EMT in vitro and in vivo.
Materials and methods
Cell culture
The human melanoma cell lines A375 and WM115 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of sciences (Shanghai, China), which were maintained in Dulbecco’ s modified Eagle’ s medium, and supplemented with 10% foetal bovine serum and Penicillin/Streptomycin (10 mg/ml solution diluted 1:100) and grown in a humidified atmosphere at 37°C under 5% CO2.
For EGFR activation treatment in vitro, EGFR activation in EA treated cells was achieved by specific agonist treatment (NSC228155, 100 µM, Selleckchem). DMSO (0.02%) was used as a control. Typically, the medium was replaced every 24 h with fresh medium. Cells were harvested 48 h after agonist treatment for further examinations.
Cytotoxicity and growth inhibition assay
The cells were plated at 2.5×103 cells per well in 96-well plates. After 12 h or 24 h, various concentrations of EA were added into the wells. The cells were incubated for 48 h at 37°C, then cell growth were assessed by solution formazan formation, WST-1 colorimetric assay (Roche, Mannheim, Germany). In each experiment, determinations were carried out in triplicate.
Cell migration and invasion assay
In order to evaluate cell migration, wound healing assay is performed. Cells were seeded into each chamber at densities of 1×105 cells/ml and grown to > 80% confluence. Then, cell monolayers were wounded by sterile plastic pipette tips and washed with PBS twice to remove floating cells. Cells were treated with serum-free medium (control), 180 µM Erlotinib Hydrochloride (also called as CP-358774, MCE, NJ) or 40 µM EA and cultured for another 48 hours. Images were analyzed using by the TScratch software, and rates of cell migration were determined by counting cells in the gaps using Image J software (NIH, Bethesda, MA).
The analysis of the invasion ability of melanoma cells was performed using transwell assay. Melanoma cells (2×104), treated with serum-free medium (control), CP 180 µM or EA 40 µM, were plated in the upper chamber, a BioCoatTM MatrigelTM Invasion Chamber (BD Biosciences) with inserts containing an 8-µm-pore-sized membrane with a thin layer of Matrigel in the 24-well Transwell plate filled with 500 µL serum-free RPMI1640 medium. In the lower chamber, 750 µL of the 10% FBS-containing medium were added. After cells were cultured for 24 h, the invaded wells were counted under microscopic observation using a Diff-Quick staining kit (Sysmex, Kobe, Japan).
Western blot
Proteins were harvested from cells and animals with M-PER® Mammalian Protein Extraction Reagent (Thermo Scientific, IL). 30 µg of proteins was separated on a 12% sodium dodecyl sulfate polyacrylamide gel and electroblotted on a PVDF membrane. The membrane was blocked overnight at 4°C with 5% fat-free dry milk in PBS and incubated with primary antibodies. Following incubation with secondary rabbit. Cross-reactivity was visualized using ECL western blotting detection reagents and then analyzed via scanning densitometry using a Tanon image system. p-EGFR (phosphor-Y1068) (ab182618, 1:200, Abcam), EGFR (ab32077, 1:1000, Abcam), β-catenin (ab6302, 1:1000, Abcam), Vimentin (ab137321, 1:1000, Abcam), E-cadherin (ab1416, 1:1000, Abcam), GAPDH (10494-1-AP, 1:1000, Proteintech).
Immunohistochemistry staining
Paraffin sections (4 µm thick) were deparaffinized in xylene and rehydrated in grade alcohol, followed by boiling in 10 mmol/L of citrate buffer (PH 6.0) for antigen retrieval. After inhibition of endogenous peroxidase activities for 30 min with methanol containing 0.3% H2O2, the sections were blocked with 2% bovine serum albumin for 30 min and incubated overnight at 4°C with primary monoclonal antibody for p-EGFR (phosphor-Y1068) (ab182618, 1:200, Abcam). After washing thrice with PBS, the slides were incubated with HRP polymer-conjugated anti-rabbit secondary antibody for 30 min, followed by reaction with diaminobenzibine and counterstaining with Mayer’s hematoxylin. Negative control was done by omission of the primary antibody and substituting it with nonspecific rabbit IgG.
Animal experiments
Procedures for animal experiments were all approved by the Institutional Animal Care and Use Committee at Jiangjin District Central Hospital. The mice were 4-6 weeks of age and housed on a 12-hour light dark cycle with food and water. All mice were fed with a standard caloric diet for their age, were randomly divided into three groups and given respectively 1×106 A375 cells by subcutaneous injection in the right flank area of the mice. Tumors were inspected 5 to 6 days after inoculation. The tumor inoculation efficiency was approximately 75%. When tumors reached nearly 2-4 mm in diameter, three groups mice were respectively split into sterile water, 70 mg/kg EA, and 150 mg/kg EA by gavage with 10 ml/kg/d.
The injections were continued for 30 days, with a total of 6 animals per group used for the experiment. The tumor dimensions were measured every three days, in order to estimate the volume we considered the tumors as prolate spheroids with volume equal to (4/3)*πab2, where a is the measurement for the long semi-axis and b is the measurement for the short semi-axis.
Statistical analysis
Data were analyzed with statistical software Graphpad prism Ver. 6.04 (GraphPad Software, San Diego, CA) and results were expressed as mean values ± SEM. Student’s t test was employed for intergroup comparison. Correlation was evaluated using Pearson’s correlative analysis. P < 0.05 was considered statistically significant.
Results
EA inhibited cell proliferation, invasion and migration of melanoma cells
To investigate the anti-cancer effect of EA (Figure 1A) on melanoma, human WM115 and A375 melanoma cells were treated with increasing concentrations (0-80 µM) of EA for 48 h. An MTT assay was used to determine cell viability and a dose-dependent decrease in cell survival was observed in A375 and WM115 cells (Figure 1B). The results revealed that 40 µM and 80 µM EA treatment resulted in death of > 50% of cells, and 40 µM EA treatment was used to subsequent experiments.
Figure 1.
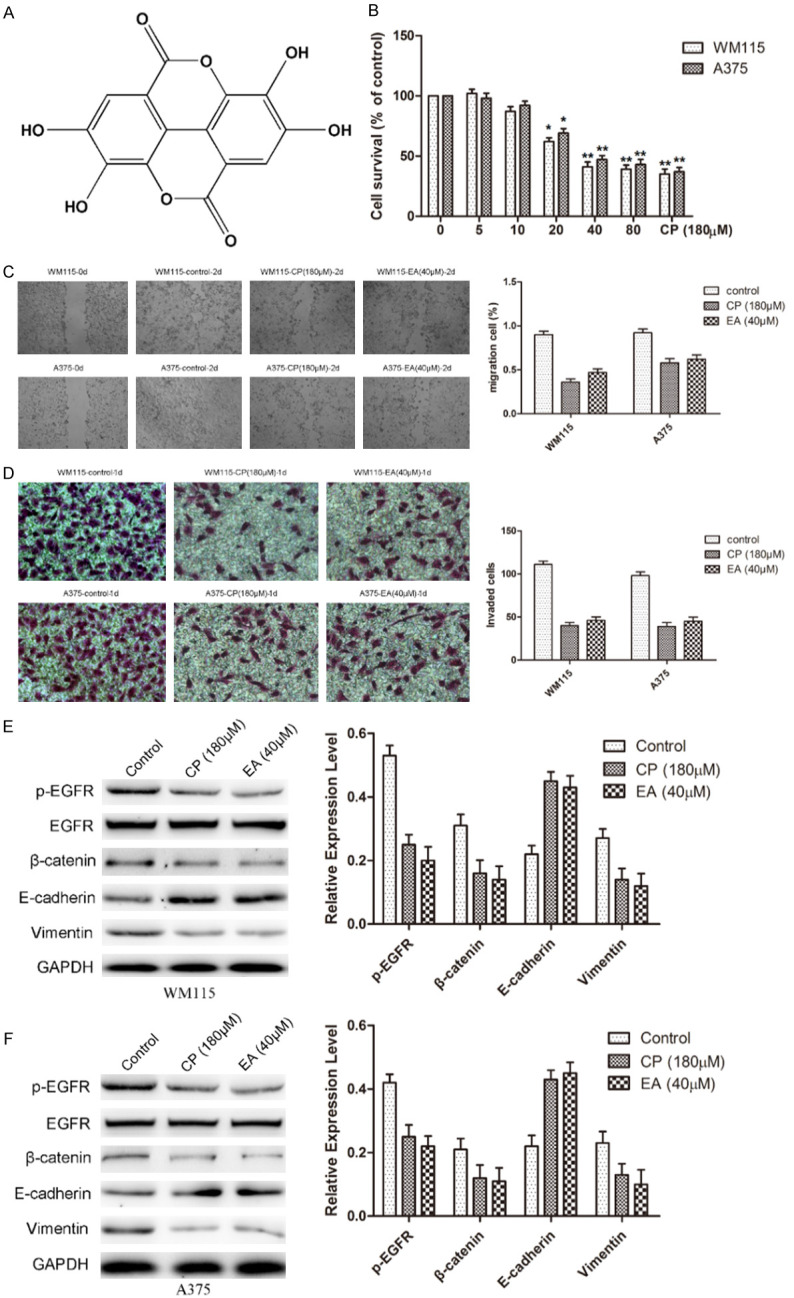
EA suppresses proliferation, migration, invasion, and the expression of EGFR and EMT markers in WM115 and A375 cells. A. The structure of EA. B. The cells were incubated with various concentration of EA for 48 h and then the cell growth was assessed by MMT assay. The results are expressed as a percentage of the untreated cell (0 µM). C. Wound healing assay was used to examine the migratory potential of WM115 and A375 cells. After 48 h for the migration assays, WM115 and A375 cells migration was reduced by EA. D. Transwell invasion assay was performed to detect the invasive potential of WM115 and A375 cells. After 24 h for the invasion assays, WM115 and A375 cells invasion was reduced by EA. E, F. Western blot was used to assess the expression and phosphorylation status of EGFR and EMT markers (β-catenin, E-cadherin, and Vimentin). Note that phosphorylation of EGFR, β-catenin, and Vimentin were reduced, and E-cadherin was increased by EA.
Wound healing assays were performed to investigate the effect of EA on malignant melanoma cell migration in vitro. Each cell line is compared as Day 0 (starting point) and Day 2 for both malignant cell lines (Figure 1C). Cells treated with CP 180 µM and EA 40 µM were compared with their respective controls. The areas of the scratch (wound) were calculated and compared to determine the statistical significance (Figure 1C, Right). We found that both inhibitors significantly reduce the wound closure of both cell lines compared to respective controls (Figure 1C, Right).
Transwell assays were performed to investigate the effect of EA on the invasion of malignant melanoma cells in vitro. Invading cells on the bottom chamber were stained with crystal violet and images were taken in randomly chosen fields as the visual representation of the invasion assay. It was showed that invading cells were respectively reduced by about 50% in CP (180 µM) or EA (40 µM) treated WM115 and A375 cells compared to the control (Figure 1D). These results suggested that both drugs are equally effective in reducing cell migration in vitro.
EA attenuated EGFR phosphorylation and cell EMT of melanoma cells
Expression levels of EGFR and EMT markers (β-catenin, E-cadherin and Vimentin) were detected by western blot from CP or EA treated WM115 and A375 cells. Western blot analysis demonstrated a significantly decreased phosphorylation of EGFR, adhesive factors β-catenin, and mesenchymal marker Vimentin, and conversely, increased expression of epithelial maker E-cadherin compared to control in WM115 and A375 cells (Figure 1E, 1F).
EGFR activation abolishes the suppression on proliferation, migration, invasion, and EMT of melanoma cells treated with EA
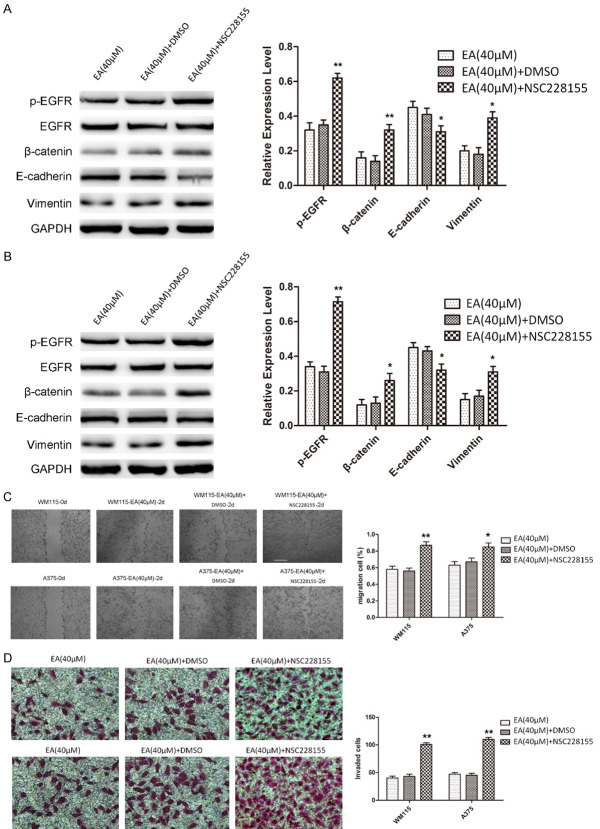
To examine the impact of EGFR on EA treated melanoma cells, we enhanced expression of EGFR in WM115 and A375 cells, which were treated with 40 µM EA, and examine the cells migration, invasion, and proliferation. As a result, the phosphorylation of EGFR was significantly increased by NSC228155 administration (Figure 2A, 2B). Elevated β-catenin and Vimentin was observed with EA (40 µM) and NSC228155 combined administration, whereas decreased E-cadherin expression in western blot analysis (Figure 2A, 2B). These results support enhanced EGFR expression participates in the EMT of melanoma cells, which was blocked by EA treatment.
Figure 2.
EGFR activation abolishes the effect of EA on migration, invasion and the expression of EGFR and EMT markers in WM115 and A375 cells. A, B. Western blot was performed to examine the phosphorylation status of EGFR and the expression of EMT markers after NSC228155 treatment in EA treated WM115 and A375 cells. Note that the phosphorylation of EGFR, β-catenin, and Vimentin were significantly increased by NSC228155 treatment, and the expression of E-cadherin was significantly decreased. C, D. Wound healing assay was performed to assess the migratory potential of EA treated WM115 and A375 cells after NSC228155 treatment. The migration potential of EA treated cells was increased by NSC228155 treatment. Transwell invasion assay was employed to assess the invasive potential of EA treated WM115 and A375 cells after NSC228155. Cell invasion of EA treated cells was increased by NSC228155 treatment.
Moreover, wound healing and transwell assays demonstrated that enhanced EGFR activation abolished the effect of EA on cell migration and invasion in WM115 and A375 cells (Figure 2C, 2D). Taken together, these data suggested that EA suppresses the migration, invasion, and EMT by decreasing activation of EGFR.
EA impairs the tumorigenesis of melanoma cells in vivo
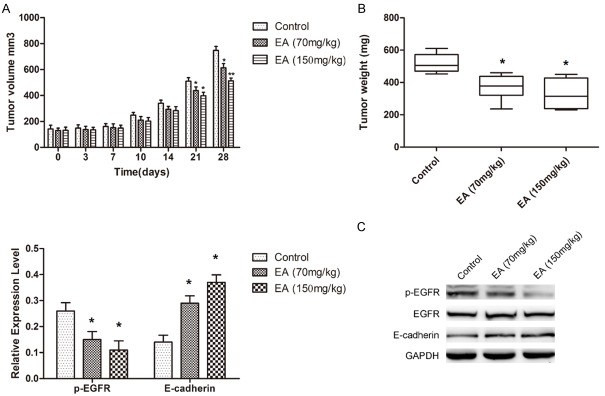
A nude-mice A375 xenograft model was used to further determine whether EA treatment affected the tumorigenesis of melanoma cells in vivo. In line with in vitro results, EA significantly reduced xenograft tumor sizes and weights in vivo (Figure 3A, 3B). Moreover, western blot also indicated that EA treatment significantly inhibited EGFR phosphorylation and increased E-cadherin in vivo (Figure 3C). These data confirmed that EA suppresses tumorigenesis via EGFR signaling pathway.
Figure 3.
EA impairs the tumorigenesis of melanoma cells in vivo. A and B. Statistical analysis of xenograft tumor sizes and weights show that a significant impairment in tumorigenesis of WM115 and A375 cells treated by EA. C. Western blot was performed to examine the phosphorylation status of EGFR and E-cadherin in xenigraft tumor samples. Note that EGFR phosphorylation was significantly reduced, and E-cadherin was significantly increased by EA in vivo.
Elevated pEGFR expression was an independent detrimental factor for melanoma patients
Our study collected totally 104 melanoma patients for further clinicopathological analysis. The cohort included 67 men and 37 women, and the age was ranging from 18 to 87 years (mean 58.70). All patients were followed up every three months and the mean survival time was 38.15 (0.5-69.2) months after diagnosis. IHC staining for pEGFR was performed with melanoma specimens, which showed 35 cases were positive for pEGFR (33.65%) (Figure 4A). Further analysis was performed for the correlations between pEGFR expression and clinicopathological parameters, which showed significant correlation with ulceration, whereas no significant correlation with age, gender, clinical stage and sentinel node metastasis (Table 1). However, univariate survival analysis indicated pEGFR expression was a prognostic factor for poor metastasis free survival (HR = 2.118, 95% CI: 1.279-3.507, P = 0.004) and overall survival (HR = 2.272, 95% CI: 1.323-3.904, P = 0.003). Multivariate analysis also supported pEGFR expression was an independent prognostic factor for poor metastasis free survival (HR = 3.010, 95% CI: 1.671-5.422, P < 0.001) and overall survival (HR = 2.146, 95% CI: 1.207-3.818, P = 0.009) (Table 2). Kaplan-Meier analysis indicated the patients with pEGFR positive tumors showed worse prognosis than those with negative ones (Figure 4B). Taken together, elevated pEGFR expression was an independent detrimental factor for melanoma patients.
Figure 4.
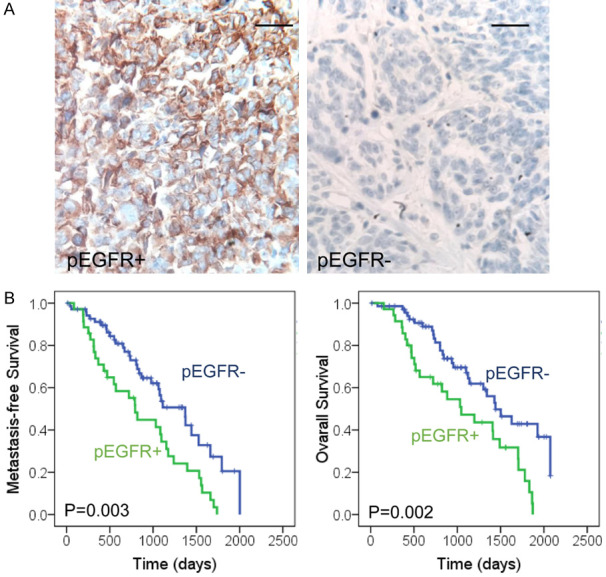
Elevated pEGFR expression is an independent detrimental factor for melanoma patients. A. IHC analysis of pEGFR expression in melanoma tissues. Representative images of melanoma tissue with positive and negative expression are presented. Scale bars represent 100 μm. B. The overall survival and metastasis-free survival of melanoma patients were analyzed with Kaplan-Meier analysis. Positive pEGFR expression was correlated with poor patient survival (P = 0.003, P = 0.002, respectively).
Table 1.
Correlation of pEGFR expression with clinicopathological characteristics of melanoma patients
| Characteristic | Cases | pEGFR | |
|---|---|---|---|
|
| |||
| Positive (%) | P-value | ||
| Total | 104 | 35 (33.65) | |
| Age | |||
| ≤ 57 | 51 | 17 (16.35) | 0.946 |
| > 57 | 53 | 18 (17.31) | |
| Gender | |||
| Male | 67 | 21 (20.19) | 0.502 |
| Female | 37 | 14 (13.46) | |
| TNM stage | |||
| I-II | 53 | 23 (22.12) | 0.367 |
| III-IV | 51 | 12 (11.54) | |
| Ulceration | |||
| No | 42 | 12 (11.54) | 0.032 |
| Yes | 62 | 23 (22.12) | |
| LN metastasis | |||
| Yes | 57 | 23 (22.12) | 0.111 |
| No | 47 | 12 (11.54) | |
Table 2.
Cox regression analysis of pEGFR expression in metastasis free survival and overall survival estimation of melanoma patients
| Metastasis free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95.0% CI | p | HR | 95.0% CI | P | |
| Univariate analysis | ||||||
| pEGFR | 2.118 | 1.279-3.507 | 0.004 | 2.272 | 1.323-3.904 | 0.003 |
| Multivariate analysis | ||||||
| Age | 1.245 | 0.736-2.105 | 0.414 | 1.049 | 0.603-1.826 | 0.865 |
| Gender | 0.930 | 0.512-1.687 | 0.810 | 1.397 | 0.78-2.499 | 0.261 |
| Stage | 4.548 | 1.305-15.852 | 0.017 | 0.692 | 0.138-3.468 | 0.654 |
| Ulceration | 1.191 | 0.703-2.016 | 0.516 | 1.888 | 1.049-3.397 | 0.034 |
| Lymph | 0.532 | 0.170-1.663 | 0.278 | 1.994 | 0.407-9.769 | 0.395 |
| pEGFR | 3.010 | 1.671-5.422 | 0.000 | 2.146 | 1.207-3.818 | 0.009 |
CI, confidence interval; HR, hazard ratios; Lymph, sentinel node metastasis. The variables were compared in the following ways: Age, ≤ 57 years vs. > 57 years; Gender, male vs. female; Stage, I-II vs. III-IV; Ulceration, no vs. yes; Lymph, no vs. yes; pEGFR, positive vs. negative.
Discussion
EA is a kind of natural Polyphenolic phytochemicals compounds which play critical functions in protecting plants from microbial infections, preventing ROS stress, or deterrence of herbivores [10-12]. Notably, growing evidence shows that EA exert its chemopreventive effect against different types of cancers in preclinical studies. However, therapeutic function of EA in melanoma remains poorly studied.
Melanoma is the most aggressive malignancy arising from skin. Although local pathological change of malignant melanoma can be cured by surgical operation, the prognosis becomes poor once metastasis occurs [13,14]. Metastasis is one of the main obstacles to improve the therapeutic effect of tumor, which led to the death of 90% of human tumors. It is well established that EMT is the initiation of tumor invasion and metastasis, during which tumor cells probably gain enhanced migration, invasion, proliferation and/or anti-apoptosis properties [15-18]. There is cumulative evidence that the expression of EGFR is related to the growth and metastasis mechanism of various tumors. EGFR has become a marker of poor prognosis in many kinds of cancer, including breast cancer [19], glioma [20], squamous cell carcinoma [21], and larynx cancer [22]. Previous reports indicated over-expressed EGFR in malignant melanoma was related to the metastasis and invasion [5,23-25]. In this study, we provided further evidences that elevated pEGFR expression was an independent detrimental factor for melanoma patients.
In the present study, we used WM115 and A375 cells to address cancer-suppressing effects of EA, and revealed a novel molecular mechanism underlying the antitumor activity of EA in WM115 and A375 cells. The data showed that EA can inhibit the proliferation of WM115 and A375 cells with time and concentration gradient, and the immunohistochemical results of Ki67 were also verified. We next confirmed migration and invasion-inhibitory function of EA via Transwell in WM115 and A375 cells. We speculated that EA might contribute to suppress cell invasion by decreasing the expression of EGFR. As we expected, EGFR was over-expressed in WM115 and A375 cells, and EA decreased the expression of EGFR. When EGFR expression was knocked down, the proliferation and invasion of WM115 and A375 cells were weakened, and EA enhanced the effect. Moreover, animal experiments further verified our speculations.
Taken together, our study revealed that EA inhibits melanoma cell proliferation and invasion via decreasing the activation of EGFR. Therefore, the administration of EA might serve as a potential adjuvant therapeutic strategy for melanoma patients.
Acknowledgements
We thank Pathology Department of Jiangjin District Central Hospital for technical assistance. This study was supported by Medical Research Program of Chongqing Health and Family Planning Commission (2017MSXM171).
Disclosure of conflict of interest
None.
Abbreviations
- EA
Ellagic acid
- EGFR
epidermal growth factor receptor
References
- 1.Hao M, Song F, Du X, Wang G, Yang Y, Chen K, Yang J. Advances in targeted therapy for unresectable melanoma: new drugs and combinations. Cancer Lett. 2015;359:1–8. doi: 10.1016/j.canlet.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 2.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 3.Guida M, Pisconti S, Colucci G. Metastatic melanoma: the new era of targeted therapy. Expert Opin Ther Targets. 2012;16(Suppl 2):S61–70. doi: 10.1517/14728222.2011.645807. [DOI] [PubMed] [Google Scholar]
- 4.Boone B, Jacobs K, Ferdinande L, Taildeman J, Lambert J, Peeters M, Bracke M, Pauwels P, Brochez L. EGFR in melanoma: clinical significance and potential therapeutic target. J Cutan Pathol. 2011;38:492. doi: 10.1111/j.1600-0560.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 5.Koprowski H, Herlyn M, Balaban G, Parmiter A, Ross A, Nowell P. Expression of the receptor for epidermal growth factor correlates with increased dosage of chromosome 7 in malignant melanoma. Somat Cell Mol Genet. 1985;11:297. doi: 10.1007/BF01534687. [DOI] [PubMed] [Google Scholar]
- 6.de Wit PE, Moretti S, Koenders PG, Weterman MA, van Muijen GN, Gianotti B, Ruiter DJ. Increasing epidermal growth factor receptor expression in human melanocytic tumor progression. J Invest Dermatol. 1992;99:168–173. doi: 10.1111/1523-1747.ep12616793. [DOI] [PubMed] [Google Scholar]
- 7.Bánkfalvi A, Krassort M, Végh A, Felszeghy E, Piffkó J. Deranged expression of the E-cadherin/beta-catenin complex and the epidermal growth factor receptor in the clinical evolution and progression of oral squamous cell carcinomas. J Oral Pathol Med. 2002;31:450–457. doi: 10.1034/j.1600-0714.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 8.Tateishi M, Ishida T, Mitsudomi T, Kaneko S, Sugimachi K. Immunohistochemical evidence of autocrine growth factors in adenocarcinoma of the human lung. Cancer Res. 1990;50:7077–7080. [PubMed] [Google Scholar]
- 9.Lai WW, Chen FF, Wu MH, Chow NH, Su WC, Ma MC, Su PF, Chen H, Lin MY, Tseng YL. Immunohistochemical analysis of epidermal growth factor receptor family members in stage I non-small cell lung cancer. Ann Thorac Surg. 2001;72:1868. doi: 10.1016/s0003-4975(01)03207-6. [DOI] [PubMed] [Google Scholar]
- 10.Kılıçgün H, Altıner D. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn Mag. 2010;6:238–241. doi: 10.4103/0973-1296.66943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan C, Yu Y, Zhou S, Wei L, Tian S, Cao S. Antioxidant activity and free radical-scavenging capacity of Gynura divaricataleaf extracts at different temperatures. Pharmacogn Mag. 2011;7:40–45. doi: 10.4103/0973-1296.75900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitrova-Dyulgerova I, Zhelev I, Mihaylova D. Phenolic profile and in vitro antioxidant activity of endemic bulgarian carduus species. Pharmacogn Mag. 2015;11(Suppl 4):S575–S579. doi: 10.4103/0973-1296.172964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 14.Lacy KE, Karagiannis SN, Nestle FO. Advances in the treatment of melanoma. Br Med J. 1966;1:752–753. [PMC free article] [PubMed] [Google Scholar]
- 15.Minzhi H, Zhiqiang C, Hongwei S, Shanyang H, Yang L, Yunping P, Chongjin F, Xinlin C, Yang Z, Millicent L. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget. 2015;6:35813–35829. doi: 10.18632/oncotarget.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayachandran A, Dhungel B, Steel JC. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol. 2016;9:74. doi: 10.1186/s13045-016-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krebs AM, Mitschke J, Losada ML, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 18.Pietilä M, Ivaska J, Mani SA. Whom to blame for metastasis, the epithelial-mesenchymal transition or the tumor microenvironment? Cancer Lett. 2016;380:359–368. doi: 10.1016/j.canlet.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Harris AL, Nicholson S, Sainsbury R, Wright C, Farndon J. Epidermal growth factor receptor and other oncogenes as prognostic markers. J Natl Cancer Inst Monogr. 1992;11:181. [PubMed] [Google Scholar]
- 20.Diedrich U, Lucius J, Baron E, Behnke J, Pabst B, Zoll B, Diedrich U. Distribution of epidermal growth factor receptor gene amplification in brain tumours and correlation to prognosis. J Neurol. 1995;242:683. doi: 10.1007/BF00866920. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa Y, Ueda M, Ando N, Ozawa S, Shimizu N, Kitajima M. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 1996;2:909. [PubMed] [Google Scholar]
- 22.Maurizi M, Scambia G, Benedetti PP, Ferrandina G, Almadori G, Paludetti G, De VR, Distefano M, Brinchi D, Cadoni G. EGF receptor expression in primary laryngeal cancer: correlation with clinico-pathological features and prognostic significance. Int J Cancer. 1992;52:862–866. doi: 10.1002/ijc.2910520605. [DOI] [PubMed] [Google Scholar]
- 23.Pietraszek-Gremplewicz K, Simiczyjew A, Dratkiewicz E, Podgorska M, Styczen I, Matkowski R, Zietek M, Nowak D. Expression level of EGFR and MET receptors regulates invasiveness of melanoma cells. J Cell Mol Med. 2019;23:8453–8463. doi: 10.1111/jcmm.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grover A, Alexander HR Jr. The past decade of experience with isolated hepatic perfusion. Oncologist. 2004;9:653–664. doi: 10.1634/theoncologist.9-6-653. [DOI] [PubMed] [Google Scholar]
- 25.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]