Abstract
Simvastatin belongs to the family of statins and is found to have some osteopromotive properties in recent years. The aim of the present study was to investigate the potential effects of simvastatin on bone formation of the expanded mid-palatal suture of rats. Forty-five Wistar rats were randomly divided into three groups: control (C), expansion (EP), and expansion plus simvastatin (ES) groups. Rats in the ES group were administrated with simvastatin (20 mg/kg/d body weight). According to the schedule of sacrifice (days 3, 7 and 14), the suture width and bone volume changes of the region of interest (ROI) were detected by micro-computed tomography during RME. Besides, morphological changes and bone morphogenetic protein 2 (BMP-2) expression in the mid-palatal suture were observed by hematoxylin and eosin (HE) and immunohistochemical staining. Kruskal-Wallis one-way analysis of variance (ANOVA) and LSD method were applied to analyze the data at P<0.05 level. By the RME appliance, the suture was successfully widened. On days 7, 14, the bone volume of ROI in the ES group was more than that in the EP group (P<0.05). Besides, histological examinations also demonstrated that more bone regeneration and capillaries in the suture in the ES group were observed than that in the EP group. The BMP-2 expression in the ES group was more (P<0.05) than that in the EP and C groups on days 3, 7, 14. Consequently, those findings showed that simvastatin can induce a favorable effect on bone regeneration in the mid-palatal suture of rats during RME.
Keywords: Simvastatin, expansion, mid-palatal suture, BMP-2, bone volume
Introduction
In clinical training, rapid maxillary expansion (RME) is one of the most common orthodontic and orthopedic treatment methods used to correct these dentofacial deformities caused by maxillary transverse deficiency, such as dental crowding, maxillary dental arch stenosis, posterior cross-bite and so on [1]. The procedure is based on the growth potential of the children or adolescents themselves and the mechanical properties and adaptability of sutures to different tensile or cyclic loading [2]. The expansion force of the RME device can be conducted to the mid-palatal suture by the molars and the alveolar bone. Next, there are some activities that have occurred in the mid-palatal suture to promote suture remodeling, including cell transformation, cellular signaling pathways, and the regulation of cell factors, growth factors and transcription factors [3-5]. However, a high incidence of post-treatment relapse is considered as the main obstacle to RME [6]. The RME results are unstable and affected by a variety of internal or external factors, such as gender, age, the magnitude of the expansion force and some exogenous factors [7-9]. Insufficient bone formation in the suture is usually accepted as a determining factor of post-expansion relapse. As a result, new bone regeneration in the mid-palatal suture plays an important role in avoiding the relapse after expanding the suture [9-11]. Exogenous stimulus-related researches are carried out to study the effects of various substances on bone metabolism via promotion of osteoblastic activity or inhibition of osteoclastic activity for improving the stability of expansion and abbreviating the retention period [12-22].
Simvastatin, which belongs to the family of statins and is the inhibitor of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase, is originally used for lowering cholesterol, decreasing the blood lipid level, and reducing the incidence of stroke in patients with coronary heart disease and hypercholesterolemia [23,24]. A new perspective on osteopromotive properties of simvastatin was introduced by the study of Mundy et al in 1999. They pointed out that simvastatin could promote bone regeneration in the calvaria defect model [25]. Subsequently, there were many studies reported regarding the pleiotropic effects of simvastatin in bone metabolism. On one hand, simvastatin could induce BMP-2 and VEGF gene expression to stimulate the differentiation of osteoblastic cells [26]. On the other hand, simvastatin could reduce the expression of TRAP and cathepsin K, prevent the fusion of osteoclast precursors, and decrease the number of active osteoclasts to inhibit bone resorption [27]. In the light of its stimulatory effects on bone formation, our aim in simvastatin administration with RME was to accelerate bone regeneration in an expanding mid-palatal suture. Consequently, the objective of this study was to investigate the effects of simvastatin on the bone formation following RME in a rat model to provide new additional insights into strategies for preventing post-expansion relapse.
Materials and methods
Animals and groups
All animal protocols were approved by the Institutional Animal Care and Use Committee of Shandong University and were carried out following the National Institutes of Health Guidelines for the Use of Laboratory Animals. With approval from the ethics committee on animal experimentation of the Medical School of Shandong University, all surgeries were performed under anesthesia, and all efforts were made to minimize the suffering of rats.
For the research, forty-five 6-week-old male Wistar rats weighing 150±10 g and supplied by the experimental animal center of Shandong University were utilized. The health of all rats was checked every day. The rats were randomly divided into the following three groups: expansion only (EP) group (n=15), expansion plus simvastatin (ES) group (n=15) and control (C) group (n=15). The expansion procedure was performed on rats of the EP and ES groups. Simvastatin was given to the rats in the ES group during the expansion period. Neither expansion procedure nor simvastatin administration was carried out in the animals of the C group.
Simvastatin obtained from Merck Sharp & Dohme Company Limited (Hangzhou, China), were crushed into powder and dissolved in normal saline. In the ES group, 20 mg simvastatin/d/kg body weight was systemically administered by intragastric injection. Dosing was performed at the same time each day until the rats were killed.
Placement of expansion appliance
For the placement of the expansion appliance, the rats in EP and ES groups were anesthetized with an intramuscular injection of 3 mg/kg xylazine hydrochloride (Shanghai ZZBIO Co. Ltd, Shanghai, China) and 35 mg/kg ketamine hydrochloride (Jiangsu Hengrui Medicine Co. Ltd, Lianyungang, China). The individual working model of the rat maxilla was first achieved to make the expander (Figure 1). An expansion appliance with two helical springs, which was fabricated from 0.014-inch Australian wire, was bonded to first and second maxillary molars on both sides to expand the mid-palatal suture. By opening the helical spring, the initial force of the expansion appliance was adjusted to 100±5 g, measured with a strain gauge (Hangzhou Aosu Medical Devicement Co., Hangzhou, China). The rats were weighed and monitored each day for signs of weight loss, infection and appliance failure throughout the study. If any complications such as mucosal infection, a rapid decrease in body weight, or detached appliance were observed, the animals were excluded from the study and replaced.
Figure 1.
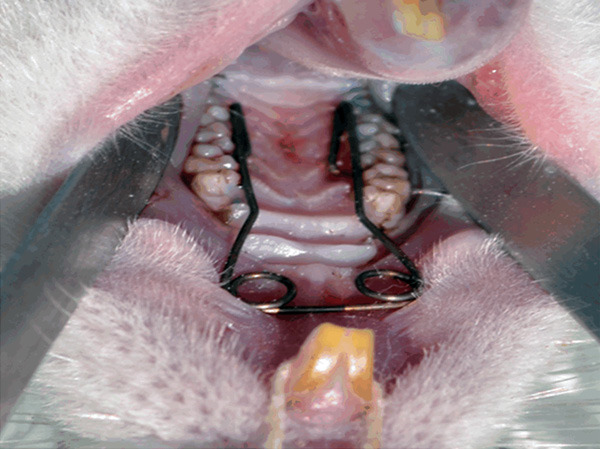
Intra-oral view of the RME appliance set on the molars of the rat.
Width and bone volume analyses of rat mid-palatal suture
Under the condition of 80 kV and 500 μA with an effective pixel size of 8.5 μm, the maxilla of the rats in each group was scanned by high-resolution micro-computed tomography (micro-CT) system (Inveon MM CT, Siemens, USA) to achieve the three- and two-dimensional radiographs of the hard palate on the 3th, 7th, 14th days. The images were reconstructed and analyzed by the software COBRA_Exxim reconstruction software (Exxim Computing Corporation, Pleasanton, California, USA) and Inveon Research Workplace (Siemens, USA) to explore the morphological changes in the mid-palatal suture. The width of the mid-palatal suture was measured at the level of the mid-coronal plane of the upper first molar (Figure 2A, 2B). Besides, bone volume changes of the region of interest (ROI, 0.60 mm × 0.90 mm × 2.00 mm) were measured (Figure 2C-E) to investigate the effects of the expansive force and simvastatin on the mid-palatal suture.
Figure 2.
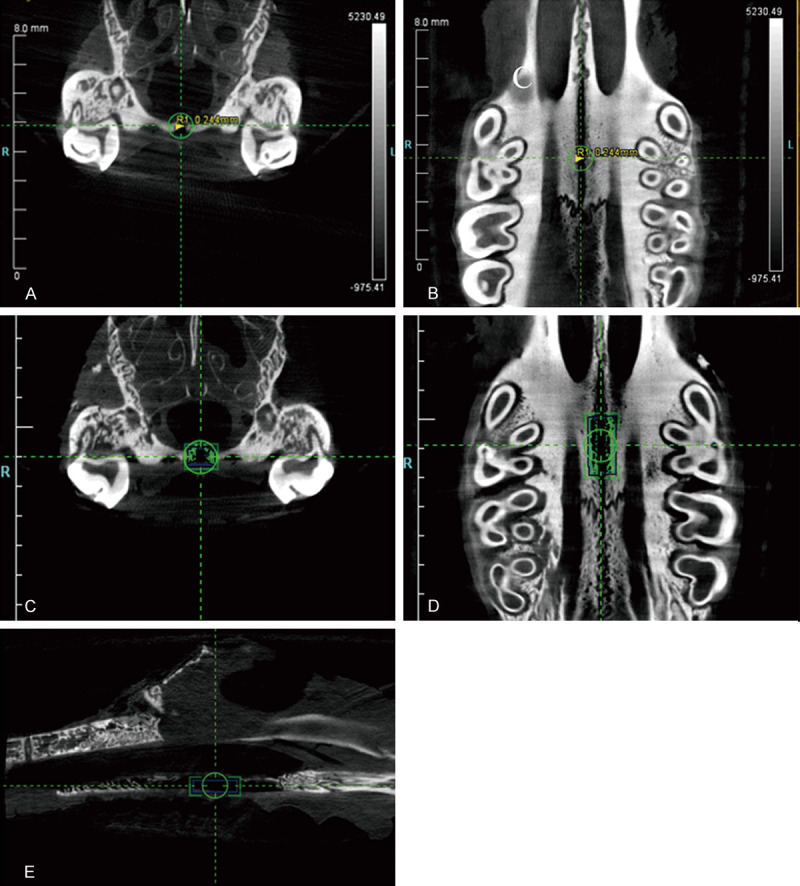
Micro-CT images of the mid-palatal suture of the rat. The width of the mid-palatal suture was measured on the mid-coronal plane of the upper first molar in the coronal view (A) and the occlusal view (B). The bone volume was measured within the region of interest (ROI, 0.60 mm × 0.90 mm × 2.00 mm) in the coronal (C), horizontal (D) and sagittal (E) plane. The blue box is ROI, and the green area is the bone volume (C-E).
Histological examination
The rats were scheduled for sacrifice in batch on the 3rd, 7th, 14th days. Their maxillae were dissected and fixed in 4% paraformaldehyde buffer at 4°C for 24 h. After the expansion appliances were removed from the maxilla, the specimens were surgically removed, trimmed and then decalcified in 10% ethylene-diaminetetraacetic acid/phosphate-buffered saline (PBS) solution at 4°C for three months. Subsequently, the tissues were dehydrated in graded ethanol dehydration and cleared with xylene, then embedded in paraffin wax.
Serial 5-μm-thick sections of the embedded tissues were cut at the level of the upper first molar in a coronal plane and mounted on 3-aminopropyl-triethoxysilane-coated slides. For histological examinations, the sections were stained with hematoxylin and eosin (HE) and then the histological changes and bone remodeling in the three groups were obtained under a light microscope (BX51, Olympus, Tokyo, Japan). The results can be observed in Figure 3.
Figure 3.
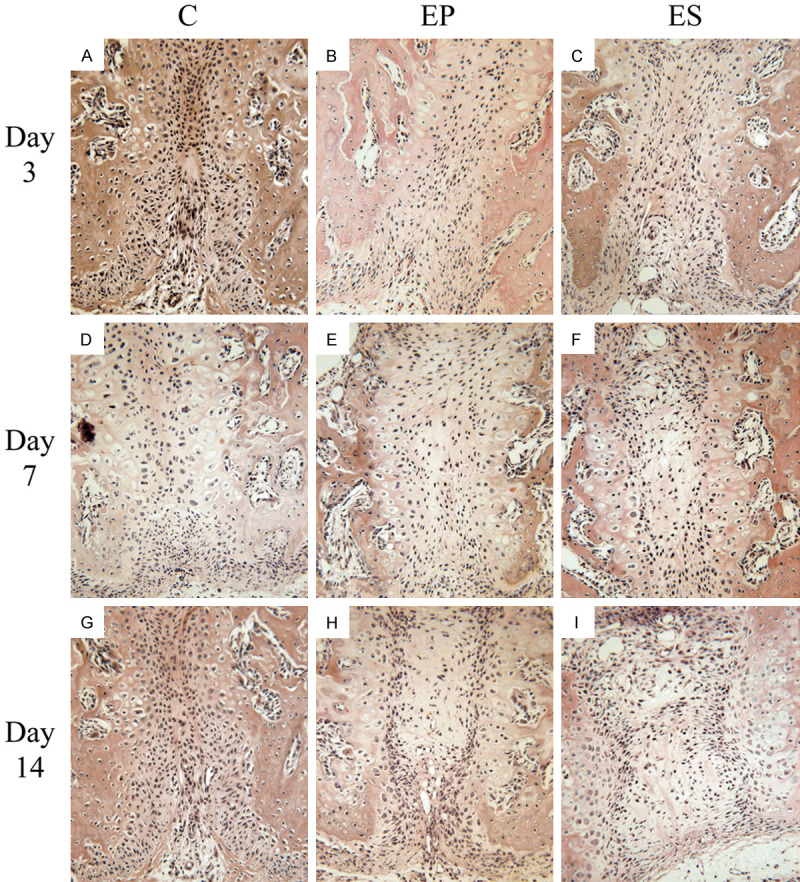
Hematoxylin and eosin staining of the histological sections of the mid-palatal suture (All images were displayed at 200 × magnification and oriented with the nasal side up and the oral side down). A. Changes of the mid-palatal suture in the C group on day 3. B. Changes of the mid-palatal suture in the EP group on day 3. C. Changes of the mid-palatal suture in the ES group on day 3. D. Changes of the mid-palatal suture in the C group on day 7. E. Changes of the mid-palatal suture in the EP group on day 7. F. Changes of the mid-palatal suture in the ES group on day 7. G. Changes of the mid-palatal suture in the C group on day 14. H. Changes of the mid-palatal suture in the EP group on day 14. I. Changes of the mid-palatal suture in the ES group on day 14.
Immunohistochemical staining of BMP-2
The sections were deparaffinized in xylene, hydrated through a graded alcohol series, and washed with PBS. Antigen retrieval was performed by treatment with 0.1% (w/v) trypsin (Zhongshan, Beijing, China) at 37°C for 10 min. The activity of endogenous tissue peroxidase was blocked with 3% H2O2 for 30 min. After pretreatment with normal goat serum for 30 min to block nonspecific binding, the sections were incubated with BMP-2 antibody (1:100 dilution; Biosynthesis Biotechnology, Beijing, China) at 4°C overnight. Subsequently, the sections were incubated with biotinylated goat anti-rabbit immunoglobulin G and StreptAvidin-BiotinComplex (Boster, Wuhan, China) at 37°C for 25 min. Diaminobenzidine solution was used to visualize localization for 2 min. Finally, the sections were lightly counterstained with hematoxylin. Sections treated with PBS instead of the primary antibody were used as negative controls.
The cells with brownish-yellow granules in the cytoplasm or nucleus were positive. The slides were analyzed by Image-Pro Plus software to determine the mean optical density (MOD) of the immunohistochemical images. A single examiner in a blind study randomly selected five high power fields (× 400) from each slide to carry out the evaluation and the results were taken as an average of five fields.
Statistical analysis
All data were presented as the mean ± SD for each group and all analysis was performed by the Prism 6 software (GraphPad Software, La Jolla, CA, USA). Kruskal-Wallis one-way analysis of variance (ANOVA) was used to compare the groups. LSD method was applied to identify intergroup differences. A threshold of P<0.05 was defined as statistically significant.
Results
The mucosal infection, dehiscence, serious weight loss, and other adverse effects were not encountered in any animals in the study period. However, one rat was excluded from the study as a result of appliance failure and was replaced with one other rat.
Body weight changes
The rats in EP and ES groups both lost weight on the first three days, but subsequently, their body weights began to increase from the fourth day. The body weights of the rats in the C group were gradually increased during the study. All animals had no diarrhea or other gastrointestinal symptoms. The mean body weights of the rats in groups EP and ES were significantly different from that in the C group on day 2 to day 7 (P<0.05). However, there were no statistically significant differences in the body weights between the three groups from the 7th day (P>0.05). No significant differences in the mean body weights of the rats between groups EP and ES were observed throughout the experiment (P>0.05) (Figure 4).
Figure 4.
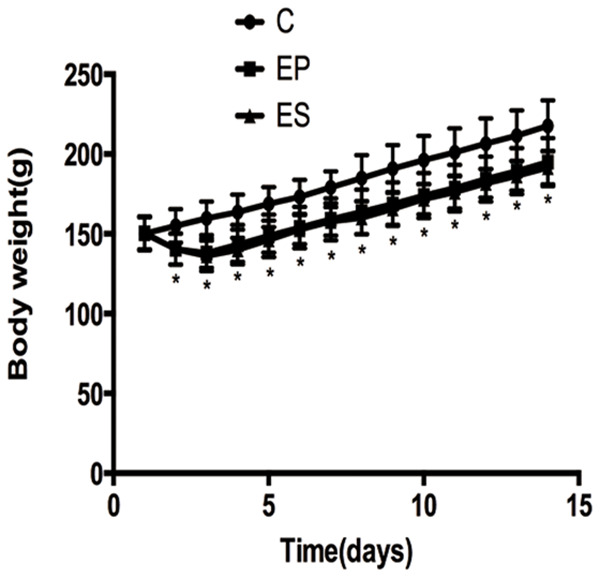
Body weight changes of rats in the three groups during the experiment. *P<0.05, significant decrease versus the C group. C, control group; EP, expansion group; ES, expansion plus simvastatin group.
Width and bone volume changes of the mid-palatal suture
There is nothing changed in the mid-palatal suture width of the C group during the experiment. In contrast, a significant increase in the width of the suture (P<0.05) in the EP and ES groups were observed on days 3, 7, and 14. We noticed the suture width in the ES group was less than that in the EP group on days 7, 14, but they did not achieve statistical significance (P>0.05) (Figure 5A). Furthermore, the change in bone volume of ROI of the C group was also no obvious. Compared with the C group, the bone volume of ROI in groups ES and EP were significantly decreasing (P<0.05). On the 7th and 14th days, the bone volume of ROI in the ES group was more than that in the EP group (P<0.05) (Figure 5B).
Figure 5.
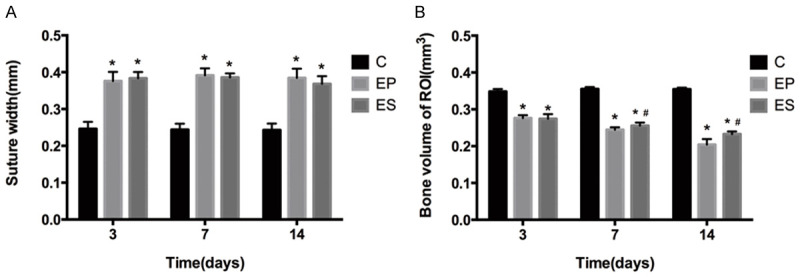
The width of the mid-palatal suture (A) and bone volume of ROI (B) in the three groups on days 3, 7, 14. *P<0.05, significant increase versus the C group. #P<0.05, significant increase versus the EP group. Data represent the mean ± SD of five rats for each group. C, control group; EP, expansion group; ES, expansion plus simvastatin group.
Histological findings
In the C group, the structure of the mid-palatal suture was shown in the slices stained with HE. There were many fibrous tissues in the center and secondary cartilage in both sides of the mid-palatal suture. Over time, only a slight increase in the number of fiber and chondrocytes was observed, and the width of the central fiber was almost unchanged on days 3, 7, and 14. When the appliances were installed in the rats of the ES and EP groups, the mid-palatal suture fiber was stretched, followed by regeneration and aggregation of some cartilaginous matrix and chondrocytes. On days 7 and 14, new bone formation was observed. Furthermore, more bone regeneration and capillaries in the mid-palatal suture in the ES group were observed than that in the EP group.
Immunohistological findings of BMP-2
As was shown in Figure 6, BMP-2 expression in the C group was very low in the mid-palatal suture on days 3, 7, and 14. In the EP and ES groups, chondrocytes, fibroblasts, osteoblasts and some vascular endothelial cells in the mid-palatal suture were found to express high levels of BMP-2. More BMP-2 were de tected in the mid-palatal suture of the rats in the EP group than that in the C group on days 3, 7, and 14. In terms of MOD value of BMP-2 expression, the difference between groups EP and C had a statistical significance (P<0.05) on day 3, but had no statistical significance on days 7, 14 (P>0.05). However, the BMP-2 MOD value of group ES was higher (P<0.05) than that of groups EP and C on days 3, 7, and 14 (Figure 7).
Figure 6.
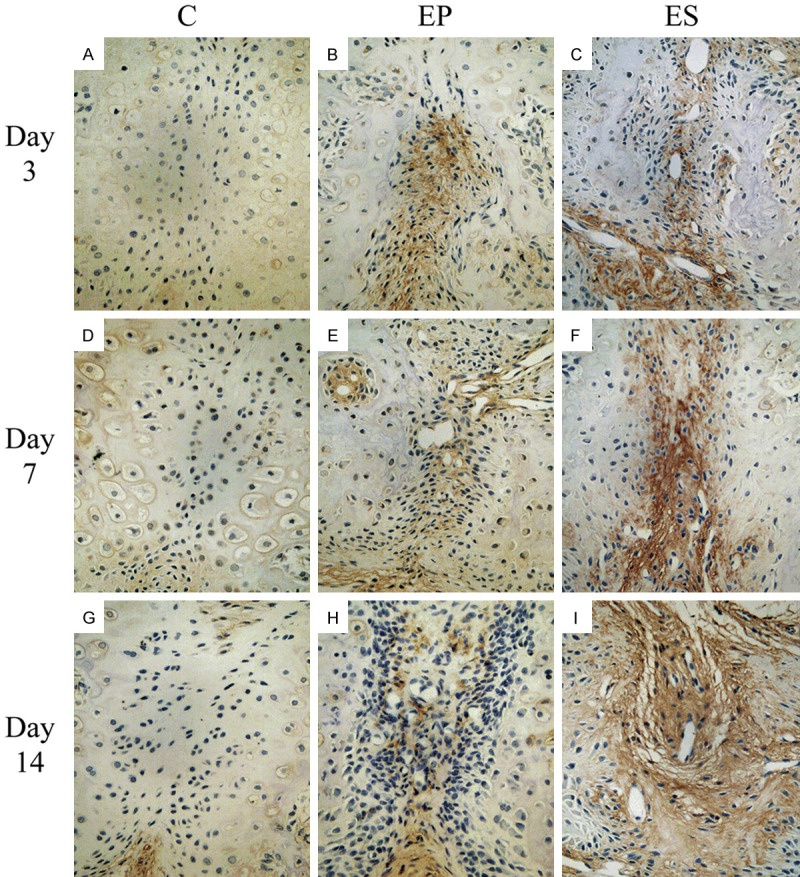
Immunohistochemical staining of BMP-2 in the mid-palatal suture of rats (All images were displayed at 400 × magnification). A. Expression of BMP2 in the C group on day 3. B. Expression of BMP2 in the EP group on day 3. C. Expression of BMP2 in the ES group on day 3. D. Expression of BMP2 in the C group on day 7. E. Expression of BMP2 in the EP group on day 7. F. Expression of BMP2 in the ES group on day 7. G. Expression of BMP2 in the C group on day 14. H. Expression of BMP2 in the EP group on day 14. I. Expression of BMP2 in the ES group on day 14.
Figure 7.
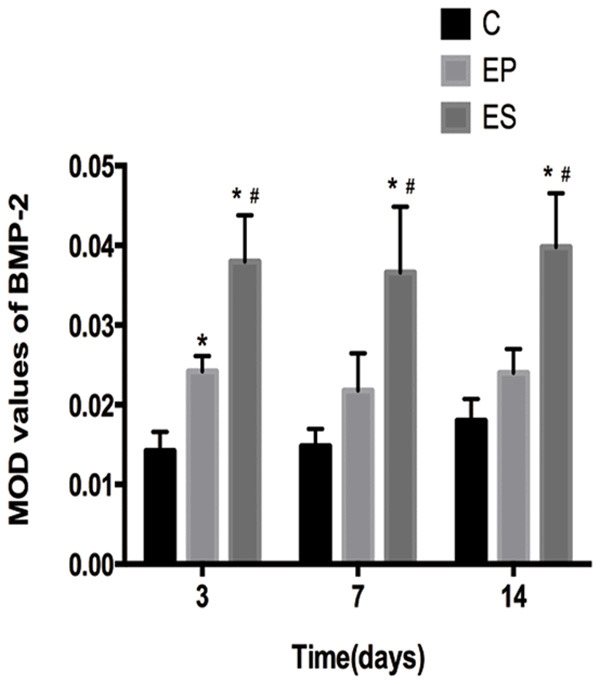
The MOD values of BMP-2 in the mid-palatal suture of the three groups on days 3, 7, 14. *P<0.05, significant increase versus the C group. #P<0.05, significant increase versus the EP group. Data represent the mean ± SD of five rats for each group. C, control group; EP, expansion group; ES, expansion plus simvastatin group. MOD, mean optical density.
Discussion
In this study, we demonstrate that the effectiveness of systemic administration of simvastatin on new bone regeneration in the expansive mid-palatal suture of the rats. Because of the reasonable price and being readily accessible, the rats were selected as an animal model [28]. Moreover, they are six weeks old as they have fast bone turnover within pubertal growth and similar to the patients whom the RME appliances were applied to in clinical training [29,30]. Therefore, six-week-old rats are selected to confirm the hypothesis of the experiment. For orthodontists and orthopedists, how to avoid the relapse after RME is a topic of great interest. Hastening new bone formation in the expanded suture would be helpful in solving the relapse problem. In current decades, more and more agents [4,13,22,31] have been investigated by researchers to accelerate the course of bone regeneration in the suture.
In the present study, the appliance that is used exploring the mid-palatal suture expansion in rats is a two-coil spring. The appliance can exert an expansive force of 100 g by opening the coil springs to widen the mid-palatal suture in a rat model. As the expansion appliance could modulate the feeding behavior of rats, the rats in the EP and ES groups were discomfortable and had difficulty in dieting, which led to weight loss in the first three days. Owing to adaption to the expansion appliance, their body weights subsequently began to increase from the fourth day. From the 7th day, there were no statistically significant differences in the body weights between the three groups, which indicates that the magnitude of 100 g force is at the range of the physiological condition and the rats can adapt themselves to the appliance. Besides, compared with that in the C group, there was a significant increase in the width of the suture in the EP and ES groups on days 3, 7, and 14. This demonstrates that the RME appliance can successfully expand the mid-palatal suture of rats. These findings are consistent with previous studies [4].
Researchers have found a relationship between simvastatin and bone regeneration. Some studies have shown that simvastatin can increase osteoblastic differentiation and stimulate neovascularization by affecting bone morphogenetic proteins and endothelial growth factor [32,33]. Some studies also have found simvastatin’s properties of osteoblast promoting, osteoclast inhibiting, anti-inflammatory, and neovascularization [34,35]. In dentistry, there are also some studies about the positive effects of simvastatin in periodontal regeneration, distraction osteogenesis, TMJ articular cartilage healing, and so on [36]. Moreover, many researchers have proved that a high dose of simvastatin increases bone formation and low dose induces bone resorption [37-40]. In this experiment, the systemically administered dosage of simvastatin we used is 20 mg simvastatin/d/kg body weight. The results we found are consistent with the above simvastatin’s effects.
The structure and microstructure of the mid-palatal suture change were represented by histology study and micro-CT measurement. In our research, histological observation of the mid-palatal suture showed that there were many mesenchymal cells, osteochondroprogenitor cells and cartilaginous cells in the growth and development period. In the C group, only a slight increase in the number of fiber and chondrocytes was observed, and the width of the central fiber was almost unchanged on days 3, 7, and 14. When the appliances were installed in the rats of the ES and EP groups, the mid-palatal suture fiber was stretched, followed by regeneration and aggregation of some cartilaginous matrix and chondrocytes. On days 7 and 14, some new bone formation was observed, which proves that expansion itself can stimulate bone formation in the mid-palatal suture. Furthermore, more bone regeneration and capillaries in the mid-palatal suture in the ES group were observed than that in the EP group. The result suggests that the appliance of simvastatin can accelerate suture regeneration. In stomatology, some researchers have also proved that the administration of simvastatin increases the value of the bone contact ratio to the implant and the bone density around the implant [41]. Meanwhile, the work of Thylin MR et al pointed out that simvastatin gel could stimulate murine cranial bone apposition [42]. The research of Chalisserry et al also found a significantly higher bone volume in the defects filled with Simvastatin loaded site by micro-CT [43]. Consistent with these, our three-dimensional measurement showed that the bone volume of ROI in group ES was more than that in group EP on the 7th and 14th days. Hence, histological examination and histomorphometric evaluation both demonstrated that the systematic application of simvastatin had the potential to promote new bone formation in the expanding mid-palatal suture. It is noticeable, however, that the suture width in the ES group was less than that in the EP group on days 7, 14, and the data did not achieve statistical significance. This is because the imaging changes will be shown when achieving a certain amount in new bone formation of the suture, which will be from the quantitative to qualitative change.
BMP-2 is known to be an important growth factor for osteogenic differentiation of stem cells [44,45]. BMP-2 also has the capacity to promote bone and cartilage formation [46,47] In view of these, the increasing of BMP-2 expression in the expansive mid-palatal suture may reflect the new bone formation of the suture to some extent. As early as 1999, Statins had been shown to increase the expression of BMP-2 gene in bone cells to enhance the new bone formation in vitro and rodents. Besides, simvastatin increased cancellous bone volume when orally administered to rats [25]. Recent studies [48-50] also found that simvastatin could stimulate osteogenesis of bone marrow mesenchymal stem cells by increasing BMP-2 expression. In our study, more BMP-2 were detected in the mid-palatal suture of the rats in the EP group than that in the C group on days 3, 7, and 14. In terms of the MOD value of BMP-2 expression, the difference between the EP and C groups had a statistical significance on day 3 but had no statistical significance on days 7, 14. This is one reason for the post-treatment replase. At the beginning of RME, the expansion can promote bone formation in the mid-palatal suture. Next, there is not enough bone formation in the suture. On days 3, 7, and 14, the BMP-2 MOD value of the ES group was higher than that of the EP and C groups, which suggests that simvastatin can enhance BMP2 expression level in the mid-palatal suture during the expansion.
In conclusion, our experimental results indicate that administration of simvastatin can induce a favorable effect on bone regeneration in the mid-palatal suture of rats during RME, which might provide new ideas and means for preventing relapse and reducing the retention time following the expansion procedures. Of course, there is still a long way to go in the appropriate utilization of simvastatin for RME patients as our study had some limitations. Our observation about simvastatin effects is only in the mid-palatal suture. There is no evaluation of the effects of simvastatin on the other parts of the body and the evidence on humans. Besides, the long-term effects of simvastatin in RME are also unknown. Thus, further studies should be designed to exploit the precise mechanism of simvastatin in RME.
Acknowledgements
The study was supported by grants from the Six Talent Peaks Project in Jiangsu Province (2019-wsw-128) and Nanjing funding project for students studying abroad (2019-B-43).
Disclosure of conflict of interest
None.
References
- 1.Bishara SE, Staley RN. Maxillary expansion: clinical implications. Am J Orthod Dentofacial Orthop. 1987;91:3–14. doi: 10.1016/0889-5406(87)90202-2. [DOI] [PubMed] [Google Scholar]
- 2.Herring SW. Mechanical influences on suture development and patency. Front Oral Biol. 2008;12:41–56. doi: 10.1159/0000115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525–532. doi: 10.1016/s0889-5406(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 4.Tang GH, Xu J, Chen RJ, Qian YF, Shen G. Lithium delivery enhances bone growth during midpalatal expansion. J Dent Res. 2011;90:336–340. doi: 10.1177/0022034510389180. [DOI] [PubMed] [Google Scholar]
- 5.Sawada M, Shimizu N. Stimulation of bone formation in the expanding mid-palatal suture by transforming growth factor-beta 1 in the rat. Eur J Orthod. 1996;18:169–179. doi: 10.1093/ejo/18.2.169. [DOI] [PubMed] [Google Scholar]
- 6.Gurel HG, Memili B, Erkan M, Sukurica Y. Long-term effects of rapid maxillary expansion followed by fixed appliances. Angle Orthod. 2010;80:5–9. doi: 10.2319/011209-22.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong T, Zhang X, Pathmanathan D, Soo C, Ting K. Craniosynostosis-associated gene nell-1 is regulated by runx2. J Bone Miner Res. 2007;22:7–18. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- 8.Chang HN, Garetto LP, Potter RH, Katona TR, Lee CH, Roberts WE. Angiogenesis and osteogenesis in an orthopedically expanded suture. Am J Orthod Dentofacial Orthop. 1997;111:382–390. doi: 10.1016/s0889-5406(97)80020-0. [DOI] [PubMed] [Google Scholar]
- 9.Altan BA, Kara IM, Nalcaci R, Ozan F, Erdogan SM, Ozkut MM, Inan S. Systemic propolis stimulates new bone formation at the expanded suture: a histomorphometric study. Angle Orthod. 2013;83:286–291. doi: 10.2319/032612-253.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uysal T, Amasyali M, Enhos S, Karslioglu Y, Yilmaz F, Gunhan O. Effect of periosteal stimulation therapy on bone formation in orthopedically expanded suture in rats. Orthod Craniofac Res. 2010;13:89–95. doi: 10.1111/j.1601-6343.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 11.Uysal T, Gorgulu S, Yagci A, Karslioglu Y, Gunhan O, Sagdic D. Effect of resveratrol on bone formation in the expanded inter-premaxillary suture: early bone changes. Orthod Craniofac Res. 2011;14:80–87. doi: 10.1111/j.1601-6343.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 12.Amini F, Najaf Abadi MP, Mollaei M. Evaluating the effect of laser irradiation on bone regeneration in midpalatal suture concurrent to rapid palatal expansion in rats. J Orthod Sci. 2015;4:65–71. doi: 10.4103/2278-0203.160237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosa CB, Habib FA, de Araujo TM, Dos Santos JN, Cangussu MC, Barbosa AF, de Castro IC, Pinheiro AL. Laser and LED phototherapy on midpalatal suture after rapid maxilla expansion: raman and histological analysis. Lasers Med Sci. 2017;32:263–274. doi: 10.1007/s10103-016-2108-3. [DOI] [PubMed] [Google Scholar]
- 14.Birlik M, Babacan H, Cevit R, Gurler B. Effect of sex steroids on bone formation in an orthopedically expanded suture in rats: an immunohistochemical and computed tomography study. J Orofac Orthop. 2016;77:94–103. doi: 10.1007/s00056-016-0021-9. [DOI] [PubMed] [Google Scholar]
- 15.Sadikoglu TB, Nalbantgil D, Ulkur F, Ulas N. Effect of hyaluronic acid on bone formation in the expanded interpremaxillary suture in rats. Orthod Craniofac Res. 2016;19:154–161. doi: 10.1111/ocr.12123. [DOI] [PubMed] [Google Scholar]
- 16.Birlik M, Kazancioglu HO, Aydin MS, Aksakalli S, Ezirganli S. Effect of energy drink on bone formation in the expanded inter-premaxillary suture. J Craniofac Surg. 2017;28:285–289. doi: 10.1097/SCS.0000000000003244. [DOI] [PubMed] [Google Scholar]
- 17.Irgin C, Corekci B, Ozan F, Halicioglu K, Toptas O, Birinci Yildirim A, Turker A, Yilmaz F. Does stinging nettle (urtica dioica) have an effect on bone formation in the expanded inter-premaxillary suture? Arch Oral Biol. 2016;69:13–18. doi: 10.1016/j.archoralbio.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Uysal T, Amasyali M, Olmez H, Enhos S, Karslioglu Y, Gunhan O. Effect of vitamin C on bone formation in the expanded inter-premaxillary suture. Early bone changes. J Orofac Orthop. 2011;72:290–300. doi: 10.1007/s00056-011-0034-3. [DOI] [PubMed] [Google Scholar]
- 19.Ozturk F, Babacan H, Inan S, Gumus C. Effects of bisphosphonates on sutural bone formation and relapse: a histologic and immunohistochemical study. Am J Orthod Dentofacial Orthop. 2011;140:e31–41. doi: 10.1016/j.ajodo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Uysal T, Ustdal A, Sonmez MF, Ozturk F. Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod. 2009;79:984–990. doi: 10.2319/112708-604.1. [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir H, Kara MI, Erciyas K, Ozer H, Ay S. Preventive effects of thymoquinone in a rat periodontitis model: a morphometric and histopathological study. J Periodontal Res. 2012;47:74–80. doi: 10.1111/j.1600-0765.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 22.Kara MI, Erciyas K, Altan AB, Ozkut M, Ay S, Inan S. Thymoquinone accelerates new bone formation in the rapid maxillary expansion procedure. Arch Oral Biol. 2012;57:357–363. doi: 10.1016/j.archoralbio.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan CJ, Gotto AM Jr, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol. 2000;35:1–10. doi: 10.1016/s0735-1097(99)00525-2. [DOI] [PubMed] [Google Scholar]
- 24.Greve AM, Dalsgaard M, Bang CN, Egstrup K, Ray S, Boman K, Rossebo AB, Gohlke-Baerwolf C, Devereux RB, Kober L, Wachtell K. Stroke in patients with aortic stenosis: the Simvastatin and Ezetimibe in Aortic Stenosis study. Stroke. 2014;45:1939–1946. doi: 10.1161/STROKEAHA.114.005296. [DOI] [PubMed] [Google Scholar]
- 25.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Matsunuma A, Kurahashi I, Yanagawa T, Yoshida H, Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J Cell Biochem. 2004;92:458–471. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- 27.Grasser WA, Baumann AP, Petras SF, Harwood HJ Jr, Devalaraja R, Renkiewicz R, Baragi V, Thompson DD, Paraklar VM. Regulation of osteoclast differentiation by statins. J Musculoskelet Neuronal Interact. 2003;3:53–62. [PubMed] [Google Scholar]
- 28.Kanekawa M, Shimizu N. Age-related changes on bone regeneration in midpalatal suture during maxillary expansion in the rat. Am J Orthod Dentofacial Orthop. 1998;114:646–653. doi: 10.1016/s0889-5406(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 29.Storey E. Tissue response to the movement of bones. Am J Orthod. 1973;64:229–247. doi: 10.1016/0002-9416(73)90017-1. [DOI] [PubMed] [Google Scholar]
- 30.Misawa Y, Kageyama T, Moriyama K, Kurihara S, Yagasaki H, Deguchi T, Ozawa H, Sahara N. Effect of age on alveolar bone turnover adjacent to maxillary molar roots in male rats: a histomorphometric study. Arch Oral Biol. 2007;52:44–50. doi: 10.1016/j.archoralbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Uysal T, Amasyali M, Enhos S, Sonmez MF, Sagdic D. Effect of ED-71, a new active vitamin D analog, on bone formation in an orthopedically expanded suture in rats. A histomorphometric study. Eur J Dent. 2009;3:165–172. [PMC free article] [PubMed] [Google Scholar]
- 32.Alam S, Ueki K, Nakagawa K, Marukawa K, Hashiba Y, Yamamoto E, Sakulsak N, Iseki S. Statin-induced bone morphogenetic protein (BMP) 2 expression during bone regeneration: an immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:22–29. doi: 10.1016/j.tripleo.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681–692. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita M, Otsuka F, Mukai T, Yamanaka R, Otani H, Matsumoto Y, Nakamura E, Takano M, Sada KE, Makino H. Simvastatin inhibits osteoclast differentiation induced by bone morphogenetic protein-2 and RANKL through regulating MAPK, AKT and Src signaling. Regul Pept. 2010;162:99–108. doi: 10.1016/j.regpep.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Sonobe M, Hattori K, Tomita N, Yoshikawa T, Aoki H, Takakura Y, Suguro T. Stimulatory effects of statins on bone marrow-derived mesenchymal stem cells. Study of a new therapeutic agent for fracture. Biomed Mater Eng. 2005;15:261–267. [PubMed] [Google Scholar]
- 36.Gupta S, Del Fabbro M, Chang J. The impact of simvastatin intervention on the healing of bone, soft tissue, and TMJ cartilage in dentistry: a systematic review and meta-analysis. Int J Implant Dent. 2019;5:17. doi: 10.1186/s40729-019-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol. 2001;21:1636–1641. doi: 10.1161/hq1001.097781. [DOI] [PubMed] [Google Scholar]
- 38.Ho ML, Chen YH, Liao HJ, Chen CH, Hung SH, Lee MJ, Fu YC, Wang YH, Wang GJ, Chang JK. Simvastatin increases osteoblasts and osteogenic proteins in ovariectomized rats. Eur J Clin Invest. 2009;39:296–303. doi: 10.1111/j.1365-2362.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri H, Naserhojjati-Roodsari R, Tahsili-Fahadan N, Khojasteh A, Mashhadi-Abbas F, Eslami B, Dehpour AR. Effect of simvastatin administration on periodontitis-associated bone loss in ovariectomized rats. J Periodontol. 2007;78:1561–1567. doi: 10.1902/jop.2007.060480. [DOI] [PubMed] [Google Scholar]
- 40.Anbinder AL, Prado Fde A, Prado Mde A, Balducci I, Rocha RF. The influence of ovariectomy, simvastatin and sodium alendronate on alveolar bone in rats. Braz Oral Res. 2007;21:247–252. doi: 10.1590/s1806-83242007000300010. [DOI] [PubMed] [Google Scholar]
- 41.Ayukawa Y, Okamura A, Koyano K. Simvastatin promotes osteogenesis around titanium implants. Clin Oral Implants Res. 2004;15:346–350. doi: 10.1046/j.1600-0501.2003.01015.x. [DOI] [PubMed] [Google Scholar]
- 42.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, Marx DB, Reinhardt RA. Effects of simvastatin gels on murine calvarial bone. J Periodontol. 2002;73:1141–1148. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 43.Chalisserry EP, Nam SY, Anil S. Simvastatin loaded nano hydroxyapatite in bone regeneration: a study in the rabbit femoral condyle. Curr Drug Deliv. 2019;16:530–537. doi: 10.2174/1567201816666190610164303. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- 45.Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119–29125. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raafat SN, Amin RM, Elmazar MM, Khattab MM, El-Khatib AS. The sole and combined effect of simvastatin and platelet rich fibrin as a filling material in induced bone defect in tibia of albino rats. Bone. 2018;117:60–69. doi: 10.1016/j.bone.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Venkatesan N, Liyanage ADT, Castro-Núñez J, Asafo-Adjei T, Cunningham LL, Dziubla TD, Puleo DA. Biodegradable polymerized simvastatin stimulates bone formation. Acta Biomater. 2019;93:192–199. doi: 10.1016/j.actbio.2019.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao PL, Wu SC, Lin ZY, Ho ML, Chen CH, Wang CZ. Alpha-5 integrin mediates simvastatin-induced osteogenesis of bone marrow mesenchymal stem cells. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030506. [DOI] [PMC free article] [PubMed] [Google Scholar]


