Abstract
Background: Endometrial carcinoma (EC) is one of the most common cancers in women, and its pathogenesis is complex. Abnormal spindle microtubule assembly (ASPM) is highly expressed in a variety of cancers and is related to poor clinical prognosis and recurrence. However, the role of ASPM in EC is still unclear. Our study was conducted to investigate the association of ASPM with tumour progression and prognosis in EC. Methods: The expression level of ASPM in EC patients was analysed by using the TCGA database and by using immunohistochemistry (IHC) to analyse EC patient samples. The relationship between ASPM expression and clinicopathological variables was analysed by the chi-square test. Survival curves were analysed by Kaplan-Meier survival analysis and log-rank test. Univariate and multivariate Cox regression analyses were performed to measure the prognosis of EC. The effects of ASPM on the proliferation, invasion and metastasis of EC cells (HEC-1A and Ishikawa) were analysed by MTT and Transwell assays. The effect of ASPM on the Wnt/β-catenin signalling pathway was detected by Western blotting. Results: ASPM was highly overexpressed in EC. Overexpression of ASPM was related to significantly worse overall survival (P<0.05) in EC patients. Univariate and multivariate Cox regression analyses suggested that upregulation of ASPM was related to poor prognosis in EC. Knockdown of ASPM inhibited the proliferation, migration and invasion of EC cells. ASPM knockdown suppressed the Wnt/β-catenin signalling pathway, while β-catenin overexpression reversed the effect of shASPM on cell activity. Conclusions: ASPM acts as an independent predictor of clinical prognosis and serves as a potential target gene for EC therapy.
Keywords: ASPM, Wnt/β-catenin, prognosis, endometrial carcinoma
Introduction
Endometrial cancer (EC) is the second most common malignancy of the female genital tract, and approximately 320,000 women worldwide are diagnosed with EC each year [1,2]. EC is caused by abnormal cell growth. It mainly occurs in women between 55 and 65 years old, but with the increase in obesity and the decrease in physical activity, the incidence of EC is on the rise in young women [3,4]. At present, surgery, chemotherapy and radiotherapy are the primary therapeutic strategies for EC. However, only a few EC patients are sensitive to these treatments, and the prognosis is relatively poor [5]. Therefore, deciphering the molecular mechanism of EC occurrence and development is crucial for the prevention, diagnosis and therapy of EC.
Wingless-type (Wnt) signalling is a signal transduction pathway. Intracellular free calcium and β-catenin are two important intracellular transducers [6,7]. The Wnt/β-catenin signalling pathway plays a key role in cell development and differentiation and is closely related to tumorigenesis, invasion, and metastasis [8]. Aberrant Wnt/β-catenin signalling is also closely related to a high incidence of various human cancers [9,10]. Chen et al. reported that aberrant activation of Wnt/β-catenin signalling plays a crucial role in the progression of colorectal cancer [11]. Yu et al. [12] pointed out that the Wnt pathway is an important signalling cascade involved in mammary organogenesis and breast oncogenesis. Early studies documented that approximately 40% of ECs have aberrant activation of the Wnt/β-catenin signalling pathway.
Abnormal spindle microtubule assembly (ASPM) is a centrosomal protein that plays a crucial role in mitotic spindle regulation, neurogenesis and brain size regulation [13,14]. ASPM is a positive regulator of the Wnt signalling pathway [15]. Studies have reported that ASPM is highly expressed in a variety of cancers and is related to poor clinical prognosis and recurrence. Pai et al. [16] reported that the expression of ASPM is increased in prostate cancer and promotes the multiplication, migration and invasion of cancer cells by enhancing Wnt-β-catenin signalling. Recent evidence has shown that the ASPM expression level in EC patients is upregulated [17]. However, the relationship between ASPM and the clinical prognosis of EC has not been reported.
Herein, we explored the expression of ASPM in EC patients by immunohistochemistry (IHC). The relationship between ASPM protein expression and the survival or clinical prognosis of EC was further studied. We present evidence that ASPM promotes EC cell proliferation, migration and invasion by promoting Wnt/β-catenin activation. ASPM can act as an independent predictor of clinical prognosis and serve as a feasible target gene for EC therapy.
Methods
TCGA datasets
ASPM expression datasets from TCGA, including 546 uterine corpus endometrial carcinoma (UCEC) tissues and 35 normal endometrial tissues, were downloaded (https://tcgadata.nci.nih.gov/tcga) to determine ASPM expression at the mRNA level and further perform Kaplan-Meier survival analysis.
Patients and specimens
EC tissues were obtained as clinical specimens from 140 EC patients who underwent primary tumour resection in Jiangsu Province Hospital. None of the patients received chemotherapy or radiotherapy before the operation. Tissues were stored in liquid nitrogen for subsequent detection. The research was supported by the Medical Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, and all patients provided consent before the research began.
Cell culture
Human EC cell lines (HEC-1A and Ishikawa) were purchased from Procell (Wuhan, China). The cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% foetal bovine serum (FBS) and then cultured at 37°C with 5% CO2. After 2-3 stable generations, logarithmic phase cells were harvested for subsequent tests.
RNA extraction and qRT-PCR
RNA was extracted from carcinoma tissues using the TRIzol Reagent Kit (Invitrogen, USA). A reverse transcription kit (Takara, Dalian, China) was used to reverse transcribe RNA to cDNA. qRT-PCR assays were executed by using SYBR Premix Ex Taq (Takara) as directed by the manufacturer, and the cycling programmes were set as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 50 s, annealing and elongation at 72°C for 10 min. The results were normalized to the expression of GAPDH. The sequences of the primers for GAPDH were 5’-CAAACTCCCCTTCTGACAGC-3’ (forward) and 5’-CCGAATCACACTGACAAACG-3’ (reverse). The ASPM primers were 5’-ACACTCCAGCTGGGACUUACAGACAAGAGCC-3’ (forward) and 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGAGCAAGG-3’ (reverse). An ABI 7500 real-time PCR system (Applied Biosystems, USA) was applied for data analysis. Each trial was performed three times.
Western blot assay
Total protein was acquired from cells by using the mammalian protein extraction reagent RIPA (Beyotime, Haimen, China) containing 1% protease inhibitor cocktail (Roche) and PMSF (Roche). Then, the proteins were separated by 10% SDS-PAGE and transferred to 0.22 mm nitrocellulose membranes (Sigma-Aldrich). The membrane was blocked with 5% milk in Tris-buffered saline at 25°C for 1 h, incubated with specific primary antibodies (ASPM, β-catenin, C-myc and cyclin D1) overnight at 4°C and then incubated with secondary antibodies for 2 h at 37°C. Finally, protein bands of interest were imaged by a Bio-Rad imaging system.
Methyl thiazolyl tetrazolium (MTT) assay
Cell proliferation ability was determined through the MTT assay. The transfected cells (HEC-1A and Ishikawa) were seeded in 96 well plates (5 × 103 cells/well). Then, 20 μl of MTT solution was added at 72 h, and the cells were further cultured for 4 h at 37°C. Absorbance at 490 nm was measured by a microplate reader. All experiments were performed three times.
Cell transfection
Cells were transfected with specific shRNA using Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. shASPM was synthesized by Invitrogen to specifically knockdown ASPM. To construct aβ-catenin-overexpressing plasmids, β-catenin cDNA was inserted into the p-MSCV-IRES-GFP plasmid. For transfection, the cells were seeded in 6-well plates and cultured for 24 h until they reached 70%-80% confluence. Then, the cells were transfected with 2 μg of plasmid using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol.
Cell migration and invasion assay
Cells were transfected with specific sh-ASPM using Lipofectamine® 3000 (Invitrogen, USA) according to the manufacturer’s protocol. After transfection for 48 h, the migration analysis was detected via Transwell insert chambers (8 mm pore size, Corning, USA). Approximately 2 × 104 cells were plated in the upper chambers in triplicate without serum. Twenty-four-well Transwell plates with 8.0 μm pore, Matrigel-coated membranes (BD Bioscience, USA) were used to detect cell invasion ability. Cells (2 × 105) were seeded into upper chambers in triplicate without serum. The lower compartment was filled with medium supplemented with 10% FBS as a chemical attractant. After 24 h of cultivation, cells in the upper chambers that did not migrate or invade were cleared by cotton swabs, and cells that migrated or invaded to the lower surface of the membrane were immobilized by cold methanol and dyed with 0.1% crystal violet. All migrated and invaded cells were counted in at least ten random regions of each membrane by light microscopy.
IHC staining and analyses
Tissue microarrays (TMAs) were constructed and stained by IHC based on previous studies [18,19]. In short, samples were incubated with 1:100 diluted anti-ASPM (goat polyclonal antibody, sc-488883, Santa Cruz Biotech Co., Ltd., USA) at 4°C overnight and then cultivated for 30 min at 25°C with goat anti-rabbit Envision System Plus-HRP (Dako Cytomation). After washing with PBS 3 times, samples were cultivated with DAB for 1 min, counterstained with Mayer haematoxylin, dehydrated, and fixed. PBS was used as the negative control.
Stained slides were evaluated by 2 independent single-blinded investigators. A semiquantitative scoring system was performed to assess the staining intensity (0, negative intensity; 1, weak intensity; 2, moderate intensity; 3, strong intensity) and the percentage of stained cells (0, <5%; 1, 5%-25%; 2, 26%-50%; 3, 51%-75%; and 4, >75%). The staining intensity score was then multiplied by the proportion of positive cells to obtain the immunoreactivity score for each sample. Acquired images were evaluated by digital sharpening. All samples were stained and evaluated in triplicate.
Luciferase reporter assay
Cells were transfected with the TCF/LEF Reporter (Baiao-Laibo, Beijing, China). After stimulation of the cells with recombinant human Wnt-3a (R&D Systems, Minneapolis, USA), the Chroma-Glo™ Luciferase Assay System (Promega, Madison, Wisconsin, USA) was used to determine the TCF/LEF reporter activity.
Statistical analysis
The Chi-squared test was performed to determine the correlation of ASPM with clinical pathological variables. Survival rates were analysed by Kaplan-Meier survival analysis, and the differences in survival curves were measured via the log-rank test. Univariate and multivariate Cox regressions were used to measure the hazard ratio of overall survival. SPSS 22 software (IBM, Chicago, IL) was used for statistical analysis. The results are shown as the mean ± S.D. (n=3). When comparing two groups, Student’s t-test was implemented to calculate differences; when comparing multiple groups, one-way ANOVA was performed. P<0.05 was identified as statistically significant and is indicated by *.
Results
Expression of ASPM is upregulated in EC tissues
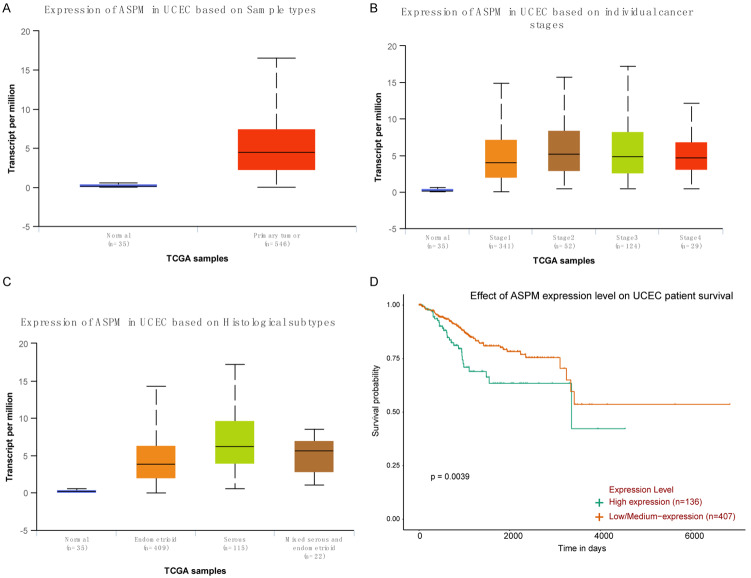
The ASPM expression levels in 546 EC patients were analysed by using the TCGA database. As indicated in Figure 1A, the transcript level of ASPM in UCEC was significantly upregulated compared with that in normal endometrial epithelium (P<0.0001). Based on individual cancer stages, ASPM was significantly highly expressed in EC tissues compared with normal tissues (P<0.0001), but only stage 1 and stage 3 were significantly different (P<0.05) between stages (Figure 1B). The expression of ASPM in UCEC based on histological subtypes was also determined, and the results revealed that ASPM was more highly expressed in serous than in normal (P<0.0001) or other types of UCEC (P<0.0001) (Figure 1C). Kaplan-Meier survival analysis suggested that patients with high ASPM expression showed a lower survival rate and poorer prognosis than patients with low ASPM expression (P=0.0039) (Figure 1D).
Figure 1.
The expression of ASPM in EC patients was analysed by using the TCGA database. A. The transcript level of ASPM in UCEC tissues and in normal endometrial epithelium. B. Expression of ASPM in different stages of UCEC. C. Expression of ASPM in different types of UCEC. D. Kaplan-Meier analyses were used to measure the survival rate of patients with high and low ASPM expression.
High expression of ASPM is associated with a lower survival rate in EC patients
To investigate the expression pattern of ASPM in EC, we performed IHC staining on tumour tissues of 102 patients with tumour resection. A semiquantitative scoring system was performed to assess the staining intensity (0, negative intensity; 1, weak intensity; 2, moderate intensity; 3, strong intensity) and the percentage of stained cells (0, <5%; 1, 5%-25%; 2, 26%-50%; 3, 51%-75%; and 4, >75%). The ASPM scores were obtained by multiplying the staining intensity score and the proportion of positive cells. ASPM expression was highly heterogeneous among ECs (Figure 2A-D). Next, the relationship of ASPM expression with overall survival (OS) in endometrial carcinoma patients was assessed by Kaplan-Meier analysis and log-rank test. EC patients with high ASPM expression had significantly shorter OS compared with those with low expression (P<0.001; Figure 2E). The results suggested that patients with a higher level of ASPM had a poorer prognosis.
Figure 2.
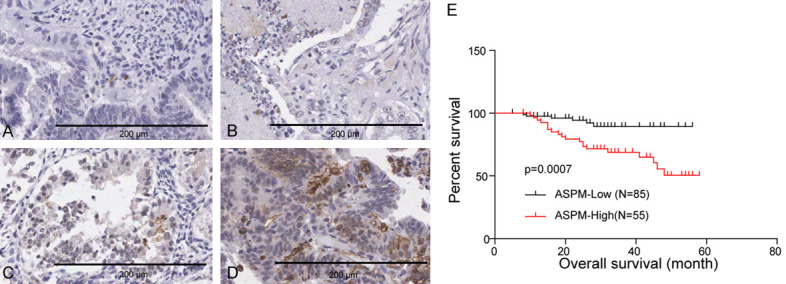
Representative IHC staining of ASPM in TMA. A. Negative intensity, 0; B. Weak intensity, 1; C. Moderate intensity, 2; D. Strong intensity, 3. Scale bar, 200 μm. E. The influence of ASPM expression on the OS of EC patients was calculated by Kaplan-Meier analyses and the log-rank test.
Upregulated ASPM is related to poor prognosis in EC
A chi-square test was performed to reveal the correlation between ASPM expression and clinicopathologic variables in EC (Table 1). No significant changes in ASPM expression were demonstrated in patients older or younger than 50 years old (P=0.863). In addition, there was no significant correlation between ASPM expression and tumour grade (P=0.534), pathological type (P=0.936) or menopausal status (P=0.617). However, tumour stage (P<0.0001) showed a significant effect on the expression of ASPM. To further explore the effect of ASPM alone and among the other predictors of EC, Cox regression models of univariate and multivariate analyses were performed to reveal the clinical prognostic value of ASPM in EC (Table 2). The univariate analysis showed that ASPM (P=0.002) and stage (P=0.008) were significantly associated with poor prognosis in EC patients. The multivariate analysis results suggested that the level of ASPM mRNA expression (P=0.017) was an independent prognostic factor for EC.
Table 1.
Correlation between the clinicopathologic characteristics and the expression of ASPM in EC
| variable | ASPM | ||
|---|---|---|---|
|
| |||
| High | Low | P | |
| age | |||
| ≤50 | 17 | 20 | 0.863 |
| >50 | 38 | 65 | |
| Stage | |||
| I | 18 | 70 | <0.0001* |
| II/III | 37 | 15 | |
| Grade | |||
| I | 16 | 29 | 0.534 |
| II/III | 39 | 56 | |
| Pathological type | |||
| Adenocarcinoma | 45 | 70 | 0.936 |
| Others | 10 | 15 | |
| Menopausal status | |||
| Premenopausal | 25 | 35 | 0.617 |
| Postmenopausal | 30 | 50 | |
indicates a statistical significance.
Table 2.
Univariate and multivariate Cox-regression analysis of ASPM expression and prognostic factors in EC patients
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| Overall survival (HR [95% CI]) | p-value | Overall survival (HR [95% CI]) | p-value | |
| Age | 1.0579 (0.459-2.436) | 0.897 | ||
| Stage | 0.333 (0.148-0.749) | 0.008* | 0.621 (0.253-1.529) | 0.3 |
| Grade | 0.738 (0.319-1.708) | 0.478 | ||
| ASPM | 0.235 (0.094-0.586) | 0.002* | 0.295 (0.108-0.806) | 0.017* |
| Pathological type | 1.946 (0.844-4.486) | 0.118 | 1.716 (0.734-4.009) | 0.213 |
| Menopausal status | 0.934 (0.428-2.039) | 0.865 | ||
Abbreviation: CI, confidence interval; HR, hazard ratio. Bold type and (*) indicate statistical significance.
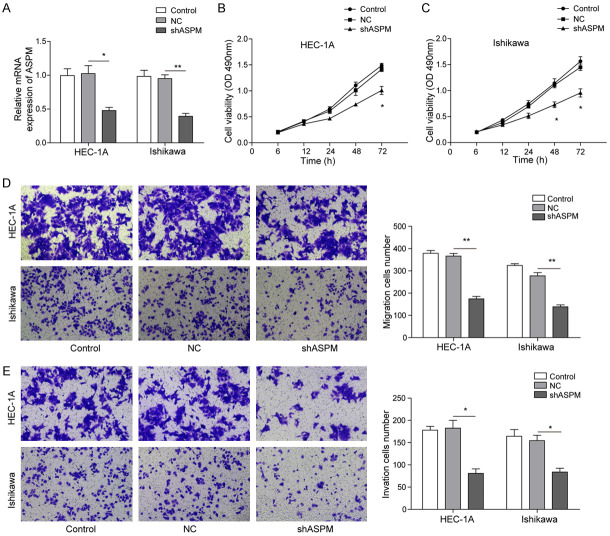
Knockdown of ASPM inhibited the proliferation, migration and invasion of EC cells
To ascertain the function of high expression of ASPM in the development of EC, we knocked down its expression by Lipofectamine® 3000-mediated RNA interference. qRT-PCR analysis demonstrated that the expression of ASPM in the control group was similar to that in the sh-NC group, while the ASPM mRNA levels in the sh-ASPM group were downregulated compared with those in the sh-NC group (Figure 3A). In addition, MTT analysis revealed that knockdown of ASPM significantly inhibited cell viability in HEC-1A and Ishikawa cell lines (Figure 3B and 3C). Meanwhile, the role of ASPM on the migration and invasion of HEC-1A and Ishikawa cells was detected via Transwell assays. The results revealed that the number of migratory and invasive cells was markedly decreased after transfection with sh-ASPM compared with transfection with sh-NC (Figure 3D and 3E). Altogether, these results demonstrated that ASPM significantly promoted the malignant nature of EC cells.
Figure 3.
ASPM knockdown blocked the proliferation, migration and invasion of EC cells. A. qRT-PCR was used to measure ASPM expression in HEC-1A and Ishikawa cells after transfection with sh-ASPM or sh-NC. B and C. MTT tests were used to measure the viability of HEC-1A and Ishikawa cells after transfection with sh-ASPM or sh-NC. D. Cell migration was performed in HEC-1A and Ishikawa cells after transfection with sh-ASPM or sh-NC. E. Cell invasion was assessed in HEC-1A and Ishikawa cells after transfection with sh-ASPM or sh-NC. The number of migrated or invaded cells was counted by analysing photographs at 100 × magnification in five random fields per chamber. All samples were stained and evaluated in triplicate. *P<0.05, **P<0.01.
Knockdown of ASPM suppresses the Wnt/β-catenin signalling pathway
To further explore the underlying mechanisms of ASPM in EC, a Western blot assay was used to explore the influence of ASPM on Wnt/β-catenin signalling. Specifically, we detected fluctuations of Wnt/β-catenin signalling pathway-related markers, such as β-catenin, C-myc, and cyclin D1, following transfection of EC cell lines (HEC-1A and Ishikawa) with sh-ASPM. The outcomes showed that β-catenin, C-myc and cyclin D1 expression was significantly inhibited in EC cells with sh-ASPM interference compared with NC cells (Figure 4). These results showed that knockdown of ASPM can inhibit the activation of the Wnt/β-catenin signalling pathway.
Figure 4.
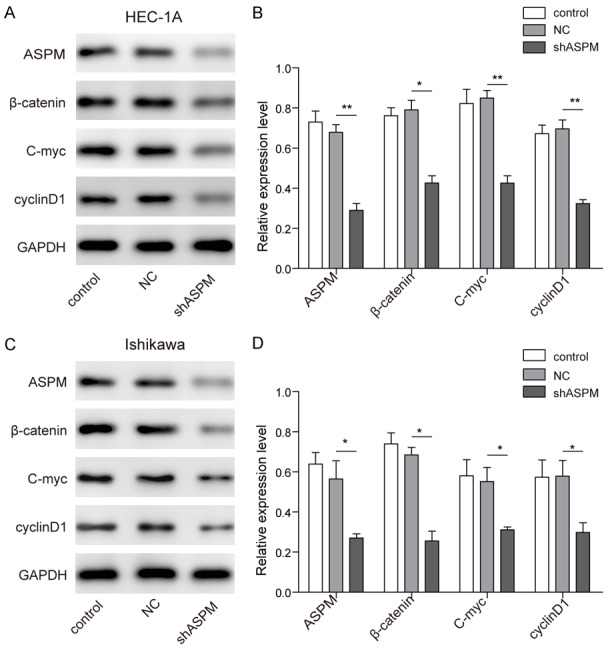
The effect of ASPM on the Wnt/β-catenin signalling pathway. A and B. The expression of Wnt/β-catenin signalling pathway-related markers was determined by Western blot assay in HEC-1A cells after transfection with sh-ASPM. C and D. The expression of Wnt/β-catenin signalling pathway-related markers was determined by Western blot assay in Ishikawa cells after transfection with sh-ASPM. Pixel densities were analysed and are presented as histograms. *P<0.05, **P<0.01.
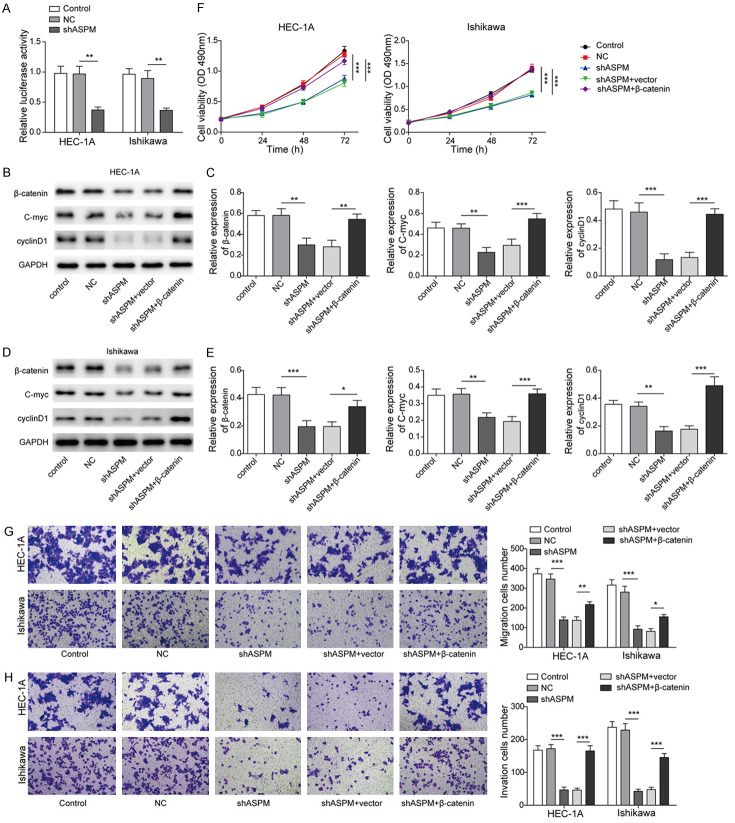
Upregulation of the Wnt/β-catenin signalling pathway can block the effect of shASPM on EC cells
To evaluate whether ASPM regulates Wnt pathway activity, we expressed Wnt reporter constructs in HEC-1A and Ishikawa cells. The luciferase activity of the shASPM treatment group was lower than that in the NC group, which indicated that sh-ASPM significantly inhibited Wnt-mediated luciferase reporter activation (Figure 5A). β-catenin is a crucial downstream component of the Wnt signalling pathway [20]. We found that knockdown of ASPM led to a substantial reduction in β-catenin protein levels, raising the possibility that ASPM can maintain Wnt pathway activity by regulating β-catenin. In addition, the expression levels of C-myc and cyclin D1 were all downregulated in the shASPM group compared with the NC group (Figure 5B-E), whereas those downregulated by shASPM were reversed by overexpression of β-catenin (Figure 5B-E). As shown in Figure 5F, compared with the effects in the NC group, the cell viability of the shASPM treatment group was decreased, but the overexpression of β-catenin reversed the effect of shASPM on cell activity. Similarly, compared with the NC group, the migration and invasion abilities of the shASPM group were decreased, while the overexpression of β-catenin reversed the effect of shASPM, resulting in the recovery of EC cell migration and invasion abilities (Figure 5G and 5H).
Figure 5.
Upregulation of the Wnt/β-catenin signalling pathway can block the effect of ASPM knockdown on the proliferation, migration and invasion of EC cells. A. Changes of Wnt-mediated luciferase activity in control- or ASPM-shRNA transduced HEC-1A or Ishikawa cells. B and C. The expression of Wnt/β-catenin signalling pathway-related markers was determined by Western blot assay in HEC-1A cells after transfection with sh-ASPM and overexpression of β-catenin. D and E. The expression of Wnt/β-catenin signalling pathway-related markers was determined by Western blot assay in Ishikawa cells after transfection with sh-ASPM and overexpression of β-catenin. F. The viability of HEC-1A and Ishikawa cells after transfection with sh-ASPM or overexpression of β-catenin. G. Cells migration were assessed in HEC-1A and Ishikawa cells after transfection with sh-ASPM and overexpression of β-catenin. H. Cells invasion were assessed in HEC-1A and Ishikawa cells after transfection with sh-ASPM and overexpression of β-catenin. *P<0.05, **P<0.01.
Discussion
EC is one of the most common cancers in women, accounting for approximately 76,000 deaths each year worldwide [21]. The morbidity and mortality rates of EC are on the rise, and the onset age has decreased, seriously jeopardizing women’s health [22]. Therefore, identifying novel biomarkers is essential for detecting EC recurrence, metastasis, and treatment and prolonging the survival rate of patients.
ASPM is a centrosomal protein that is related to poor clinical prognosis in various tumours. Vange and his colleagues [23] revealed that ASPM was a potential gastric stem/progenitor cell marker. Wang et al. [24] pointed out that ASPM was overexpressed in gastric cancer, and the expression of ASPM in poorly differentiated tumours was significantly lower than that in moderately differentiated tumours (P=0.0204). Freitas et al. reported that overexpression of ASPM may be associated with the occurrence of medulloblastoma, as it could alter the differentiation ability of stem cells during the development of the central nervous system [25]. In this study, ASPM showed a higher expression level in EC tissues than in normal endometrial epithelium tissues (P<0.0001). The Kaplan-Meier analysis and log-rank test suggested that patients with a higher level of ASPM had a worse OS. Univariate and multivariate Cox regression analyses revealed that the upregulation of ASPM is related to poor prognosis in EC. We provided convincing evidence that ASPM can act as an independent predictor of EC. To the best of our knowledge, this is the first time that ASPM has been identified as an independent predictor of EC.
Wnt signalling is crucial in embryogenesis, healing and tissue homeostasis, playing an important role in major axis formation, organogenesis, and cell multiplication [26,27]. Wnt signalling can also induce epithelial-mesenchymal transition (EMT) and promote the metastasis of cancer cells. Stemmer et al. reported that the Wnt signalling pathway can increase the expression of the transcription factors Slug, Snail, and Twist and decrease the expression of E-cadherin, resulting in the polarity of the cell epithelium and the absence of junctions, which promotes EMT and enhances cell migration and invasion [28]. Yamamoto et al. [29] pointed out that Wnt5a promoted the aggressiveness of prostate cancer. Activation of the Wnt/β-catenin pathway is frequently observed in ECs, and many studies have confirmed the role of Wnt/β-catenin signalling in EC. McConechy et al. [30] suggested that β-catenin mutation occurred in 20-25% of endometrioid ECs. A more reliable IHC study reported activation of β-catenin in 12-31% of endometrioid EC [31]. Chan et al. documented that approximately 40% of ECs have aberrant activation of the Wnt/β-catenin signalling pathway [32]. All of this evidence suggests that activation of the Wnt pathway accelerates the development of EC. Here, the in vitro experiment demonstrated that the expression of Wnt/β-catenin was significantly inhibited in EC cells after ASPM knockdown. To investigate the physiological function of ASPM, ASPM knockdown in HEC-1A and Ishikawa cells was performed via a specific sh-ASPM. MTT and Transwell assay results indicated that silencing ASPM inhibited cell proliferation, migration and invasion. To evaluate whether ASPM regulates Wnt pathway activity, we expressed Wnt reporter constructs in HEC-1A and Ishikawa cells. The luciferase activity in the shASPM treatment group was lower than that in the NC group, which indicated that the knockdown of ASPM significantly inhibited Wnt-mediated luciferase reporter activation. In addition, the expression of β-catenin, C-myc, and cyclin D1 and the cell activity, migration and invasion ability in the shASPM group were all decreased compared with those in the NC group, and overexpression of β-catenin reversed this effect.
Conclusions
These data show that ASPM is markedly overexpressed in EC tissues and that ASPM overexpression can augment Wnt/β-catenin expression, promoting the proliferation, migration and invasion of EC cells. ASPM acts as an independent predictor of clinical prognosis and serves as a possible target gene for EC therapy.
Disclosure of conflict of interest
None.
Abbreviations
- EC
Endometrial carcinoma
- ASPM
Abnormal spindle microtubule assembly
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Foetal bovine serum
- qRT-PCR
Quantitative real-time PCR
- MTT
Methyl thiazolyl tetrazolium
- TMA
Tissue microarrays
- IHC
Immunohistochemistry
- OS
Overall survival
- CI
Confidence interval
- HR
Hazard ratio
- Wnt
Wingless-type
References
- 1.Jin C, Liang R. miR-205 promotes epithelial-mesenchymal transition by targeting AKT signaling in endometrial cancer cells. J Obstet Gynaecol Res. 2015;41:1653–1660. doi: 10.1111/jog.12756. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Jia N, Lyv T, Wang C, Tao X, Wong K, Li Q, Feng W. Paired box 2 promotes progression of endometrial cancer via regulating cell cycle pathway. J Cancer. 2018;9:3743–3754. doi: 10.7150/jca.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Li H, Zhao C, Jia H. MicroRNA-101 inhibits angiogenesis via COX-2 in endometrial carcinoma. Mol Cell Biochem. 2018;448:61–69. doi: 10.1007/s11010-018-3313-0. [DOI] [PubMed] [Google Scholar]
- 4.Cao X, He GZ. Knockdown of CLDN6 inhibits cell proliferation and migration via PI3K/AKT/mTOR signaling pathway in endometrial carcinoma cell line HEC-1-B. Onco Targets Ther. 2018;11:6351–6360. doi: 10.2147/OTT.S174618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev. 2012:CD003915. doi: 10.1002/14651858.CD003915.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thrasivoulou C, Millar M, Ahmed A. Activation of intracellular calcium by multiple Wnt ligands and translocation of beta-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/beta-catenin pathways. J Biol Chem. 2013;288:35651–35659. doi: 10.1074/jbc.M112.437913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien AJ, Moon RT. WNTS and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–457. doi: 10.2741/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Zhu D, Zhang H, Han C, Xue G, Zhu T, Luo J, Kong L. YAP-dependent ubiquitination and degradation of beta-catenin mediates inhibition of Wnt signalling induced by Physalin F in colorectal cancer. Cell Death Dis. 2018;9:591. doi: 10.1038/s41419-018-0645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu QC, Verheyen EM, Zeng YA. Mammary development and breast cancer: a Wnt perspective. Cancers (Basel) 2016;8 doi: 10.3390/cancers8070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikeye SN, Colin C, Marie Y, Vampouille R, Ravassard P, Rousseau A, Boisselier B, Idbaih A, Calvo CF, Leuraud P, Lassalle M, El Hallani S, Delattre JY, Sanson M. ASPM-associated stem cell proliferation is involved in malignant progression of gliomas and constitutes an attractive therapeutic target. Cancer Cell Int. 2010;10:1. doi: 10.1186/1475-2867-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchman JJ, Durak O, Tsai LH. ASPM regulates Wnt signaling pathway activity in the developing brain. Genes Dev. 2011;25:1909–1914. doi: 10.1101/gad.16830211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Major MB, Roberts BS, Berndt JD, Marine S, Anastas J, Chung N, Ferrer M, Yi X, Stoick-Cooper CL, von Haller PD, Kategaya L, Chien A, Angers S, MacCoss M, Cleary MA, Arthur WT, Moon RT. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Sci Signal. 2008;1:ra12. doi: 10.1126/scisignal.2000037. [DOI] [PubMed] [Google Scholar]
- 16.Pai VC, Hsu CC, Chan TS, Liao WY, Chuu CP, Chen WY, Li CR, Lin CY, Huang SP, Chen LT, Tsai KK. ASPM promotes prostate cancer stemness and progression by augmenting Wnt-Dvl-3-beta-catenin signaling. Oncogene. 2019;38:1340–1353. doi: 10.1038/s41388-018-0497-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Chen F, Xiu A, Du B, Ai H, Xie W. Identification of key candidate genes and pathways in endometrial cancer by integrated bioinformatical analysis. Asian Pac J Cancer Prev. 2018;19:969–975. doi: 10.22034/APJCP.2018.19.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G, Chen Z, Li J, Ye F, Chen G, Fan Q, Dong H, Yuan S, Zhu X. NOTCH4 is a novel prognostic marker that correlates with colorectal cancer progression and prognosis. J Cancer. 2018;9:2374–2379. doi: 10.7150/jca.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie JJ, Zhuo YJ, Zheng Y, Mo RJ, Liu ZZ, Li BW, Cai ZD, Zhu XJ, Liang YX, He HC, Zhong WD. High expression of ASPM correlates with tumor progression and predicts poor outcome in patients with prostate cancer. Int Urol Nephrol. 2017;49:817–823. doi: 10.1007/s11255-017-1545-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Zhang J, Li K, Yang J, Yu H, Duan S, Jiang K, Li X. beta-Catenin regulates membrane potential in muscle cells by regulating the alpha2 subunit of Na,K-ATPase. Eur J Neurosci. 2014;40:2216–2224. doi: 10.1111/ejn.12564. [DOI] [PubMed] [Google Scholar]
- 21.Weiderpass E, Labreche F. Malignant tumors of the female reproductive system. Saf Health Work. 2012;3:166–180. doi: 10.5491/SHAW.2012.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Zhou Q, Gao S, Li W, Li X, Liu Z, Jin P, Jiang J. Silibinin inhibits endometrial carcinoma via blocking pathways of STAT3 activation and SREBP1-mediated lipid accumulation. Life Sci. 2019;217:70–80. doi: 10.1016/j.lfs.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Vange P, Bruland T, Beisvag V, Erlandsen SE, Flatberg A, Doseth B, Sandvik AK, Bakke I. Genome-wide analysis of the oxyntic proliferative isthmus zone reveals ASPM as a possible gastric stem/progenitor cell marker over-expressed in cancer. J Pathol. 2015;237:447–459. doi: 10.1002/path.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Li J, Liu J, Zhao Q. Controversial role of the possible oxyntic stem cell marker ASPM in gastric cancer. J Pathol. 2017;241:559–561. doi: 10.1002/path.4863. [DOI] [PubMed] [Google Scholar]
- 25.Vulcani-Freitas TM, Saba-Silva N, Cappellano A, Cavalheiro S, Marie SK, Oba-Shinjo SM, Malheiros SM, de Toledo SR. ASPM gene expression in medulloblastoma. Childs Nerv Syst. 2011;27:71–74. doi: 10.1007/s00381-010-1252-5. [DOI] [PubMed] [Google Scholar]
- 26.Coopes A, Henry CE, Llamosas E, Ford CE. An update of Wnt signalling in endometrial cancer and its potential as a therapeutic target. Endocr Relat Cancer. 2018 doi: 10.1530/ERC-18-0112. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Dellinger TH, Planutis K, Tewari KS, Holcombe RF. Role of canonical Wnt signaling in endometrial carcinogenesis. Expert Rev Anticancer Ther. 2012;12:51–62. doi: 10.1586/era.11.194. [DOI] [PubMed] [Google Scholar]
- 28.Stemmer V, de Craene B, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene. 2008;27:5075–5080. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 30.McConechy MK, Ding J, Senz J, Yang W, Melnyk N, Tone AA, Prentice LM, Wiegand KC, McAlpine JN, Shah SP, Lee CH, Goodfellow PJ, Gilks CB, Huntsman DG. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 2014;27:128–134. doi: 10.1038/modpathol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholten AN, Creutzberg CL, van den Broek LJ, Noordijk EM, Smit VT. Nuclear beta-catenin is a molecular feature of type I endometrial carcinoma. J Pathol. 2003;201:460–465. doi: 10.1002/path.1402. [DOI] [PubMed] [Google Scholar]
- 32.Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–1556. doi: 10.18632/oncotarget.667. [DOI] [PMC free article] [PubMed] [Google Scholar]