Dear Editor,
We read with great interest the recent article “COVID-19 Infection in a Patient with End-Stage Kidney Disease” by Fu et al. [1]. Previous studies have reported that ∼10% of infected patients may develop acute kidney injury (AKI), which is a strong prognostic factor increasing risk of death [2, 3, 4]. We agree with the authors that SARS-CoV2 affects the kidney function and special care of renal function should be taken into account in COVID-19 patients. However, the current definition of AKI does not provide a measurement of loss of kidney function because serum creatinine level is not a sensitive marker of early tubular injury (elevation of serum creatinine requires damage/dysfunction of >50% of the nephron mass), whereas biomarkers of tubular injury provide information on early kidney injury and response to noxious stimuli [5].
All COVID-19 infection patients without a prior history of chronic kidney disease included in our study (n = 32) were consecutively admitted to our hospital in February, who were confirmed, classified as 3 subtypes (common, severe, and critical), and discharged from our hospital based on the guidelines for the diagnosis and treatment of novel coronavirus disease (version 6) [6].
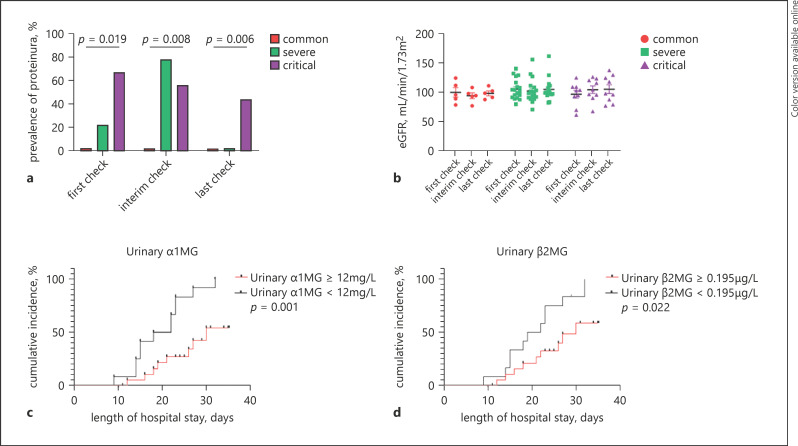
Most of these patients had mean levels of estimated glomerular filtration rate (eGFR) within the normal range, whereas 31.3% (n = 10) had proteinuria, 9.4% (n = 3) had macroalbuminuria, and 12.5% (n = 4) had microalbuminuria (Table 1). The proportion of patients with increased urinary levels of β2-microglobulin (β2MG), α1-microglobulin (α1MG), retinol binding protein (RBP), and N-acetyl-β-d-glucosaminidase (NAG) levels were 20, 20, 10, and 10%, respectively. On the first day of hospital admission, there were no significant differences in mean levels of serum creatinine, blood urea nitrogen, and eGFR among the common, severe, and critical subtypes. However, the proportion of albuminuria as well as the levels of urinary β2MG-creatinine ratio, α1MG-creatinine ratio, RBP-creatinine ratio, and NAG-creatinine ratio significantly increased according to the severity of disease. During the hospital stay, the proportion of proteinuria (dipstick >1+) in critically ill COVID-19 patients was significantly higher than that observed in common COVID-19 patients on the first check and gradually improved during the patients' hospital admission (Fig. 1). No significant differences were observed in the mean levels of eGFR both on the first day of admission and during the hospital stay amongst the 3 patient subtypes. Furthermore, Kaplan-Meier survival curves showed that patients with elevated urinary β2MG and α1MG levels had significantly lower rates of hospital discharge compared to those with normal urinary β2MG and α1MG levels.
Table 1.
Baseline characteristics of patients with COVID-19 infection
| All patients | Disease subtypes | |||||||
|---|---|---|---|---|---|---|---|---|
| n = 32 | common subtype (n = 5) | severe subtype (n = 18) | critical subtype (n = 9) | p value | ||||
| Demographic parameters | ||||||||
| Age | 61 (54–73) | 61 (55–73) | 64 (55–72) | 55 (53–81) | 0.957 | |||
| Male sex, n (%) | 20 (62.5) | 3 (60) | 10 (55.6) | 7 (77.8) | 0.608 | |||
| Clinical parameters | ||||||||
| Fever, n (%) | 17 (53.1) | 1 (20) | 12 (66.7) | 4 (44.4) | 0.159 | |||
| Heart rate, beats per minute | 82 (76–94) | 82 (72–87) | 85 (76–96) | 79 (68–94) | 0.692 | |||
| Respiratory rate, breaths per minute | 20 (20–22) | 20 (18–20) | 20 (20–23) | 21 (19–33) | 0.155 | |||
| Systolic blood pressure, mm Hg | 136 (124–144) | 138 (132–157) | 129.5 (119–144) | 139 (130–167) | 0.102 | |||
| Diastolic blood pressure, mm Hg | 79 (72–82) | 82 (75–88) | 75 (68–82) | 82 (74–87) | 0.091 | |||
| Hypertension, n (%) | 10 (31.3) | 2 (40) | 5 (27.8) | 3 (33.3) | 0.879 | |||
| Diabetes, n (%) | 5 (15.6) | 1 (20) | 3 (16.7) | 1 (11.1) | 1,000 | |||
| CRRT, n (%) | 1 (3.1) | 0 | 0 | 1 (11.1) | 0.438 | |||
| Invasive ventilation, n (%) | 9 (28.1) | 0 | 1 (5.6) | 8 (88.9)b, c | <0.001 | |||
| ECMO, n (%) | 4 (12.5) | 0 | 0 | 4 (44.4)c | 0.006 | |||
| Length of hospital stay, days | 23 (17–27) | 14 (11–15) | 22 (18–26)a | 27 (24–35)b, c | <0.001 | |||
| Laboratory parameters | ||||||||
| Leukocyte count, ×109/L | 7.7 (5.7–11.8) | 5.9 (5.5–12.7) | 7.2 (5.2–11.4) | 8.2 (7.5–12.1) | 0.466 | |||
| Lymphocyte count, ×109/L | 0.8±0.4 | 0.9±0.4 | 0.9±0.3 | 0.6±0.4 | 0.183 | |||
| Hemoglobin, g/L | 124.6±17.4 | 117.4±16.9 | 123.9±16.2 | 130±20.2 | 0.431 | |||
| Platelet count, ×109/L | 229.4±100.3 | 353.0±109.7 | 226.8±80.1a | 166.1±72.7b | 0.002 | |||
| C-reactive protein ≥10 mg/L, n (%) | 29 (90.6) | 4 (80) | 16 (88.9) | 9 (100) | 0.395 | |||
| Alanine aminotransferase, U/L | 24 (20–42) | 39 (20–85) | 23.5 (19–27) | 42 (22–102) | 0.197 | |||
| Aspartate aminotransferase, U/L | 31 (25–61) | 28 (21–48) | 30 (25–35) | 57 (39–96)c | 0.008 | |||
| Lactose dehydrogenase, U/L | 401±151 | 257±77 | 365±103 | 553±145b, c | <0.001 | |||
| Creatinine kinase, U/L | 70 (56–207) | 64 (41–75) | 64 (54–172) | 164 (72–361) | 0.037 | |||
| Serum albumin, g/L | 31.6 (29–35) | 32.7 (29–37) | 31.6 (29–35) | 30.5 (29–33) | 0.618 | |||
| Blood urea nitrogen, mg/dL | 15.6±6.7 | 17.6±5.7 | 14.3±7.9 | 17.1±3.8 | 0.475 | |||
| Serum creatinine, mg/dL | 0.7±0.1 | 0.8±0.1 | 0.7±0.2 | 0.8±0.1 | 0.566 | |||
| eGFR, mL/min/1.73 m2 | 100.7±20.3 | 99.8±18.3 | 103.1±21.7 | 96.4±20.0 | 0.731 | |||
| Urinary β2MG ≥0.195 µg/mL, n (%) | 20 (62.5) | 2 (40) | 11 (61.1) | 7 (77.8) | 0.417 | |||
| Urinary α1MG ≥12 mg/L, n (%) | 20 (62.5) | 1 (20) | 12 (66.7) | 7 (77.8) | 0.086 | |||
| Urinary RBP ≥0.7 µg/mL, n (%) | 10 (31.3) | 0 | 4 (22.2) | 6 (66.7) | 0.019 | |||
| Urinary NAG ≥14.6 U/L, n (%) | 10 (31.3) | 1 (20) | 4 (22.2) | 5 (55.6) | 0.191 | |||
| Urinary β2MG-creatinine ratio, mg/g | 0.4 (0.1–2.1) | 0.2 (0.1–0.5) | 0.2 (0.1–1.0) | 4.8 (0.4–150)c | 0.024 | |||
| Urinary α1MG-creatinine ratio, mg/g | 16.3 (8.1–37.6) | 7.8 (3.7–15.9) | 14.2 (7.5–28.8) | 222 (26.6–593)b, c | 0.001 | |||
| Urinary RBP-creatinine ratio, mg/g | 0.4 (0.2–4.7) | 0.1 (0.1–0.3) | 0.3 (0.2–0.6) | 24.3 (0.5–166)b, c | 0.001 | |||
| Urinary NAG-creatinine ratio, mg/g | 8.1 (4.6–17.0) | 6.6 (4.5–8.4) | 7.5 (4.6–13.8) | 68.1 (6.1–172)c | 0.049 | |||
| Urinary ACR category, n (%) | 0.002 | |||||||
| ACR ≥30 mg/g | 7 (21.9) | 0 | 1 (5.6) | 6 (66.7) | ||||
| ACR <30 mg/g | 25 (78.1) | 5 (100) | 17 (94.4) | 3 (33.3) | ||||
| Proteinuria, n (%) | 0.019 | |||||||
| Negative | 22 (68.8) | 5 (100) | 14 (77.8) | 3 (33.3) | ||||
| Positive | 10 (31.2) | 0 | 4 (22.2) | 6 (66.7) | ||||
| Clinical outcome | ||||||||
| Remained in hospital, n (%) | 12 (37.5) | 0 | 6 (33.3) | 6 (66.7) | 0.036 | |||
| Discharged, n (%) | 20 (62.5) | 5 (100) | 12 (66.7) | 3 (33.3) | ||||
Data are expressed as numbers (percentages) for categorical variables, means±SD for normally distributed continuous variables, and medians (interquartile ranges) for skewed distributed continuous variables. Differences between the groups were tested either by one-way ANOVA analysis (Bonferroni correction for comparisons) and the Kruskal-Wallis test (for continuous variables) or by the χ2 test and Fisher's exact test (for categorical variables). ACR, albumin to creatinine ratio; CRRT, continuous renal replacement therapy; eGFR, estimated glomerular filtration rate; ECMO, extracorporeal membrane oxygenation; RBP, retinol binding protein; NAG, N-acetyl-β-D-glucosaminidase; β2MG, β2-microglobulin; α1MG, α1-microglobulin.
p < 0.05 between common and severe subtype.
p < 0.05 between common and critical subtype.
p < 0.05 between severe and critical subtype.
Fig. 1.
The proportion of proteinuria (a) and the mean levels of eGFR (b) across different disease subtypes of COVID-19 patients during the hospital stay; Kaplan-Meier curves for cumulative hospital discharge rates of COVID-19 patients stratified by urinary levels of kidney injury biomarkers (urinary α1MG (c); urinary β2MG (d)). eGFR, estimated glomerular filtration rate; α1MG, α1-microglobulin; β2MG, β2-microglobulin.
In conclusion, we suggest that COVID-19 infection may induce early development of abnormal albuminuria and impair kidney tubular function. Because SARS-CoV-2 has been isolated from urinary samples of an infected patient and the receptor of this virus is the angiotensin converting enzyme II which is expressed on podocytes and proximal straight tubule cells [4, 7]. Notably, podocytes and proximal straight tubule cells are particularly vulnerable to viral attacks, and our findings suggested that the excretion of these urinary biomarkers may be related to the severity of the infection. Therefore, more careful medical surveillance of urinary biomarkers of early AKI is required in COVID-19-infected patients because early detection and treatment can slow or prevent progression of kidney disease.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by grants from the National Natural Science Foundation of China (81500665), High Level Creative Talents from Department of Public Health in Zhejiang Province and Project of New Century 551 Talent Nurturing in Wenzhou. G.T. is supported in part by grants from the School of Medicine, University of Verona, Verona, Italy. C.D.B. is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK.
Author Contributions
Dan-Qin Sun and Ming-Hua Zheng conceived and designed the study; Ting-Yao Wang and Yong-Ping Chen collected the data; Dan-Qin Sun and Ting-Yao Wang analyzed and interpreted the data; Dan-Qin Sun and Kenneth I. Zheng drafted the manuscript; Giovanni Targher and Christopher D. Byrne reviewed and edited the manuscript. All authors contributed to the manuscript for important intellectual content and approved the submission.
References
- 1.Fu D, Yang B, Xu J, Mao Z, Zhou C, Xue C. COVID-19 infection in a patient with end-stage kidney disease. Nephron. 2020 doi: 10.1159/000507261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Liang Y. Potential risk of the kidney vulnerable to novel coronavirus 2019 infection. Am J Physiol Ren Physiol. 2020;318((5)):F1136–F7. doi: 10.1152/ajprenal.00085.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395((10223)):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97((5)):824–8. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desanti De Oliveira B, Xu K, Shen TH, Callahan M, Kiryluk K, D'Agati VD, et al. Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol. 2019;15((10)):599–612. doi: 10.1038/s41581-019-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health Commission of the PRC COVID-19 Management Guidance. (6th Edition) Published Feburary 19, 2020. Accessed May 20, 2020.
- 7.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]