Abstract
Accurate transcription of audio recordings in psychotherapy would improve therapy effectiveness, clinician training, and safety monitoring. Although automatic speech recognition software is commercially available, its accuracy in mental health settings has not been well described. It is unclear which metrics and thresholds are appropriate for different clinical use cases, which may range from population descriptions to individual safety monitoring. Here we show that automatic speech recognition is feasible in psychotherapy, but further improvements in accuracy are needed before widespread use. Our HIPAA-compliant automatic speech recognition system demonstrated a transcription word error rate of 25%. For depression-related utterances, sensitivity was 80% and positive predictive value was 83%. For clinician-identified harm-related sentences, the word error rate was 34%. These results suggest that automatic speech recognition may support understanding of language patterns and subgroup variation in existing treatments but may not be ready for individual-level safety surveillance.
Subject terms: Translational research, Depression
Introduction
Although psychotherapy has proven effective at treating a range of mental health disorders, we have limited insight into the relationship between the structure and linguistic content of therapy sessions and patient outcomes1–6. This gap in knowledge limits insights into causal mechanisms of patient improvement, the evaluation and refinement of treatments, and the training of future clinicians7. Many patient and therapist factors have been assessed in psychotherapy (e.g., patient diagnosis, therapist experience, and theoretical orientation). However, there is little consensus as to which specific therapist behaviors contribute to patients’ symptom improvement or deterioration2.
Understanding what patients and therapists say during therapy, in conjunction with pre- and post-symptom assessment, may surface markers of good psychotherapy. Psychotherapy transcripts have long been used to search for objective, reproducible characteristics of effective therapists8. Also, analysis of psychotherapy transcripts has been used to generate theories and test hypotheses of specific mechanisms of action, but has been limited in part by technological capacity9–11. Discourse analysis is not common in controlled trials or effectiveness studies, and psychotherapy is rarely recorded outside of training settings or clinical trials. When it is recorded, a transcription is typically completed by a person, after which qualitative or quantitative analyses are undertaken. Manual transcription is expensive and time consuming12, leaving most psychotherapy unscrutinized3.
Automatic speech recognition (ASR) is being explored to augment clinical documentation and clinician interventions3,13. Evaluations of medical ASR systems often focus on individual dictation rather than modeling conversational discourse14, which is far more complex15,16. Prior literature estimates the word error rate of conversational medical ASR systems between 18 and 63%17,18. Although patient language analysis can inform diagnosis19, and clinician language use can inform treatment evaluation12,20, few approaches exist for transcribing clinical therapy sessions en masse. Although potentially useful, the need to audit emerging machine-learning systems has been highlighted by research showing that many ASR systems have worse performance for ethnic minorities21. Given existing health disparities in mental health treatment, there is a need to redress, rather than intensify equitable treatment across diverse groups22,23. Thus, methods to assess the performance of ASR systems in the mental health domain are needed.
In this work, we present an assessment of ASR performance in psychotherapy discourse. Using a sample of patient-therapist audio recordings collected as part of a US-based clinical trial24, we compare transcriptions generated by humans, which we consider the reference standard, to transcriptions generated by a commercial, cloud-based ASR service (Google Cloud Speech-to-Text)25. We quantify errors using three approaches. First, we analyze ASR performance using standard, domain-agnostic evaluation metrics such as word error rate. Second, we analyze patient symptom-focused language using a metric derived from a common depression symptom reporting tool, the Patient Health Questionnaire (PHQ-9)26. Third, we identify individual crisis moments related to self-harm and harm to others, and evaluate ASR’s performance in identifying these moments. Our evaluation, which uses a scalable HIPAA-compliant workflow for analyzing patient recordings, lays the foundation for future work using computational methods to analyze psychotherapy.
Results
The study used a total of 100 therapy sessions between April 2013 and December 2016 containing 100 unique patients and 78 unique therapists. Among 100 patients for whom age was available (91%), the average age was 23 years (median 21; range, 18–52; SD, 5). A total of 87% of patients were female (Table 1). The average therapy session was 45 min (median, 47; range, 13–69; SD, 11) in length. During a session, the therapist spoke an average of 2909 words (median, 2,886; range, 547–6,213; SD, 1,128) over 20 min (median, 19; range, 4–41; SD, 8). The patient spoke an average of 3,665 words (median, 3,555; range, 277–7,043; SD, 1,550) over 25 min (median, 25; range 2–46; SD, 9). To characterize ASR in psychotherapy, a three-pronged evaluation framework is used: domain agnostic performance, depression symptom-specific performance, and harm-related performance.
Table 1.
Patient demographics and therapy session information.
| Patient demographics | Average | Standard deviation | Median | Min | Max |
|---|---|---|---|---|---|
| Number of patients | 100 | – | – | – | – |
| Female (%) | 87 | – | – | – | – |
| Age (years) | 23 | 5 | 21 | 18 | 52 |
| Session information | |||||
| Length | |||||
| Minutes | 45 | 11 | 47 | 13 | 69 |
| Number of words | 6574 | 2102 | 6387 | 824 | 11,310 |
| Time talking per session (min) | |||||
| Patient | 25 | 9 | 26 | 2 | 46 |
| Therapist | 20 | 8 | 19 | 4 | 41 |
| Words spoken per session (n) | |||||
| Patient | 3665 | 1550 | 3555 | 277 | 7043 |
| Therapist | 2909 | 1128 | 2886 | 547 | 6213 |
Domain agnostic performance
The first prong of our evaluation is domain agnostic, which uses word error rate and semantic distance to determine errors. The average word error rate of the speech recognition system was 25% (median, 24%; range, 8–74%; SD, 12%) (Table 2). Semantic distance is a proxy for the similarity of meaning between two sentences, based on computing a vector representation for the words in each sentence and looking at the distance between these vectors in Euclidean space27. The average semantic distance between human-transcribed and ASR-transcribed sentences was 1.2 points (median, 1.1; range, 0.5–2.4; SD, 0.3). For reference, the semantic distance between random words, random sentences, and human-selected paraphrases is 4.14, 2.97, and 1.14, respectively (Supplementary Tables 1 and 2).
Table 2.
Similarity between the human-transcribed reference standard and ASR-transcribed sentences.
| Word overlap | Semantic similarity | ||||||
|---|---|---|---|---|---|---|---|
| Group | n | Error Rate, % | Shapiro–Wilk | p value | Semantic distance, pts | Shapiro–Wilk | p value |
| Aggregate | |||||||
| Total | 100 | 25% ± 12% | 0.93 | <0.001 | 1.20 ± 0.31 | 0.97 | 0.03 |
| Speaker | |||||||
| Patient | 100 | 25% ± 12% | 0.86 | <0.001 | 1.19 ± 0.33 | 0.94 | <0.001 |
| Therapist | 100 | 26% ± 11% | 0.88 | <0.001 | 1.20 ± 0.29 | 0.99 | 0.57 |
| Patient gender | |||||||
| Male | 13 | 24% ± 9% | 0.95 | 0.55 | 1.17 ± 0.30 | 0.95 | 0.55 |
| Female | 87 | 25% ± 13% | 0.84 | <0.001 | 1.19 ± 0.33 | 0.94 | <0.001 |
Plus/minus values denote standard deviation. Lower error rate is better. Lower semantic distance is better. Shapiro–Wilk tests were conducted to test the normality assumption (Supplementary Fig. 2). Low p values indicate the data are not normally distributed.
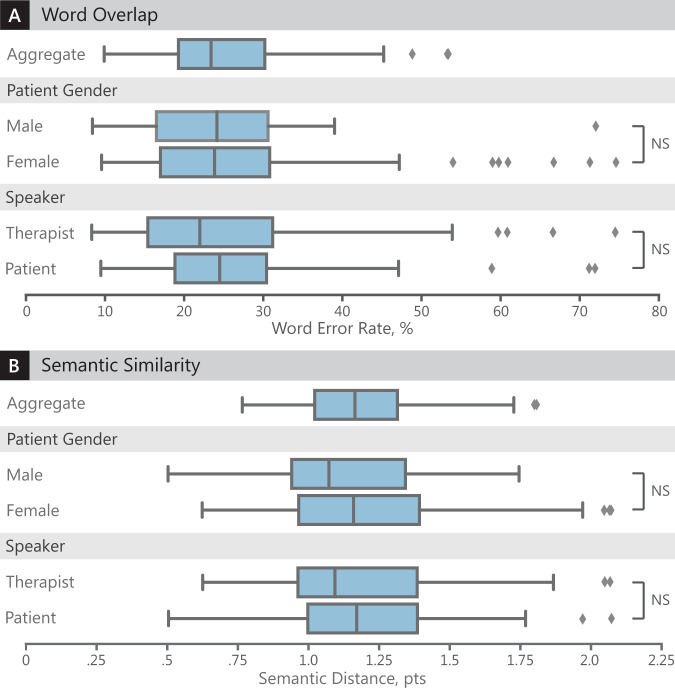
Transcription of patients’ speech was not significantly different from therapists’ speech (25% vs 26% error rate, two-tailed Mann–Whitney U-test, p = 0.21) (Fig. 1). In addition, transcription of male speech was not significantly different from female speech (24% vs 25% error rate, two-tailed Welch’s t-test, p = 0.69).
Fig. 1. Automatic speech recognition performance, overall and by subgroup.
Evaluation of ASR transcription performance compared to the human-generated reference transcription. Each box denotes the 25th and 75th percentile. Box center-lines denote the median. Whiskers denote the minimum and maximum values, excluding any outliers. Outliers, denoted by diamonds, are defined as any point further than 1.5× the interquartile range from the 25th or 75th percentile. Sample sizes are listed in Table 2. NS not significant means the difference is not statistically significant. a Comparison of word overlap (i.e., word error rate). Lower word error rate is better. b Comparison of semantic similarity (i.e., semantic distance). Lower semantic distance is better.
Depression symptom specific performance
The second prong of our evaluation is depression-specific. Across medical terms from the Patient Health Questionnaire26, the average sensitivity (i.e., recall) was 80% and positive predictive value (i.e., precision) was 83% (Table 3). The PHQ category with the highest sensitivity was category 2 (depression) with a sensitivity of 85%. The categories with the highest positive predictive value were categories 5 (overeating) and 7 (mindfulness) with a positive predictive value of 100%. Results are presented for each medical term in Supplementary Table 3.
Table 3.
Performance on clinically-relevant utterances by patients.
| PHQ | Keywordsa | Number of positives | True positives | False negatives | False positives | Sensitivity | Positive predictive value |
|---|---|---|---|---|---|---|---|
| 1 | Interest, interested, interesting, interests, pleasure | 169 | 127 | 42 | 38 | 75% | 77% |
| 2 | Depressed, depressing, feeling down, hopeless, miserable | 74 | 63 | 11 | 12 | 85% | 84% |
| 3 | Asleep, drowsy, sleepiness, sleeping, sleepy | 114 | 85 | 29 | 19 | 75% | 82% |
| 4 | Energy, tired | 143 | 115 | 28 | 22 | 80% | 84% |
| 5 | Overeat, overeating | 5 | 3 | 2 | 0 | 60% | 100% |
| 6 | Bad, badly, poorly | 405 | 336 | 69 | 56 | 83% | 86% |
| 7 | Mindfulness | 11 | 9 | 2 | 0 | 82% | 100% |
| 8 | Fidget, fidgety, restless, slow, slowing, slowly | 39 | 28 | 11 | 13 | 72% | 68% |
| 9 | Dead, death, depression, died, suicide | 103 | 86 | 17 | 18 | 83% | 83% |
| Weighted average | 1063 | 852 | 211 | 178 | 80% | 83% |
aFor each question of the Patient Health Questionnaire (PHQ-9), relevant keywords were identified by querying the Unified Medical Language System using each PHQ question to generate search terms. Each table row denotes a different question from the PHQ-9. Number of occurrences refer to how often the keywords appear in our transcribed therapy sessions. True positives refer to a correct transcription by the automatic speech recognition system. False negatives and false positives denote incorrect transcriptions. Sample size is denoted by the number of positives.
Harm-related performance
The third prong of our evaluation centers on harm-related performance. A total of 97 clinician-identified harm-related sentences were identified. Half of the manually annotated sessions (50%; 10 of 20) had at least one harm-related utterance. These sentences demonstrated an average error rate of 34% (median, 16%; range 0–100%; SD, 37%) and average semantic distance of 0.61 (median, 0.30; range 0–2.62; SD, 0.75). Compared with performance across all therapy sentences, harm-related sentences demonstrated a higher word error rate (34% vs 25% error rate, two-tailed Mann–Whitney U-test, p = 0.07) but a significantly lower semantic distance (0.61 vs 1.20, two-tailed Mann–Whitney U-test, p < 0.001).
For the 45 harm-related sentences spoken by the therapist, the average error rate was 36% (median, 20%; range, 0–100%; SD, 39%). For the 52 harm-related sentences spoken by the patient, the average error rate was 32% (median, 13%; range 0–100%; SD, 35%). Sentences spoken by the patient were not significantly different from sentences spoken by the therapist in terms of word error rate (32% vs 36%, two-tailed Mann–Whitney U-test, p = 0.60) and semantic distance (0.62 vs 0.58, two-tailed Mann–Whitney U-test, p = 0.59). Table 4 illustrates the importance of semantic distance, in the context of transcription errors. Several sentences are categorized by the type of their transcription error, thus demonstrating the clinical relevance of surface differences in words, or phonetics, versus deeper semantic errors.
Table 4.
Transcription errors made by the automatic speech recognition system.
| Meaning (semantics) | |||
|---|---|---|---|
| Similar to reference standard | Different from reference standard | ||
| Form (Words or Phonetics) | Similar to reference standard |
1. Tuesday, I had found out about that my grandmother had died is dying. 2. Came back and ate eat some more. |
1. I have still been feeling depressed the preston. 2. Do you have any plans to hurt dirt yourself? |
| Different from reference standard |
1. Depends on like what I eat or what I’ve been eating have been made. 2. Comfortable to expressing his these negative emotions. |
1. It still stings. It doesn’t hurt as much as it did wasn’t hers do you still feel like. 2. I’m going to try to appeal kill the schools. |
|
Each numbered sentence is a different sentence containing both the reference standard and ASR transcription. Strikethrough denotes the human-generated reference standard. Underline denotes the speech recognition system’s erroneous output. Black text denotes agreement.
Discussion
We proposed the use of semantic distance, clinical terminology, and clinician-labeled utterances to better quantify ASR performance in psychotherapy. This is more comprehensive than word error metrics alone, which treat all errors (e.g., word substitutions, additions) as equal. Our evaluation found a general error rate of 25%, which varied by use case (e.g., symptom detection vs harm-related utterances). When evaluated using semantic similarity and not error rate, the ASR system was significantly better at transcribing clinician-labeled sentences related to harm than other sentences spoken during the session. This suggests that acceptable performance may vary depending on clinical use case and choice of evaluation framework.
Given these findings, using ASR to passively collect symptom information may be possible, as currently only 20% of mental health practitioners use measurement-based care28. Creating transcripts is important because their inspectability offers a benefit for clinician training and supervision compared to using black-box deep learning models29,30, which may have predictive validity, but are challenging to interpret31. However, critical words used to diagnose depression had different rates of performance (Table 3), ranging from 60 to 100%. More research is warranted in symptom-focused accuracy, as culturally sensitive diagnostic accuracy will be crucial if ASR is to aid in clinical documentation. Special attention to algorithmic performance is especially crucial in healthcare settings to ensure equitable performance across patient and provider subgroups (e.g. age, race, ethnicity, gender, diagnosis)32–34. Although ASR is unlikely to be first used to detect harm-related utterances in clinical settings, assessing risk of harm to self or others is a cornerstone of clinician duty. Thus, recognizing harm-related phrases is crucial to any downstream processes and merits special attention.
A known bottleneck in psychotherapy research is that psychotherapy sessions are rarely examined in their entirety, which impedes analysis of practice patterns35. Despite assumptions of provider uniformity in randomized clinical trials and naturalistic investigations36,37, therapist effects–that some therapists consistently achieve better results than others–is well documented38,39. Accurate transcriptions would facilitate more rigorous quality assessment than is currently feasible6,40. ASR provides a potential avenue to study such effects using computational approaches.
Although ASR is not perfect, it may enable better therapist training. For instance, ASR may quickly surface illustrations of patient idioms of distress41, or effective examples of appropriate and inappropriate clinician responses. Similarly, ASR-generated transcripts could aid in linking speech acts to theoretically important phenomenon such as therapeutic alliance, the most consistent predictor of psychotherapeutic outcome42. Although these applications may seem distant, a more proximal application of this technology could be to facilitate the supervision of trainees, in which licensed clinicians review trainees’ transcripts. ASR can accelerate this process, however, integrating ASR into clinical practice will require thoughtful design and implementation6. Additional use cases of ASR in medicine extend to patient symptom documentation13,18, exploring communication-based ethnic disparities in treatment40,43,44, assessing dissemination efforts of evidence-based practices45,46, pooling, and standardizing transcripts from psychotherapy studies40, and monitoring harmful or illegal clinician behavior47.
Our work has limitations. First, we analyzed ASR performance on outpatient psychotherapy sessions between therapists and college-aged participants. These results may not generalize to other patient or provider populations48. Second, our evaluation uses transcriptionist-generated timestamps for each spoken phrase. These transcriptionists may provide inaccurate timestamps due to delayed reaction times or other human errors. Third, to maximize reproducibility, we limit our analysis to words directly from the PHQ-9 and Unified Medical Language System (Table 3)49. These lists are not meant to be exhaustive, and future research should seek to expand this list to additional clinically-relevant terminology50–56. Fourth, while our evaluation method analyzed ASR performance broken down by the role of patient versus therapist, such role annotations were only available in the human-annotated transcriptions. It is unknown how well ASR performs role assignment (i.e., speaker diarization). Fifth, it is possible that the human-generated transcripts had inaccuracies. As a result, our estimates are likely conservative. Sixth, we note that while we did choose a state-of-the-art tool for automatic transcription, other ASR systems may perform differently21. Assessing transcription accuracy across tools and clinical settings is a crucial next step21. Seventh, we use one method for computing word embeddings (Word2Vec27) and sentence embeddings (earth mover distance57) to establish this baseline, however other appropriate options exist and should be assessed in future work (e.g., BioBERT, GloVe)58–60. However, by establishing a three-pronged evaluation framework, we enable a more nuanced comparison of ASR systems than currently allowed by word error rate-based approaches.
ASR will likely be useful before it is perfect. Thus, it is crucial to design evaluations that differentiate between the types of errors, assess clinical impact, and detail performance for legally mandated situations such as self-harm61,62. ASR holds promise to convert psychotherapy sessions into computable data at scale; and with enough data, characteristics of effective therapy may be uncovered via supervised machine learning and discourse analysis. However, claims regarding the potential of artificial intelligence should be tempered in the context of real performance metrics, and challenges in fairness, maintaining privacy, and trust63–66. ASR may offer a cost-effective and reproducible way to transcribe sensitive conversations, but collecting and analyzing intimate data at unprecedented scales demands improved governance around limiting unintended use and tracking provenance of the conclusions drawn67–75.
The National Institute of Mental Health has called for computational approaches to understand trajectories of mental illness and to create standardized data elements76. With improved accuracy and the development of agreed-upon thresholds for acceptable performance, mechanisms of action in psychotherapy would be easier to uncover. Our work, which uses a scalable, HIPAA-compliant workflow for analyzing patient recordings, lays the foundation for future work using computational methods to analyze psychotherapy. By facilitating better descriptions of psychotherapeutic encounters associated with good outcomes, ASR can help illuminate precise interventions that improve psychotherapy effectiveness and allow us to revisit long-held ideas of psychotherapy with more objective, inspectable, and scalable analyses.
In conclusion, we outlined a three-pronged evaluation framework spanning domain agnostic performance, clinical terminology, and clinician-identified phrases to characterize ASR performance in psychotherapy. Compared to human-generated transcripts, ASR software demonstrated a word error rate of 25% and a mean semantic distance of 1.2, which is likely sufficient to enable research aimed at understanding existing treatments and to augment clinician training. However, accuracy, in terms of word error rate and semantic distance, varied for depression-related words and for harm-related phrases, suggesting a need for both improved accuracy and the development of agreed-upon thresholds for use in safety monitoring. ASR can potentially enable psychotherapy effectiveness research but requires further improvement before use in safety monitoring. Our work lays the foundation for using computational methods to analyze psychotherapy at scale.
Methods
Study design
This study is a secondary analysis of audio recordings of 100 therapy sessions from a cluster randomized trial. Audio recordings of college counseling psychotherapy were gathered per protocol during the trial, which had a primary aim of studying two clinician training strategies24. Written consent was obtained per protocol in the original trial from both patients and therapists. The primary objective of the current study is to quantify the accuracy of automatic speech recognition software via a comparison with the human-generated transcripts on overall accuracy, depression-specific language, and harm-related conversations.
This study was conceptualized and executed after the design and launch of the original study. All research procedures for this study were reviewed and approved by the Institutional Review Board at Stanford University. During the original trial, all therapists were consented by Washington University in St. Louis, and all patients involved in the study were consented by their local institutions. The Stanford University Institutional Review Board approved all consent procedures. Although approaches will vary between organizations, we describe our process for establishing a HIPAA-compliant ASR process in Supplementary Note 1.
Clinical setting and data collection
This study assessed audio recordings of 100 therapy sessions from 100 unique patient-therapist dyads. The sessions took place between April 2013 and December 2016 at 23 different college counseling sites across the United States. Audio recordings were collected in the original study for humans to review and assess therapist quality.
Corpus creation
In order to compare the ASR to human-generated transcripts, two transcriptions were done: one using industry-standard manual transcription services, and the other using a commercially-available ASR software25. A third-party transcription company was paid to create the transcriptions by listening to the original audio. Scribes transcribed all words including “filler words” (e.g., -huh-, -mm-hm-). The protocol for manual transcription is provided in Supplementary Note 2. Each utterance was “diarized” (i.e., ascribed to a speaker: therapist, patient, or unknown) and each change of speaker was timestamped in minutes and seconds. The human-generated transcripts were used as the reference standard for all comparisons. Data storage, transmission, and access were assessed and approved by the Stanford University Information Security Office and the Stanford University Institutional Review Board.
Measures of automatic speech recognition performance
There are currently no standard approaches to assessing ASR quality in psychotherapy. We propose three approaches: (1) a general, commonly used domain agnostic evaluation; (2) examining symptom-specific language; and (3) examining crucial phrases related to self-harm or harm to others.
Domain agnostic evaluation measures: The standard evaluation metric for speech recognition systems is word error rate (WER)77,78, defined as the total number of word substitutions (S), deletions (D), and insertions (I) in the transcribed sentences, divided by the total number of words (N) in the reference sentence (i.e., human-transcription). That is, WER = (S + D + I)/N. The word error rate requires an exact word match to be considered correct. Homophones (i.e., words that sound the same but have different meanings like “buy” and “bye”) were measured as inaccuracies.
One shortcoming of word error rate is how it assigns equal importance to all words. Transcribing the word “death” into “dead” will be registered as an error. However, such an error may not significantly change the meaning of the sentence and in fact may be sufficiently correct for clinical use. This can be partly mitigated by using relative word importance to re-weight the final metric accordingly79,80. However, this still measures word-level equivalence rather than sentence-level resemblance81.
To address these shortcomings, we propose measuring semantic distance between each ASR-generated transcription and human-generated transcript. While subjective measures of semantic similarity for machine translation and paraphrase detection exist82–85, large-scale manual review by humans is generally infeasible. Therefore, we used word2vec embeddings27 to extract word-level embeddings followed by mean-pooling to compute a sentence-level embedding86. The sentence embeddings of the human-generated transcripts were compared to the ASR-generated embeddings using earth mover distance57. A comparison of earth mover and cosine distance is shown in Supplementary Fig. 1. A smaller value of semantic distance indicates higher similarity, with zero semantic distance indicating perfect similarity.
Depression-specific evaluation: Assessing domain-specific vocabulary in health contexts has been called for by researchers from the Centers for Disease Control and Prevention and the U.S. Food and Drug Administration87. To evaluate depression-specific vocabulary, we selected clinically-relevant words directly from a commonly used depression screen, the Patient Health Questionnaire (PHQ-9)26. Keywords from the PHQ-9 (e.g., sleep, mood, suicide) were extended to a larger list using the Unified Medical Language System, a medical terminology system maintained by the U.S. National Library of Medicine88. This is similar to previous approaches used to search for medical subdomain language89. While there are methods to expand the vocabulary to synonyms and informal phrases90, in this work, our goal was to provide a baseline that allows for simplicity and reproducibility87. Our approach using the Unified Medical Language System was selected to prioritize false negatives (Type II errors) over false positives (Type I errors) for symptom detection. This approach may differ across use cases.
Once the list of clinically-relevant words was determined, sensitivity and positive predictive values were computed from the perspective of binary classification. Clinically-relevant words were treated as positive examples and all other words were treated as negative examples. For each clinical word, transcription performance was measured across all therapy sessions. For each word (positive example), the number of negative examples is large, consisting of the set of every other word in the English language, thus leading to very high specificity rates (i.e., above 99.9%). Because it would not meaningfully differentiate performance, we do not report specificity.
Harm-related evaluation: A licensed clinical psychologist (Author: A.S.M.) randomly sampled and retrospectively read 20 transcripts from the dataset and annotated any harm-related phrases spoken by the patient or therapist (e.g., “I want to hurt myself”). The harm-related sentences are a subset of the full dataset in Table 1. We then assessed the accuracy of ASR on this subset. This assessment was of historical data, and thus no safety concerns were shared with law enforcement or other mandated reporting agencies.
Statistical analyses
Before testing for a difference of means, subgroups were tested against the normality assumption and their variance was assessed. To test the normality assumption, the Shapiro–Wilk test was used (Supplementary Fig. 2). To test for equal subgroup variance, the Levene test was used. Depending on the Shapiro–Wilk and Levene test results, one of the following difference tests were used: two-tailed Welch’s t-test or two-tailed Mann–Whitney U-test. The significance threshold was p = 0.01. All statistical analyses were implemented in Python (version 3.7; Python Software Foundation) with the SciPy software library91. Covariates were the word error rate and semantic distance.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
A.S.M. was supported by grants from the National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Award (KL2TR001083 and UL1TR001085), the Stanford Department of Psychiatry Innovator Grant Program, and the Stanford Human-Centered AI Institute. S.L.F. was supported by a Big Data to Knowledge (BD2K) grant from the National Institutes of Health (T32 LM012409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
A.S.M. and A.H. contributed equally as co-first authors. A.S.M., A.H., B.A.A., W.S.A., N.H.S., J.A.F., and S.L.F. conceptualized and designed the study. W.S.A., D.E.W., G.T.W., A.S.M., A.H., J.A.F., S.L.F., B.A.A., and N.H.S. acquired, analyzed or interpreted the data. A.S.M., A.H., J.A.F., S.L.F., A.M., D.J., B.A.A., W.S.A., L.F-F., and N.H.S. drafted the manuscript. All authors performed critical revision of the manuscript for important intellectual content. A.S.M., A.H., J.A.F., S.L.F., D.J., and N.H.S. performed statistical analysis. B.A.A. and N.H.S. provided administrative, technical, and material support. L.F-F., B.A.A., W.S.A., and N.H.S. supervised the study. A.S.M and A.H. had full access to all the data. A.S.M. and A.H. take responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability
The dataset is not publicly available due to patient privacy restrictions, but may be available from the corresponding author on reasonable request.
Code availability
The code used in this study can be found at:https://github.com/som-shahlab/psych-audio.
Competing interests
L.F-F. served as Chief Scientist at Google Cloud from 2017 to 2018. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Adam S. Miner, Albert Haque.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41746-020-0285-8.
References
- 1.Merz Jasmin, Schwarzer Guido, Gerger Heike. Comparative Efficacy and Acceptability of Pharmacological, Psychotherapeutic, and Combination Treatments in Adults With Posttraumatic Stress Disorder. JAMA Psychiatry. 2019;76(9):904. doi: 10.1001/jamapsychiatry.2019.0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castonguay, L. G. & Hill, C. E. How and why are some therapists better than others?: Understanding Therapist Effects Vol. 356 (American Psychological Association, 2017).
- 3.Imel ZE, Steyvers M, Atkins DC. Computational psychotherapy research: scaling up the evaluation of patient-provider interactions. Psychotherapy. 2015;52:19–30. doi: 10.1037/a0036841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes EA, et al. The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry. 2018;5:237–286. doi: 10.1016/S2215-0366(17)30513-8. [DOI] [PubMed] [Google Scholar]
- 5.Kazdin AE. Addressing the treatment gap: a key challenge for extending evidence-based psychosocial interventions. Behav. Res. Ther. 2017;88:7–18. doi: 10.1016/j.brat.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Miner AS, et al. Key considerations for incorporating conversational AI in psychotherapy. Front. Psychiatry. 2019;10:746. doi: 10.3389/fpsyt.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfried MR. Obtaining consensus in psychotherapy: what holds us back? Am. Psychol. 2019;74:484–496. doi: 10.1037/amp0000365. [DOI] [PubMed] [Google Scholar]
- 8.Rogers CR. The use of electrically recorded interviews in improving psychotherapeutic techniques. Am. J. Orthopsychiatry. 1942;12:429–434. [Google Scholar]
- 9.Gelo, O., Pritz, A. & Rieken, B. Psychotherapy Research: Foundations, Process, and Outcome (Springer, 2016).
- 10.Gelo OCG, Salcuni S, Colli A. Text Analysis within quantitative and qualitative psychotherapy process research: introduction to special issue. Res. Psychother. 2012;15:45–53. [Google Scholar]
- 11.Ewbank Michael P., Cummins Ronan, Tablan Valentin, Bateup Sarah, Catarino Ana, Martin Alan J., Blackwell Andrew D. Quantifying the Association Between Psychotherapy Content and Clinical Outcomes Using Deep Learning. JAMA Psychiatry. 2020;77(1):35. doi: 10.1001/jamapsychiatry.2019.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao B, Imel ZE, Georgiou PG, Atkins DC, Narayanan SS. ‘Rate My Therapist’: Automated detection of empathy in drug and alcohol counseling via speech and language processing. PLOS ONE. 2015;10:e0143055. doi: 10.1371/journal.pone.0143055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin SY, Shanafelt TD, Asch SM. Reimagining clinical documentation with artificial intelligence. Mayo Clin. Proc. 2018;93:563–565. doi: 10.1016/j.mayocp.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Blackley SV, Huynh J, Wang L, Korach Z, Zhou L. Speech recognition for clinical documentation from 1990 to 2018: a systematic review. J. Am. Med. Inform. Assoc. 2019;26:324–338. doi: 10.1093/jamia/ocy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu, C.-C. et al. Speech recognition for medical conversations. Interspeech.10.21437/Interspeech.2018-40 (2018).
- 16.Labov, W. & Fanshel, D. Therapeutic Discourse: Psychotherapy as Conversation (Academic Press, 1977).
- 17.Kodish-Wachs J, Agassi E, Kenny P, 3rd, Overhage JM. A systematic comparison of contemporary automatic speech recognition engines for conversational clinical speech. AMIA Annu. Symp. Proc. 2018;2018:683–689. [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkomar Alvin, Kannan Anjuli, Chen Kai, Vardoulakis Laura, Chou Katherine, Cui Claire, Dean Jeffrey. Automatically Charting Symptoms From Patient-Physician Conversations Using Machine Learning. JAMA Internal Medicine. 2019;179(6):836. doi: 10.1001/jamainternmed.2018.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marmar Charles R., Brown Adam D., Qian Meng, Laska Eugene, Siegel Carole, Li Meng, Abu‐Amara Duna, Tsiartas Andreas, Richey Colleen, Smith Jennifer, Knoth Bruce, Vergyri Dimitra. Speech‐based markers for posttraumatic stress disorder in US veterans. Depression and Anxiety. 2019;36(7):607–616. doi: 10.1002/da.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mieskes, M. & Stiegelmayr, A. Preparing data from psychotherapy for natural language processing. In International Conference on Language Resources and Evaluation (European Language Resources Association, 2018).
- 21.Koenecke A, et al. Racial disparities in automated speech recognition. Proc. Natl Acad. Sci. USA. 2020;117:7684–7689. doi: 10.1073/pnas.1915768117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen IY, Szolovits P, Ghassemi M. Can AI help reduce disparities in general medical and mental health care? AMA J. Ethics. 2019;21:E167–E179. doi: 10.1001/amajethics.2019.167. [DOI] [PubMed] [Google Scholar]
- 23.Schueller Stephen M., Hunter John F., Figueroa Caroline, Aguilera Adrian. Use of Digital Mental Health for Marginalized and Underserved Populations. Current Treatment Options in Psychiatry. 2019;6(3):243–255. [Google Scholar]
- 24.Wilfley DE, et al. Training models for implementing evidence-based psychological treatment for college mental health: a cluster randomized trial study protocol. Contemp. Clin. Trials. 2018;72:117–125. doi: 10.1016/j.cct.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Google. Cloud Speech-to-Text (Google, 2020).
- 26.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikolov T, Sutskever I, Chen K, Corrado GS, Dean J. Distributed representations of words and phrases and their compositionality. Adv. Neural Inform. Process. Syst. 2013;26:3111–3119. [Google Scholar]
- 28.Lewis Cara C., Boyd Meredith, Puspitasari Ajeng, Navarro Elena, Howard Jacqueline, Kassab Hannah, Hoffman Mira, Scott Kelli, Lyon Aaron, Douglas Susan, Simon Greg, Kroenke Kurt. Implementing Measurement-Based Care in Behavioral Health. JAMA Psychiatry. 2019;76(3):324. doi: 10.1001/jamapsychiatry.2018.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteva A, et al. A guide to deep learning in healthcare. Nat. Med. 2019;25:24–29. doi: 10.1038/s41591-018-0316-z. [DOI] [PubMed] [Google Scholar]
- 30.Haque, A., Guo, M., Miner, A. S. & Fei-Fei, L. Measuring depression symptom severity from spoken language and 3D facial expressions. In: Thirty-second Conference on Neural Information Processing Systems, Machine Learning for Health workshop. Preprint at: arXiv:1811.08592 (Montreal, Canada, 2018).
- 31.Hutson M. Has artificial intelligence become alchemy? Science. 2018;360:478. doi: 10.1126/science.360.6388.478. [DOI] [PubMed] [Google Scholar]
- 32.Goodman SN, Goel S, Cullen MR. Machine learning, health disparities, and causal reasoning. Ann. Intern. Med. 2018;169:883–884. doi: 10.7326/M18-3297. [DOI] [PubMed] [Google Scholar]
- 33.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366:447–453. doi: 10.1126/science.aax2342. [DOI] [PubMed] [Google Scholar]
- 34.Caliskan A, Bryson JJ, Narayanan A. Semantics derived automatically from language corpora contain human-like biases. Science. 2017;356:183–186. doi: 10.1126/science.aal4230. [DOI] [PubMed] [Google Scholar]
- 35.Norcross JC, Wampold BE. Evidence-based therapy relationships: research conclusions and clinical practices. Psychotherapy. 2011;48:98–102. doi: 10.1037/a0022161. [DOI] [PubMed] [Google Scholar]
- 36.Elkin I. A major dilemma in psychotherapy outcome research: disentangling therapists from therapies. Clin. Psychol.: Sci. Pract. 1999;6:10–32. [Google Scholar]
- 37.Kim D-M, Wampold BE, Bolt DM. Therapist effects in psychotherapy: a random-effects modeling of the National Institute of Mental Health Treatment of Depression Collaborative Research Program data. Psychother. Res. 2006;16:161–172. [Google Scholar]
- 38.Baldwin, S. A. & Imel, Z. E. Therapist effects: findings and methods. In: Bergin and Garfield's Handbook of Psychotherapy and Behavior Change. 258–297 (Wiley, 2013).
- 39.Johns RG, Barkham M, Kellett S, Saxon D. A systematic review of therapist effects: a critical narrative update and refinement to review. Clin. Psychol. Rev. 2019;67:78–93. doi: 10.1016/j.cpr.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Owen J, Imel ZE. Introduction to the special section ‘Big’er’ Data’: Scaling up psychotherapy research in counseling psychology. J. Couns. Psychol. 2016;63:247–248. doi: 10.1037/cou0000149. [DOI] [PubMed] [Google Scholar]
- 41.Cork C, Kaiser BN, White RG. The integration of idioms of distress into mental health assessments and interventions: a systematic review. Glob. Ment. Health. 2019;6:e7. doi: 10.1017/gmh.2019.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castonguay LG, Beutler LE. Principles of therapeutic change: a task force on participants, relationships, and techniques factors. J. Clin. Psychol. 2006;62:631–638. doi: 10.1002/jclp.20256. [DOI] [PubMed] [Google Scholar]
- 43.Gordon HS, Street RL, Jr., Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J. Clin. Oncol. 2006;24:904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 44.Hook JN, et al. Cultural humility and racial microaggressions in counseling. J. Couns. Psychol. 2016;63:269–277. doi: 10.1037/cou0000114. [DOI] [PubMed] [Google Scholar]
- 45.Asch SM, et al. Who is at greatest risk for receiving poor-quality health care? N. Engl. J. Med. 2006;354:1147–1156. doi: 10.1056/NEJMsa044464. [DOI] [PubMed] [Google Scholar]
- 46.Stirman SW, Crits-Christoph P, DeRubeis RJ. Achieving successful dissemination of empirically supported psychotherapies: A synthesis of dissemination theory. Clin. Psychol.: Sci. Pract. 2004;11:343–359. [Google Scholar]
- 47.Drescher J, et al. The growing regulation of conversion therapy. J. Med Regul. 2016;102:7–12. [PMC free article] [PubMed] [Google Scholar]
- 48.Vessey, J. T. & Howard, K. I. Who seeks psychotherapy? (Group Dynamics,1993).
- 49.Park J, et al. Detecting conversation topics in primary care office visits from transcripts of patient-provider interactions. J. Am. Med. Inform. Assoc. 2019;26:1493–1504. doi: 10.1093/jamia/ocz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraus DR, Castonguay L, Boswell JF, Nordberg SS, Hayes JA. Therapist effectiveness: implications for accountability and patient care. Psychother. Res. 2011;21:267–276. doi: 10.1080/10503307.2011.563249. [DOI] [PubMed] [Google Scholar]
- 51.Institute of Medicine. Vital Signs: Core Metrics for Health and Health Care Progress (National Academies Press, 2015). [PubMed]
- 52.Pérez-Rojas AE, Brown R, Cervantes A, Valente T, Pereira SR. ‘Alguien abrió la puerta:” The phenomenology of bilingual Latinx clients’ use of Spanish and English in psychotherapy. Psychotherapy. 2019;56:241–253. doi: 10.1037/pst0000224. [DOI] [PubMed] [Google Scholar]
- 53.Yu, Z., Cohen, T., Wallace, B., Bernstam, E. & Johnson, T. Retrofitting word vectors of mesh terms to improve semantic similarity measures. In: Workshop on Health Text Mining and Information Analysis. 43–51. 10.18653/v1/W16-6106 (2016).
- 54.Aronson, A. R. Effective mapping of biomedical text to the UMLS Metathesaurus: the MetaMap program. In: Proc. AMIA Symp. 17–21 (American Medical Informatics Association, 2001). [PMC free article] [PubMed]
- 55.Savova GK, et al. Mayo clinical text analysis and knowledge extraction system (cTAKES): architecture, component evaluation and applications. J. Am. Med. Inform. Assoc. 2010;17:507–513. doi: 10.1136/jamia.2009.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soysal E, et al. CLAMP–a toolkit for efficiently building customized clinical natural language processing pipelines. J. Am. Med. Inform. Assoc. 2018;25:331–336. doi: 10.1093/jamia/ocx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubner, Y., Tomasi, C. & Guibas, L. J. A metric for distributions with applications to image databases. In: International Conference on Computer Vision.10.1109/ICCV.1998.710701 (IEEE, 1998).
- 58.Amir S, Coppersmith G, Carvalho P, Silva MJ, Wallace BC. Quantifying mental health from social media with neural user embeddings. Mach. Learn. Healthc. Conf. 2017;68:306–321. [Google Scholar]
- 59.Lee J, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234–1240. doi: 10.1093/bioinformatics/btz682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pennington, J., Socher, R. & Manning, C. D. Glove: Global vectors for word representation. In Conference on Empirical Methods in Natural Language Processing. 1532–1543. 10.3115/v1/D14-1162 (2014).
- 61.Tatman, R. Gender and dialect bias in YouTube’s automatic captions. In Workshop on Ethics in Natural Language Processing 53–59 (ACL, 2017).
- 62.Garg N, Schiebinger L, Jurafsky D, Zou J. Word embeddings quantify 100 years of gender and ethnic stereotypes. Proc. Natl Acad. Sci. USA. 2018;115:E3635–E3644. doi: 10.1073/pnas.1720347115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen JH, Asch SM. Machine learning and prediction in medicine - beyond the peak of inflated expectations. N. Engl. J. Med. 2017;376:2507–2509. doi: 10.1056/NEJMp1702071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emanuel Ezekiel J., Wachter Robert M. Artificial Intelligence in Health Care. JAMA. 2019;321(23):2281. doi: 10.1001/jama.2019.4914. [DOI] [PubMed] [Google Scholar]
- 65.Doraiswamy PM, Blease C, Bodner K. Artificial intelligence and the future of psychiatry: insights from a global physician survey. Artif. Intell. Med. 2020;102:101753. doi: 10.1016/j.artmed.2019.101753. [DOI] [PubMed] [Google Scholar]
- 66.Hsin H, et al. Transforming psychiatry into data-driven medicine with digital measurement tools. NPJ Digit Med. 2018;1:37. doi: 10.1038/s41746-018-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts, L. W. A Clinical Guide to Psychiatric Ethics (American Psychiatric Publication, 2016).
- 68.Martinez-Martin N, Kreitmair K. Ethical issues for direct-to-consumer digital psychotherapy apps: addressing accountability, data protection, and consent. JMIR Ment. Health. 2018;5:e32. doi: 10.2196/mental.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He J, et al. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J. Gen. Intern. Med. 2019 doi: 10.1007/s11606-019-05035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Brien BC. Do you see what i see? Reflections on the relationship between transparency and trust. Acad. Med. 2019;94:757–759. doi: 10.1097/ACM.0000000000002710. [DOI] [PubMed] [Google Scholar]
- 72.Kazdin AE, Rabbitt SM. Novel models for delivering mental health services and reducing the burdens of mental illness. Clin. Psychol. Sci. 2013;1:170–191. [Google Scholar]
- 73.Roberts LW, Chan S, Torous J. New tests, new tools: mobile and connected technologies in advancing psychiatric diagnosis. npj Dig. Med. 2018;1:20176. doi: 10.1038/s41746-017-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The Lancet Digital Health Walking the tightrope of artificial intelligence guidelines in clinical practice. The Lancet Digital Health. 2019;1(3):e100. doi: 10.1016/S2589-7500(19)30063-9. [DOI] [PubMed] [Google Scholar]
- 75.Nebeker C, Torous J, Bartlett Ellis RJ. Building the case for actionable ethics in digital health research supported by artificial intelligence. BMC Med. 2019;17:137. doi: 10.1186/s12916-019-1377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Institute of Mental Health. Strategic Objective 3: Strive for Prevention and Cures. NIMH Strategic Plan for Research.https://www.nimh.nih.gov/about/strategic-planning-reports/strategic-objective-3.shtml (2019).
- 77.Zhou L, et al. Analysis of errors in dictated clinical documents assisted by speech recognition software and professional transcriptionists. JAMA Netw Open. 2018;1:e180530. doi: 10.1001/jamanetworkopen.2018.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jurafsky, D. & Martin, J. H. Speech and Language Processing. (Prentice Hall, 2008).
- 79.Nanjo, H. & Kawahara, T. A new ASR evaluation measure and minimum Bayes-risk decoding for open-domain speech understanding. In: International Conference on Acoustics, Speech, and Signal Processing.10.1109/ICASSP.2005.1415298 (IEEE, 2005).
- 80.Kafle S, Huenerfauth M. Predicting the understandability of imperfect english captions for people who are deaf or hard of hearing. ACM Trans. Access. Comput. 2019;12:7:1–7:32. [Google Scholar]
- 81.Spiccia, C., Augello, A., Pilato, G. & Vassallo, G. Semantic word error rate for sentence similarity. In: International Conference on Semantic Computing. 266–269. 10.1109/ICSC.2016.11 (2016).
- 82.Mishra, T., Ljolje, A. & Gilbert, M. Predicting human perceived accuracy of ASR systems. In: 12th Annual Conference of the International Speech Communication Association. 1945–1948. https://www.iscaspeech.org/archive/interspeech_2011/i11_1945.html(Florence, Italy, 2011).
- 83.Levit, M., Chang, S., Buntschuh, B. & Kibre, N. End-to-end speech recognition accuracy metric for voice-search tasks. In International Conference on Acoustics, Speech and Signal Processing. 5141–5144. 10.1109/ICASSP.2012.6289078 (2012).
- 84.Kiros R, et al. Skip-thought vectors. Adv. Neural Inform. Process. Syst. 2015;28:3294–3302. [Google Scholar]
- 85.Wieting, J., Bansal, M., Gimpel, K. & Livescu, K. Towards universal paraphrastic sentence embeddings. In: Proceedings of the International Conference on Learning Representations, Preprint at: arXiv:1511.08198 (San Juan, Puerto Rico, 2016).
- 86.Shen, D. et al. Baseline needs more love: on simple word-embedding-based models and associated pooling mechanisms. In Annual Meeting of the Association for Computational Linguistics. 440–450. 10.18653/v1/P18-1041 (2018).
- 87.Kreimeyer K, et al. Natural language processing systems for capturing and standardizing unstructured clinical information: a systematic review. J. Biomed. Inform. 2017;73:14–29. doi: 10.1016/j.jbi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267–D270. doi: 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weng W-H, Wagholikar KB, McCray AT, Szolovits P, Chueh HC. Medical subdomain classification of clinical notes using a machine learning-based natural language processing approach. BMC Med. Inform. Decis. Mak. 2017;17:155. doi: 10.1186/s12911-017-0556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hill, F., Cho, K., Jean, S., Devin, C. & Bengio, Y. Embedding word similarity with neural machine translation. In: International Conference on Learning Representations, Preprint at: arXiv:1412.6448 (San Diego, CA, USA, 2015).
- 91.Virtanen P, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset is not publicly available due to patient privacy restrictions, but may be available from the corresponding author on reasonable request.
The code used in this study can be found at:https://github.com/som-shahlab/psych-audio.