Abstract
The discovery and development of multistage antimalarial drugs targeting intra-erythrocytic asexual and sexual Plasmodium falciparum parasites is of utmost importance to achieve the ambitious goal of malaria elimination. Here, we report the validation of naphthylisoquinoline (NIQ) alkaloids and their synthetic analogues as multistage active antimalarial drug candidates. A total of 30 compounds were tested, of which 17 exhibited IC50 values <1 μM against drug-sensitive P. falciparum parasites (NF54 strain); 15 of these retained activity against a panel of drug-resistant strains. These compounds showed low in vitro cytotoxicity against HepG2 cells, with selectivity indices of >10. The tested compounds showed activity in vitro against both early- and late-stage P. falciparum gametocytes while blocking male gamete formation (>70% inhibition of exflagellation at 2 μM). Additionally, five selected compounds were found to have good solubility (≥170 μM in PBS at pH 6.5), while metabolic stability towards human, mouse, and rat microsomes ranged from >90% to >7% after 30 min. Dioncophylline C (2a) emerged as a front runner from the study, displaying activity against both asexual parasites and gametocytes, a lack of cross-resistance to chloroquine, good solubility, and microsomal stability. Overall, this is the first report on the multistage activity of NIQs and their synthetic analogues including gametocytocidal and gametocidal effects induced by this class of compounds.
Keywords: Naphthylisoquinoline alkaloids, Natural products, Hit validation, Plasmodium falciparum, Malaria, Multistage active antimalarial drug candidates
Graphical abstract
Highlights
-
•
Naphthylisoquinolines (NIQs) validated as antimalarial hit candidates.
-
•
First report on transmission-blocking properties of NIQs and analogues.
-
•
15 compounds active across 9 P. falciparum strains, with acceptable RI <10 and SI >10.
-
•
5 compounds show good solubility and microsomal stability.
-
•
Dioncophylline C is the frontrunner antimalarial candidate with multistage activity.
1. Introduction
Malaria continues to be a significant global health burden causing over 228 million clinical cases and 405 000 fatalities annually (WHO, 2019). The World Health Organisation African Region, accounts for over 90% of all of these cases (WHO, 2019). The perpetual challenge posed by the emergence of drug-resistant Plasmodium falciparum strains hampers the fight against malaria and underscores the need to discover and develop new antimalarial drugs (Haldar et al., 2018; Otienoburu et al., 2019). To achieve the goal of malaria elimination, these new drug entities ought to be active against multiple life cycle stages of Plasmodium parasites including the proliferative asexual forms that cause pathology and also should be able to interrupt parasite transmission, both human to mosquito (targeting sexual gametocytes) and mosquito to human (targeting hepatic asexual parasites) (Burrows et al., 2017).
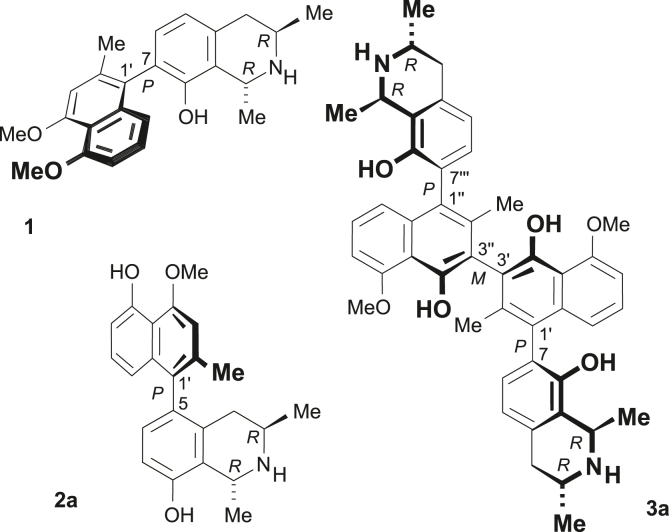
Naphthylisoquinoline (NIQ) alkaloids are secondary metabolites isolated from lianas that belong to the tropical plant families Ancistrocladaceae and Dioncophyllaceae exclusively. All representatives of this class of natural products are built up from a naphthalene moiety and an isoquinoline subunit, which are linked via a biaryl axis. This leads to a broad structural diversity of the compounds, among them C,C- and N,C-coupled NIQs, but also dimeric representatives (Bringmann et al., 2006; Bringmann and Pokorny, 1995; François et al., 1995; Ibrahim and Mohamed, 2015; Lombe et al., 2019). NIQs display outstanding anti-infective activities against parasitic pathogens causing diseases such as leishmaniasis (Bringmann et al., 2006; Ponte-Sucre et al., 2007) and trypanosomiasis (Bringmann et al., 2000). Moreover, these agents have been shown to be potent antiplasmodial compounds with in vitro and even in vivo activity against the exo-erythrocytic (hepatic) stages of Plasmodium (François et al., 1997a, 1997b) and both chloroquine-sensitive and -resistant intra-erythrocytic asexual stage parasites (Bringmann et al., 2006, 2013; François et al., 1994, 1996, 1997a, 1997b). Dioncophylline A (1), dioncophylline C (2a), and jozimine A2 (3a) are promising candidates for more in-depth investigations as possible lead compounds for the development of new antimalarial agents (Fig. 1). Dioncophylline A (1) is the most potent NIQ against exo-erythrocytic stages in vitro (François et al., 1997a, 1997b) whilst dioncophylline C (2a) is efficient both in vitro and in vivo against the murine P. berghei parasite, where parasitaemia was decreased (99.6%) after a single oral dose and a radical cure was achieved after a 4-day Peter's suppressive test (François et al., 1997c). The dimeric alkaloid jozimine A2 (3a) selectively kills asexual P. falciparum in vitro (IC50 of 0.0014 μM) with mammalian cells unaffected even at >11 000-fold increased doses (Bringmann et al., 2013).
Fig. 1.
Structures of dioncophyllines A (1) and C (2a) and of the dimeric alkaloid jozimine A2 (3a).
Despite their outstanding antiplasmodial activities, NIQs are usually difficult to isolate, also due to the rare plant resources. Moreover, in view of their special chirality, and they are complex to synthesise, which leads to limited accessible quantities of these compounds. To mitigate this, quantitative structure-activity relationship (QSAR) guided studies were used to synthesise simplified and easily accessible novel antiplasmodial NIQ analogues (Bringmann et al., 2010). To advance NIQs in a hit-to-lead strategy, we report here the further, mechanistic validation of selected NIQs as well as their synthetic analogues, with activity against both asexual (Target Candidate Profile, TCP-1) and gametocyte stages (TCP-5) of P. falciparum. Additionally, we describe the effect of a selection of these natural products and their analogues on β-haematin formation, their solubility and microsomal stability.
2. Material and methods
2.1. Selection of compounds
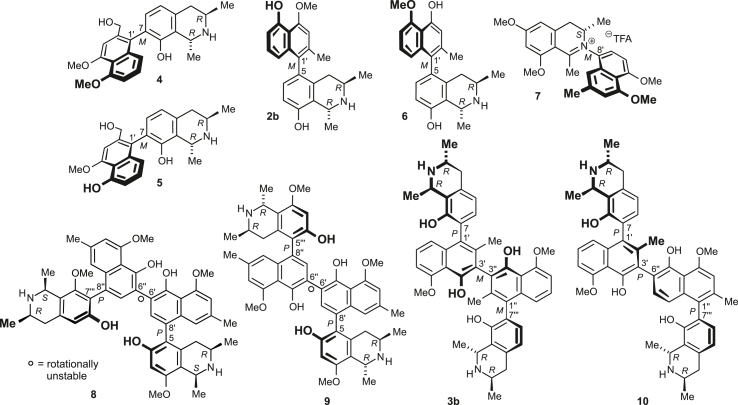
We selected prominent representatives of the different NIQ subclasses based on previously described antiplasmodial activities: dioncophylline A (1), dioncophylline C (2a), and jozimine A2 (3a), and a series of related substances (Fig. 2). While the 7,1′-coupled monomeric C,C-coupled alkaloids habropetaline A (4) and dioncopeltine A (5) are closely related to dioncophylline A (1), 5-epi-dioncophylline C (2b) and its likewise 5,1′-coupled regioisomer 6 are derivatives of dioncophylline C (2a). Compounds 2b and 6 were obtained by total synthesis using a Suzuki-Miyaura coupling approach, as described in detail in the Supplementary Information. As a representative of N,C-coupled NIQs we included ancistrocladinium A (7) into our investigations. Furthermore, four dimeric C,C-coupled analogues were studied more closely, namely ealapasamine C (8) from the Central African liana Ancistrocladus ealaensis, the synthetic 8,8″-O,O-dimethyl analogue 9 of michellamine A, jozibrevine C (3b), and jozilebomine A (10) from A. ileboensis. Two of these compounds, 8 and 9, are 6′,6″-coupled dimers, consisting of either two 5,8′-linked halves as in the case of 9 or a 5,8′- and a 7,8′-coupled naphthylisoquinoline portion as in the case of the ‘mixed’ dimer 8. In contrast to 3b and 10, their central axis is not an additional element of chirality but can rotate freely. The 3′,3″-coupled jozibrevine C (3b) and the 3′,6″-linked jozilebomine A (10) are characterised by the presence of even three consecutive chiral biaryl axes. All these molecules are genuine natural products, except for 2b, 6, and 9, which have not been isolated from plant material yet but were synthesised as derivatives closely related to their lead structures dioncophylline C (2a) for 2b and 6 and, akin to the michellamines, for the synthetic 6′,6″-coupled dimer 9.
Fig. 2.
The investigated natural and synthetic monomeric C,C- and N,C-coupled, and dimeric NIQ alkaloids.
Furthermore, we investigated the simplified analogues 11–28, as structurally derived from NIQs (Fig. 3). Their molecular design was based on QSAR studies and they had previously been shown to exert antiplasmodial activities (Bringmann et al., 2010).
Fig. 3.
Structurally simplified unnatural analogues of NIQs.
2.2. In vitro continuous culture of intra-erythrocytic asexual P. falciparum parasites and gametocytogenesis induction
In vitro culturing of intra-erythrocytic P. falciparum parasites and volunteer blood donation for human erythrocytes holds ethics approval from the University of Pretoria Faculty of Natural and Agricultural Sciences Ethics Committee (EC120821-077). P. falciparum parasite strains and clinical isolates (van der Watt et al., 2018) were cultured to allow intra-erythrocytic asexual development in complete RPMI 1640 culture medium (supplemented with 25 mM HEPES, 20 mM D-glucose, 200 μM hypoxanthine, 0.2% sodium bicarbonate, 24 μg/ml gentamicin, and 0.5% AlbuMAX II) at 37 °C in human erythrocytes (O+) under hypoxic conditions (90% N2, 5% O2, and 5% CO2) (Verlinden et al., 2011). Parasites were synchronised to a >95% ring-stage population using D-sorbitol (5% w/v) (Verlinden et al., 2011).
Gametocytogenesis was induced from synchronised asexual P. falciparum parasites of the transgenic luciferase-expressing strain NF54-Pfs16-GFP-Luc (kind gift from D. A. Fidock, Columbia University, USA) and cultured as previously reported (Reader et al., 2015).
2.3. In vitro assessment of activity against intra-erythrocytic asexual P. falciparum parasites
In vitro activity against asexual P. falciparum parasites was assessed using SYBR Green I fluorescence (Verlinden et al., 2011). All compounds were assessed for auto-fluorescence (Supplementary Information Table S1). Compounds were serially diluted (two-fold) in sterile 96-well plates (100 μl/well) followed by seeding with 100 μl/well of ring-stage P. falciparum parasites suspension (1% parasitaemia, 1% haematocrit), with a 96 h drug pressure at 37 °C. Chloroquine served as a positive control reference drug for inhibition of asexual parasites proliferation. Dose-response curves were obtained with GraphPad Prism (v6), from which half-maximal inhibitory concentration (IC50) values were deduced. Unless stated otherwise, assays were performed in technical triplicate for ≥2 biological repeats. Additionally, the IC50 value for jozimine A2 (3a) was confirmed with a parasite lactate dehydrogenase (pLDH) assay, as described elsewhere (Moyo et al., 2016).
2.4. In vitro cytotoxicity assessment
The lactate dehydrogenase (LDH) leakage assay was used to examine the in vitro cytotoxicity of NIQs and their analogues against cells of a human hepatocellular liver carcinoma cell line (HepG2), seeded at 20000 cells/well (Coertzen et al., 2018). The cytotoxic natural product emetine and the antimalarial drug chloroquine served as references with assay set up and readout as described earlier (Coertzen et al., 2018). Assays were performed in duplicate technical repeats for ≥2 biological repeats. Sigmoidal dose-response curves were plotted using GraphPad Prism (v6), from which the half-maximal effective concentration (EC50) values were determined.
2.5. β-Haematin inhibition assay
Based on a method described earlier (Carter et al., 2010; Sandlin et al., 2011), the detergent mediated assay for β-haematin inhibition was performed using 96-well plates. The pyridine-ferrochrome method developed in the literature was used to measure unreacted haematin (Ncokazi and Egan, 2005). The UV-vis absorbances were monitored at 405 nm on a Thermo Scientific Multiskan GO plate reader (Thermo Scientific, Waltham, MA, USA). Data are from two independent repeats, each performed in technical duplicates. To calculate the IC50 values of each compound, sigmoidal dose-response curves were plotted using GraphPad Prism on means ± SD. (v6).
2.6. In vitro assessment of gametocytocidal activity and inhibition of male gamete exflagellation
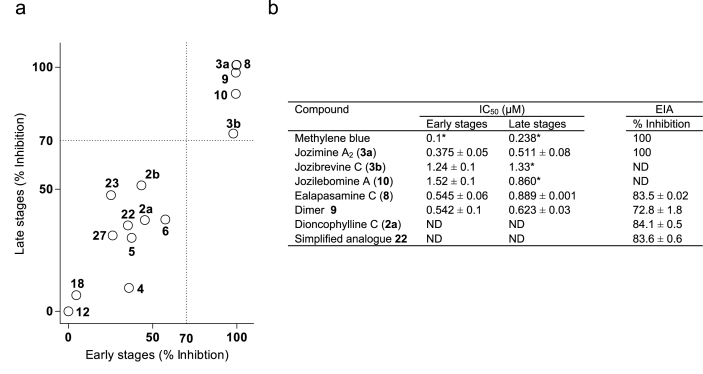
Early- (>85% stage II/III) and late-stage gametocyte (>85% stage IV/V) assays (full dose-response investigations for two independent biological repeats, in technical triplicates) were performed as previously reported (Reader et al., 2015) with a luciferase reporter assay after 48 h drug pressure. To investigate for inhibition of male gamete formation, mature gametocytes (>95% stage V) were placed under 48 h drug pressure (single point screen at 2 μM for two independent biological repeat experiments) and then the exflagellation inhibition assay (EIA) was performed on gametes in a carry-over drug pressure format as described elsewhere (Coetzee et al., 2020). Methylene blue served as a reference compound for inhibition for both in vitro gametocyte viability tests and for EIAs.
2.7. Solubility and metabolic stability of NIQ alkaloids and their analogues
Solubility of the compounds at pH 6.5 was assessed using an adapted miniaturised shake-flask method in 96-well plate format (Abay, 2013; Kerns and Di, 2008). Briefly, 4 μl of a 10 mM stock in 100% DMSO were added to a 96-well plate and evaporated using a GeneVac system. Phosphate-buffered saline (PBS) (pH 6.5) was added to the wells and the plate was incubated for 24 h at 25 °C with shaking. At the end of this incubation, the samples were centrifuged at 3500 g for 15 min and then transferred to an analysis plate. A calibration curve for each sample between 10 and 220 μM in DMSO was prepared and included in the analysis plate. Analysis was then performed by HPLC-DAD and solubility of each sample determined from the corresponding calibration curve.
The metabolic stability was investigated using a single-point assay (Di et al., 2004) optimised for phase I metabolic reactions. NIQ alkaloids and their analogues were incubated at 1 μM in human (mixed gender, Xenotech), rat (male rats IGS, Xenotech), and mouse (male mice CD1, Xenotech) liver microsomes (0.4 mg/ml) for 30 min at 37 °C. Reactions were quenched by adding ice-cold acetonitrile containing internal standard. The samples were then centrifuged and analysed by LC-MS/MS for the disappearance of parent compound. Intrinsic clearance was determined using standard equations (Di et al., 2004; Obach, 1999).
3. Results
3.1. Antiplasmodial activity of NIQs and their synthetic analogues
The in vitro activities of NIQs and their synthetic analogues were first assessed against drug-sensitive intra-erythrocytic asexual P. falciparum (NF54 strain) parasites using an SYBR Green I fluorescence assay (Table 1 and Supplementary Table S1). Additionally, all compounds with sub-micromolar activity against NF54 were screened primarily against the multidrug-resistant W2 strain and further against a panel of seven drug-resistant strains and clinical isolates of African origin (Supplementary Information Tables S2 and S3).
Table 1.
Antiplasmodial and cytotoxic activity of NIQs and their synthetic analogues.
| Compound | Inhibition of parasite proliferation |
Cytotoxicity |
|||
|---|---|---|---|---|---|
| IC50 (μM)a |
EC50 (μM)a |
||||
| NF54 | W2 | RIb | HepG2 | SIc | |
| Chloroquine | 0.013 ± 0.001 | 0.382 ± 0.09 | 29.5 | 37.0 ± 4.5 | 2855.4 |
| Monomeric NIQs | |||||
| Dioncopeltine A (5) | 0.008 ± 0.002 | 0.304 ± 0.06 | 38.1 | 39.0 ± 13.1 | 4890.8 |
| Habropetaline A (4) | 0.015 ± 0.002 | 0.084 ± 0.03 | 5.4 | 45.5 ± 12.1 | 2922.9 |
| Dioncophylline C (2a) | 0.038 ± 0.0002 | 0.112 ± 0.01 | 2.9 | 21.2 ± 4.0 | 552.6 |
| Analogue 2bd | 0.234 ± 0.023 | 0.556 ± 0.11 | 2.4 | >50 | >50 |
| Analogue 6d | 0.042 ± 0.003 | 0.138 ± 0.02 | 3.3 | 25.0 ± 11.6 | 602.4 |
| Dimeric NIQs | |||||
| Jozimine A2 (3a) | 0.151 ± 0.017 | 0.243 ± 0.09 | 1.6 | 4.1 ± 1.2 | 27.4 |
| Jozibrevine C (3b) | 0.265 ± 0.011 | ND | ND | ND | ND |
| Jozilebomine A (10) | 0.167 ± 0.032 | ND | ND | ND | ND |
| Ealapasamine C (8) | 0.063 ± 0.01 | 0.134 ± 0.004 | 2.1 | 13.1 ± 3.5 | 208.6 |
| Dimer 9 | 0.088 ± 0.003 | 0.184 ± 0.06 | 2.1 | 30.5 ± 9.0 | 347.1 |
| Simplified NIQse | |||||
| Compound 12 | 0.990 ± 0.105 | 0.962 ± 0.27 | 1.0 | >50 | >50 |
| Compound 18 | 0.740 ± 0.129 | 0.866 ± 0.37 | 1.2 | >50 | >50 |
| Compound 22 | 0.715 ± 0.084 | 0.238 ± 0.07 | 0.3 | >50 | >50 |
| Compound 23 | 0.517 ± 0.018 | 0.285 ± 0.03 | 0.6 | >50 | >50 |
| Compound 27 | 0.576 ± 0.027 | 0.796 ± 0.38 | 1.4 | 32.3 ± 5.6 | 56.0 |
ND, not determined.
IC50 and EC50 values are from two or more independent biological experiments (n ≥ 2) with data presented as mean ± SEM.
Resistance Index (RI) = IC50 for W2/IC50 for NF54.
Selectivity Index (SI) = EC50 for HepG2/IC50 for NF54.
Derivatives of dioncophylline C (2a).
Molecular design based on QSAR-guided studies.
Among the 30 compounds evaluated, 15 exhibited sub-micromolar potency across all strains and clinical isolates (Table 1 and Supplementary Table S1). Although dioncopeltine A (5) was the most potent NIQ against the NF54 strain (IC50 = 0.008 μM), it showed diminished potency against the multidrug-resistant W2 strain (IC50 = 0.304 μM; Resistance Index (RI) = 38.1). Interestingly, habropetaline A (4) (NF54 IC50 = 0.015 μM), which structurally differs from dioncopeltine A (5) only at the 5′-carbon, by having a methoxy instead of a hydroxy substituent in the naphthalene portion (Fig. 2), retained an acceptable RI of 5.4 against the W2 strain. Dioncophylline C (2a) (IC50 = 0.038 μM) was equipotent (against the NF54 strain) to its closely related derivative 6 (IC50 = 0.042 μM), which differed from 2a by its opposite methoxy-hydroxy pattern in the naphthalene portion and by the configuration at the biaryl axis. However, both compounds had an approximately 5-fold difference in potency compared to 2b (IC50 = 0.234 μM), the actual atropo-diastereomer of 2a.
Regarding the dimeric compounds jozimine A2 (3a), its atropisomer, jozibrevine C (3b), as well as its regioisomer jozilebomine A (10), comparable values were obtained against the NF54 strain, with IC50 of 0.151 μM (IC50 = 0.178 μM, measured by using the pLDH assay), 0.167 μM and 0.265 μM, respectively, while ealapasamine C (8) and the michellamine-type dimer 9 scored even better. Similar results were observed in terms of their activity against the drug-resistant strains.
In agreement with the fact that the simplified analogues were structurally quite different from one another, their IC50 values varied to a high degree, ranging from 0.517 μM to 25.4 μM against the NF54 strain with three synthetic analogues (phenylisoquinoline 12 and the naphthyldihydroisoquinolines 22 and 23), showing no in vitro cross-resistance with chloroquine (Table 1 and Supplementary Information Table S2). Some structural modifications were observed to either enhance or diminish the potency of synthetic analogues (Supplementary Information Fig. S1). For instance, comparing the activities of the naphthyldihydroisoquinolines 19 with 20 and 22 with 24 (Fig. 3) showed that the substitution of a hydroxy function, as in 20 (IC50 = 1.63 μM) and 22 (IC50 = 0.715 μM), with a methoxy group, as in 19 (IC50 = 14.5) and 24 (IC50 = 6.09 μM), was accompanied by a loss in activity (>8.5-fold variation in IC50 values). The methyl substituent in para position to the biaryl axis of 12 (IC50 = 0.99 μM) led to a > 2-fold decrease in IC50 value compared to compounds in which substituents were either a hydrogen (as in 11; IC50 = 5.09 μM), a trifluoromethyl group (as in 14; IC50 = 4 μM) or a chlorine substituent (as for 16; IC50 = 21.9 μM). Placement of a chlorine substituent in either the para (as for 16) or the meta (as for 17; IC50 = 1.93 μM) position resulted in significantly different potency levels (>10-fold difference in IC50 values).
All compounds with sub-micromolar activity against asexual P. falciparum parasites exhibited minimal to no cytotoxicity against HepG2 cells (selectivity indices (SI) > 10) with five of the structurally simplified analogues inactive even at 50 μM (Table 1).
3.2. Mechanistic studies
Previous investigations wherein dioncophylline C (2a) had been shown to form a complex with haem using model studies (Schwedhelm et al., 2007), had led to the hypothesis that inhibition of haemozoin formation might be responsible for part of its activity. To investigate this assumption, inhibition of β-haematin formation (synthetic haemozoin) was examined for dioncophylline C (2a) and its regioisomer 6, which should also hit the same target as 2a, as assumed based on its structural similarity and its activity profile against intra-erythrocytic asexual parasites. Indeed, both compounds (IC50 = 19.9 ± 3.1 μM for 2a and 46.6 ± 4.7 μM for 6) inhibited β-haematin formation with a potency that was quite similar to that of the reference compound used in the assay, chloroquine (IC50 = 13.9 ± 1.09 μM). In view of the cross-resistance of dioncopeltine A (5) to chloroquine, we furthermore hypothesised that it could also inhibit β-haematin formation, however 5 exhibited an IC50 value of 134.1 ± 2.4 μM, i.e. above the cut-off threshold of IC50 < 100 μM, which excluded haemozoin formation as its target.
3.3. Inhibition of viability of gametocyte stages and EIAs
Driven by the goal to identify dual-active NIQs and synthetic analogues, we assessed the transmission-blocking activity of the 15 intra-erythrocytic asexual stage potent compounds by screening them in vitro against early- (>85% stage II/III) and late-stage (>85% stage IV/V) P. falciparum gametocytes (Table 1). Interestingly, of the 15 compounds examined, only the large-sized dimeric compounds jozimine A2 (3a), jozibrevine C (3b), ealapasamine C (8), dimer 9, and jozilebomine A (10), consistently showed >70% inhibition of viability of both gametocyte stages at 5 μM (Fig. 4a), with 3a, 8, and 9 displaying appreciable sub-micromolar in vitro gametocytocidal activities against both gametocyte stages (Fig. 4b). Among the NIQ dimers, jozimine A2 (3a) was the most active gametocytocidal compound with an IC50 value of 0.3–0.5 μM (Fig. 4b).
Fig. 4.
In vitro gametocytocidal and gametocidal activity of NIQs and their synthetic analogues. The luciferase reporter line NF54-Pfs16-GFP-Luc was used to assess in vitro early- (>85% stage II/III) and late-stage (>85% stage IV/V) gametocytocidal activity (a) single-point screen (5 μM, n = 1, gametocytaemia of 2% at final haematocrit of 1%) and (b) full dose-response (n = 2, mean ± SEM, *n = 1, gametocytaemia of 2% at final haematocrit of 1%) investigations of compounds for 48 h incubation period and single-point screen (2 μM) EIA, n = 2, mean ± SEM. Methylene blue served as a reference compound inhibiting >90% in vitro gametocyte (both early and late stages) viability at 5 μM. ND, not determined.
To examine if the observed inhibition of gametocyte viability was due to a blocking of male gamete formation, the three dimeric compounds, jozimine A2 (3a), ealapasamine C (8), and dimer 9, that had been shown to have sub-micromolar activity against both early- and late-stage gametocytes, were assessed in the EIA. For this analysis we additionally included dioncophylline C (2a) as the most potent gametocytocidal monomeric NIQ (highly active against both early- and late-stage gametocytes) and the simplified synthetic analogue 22, to examine if their observed moderate potency against gametocytes could be due to compromised functional viability of mature male gametocytes. Encouragingly, all five compounds, 2a, 3a, 8, 9, and 22 (at 2 μM), inhibited male gamete formation at more than 70%, with jozimine A2 (3a) being the most active one, completely compromising male gametogenesis (Fig. 4b). Taken together, our data point to dimeric compounds being multistage active targeting asexual parasites, gametocytes, and male gametes. On the other hand, the simplified analogues were selective towards asexual parasites and male gametes, with moderate activity against gametocytes.
3.4. Solubility and microsomal stability
For solubility and microsomal stability assays we prioritised dioncophylline C (2a) as the front runner compound based on its potency against asexual parasites, both drug-sensitive and resistant strains, and gamete activity. To investigate if stereochemical features have an influence on either solubility or microsomal stability of this class of compounds, we included its atropisomer 2b for comparison of its data to that of its parent compound, dioncophylline C (2a). Additionally, to obtain a generalised overview on the solubility and microsomal stability of NIQs and their synthetic analogues, we selected a batch comprising a monomeric NIQ, dioncopeltine A (5), a dimer, compound 9, and a synthetic analogue, compound 12, thus, a top representative of each subclass. All compounds selected demonstrated good kinetic solubility in PBS at pH 6.5 (Table 2). Moreover, the five tested compounds, 2a, 2b, 5, 9, and 12, showed divergent stabilities towards different microsomes. Dioncophylline C (2a) and the michellamine-type dimer 9 displayed good microsomal stability when incubated with human, mouse, or rat microsomes, as observed by minimal clearance of the parent compounds. The atropo-diastereomer 2b of dioncophylline C (2a) and the structurally simplified analogue 12 displayed poor stability on exposure to rat and mouse microsomes with high rates of in vitro intrinsic clearance, while 5 was unstable only against mouse microsomes (Table 2).
Table 2.
Solubility and microsomal stability of NIQs and their analogues.
| Compound | Solubility |
% Remaining after 30 min |
Microsomal clearance (μl/min/mg) |
||||
|---|---|---|---|---|---|---|---|
| PBS (pH 6.5) | Human | Mouse | Rat | Human | Mouse | Rat | |
| Reserpinea | <5 | – | – | – | – | – | – |
| Hydrocortisonea | 190 | – | – | – | – | – | – |
| Midazolama | – | <7 | <7 | <7 | >250 | >250 | >250 |
| Propranolola | – | 63 | 32 | <7 | 39.6 | 93.9 | >250 |
| MMV390048a | – | 92 | >99 | 97 | <11.6 | <11.6 | <11.6 |
| Dioncophylline C (2a) | 170 | 94 | 95 | 95 | <11.6 | <11.6 | <11.6 |
| Dioncopeltine A (5) | 200 | 98 | <7 | >99 | <11.6 | >250 | <11.6 |
| Atropisomer 2b | 180 | 83 | 32 | 36 | 15.4 | 95.1 | 86.6 |
| Synthetic dimer 9 | 200 | 98 | 99 | 96 | <11.6 | <11.6 | <11.6 |
| Simplified analogue 12 | 180 | 71 | <7 | <7 | 28.2 | >250 | >250 |
Reference compounds used in respective assays.
4. Discussion
In this paper, we provide a first report on the multistage potency of NIQs and their synthetic analogues against intra-erythrocytic asexual and sexual stage P. falciparum parasites in vitro. Their activity profile fundamentally marks this class of compounds as chemical scaffolds with potential for the development of multistage-active antimalarial drugs that could emerge as a potent tool in the endeavour to eliminate malaria. Currently, no available antimalarial drug has this activity profile, since most such agents primarily target intra-erythrocytic asexual P. falciparum parasites (Delves et al., 2012). Our data show stage-specific sensitivity to the compounds examined in the study with asexual-stage Plasmodium parasites being the most potently affected. The structurally unique dimeric NIQs, particularly jozimine A2 (3a), were pharmacologically efficient being highly active across all three distinct stages examined in the study. However, undesirable physicochemical properties, including high molecular weight (>500 g/mol) and clogP (ranging between 7.36 and 7.45), could potentially limit their progress to become lead antimalarial candidates. On the other hand, the monomeric NIQs and simplified analogues were distinctively selective towards asexual parasites and male gametes with two compounds, dioncophylline C (2a) and 22, emerging as validated multistage-active (TCP-1 and TCP-5) hits.
Five compounds, 2a, 3a, 8, 9, and 22, displayed notable activity in blocking exflagellation of male gametes in vitro. This is the first report detailing the biological profile of this chemotype, making exploration of NIQs and their analogues, as a starting point for the development of gametocidal drugs for consideration as TCP-5 candidates, a rewarding goal. As the EIA correlates well with the standard membrane feeding assay (Colmenarejo et al., 2018), which is the gold standard assay for confirmation of transmission blocking of drugs, these results further enhance the credentials of NIQs and their simplified analogues as transmission-blocking agents. It was surprising to note that natural dioncophylline C (2a) and the unnatural naphthyldihydroisoquinoline 22 both inhibited male gamete exflagellation, while demonstrating moderate gametocytocidal activity. However, this stage-specific profile is not new with the antifolates pyrimethamine and cycloguanil, which are known to have a similar activity profile (Ruecker et al., 2014). In the case of dioncophylline C (2a) and the simplified analogue 22, inhibition of exflagellation may be explained by these compounds either having a sterilising or a contraceptive effect on mature gametocytes resulting in their compromised functional viability, which has been described for other compounds earlier (Ruecker et al., 2014). While the dimeric NIQs jozimine A2 (3a), ealapasamine C (8), and the michellamine-type dimer 9 were the most potent agents against gametocytes, these compounds were even 2.4 to 14-fold more active against intra-erythrocytic asexual stages of P. falciparum, demonstrating selectivity towards the aforementioned proliferative parasite stages.
The divergent stage-specific profiles of structurally related compounds investigated in this study raised pertinent questions from a drug-target perspective concerning the putative mode of action (MoA) of the most promising monomeric agent, dioncophylline C (2a). While our data strongly point to an inhibition of haemozoin formation as a possible MoA of this compound, given the β-haematin inhibition assay results, further investigations still need to confirm this activity intracellularly. Given the activity of this compound against male gametes (stages of the parasites devoid of the haem degradation biological process), our data hint at an inhibition of haemozoin formation as being only partly contributing to the overall MoA of dioncophylline C (2a). On the other hand, it is remarkable that dioncopeltine A (5), which had a resistance profile similar to that of chloroquine, did not inhibit β-haematin formation, suggesting possible different targets for this compound within the parasite. Indeed, since habropetaline A (4), although structurally closely related to dioncopeltine A (5), is more active to chloroquine-resistant parasites, it would be interesting to test its activity in the β-haematin assay as well. If it also lacked activity similar to dioncopeltine A (5), it would point to a different MoA of these compounds and that the chloroquine-resistance profile for dioncopeltine A (5) is not linked.
Although the antiplasmodial activity of NIQs had been demonstrated previously, from our data the activities of the most notable compounds investigated including dioncophylline C (2a), habropetaline A (4), dioncopeltine A (5), as well as the simplified analogues 22, 23, and 27, matched quite well those obtained in previous studies (Bringmann et al., 2003, 2006, 2010, 2013; François et al., 1995, 1996, 1997b, 1997c; Lombe et al., 2019; Tshitenge et al., 2017). A major discrepancy (ca. 100-fold difference in the observed IC50 values) was noted for the dimeric alkaloid jozimine A2 (3a). Interestingly, the activity of ealapasamine C (8) against the NF54 strain was consistent to that from an earlier investigation (Tshitenge et al., 2017), while strikingly a 22-fold difference in the IC50 values measured against the K1 strain was observed. These variances could be attributed to differences in assay readouts and strains used in the respective studies.
Although bearing the typical functional groups of their highly potent NIQ parent compounds, generally most of the simplified analogues were less potent. Only a handful of them showed marked activity with IC50 <1 μM against asexual Plasmodium parasites, with the achiral derivatives emerging as the most active. However, a most encouraging activity profile of the simplified analogues was their low-to-no cross resistance against multidrug-resistant strains, suggesting a different MoA, with minimal to no cytotoxicity observed against cells of the mammalian cell line. Furthermore, just like the monomeric NIQs, the simplified analogues have a predicted lipophilicity of >4.2, which compares fairly well to that of preclinical and clinical antimalarial candidates (average value of 4.0) currently in development. This, in theory, implied that the compounds meet the new target product profiles of desired antimalarial drugs with particular reference to long half-life times (Charman et al., 2020). A high lipophilicity provides the option of having long half-lives, enabling them to be used in a single-dose regimen (Charman et al., 2020).
It is quite striking to note the impact of axial chirality on the microsomal stability differences between dioncophylline C (2a) and its metabolically less stable (M)-configured synthetic atropo-diastereomer, compound 2b. This finding is in agreement with previous investigation where atropisomers were shown to have varied metabolic stability (Hasegawa et al., 2017; Wang et al., 2017), although also contrary results have likewise been observed (Wang et al., 2017). In a similar way, atropo-diastereomeric NIQ alkaloids had previously shown substantially different antiplasmodial activities (Bringmann et al., 2013; Fayez et al., 2019).
5. Conclusion
In the present study, NIQs have been - for the first time - validated as hit antiplasmodial compounds with in vitro activity against both early- and late-stage gametocytes of P. falciparum while also blocking exflagellation of male gametes. This is a significant finding as it implies that these compounds are active across all stages of the malaria parasite found in the human host, marking them as potential multistage-active antimalarial agents. In view of the goal of malaria elimination, this class of compounds could certainly play a vital role and as such deserves more attention. Among the investigated compounds, dioncophylline C (2a) was the most promising candidate for further exploration, as it consistently maintained good activity across all stages while also demonstrating good solubility and metabolic stability.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgements
We thank Dr. Raina Seupel, Dr. Jun Li, Dr. Christine Schies, Prof. Dr. Dieudonné Tshitenge, and Dr. Sebastian Bischof for providing substances tested in this study. The UP ISMC acknowledges the SAMRC as Collaborating Centre for Malaria Research. This work was funded by the South African Research Chairs Initiative of the Department of Science and Technology, administered through the South African National Research Foundation to LMB (UID 84627), and supported by the SFB 630 “Agents against Infectious Diseases” funded by the Deutsche Forschungsgesellschaft (DFG) to GB. ACCdS and TJE acknowledge support of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under grant number R01AI143521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.05.003.
Contributor Information
Gerhard Bringmann, Email: bringman@chemie.uni-wuerzburg.de.
Lyn-Marie Birkholtz, Email: lbirkholtz@up.ac.za.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abay S. Blocking malaria transmission to Anopheles mosquitoes using artemisinin derivatives and primaquine: a systematic review and meta-analysis. Parasites Vectors. 2013;6:278. doi: 10.1186/1756-3305-6-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann G., Bischof S.K., Müller S., Gulder T., Winter C., Stich A., Moll H., Kaiser M., Brun R., Dreher J., Baumann K. QSAR guided synthesis of simplified antiplasmodial analogs of naphthylisoquinoline alkaloids. Eur. J. Med. Chem. 2010;45:5370–5383. doi: 10.1016/j.ejmech.2010.08.062. [DOI] [PubMed] [Google Scholar]
- Bringmann G., Hamm A., Günther C., Michel M., Brun R., Mudogo V. Ancistroealaines A and B, two new bioactive naphthylisoquinolines, and related naphthoic acids from Ancistrocladus ealaensis. J. Nat. Prod. 2000;63:1465–1470. doi: 10.1021/np000247+. [DOI] [PubMed] [Google Scholar]
- Bringmann G., Kajahn I., Reichert M., Pedersen S.E.H., Faber J.H., Gulder T., Brun R., Christensen S.B., Ponte-Sucre A., Moll H., Heubl G., Mudogo V. Ancistrocladinium A and B, the first N,C-coupled naphthyldihydroisoquinoline alkaloids, from a Congolese Ancistrocladus species. J. Org. Chem. 2006;71:9348–9356. doi: 10.1021/jo061626w. [DOI] [PubMed] [Google Scholar]
- Bringmann G., Messer K., Schwöbel B., Brun R., Aké Assi L. Habropetaline A, an antimalarial naphthylisoquinoline alkaloid from Triphyophyllum peltatum. Phytochemistry. 2003;62:345–349. doi: 10.1016/s0031-9422(02)00547-2. [DOI] [PubMed] [Google Scholar]
- Bringmann G., Pokorny F. The naphthylisoquinoline alkaloids. In: Cordell G.A., editor. In: the Alkaloids: Chemistry and Pharmacology. 1995. pp. 127–271. New York. [Google Scholar]
- Bringmann G., Zhang G., Büttner T., Bauckmann G., Kupfer T., Braunschweig H., Brun R., Mudogo V. Jozimine A2: the first dimeric Dioncophyllaceae‐type naphthylisoquinoline alkaloid, with three chiral axes and high antiplasmodial activity. Chem. Eur J. 2013;19:916–923. doi: 10.1002/chem.201202755. [DOI] [PubMed] [Google Scholar]
- Burrows J.N., Duparc S., Gutteridge W.E., van Huijsduijnen R.H., Kaszubska W., Macintyre F., Mazzuri S., Möhrle J.J., Wells T.N. New developments in anti-malarial target candidate and product profiles. Malar. J. 2017;16:26. doi: 10.1186/s12936-016-1675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M.D., Phelan V.V., Sandlin R.D., Bachmann B.O., Wright D.W. Lipophilic mediated assays for beta-hematin inhibitors. Comb. Chem. High Throughput Screen. 2010;13:285–292. doi: 10.2174/138620710790980496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman S.A., Andreu A., Barker H., Blundell S., Campbell A., Campbell M., Chen G., Chiu F.C.K., Crighton E., Katneni K., Morizzi J., Patil R., Pham T., Ryan E., Saunders J., Shackleford D.M., White K.L., Almond L., Dickins M., Smith D.A., Moehrle J.J., Burrows J.N., Abla N. An in vitro toolbox to accelerate anti-malarial drug discovery and development. Malar. J. 2020;19:1. doi: 10.1186/s12936-019-3075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coertzen D., Reader J., van der Watt M., Nondaba S.H., Gibhard L., Wiesner L., Smith P., D'Alessandro S., Taramelli D., Wong H.N., du Preez J.L., Wu R.W.K., Birkholtz L.-M., Haynes R.K. Artemisone and artemiside are potent panreactive antimalarial agents that also synergizeredox imbalance in Plasmodium falciparum transmissible gametocyte stages. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02214-17. e02214-02217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee N., von Grüning H., Opperman D., van der Watt M., Reader J., Birkholtz L.-M. Epigenetic inhibitors target multiple stages of Plasmodium falciparum parasites. Sci. Rep. 2020;10:2355. doi: 10.1038/s41598-020-59298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenarejo G., Lozano S., González-Cortés C., Calvo D., Sanchez-Garcia J., Matilla J.-L.P., Leroy D., Rodrigues J. Predicting transmission blocking potential of anti-malarial compounds in the mosquito feeding assay using Plasmodium falciparum male gamete inhibition assay. Sci. Rep. 2018;8:7764. doi: 10.1038/s41598-018-26125-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves M., Plouffe D., Scheurer C., Meister S., Wittlin S., Winzeler E.A., Sinden R.E., Leroy D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L., Kerns E.H., Gao N., Li S.Q., Huang Y., Bourassa J.L., Huryn D.M. Experimental design on single‐time‐point high‐throughput microsomal stability assay. J. Pharmaceut. Sci. 2004;93:1537–1544. doi: 10.1002/jps.20076. [DOI] [PubMed] [Google Scholar]
- Fayez S., Li J., Feineis D., Aké Assi L., Kaiser M., Brun R., Anany M.A., Wajant H., Bringmann G. A near-complete series of four atropisomeric jozimine A2-type naphthylisoquinoline dimers with antiplasmodial and cytotoxic activities and related alkaloids from Ancistrocladus abbreviatus. J. Nat. Prod. 2019;82:3033–3046. doi: 10.1021/acs.jnatprod.9b00589. [DOI] [PubMed] [Google Scholar]
- François G., Bringmann G., Dochez C., Schneider C., Timperman G., Aké Assi L. Activities of extracts and naphthylisoquinoline alkaloids from Triphyophyllum peltatum, Ancistrocladus abbreviatus and Ancistrocladus barteri against Plasmodium berghei (Anka strain) in vitro. J. Ethnopharmacol. 1995;46:115–120. doi: 10.1016/0378-8741(95)01240-e. [DOI] [PubMed] [Google Scholar]
- François G., Bringmann G., Phillipson J.D., Aké Assi L., Dochez C., Rübenacker M., Schneider C., Wéry M., Warhurst D.C., Kirby G.C. Activity of extracts and naphthylisoquinoline alkaloids from Triphyophyllum peltatum, Ancistrocladus abbreviatus and A. barteri against Plasmodium falciparum in vitro. Phytochemistry. 1994;35:1461–1464. [Google Scholar]
- François G., Steenackers T., Timperman G., Aké Assi L., Haller R., Bär S., Isahakia M., Robertson S., Zhao C., De Souza N. Retarded development of exoerythrocytic stages of the rodent malaria parasite Plasmodium berghei in human hepatoma cells by extracts from Dioncophyllaceae and Ancistrocladaceae species. Int. J. Parasitol. 1997;27:29–32. doi: 10.1016/s0020-7519(96)00171-3. [DOI] [PubMed] [Google Scholar]
- François G., Timperman G., Eling W., Aké Assi L., Holenz J., Bringmann G. Naphthylisoquinoline alkaloids against malaria: evaluation of the curative potentials of dioncophylline C and dioncopeltine A against Plasmodium berghei in vivo. Antimicrob. Agents Chemother. 1997;41:2533–2539. doi: 10.1128/aac.41.11.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François G., Timperman G., Holenz J., Aké Assi L., Geuder T., Maes L., Dubois J., Hanocq M., Bringmann G. Naphthylisoquinoline alkaloids exhibit strong growth-inhibiting activities against Plasmodium falciparum and P. berghei in vitro -structure-activity relationships of dioncophylline C. Ann. Trop. Med. Parasitol. 1996;90:115–123. doi: 10.1080/00034983.1996.11813035. [DOI] [PubMed] [Google Scholar]
- François G., Timperman G., Steenackers T., Aké Assi L., Holenz J., Bringmann G. In vitro inhibition of liver forms of the rodent malaria parasite Plasmodium berghei by naphthylisoquinoline alkaloids – structure-activity relationships of dioncophyllines A and C and ancistrocladine. Parasitol. Res. 1997;83:673–679. doi: 10.1007/s004360050318. [DOI] [PubMed] [Google Scholar]
- Haldar K., Bhattacharjee S., Safeukui I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 2018;16:156–170. doi: 10.1038/nrmicro.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa F., Kawamura K., Tsuchikawa H., Murata M. Stable C–N axial chirality in 1-aryluracil scaffold and differences in in vitro metabolic clearance between atropisomers of PDE4 inhibitor. Bioorg. Med. Chem. 2017;25:4506–4511. doi: 10.1016/j.bmc.2017.06.042. [DOI] [PubMed] [Google Scholar]
- Ibrahim S.R., Mohamed G.A. Naphthylisoquinoline alkaloids potential drug leads. Fitoterapia. 2015;106:194–225. doi: 10.1016/j.fitote.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Kerns E.H., Di L. Chapter 25 - solubility methods. In: Kerns E.H., Di L., editors. Drug-like Properties: Concepts, Structure Design and Methods. Academic Press; San Diego: 2008. pp. 276–286. [Google Scholar]
- Lombe B.K., Feineis D., Bringmann G. Dimeric naphthylisoquinoline alkaloids: polyketide-derived axially chiral bioactive quateraryls. Nat. Prod. Rep. 2019;36:1513–1545. doi: 10.1039/c9np00024k. [DOI] [PubMed] [Google Scholar]
- Moyo P., Botha M.E., Nondaba S., Niemand J., Maharaj V.J., Eloff J.N., Louw A.I., Birkholtz L. In vitro inhibition of Plasmodium falciparum early and late stage gametocyte viability by extracts from eight traditionally used South African plant species. J. Ethnopharmacol. 2016;185:235–242. doi: 10.1016/j.jep.2016.03.036. [DOI] [PubMed] [Google Scholar]
- Ncokazi K.K., Egan T.J. A colorimetric high-throughput β-hematin inhibition screening assay for use in the search for antimalarial compounds. Anal. Biochem. 2005;338:306–319. doi: 10.1016/j.ab.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Obach R.S. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]
- Otienoburu S.D., Suay I., Garcia S., Thomas N.V., Srisutham S., Björkman A., Humphreys G.S. An online mapping database of molecular markers of drug resistance in Plasmodium falciparum: the ACT Partner Drug Molecular Surveyor. Malar. J. 2019;18:12. doi: 10.1186/s12936-019-2645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte-Sucre A., Faber J.H., Gulder T., Kajahn I., Pedersen S.E.H., Schultheis M., Bringmann G., Moll H. Activities of naphthylisoquinoline alkaloids and synthetic analogs against Leishmania major. Antimicrob. Agents Chemother. 2007;51:188–194. doi: 10.1128/AAC.00936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader J., Botha M., Theron A., Lauterbach S.B., Rossouw C., Engelbrecht D., Wepener M., Smit A., Leroy D., Mancama D. Nowhere to hide: interrogating different metabolic parameters of Plasmodium falciparum gametocytes in a transmission blocking drug discovery pipeline towards malaria elimination. Malar. J. 2015;14:1. doi: 10.1186/s12936-015-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruecker A., Mathias D.K., Straschil U., Churcher T.S., Dinglasan R.R., Leroy D., Sinden R.E., Delves M.J. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob. Agents Chemother. 2014;58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandlin R.D., Carter M.D., Lee P.J., Auschwitz J.M., Leed S.E., Johnson J.D., Wright D.W. Use of the NP-40 detergent-mediated assay in discovery of inhibitors of β-hematin crystallization. Antimicrob. Agents Chemother. 2011;55:3363. doi: 10.1128/AAC.00121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedhelm K.T., Horstmann M, Faber J.F., Reichert Y, Bringmann G, Faber C. The Novel Antimalarial Compound Dioncophylline C Forms a Complex with Heme in Solution. ChemMedChem. 2007;2:541–548. doi: 10.1002/cmdc.200600263. [DOI] [PubMed] [Google Scholar]
- Tshitenge D.T., Feineis D., Mudogo V., Kaiser M., Brun R., Bringmann G. Antiplasmodial ealapasamines A-C,‘mixed’ naphthylisoquinoline dimers from the Central African liana Ancistrocladus ealaensis. Sci. Rep. 2017;7:5767. doi: 10.1038/s41598-017-05719-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Watt M.E., Reader J., Churchyard A., Nondaba S.H., Lauterbach S.B., Niemand J., Abayomi S., van Biljon R.A., Connacher J.I., van Wyk R.D. Potent Plasmodium falciparum gametocytocidal compounds identified by exploring the kinase inhibitor chemical space for dual active antimalarials. J. Antimicrob. Chemother. 2018;73:1279–1290. doi: 10.1093/jac/dky008. [DOI] [PubMed] [Google Scholar]
- Verlinden B.K., Niemand J., Snyman J., Sharma S.K., Beattie R.J., Woster P.M., Birkholtz L.-M. Discovery of novel alkylated (bis) urea and (bis) thiourea polyamine analogues with potent antimalarial activities. J. Med. Chem. 2011;54:6624–6633. doi: 10.1021/jm200463z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zeng W., Li S., Shen L., Gu Z., Zhang Y., Li J., Chen S., Jia X. Discovery and assessment of atropisomers of (±)-lesinurad. ACS Med. Chem. Lett. 2017;8:299–303. doi: 10.1021/acsmedchemlett.6b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2019. World Malaria Report 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.