Abstract
Background
Nerve regeneration after an injury should occur in a timely fashion for function to be restored. Current methods cannot monitor regeneration prior to muscle reinnervation. Diffusion tensor imaging has been previously shown to provide quantitative indices after nerve recovery. The goal of this study was to validate the use of this technology following nerve injury via a series of rat sciatic nerve injury/repair studies.
Methods
Sprague-Dawley rats were prospectively divided by procedure (sham, crush, cut/repair), and time points (1, 2, 4, and 12 weeks post-surgery). At the appropriate time point, each animal was euthanized, the sciatic nerve harvested and fixed. Data were obtained using a 7T Magnetic Resonance Imaging system. For validation, our findings were compared to behavioral testing (foot fault asymmetry and sciatic function index) and cross-sectional axonal counting of toluidine blue stained sections examined under light microscopy.
Results
Sixty-three rats were divided into 3 treatment groups (sham=21, crush=23, cut/repair=19). Fractional anisotropy was able to differentiate between recovery following sham, crush, and cut/repair injuries as early as 2 weeks (p<0.05), with more accurate differentiation thereafter. More importantly, the difference in anisotropy between distal and proximal regions (ΔFA) recognized animals with successful and failed recoveries according to behavioral analysis, especially at 12 weeks. In addition, diffusion tension imaging based tractography provided a visual representation of nerve continuity in all treatment groups.
Conclusions
Diffuse tensor imaging is an objective and noninvasive tool for monitoring nerve regeneration, which could facilitate earlier detection of failed repairs to potentially help improve outcomes.
INTRODUCTION
In the Unites States, the prevalence of traumatic peripheral nerve injury in level I trauma centers is about 2.8%.1 This can result from penetrating injuries, lacerations, stretch, ischemia and/or crush mechanisms.2 Primary repair after traumatic peripheral nerve injury is recommended when the severed nerve endings can be approximated without tension, representing approximately half of all these injuries.3,4,5 Regardless of the quality of nerve repair, regeneration is a protracted process that occurs at a rate of 1mm/day, taking months to reinnervate target muscles.6 Previous studies claim that reinnervation has to be completed before 16 months post-injury to avoid irreversible muscular atrophy.7, 8 Additionally, not all interventions are successful upon the first attempt, and a secondary repair may be required.9
Currently, the gold-standard test to assess nerve repairs for extremities is electromyography and nerve conduction studies.10 In addition to being invasive and uncomfortable for patients, perhaps the most important limitation of these two modalities is that they can only detect valuable motor information when the nerve axons reach their muscular end plate. This has critical implications in recovery of patients since it obligates physicians to adopt a “watch and wait” approach, leading to significant delays in verifying the diagnosis which can result in irreversible atrophy. Consequently, a technique which allows early accurate evaluation of the nerve recovery that would help to make a timely decision to salvage the repair could lead to improve outcomes by allowing earlier revision coaptation, nerve grafting, and/or nerve transfers.
Diffusion tensor imaging (DTI) is a magnetic resonance modality that provides information about tissues by measuring the random movement of water molecules at a microstructural level.11 Water diffusion is isotropic (i.e.,the mean-squared displacement of water molecules is the same in all directions) when there are no constrained boundaries.7,12 When water is constrained by tissue structures (e.g.,packed cylindrical axons), the apparent diffusivity becomes direction-dependent or anisotropic. Peripheral nerve studies indicated that apparent diffusivity is lower perpendicular to axons than parallel to them due to the arrangement of nerve fibers.7,8,12 Previous authors have shown that fractional anisotropy may be a viable biomarker of nerve degeneration and regeneration.13–18 Although previous DTI studies have predicted the severity of nerve injury, no studies to date, have quantified the ability of this technology to stratify successful or failed surgical interventions. 16 In this study, high-resolution ex-vivo imaging of rat sciatic nerves following different injuries at different time points, are validated to behavioral and histological data to demonstrate the techniques’ ability to follow nerve recover after repair. Interestingly, the technique can detect distal nerve injury/ lack of re-innervation at early time points which might help surgeons determine which nerve repairs will require reinnervation at an earlier time point.
METHODS
Experimental Design
All procedures were reviewed and approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee, which adhere to the Guide for Care and Use of Laboratory Animals to minimize pain and suffering. Sprague-Dawley female rats, between the ages of 8 to 12 weeks, were prospectively divided into groups according to their surgical intervention. Animals were euthanized at different time points after each intervention (1, 2, 4, and 12 weeks), at which point ex-vivo scanning was performed. Behavioral tests with foot fault asymmetry score and the sciatic function index were performed before each surgical intervention, on postoperative day 3, and weekly thereafter until each animal s end point.
Sciatic Nerve Injury
Isoflurane 2% at a dose of 3mL/min was used for induction and maintenance of general anesthesia. Hypothermia was avoided during the procedure. A single surgeon (MN) performed all the procedures. The surgical area was prepped, and a 3 cm longitudinal incision was made from the ischial notch down distally, parallel to the femur. Using a split muscle technique of biceps femoris, the sciatic nerve was identified. The muscle was transected, and the nerve was freed proximally and distally up to its trifurcation. At this point, each nerve was treated with sham, crush, or cut/repair. The sham consisted on no further intervention. An area 1 cm proximal from the trifurcation was selected. For the crush group, a Hemostat was applied to the nerve for 10 seconds. For the cut/repair group, the nerve was completely transected and immediately repaired in an end-to-end fashion with interrupted epineurial 9–0 nylon sutures (Ethicon, Somerville, NJ). All wound was irrigated thoroughly and closed in 2 layers using 5–0 monocryl suture (Ethicon, Somerville, NJ). The animals were allowed to recover. Upon reaching their time points, each rat was euthanized with an intra-cardiac dose of Euthasol (Virbac AH, Fort Worth, Texas) at a dose of 120mg/kg and the nerves were harvested to undergo imaging.
Tissue Sample Preparation
All nerve samples were washed for at least 1 week in Phosphate-buffered saline to remove excess fixation and then immersed in 1mM Gadolinium diethylene triamine pentaacetic acid (Magnevist, Bayer healthCare) at 4°C for 36 hours before imaging to reduce spin-lattice relaxation (and corresponding scan) times. Prior to imaging, all samples were trimmed to 1cm in length, with crush and cut regions at the center of each nerve segment. These were placed in 1.75mm glass capillary tubes filled with Fomblin to prevent dehydration without disrupting the magnetic resonance signal. Six nerves were imaged simultaneously in a hexagonal arrangement.
Diffusion Tensor Imaging Acquisition
Data was acquired in a 7-T, 16cm bore Bruker Biospec console (Rheinstetten, Germany) using a 25mm quadrature Doty Scientific for transmission and reception. High-resolution scans were performed using a TE/TR of 22/425ms, FOV of 60×60×160mm3, for a nominal resolution of 125×125×372μm3. DTI was performed using a 3D diffusion-weighted spin-echo sequence, with a δ/Δ of 4/12msec, 2 averages, 20 directions with b of 2000s/mm2 (and one b of 0 image), and a scan time of 7 hours and 40 minutes.
Diffusion Tensor Imaging Analysis
Analysis was performed on a voxel-wise basis using a linear regression approach written in MATLAB (Mathworks).19 Fractional anisotropy was estimated from this analysis. Each slice was classified relative to the injury site (proximal to injury, within zone of injury, distal to injury), and a region-of-interest was drawn manually at each slice to measure the mean fractional anisotropy along each sciatic nerve. In addition, fiber tracts were reconstructed using the ExploreDTI Toolbox in MATLAB with an fractional anisotropy threshold of 0.4.20 Recovery of fractional anisotropy in the distal region with respect to the proximal region (ΔFA) was obtained by subtracting each individual fractional anisotropy value of the distal region from the mean fractional anisotropy in the proximal region of the same nerve. This analysis aimed to eliminate possible inconsistencies across samples. Also, normalization of each nerve’s distal fractional anisotropy value to its proximal value provides a quantifiable measurement of nerve recovery.
Behavioral Testing
We obtained measures of foot fault asymmetry score and sciatic function index, as described in previous studies.21 An index>−10 was considered as recovered, between −30 and −10 partially recovered and <−30 as not recovered.
Histology
Following MRI, samples were post-fixed in 2% paraformaldehyde/3% glutaraldehyde, counterstained with 1% OsO4 solution, dehydrated in increasing concentrations of ethanol, and then embedded in resin. Samples were sectioned at 1μm and stained with 1% toluidine blue for examination with light microscopy (Olympus Vanox-T AH-2).
Total axonal cross-sectional areas and axon counts were measured using Image Pro Plus 7.0 (Media Cybernetics, Bethesda, MD). Within each segment of each nerve, axon density was calculated in triplicate by manually counting axons within three randomly selected areas at 40x magnification, by a single examiner which was blinded to the experimental group. Total axons were calculated by multiplying each axon density by the nerve cross-sectional area measurement, and then averaging all 3 to avoid selection bias. Total axon counts were normalized by dividing distal measurements by its corresponding proximal measurements, except for the sham group, which the ratio was obtained by dividing the individual measurement by the average of all shams within each time point. Mean differences in axon counts across injury types were compared.
Statistical Analysis
Using R version 3.3.2, Mann-Whitney U test was performed to evaluate differences between sham, crush, and cut/repair injury types. A p-value < 0.05 were considered significant.
RESULTS
A total of 63 Sprague-Dawley rats underwent surgical intervention. They were prospectively divided into 3 groups; sham surgery, crush injury, vs complete transection with primary repair. The unbalanced number of our sample groups is the result of technical difficulties with prolonged fixative exposure, which resulted in some nerves unfit to be scanned. The 3 groups were compared via ex-vivo DTI performed in nerve samples at 1, 2, 4, and 12 weeks postoperatively. (See Figure, Supplemental digital content 1, which shows the Median fractional anisotropy (FA) of procedure groups at all time points. Sham (green), crush (red), and cut/repair (blue). At week 1, all three groups had the same values of fractional anisotropy. As time progressed, there was an increase in fractional anisotropy in all groups, which was more noticeable in the sham and crush groups as early as 2 weeks. At week 2 and 4, sham was higher than crush, p<0.05 (##,$ $). Cut/repair group differed from both sham and crush at all time points, p<0.05 (*, #, $, ^) and was not able to reach baseline values at 12 weeks, INSERT HYPER LINK) compares the median fraction anisotropy of all groups at different time points. In comparison to the crush and cut/repair groups, the sham group displayed the fastest increase in fraction anisotropy, reaching recovery and a plateau by week 4. As expected, the crush group exhibited the second fastest nerve recovery with a path very similar to the sham group, but full recovery was not achieved until week 12. Of note, at 1 week postoperatively, the distal fractional anisotropy between the sham, crush & cut/repair groups showed no statistically significant differences. In contrast, cut/repaired nerves were the slowest with almost no growth up to week 4. At 12 weeks, the distal mean fraction anisotropy values for cut/repair were approximately half that of the sham and crush groups.
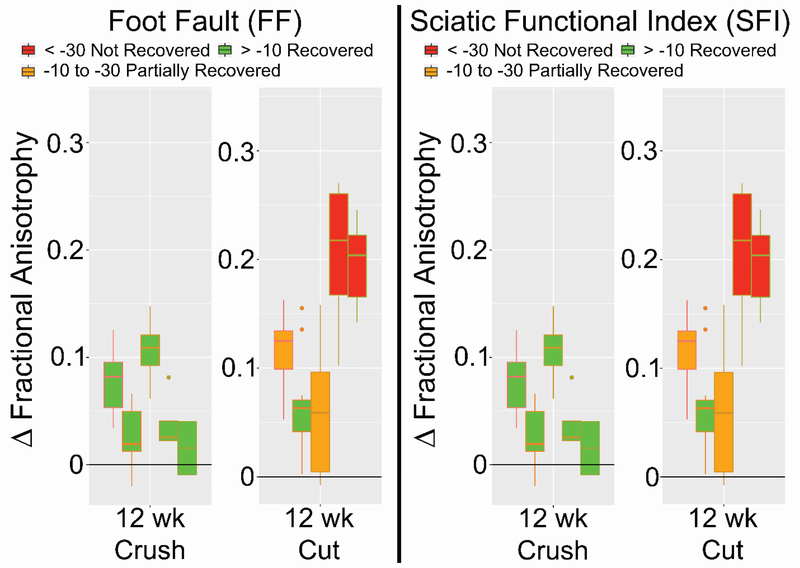
Verification of DTI metrics as a viable biomarker of nerve regeneration was attempted by correlating its results with behavioral studies for each nerve injury, as shown in Figure 1. At week 12, there was a clear correlation between FA and behavioral assessments, as recovered (green) and partially recovered (orange) nerves, expressed the lowest ΔFA. Non-recovered nerves (red) expressed the highest values of ΔFA and were only present in the cut/repair population at 12 weeks. Thus, a clear contrast in ΔFA values was noted between recovered and non-recovered nerves in this time period.
Figure 1.
The differential between proximal and distal fractional anisotropy (ΔFA) of crush nerves (bottom) and cut/repair nerves (top) compared to Foot Fault Asymmetry and Sciatic Function Index at different time points. All nerves assessed as recovered (> −10) or partially recovered (> −30) by the behavioral data are color-coded as green or orange, respectively. At 12 weeks, when regeneration and recovery are possibly achieved, in subjects that did not recover clinically there was a significant correlation between high ΔFA values and low clinical scores (< - 30 on Foot fault and SFI) in the cut/repair group represented by the color red.
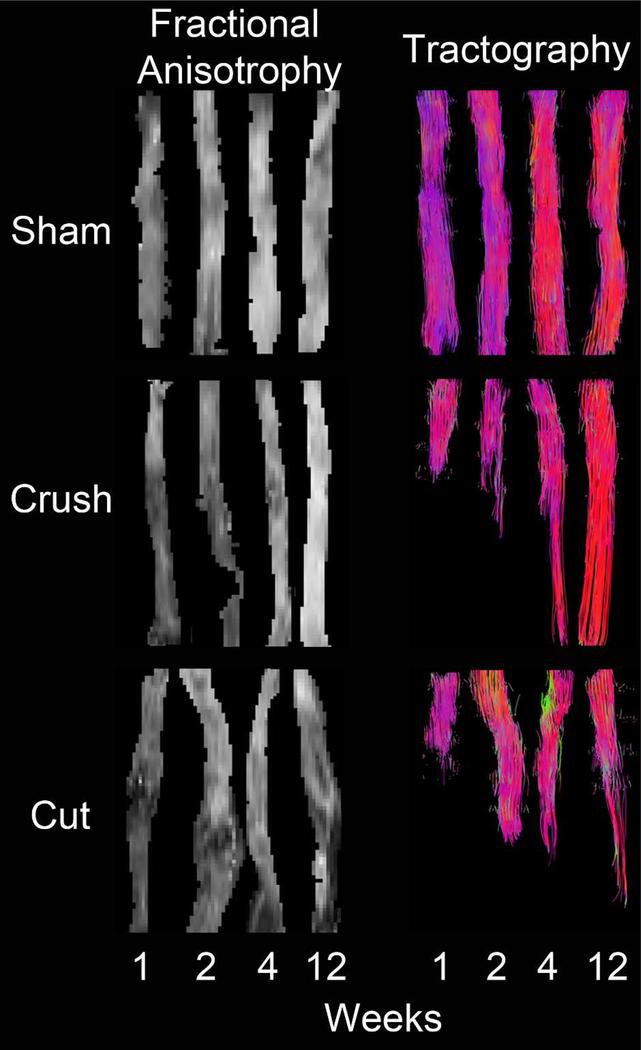
Maps of reconstructed fiber tracts with fraction anisotropy overlays, also known as tractography, of the sciatic nerves for each time point and intervention are depicted in Figure 2. In the sham nerves, continuous fiber tracts were noted, the fraction anisotropy values were homogeneously distributed along the nerves, and there was a gradual increase in fraction anisotropy with time until it reached full recovery at 4 weeks. Fiber tracts of crush nerves showed three different behaviors. First, a large decrease in fraction anisotropy was observed at the injury site that was noticeable at weeks 1, 2, and 4, but totally disappeared by 12 weeks. Second, the distal region fraction anisotropy values demonstrated a slight decrease at week 1, with a gradually recovery through weeks 2 and 4, reaching full recovery at 12 weeks. It was also noted that fraction anisotropy intensities in the proximal region were very similar to those of sham nerves at corresponding times. Third, fiber tracts demonstrated a continuous recovery of the fibers over time with a few fibers completely recovered by week 4, and total recovery by 12 weeks. However, for cut/repair nerves, the coaptation region showed a protuberance at weeks 1 and 2, with a marked decrease in the fraction anisotropy intensity distally. At weeks 4 and 12, the protuberance almost faded entirely; however, fraction anisotropy beyond the repair, never reached values of sham or crushed nerves. Additionally, at the distal region, the fraction anisotropy was very low at 1 week and recovery was not perceptible until week 12. Finally, tractography images showed that tracing of the new fibers began at week 4, and it was even more noticeable at week 12.
Figure 2.
Representative fraction anisotropy and tractography at each time point and divided by treatment. There was no disruption of tracing throughout the different time points in the sham group. For the crush cohorts, there was progression of tracings throughout the time points that can be translated as recovery. At 12 weeks, there was no difference between the sham and crush tractrographies. In the cut/repair group, there was tracing beyond the area corresponding to the coaptation, which correlates with regeneration.
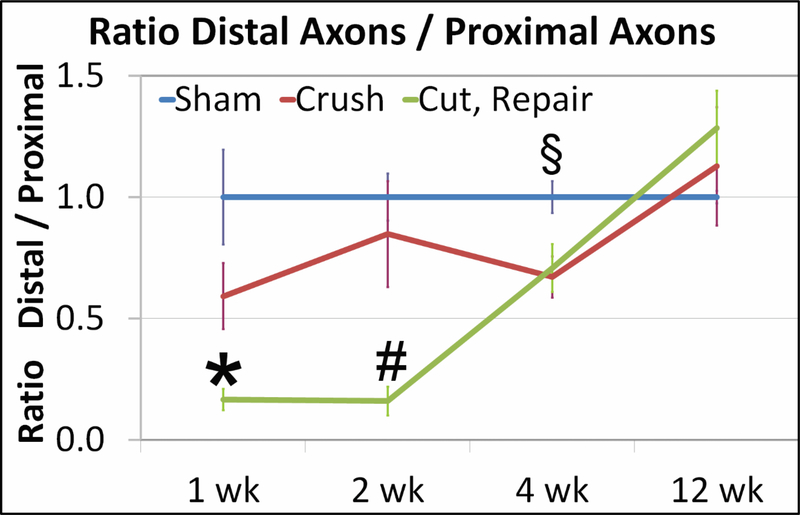
Histologically, in quantitative analysis (Figure 3), at weeks 1 and 2 there was a significant difference between sham and crush axon counts with the cut/repair group (p<0.05), while no difference existed between crush and sham axons. By week 4, there was a statistical difference between sham and both crush and cut/repair (p<0.05); however, no difference existed between crush and cut/repair. Finally, at 12 weeks there was no difference between any treatments.
Figure 3.
The graph depicts Distal/Proximal total axon ratios for each treatment group at each time point. The sham ratio (blue) is 1 because the amount of axons distally is the same amount as proximal. At week 1, there were statistical differences between cut/repair (green) versus sham and crush (red) groups, mostly due to Wallerian degeneration (*: p<0.05). At week 2, the significant discrepancies still remained (#: p<0.05). At 4 weeks, there was an increase in the ratio of cut/repair group, equalizing the crush group, but below the sham (§: p<0.05) (sham versus crush and cut). At 12 weeks, the intervention groups were higher than the sham, due to regeneration and recovery. No statististical significance was observed at this time point. Bar represents SE. Scale bar = 100μm.
DISCUSSION
This prospective animal study has quantified the ability of ex vivo DTI to stratify successful or failed surgical interventions after nerve injuries. Earlier work by Yamasaki et al. examined crush injuries in rabbit sciatic nerves and compared them with the normal contralateral nerves.22 They compared DTI results with clinical and histological data, and were able to correlate fractional anisotropy with nerve integrity. A decrease in fractional anisotropy in their earlier time points was attributable to myelin sheath disruption. Other studies have reported a decrease in fractional anisotropy in the central nervous system due to chronic degenerative disease, and in the peripheral nervous system due to compressive entities or Wallerian degeneration.17,23,24 Our analysis has demonstrated that inflammation and edema following the injury can affect the assessment of severity with this technique. This is attributed to the abnormal behavior of water in the different cellular compartments of the nerves, resulting in indistinguishable fraction anisotropy values in all 3 injury types at week 1. However, even in early stages after the injury, tractography can at least differentiate between sham and the other injury types (Figure 2).
Tractography is considered a qualitative tridimensional axonal representation that can reveal abnormalities beyond magnetic resonance resolution, and allows early noninvasive nerve regeneration monitoring, better than fractional anisotropy maps.25 At week 2, edema starts to diminish and slight differences between the injury types can be seen with fractional anisotropy. Lehmann et al. evaluated sciatic nerves in a murine study and showed a higher fractional anisotropy in the control and crush groups when compared to the transected group.13 In addition, Takagi et al. examined crush sciatic nerve injuries in rats with fractional anisotropy 5mm proximal to the injury epicenter, at the epicenter, and 5mm caudal to the injury. When compared to normal contralateral nerves, the injured nerves showed a drastic drop of fractional anisotropy at the epicenter 4 days after the injury, with recovery to baseline levels at 3 weeks. These results correlated with axon density and behavioral tests (e.g., leg muscle contraction); however, this work was limited to crush injuries only.17 Our findings suggest that the ideal time to distinguish between different types of nerve injury with DTI is at 4 weeks, when inflammation has subsided, crushed axonal recovery is mostly completed, and repaired nerves are beginning to regenerate. This may have major implications for nerve injury patients as this is a potentially powerful noninvasive tool for assessing failure of nerve recovery early after repair. For proximal injuries, this could allow for earlier detection of failed repairs before distal targets atrophy or become nonreceptive for re-innervation. This novel discovery could be animal specific and future studies must be designed to prove if this finding could be translated across species.
In our study, it was clear from the correlation of the behavioral and imaging data that all crush injuries returned to normal at 12 weeks. Conversely, clinical improvement of cut/repair injuries was not consistent, with nerves demonstrating a wide spectrum of recovery. After utilizing the differential fractional anisotropy between the distal and proximal to the injury, it was possible to correlate imaging with clinical recovery. This single parameter predicted which rats recovered clinically from both injuries at 12 weeks. Performing supplemental tractography also confirmed which nerves regenerated at that time point.
Previous studies advocated that an increase in fractional anisotropy was correlated with an increase in number of axons.14 Likewise, our data showed an increase in the number of axons when fractional anisotropy increased in all groups, but there were no statistical differences between the 3 groups at 12 weeks. At this time point, DTI recognized and classified in a noninvasive fashion the types of injury, regeneration, and even clinical recovery. An increase in axonal population was noted after inflammation subsided and regeneration or recovery was completed. In some cases, the number of axons did not correlate with behavioral improvement at 12 weeks. Riley et al. showed no clinical improvement at this same time period, despite an increased axon count in rat sciatic nerve treated with repair plus an axonal fusion agent. This could be explained by the failure of reestablishing a successful connection to the targeted muscle.26 Another theory could explain this phenomenon is the possibility of axonal sprouting trying to reach the target muscle. In the future we will try to follow this injured/repaired axons distally with tractography, trying to decipher this dilemma. Also, we will include other histomorphometric parameters in our subsequent studies; such as myelin thickness or percentage of nerve tissue. Gathering more information at the cellular level could enlighten our knowledge about this situation.
This study has some limitations that merit discussion. First, performing prolonged scans was only possible with rat sciatic nerves after harvest, which eliminated motion artifacts and prolonged use of anesthetics. Ideally, it will be more accurate to monitor the same injured subject through time, which we have developed a protocol for our ongoing human DTI/MRI trials. Additionally, DTI offers more parameters (e.g., axial diffusivity, radial diffusivity) that can also evaluate recovery other than fractional anisotropy. Further investigations are needed to determine the value of these DTI parameters compared to our findings.
In conclusion, our analysis demonstrated that following traumatic nerve injury; high-resolution DTI measurements could monitor regeneration by identifying injury type and surgical recovery in a rat model. To the best of our knowledge, this is the first study to date to correlate imaging findings with both behavioral and histologic evaluation in crush and cut/repair models. In addition, by calculating the recovery of fractional anisotropy in the distal nerve region with respect to the proximal region, we were able to eliminate inconsistencies between samples, minimize the diffuse effect of edema, and more accurately quantify nerve recovery. The DTI MRI technology has a great potential to efficiently predict non-invasively which nerve injuries might require an intervention. This is critically important for patients with a failed repair of a proximal nerve injury, in which an early intervention could avoid muscular atrophy.
Supplementary Material
Acknowledgments
Financial Disclosure:
This work was supported by U.S. Army Medical Research and Materiel Command, Contract Number W81XWH-15-JPC-8/CRMRP-NMSIRA, Grant number: MR150075. Additional support provided by NIH/NINDS R01 NS97821 (RDD).
Footnotes
Financial Disclosure:
“None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.”
REFERENCES
- 1.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45(1):116–122. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381–385. [DOI] [PubMed] [Google Scholar]
- 3.Moore AM, Wagner IJ, Fox IK. Principles of nerve repair in complex wounds of the upper extremity. Semin PlastSurg. 2015;29(l):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo-Galvan ML, Martinez-Ruiz FM, de la Garza-Castro O, Elizondo-Omana RE, Guzman-Lopez S. [Study of peripheral nerve injury in trauma patients]. Gac Med Mex. 2014;150(6):527–532. [PubMed] [Google Scholar]
- 5.Li A, Hokugo A, Yalom A, et al. A bioengineered peripheral nerve construct using aligned peptide amphiphile nanofibers. Biomaterials. 2014;35(31):8780–8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ova lie F, Patel A, Pollins A, et al. A simple technique for augmentation of axonal ingrowth into chondroitinase-treated acellular nerve grafts using nerve growth factor. Ann PlastSurg. 2012;68(5):518–524. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. [DOI] [PubMed] [Google Scholar]
- 8.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2007;4(3):316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vastamaki M, Kallio PK, Solonen KA. The results of secondary microsurgical repair of ulnar nerve injury. J HandSurg Br. 1993;18(3):323–326. [DOI] [PubMed] [Google Scholar]
- 10.Han D, Lu J, Xu L, Xu J. Comparison of two electrophysiological methods for the assessment of progress in a rat model of nerve repair. IntJ Clin Exp Med. 2015;8(2):2392–2398. [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu C The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. [DOI] [PubMed] [Google Scholar]
- 12.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann HC, Zhang J, Mori S, Sheikh KA. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol. 2010;223(l):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisaki S, Kawai Y, Umeda M, et al. In vivo assessment of peripheral nerve regeneration by diffusion tensor imaging. J Magn Reson Imaging. 2011;33(3):535–542. [DOI] [PubMed] [Google Scholar]
- 15.Stanisz GJ, Midha R, Munro CA, Henkelman RM. MR properties of rat sciatic nerve following trauma. Magn Reson Med. 2001;45(3):415–420. [DOI] [PubMed] [Google Scholar]
- 16.Boyer RB, Kelm ND, Riley DC, et al. 4.7-T diffusion tensor imaging of acute traumatic peripheral nerve injury. Neurosurg Focus. 2015;39(3):E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi T, Nakamura M, Yamada M, et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage. 2009;44(3):884–892. [DOI] [PubMed] [Google Scholar]
- 18.McAllister RM, Gilbert SE, Calder JS, Smith PJ. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J HandSurg Br. 1996;21(1):4–13. [DOI] [PubMed] [Google Scholar]
- 19.MATLAB Release 2016. In. Natick Massachusetts: Mathworks Inc; 2016. [Google Scholar]
- 20.Leemans A ExploreDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MR data. In. Vol 3536. Proceedings of the 17th annual Meeting of international Society for Magnetic Resonance in Medicine Honolulu, Hawaii: Curran Associates Inc; 2009. [Google Scholar]
- 21.Nguyen L, Afshari A, Kelm ND, et al. Bridging the Gap: Engineered Porcine-derived Urinary Bladder Matrix Conduits as a Novel Scaffold for Peripheral Nerve Regeneration. Ann Plast Surg. 2017;78(6S Suppl 5):S328–S334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamasaki T, Fujiwara H, Oda R, et al. In vivo evaluation of rabbit sciatic nerve regeneration with diffusion tensor imaging (DTI): correlations with histology and behavior. Magn Resort Imaging. 2015;33(1):95–101. [DOI] [PubMed] [Google Scholar]
- 23.Kimura-Ohba S, Yang Y, Thompson J, et al. Transient increase of fractional anisotropy in reversible vasogenic edema. J Cereb Blood Flow Metab. 2016;36(10):1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon T, Fung MM, Koch KM, Tan ET, Sneag DB. Peripheral nerve diffusion tensor imaging: Overview, pitfalls, and future directions. J Magn Reson Imaging. 2018;47(5):1171–1189. [DOI] [PubMed] [Google Scholar]
- 25.Khalil C, Budzik JF, Kermarrec E, Balbi V, Le Thuc V, Cotten A. Tractography of peripheral nerves and skeletal muscles. Ear J Radiol. 2010;76(3):391–397. [DOI] [PubMed] [Google Scholar]
- 26.Riley DC, Boyer RB, Deister CA, et al. Immediate Enhancement of Nerve Function Using a Novel Axonal Fusion Device After Neurotmesis. Annals of plastic surgery. 2017;79(6):590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.