Abstract
The impact of multisite acquisition on resting-state functional MRI (rsfMRI) connectivity has recently gained attention. We provide consistency values (Pearson's correlation) between rsfMRI connectivity maps of an adult volunteer (Csub) scanned 25 times over 3.5 years at 13 sites using the Canadian Dementia Imaging Protocol (CDIP, www.cdip-pcid.ca). This dataset was generated as part of the following article: Multivariate consistency of resting-state fMRI connectivity maps acquired on a single individual over 2.5 years, 13 sites and 3 vendors [1]. Acquired on three 3T scanner vendors (GE, Siemens and Philips), the Csub dataset is part of an ongoing effort to monitor the quality and comparability of MRI data collected across the Canadian Consortium on Neurodegeneration in Aging (CCNA) imaging network. The participant was scanned 25 times in the above-mentioned article: multiple times at six sites over a period of 2.5 years, and once at the remaining seven sites. Since then the participant was scanned an additional 45 times, allowing us to extend the dataset to 70 rsfMRI scans over a period of >4 years.
In addition, we provide intra- and inter-subject consistency values of rsfMRI connectivity maps derived from 26 adult participants belonging to the publicly released Hangzhou Normal University dataset (HNU1). All HNU1 participants underwent 10 rsfMRI scans over one month on a single 3T scanner (GE).
Connectivity maps of seven canonical networks were generated for each scan in the two datasets (Csub and HNU1). All consistency values, along with the scripts used to preprocess the rsfMRI data and generate connectivity maps and pairwise consistency values, have been made available on two public repositories, Github and Zenodo. We have also made available four Jupyter notebooks that use the provided consistency values to (a) generate interactive graphical summaries – 1 notebook, (b) perform statistical analyses - 2 notebooks, and (c) perform data-driven cluster analysis for the recovery of subject identity (i.e. rsfMRI fingerprinting) – 1 notebook. In addition, we provide two interactive dashboards that allow visualization of individual connectivity maps from the two datasets. Finally, we also provide minimally preprocessed rsfMRI data in Brain Imaging Data Standard (BIDS) format on all 70 scans in the extended dataset.
Keywords: Resting-state fMRI, Multisite, Consistency values, Fingerprinting
Specifications Table
| Subject area | Neuroscience |
| More specific subject area | Brain Imaging |
| Type of data | Human brain imaging, consistency estimates of functional connectivity, interactive visualization dashboard |
| How data was acquired | resting-state functional MRI (GE, Philips & Siemens 3T scanners) |
| Data format | Raw, preprocessed and analyzed data |
| Experimental factors | Repeated resting-state functional MRI scans |
| Experimental features | Repeated resting-state functional MRI scan of human participants at multiple sites in Canada, and a single site in China |
| Data source location |
Raw data: International Neuroimaging Data-Sharing Initiative at Child Mind Institute athttp://fcon_1000.projects.nitrc.org/indi/ Analyzed data: Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), Montreal, Canada |
| Data accessibility |
Raw Data - Structural and resting-state functional MRI data is available online at the following sites: Csub dataset: http://fcon_1000.projects.nitrc.org/indi/retro/SIMON.html HNU1 dataset: http://fcon_1000.projects.nitrc.org/indi/CoRR/html/hnu_1.html Minimally preprocessed resting-state functional MRI data http://fcon_1000.projects.nitrc.org/indi/retro/SIMON.html Analyzed data is available online at the following sites: Github:https://github.com/SIMEXP/cdip_human_phantomzenado:https://doi.org/10.5281/zenodo.3350885 |
| Related research article | Badhwar et al. “Multivariate consistency of resting-state fMRI connectivity maps acquired on a single individual over 2.5 years, 13 sites and 3 vendors”[1] |
Value of the data
-
•
Matlab scripts provided can be adapted to provide consistency values in other datasets
-
•
Minimally preprocessed resting-state fMRI data of the extended dataset of 70 scans (an additional 45 scans) can use used to conduct a larger multisite consistency study
-
•
Jupyter notebooks can be used to perform graphical visualization, statistical and fingerprinting analyses on consistency values derived from any neuroimaging pipeline
-
•
Consistency values can be compared or pooled with values from other datasets, when similarly processed with the code provided
1. Data
Raw data, specifically the T1-weighted (T1w) anatomical and T2*-weighted functional blood-oxygen level dependent (BOLD) MRI scans, for the Csub dataset have been made publicly available as part of the Single Individual volunteer for Multiple Observations across Networks (SIMON) MRI dataset1. T1-weighted anatomical and T2*-weighted functional BOLD scans from the HNU1 dataset are also publicly available2 as indicated in the Specifications Table. Scan parameters for the Csub and HNU1 datasets are described below in Sections 2.1 and 2.2, respectively.
Analyzed data release contains the following: (a) two csv files containing numerical consistency values from Csub and HNU1 datasets, (b) one csv file containing values for head motion, scan time, and temporal signal-to-noise ratio (c) eight Matlab scripts used to preprocess the resting-state functional MRI (rsfMRI) data, generate connectivity maps, and calculate pairwise Pearson's correlation (or consistency) values between connectivity maps, (d) four Jupyter notebooks, (e) two dashboards that provide interactive illustrations of our findings, and (f) minimally preprocessed rsfMRI data, using the NeuroImaging Analysis Kit (NIAK) pipeline, on all 70 rsfMRI scans in the extended dataset.
The Jupyter notebooks can be executed online via the binder platform3. One notebook (graphs.ipynb) generates the following interactive graphs: Csub intra-site, inter-scan consistency over time (2.5 years); Csub intra- and inter-vendor consistency; and HNU1 intra-individual consistency per network, and across all networks. The second notebook (stats_repro.ipynb) generates the statistics used to assess the consistency of individual rsfMRI measures within/between sites. The third notebook (stats_tsnr_time_motion.ipynb) generates the statistics used to assess the relationship of head motion, scan time, and tSNR (or temporal signal to noise ratio) with time and scanner vendors. The fourth notebook (stats_fingerprinting.ipynb) can be used to (a) generate the statistics used to assess the consistency of connectivity maps within/between subjects, and (b) to assess the ability of a data-driven cluster analysis to recover participant identity from connectivity maps.
Of the two dashboards released, one displays the connectivity maps of Csub's multiple retest visits for each of the seven canonical rsfMRI networks. The second dashboard displays the connectivity maps of HNU1 participants and Csub for each of the seven canonical rsfMRI networks. Further provided in both dashboards is the average connectivity map per network, and the atlas parcellation that was used to generate them.
2. Experimental Design, Materials and Methods
2.1. Canadian subject dataset (Csub)
All brain imaging data were acquired from a volunteer Csub: a healthy male with no history of (a) psychiatric and/or neurological illnesses, (b) psychoactive drug usage, or (c) contraindications to MRI. Csub was 42 years old at the start of data collection (2014). The data was acquired as part of an ongoing effort to monitor the quality and comparability of MRI data collected across the Canadian Consortium on Neurodegeneration in Aging (CCNA4) imaging network. The schedule of visits did not follow a strict design, with an approximate goal of one visit a year, starting at site qualification. In total, the participant underwent 25 scanning sessions at 13 CCNA imaging sites (only data from 24 scans were used); using scanners from three manufacturers (Philips, Siemens and GE), see Graphical Abstract in [1]. For additional information, please refer to Badhwar et al. [1]. Note that following the above-mentioned 25 scans, Csub underwent an additional 45 rsfMRI scans, bringing the total to 70 rsfMRI scans at 23 CCNA imaging sites, over a period of >4 years. Ethics approval was obtained from the institutional review board of each participating institution prior to scanning. Informed consent was obtained from the subject for the overall scans and before every scan session.
Anatomical scans included T1w imaging to assess fine anatomical detail with high resolution (voxel size = 1.0 × 1.0 × 1.0 mm3) and acceleration factor of 2 (Siemens: MP-RAGE; GE: FSPGR; Philips: T1-TFE). Functional T2*-weighted images were obtained using a BOLD sensitive single-shot echo-planar (EPI) sequence. Detailed information on the imaging parameters is provided in Badhwar et al. [1]. The full dataset containing T1w anatomical and 70 functional T2*-weighted scans has been made publicly available in Brain Imaging Data Structure (BIDS, https://bids.neuroimaging.io/) format as part of the Single Individual volunteer for Multiple Observations across Networks (SIMON) MRI dataset.
2.2. Publicly available Hangzhou Normal University dataset (HNU1)
The HNU1 dataset includes 30 healthy adults 20-30 years of age (mean age 24.4 years), each receiving 10 scans across one month (one scan every three days) on a single 3T GE Discovery MR750 scanner [2]. Anatomical scans included T1w imaging (voxel size = 1.0 × 1.0 × 1.0 mm3 and acceleration factor of 2). Functional T2*-weighted images were obtained using a 10 min BOLD-sensitive single-shot EPI sequence: 3.4 mm isotropic voxels, 90 deg flip angle, 64 × 64 matrix size, 30 ms TE, 2000 ms TR, 300 time points5.
2.3. Computational environment
The datasets were preprocessed and analyzed using NIAK, version 1.1.3 (NIAK-COG)6 [3]), executed within an Ubuntu 16.0.4 Singularity7 container, running GNU Octave8 version 4.2.1, and the MINC toolkit9 version 1.9.15. Python packages used in the Jupyter notebooks include Numpy [4], Pandas [5], Matplotlib [6], Scikit-learn [7], SciPy [8], Seaborn10 and StatsModel [9]. Interactive plots in the Jupyter notebooks were generated using Plotly11. All notebooks can be executed online via the binder platform12, and run in a docker container13 built from a public configuration file.
2.4. Pre-processing and quality control of MRI data
MRI data underwent preprocessing and quality control as described in Badhwar et al. [1]. The minimally preprocessed Csub (25 scans) and Csub ‘extended’ (70 scans) resting-state data has been made publicly available in BIDS, a simple and easy to adopt way of organizing neuroimaging data [10]. Although BIDS does not make a formal recommendation for the organization of derivatives beyond that they are stored separately from the raw data [10], we have attempted to provide the minimally processed data in a format that is in accordance with BIDS standards. Adhering as close as possible to BIDS file types and naming conventions for derivatives allows for intuitive file identification and ease of file use for additional processing.
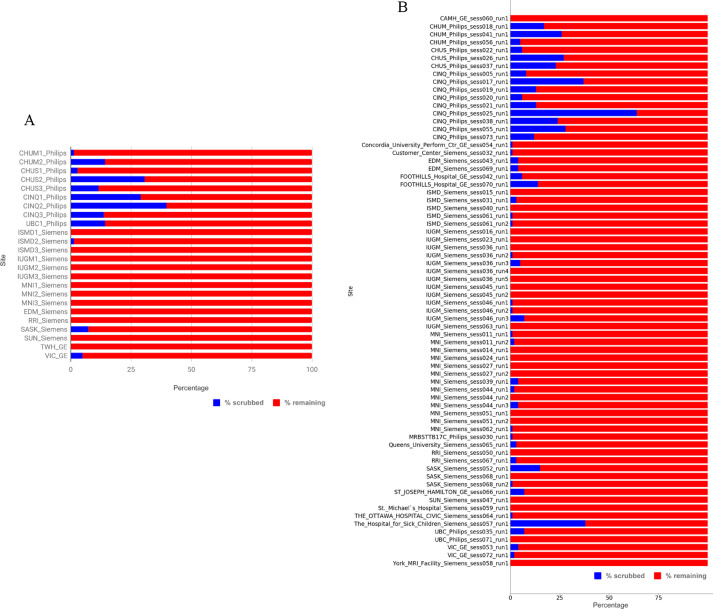
Figure 1A shows per scan, the percent of volumes scrubbed due to excessive motion (displacement > 0.5 mm) and the percent of volumes remaining for generation of connectivity maps from 24 rsfMRI scans used in the original dataset. In Figure 1B, we show the number of volumes scrubbed for the larger dataset released in this article (70 rsfMRI scans in total).
Figure 1.
Volumes: percent scrubbed and remaining per scan (a) 24 rsfMRI scans used in the original Csub dataset (b) extended Csub dataset consisting of 70 rsfMRI scans.
2.5. Connectivity maps
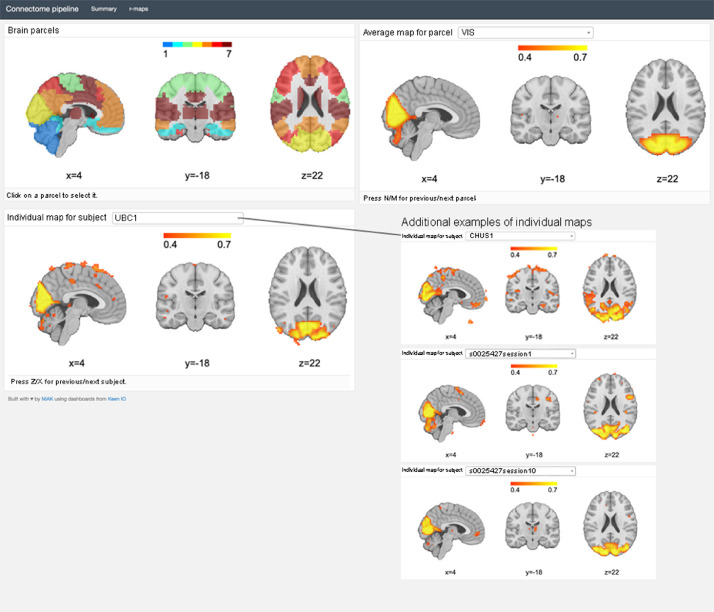
Using NIAK's connectome pipeline, for each rsfMRI scan (from both Csub and HNU1 datasets), we computed voxel-wise connectivity maps associated with each of the seven network templates defined in the MIST group-level functional brain atlas [11]. In depth details are described in Badhwar et al. [1]. Figure 2 shows how an individual connectivity map (from both Csub and HNU1 datasets) can be viewed on a dashboard for any of the seven canonical rsfMRI networks.
Figure 2.
Interactive dashboard view of an individual connectivity map (Csub rsfMRI scan UBC) for the visual network, along with examples of a few other connectivity maps (bottom right). The dashboard allows for interactive viewing of connectivity maps of all seven rsfMRI networks per scan data, for all scan data. Also shown in the dashboard is the average connectivity map per network, and the MIST atlas parcellation that was used to generate them. x, y, and z Montreal Neurological Institute (MNI) coordinates are given for sagittal, coronal, and axial slices.
2.6. Consistency values and statistical analyses
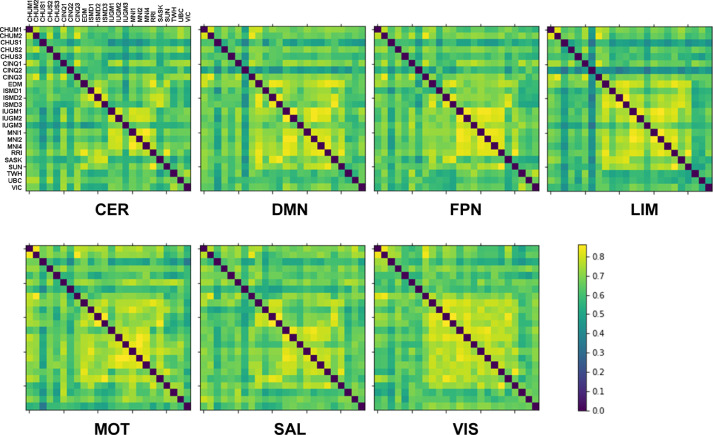
For each of the seven rsfMRI networks, a scan by scan similarity (Pearson's correlation) matrix was generated to summarize the consistency of connectivity maps present in the Csub (Figure 3) and Csub+HNU1 datasets.
Figure 3.
Matrix of scan by scan similarity (Pearson's correlation) for each network for the Csub dataset - consisting of 24 rsfMRI scans. Abbreviations: cerebellar (CER), default-mode (DMN), frontoparietal (FPN), limbic (LIM), motor (MOT), salience (SAL), and visual (VIS). The more yellow the color, the higher the similarity value.
To statistically compare these consistency values we implemented two general linear models (GLMs): (1) to assess consistency of individual rsfMRI measures within/between sites, based only on data from the Csub dataset; and (2) to assess consistency of individual rsfMRI measures within/between subjects, based on data from Csub and the HNU1 datasets. Table 1 provides, per network, the outputs of two within/between sites GLM analyses with the following explanatory variables: (Table 1A) time between scans, expressed in years and corrected to a zero mean; dummy variables encoding intra-vendor comparisons (three covariates: GE, Siemens and Philips); dummy variables encoding intra-site comparisons (six covariates: CHUM, CHUS, CINQ, ISDM, IUGM, MNI; the other sites did not have multiple retest data available); and (Table 1B) All variables mentioned in 1A as well as frame displacement (FD); temporal signal-to-noise ratio (tSNR); and number of volumes remaining after scrubbing (n_vols).
Table 1.
Outputs of the within/between sites GLMs analyses
 |
A linear mixture of the explanatory variables were adjusted on the inter-scan consistency measures (dependent variable) using ordinary least squares, for each network separately. Additional information about the statistical analysis can be obtained from Badhwar et al. [1].
2.7. Fingerprinting of HNU1 participants and Csub
We assessed the ability of a simple data-driven cluster analysis to recover the identity of participants (i.e. rsfMRI fingerprinting) based on connectivity maps of a single network, mixing the Csub single participant with the HNU1 participants. The fingerprinting analysis is described in depth in Badhwar et al. [1]. The fingerprinting procedure was repeated B=10000 times using random scan selections, and independently for each network. The average accuracy of fingerprinting for a given subject and network was derived as the proportion of successful fingerprinting experiments.
Acknowledgements
We would like to thank Dr Bratislav Misic for suggesting the fingerprinting experiment, as well as the CCNA LORIS platform (Imaging, Database & Information Technology) for organizing the data. A.B. is currently supported by a Canadian Institute for Health Research (CIHR) Postdoctoral Fellowship (#152548), the CCNA, and the Courtois Foundation. At the start of the project A.B was supported by the Alzheimer Society of Canada Postdoctoral Fellowship. P.B. is supported by the CCNA and the Courtois Foundation. Financial support for I.C. and O.P. was obtained from the Alzheimer's Society of Canada (#13-32), the CIHR (#117121), and the Fonds de recherche du Québec – Santé / Pfizer Canada - Pfizer-FRQS Innovation Fund (#25262). J.V. is supported by a Vanier Canada Graduate Studies Doctoral scholarship. S.D. is a Research Scholar from the Fonds de recherche du Québec – Santé (#30801). The Consortium d'identification précoce de la maladie d'Alzheimer – Québec is financed through the Fonds de recherche du Québec – Santé / Pfizer Canada Innovation Fund (#25262). The Canadian Consortium on Neurodegeneration in Aging is supported by a grant from the Canadian Institutes of Health Research with funding from several partners including the Alzheimer Society of Canada, Sanofi, and Women's Brain Health Initiative.
Footnotes
References
- 1.Badhwar A., Collin-Verreault Y., Orban P., Urchs S., Chouinard I., Vogel J., Potvin O., Duchesne S., Bellec P. Multivariate consistency of resting-state fMRI connectivity maps acquired on a single individual over 2.5 years, 13 sites and 3 vendors. Neuroimage. 2019 doi: 10.1016/j.neuroimage.2019.116210. [DOI] [PubMed] [Google Scholar]
- 2.Zuo X.-N., Anderson J.S., Bellec P., Birn R.M., Biswal B.B., Blautzik J., Breitner J.C.S., Buckner R.L., Calhoun V.D., Castellanos F.X., Chen A., Chen B., Chen J., Chen X., Colcombe S.J., Courtney W., Craddock R.C., Di Martino A., Dong H.-M., Fu X., Gong Q., Gorgolewski K.J., Han Y., He Y., He Y., Ho E., Holmes A., Hou X.-H., Huckins J., Jiang T., Jiang Y., Kelley W., Kelly C., King M., LaConte S.M., Lainhart J.E., Lei X., Li H.-J., Li K., Li K., Lin Q., Liu D., Liu J., Liu X., Liu Y., Lu G., Lu J., Luna B., Luo J., Lurie D., Mao Y., Margulies D.S., Mayer A.R., Meindl T., Meyerand M.E., Nan W., Nielsen J.A., O'Connor D., Paulsen D., Prabhakaran V., Qi Z., Qiu J., Shao C., Shehzad Z., Tang W., Villringer A., Wang H., Wang K., Wei D., Wei G.-X., Weng X.-C., Wu X., Xu T., Yang N., Yang Z., Zang Y.-F., Zhang L., Zhang Q., Zhang Z., Zhang Z., Zhao K., Zhen Z., Zhou Y., Zhu X.-T., Milham M.P. An open science resource for establishing reliability and reproducibility in functional connectomics. Sci Data. 2014;1 doi: 10.1038/sdata.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellec P., Carbonell F.M., Perlbarg V., Lepage C., Lyttelton O., Fonov V., Janke A., Tohka J., Evans A.C. Proceedings of the 17th International Conference on Functional Mapping of the Human Brain. 2011. A neuroimaging analysis kit for Matlab and Octave; pp. 2735–2746. [Google Scholar]
- 4.Oliphant T.E. Trelgol Publishing; USA: 2006. A guide to NumPy. [Google Scholar]
- 5.McKinney W. Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference; Austin, TX; 2010. pp. 51–56. [Google Scholar]
- 6.Hunter J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007;9:90–95. [Google Scholar]
- 7.Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V., Vanderplas J., Passos A., Cournapeau D., Brucher M., Perrot M., Duchesnay É. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 8.E. Jones, T. Oliphant, P. Peterson, others. SciPy: Open source scientific tools for Python. 2001, URLHttp://www.Scipy.Org. (2016).
- 9.Seabold S., Perktold J. Statsmodels: Econometric and statistical modeling with python, in: Proceedings of the 9th Python in Science Conference. SciPy society Austin. 2010:61. [Google Scholar]
- 10.Gorgolewski K., Auer T., Calhoun V. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data. 2016;3 doi: 10.1038/sdata.2016.44. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urchs S., Armoza J., Benhajali Y., St-Aubin J., Orban P., Bellec P. MIST: A multi-resolution parcellation of functional brain networks. MNI Open Res. 2017;1:3. [Google Scholar]